Abstract
Hemicellulose from a wide range of sources and abundant reserves shows good biocompatibility, degradability, and renewability. Because of its low degree of polymerization and a large amount of hydroxyl groups, hemicellulose-based films exhibit low strength and tend to be hydrophilic, which hinders some potential applications. Therefore, hemicellulose-based films with improved strength and barrier properties are needed. In this study, nano-SiO2 was incorporated into polyvinyl alcohol/xylan matrix for the purpose of preparing an inorganic-organic hybrid composite film with elevated mechanical and barrier performance. The addition of nano-SiO2 can serve this purpose. A 1% nano-SiO2 loading resulted in an increase of contact angle of the composite film from 89.6° to 110.4°, an increase of the tensile strength from 11.2 to 14.8 MPa, and a decrease of oxygen permeability from 1.83 to 0.27 (cm3×µm)/(m2·d·kPa), which corresponds to a contact angle that was increased by 23%, tensile strength increased by 32%, and oxygen permeability decreased by 85%. These results indicated that the nano-SiO2 modified xylan film might have great application prospects as a barrier film in food packaging.
Download PDF
Full Article
Nano-SiO2-modified Xylan-PVOH-based Composite Films: Mechanical and Barrier Properties Investigation
Guoshuai Liu,a Kang Shi,a Hui Sun,a,b,* Biao Yang,a and Yunxuan Weng a,b,*
Hemicellulose from a wide range of sources and abundant reserves shows good biocompatibility, degradability, and renewability. Because of its low degree of polymerization and a large amount of hydroxyl groups, hemicellulose-based films exhibit low strength and tend to be hydrophilic, which hinders some potential applications. Therefore, hemicellulose-based films with improved strength and barrier properties are needed. In this study, nano-SiO2 was incorporated into polyvinyl alcohol/xylan matrix for the purpose of preparing an inorganic-organic hybrid composite film with elevated mechanical and barrier performance. The addition of nano-SiO2 can serve this purpose. A 1% nano-SiO2 loading resulted in an increase of contact angle of the composite film from 89.6° to 110.4°, an increase of the tensile strength from 11.2 to 14.8 MPa, and a decrease of oxygen permeability from 1.83 to 0.27 (cm3⋅µm)/(m2·d·kPa), which corresponds to a contact angle that was increased by 23%, tensile strength increased by 32%, and oxygen permeability decreased by 85%. These results indicated that the nano-SiO2 modified xylan film might have great application prospects as a barrier film in food packaging.
DOI: 10.15376/biores.18.2.4195-4211
Keywords: Hemicellulose; Nano-SiO2; Mechanical properties; Barrier properties
Contact information: a: College of Chemistry and Materials Engineering, Beijing Technology and Business University, Beijing 100048, China; b: Beijing Key Laboratory of Quality Evaluation Technology for Hygiene and Safety of Plastics, Beijing Technology and Business University, Beijing 100048, China;
* Corresponding author: sunhui@th.btbu.edu.cn; wyxuan@th.btbu.edu.cn
GRAPHICAL ABSTRACT

INTRODUCTION
At a time of increasingly scarce global petrochemical resources and environmental problems caused by the widespread use of petroleum-based materials, the development and utilization of renewable and degradable biomass resources is urgent (Thakur et al. 2014; Tayeb et al. 2018; Shao et al. 2020). Biomass resources are natural energy carriers that store solar energy in the form of chemical energy. They are the most abundant renewable resources on the planet, including crop straw, wood, aquatic plants, etc. The effective development of biomass resources is expected to meet human demand for energy, fine chemicals, and materials. With regard to food packaging, commonly used packaging materials include glass, plastic, and metal, etc. The extensive use of conventional plastic materials inevitably produces a lot of packaging waste (Nesic et al. 2020). Therefore, the development of biodegradable or bio-based materials as packaging alternatives is an effective method to tackle the problem (Asgher et al. 2020).
Lignocellulosic biomass is mainly composed of cellulose, hemicellulose, and lignin, among which hemicellulose is the second most abundant polysaccharide. Hemicellulose is a main component of plant cell walls, accounting for 20 to 35% of the total weight (Pauly and Keegstra 2008). Its properties, including good biocompatibility, degradability, and renewability, give hemicellulose a natural advantage in food packaging. However, its low degree of polymerization and the large number of hydroxyl groups in the main chain and side groups (Peng et al. 2011; Escalante et al. 2012) lead to poor mechanical and barrier properties, which are disadvantages for food packaging (Hansen and Plackett 2008). Therefore, physical or chemical modification of hemicellulose has long been a focus of research. Plasticizers and reinforcing agents improve the film-forming properties and mechanical properties of hemicellulose-based films. Commonly used plasticizers include glycerin and sorbitol (Mikkonen et al. 2012). Some chemical modification methods of hemicellulose include esterification (Egues et al. 2014; Gordobil et al. 2014), etherification (Hartman et al. 2006a; Velkova et al. 2015), crosslinking (Zoldners and Kiseleva 2013; Azeredo et al. 2015), and grafting (Borjesson and Westman 2016; Du et al. 2018).
Xylan is a major component of hemicellulose. This polar polymer with good oxygen barrier properties (Hartman et al. 2006b) has been studied for food packaging applications. Good mechanical and gas barrier properties are among the requirements for food packaging films to ensure the integrity and safety of food (Nesic et al. 2020). As mentioned earlier, hemicellulose films still have some disadvantages with regard to its use as food packaging, such as poor mechanical properties, high hydrophilicity, or poor barrier properties. In this context, extensive research has been directed to enhancing these properties of hemicellulose-based composite films (Reddy et al. 2013). An example of such attempts was the addition of nanoparticles. Xu et al. (2019) used nanocellulose to improve the mechanical properties of hemicellulose-based films. Chen et al. (2015) found improvements in barrier properties of hemicellulose-based composite films by blending with montmorillonite. At present, nano-silica (nano-SiO2) has received extensive attention in the field of food packaging. For example, Zhang et al. (2018) prepared nano-silica/potato starch films; Kariminejad et al. (2022) studied the effect of nano-silica on the structure and physicochemical properties of polyvinyl alcohol (PVOH)/chitosan composite films.
Although nanoparticles could be used to improve the mechanical properties of hemicellulose films, intended for use as food packaging materials, the safety of nanomaterials has been considered a matter of concern. Packaging materials that come into contact with food should be inactive to ensure that they do not adversely affect food quality and human safety (Sothornvit 2019). The risk of nanomaterials to human body can be expected to depend on the size, morphology, toxicity, migration rate, and consumption of nanomaterials, but such issues are still under debate. Recently, the European Food Safety Agency (EFSA) issued a risk assessment guide on the application of nanoscience and nanotechnology in the food and feed chain in human and animal health. The physical and chemical properties, exposure assessment and hazard characterization of nanomaterials were introduced (More et al. 2021).
Nano-SiO2 is a non-toxic, odorless, amorphous white powder. Nano-SiO2 has the advantages of high stability and high food safety (Yu et al. 2012). Because nano-SiO2 is cheaper than most other nanoparticles and is derived from readily available natural resources, it can be produced in existing production facilities. Nanocomposites show broad application prospects in food processing and preservation because of their lower cost and simpler preparation than current common multilayer food packaging (Mitrano et al. 2015; Zhang et al. 2016). Because of its high surface energy, nano-SiO2 has a strong tendency to aggregate in water, but the inorganic anionic surfactant sodium hexametaphosphate (SHMP) changes the surface charge distribution of nanoparticles (Aruna et al. 2014), reduces the aggregation of nanoparticles, and improves the dispersion in the hemicellulose-based composite film.
This article investigated xylan-based films for use in food packaging. To this end, polyvinyl alcohol (PVOH) was used as co-matrix and nano-SiO2 was incorporated to form a physically cross-linked structure, using sorbitol as plasticizer. A solution-casting method was used to prepare a PVOH/xylan composite film. The effects nano-SiO2 on the surface hydrophilicity, mechanical properties and gas barrier properties of the composite film were studied.
EXPERIMENTAL
Beech xylan (Mw is 20000 to 30000 g/mole, Shanghai Yuanye Bio-Technology Co., Ltd., Shanghai, China), PVOH (polyvinyl alcohol with a degree of polymerization of 1700 and alcoholysis degree 99%, Shanghai Titan Scientific Co., Ltd., Shanghai, China), D-sorbitol (Mw is 182.17 g/mole, particle size 50 nm, Shanghai Yuanye Bio-Technology Co., Ltd., Shanghai, China), nano-SiO2 (Mw is 60.08, Shanghai Macklin Biochemical Technology Co., Ltd., Shanghai, China), sodium hexametaphosphate (Mw is 611.77 g/mole, Fuchen Chemical Reagent Co., Ltd., Tianjin, China) were used as received without further purification.
Preparation of Nano-SiO2 Modified Hemicellulose-Based Composite Films
PVOH was completely dissolved in 0.05% sodium hexametaphosphate solution at 95 °C. A predetermined amount of nano-SiO2 (0 to 2%, weight ratio, based on the total weight of PVOH and beech xylan) was dispersed in a 0.05% sodium hexametaphosphate solution, and then sonicated for 30 min at room temperature. The PVOH solution was cooled to 75 °C, and xylan and sorbitol were added. The mixture was stirred for 15 min, and nano-SiO2 dispersion was added dropwise. After reaction at 75 °C for 2 h, the mixture was cooled to room temperature and sonicated for 30 min. The mixture was poured into a polystyrene dish (13 cm × 13 cm) and dried under ambient conditions to form a film. The composition of each film is shown in Table 1. Before all measurements, the dried films were placed in an oven at 25 °C for 48 h.
Table 1. Nomenclature and Composition of Films
Analytical Methods
Fourier transform infrared spectroscopy (FT-IR) was performed with a Nicolet LSJ20 infrared spectrometer (Waltham, MA, USA) with scanning range of 440 to 4000 cm-1, 32 scans, and a resolution of 4 cm-1.
For scanning electron microscope (SEM) analysis, the film sample was cut into a small square piece, and a thin layer of gold was plated on the surface. The surface morphology of the film was observed with a scanning electron microscope (Quanta 250 FEG, FEI, Hillsboro, OR, USA) using an acceleration voltage of 10 kV.
The X-ray diffraction (XRD) analysis of xylan and nano-SiO2 modified and PVOH/xylan composite films were performed by using an X-ray diffractometer (D8 Advance, AXS, Bruker, Karlsruhe, Germany). The diffraction angle (2θ) varied in the range of 10 to 80° at a scanning speed of 5°/min.
The contact angle of the films was measured using a contact angle measuring instrument (OCA35, Data Physics Instruments, Charlotte, NC, USA). Water droplets of 2 μL were dropped on the surface of the film. The instrument software was used to capture the image of the droplet, and the contact angle was calculated from the image. Five different positions of each sample were measured, and the average value was reported as the contact angle of the sample (Zhao and Jiang 2018).
According to standard GB/T1040.2-2006 (2006), the film was cut into a rectangular sample of 10 mm × 80 mm. The thickness of the sample was measured using a thickness gauge. A microcomputer-controlled electronic universal testing machine (CMT6104, MTS Systems Co. Ltd., China) was used to test the tensile properties of the samples at a tensile speed of 5 mm/min with the distance between the clamps set at 60 mm. 5 rectangular specimens were cut from each film. Tensile strength and elongation at break of the film were the average of the test results of the 5 rectangular specimens.
According to China national standard GB/T 1038-2000 (2000), the oxygen permeability of the film was measured with a differential pressure gas permeameter (VAC-V2, Labthink) after the film sample was cut into slices with a diameter of 10 cm. Measurements were performed at 23 °C under relative humidity of 50%. The average value of 3 samples was taken.
Thermogravimetric analysis (TGA) was conducted on a thermogravimetric analyzer (STA7200, Hitachi, Tokyo) in temperature range of 40 to 600 °C with a heating rate of 20 °C/min, and a nitrogen flow rate of 20 mL/min.
RESULTS AND DISCUSSION
Preparation of Xylan-based Film
Xylan has a short molecular chain, low molecular weight, high glass transition temperature, and poor film-forming properties (Hanani et al. 2013). Blending xylan with other polymers improves its film-forming properties. Polyvinyl alcohol (PVOH) is a linear polymer containing a large number of hydroxyl groups. PVOH has high polarity and is suitable for blending with natural polymers (Chiellini et al. 2001). Its good mechanical properties, resistance to organic solvents, heat resistance, and gas barrier properties (Tang and Alavi 2011) offer this polymer unique advantages for food packaging. Incorporation of PVOH as co-substrate with other biopolymers has been reported.
Mixing PVOH with xylan to prepare a film can increase the possibility of application of xylan as film materials. Gao et al. (2014) blended xylan with PVOH to prepare composite films. Sorbitol was added to hemicellulose as a plasticizer to improve the processability of hemicellulose and the flexibility of hemicellulose-based composite films.
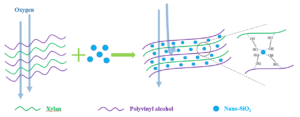
Sodium hexametaphosphate was used to reduce the surface energy of nano-SiO2 particles, and an ultrasonic disperser was also used to improve the dispersibility of nano-SiO2 in the film, as shown in Fig. 1.
Fig. 1. Physical cross-linking reaction of the matrix (PVOH-Xylan) and nano-SiO2
Structural Analysis
The FT-IR spectra of the nano-SiO2 modified hemicellulose film are shown in Fig. 2. The peak at 3278 cm-1 is attributed to O–H stretching vibration in xylan and PVOH. The peak at 2939 cm-1 is assigned to the alkane C–H stretching vibration. The peak located at 1617 cm-1 originated from the stretching vibration of –COO– of uronic acid in hemicellulose (Chen et al. 2015). The stretching vibration of C–O accounts for the peak at 1037 cm-1.
Fig. 2. FT-IR spectra of nano-SiO2 modified xylan films
After the addition of nano-SiO2, peaks at 779 cm-1 and 462 cm-1 emerged in the H-0.4 to H-2 composite films, which are an indication of Si–O–Si bond stretching vibration and bending vibration absorption (Rodgers et al. 2008). Another observation was the shift of hydroxyl absorption peak from 3278 cm-1 to higher (3299 cm-1) in the nano-SiO2/xylan/PVOH composite film, indicating the occurrence of hydrogen bonding interaction between the nano-SiO2 particles and the film matrix.
The surface morphology of the hemicellulose-based composite film with different addition of nano-SiO2 is shown in Fig. 3. The film without nano-SiO2 addition (H-0) had a relatively smooth surface, with good compatibility between PVOH and xylan. With nano-SiO2 addition, particle-shaped protrusions appeared on the surface of composite films, displaying rough surface morphology.
Fig. 3. SEM images of nano-SiO2 modified xylan films
At an addition below 1.2%, nano-SiO2 particles were dispersed in the film relatively uniformly. At a loading of 1.5%, nano-SiO2 particles showed a certain degree of aggregation on the surface of the film, with the appearance of large size oval particles observed. At higher SiO2 loading of 2%, the particles agglomeration phenomenon became more obvious. The higher the content of nano-SiO2, the more attractive the nanoparticles were to each other. The formation of larger and more irregular clumps (Phothisarattana and Harnkarnsujarit 2022) resulted in an increased surface roughness. For non-homogeneous nanocomposites, the increase of the aggregate size of nanoparticles reduced the mechanical and barrier properties of the films (Kanmani and Rhim 2014; Dehghani et al. 2019).
The X-ray diffraction patterns of xylan, PVOH, nano-SiO2, and hemicellulose composite films are shown in Fig. 4. The characteristic diffraction peak of xylan in hemicellulose composite films shifted from 2θ = 18.6° to 2θ =21.1°, and that of PVOH shifted from 2θ =19.6° to 2θ =17.7°. This may be due to the change in crystal structure of the two polymers through hydrogen bonding during the formation of hemicellulose composite films (Liu et al. 2019b). Nano-SiO2 had a wide diffraction peak at 2θ =21.9°, indicating that it was amorphous (Tavakolian et al. 2021). After the addition of nano-SiO2, no new crystallization peak appeared in the composite films H-1 and H-2, but the crystallization peak intensity of xylan/PVOH matrix at 2θ = 17.7° and 21.1° decreased compared with that of H-0. PVOH and xylan were semi-crystalline due to the hydroxyl groups on the side chains. When the nano-SiO2 interacted with the hydroxyl groups of the film matrix, the number of hydroxyl groups for forming intermolecular hydrogen bonds of xylan and PVOH decreased, leading to the decrease in crystallinity (Liu et al. 2019a).
Fig. 4. XRD patterns of PVOH, xylan and nano-SiO2 (a) and nano-SiO2 modified xylan films (b)
Surface Wettability
Advancing contact angles of the composite films were measured for evaluation of surface hydrophobicity. As shown in Fig. 5, the contact angles of H-0, H-0.4, H-0.7, H-1, H-1.2, H-1.5, and H-2 were 89.6°, 101.3°, 105.9°, 110.4° 105.1°, 100.2°, and 93.4°, respectively.
Film surfaces with contact angles greater than 90° can be considered as hydrophobic surfaces. It can be seen from the figure that the advancing contact angle of the composite film was increased after nano-SiO2 addition, and the hemicellulose-based composite film was a hydrophobic film. The content of nano silica increased from 0 to 1%, and the contact angle increased from 89.6 ° to 110.4 °. The change trend of the contact angle indicated that nano-SiO2 was able to increase the surface hydrophobicity of the hemicellulose-based composite film. After adding nano-SiO2 particles, some of the nano-SiO2 particles appeared on the surface of the film, which might form a large number of micro/nano structures, increasing the surface roughness of the composite film (Song and Zheng 2013). At this point, the rough depressions on the film surface might be narrow and deep enough that each depression seemed bottomless, similar to a porous surface (Cassie and Baxter 1944; Hubbe et al. 2015). The depression between the nano-SiO2 particles were filled with air, and the contact area between the film surface and the water droplets was further reduced. The tension between the surface of the film and the air layer was very low, which prevented the penetration of water droplets on the surface of the film (Mohammadi et al. 2012). However, when the amount of nano-SiO2 exceeds 1%, the surface of the film was not uniform due to the agglomeration of nanoparticles on the surface of the film. In addition, nano-SiO2 was hydrophilic. The surface of nano-SiO2 with high content contained more hydrophilic hydroxyl groups. With the continuous increase of nano-SiO2 addition, the improvement effect of nanoparticle on the hydrophobicity of xylan/PVOH composite films became weaker (Phothisarattana et al. 2022). This observation was consistent with the analysis of the surface morphology of the film in Fig. 3.
Fig. 5. Contact angle results of nano-SiO2 modified xylan films
Mechanical properties of the nano-SiO2 modified xylan films were tested, and the results are shown in Table 2. At the initial stage (nano-SiO2 addition less than 1%) tensile strength values of the composite film were found to rise with increasing nano- SiO2 addition, which declined at higher (above 1% nano-SiO2 loadings. The elongation at break was found to change in the opposite way.
A maximal tensile strength of 14.78 MPa was achieved at 1% nano-SiO2 loading, which is 32% higher than that of blank xylan/PVOH film. However, its elongation at break dropped to 4.65% at this nano-SiO2 loading. Nano-SiO2 of lower loadings (below 1.2%) showed good dispersion effect in composite film. The high surface energy and large specific surface area of the uniformly dispersed nano-SiO2 particles could generate a huge interfacial area in the polymer matrix and improve the interfacial bonding force between the nanoparticles and the polymer matrix. At the same time, hydroxyl groups on the surface of small size rigid nano-SiO2 particles and the hydroxyl groups in the polymer formed hydrogen bonds, which restricted the free movement of polymer chains (Wadaugsorn et al. 2022). The formation of hydrogen bonds raised the tensile strength (de Azeredo 2009; Liu et al. 2018) and reduced the flexibility of the film. Higher nano-SiO2 loading reduced tensile strength but raised elongation at break of the composite film. Film H-2 showed a tensile strength of 10.95 MPa, and the elongation at break of 8.68%. At an addition of more than 1.5%, nano-SiO2 exhibited a certain degree of agglomeration in the film matrix, resulting in stress concentration points, poor dispersion, and weakening of the interaction between nano-SiO2 and polymer. In addition, the elongation at break of xylan composite films with nano-SiO2 content more than 1.2% was higher than that of film H-0. At this level of addition, the agglomeration of nano-SiO2 diminished the surface free energy, reduced the hydrogen bonding between nano-SiO2 and xylan/PVOH matrix, displaying an effect similar to that of a plasticizer (Liu et al. 2019a). Large-size nano-SiO2 aggregates increased the distance between xylan chains and PVOH chains, weakened the interaction between xylan and PVOH molecules, and made their molecular chains slide more easily.
Table 2. Tensile Test Results of the Nano-SiO2 Modified Xylan Films
Oxygen Barrier Properties
With regard to food packaging, the transmission of oxygen will lead to deteriorated food quality and shortened shelf life. Therefore, good oxygen barrier performance is a prerequisite for food packaging materials (Soni et al. 2016). Under the conditions of a temperature of 23 °C and a relative humidity of 50%, the oxygen permeability of the hemicellulose composite films with different nano-SiO2 additions and some typical packaging materials are shown in Table 3.
Table 3. Comparison of Oxygen Permeability of the Packaging Films in the Literature and this Work
As shown in Table 3, the oxygen permeability of the hemicellulose-based composite films was much lower than those of the common packaging materials LDPE, HDPE, and EVA, demonstrating that an appropriate amount of nano-SiO2 addition can improve the oxygen barrier performance as indicated by the reduced oxygen permeability. At a 1% nano-SiO2 addition, the oxygen permeability of the film reached the minimum [0.27 (cm3·µm)/(m2·d·kPa)], which was 85% lower than that of the blank xylan/PVOH film. This was mainly due to the small size of nano-SiO2, which was uniformly dispersed in xylan and PVOH matrix and filled the voids inside the composite film (Li et al. 2016). In addition, the nanoparticles were present in the molecular chains of xylan and PVOH and had strong intermolecular interactions with them (Rukmanikrishnan et al. 2021), reducing the mobility and free volume between the chains and making the film matrix more compact. However, the nano-SiO2 particles of higher addition had serious agglomeration, and the agglomerated nanoparticles had more particle-particle interactions (Reddy et al. 2018). The oxygen barrier properties of the hemicellulose films were decreased due to the decrease of the compactness of the film.
An oxygen-barrier polymer can be defined as a polymer having an OP value of lower than 38.9 (cm3·µm)/(m2·d·kPa) at 23 °C (Hong and Krochta 2006). With an OP value of lower than 2 (cm3·µm)/(m2·d·kPa), nano-SiO2 modified xylan film showed a great potential for application in food packaging.
Thermal Stability
A thermogravimetric analyzer (TGA) was used to study the thermal stability of nano-SiO2 reinforced xylan film at 40 to 600 °C. As shown in Table 4 and Fig. 6, the weight loss of the film was mainly divided into the following three stages: 60 to 210 °C, 210 to 340 °C, and 340 to 520 °C. In the first stage of the film, the weight loss was caused by the evaporation of water vapor adsorbed on the surface of the film and the evaporation of internal moisture. Due to the strong hydrophilicity of nano-SiO2 containing a large number of hydroxyl groups on the surface, the modified film contains relatively large amount of water. The weight loss of the modified film in the first stage was higher than that of the unmodified film. In the second stage the films showed the most weight loss due to degradation of the polymers (xylan, PVOH, and sorbitol) (Huang et al. 2018). The weight loss of the film at this stage was mainly due to the degradation of the side chains of xylan and PVOH. The weight loss of the film in the third stage was due to the carbonization of the polymer and the C–C backbone of xylan and PVOH was cleaved in this temperature range. At 600 °C, the unmodified film had the lowest carbon residue percentage of only 18.96%.
Table 4. Thermal Properties of the Nano-SiO2 Modified Xylan Films
Fig. 6. Thermogravimetric (TGA) curves (left) and derivative thermogravimetry (DTG) curves (right) of nano-SiO2 modified xylan film
In the second and third stages of weight loss, the thermal degradation temperature of the hemicellulose composite film with nano-SiO2 particles was higher than that of the pure PVOH/xylan composite film. At a weight loss rate of 50%, unmodified film showed a degradation temperature of 313 °C, while the composite films exhibited degradation temperatures in the range of 318 to 322 °C. The temperature corresponding to the peak thermal decomposition rate of the xylan film (Tmax2 and Tmax3) was increased after the addition of nano-SiO2 particles. The nano-SiO2 particles improved the thermal stability of the composite film in the second and third stages. This was mainly attributed to the generation of hydrogen bonding forces between nano-SiO2, PVOH and xylan. A partial hydrogen bond network (Ahmadizadegan et al. 2018) formed inside the hemicellulose film, leading to an increase in the energy required for thermal degradation. Because nano-SiO2 was not degraded at high temperature, the residual amount of the nano-SiO2 modified xylan film at 600 °C was higher than that of the pure PVOH/xylan film.
CONCLUSIONS
- The addition of nano-SiO2 improved the mechanical strength and barrier properties of xylan/PVOH film because of the hydrogen bond interaction between nano- SiO2 and xylan/PVOH matrix. A 1% nano-SiO2 loading afforded the best improvement in the properties.
- The uniform dispersion of nano-SiO2 resulted in rougher surface and raised the static contact angle of composite (over 90°), which was considered an effective approach to the preparation of hydrophobic hemicellulose-based nanocomposite film.
- The prepared composite film may be used as an alternative to, or substitute for traditional petrochemical-based food packaging materials due to the overall performance, especially good oxygen barrier properties.
ACKNOWLEDGMENTS
Financial support from National Natural Science Foundation of China (grant numbers: 31570575) is gratefully acknowledged.
REFERENCES CITED
Ahmadizadegan, H., Esmaielzadeh, S., Ranjbar, M., Marzban, Z., and Ghavas, F. (2018). “Synthesis and characterization of polyester bionanocomposite membrane with ultrasonic irradiation process for gas permeation and antibacterial activity,” Ultrasonics Sonochemistry 41, 538-550. DOI: 10.1016/j.ultsonch.2017.10.020
Aruna, S. T., Anandan, C., and Grips, V. K. W. (2014). “Effect of probe sonication and sodium hexametaphosphate on the microhardness and wear behavior of electrodeposited Ni-SiC composite coating,” Applied Surface Science 301, 383-390. DOI: 10.1016/j.apsusc.2014.02.087
Asgher, M., Qamar, S. A., Bilal, M., and Iqbal, H. M. N. (2020). “Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials,” Food Research International 137, article 109625. DOI: 10.1016/j.foodres.2020.109625
Azeredo, H. M. C., Kontou-Vrettou, C., Moates, G. K., Wellner, N., Cross, K., Pereira, P. H. F., and Waldron, K. W. (2015). “Wheat straw hemicellulose films as affected by citric acid,” Food Hydrocolloids 50, 1-6. DOI: 10.1016/j.foodhyd.2015.04.005
Borjesson, M., and Westman, G. (2016). “Branching of hemicelluloses through an azetidinium salt ring-opening reaction,” Carbohydrate Research 428, 23-30. DOI: 10.1016/j.carres.2016.04.005
Borrega, M., and Orelma, H. (2019). “Cellulose nanofibril (CNF) films and xylan from hot water extracted birch kraft pulps,” Applied Sciences-Basel 9(16), DOI: 10.3390/app9163436
Cassie, A. B. D., and Baxter, S. (1944). “Wettability of porous surfaces,” Trans. Faraday Soc 40, 546. DOI: 10.1039/tf9444000546
Chen, G. G., Qi, X. M., Guan, Y., Peng, F., Yao, C. L., and Sun, R. C. (2016). “High strength hemicellulose-based nanocomposite film for food packaging applications,” ACS Sustainable Chemistry & Engineering 4(4), 1985-1993. DOI: 10.1021/acssuschemeng.5b01252
Chen, G.-G., Qi, X.-M., Li, M.-P., Guan, Y., Bian, J., Peng, F., Yao, C.-L., and Sun, R.-C. (2015). “Hemicelluloses/montmorillonite hybrid films with improved mechanical and barrier properties,” Scientific Reports 5, 16405. DOI: 10.1038/srep16405
Chiellini, E., Cinelli, P., Fernandes, E. G., Kenawy, E. R. S., and Lazzeri, A. (2001). “Gelatin-based blends and composites. Morphological and thermal mechanical characterization,” Biomacromolecules 2(3), 806-811. DOI: 10.1021/bm015519h
de Azeredo, H. M. C. (2009). “Nanocomposites for food packaging applications,” Food Research International 42(9), 1240-1253. DOI: 10.1016/j.foodres.2009.03.019
Dehghani, S., Peighambardoust, S. H., Peighambardoust, S. J., Hosseini, S. V., and Regenstein, J. M. (2019). “Improved mechanical and antibacterial properties of active LDPE films prepared with combination of Ag, ZnO and CuO nanoparticles,” Food Packaging and Shelf Life 22, 100391. DOI: 10.1016/j.fpsl.2019.100391
Du, J., Li, C., Zhao, Y. D., and Wang, H. S. (2018). “Hemicellulose isolated from waste liquor of viscose fiber mill for preparation of polyacrylamide-hemicellulose hybrid films,” International Journal of Biological Macromolecules 108, 1255-1260. DOI: 10.1016/j.ijbiomac.2017.11.036
Egues, I., Stepan, A. M., Eceiza, A., Toriz, G., Gatenholm, P., and Labidi, J. (2014). “Corncob arabinoxylan for new materials,” Carbohydrate Polymers 102, 12-20. DOI: 10.1016/j.carbpol.2013.11.011
Escalante, A., Goncalves, A., Bodin, A., Stepan, A., Sandstrom, C., Toriz, G., and Gatenholm, P. (2012). “Flexible oxygen barrier films from spruce xylan,” Carbohydrate Polymers 87(4), 2381-2387. DOI: 10.1016/j.carbpol.2011.11.003
Gao, C.-d., Ren, J.-l., Wang, S.-y., Sun, R.-c., and Zhao, L.-h. (2014). “Preparation of polyvinyl alcohol/xylan blending films with 1,2,3,4-butane tetracarboxylic acid as a new plasticizer,” Journal of Nanomaterials 2014, 764031. DOI: 10.1155/2014/764031
Gordobil, O., Egues, I., Urruzola, I., and Labidi, J. (2014). “Xylan-cellulose films: Improvement of hydrophobicity, thermal and mechanical properties,” Carbohydrate Polymers 112, 56-62. DOI: 10.1016/j.carbpol.2014.05.060
Hanani, Z. A. N., McNamara, J., Roos, Y. H., and Kerry, J. P. (2013). “Effect of plasticizer content on the functional properties of extruded gelatin-based composite films,” Food Hydrocolloids 31(2), 264-269. DOI: 10.1016/j.foodhyd.2012.10.009
Hansen, N. M. L., and Plackett, D. (2008). “Sustainable films and coatings from hemicelluloses: A review,” Biomacromolecules 9(6), 1493-1505. DOI: 10.1021/bm800053Z
Hartman, J., Albertsson, A. C., Lindblad, M. S., and Sjoberg, J. (2006a). “Oxygen barrier materials from renewable sources: Material properties of softwood hemicellulose-based films,” Journal of Applied Polymer Science 100(4), 2985-2991. DOI: 10.1002/app.22958
Hartman, J., Albertsson, A. C., and Sjoberg, J. (2006b). “Surface- and bulk-modified galactoglucomannan hemicellulose films and film laminates for versatile oxygen barriers,” Biomacromolecules 7(6), 1983-1989. DOI: 10.1021/bm060129m
Hong, S. I., and Krochta, J. M. (2006). “Oxygen barrier performance of whey-protein-coated plastic films as affected by temperature, relative humidity, base film and protein type,” Journal of Food Engineering 77(3), 739-745. DOI: 10.1016/j.jfoodeng.2005.07.034
Huang, B. B., Tang, Y. J., Pei, Q. Q., Zhang, K. J., Liu, D. D., and Zhang, X. M. (2018). “Hemicellulose-based films reinforced with unmodified and cationically modified nanocrystalline cellulose,” Journal of Polymers and the Environment 26(4), 1625-1634. DOI: 10.1007/s10924-017-1075-5
Hubbe, M. A., Gardner, D. J., and Shen, W. (2015). “Contact angles and wettability of cellulosic surfaces: A review of proposed mechanisms and test strategies,” BioResources 10(4), 8657-8749. DOI: 10.15376/biores.10.4.Hubbe_Gardner_Shen
Kanmani, P., and Rhim, J.-W. (2014). “Properties and characterization of bionanocomposite films prepared with various biopolymers and ZnO nanoparticles,” Carbohydrate Polymers 106, 190-199. DOI: 10.1016/j.carbpol.2014.02.007
Kariminejad, M., Zibaei, R., Kolahdouz-Nasiri, A., Mohammadi, R., Mortazavian, A. M., Sohrabvandi, S., Khanniri, E., and Khorshidian, N. (2022). “Chitosan/polyvinyl alcohol/SiO2 nanocomposite films: Physicochemical and structural characterization,” Biointerface Research in Applied Chemistry 12(3), 3725-3734. DOI: 10.33263/Briac123.37253734
Li, D., Zhang, J., Xu, W., and Fu, Y. (2016). “Effect of SiO2/EVA on the mechanical properties, permeability, and residual solvent of polypropylene packaging films,” Polymer Composites 37(1), 101-107. DOI: 10.1002/pc.23159
Liu, X., Chen, X., Ren, J., and Zhang, C. (2018). “TiO2-KH550 nanoparticle-reinforced PVA/xylan composite films with multifunctional properties,” Materials 11(9), 1589. DOI: 10.3390/ma11091589
Liu, X. X., Chen, X. F., Ren, J. L., Chang, M. M., He, B., and Zhang, C. H. (2019a). “Effects of nano-ZnO and nano-SiO2 particles on properties of PVA/xylan composite films,” International Journal of Biological Macromolecules 132, 978-986. DOI: 10.1016/j.ijbiomac.2019.03.088
Liu, Z., Liu, R., Yi, Y., Han, W., Kong, F., and Wang, S. (2019b). “Photocatalytic degradation of dyes over a xylan/PVA/TiO2 composite under visible light irradiation,” Carbohydrate Polymers 223, 115081. DOI: 10.1016/j.carbpol.2019.115081
Mikkonen, K. S., Heikkila, M. I., Willfor, S. M., and Tenkanen, M. (2012). “Films from glyoxal-crosslinked spruce galactoglucomannans plasticized with sorbitol,” International Journal of Polymer Science 2012, 482810. DOI: 10.1155/2012/482810
Mitrano, D. M., Motellier, S., Clavaguera, S., and Nowack, B. (2015). “Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products,” Environment International 77, 132-147. DOI: 10.1016/j.envint.2015.01.013
Mohammadi Nafchi, A., Alias, A. K., Mahmud, S., and Robal, M. (2012). “Antimicrobial, rheological, and physicochemical properties of sago starch films filled with nanorod-rich zinc oxide,” Journal of Food Engineering 113(4), 511-519. DOI: 10.1016/j.jfoodeng.2012.07.017
More, S., Bampidis, V., Benford, D., Bragard, C., Halldorsson, T., Hernandez-Jerez, A., Bennekou, S. H., Koutsoumanis, K., Lambre, C., Machera, K., Naegeli, H., Nielsen, S., Schlatter, J., Schrenk, D., Silano, V., Turck, D., Younes, M., Castenmiller, J., Chaudhry, Q., Cubadda, F., Franz, R., Gott, D., Mast, J., Mortensen, A., Oomen, A. G., Weigel, S., Barthelemy, E., Rincon, A., Tarazona, J., Schoonjans, R., and Comm, E. S. (2021). “Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: human and animal health,” Efsa Journal 19(8), 6768. DOI: 10.2903/j.efsa.2021.6768
Nesic, A., Cabrera-Barjas, G., Dimitrijevic-Brankovic, S., Davidovic, S., Radovanovic, N., and Delattre, C. (2020). “Prospect of polysaccharide-based materials as advanced food packaging,” Molecules 25(1), 135. DOI: 10.3390/molecules25010135
Pauly, M., and Keegstra, K. (2008). “Cell-wall carbohydrates and their modification as a resource for biofuels,” Plant Journal 54(4), 559-568. DOI: 10.1111/j.1365-313X.2008.03463.x
Peng, X. W., Ren, J. L., Zhong, L. X., and Sun, R. C. (2011). “Nanocomposite films based on xylan-rich hemicelluloses and cellulose nanofibers with enhanced mechanical properties,” Biomacromolecules 12(9), 3321-3329. DOI: 10.1021/bm2008795
Phothisarattana, D., and Harnkarnsujarit, N. (2022). “Migration, aggregations and thermal degradation behaviors of TiO2 and ZnO incorporated PBAT/TPS nanocomposite blown films,” Food Packaging and Shelf Life 33, 100901. DOI: 10.1016/j.fpsl.2022.100901
Phothisarattana, D., Wongphan, P., Promhuad, K., Promsorn, J., and Harnkarnsujarit, N. (2022). “Blown film extrusion of PBAT/TPS/ZnO nanocomposites for shelf-life extension of meat packaging,” Colloids and Surfaces B-Biointerfaces 214, 112472. DOI: 10.1016/j.colsurfb.2022.112472
Reddy, J. P., Rajulu, A. V., Rhim, J.-W., Seo, J. (2018). “Mechanical, thermal, and water vapor barrier properties of regenerated cellulose/nano-SiO2 composite films,” Cellulose 25(12), 7153-7165. DOI: 10.1007/s10570-018-2059-x
Reddy, M. M., Vivekanandhan, S., Misra, M., Bhatia, S. K., and Mohanty, A. K. (2013). “Biobased plastics and bionanocomposites: Current status and future opportunities,” Progress in Polymer Science 38(10-11), 1653-1689. DOI: 10.1016/j.progpolymsci.2013.05.006
Rodgers, M. P., Shi, Z. Q., and Holdcroft, S. (2008). “Transport properties of composite membranes containing silicon dioxide and Nafion (R),” Journal of Membrane Science. 325(1), 346-356. DOI: 10.1016/j.memsci.2008.07.045
Rukmanikrishnan, B., Ramalingam, S., Kim, S. S., and Lee, J. (2021). “Rheological and anti-microbial study of silica and silver nanoparticles-reinforced k-carrageenan/hydroxyethyl cellulose composites for food packaging applications,” Cellulose 28(9), 5577-5590. DOI: 10.1007/s10570-021-03873-z
Shao, H., Zhao, Y. L., Sun, H., Yang, B., Fan, B. M., Zhang, H. J., Weng, Y. X. (2020). “Barrier film of etherified hemicellulose from single-step synthesis,” Polymers 12(10), 2199. DOI: 10.3390/polym12102199
Song, H., and Zheng, L. (2013). “Nanocomposite films based on cellulose reinforced with nano-SiO2: microstructure, hydrophilicity, thermal stability, and mechanical properties,” Cellulose 20(4), 1737-1746. DOI: 10.1007/s10570-013-9941-3
Soni, B., Hassan, E., Schilling, M. W., and Mahmoud, B. (2016). “Transparent bionanocomposite films based on chitosan and TEMPO-oxidized cellulose nanofibers with enhanced mechanical and barrier properties,” Carbohydrate Polymers 151, 779-789. DOI: 10.1016/j.carbpol.2016.06.022
Sothornvit, R. (2019). “Nanostructured materials for food packaging systems: New functional properties,” Current Opinion in Food Science 25, 82-87. DOI: 10.1016/j.cofs.2019.03.001
Tang, X. Z., and Alavi, S. (2011). “Recent advances in starch, polyvinyl alcohol based polymer blends, nanocomposites and their biodegradability,” Carbohydrate Polymers 85(1), 7-16. DOI: 10.1016/j.carbpol.2011.01.030
Tavakolian, S., Ahari, H., Givianrad, M. H., and Hosseini, H. (2021). “Improving the barrier properties of food packaging by Al2O3@TiO2 & Al2O3@SiO2 nanoparticles,” Food and Bioprocess Technology 14(7), 1287-1300. DOI: 10.1007/s11947-021-02635-w
Tayeb, A. H., Amini, E., Ghasemi, S., and Tajvidi, M. (2018). “Cellulose nanomaterials-Binding properties and applications: A review,” Molecules 23(10), 2684. DOI: 10.3390/molecules23102684
Thakur, V. K., Thakur, M. K., Raghavan, P., and Kessler, M. R. (2014). “Progress in green polymer composites from lignin for multifunctional applications: A review,” ACS Sustainable Chemistry & Engineering 2(5), 1072-1092. DOI: 10.1021/sc500087z
Velkova, N., Doliska, A., Zemljic, L. F., Vesel, A., Saake, B., and Strnad, S. (2015). “Influence of carboxymethylation on the surface physical-chemical properties of glucuronoxylan and arabinoxylan films,” Polymer Engineering and Science 55(12), 2706-2713. DOI: 10.1002/pen.24059
Wadaugsorn, K., Panrong, T., Wongphan, P., and Harnkarnsujarit, N. (2022). “Plasticized hydroxypropyl cassava starch blended PBAT for improved clarity blown films: Morphology and properties,” Industrial Crops and Products 176, 114311. DOI: 10.1016/j.indcrop.2021.114311
Xu, J., Xia, R., Zheng, L., Yuan, T., and Sun, R. (2019). “Plasticized hemicelluloses/ chitosan-based edible films reinforced by cellulose nanofiber with enhanced mechanical properties,” Carbohydrate Polymers 224, article 115164. DOI: 10.1016/j.carbpol.2019.115164
Yu, Y. W., Zhang, S. Y., Ren, Y. Z., Li, H., Zhang, X. N., and Di, J. H. (2012). “Jujube preservation using chitosan film with nano-silicon dioxide,” Journal of Food Engineering 113(3), 408-414. DOI: 10.1016/j.jfoodeng.2012.06.021
Zhang, H. T., Guo, Z., Chen, Q., Wang, X. W., Wang, Z. D., and Liu, Z. W. (2016). “Deposition of silicon oxide coatings by atmospheric pressure plasma jet for oxygen diffusion barrier applications,” Thin Solid Films 615, 63-68. DOI: 10.1016/j.tsf.2016.06.042
Zhang, R., Wang, X., and Cheng, M. (2018). “Preparation and characterization of potato starch film with various size of nano-SiO2,” Polymers 10(10), 1172. DOI: 10.3390/polym10101172
Zhao, T. Y., and Jiang, L. (2018). “Contact angle measurement of natural materials,” Colloids and Surfaces B – Biointerfaces 161, 324-330. DOI: 10.1016/j.colsurfb.2017.10.056
Zhao, Y., Sun, H., Yang, B., Fan, B., Zhang, H., and Weng, Y. (2021). “Enhancement of mechanical and barrier property of hemicellulose film via crosslinking with sodium trimetaphosphate,” Polymers 13(6), 927. DOI: 10.3390/polym13060927
Zhong, Y., Janes, D., Zheng, Y., Hetzer, M., and De Kee, D. (2007). “Mechanical and oxygen barrier properties of organoclay-polyethylene nanocomposite films,” Polymer Engineering and Science 47(7), 1101-1107. DOI: 10.1002/pen.20792
Zoldners, J., and Kiseleva, T. (2013). “Modification of hemicelluloses with polycarboxylic acids,” Holzforschung 67(5), 567-571. DOI: 10.1515/hf-2012-0183
Article submitted: March 3, 2023; Peer review completed: April 1, 2023; Revised version received: April 19, 2023; Further revised version received and accepted: April 22, 2023; Published: April 28, 2023.
DOI: 10.15376/biores.18.2.4195-4211
