Abstract
This review article was prompted by a remarkable growth in the number of scientific publications dealing with the use of nanocellulose (especially nanofibrillated cellulose (NFC), cellulose nanocrystals (CNC), and bacterial cellulose (BC)) to enhance the barrier properties and other performance attributes of new generations of packaging products. Recent research has confirmed and extended what is known about oxygen barrier and water vapor transmission performance, strength properties, and the susceptibility of nanocellulose-based films and coatings to the presence of humidity or moisture. Recent research also points to various promising strategies to prepare ecologically-friendly packaging materials, taking advantage of nanocellulose-based layers, to compete in an arena that has long been dominated by synthetic plastics. Some promising approaches entail usage of multiple layers of different materials or additives such as waxes, high-aspect ratio nano-clays, and surface-active compounds in addition to the nanocellulose material. While various high-end applications may be achieved by chemical derivatization or grafting of the nanocellulose, the current trends in research suggest that high-volume implementation will likely incorporate water-based formulations, which may include water-based dispersions or emulsions, depending on the end-uses.
Download PDF
Full Article
Nanocellulose in Thin Films, Coatings, and Plies for Packaging Applications: A Review
Martin A. Hubbe,a Ana Ferrer,b Preeti Tyagi,a Yuanyuan Yin,a.c Carlos Salas,a Lokendra Pal,a and Orlando J. Rojas a,d
This review article was prompted by a remarkable growth in the number of scientific publications dealing with the use of nanocellulose (especially nanofibrillated cellulose (NFC), cellulose nanocrystals (CNC), and bacterial cellulose (BC)) to enhance the barrier properties and other performance attributes of new generations of packaging products. Recent research has confirmed and extended what is known about oxygen barrier and water vapor transmission performance, strength properties, and the susceptibility of nanocellulose-based films and coatings to the presence of humidity or moisture. Recent research also points to various promising strategies to prepare ecologically-friendly packaging materials, taking advantage of nanocellulose-based layers, to compete in an arena that has long been dominated by synthetic plastics. Some promising approaches entail usage of multiple layers of different materials or additives such as waxes, high-aspect ratio nano-clays, and surface-active compounds in addition to the nanocellulose material. While various high-end applications may be achieved by chemical derivatization or grafting of the nanocellulose, the current trends in research suggest that high-volume implementation will likely incorporate water-based formulations, which may include water-based dispersions or emulsions, depending on the end-uses.
DOI: 10.15376/biores.12.1.2143-2233
Keywords: Barrier properties; Water vapor transmission; Food shelf life; Oxygen transmission; Packages; Cellulose nanomaterials
Contact information: a: Department of Forest Biomaterials, College of Natural Resources; North Carolina State University, Raleigh, NC 27695, USA; b: Nalco Champion, an Ecolab Company, 7705 Highway 90-A, Sugar Land, TX 77478, USA; c: School of Clothing and Textiles, Jiangnan University, Wuxi, Jiangsu, China; d: Department of Bioproducts and Biosystems, School of Chemical Engineering, Aalto University, PO Box 16300, FI-00076 Aalto, Finland;
* Corresponding author: hubbe@ncsu.edu
Contents
INTRODUCTION
There has been explosive growth in the publication of peer-reviewed articles that combine key words related to “packaging” and “cellulose,” in combination with the terms “nanocellulose,” “nanocrystal*,” or “nanofibril*”. As of November 2016, a search of this combination of terms showed about as many publications since the start of 2015, compared to all preceding years combined. Given such an acceleration of research around the world, it makes sense to ask whether this high amount of research effort has yet borne significant fruit. In light of this question, the emphasis of this review article is on research publications that shed light on known challenges to the successful implementation of nanocellulose products to enhance the performance of packaging.
In principle, a nanocellulose-based film, coating, or intermediate layer, in addition to being light in weight, can provide benefits of renewability, recyclability, processability, and compatibility with health and the environment. In particular, very high performance, relative to plastic-based materials, has been reported for the oxygen permeation resistance of certain nanocellulose-based films (Fukuzumi et al. 2009; Syverud and Stenius 2009; Aulin et al. 2010a; Hult et al. 2010; Plackett et al. 2010; Chinga-Carrasco and Syverud 2012; Rodionova et al. 2012a,b; Shimizu et al. 2016). While a high level of resistance to oxygen permeation has been reported, many other studies have revealed much lower performance of cellulose-based films and coatings in terms of resistance to water vapor transmission. In many applications it would be very important to hold out gases and water vapor under both dry and humid or wet conditions. Future successful implementations of nanocellulose-based films are most likely to take advantage of inherent positive attributes of cellulose-based films, while compensating for or overcoming product requirements that are inherently difficult or expensive to achieve with a nanocellulose-based film structure.
Several important review articles provide a starting platform and raise some important issues to be further considered in this article. The general subject area of packaging materials involving biomaterials has been the focus of numerous review articles and monographs (Lagaron et al. 2004; Rhim 2007; Rhim and Ng 2007; Chiellini 2008; Johansson et al. 2012; Tang et al. 2012; Paunonen 2013a). For example, Krochta and DeMulderJohnston (1997) reviewed research related to edible and biodegradable films for packaging applications. Also, there has been much interest and research related to cellulose fiber usage in composite materials for packaging (Johansson et al. 2012; Faruk et al. 2014). Research related to the use of nanocellulose in packaging applications also has been reviewed (Turbak et al. 1983; Dufresne 2008, 2012; Hubbe et al. 2008; Azeredo 2009; Eichhorn et al. 2009; Oksman et al. 2009; Habibi et al. 2010; Siqueira et al. 2010; Siro and Plackett 2010; Moon et al. 2011; Olsson et al. 2011; Petersen and Gatenholm 2011; Faruck et al. 2012; Huber et al. 2012; Khalil et al. 2012, 2014; Lavoine et al. 2012; Freire et al. 2013; Lopacka 2013; Paunonen 2013a,b; Sandquist 2013; Cowie et al. 2014; Khan et al. 2014a; Tammelin and Vartiainen 2014; Mihindukulasuriya and Lim 2014; Azizi Samir et al. 2015; Hannon et al. 2015; Li et al. 2015a; Simao et al. 2015; Gomez et al. 2016; Khalil et al. 2016). In particular, Lindström and Aulin (2014) reviewed research progress up to 2014, emphasizing some of the key unmet issues that are likely to continue to slow down progress in production-scale implementation of nanocellulose in packaging. The cited article will be used in the present article as a kind of benchmark by which to judge whether or not meaningful progress has been achieved more recently. Near to the end of this article, a list of unresolved issues highlighted by Lindström and Aulin (2014) will be considered again, with attention to whether or not the challenges have been addressed in the intervening two years. While the articles mentioned in this paragraph mainly concerned technical feasibility, Shatkin et al. (2014) reviewed the potential market projections for cellulose nanomaterials and came to the conclusion that the greatest volume potential for nanocellulose lies in paper and packaging applications.
Motivations to Employ Nano-scale Cellulosic Particles in Packaging
The aforementioned published literature points to two classes of motivating factors favoring efforts to use cellulosic nanomaterials for enhancement of packaging. Firstly, there is a widespread desire to replace petroleum-based materials with renewable, biodegradable, and life-friendly nature-based materials. In addition, there is fast-accumulating information regarding the performance of nanocellulose-containing packaging structures relative to end-use requirements such as barrier properties, appearance, and strength. Some general directions for the development of barrier films already had been well established before nanocellulose films were even considered for such applications (Lagaron et al. 2004), and these goals have been extended to systems that can involve cellulosic nanomaterials (Moon et al. 2011; Paunonen 2013b). The subsections below further expand upon such aspects.
Mechanical and barrier properties
During the early development of cellulosic nanomaterials there was much attention paid to the superior tensile modulus and other strength attributes that can be achieved upon the drying of such materials (Nakagaito and Yano 2004). The individual crystals of nanocellulose exhibit elastic modulus and breaking strength characteristics that are among the highest listed, per unit mass, for common materials (Eichhorn et al. 2009). Furthermore, as a result of extensive hydrogen bonding and high density, the tensile strength of nanocellulose-based thin films can reach values that approach those of metals and advanced synthetic polymer materials (Qing et al. 2012).
A high proportion of articles dealing with nanocellulose materials for packaging have been focused on barrier properties. Figure 1 represents four kinds of barrier properties that have potential to be important in various applications of nanocellulose films in packaging.
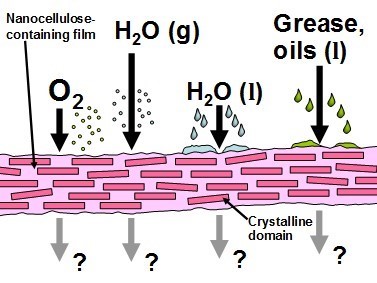
Fig. 1. Four types of barrier performance often studied relative to packaging film requirements
Oxygen barrier
Foremost among the concerns regarding packaging applications has been a motivation to limit the permeation of oxygen and other gases and volatile compounds through the barrier layers of packages. In this way, nanocellulose-related films have the potential to either increase the shelf life of foods, to prevent the accumulation of unwanted odors or contamination of the food, or conversely to avoid the escape of food odors. The following articles indicate strong performance of nanocellulose-containing films as oxygen barriers (George et al. 2005; Pääkkö et al. 2007; Fukuzumi et al. 2009; Syverud and Stenius 2009; Aulin et al. 2010a; 2012; Edlund et al. 2010; Hult et al. 2010; Plackett et al. 2010; Sanchez-Garcia and Lagaron 2010; Yang et al. 2011; Charani et al. 2013; Chinga-Carrasco and Syverud 2012; Fortunati et al. 2012b; Martínez-Sanz et al. 2012; Rodionova et al. 2012a,b; Savadekar et al. 2012; Stevanic et al. 2012; Espino-Pérez et al. 2013; Li et al. 2013a; Martínez-Sanz et al. 2013a; Österberg et al. 2013; Paunonen 2013a,b; Iotti 2014; Kumar et al. 2014; Lavoine et al. 2014c; Liu et al. 2014; Miettinen et al. 2014; Ibn Yaich et al. 2015; Rojo et al. 2015; Cheng et al. 2016; Shimizu et al. 2016).
Oils, grease barrier
In a fundamental sense, the hold-out of greases and oils is closely related to the holdout of nonpolar gases such as oxygen. The key is that neither oxygen gas nor greases and oils interact strongly with the hydrogen-bonded structure of a cellulose-based barrier film. Due to the importance of the topic for such applications as food packaging, several studies have focused on oil resistance (Aulin et al. 2009b, 2010a; Österberg et al. 2013; Kumar et al. 2014; Sirviö et al. 2014; Kisonen et al. 2015; Raghu 2015). Interestingly, several of these studies showed that the same systems providing superior oil hold-out also acted as superior barriers for oxygen permeation (Aulin et al. 2010a; Österberg et al. 2013; Kisonen et al. 2015). Researchers also have made efforts to further improve oil-holdout from nanocellulose-based films by rendering them oleophobic. This can be achieved by derivatization with very low surface energy substances, such as fluorocarbons; such effects can be enhanced if a surface is pretreated with nanoparticles to impart nano-scale roughness prior to perfluorosilane treatment (Kisonen et al. 2015).
Water vapor barrier
It would be a great advantage if a thin, eco-friendly barrier layer would also provide full resistance to moisture and high humidity. Cellulosic materials are inherently sensitive to the presence of both gaseous and liquid water (Spence et al. 2010a,b, 2011a,b; Belbekhouche et al. 2011; Ferrer et al. 2012a,b; Abdollahi et al. 2013a; Bai et al. 2015; Ferrer et al. 2015, 2016b; Rojo et al. 2015; Lundahl et al. 2016). There also have been efforts to modify nanocellulose-based systems so as to improve barrier performance in key areas. It is a challenge to prevent permeation of water vapor (Belbekhouche et al. 2011; Spence et al. 2011b; Paunonen 2013b; Lu et al. 2014, 2015). At high humidity, or when wet, typical cellulose-based films lose much of their ability to prevent the permeation of oxygen (Aulin et al. 2010a). Studies addressing these issues will be considered in the course of this review article.
Moisture sensitivity also can be a key concern when water-soluble polymeric substances are used to prepare thin films and their materials. In some such cases the inclusion of cellulosic reinforcing materials, at suitable levels, has been shown to reduce moisture-sensitivity (Cao et al. 2008; Azeredo et al. 2009; Bilbao-Sáinz et al. 2010; Sanchez-Garcia et al. 2010; George and Siddaramaiah 2012; Johnsy and Siddaramaiah 2012; Savadekar et al. 2012; Follain et al. 2013; Dehnad et al. 2014a; Peresin et al. 2014; Santos et al. 2014). Similar effects have been found when cellulose nanocrystals were used to reinforce a natural rubber matrix (Bras et al. 2010) or poly-lactic acid (PLA) (Sanchez-Garcia and Lagaron 2010; Hossain et al. 2011; Fortunati et al. 2012b; Martínez-Sanz et al. 2012; Song et al. 2014). However, Pereda et al. (2011) reported no beneficial effects relative to water vapor penetration or other attributes when including nanocellulose in a sodium caseinate-type protein film. Presumably any beneficial effects of reinforcements in limiting water vapor transmission may be due to either an improvement in film integrity, such as resistance to swelling in moist environments, or to the vapor-impermeable nature of crystalline cellulose.
Aqueous liquid barrier
Resistance to penetration of packaging materials by aqueous solutions is important in many applications, and several studies involving nanocellulose have focused on this issue (Choi and Simonson et al. 2006; Chinga-Carrasco et al. 2012; Yang et al. 2012; Follain et al. 2013; Kisonen et al. 2015; Shimizu et al. 2016). Liquid water is an especially challenging fluid from the perspective of cellulose-based films due to the fact that it has the potential to invade and replace hydrogen bonds connecting adjacent cellulosic surfaces in the film. It follows that it is not sufficient just to focus on achieving a dense layer without large pores. Rather, efforts to minimize penetration by aqueous fluids generally have focused on decreasing the water-wettability of the nanocellulose-based barrier films (Yang et al. 2012; Kisonen et al. 2015). Measurements of the contact angle of water have been employed as a criterion for identifying promising formulations to achieve resistance to liquid water (Spence et al. 2010b; Rodionova et al. 2011, 2012a; Yang et al. 2012; Pereda et al. 2014; Kisonen et al. 2015; Rojo et al. 2015; Visanko et al. 2015).
Drug release and antimicrobial packaging
The controlled release of drugs is another application for which the use of nanocellulose barrier layers has been considered (Kolakovic et al. 2012; Lavoine et al. 2014b, 2016). In these applications, the nanocellulose-based film appears to function as a barrier to the contained pharmaceutical compounds. Kolakovic et al. (2012) used a filtration procedure to form the nanocellulose film and then to collect a model drug compound. Lavoine et al. (2014b) coated nanofibrillated cellulose onto a caffeine-impregnated paper base. In these studies, the rate of release of the confined material was shown to be slowed down by the presence of a nanocellulose-based layer.
Several researchers have evaluated strategies to impart antimicrobial properties to packaging with systems that involve nanocellulose (Andresen et al. 2007; Dobre et al. 2012; Boumail et al. 2013a,b; Cozzolino et al. 2013; Costa et al. 2014; Dehnad et al. 2014b; Salmieri et al. 2014a,b; El-Wakil et al. 2015; Saini et al. 2015, 2016a,b; Amini et al. 2016; Hu and Wang 2016; Jebel and Almasi 2016; Padrao et al. 2016; Yan et al. 2016). Of particular interest are treatments with food-grade compounds such as sorbic acid (Dobre et al. 2012) or the bio-based cationic polymer chitosan (Tome et al. 2013; Velasquez-Cock et al. 2014; Li et al. 2015b), which nevertheless can improve the ability of the package to protect the food inside it against decay. Also, there has been much interest in utilizing nanomaterials such as colloidal silver particles in combination with nanocellulose for antimicrobial activity in packaging (Amini et al. 2016; Yan et al. 2016).
Transparency
In addition to the barrier properties and related functional capabilities of nanocellulose-based layers, much research has focused on desirable attributes such as transparency (Yano et al. 2005; Petersson and Oksman 2006; Nordqvist et al. 2007; Shimazaki et al. 2007; Ayuk et al. 2009; Fernandes et al. 2009, 2010; Fukuzumi et al. 2009; Kim et al. 2009; Nogi et al. 2009; Petersson et al. 2009; Sehaqui et al. 2010; Hassan et al. 2011; Pereda et al. 2011, 2014; Stevanic et al. 2011; Yang et al. 2011; Aulin et al. 2012; Hu et al. 2013; Li et al. 2013a; Tome et al. 2013; Khan et al. 2014b; Kumar et al. 2014; Kurihara and Isogai 2014; Tammelin and Vartiainen 2014; Ambrosio-Martin et al. 2015b; Honorato et al. 2015; Oun and Rhim 2015; Toivonen et al. 2015a,b). Hu et al. (2013) showed that nanocellulose films could be rendered conductive by deposition of tin-doped indium oxide, while still retaining their transparency; solar cells prepared with such films were successfully demonstrated. In general, it has been found that good transparency can be achieved as long as the cellulosic material is small enough, fully wetted by the matrix material (if any) in the layer, and not clumped up or entangled. Simao et al. (2015) carried out related work in which the band gap of optical absorption was determined for nanocellulose thin films. By contrast, more opaque films have been achieved in cases where cellulose reinforcements were either poorly wetted, agglomerated (Santos et al. 2014; Ambrosio-Martin et al. 2015b), or simply large relative to the wavelength of light (Kumar et al. 2014). Toivonen et al. (2015a) demonstrated for the first time that transparent films can be achieved even in the case where aerogel technology had been used in the initial film formation; subsequent compaction yielded transparent, flexible films.
Edibility
In potential applications where a nanocellulose-based film is applied directly to food, researchers have been concerned about the edibility of such films (Dogan and McHugh 2007; Azeredo et al. 2009, 2010; Bilbao-Sáinz et al. 2010; George and Siddaramaiah 2012; Johnsy and Siddaramaiah 2012; Pereda et al. 2014; Oun and Rhim 2015; George et al. 2016). In none of these cited studies was edibility actually evaluated; rather edibility was assumed based on the ubiquitous character and natural source of the cellulose.
Biodegradation properties
Nanocellulose is generally regarded as biodegradable for two reasons: As a type of cellulose, one can expect it to be susceptible to cellulase-producing fungi and bacteria, which are present throughout the biosphere (Rabinovich et al. 2002; Sukumaran et al. 2005). Secondly, the tiny dimensions of nanocellulose imply a high exposure to its surroundings. The issue of biodegradation has been emphasized in studies in which nanocellulose was used in composite structures with other natural film-forming materials (Lu et al. 2008; Ma et al. 2008; Cheng et al. 2009; Wan et al. 2009; Azeredo et al. 2010; Bras et al. 2010; Khan et al. 2010, 2012, 2014b; Siro and Plackett 2010; Chinga-Carrasco and Syverud 2012; da Silva et al. 2012; George and Siddaramaiah 2012; Hassan et al. 2012; Johnsy and Siddaramaiah 2012; Tang et al. 2012; Baheti and Militky 2013; Chinga-Carrasco et al. 2013; Ollier et al. 2013; Bhardwaj et al. 2014; Dehnad et al. 2014a; Fortunati et al. 2014; Ghaderi et al. 2014; Khalil et al. 2014; Kumar et al. 2014; Lu et al. 2014; Marais et al. 2014; Rafieian and Simonsen 2014; Reddy and Rhim 2014; Song et al. 2014; Yang et al. 2014; Azizi Samir et al. 2015; Feng et al. 2015a; Figueiredo et al. 2015; Honorato et al. 2015; Lavoine et al. 2015; Li et al. 2015a; Lu et al. 2015; Youssef et al. 2015; Cheng et al. 2016; Shankar and Rhim 2016). According to Lindström and Aulin (2014), biodegradability can be regarded as a more important issue for packaging, when compared to the displacement of petroleum-derived plastic materials.
Types of Nanocellulose to Consider for Packaging
Up to this point in this article there has been little emphasis on the different available types of nanocellulose. Now, to lay the groundwork for a discussion of how to improve the performance of nanocellulose applications in packaging, some more attention will be paid to that issue. As mentioned before, the three types of nanocellulose products that mainly are being studied for packaging are cellulose nanocrystals (CNCs), nanofibrillated cellulose (NFC), and bacterial cellulose (BC). Though the term “cellulose nanofibrils” has sometimes been used as an alternative to NFC, the latter term is preferred in the present article to emphasize the fact that the nanofibrillated material is often not completely separated into individual fibrils. These categories of nanocellulose, which will be discussed below, are sketched in Fig. 2. Overviews that describe all three of these products have been published (Siro and Plackett 2010; Nelson et al. 2016).
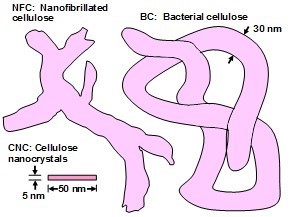
Fig. 2. Sketches of the three major types of nanocellulose, showing some typical dimensions. Please note that the lengths of some NFC and especially BC can be many times longer than what is represented in the figure. Also, CNCs in some cases can exceed 1000 nm in length.
Cellulose nanocrystals
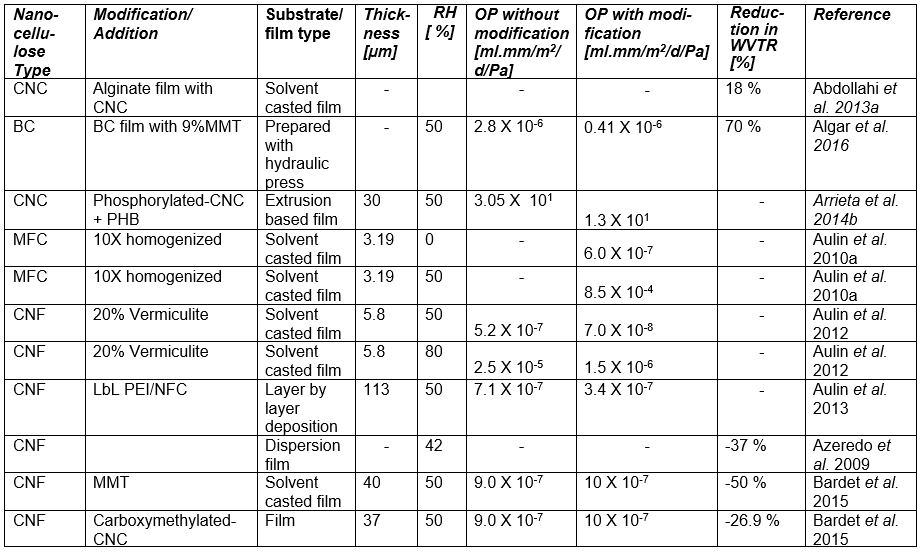
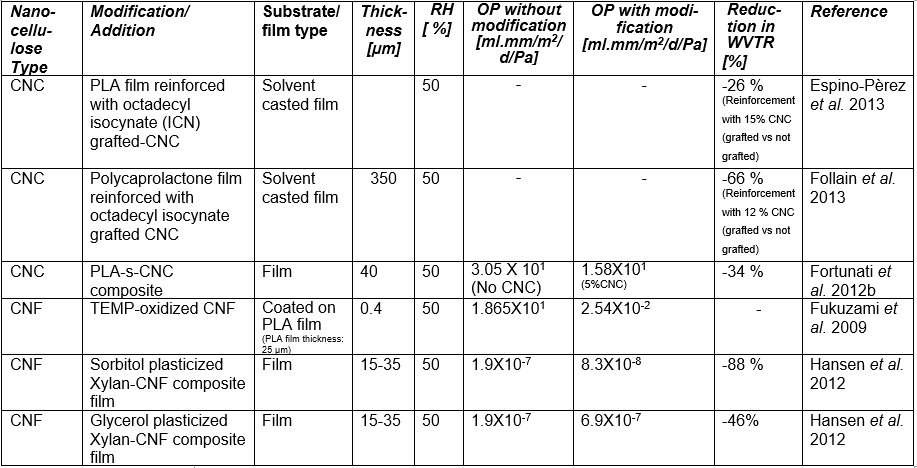
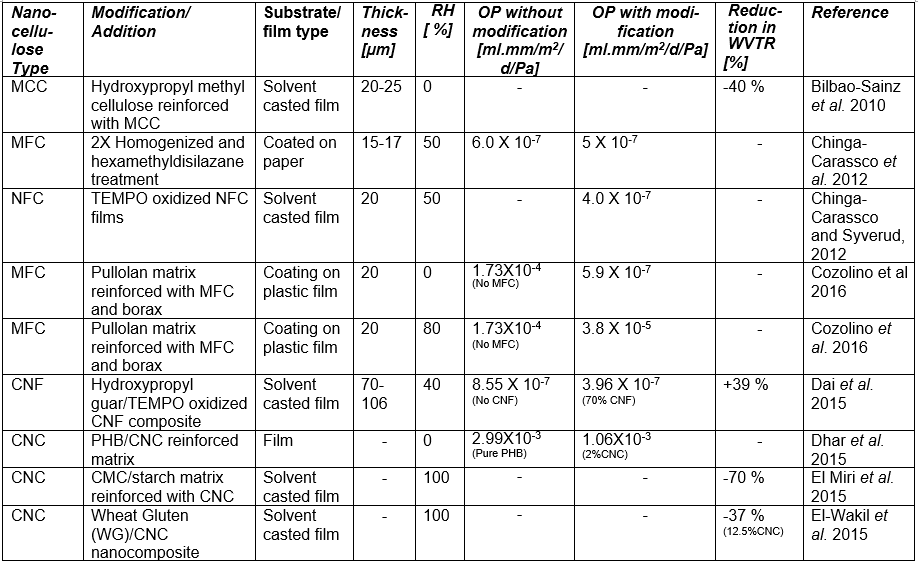
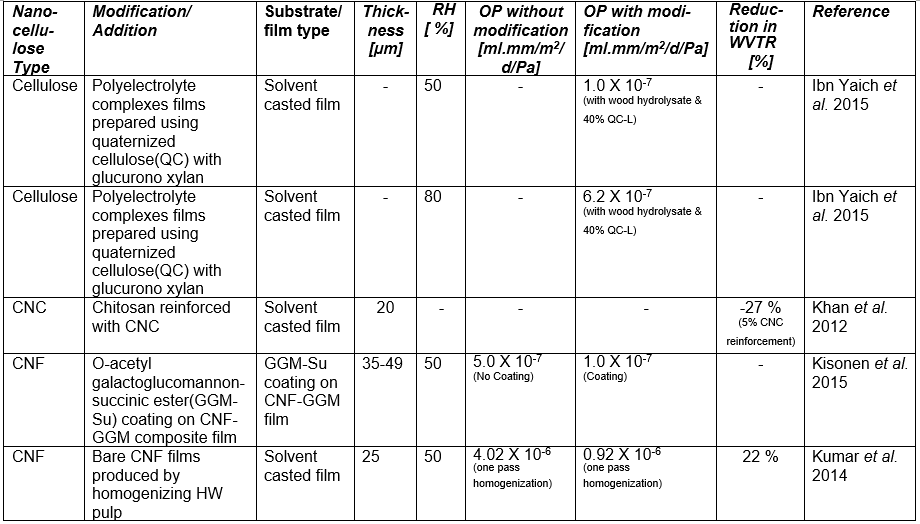
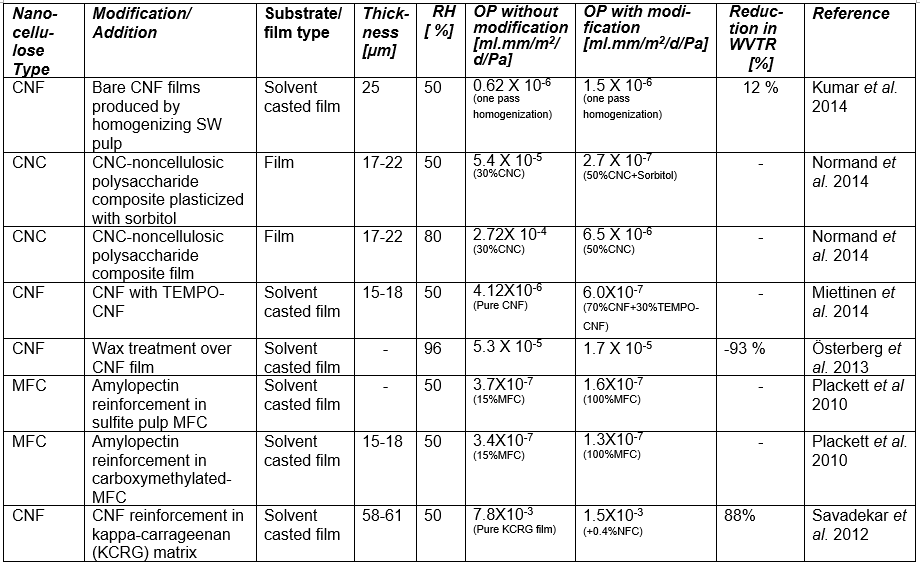
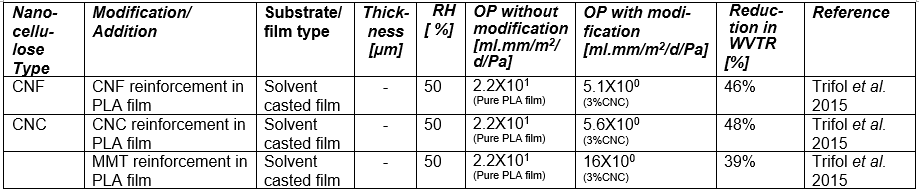
If one processes plant-based material to remove the lignin and then treats the isolated cellulose with a strong acid or other suitable reagents to degrade and remove the less crystalline domains and any residual hemicelluloses, then, by optimizing the conditions of treatment, one can obtain a suspension of cellulose crystallites (Mariano et al. 2014; Nelson et al. 2016). Typical sizes of cellulose crystallites range from about 3 to 30 nm in thickness and few hundreds of nm in length, depending on the plant source (Elazzouzi-Hafraoui et al. 2008; Eichhorn 2011). Nanocellulose crystals also can be obtained from other cellulose sources such as algae (Feng et al. 2015b; Hai et al. 2015; Chen et al. 2016b), tunicin (Dufresne 2012; Piao and Zhang 2016), and bacteria (discussed in a different section). Studies related to the use of CNCs in packaging are listed in Table A in the Appendix of this article (see first column, in which the type of nanocellulose is identified). Table B in the Appendix provides a summary of barrier performance findings for oxygen and water vapor transmission in films composed primarily of nanofibrillated cellulose.
Relative to the production of cellulose-containing packaging materials, CNCs represent the smallest, most fundamental option. The crystalline content of cellulose in raw biomass ranges from about 25 to 75% (Xu et al. 2013), whereas the crystallinity of CNCs has been reported as about 85% according to X-ray diffraction tests (Aulin et al. 2009a). The CNC particles resulting from the hydrolysis of native cellulose are rigid and relatively straight, with aspect ratios generally in the range of 11 to 67 (Bras et al. 2011). These dimensions have two implications regarding thin film structures. On the one hand, they set a practical lower limit on the conceivable thickness of thin films comprising CNCs. On the other hand, they entail a large ratio of surface area to mass; this implies that any surface treatments of the CNCs are likely to be demanding, if needed.
The surface chemistry of CNCs has been found to be dependent on the mode of isolation. Sulfuric acid digestion of cellulose to obtain CNCs yields a negative surface charge, which is due to the presence of sulfate half-ester groups (Mascheroni et al. 2016). The cited authors showed that higher negative charge density can be achieved by using ammonium persulfate as the oxidant during the treatment of cellulose to release the nanocrystals. In that case, dissociation of surface carboxylic acid groups would account for the negative charge. Alternatively, a negative charge can be imparted by phosphorylation (Naderi et al. 2016). The negative charges can be beneficial in aqueous media as a means of keeping the CNCs in stable suspension. Other modes of digestion such as HCl or enzymes do not impart the negative charge to the surfaces, though negative charges can result from secondary treatment, as with TEMPO-mediated oxidation (George et al. 2010). Chen et al. (2016a) employed difunctional carboxylic acids to impart a strong negative charge to both CNC and NFC.
Highly fibrillated cellulose
The term “highly fibrillated” is used here in recognition of the difficulty in drawing a clear differentiation within a broad, continuous range of possible mechanical treatments (Kangas et al. 2014; Khalil et al. 2014). In addition to refining, homogenizing, and grinding procedures, NFC also can be prepared by “counter-collision” of aqueous streams (Jiang et al. 2016). The terms “nanofibrillated cellulose” (NFC) (Siro and Plackett 2010; Lavoine et al. 2012; Sandquist 2013) and “microfibrillated cellulose” (MFC) (Aulin et al. 2012; Österberg et al. 2013; Khalil et al. 2014; Simao et al. 2015) are both used in the literature, with an implied understanding that the widths of fibrils ought to determine which term is more appropriate. In either case, both the lengths and widths of component fibrils are substantially larger than those of the CNCs already discussed. According to Chinga-Carrasco and Syverud (2010), the individual fibrils within NFC are typically in the range of 20 to 30 nm in width. Aulin et al. (2009a) found highly fibrillated cellulose samples to have crystallinities in the range of 60 to 70%. Another difference is that highly fibrillated fibers will clearly contain higher amounts of non-crystalline cellulosic matter. Thus, in general, highly fibrillated celluloses will tend to be more flexible in the wet state when compared to a crystalline cellulose structure. The term “nanocellulose aggregate” has sometimes been used to draw attention to some preparations of highly fibrillated cellulose in which bunches of fibrils remain attached together (Ambrosio-Martin et al. 2015a). According to Cowie et al. (2014), the market potential of highly fibrillated cellulose products is much greater than that of CNCs.
Regarding the preparation of nanocellulose films, various studies have indicated that the flexible nature of NFC gives it the potential to achieve high density in cross-linked structures that are formed, achieving low porosity and high resistance to air permeation (Aulin et al. 2010a). Belbekhouche et al. (2011) observed higher resistance to air permeation in films prepared from NFC relative to films prepared from CNC suspensions. Table A lists some essential information about numerous studies in which highly fibrillated cellulose (MFC or NFC) has been considered for films or layers for packaging.
Bacterial cellulose
In theory, bacterial cellulose (BC) has some important inherent advantages in terms of preparation of nanocellulose material. Unlike biomass derived from wood and other plant sources, bacterial cellulose contains neither lignin nor heteropolysaccharides (Feng et al. 2015c). The properties of BC are highly dependent on the bacterial source, and fibrils having widths in the range of 10 to 50 nm have been reported (Moon et al. 2011). The BC particles are very long and flexible in the wet state. Studies in which bacterial cellulose was evaluated for its properties in packaging applications are listed in Table A.
Because certain purified bacterial cellulose products have been regarded as “food grade,” BC has been considered for direct application in food items, such as vegetables, fruits, and meat, as edible films (George and Siddaramaiah 2012; Johnsy and Siddaramaiah 2012; George et al. 2016; Padrao et al. 2016).
NANOCELLULOSE IN FILMS AND COATINGS
An important function of a package can be to inhibit the passage of gases or liquids into or out from the contained products. Nanocellulose has potential usage in various layers or coatings, which may contribute to barrier properties. This section will deal with some contrasting types of nanocellulose-based layer types and some markedly different means of achieving them. Four of the most widely studied means of preparing these films or “nanopapers” that contain nanocellulose are illustrated schematically in Fig. 3.
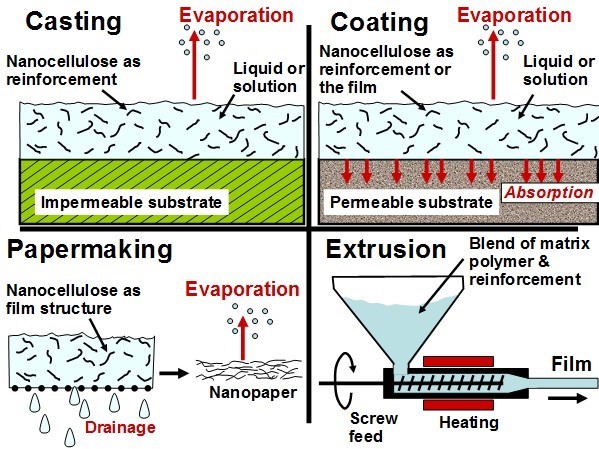
Fig. 3. Schematic illustration of four primary means of preparing thin films that contain nanocellulose, emphasizing differences in how liquid (if any) is mainly withdrawn during preparation of the film or nanopaper
These ways of forming films can be called casting, coating, papermaking, and extrusion. Extrusion will be considered first, then cast film processes, processes that resemble papermaking, and then coating processes.
Extruded Films
Extrusion can be defined as a process in which a substance or mixture is forced through a die at high pressure and temperature to form a sheet, fiber, filament, or other continuous form. This type of forming is widely used in preparation of lamination for packaging based on synthetic plastic materials. When employing nanocellulose for such strategies, some key issues might include breakage, thermal degradation, alignment of nanocellulose, and high viscosity due to the high aspect ratio of the particles. Another key aspect is the manner in which one achieves a solid-type layer after having essentially handled the composition as a liquid in the course of the extrusion. Thus, the subsections that follow will deal with such options as cooling (after having melted a matrix material) or undergoing a chemical reaction to cure the composition. In other words, the resulting layer may be either a thermoplastic or a reactive system.
Melt extrusion
Melt processing has been employed in numerous research projects in which nanocellulose was used as a minor component to reinforce a thermoplastic resin (Seydibeyoglu and Oksman 2008; Martínez-Sanz et al. 2012, 2013a,b,c; Suzuki et al. 2013, 2014; Fortunati et al. 2014; Ambrosio-Martin et al. 2015a; Arrieta et al. 2015; Ferrer et al. 2016a; Herrera et al. 2016; Lendvai et al. 2016). After emerging from the die of the extruder, the material cools below the melting point of the matrix polymer. Generally, it has been found that inclusion of nanocellulose increases the modulus of elasticity in such applications. Relatively low-melting polymers are often preferred in order to avoid thermal damage to the cellulosic reinforcement during compounding. Alternatively, researchers who want to utilize nature-based products have selected poly-(lactic acid) (Martínez-Sanz et al. 2012) or thermoplastic starch mixtures (Lendvai et al. 2016) as the matrix. Herrera et al. (2016) showed that the results were strongly affected by the rate of cooling in the case of poly-lactic acid film reinforced with CNC. Rapid cooling yielded more amorphous, transparent, and compliant films.
Curing
By relying upon a reaction, rather than cooling to solidify the extruded film, there can be an opportunity to avoid the high temperatures required for melting or the high shear stresses associated with the high viscosity of a fully polymerized matrix. Aulin and Ström (2013) considered such a system in which autoxidation of an extruded film brought about solidification. A patent by Schade et al. (2015) lists a “curing agent” as an option to cure a nanocellulose-reinforced film in the course of its extrusion. Curable resins such as epoxy also have been impregnated into pre-formed cellulosic films (Lee et al. 2012)
Pre-milling and pre-mixing
As a way to improve the performance of extrusion operations, efforts to improve the initial blending of ingredients can be important. Ambrosio-Martin et al. (2015b) used ball milling to improve the incorporation of freeze-dried CNC aggregates in a more fully dispersed form. In a related work (Ambrosio-Martin et al. 2015a), it was shown that more favorable properties could be achieved by pre-blending reinforcement with the matrix prior to extrusion. Though freeze drying is preferred as a means of minimizing aggregation of nanocellulose, the relatively pure cellulosic surfaces of CNC are highly prone to the development of mutual hydrogen bonding upon drying (Sanchez-Garcia and Lagaron 2010; Baez et al. 2014; Lindström and Aulin 2014).
Casting of Nanocellulose Films from Liquids
When one’s goal is to prepare a nano-cellulose-based film or layer having either 100% or a high proportion of nanocellulose in it, then extrusion may not be practical due to poor flow characteristics at moderate to high solids levels. Instead, it makes sense to suspend the nanocellulose in a suitable liquid or solution that can be subsequently evaporated. Two main classes of such “casting from solution” systems can be differentiated: casting from aqueous solution (or pure water) and casting from a non-aqueous liquid. The former case, using water, has the potential advantage of allowing strong hydrogen bonding to take place among the cellulose nanoparticles during the course of drying. Alternatively, non-aqueous casting systems have a potential advantage of allowing dissolution of various water-insoluble matrix materials that may influence the properties of a resulting layer or film. In either case, the proportion of solids relative to the evaporable liquid will depend on such factors as being able to uniformly disperse particles of relatively high aspect ratio, while on the other hand having to evaporate a lot of liquid.
Aqueous media
Numerous researchers have prepared nanocellulose-based films from aqueous media, including aqueous solutions and suspensions. The following studies pertain to preparation of relatively pure cellulose films, using plain water as a casting medium: (Dufresne et al. 1997; Yano and Nakahara 2004; Fukuzumi et al. 2009; Aulin et al. 2010a; Minelli et al. 2010; Rodionova et al. 2012a,b; Tammelin et al. 2013; Palaninathan 2014; Lu et al. 2015).
Notably, Dufresne et al. (1997) discovered that the pectin component of their highly fibrillated sugar beet cellulose played a key role in strength development of the sheets formed when the cast film was dried. Yano and Nakahara (2004) observed a doubling of yield strain and bending strength upon addition of 2% oxidized starch to the formulation, on a dry basis. Thus, even in compositions that are mostly cellulose, it can be advantageous to have some amount of water-soluble or water-swellable polymer present that can function as a binder. It appears that more research related to this topic is merited.
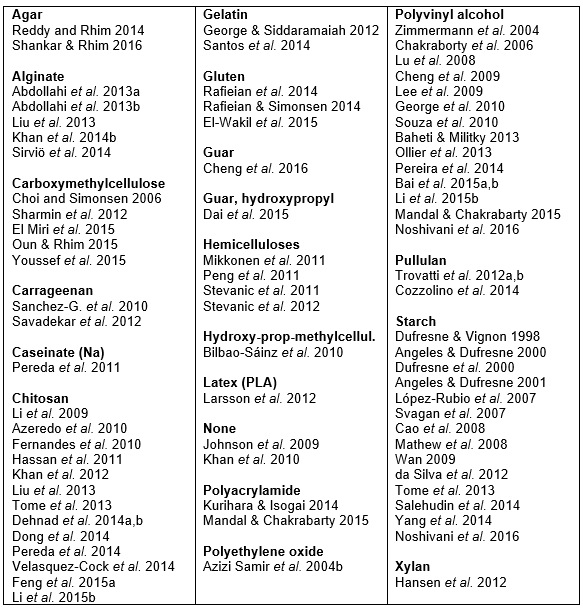
When an aqueous solution of a soluble polymer is employed in a casting and evaporation procedure, the dissolved matter becomes incorporated into a composite product. Many studies related to this were found in the present search of the literature. Table 1 lists such studies according to the type of solvent (if any) that was dissolved in the aqueous solution.
The presence of nanocellulose as reinforcement in a polymer film can have diverse effects on the subsequent processing and properties. López-Rubio et al. (2007) discovered that microfibrillated cellulose could play a role analogous to that of a plasticizer, replacing glycerol in facilitating the preparation of high-quality films from amylopectin. In the absence of MFC it was not possible to prepare the highly uniform, strong films without the addition of glycerol. Tammelin et al. (2013) described how the application of a water-based nanocellulose formulation to a support surface, followed by drying in place, can be a convenient way to avoid problems of shrinkage in separately-prepared films. Toivonen et al. (2015a) showed that the transparency of films could be retained effectively by using a solvent-exchange process as a means of drying. The nanocellulose in those products made it possible to maintain a stable mesoporous structure, and the films showed promise for use in air filtration.
Table 1. Solutes Employed in Studies of Aqueous “Cast Film” Procedures
Non-aqueous media
When the matrix phase to be reinforced by nanocellulose is too hydrophobic to dissolve in water, non-aqueous solvents have been employed in the preparation of such films and coatings (Grunert and Winter 2002; Petersson and Oksman 2006; Sanchez-Garcia et al. 2008; Ayuk et al. 2009; Petersson et al. 2009; Hossain et al. 2011; Fortunati et al. 2012b; Hassan et al. 2012; Salmieri et al. 2014b; Song et al. 2014; Fortunati et al. 2015; Kiziltas et al. 2015; Urbina et al. 2016). By employing a solvent with a sufficiently low boiling point, the film preparation can be carried out with good distribution of the reinforcement, suitably low viscosity to allow easy spreading of the film, ready evaporation of the solvent from the film, and avoidance of the need for elevated temperatures, since the matrix polymers are dissolved rather than melted. Likewise, Aulin et al. (2013) regarded solvent-cast poly-lactic acid films as a leading “benchmark” of performance, which they attempted to improve upon by further surface treatments.
One of the challenges faced by researchers employing non-aqueous solvent-casting with the inclusion of nanocellulose solids is the incompatibility of ordinary cellulose surfaces with relatively non-polar matrix polymers such as poly-lactic acid (PLA) and cellulose acetate butyrate (CAB). Grunert and Winter (2002) pioneered the modification of nanocellulose for such systems, using trimethylsilane to make the surface of bacterial cellulose less hydrophilic. Several other research groups have employed related strategies to hydrophobically modify nanocellulose materials for use in solvent-casting with a hydrophobic matrix (Hassan et al. 2012; Song et al. 2014). Alternatively, Fortunati et al. (2012b) found that uniform mixing of un-modified nanocellulose in such a system could be achieved by adding a surfactant, due to reduction in surface energy of nanocellulose by surfactants.
Filtration and Papermaking Processes
Papermaking can be described as a process in which a suspension of cellulosic fibrous matter is collected on a screen, followed by drying and the development of inter-fiber hydrogen bonding. A number of researchers have demonstrated such a process when using nano-fibrillated cellulose (Nakagaito and Yano 2004, 2005, 2008a,b; Henriksson et al. 2008; Nogi et al. 2009; Syverud and Stenius 2009; Sehaqui et al. 2010, 2011; Larsson et al. 2012; Hu et al. 2013; Rojo et al. 2015). Keshvarzi et al. (2015) prepared paper-like films from a gelled mixture of nanocellulose and zeolites. These films were found to have a high ability to absorb odors. Such preparation methods generally can achieve relatively high strength, high resistance to oxygen, and high transparency (Klemm et al. 2011; Lindström and Aulin 2014).
Alternatively, nanofibrillated or microfibrillated cellulose has been added to suspensions of ordinary wood-pulp fibers in order to achieve higher strength of the resulting paper (Ahola et al. 2008a,b; Eriksen et al. 2008; Schlosser 2008; Syverud et al. 2009; Guimond et al. 2010; Song et al. 2010; Taipale et al. 2010; Gao et al. 2011; Husband et al. 2011; González et al. 2012; Johansson et al. 2012; Charani et al. 2013; Ankerfors et al. 2014). Slower dewatering during paper forming has been observed (Taipale et al. 2010), though residual lignin and added cationic polymers have been found to be helpful to promote dewatering in such cases. Otherwise, the dewatering is too slow due to the high surface area of nanocellulose, which has more capacity to hold water than conventional cellulose (Taipale et al. 2010; Rojo et al. 2015).
Coating Processes
A coating process can be defined as the application of a slurry onto a porous surface, such that the solid contents are converted into a film that has good adhesion to the underlying matter. The liquid suspending medium may be partly absorbed into the underlying material, and the rest is typically evaporated directly. According to Kiviranta (2000), most of the paper and board products currently being used for food packaging already have some kind of coating. Accordingly, the presence of nanocellulose in coatings for paper deserves consideration.
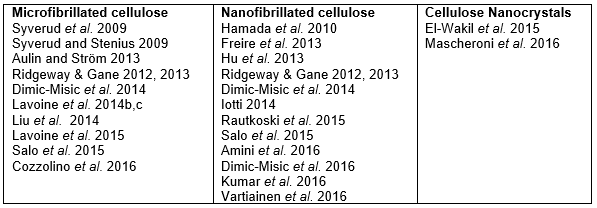
In cases of particular interest to packaging, the support surface can be paper or paperboard, and the absorption of solution into the pores can provide a primary means of initially draining the suspending medium from the coating layer (Lindström and Aulin 2014). Coatings of nanocellulose suspensions onto paperboard have been reported, and the topic has been reviewed by Rastogi and Samyn (2015). Table 2 lists such studies based on the type of nanocellulose.
Table 2. Studies in which Micro- or Nanocellulose Was Applied as a Coating
In addition to the listed studies, Nygårds et al. (2011) employed an offset printing approach to apply various nanocellulose-based barrier coatings. A potential advantage of this kind of system is that absorption of water by the underlying paper can rapidly immobilize the coating. Kumar et al. 2016) demonstrated the feasibility of a roll-to-roll coating system with non-contact infra-red drying and air drying to apply a CNF coating to paper at speeds up to 30 m/min.
According to Lindström and Aulin (2014), the inherently high viscosity of suspensions of nanofibrillated cellulose pose great challenges to the industrial implementation of such technology. In some coating systems it may be necessary to lower the solids in the formulation in order to achieve suitable flow properties. Salo et al. (2015) found that highly fibrillated cellulose could serve the role of “water retention aid” in a coating formulation, helping to promote the leveling of the coating after its application to a paper surface. As noted by Syverud and Stenius (2009), such coating layers can increase paper strength and reduce oxygen transmission through the paper. Charani et al. (2013) compared the effects of adding microfibrillated cellulose to the fiber slurry or as a coating on paper. The coating approach was found to be more effective in reducing air permeability.
Due to their lower aspect ratios, typical cellulose nanocrystals do not pose such great challenges related to rheology of their suspensions as do highly fibrillated cellulose materials at similar solids levels. Li et al. (2013a) applied cellulose nanocrystal suspensions to various substrates, including regenerated cellulose film. Liu et al. (2015) prepared composites of nano-Fe3O4 with CNC and used these nanocomposites to prepare conductive paper by a coating method. Yang et al. (2014) reported the preparation of starch solutions containing CNC at the 0.3% level on starch solids; their application to paper in a size press operation had a favorable effect on paper strength and resistance to air permeation.
Coating from non-aqueous solution onto a paper substrate has already been mentioned in the context of casting of films (Song et al. 2014). The cited authors applied blended mixtures of surface-hydrophobized nanocellulose in a solution of poly-lactic acid onto paper. The presence of 1% CNC in the cast-coated PLA film resulted in a low water vapor transmission rate.
Impregnation of Nanocellulose Films
Several researchers have explored procedures similar to coating in which the applied fluid was able to permeate a previously-prepared film of nanocellulose. For instance, the following authors reported improvements in various barrier and strength properties of the thin films achieved through such treatments (Wan et al. 2009; Lee et al. 2012; Aulin and Ström 2013; Barud et al. 2013). Nakagaito and Yano (2004, 2005, 2008a) reported similar work in which fibrillated kraft pulp impregnated with phenolic resin was compressed under very high pressure to make high-strength nanocomposites. By using impregnation of an existing nanocellulose film with nanofillers, high contents of cellulose can be achieved. Also, the impregnant may be able to aid in sealing off some pores.
Layer-by-Layer Processes
Using a sequential adsorption of oppositely charged polyelectrolytes, it is possible to build up well-organized multilayers having very uniform and controllable thickness (Decher 1997). Some researchers have applied the same approach to preparing films incorporating nanocellulose, essentially substituting the nanocellulose suspension in place of the negatively charged polyelectrolyte solutions typically used in such procedures (Wågberg et al. 2008; Aulin et al. 2009b, 2010b, 2013; de Mesquita et al. 2010; Li et al. 2013b; Marais et al. 2014). Alternatively, it is possible to convert nanocellulose to a positively charged form and make the opposite substitution (Aulin et al. 2010b). Aulin et al. (2010b) discovered that such films exhibited considerable strength even before the film was dried, i.e. a kind of “green strength.” The cohesion of the undried films was attributed to electrostatic attraction between oppositely charged surfaces. Aulin et al. (2013), who prepared 50-pair-layer structures (nanofibrillated cellulose and polyethyeneimine in each bilayer), were able to exceed the oxygen permeability resistance of solvent-cast NFC films through this route. Ankerfors et al. (2016) demonstrated the application of microfibrillated cellulose layers onto mechanical pulp fibers, with the incorporation of charged starch derivatives and poly-(amidoamine epichlorohydrin) wet-strength resins. The CTMP pulp, treated in this way, gave high levels of strength to the paper.
A positive attribute of polyelectrolyte multilayer deposition as a way to create nanocellulose-containing thin films is its great flexibility to achieve a wide range of properties, which can be extremely uniform down to nanometer dimensions. For instance, Aulin et al. (2009b) prepared superoleophobic (contact angle with glycerol >90) films by deposition of cellulose nanocrystals onto a silica substrate, followed by a layer of fluoropolymer. Aulin et al. (2013) were able to tune the barrier properties of the films by varying the procedural details of successive adsorption of polyethyleneimine and nanocellulose. Marais et al. (2014) demonstrated unusually high straining ability of such films. They also demonstrated a large difference in properties, depending on which of the successive layers was the last to be deposited in a multilayer film structure. By incorporating tin-doped indium oxide, carbon nanotubes, and silver nanowires into a multilayer structure with nanocellulose, Hu et al. (2013) prepared films that were electrically conductive, in addition to being transparent.
Application procedures for polyelectrolyte multilayer deposition are inherently slow, relative to high-speed manufacturing processes. Time is required at each step for the respective polyelectrolyte to diffuse from a solution and form a charged complex with the opposite charges of the substrate or of a previous deposited layer. Most procedures used for polyelectrolyte multilayer preparation call for a rinsing step between each adsorption step.
Foam Structure Preparation
Many of the same factors already discussed in the context of thin films also have relevance to the preparation and properties of foam materials, which can be envisioned as structures formed from the thin film walls of bubbles (Lindström and Aulin 2014; Ago et al. 2016). For instance, Tchang Cervin et al. (2014) patented the preparation of a foam composition including nanofibrillated cellulose. Through the incorporation of a hydrophobic amine additive, the foam was rendered resistant to water, and it also was claimed to be effective as a gas barrier. The incorporation of the nanocellulose into a foam wall structure provides rigidity to the material (Pääkkö et al. 2008; Srinivasa et al. 2015). To avoid the collapse of such foams during drying, freeze-drying is the usual approach in the cited works.
Curing Processes
As a means to achieve the desired properties, some form of heating or drying is often the final step in the preparation of a thin film incorporating nanocellulose. Such “curing” steps are considered below.
Several research teams have described procedures whereby nanocellulose-containing films have been heated or subjected to photo-initiation in order to bring about chemical reactions that cure the films (Shimazaki et al. 2007; Stoica-Guzun et al. 2013; Bai et al. 2015a,b; Schade et al. 2015). For example, the article and patent by Bai et al. (2015a,b) describe the use of UV light to cure a formulation that included nanofibrillated cellulose, polyvinyl alcohol, a cross-linking agent, and a photo-initiator. A patent by Schade et al. (2015) describes a similar approach, but the curable matrix consists of either a phenol-formaldehyde resin or an isocyanate formulation.
Hot-pressing is another well-known curing strategy, which recently has been applied in the preparation of films and layers containing nanocellulose (Larsson et al. 2012, 2016; Qing et al. 2012; Khan et al. 2013; Österberg et al. 2013; Figueiredo et al. 2015; Schade et al. 2015). Larsson et al. (2012) and Figueiredo et al. (2015) took advantage of the thermoplastic nature of polylactide and polycaprolactone, respectively, in heat-preparation of a composite films with nanocellulose. Österberg et al. (2013) found that heating was able to increase the water-resistance of films prepared from nanofibrillated cellulose, and that water-resistance could be further enhanced by wax coating.
A specialized drying procedure can sometimes be used if there is a motivation to preserve a high surface area or other specialized effects in a nanocellulose-based thin film. Thus, Sehaqui et al. (2011) exchanged the water in nanofibrillated cellulose hydrogels with liquid CO2, supercritical CO2, and tert-butanol, followed by drying by sublimation. The resulting films had a high porosity (e.g. 56%) and a high specific surface area (as high as 480 m2/g). Bardet et al. (2015) used vacuum drying (75 °C, 1 h) to remove sulfate ester groups from the CNC surfaces in the film. Rodionova et al. (2012a) discussed hornification effects, i.e. a loss of re-swelling ability, which can take place during routine drying of nanocellulose-based films. Effects of different drying methods on the properties of bacterial cellulose were reported by Feng et al. (2015a). Freeze drying, relative to conventional oven drying, yielded much lower watering holding capacity (about 6000% in comparison to 12,000%) but a much higher water absorption rate (about 880% vs. about 140%).
As noted by Tammelin et al. (2013), nanocellulose-based films have the potential to shrink greatly during evaporative drying from an aqueous suspension. To avoid this, Baez et al. (2014) evaluated different modes of restraint of the dimensions during drying. Stretching of the films prior to drying yielded the highest alignment of fibrils within the structure. It also yielded the highest directional strength and stiffness in the stretched direction. Fully restrained drying achieved a non-aligned film with relatively high strength characteristics relative to an unrestrained sample. Lindström et al. (2012) investigated the effects of cyclic exposure to different humidities. The nanocellulose films and aerogels showed substantial creep behavior, indicating that creep was dominated by local events within the film during changes in humidity.
BARRIERS AND PATHS TO IMPLEMENTATION
Based on literature already cited, there are many ongoing concerns related to performance issues of nanocellulose-based films, coatings, and layers. These are summarized in Table 3, along with some ways that researchers have attempted to address those concerns. The final section of the review article will discuss progress that has been made relative to the challenges posed in Table 3. Emphasis will be placed on work related to resistance to oxygen and water vapor diffusion through the films, especially in humid or wet environments.
Oxygen Barrier Performance Concerns
Oxygen barrier issues will be considered first here, mainly in recognition of the outstanding performance levels reported by some authors (Fukuzimi et al. 2009; Syverud and Stenius 2009; Aulin et al. 2010a; Cozzolino et al. 2014; Kisonen et al. 2015). It seems likely that at least part of the recent spurt of academic and industrial attention directed toward nanocellulose films in packaging can be traced to a superior ability to block the passage of oxygen.
A high level of success, in any arena, can entail a subsequent higher level of scrutiny. There can be concerns that maybe, by modest modifications in procedures or composition, it might be possible to make very large improvements in the first-generation films created in the laboratory thus far. On the other hand, high levels of oxygen hold-out achieved in the lab might not hold true when attempting to implement the same scheme on an industrial scale. For instance, defects and leakage past the barrier might become an important issue when trying to coat large areas or when trying to form films rapidly. Issues related to effects of humidity and moisture will be considered subsequently.
As can be recognized by inspecting the corresponding entries in Table A (see Appendix), a great many researchers have reported on the ability of nanocellulose-based films to limit or to almost prevent the passage of oxygen. Some key examples are listed by Aulin et al. (2010a). The performance of various nanocellulose-based films to resist permeation by oxygen is summarized in Table B of the Appendix.
Table 3. Challenges Concerning the Performance of Nanocellulose-based Thin Films, Coatings, and Layers for Packaging
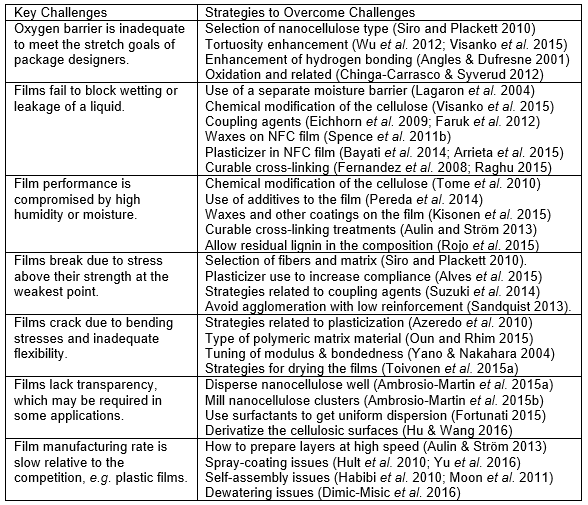
Some key factors accounting for the ability of nanocellulose films to block gas transport will be summarized. As noted already by Lagaron et al. (2004), one of the essential principles underlying resistance to diffusion of gases through packaging materials is to have a very low solubility of the gas in the material. In general terms, very low solubility implies a large difference in factors such as hydrogen bonding ability. Both oxygen and nitrogen gases are non-polar compounds, whereas cellulose is rich in polar, hydrogen-bonding –OH groups. The other key factor noted by Lagaron et al. (2004) is a high cohesive energy density, which again can be attributed to the hydrogen bonding. According to Lagaron et al. (2004), resistance to gas permeation through a solid film is often correlated with the glass transition temperature; a high Tg value implies a generally immobile nature of molecular segments, so that gas diffusion through the material is not facilitated. As long as the film is uniform and defect-free, effective barrier performance can be expected.
As noted by Aulin et al. (2010a), nanofibrillated cellulose, when dried as a film from aqueous solution, can form a very dense structure. The high level of hydrogen bonding within the structure, involving a high density of cohesive energy, may be important not only in achieving higher film density, but also in avoiding gaps in such films. As illustrated informally in Fig. 4, due to the hydrogen bonding between the molecular chains, cellulose has sufficient cohesive energy density to hold the material together as a film that is sufficiently dense, on a molecular level, to be able to block gas molecules. Such film characteristics can be effective for impeding the permeation of oils as well as gases (Aulin et al. 2010a).

Fig. 4. Concept of hydrogen bonding within a nanocellulose film, leading to a highly dense structure that can block gas molecules
Hansen et al. (2012) found that whereas pure cellulose films, prepared from NFC, were highly resistant to oxygen, such resistance was seriously hurt by the incorporation of plasticizers in the formulation of the films. Such observations can serve as confirmation of the hypothesis that hydrogen bonding and a high energy of interaction among the cellulosic elements in the film are mainly responsible for the ability to resist oxygen. A plasticizer, which by its nature has much less ability to hydrogen bond, would provide less-bonded regions that could locally reduce the solid density, which would be more conducive for the diffusion of gas. This concept is illustrated in Fig. 5.

Fig. 5. Concept that the presence of plasticizer molecules can be expected to interrupt the network of hydrogen bonding within a nanocellulose film
Type of nanocellulose vs. O2 barrier performance
Belbekhouche et al. (2011) prepared thin films from cellulose nanocrystals and microfibrillated cellulose, both obtained from sisal fibers. The film prepared from the CNC was much more permeable to gases, and the difference was attributed to a more porous nature of such films. Thus, the inherently higher flexibility of nanofibrillated cellulose may be essential if the goal is to achieve high resistance to air permeation through a film composed only of cellulose. Indeed, high levels of resistance to oxygen have been reported from studies focusing on NFC (Fukuzimi et al. 2009; Syverud and Stenius 2009; Aulin et al. 2010a; Cozzolino et al. 2014; Kisonen et al. 2015).
In light of the findings just cited, one might expect that such resistance might increase with increased fibrillation. Siro et al. (2011) found otherwise. Oxygen permeability was not significantly influenced by the number of times the material had been passed through a homogenizer. Such results suggest that the lowest level of microfibrillation considered by the cited authors was already sufficient to impart enough flexibility into the wet material in order to be able to form a dense, air-impermeable structure upon drying.
Oxidation and negative surface charges vs. O2 barrier performance
Many researchers aiming to achieve oxygen barriers with nanocellulose particles have employed underivatized NFC. Visanko et al. (2015) followed a contrasting approach, starting with CNC, then derivatizing the cellulose with TEMPO-mediated oxidation, followed by reductive amination to connect butylamino groups. High resistance to oxygen transport was observed even at 80% relative humidity. Likewise, Chinga-Carrasco and Syverud (2012) employed TEMPO oxidation to produce CNF, which was then used to form films that exceeded the oxygen barrier performance of parallel samples prepared without oxidation. The authors attributed the high performance to the dense nature of the resulting films. Both of these effects, as cited above, may be regarded as consequences of the polar nature of carboxylic acid groups, which in principle might give a higher cohesive energy density to the film material.
Mascheroni et al. (2016) observed strong resistance to oxygen permeation when CNC was applied as a coating to poly(ethylene terephthalate) films. Notably, better blocking of oxygen transport was achieved by the use of CNCs produced by ammonium persulfate (APS), rather than the usual sulfuric acid. The results were attributed to a higher negative charge density induced by the APS, in the form of carboxylate groups on the CNC. Related and confirmatory results were obtained by Naderi et al. (2016), who phosphorylated NFC in order to increase its negative surface charge. Again, substantially lower oxygen permeabilities were observed when compared to the default NFC.
One can hypothesize that negative charges induced by the oxidation would inhibit early strong adhesion between cellulosic surfaces, thus allowing adjacent chains to organize themselves more densely during the gradual drying of a film (Hubbe and Rojas 2008). In other words, charge-charge repulsion between particles helps the particles to avoid forming clumps, and thus a denser, less permeable dry coating layer will have been formed once the drying process has been completed.
Tortuosity enhancement vs. O2 barrier performance
It has been widely assumed that gas diffusion cannot take place at all within or through crystalline domains of cellulose. It is reasonable to suspect that the direct blockage of gas diffusion by crystalline regions can account for at least part of the observed ability of nanocellulose films to impede passage of oxygen (Fukuzimi et al. 2009; Syverud and Stenius 2009; Aulin et al. 2010a; Lindstrom and Aulin 2014).
Figure 6 presents a view of how tortuosity may play a significant role in limiting gas diffusion through nanocellulose films. In this figure the film is assumed to be comprised of NFC. Each fibril in the structure is understood to be made up of cellulose nanocrystals (shown with darker shading) that are connected or surrounded by non-crystalline regions (shown without shading). The structure as a whole is assumed to be tightly bonded together, on account of the high flexibility of the individual NFCs in the wet state, before drying of the film. A gas molecule, when passing through the film, would be blocked each time that it encounters a crystalline region, which may occupy the majority of the volume. The lengthening of the diffusion paths would imply a slower rate of gas transport.
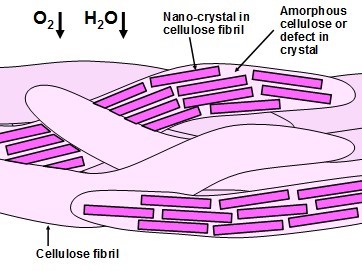
Fig. 6. Concept that crystalline regions inherent in native cellulosic structures are likely to play a role in impeding the diffusion and permeance of oxygen and water vapor, both of which would have to pass around the outsides of any crystalline domains
The very thin, platy nature of montmorillonite (i.e. bentonite) particles can be used advantageously in various films to reduce the permeation of gases (Rhim et al. 2013). The idea is that the crystalline nature of the particles precludes air passage through the particles, and their wide, flat shapes mean that gas molecules must take a longer path during their diffusion through the matrix polymer. Wu et al. (2012) found montmorillonite particles to be highly effective for decreasing the oxygen-permeability of films formed from TEMPO-oxidized NCFs.
Induced crystallinity vs. O2 barrier performance
Various researchers have shown that the addition of nanocellulose reinforcement in polymer films also has the ability to decrease gas permeation (Paunonen 2013a; Ambrosio-Martin et al. 2015a; Dhar et al. 2015). As discussed above, such results might be partly attributed to the relatively high crystallinity of most types of nanocellulose (Aulin et al. 2009a; Xu et al. 2013), which can help to explain their apparent ability to block oxygen permeation when present in certain polymeric matrices (Ambrosio-Martin et al. 2015a). Dhar et al. (2015) attributed the strong gas-barrier properties to the effective hydrogen bonding between cellulose and the matrix, as well as the more tortuous path required for diffusing gas molecules. Visanko et al. (2015) observed resistance to O2 permeability even at relatively high humidity when using periodate-oxidized-aminated CNC to form a single-component film. The promising results were attributed to tortuosity, giving rise to longer diffusion paths through the dense film.
Though mechanisms based on tortuosity have been shown to be valid in other circumstances, it seems doubtful that CNC would be greatly effective in blocking the progress of gas molecules when present at a relatively low percentage in a polymer matrix. The columnar shape of a CNC particle does not seem nearly as well suited for such purposes as, for instance, montmorillonite clay (Liu and Berglund 2012). Another possible explanation for enhanced barrier properties upon addition of CNC to a matrix polymer melt is that the crystalline regions could have functioned as nucleation sites for crystallization of the matrix polymer. If such a process leads to higher overall crystallinity, while still preserving a defect-free structure, then the barrier properties might be enhanced. In support of this concept, Camarero-Espinoza et al. (2015) reported that CNC induced increased crystallinity of poly-lactide after heating. Gas permeability was not measured in that study, though higher storage modulus was observed. Lu et al. (2016) also observed an increased rate of crystallization of poly-(lactic acid) with the inclusion of nanocellulose formate in the composite. Further studies are needed in order to determine whether such induced crystallinity can be used as a strategy to decrease gas permeability, when using nanocellulose reinforcement in thermoplastic matrix polymers.
Additives and coatings vs. O2 barrier performance
Increased resistance to oxygen transmission has been achieved by adding shellac, along with MFC (Hult et al. 2010). Though shellac forms a relatively water-repellent film, the main ingredients are rich in hydrophilic carboxyl and hydroxyl groups (Sharma et al. 1983). Hansen et al. (2012) found that high oxygen resistance was maintained when preparing composite films of xylan (hemicellulose) with NFC. These are examples of combining two ingredients, both of which have substantial hydrogen bonding ability, to achieve high-performing films.
Hambardzumyan et al. (2015) showed that films with promising O2-barrier performance could be prepared from lignins in combination with CNC. Fenton’s reagent was used to promote grafting of lignin onto the CNC surfaces. Related work was reported by Yang et al. (2016), who used CNC and lignin nanoparticles together in poly-(lactic acid) matrix; the two types of nano-reinforcement appeared to be acting synergistically.
Promising results have been found in some cases where a thin coating was applied on top of highly fibrillated cellulose films. Spence et al. (2011b) found that the application of a surface coating of wax on top of a microfibrillated cellulose layer achieved oxygen-resistance that exceeded that of low-density polyethylene. Kisonen et al. (2015) applied coatings of either native galactoglucomannan (GM) or a succinic ester of GM onto the surface of composite films of NFC and O-acetyl galactoglucomannan. Both coatings increased resistance to oxygen permeation, and they also more effectively prevented grease penetration. Such results suggest that NFC and the hemicellulose layer may constitute a synergistic pair, in which the NFC provides a stable structure and the hemicellulose, being more fluid, can seal any holes that may be present in the NFC film structure. This concept is illustrated in Fig. 7, where a wax is assumed. The promising nature of the results described in this paragraph, coupled with the simplicity of the preparation of such bilayer systems, suggests that this is a very promising area for future research.
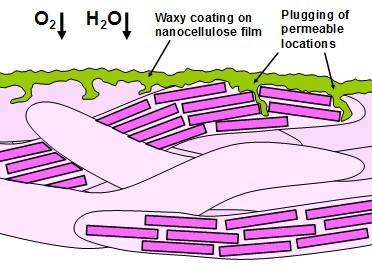
Fig. 7. Concept of a coating material that serves to “plug” locations in a nanocellulose film that may be defective or may offer higher gas permeability due to their amorphous nature
Fortunati et al. (2012a,b, 2013, 2015) obtained promising results for surfactant-modification of CNC. The surfactant employed was an acid phosphate ester of ethoxylated nonylphenol. The modified CNC was used as a reinforcement in poly-(lactic acid) (PLA) films. The surfactant tended to improve the distribution of CNC in the matrix, and the system also led to increased crystallinity of the PLA.
Polyelectrolyte complexes vs. O2 barrier performance
Some studies have reported enhancements of oxygen barrier performance with the formation of polyelectrolyte complexes (PECs) from aqueous solutions during the preparation of the film. For instance, in the following studies, the nanocellulose reinforcements had an opposite ionic charge from a polyelectrolyte, which would eventually play the role of matrix in the formed composite films (de Mesquita et al. 2010; Khan et al. 2012; Liu et al. 2013; Dong et al. 2014; Velasquez-Cock et al. 2014; Ibn Yaich et al. 2015; Li et al. 2015b). However, only in a few of these cases were the oxygen or grease permeability evaluated (Ibn Yaich et al. 2015; Shimizu et al. 2016). Liu et al. (2013) reported a system in which the positively charged polyelectrolyte chitosan was precipitated onto the surface of bacterial cellulose by adding phosphate ions; then, the chitosan-treated BC was combined with the anionic polyelectrolyte sodium alginate. Good strength and antibacterial effects were reported. Sirviö et al. (2014) used calcium ions to create complexation within a mixture of negatively charged sodium alginate and strongly negatively charged (carboxylated) NFCs. Excellent resistance to grease and water vapor permeation were observed. Shimizu et al. (2016) created complexes by adding multivalent cation solutions to TEMPO-oxidized NFC and achieved very low oxygen permeability through the resulting films.
Presumably, the strong contribution of opposite ionic charges to bonding might be expected to enhance the cohesive energy density of a PEC film, thus making it more difficult for gas molecules to pass. The concept is illustrated in Fig. 8, which emphasizes the ionic interaction between positively charged ionic groups on a polyelectrolyte and anionic groups at the surface of nanocellulose particle. Because their energy content can be much larger than hydrogen bonds, ionic interactions seem worthy of consideration, as a way to achieve high bonding within a nanocellulose-containing film. However, since the oxygen or grease permeability was evaluated only in a few cases (Ibn Yaich et al. 2015; Shimizu et al. 2016), there is a need for further research.
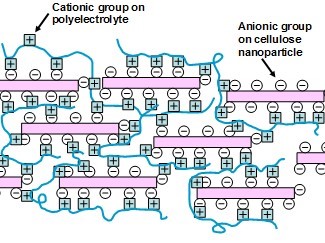
Fig. 8. Conceptual sketch of ionic bonding within a film comprised of anionically substituted nanocellulose particles complexed with a cationic polyelectrolyte, then dried
Plasticizers vs. O2 barrier performance
Some conflicting results have been obtained regarding the effects of plasticizing agents on oxygen permeability in different circumstances. As a general rule, plasticizers tend to hurt the cohesive energy density of a material. As a result, they tend to hurt resistance to permeation (Lagaron et al. 2004). Positive effects of plasticizers on oxygen or water vapor barrier performance have been reported by some authors (Bayati et al. 2014; Arrieta et al. 2015). Hansen et al. (2012) found that various hydrophilic plasticizers increased the equilibrium moisture content as a function of relative humidity, but that one of the plasticizers that they tested (sorbitol) reduced O2 permeability. In systems containing only MFC and optional glycerol, Minelli et al. (2010) found that glycerol was helpful for achieving uniform films. These films showed high resistance to gas permeability under very dry conditions. However, under humid conditions the barrier performance was decreased dramatically. Such varied findings suggest that there is a subtle balance between the conflicting effects of surfactants – at once tending to improve the uniform distribution of the cellulosic nanoreinforcement but simultaneously tending to interrupt the dense hydrogen bonded film structure depending on the chemical structure of plasticizers.
Moisture Sensitivity Concerns
Moisture sensitivity of O2 barrier performance
Sensitivity to moisture, including the effects of high humidity, is clearly a major obstacle to many potential applications of nanocellulose-based barrier films. Numerous researchers have discovered that resistance to oxygen permeation falls rapidly when the relative humidity rises (Martínez-Sanz et al. 2013a; Cozzolino et al. 2014; Miettinen et al. 2014; Tammelin and Varianen 2014). For instance, Cozzolino et al. (2014) found roughly 20 times higher oxygen transmission through MFC-based films at 80% relative humidity in comparison to completely dry conditions.
Lagaron et al. (2004) attributed such effects to the plasticizing effect of water within the cellulosic material; just like an organic plasticizer, the level of water molecules associated with high humidity conditions can be sufficient to weaken the film cohesion, thus speeding up the rate of gas diffusion. This effect is depicted schematically in Fig. 9. Though water molecules would be expected to be involved in hydrogen bonding, such bonds are not drawn into Fig. 9 due to their expected short lifetimes, compared with the stability of hydrogen bonds that are associated with dense polysaccharide structures. Miettinen et al. (2014) noted that film thickness tended to increase with increasing relative humidity, i.e. a swelling effect. The observed increasing oxygen permeability was consistent with the increased molecular-scale porosity of the NFC films that they studied.
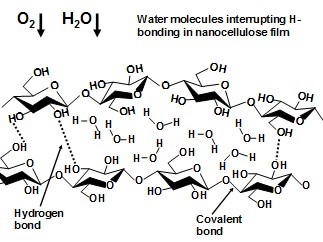
Fig. 9. Schematic picture of how the presence of water molecules within a nanocellulose film can be expected to interrupt the hydrogen bonds among –OH groups along the polymer chains
Using layers to overcome moisture sensitivity
A number of researchers have demonstrated packaging strategies in which cellulosic material is employed in a layered system, isolated from a humid environment, and thereby protecting the vulnerable material from the effects of moisture (Lagaron et al. 2004; Boumail et al. 2013b; Chinga-Carrasco et al. 2013; Österberg et al. 2013; Shade et al. 2015; Vartiainen et al. 2016). Despond et al. (2005) protected a bilayer of chitosan on a paper substrate by applying an outer layer of wax. The patent by Schade et al. (2015) describes the use of a multi-layered structure in which a layer with NFC is protected by water-resistant plastic layers. Österberg et al. (2013) found that very high resistance to oxygen could be maintained, even at high relative humidity, by coating of an NFC film with wax. Such systems are represented, in cross-sectional view, in Fig. 10.

Fig. 10. Schematic views of “sandwich” type film structures in which a nanocellulose film (offering high resistance to oxygen permeation when dry) is sandwiched between a pair of highly water-resistant layers
A question that appears to merit further research is whether such structures, as illustrated in Fig. 10, will gradually fail during long-term exposure due to eventual equilibration with a humid environment, even when the rates of water vapor permeability through the outer layers may be very low. It is reasonable to expect the moisture content of a thin nanocellulose layer to change significantly, even with just a minor influx of water molecules, due to the layer’s low mass.
Chemical modification to overcome moisture sensitivity
Ibn Yaich et al. (2015), who studied polyelectrolyte complexes involving cationically modified cellulose and an anionic xylan in aqueous solution, reported low oxygen permeability even at a relative humidity of 80%. Likewise, Shimizu et al. (2016) reported the persistence of strong resistance to oxygen passage at high humidity in the case of a TEMPO-oxidized cellulose film that had been soaked in solutions of salts of various multivalent ions, thus forming complexes. These results seem surprising given the tendency of many polyelectrolyte complexes to absorb water (Bajpai et al. 2016). Clearly more experimental investigation is warranted.
Balan et al. (2015) carried out studies in which MFC was used in combination with different types of the chitosan. Though chitosan has a positive charge, one of the modifications involved quaternization, making the polymer yet more cationic and fully water-soluble. Alternatively, chitosan was derivatized with C8 alkyl chains (alkyl chitosan) or carboxymethyl cellulose (CMC chitosan). Both the alkyl chitosan and the CMC chitosan improved the barrier to water vapor.
Another chemical approach that achieved high resistance to oxygen permeation even at high relative humidity was the butylamino functionalization of CNC (Visanko et al. 2015). From one perspective, the more hydrophobic nature of the modified nanocellulose would render it less sensitive to water molecules. But on the other hand, one is left to wonder how such modified nanocellulose would still be capable of achieving a high cohesive energy density, which is essential in order to effectively block oxygen gas (Lagaron et al. 2004).
Dufrense et al. (2000) observed beneficial effects of glycerol, as a plasticizer, on the water vapor barrier performance of starch films formed with or without NFC reinforcement. Performance increased by a factor of about five as the level of NFC was increased from zero to 20%. Incorporation of 30% glycerol in the formulation led to a further decrease in moisture diffusion by as much as a factor of five. Minelli et al. (2010) found that although glycerol plasticizer improved water vapor sorption at low levels of humidity, it had the opposite effect when the humidity was high.
Nanocellulose reinforcement to overcome moisture sensitivity
Under some circumstances the reinforcement of a thermoplastic matrix with nanocellulose has been found to decrease the film’s sensitivity to moisture. For instance, the presence of bacterial cellulose reinforcement in a thermoplastic poly-lactic acid (PLA) matrix was found to improve resistance to oxygen permeation at a relative humidity of 80% (Ambrosio-Martin et al. 2015a). The cited authors proposed that the effect was due to blockage of gas molecules by the cellulose itself. However, the content of cellulose in the composite films was, at most, 5%. Hence, it appears that the nano-reinforcement within the PLA matrix aided in the formation of a dense, defect-free layer, and that blockage of gas by cellulose crystalline domains probably played only a supplementary role. In addition, as mentioned earlier, the crystalline nature of the nanocellulose might have been affecting the extent of crystallinity in the matrix material as it cooled.
Related results have been achieved with elongated CNC, i.e. tunicin whiskers. Angles and Dufresne (2001) found that such reinforcement in a starch matrix helped achieve a strong and continuous film. The cited authors proposed that the good film properties, when combining starch with cellulosic nanoreinforcement, could be attributed to hydrogen bonding. Da Silva et al. (2012) used CNCs to reinforce cassava starch in the presence of sugars as plasticizers; the nanocellulose helped to reduce the extent of swelling and solubility in water. Follain et al. (2013) observed improved resistance to water vapor with the addition of CNC to poly(epsilon-caprolacone) films; the effect was attributed to tortuosity, i.e. a longer diffusion path for water molecules in the film. Saxena and Ragauskas (2009) showed that incorporation of CNC in a xylan film reduced the water vapor transmission by 74%. Cozzolino et al. (2014) observed excellent oxygen barrier performance with a film prepared from pullulans with an MFC reinforcement. However, the presence of the MFC did not prevent a sharp lessening of resistance to oxygen permeability at higher humidity levels, such as 65% and 80%. Dhar et al. (2015) found that CNC improved the oxygen barrier properties of poly(3-hydroxybutyrate) films, an effect that they attributed to good hydrogen bonding between the cellulose and the matrix.
To account for the effects in the reports cited above, one likely explanation is illustrated in Fig. 11. In part A of the figure, the polymer matrix is shown as being defective in some way, possibly due to its brittleness or due to problems related to film formation. In part B of the figure, the CNC particles are envisioned as helping to tie the nanostructure together as a defect-free continuum. In this type of mechanism, the level of CNC would probably be insufficient to have a significant effect on the lengths of diffusion paths by which gas molecules can pass through the film. Rather, the effect of the CNC on barrier performance would be attributed to a reduction in pores, cracks, or other defects in the film.
Fig. 11. Concept of how CNC reinforcement may, in some cases, improve barrier performance of a polymer film by decreasing the frequency of defects in the film
Montmorillonite and water-resistance
Several researchers have studied the influence of montmorillonite clay or related materials on the water-resistant properties of thin films that involved cellulose nanomaterials (Liu and Berglund 2012; Abdollahi et al. 2013b; Gamelas and Ferraz 2015; Noshirvani et al. 2016). Aulin et al. (2012) found greater oxygen resistance even at relatively high humidity when vermiculite nanoparticles were incorporated into NFC films. Liu and Berglund (2012) reported much lower oxygen transmission rates at 90% relative humidity upon the addition of either montmorillonite clay or a combination of the nanoclay and chitosan. Abdollahi et al. (2013b) found that both the CNC and the nanoclay decreased the water-solubility of alginate films, though the nano-clay was more effective for this purpose. Noshirvani et al. (2016) compared CNC and montmorillonite in starch-polyvinyl alcohol films and judged that the montmorillonite was more effective for improving strength and thermal stability.
Curing for water-resistance
Chemical reactions to cure and insolubilize a matrix polymer, usually by some form of cross-linking, have been used as a means to decrease the moisture-sensitivity of nanocellulose-containing barrier layers (Fernandez et al. 2008; Aulin and Ström 2013; Raghu 2015). Fernandez et al. (2008) employed gamma irradiation to cure ethylene vinyl alcohol films incorporating MFC as a reinforcement. The water barrier properties of the films were enhanced by the gamma radiation treatment. Aulin and Ström (2013) employed alkyd resins and cured the film by auto-oxidation. Choi and Simonsen (2006) found that simply heating a carboxymethyl cellulose (CMC) film, prepared with microcrystalline cellulose, resulted in water resistance. One possible explanation is that the heat was sufficient to promote esterification within the film, including the possibility of ester linkages between the CMC and the cellulose. Österberg et al. (2013) observed that the simple heating of NFC films imparted wet strength. Formation of ester cross-linking would seem unlikely in such a system, though the observed effects may have been due to coalescence of the adjacent cellulosic surfaces, an effect sometimes termed “aggregation” of the nanocellulose fibrils (Pönni et al. 2012).
Susceptibility to Wetting by Liquids
Hydrophobization to overcome adverse effects of wetting by water
If a liquid is not able to wet a porous barrier layer, then, as long as there are no serious defects, theories of capillary penetration predict that the liquid will not be able to pass through it (Hubbe et al. 2015a). Accordingly, various researchers have explored ways to render cellulose-based films resistant to wetting (Aulin et al. 2009b; Spence et al. 2010b; Rodionova et al. 2011; Yang et al. 2012; Abdollahi et al. 2013b; Kisonen et al. 2015; Visanko et al. 2015). Spence et al. (2010b) and Rojo et al. (2015) showed that contact angles of water on cellulosic films tend to increase with increasing lignin content within the range from about 1 to 14% lignin. Contact angles were reduced, thereby increasing water-wettability in cases where the films had been extracted with a benzene/ethanol mixture. Thus, if one’s aim is to resist water wetting, it makes sense to prepare highly fibrillated cellulose from raw biomass that still has lignin and extractive materials present in it.
Even though CNCs are composed of cellulose, which is very rich in water-loving –OH groups, the use of CNC’s as reinforcement in a hydrophilic polymer film sometimes can decrease the wettability. Thus, Abdollahi et al. (2013b) rendered alginate films increasingly resistant to wetting by incorporation of CNCs that had been prepared by sulfuric acid digestion of microcrystalline cellulose. The water contact angle increased from about 41° to about 74° as the CNC content was raised from 0 to 5%. Notably, the addition of sodium montmorillonite, over the same range, merely made the alginate film slightly more wettable by water. This may be another case in which the observed changes might be explained by the ability of the crystalline cellulosic material to induce increased crystallinity in the matrix material; it would be interesting to carry out additional research to determine whether the alginate films were more highly crystalline after having been dried in the presence of the CNCs. Pereda et al. (2014) achieved strong resistance to aqueous wetting by a combination of CNC incorporation and olive oil treatment of chitosan films.
Derivatization to resist wetting
As has been described in more detail in earlier review articles (Hubbe et al. 2015a,b), cellulosic surfaces can be changed from hydrophilic to hydrophobic by chemical derivatization. The following authors reported the hydrophobization of nanocellulose-based thin films by the covalent attachment of hydrophobic groups to the cellulose (Rodionova et al. 2011; Chinga-Carrasco et al. 2012; Yang et al. 2012; Follain et al. 2013; Visanko et al. 2015). Tchang Cervin et al. (2014) used a similar approach to achieve a hydrophobic solid foam of NFC; the hydrophobic treatment of the cellulose rendered the product hydrophobic.
Tome et al. (2010) derivatized bacterial cellulose membranes by esterification with hexanoyl chloride. The water contact angle was increased, along with resistance to water and gas diffusion. Rodionova et al. (2011) rendered MFC hydrophobic by acetylation; remarkably, this treatment did not seem to hurt inter-fibril bonding within the MFC films. Related results were obtained by Yu et al. (2014b) and Trifol et al. (2016a,b). Hu and Wang (2016) achieved hydrophobicity of polyvinyl alcohol films by reinforcing them with MFC that had been derivatized with hydroxypropyltrimethylammonium chloride. The derivatized CNC retained sufficient hydrogen bonding capability so that good dispersion was obtained, leading to increased strength. Kisonen et al. (2015) achieved a much higher level of hydrophobicity by coating a film of NFC and hemicellulose by use of an alkylsuccinic ester of the hemicellulose.
Superhydrophobicity and related
Extremely high resistance to wetting often can be achieved by a combination of nano-scale roughness and low-energy surface modification (Song and Rojas 2013; Hubbe et al. 2015a,b). Yang et al. (2012) demonstrated such an approach using CNC that was derivatized in various ways. Final treatment with a silane reagent rendered the films superhydrophobic by covalently bonding CNC and acrylic acid chains via silane bridges, meaning that contact angles of water droplets were greater than 150 degrees. Aulin et al. (2009b), taking a much more aggressive approach, used a polyelectrolyte layer-by-layer deposition approach to incorporate CNCs into a thin film. This was followed by treatment with fluorinated trichlorosilane. The result was a super-oleophobic surface that was able to even prevent wetting by oils.
More recent work (Guo et al. 2016) presents a facile process for tailoring the surface wettability and functionality of NFC films by a fast and versatile approach. Firstly, the NFC films were coated with a layer of reactive nanoporous silicone nanofilament by polycondensation that afforded reactive vinyl groups, thereby enabling simple UV-induced functionalization with various thiol-containing molecules via the photo “click” thiol-ene reaction. Modification with perfluoroalkyl thiols resulted in robust superhydrophobic surfaces, which could then be further transformed into transparent slippery lubricant-infused NFC films that displayed repellency against both aqueous and organic liquids with surface tensions as low as 18 mN·m-1. Transparent and flexible NFC films incorporated hydrophilic micropatterns by modification with OH, NH2, or COOH surface groups, enabling space-resolved superhydrophobic-hydrophilic domains.
Weakness of Barrier Films
In addition to holding out gases or liquids, another realistic expectation of nanocellulose-based barrier films is that they should have sufficient strength. These issues will be considered in three parts, starting with situations where failure might be caused by tensile or shearing stresses above a critical value, continuing with concerns about the modulus of elasticity, and finishing with concerns about toughness and stretchability.
Concerns about tensile stress to breakage
In some applications a barrier film may have to contribute to the resistance to tensile stresses. Authors have shown that, when suitably formed, nanocellulose-based films can have remarkably high ability to resist such stresses. Qing et al. (2012) reported that neat NFC films were able to resist up to 232 MPa of tensile stress. Yano and Nakahara (2004) reported bonding strength up to 250 MPa in plant microfiber structures, even without the use of a binder. Addition of just 2% oxidized starch improved that value to 310 MPa.
In light of these high values, obtained under ideal conditions, it is important to emphasize that failure is likely to occur at points of weakness or defects, such as thin areas, places where the film or underlying material may have been cut, or places where the structure may have become wet, etc. Thus, the uniformity of the preparation of barrier layers could be important for product strength in some applications.
Percolation threshold and agglomeration issues
When nanocellulose is being used as a reinforcement within a continuous polymeric matrix, many studies have reported a maximum of strength at a certain level of reinforcement, followed by a decline in strength at higher levels of nanocellulose (Zimmermann et al. 2004; Martínez-Sanz et al. 2013c; Rafieian and Simonsen 2014). The optimum point is often said to be associated with a percolation threshold, i.e. the proportion of fibers, of a given aspect ratio, that is just sufficient to fill the volume with an inter-connecting structure (Dufresne 2008; Moon et al. 2011; George and Siddaramaiah 2012; Baheti and Militky 2013). The fall-off at higher levels of the reinforcement is sometimes attributed to “agglomeration” (Abdollahi et al. 2013a; Sandquist 2013; Rafieian and Simonsen 2014), which appears to be a problem especially in systems where there is incomplete wetting and contact between the phases. This situation is illustrated schematically in Fig. 12.
In practical terms, a cluster of cellulosic particles or fibrils within a composite structure can be expected to introduce points of weakness, especially if there are air-filled gaps or direct contact between the cellulosic particles with no intervening matrix polymer. Since a continuous network of fibers becomes highly probable above the percolation threshold, it also makes sense to expect more entanglements or flocs among those fibers, especially when flow is present. As the mixture is sheared, elongated particles are forced to collide and become entangled, leading to increased levels of clustering. Composites based on thermoplastic polymers such as polyethylene are typically prepared under dry conditions in which there is little opportunity for the cellulosic particles to become held together by extensive hydrogen bonding. This is a different situation compared to a sheet of paper, wherein extensive hydrogen bonding contributes to the inter-fiber bonding (Davison 1980). Thus, cellulose-to-cellulose contact within a composite prepared under nonaqueous conditions can be expected to contribute points of weakness in the structure.
Fig. 12. Schematic illustration of common finding that agglomeration of fibrillary reinforcement particles becomes important above a threshold level, such that the elastic modulus is typically highest at an intermediate level of reinforcement
Concerns about elastic modulus
The achievement of a high modulus of elasticity in a barrier film may be important in some applications where the package, as a whole, needs to be stiff, or where the film itself needs to resist stretching or sagging. Factors affecting the modulus of elasticity (MoE) of nanocellulose-based films have been widely studied (Dufresne et al. 1997; Yano and Nakahara 2004; Nakagaito and Yano 2005; 2008a; Yano et al. 2005; Nogi et al. 2009; Qing et al. 2012; Visanko et al. 2015; Shimizu et al. 2016). Also, several studies have shown that the Young’s modulus of different polymeric films can be increased by the addition of nanocellulose reinforcements (Cao et al. 2008; Azeredo et al. 2009, 2010; Martins et al. 2009; Bras et al. 2010; Fernandes et al. 2010; Bilbao-Sáinz et al. 2011; Mikkonen et al. 2011; Bulota et al. 2012; Trovatti et al. 2012a,b; Wu et al. 2012; Tome et al. 2013; Dehnad et al. 2014a; Kurihara and Isogai 2014; Pereira et al. 2014; Salehudin et al. 2014; El-Wakil et al. 2015). In general, these studies showed increases in the elastic modulus upon the addition of optimized levels of nanocellulose to a matrix polymer. Notably, however, Nordqvist et al. (2007) found that MFC had little or no effect on the modulus of chitosan-based films. Capadona et al. (2009), who prepared CNC-reinforced nanocomposites in such a way as to avoid the possibility of CNC agglomerate formation, observed a continuous increase in modulus with increasing CNC content, and the results were in reasonable agreement with predictions based on a percolation model.
Brittleness of Reinforced Matrix
Solid materials also fail as a result of being changed in dimensions beyond their limits of stretching or compression. In other words, they may be too brittle to meet the requirements of certain applications such as folding cartons. This is a particularly important issue in the case of fiber-reinforced plastic composites that combine cellulosic fibers and relatively soft matrix materials. In such cases, though the composites might have much higher modulus than the polymer alone, the elongation or distortion before failure can be much lower (Martins et al. 2009; Pereda et al. 2011; George et al. 2012; Rafieian et al. 2014; Salehudin et al. 2014; Santos et al. 2014; Oun and Rhim 2015).
By contrast, certain other composites incorporating cellulose display high toughness, meaning that a high amount of energy is expended in bringing about their fracture (Zimmerman et al. 2004; Qing et al. 2012). Zimmerman et al. (2004) attributed the toughness of their specimens to the compliant nature of their matrix polymer, hydroxypropyl cellulose, which was plasticized by the presence of water. On the other hand, Nakagaito and Yano (2008b) showed that higher work to failure could be achieved by NaOH treatment of microfibrillated cellulose; those results were attributed to changes in the amorphous regions of the cellulose, i.e. formation of a highly networked structure.
Use of plasticizer vs. mechanical poperies
The tolerance of many polymeric materials to changes in dimension before breakage can be increased by the addition of plasticizing agents, which can usually be described as organic compounds having a high solubility in the polymer. Several studies have shown that plasticizers can be used at controlled levels to adjust the degree of compliance or rigidity of films that contain nanocellulose reinforcements (Angles and Dufresne 2000; Dufresne et al. 2000; López-Rubio et al. 2007; Svagan et al. 2007; Azeredo et al. 2010; Mikkonen et al. 2011; Peng et al. 2011; Hansen et al. 2012; Trovatti et al. 2012ab; Barud et al. 2013; Fortunati et al. 2014; Pereda et al. 2014; Alves et al. 2015).
The importance of glycerol as a plasticizer was shown in a study by Angles and Dufresne (2000), who prepared starch films reinforced by cellulose nano-whiskers (unusually long nanocrystals) obtained from tunicins. Without the plasticizer, the starch would have been too rigid. The glycerol was found to be enriched in the interfacial zone close to the nanocellulose. Azeredo et al. (2010) found that chitosan films reinforced with nanocellulose achieved their best overall properties when the glycerol content was about 18%. Fortunati et al. (2014) showed that the addition of limonene to poly-lactic acid (PLA) films reinforced with CNC tended to decrease the glass transition temperature of the PLA. Thus, by adjusting the levels of plasticizer and reinforcement, one can obtain various levels of compliance before failure, as well as other properties.
López-Rubio et al. (2007) found that it was not possible to cast neat amylopectin films of high uniformity with less than 38% glycerol plasticizer. But when MFC was added, it appeared to play the role of plasticizer, making it possible to achieve high-quality films with no glycerol at all. Moreover, the MFC-reinforced films without glycerol were not brittle. The researchers attributed this to the increased moisture associated with the cellulose. Because it is not usual to achieve plasticizer-like effects by the use of nanomaterials, future studies are recommended in order to more fully illuminate the underlying mechanisms.
Degraded Transparency When Adding Reinforcement
In some potential applications it might be important to preserve the transparency of composite films. As mentioned earlier, many previous reports have made assertions regarding the high transparency achieved with the use of nanofibrillated cellulose (see, for instance, Yano et al. 2005).
In principle, as long as the matrix polymer by itself can form a transparent film, the next step is to incorporate the reinforcement particles in such a way as to not disturb that desired condition. Light scattering generally can be avoided if there are no air gaps between the phases and if the refractive indices of the phases are similar. Since the refractive index of cellulose (about 1.6) is similar to that of commonly used plastics, it follows that incompatibility between the phases, leading to gaps, may be a main contributor to the opacity of reinforced composite films.
Another principle is that light scattering becomes significant when particles in a continuous medium are larger than about 0.2 times the wavelength of light (Hu et al. 2013), which works out to about 80 to 140 nm within the visible range of light. Thus, superior transparency of films incorporating fibrillated cellulose has been reported mainly in cases where the cellulose has been thoroughly broken down to a very small size (Nogi et al 2009; Plackett et al. 2010; Siro et al. 2011). Siro et al. (2011) found that multiple passes through a homogenizer device during the preparation of NFC tended to improve the transparency of the resulting films. Ambrosio-Martin et al. (2015b) ensured that nanocellulose was present as individual particles rather than as clusters by the ball-milling of bacterial cellulose before its incorporation into poly-lactide films.
Progress has been achieved with respect to ensuring that nanocellulose is truly of “nano” size when applied as a reinforcement. Bilbao-Sáinz et al. (2011) showed that CNCs could be incorporated in a hydroxypropylmethylcellulose (HPMC) film with only 3 to 6% loss in transparency.
High transparency has been especially achieved when NFC films are formed separately, rather than incorporating the nanocellulose within a matrix. This approach has been extended in a few studies in which a pure NFC film was used as a coating or layer. Cozzolino et al. (2014) found that better transparency could be achieved when MFC was used as a coating, rather than incorporating it into a pullulan film. Aulin et al. (2013) suggested that the high transparency of films prepared from nanofibrillated cellulose may be due to the high nano-scale uniformity that can be achieved in such films.
End of Life Issues
Packaging materials are well represented not only in typical batches of household garbage, but also in roadside litter. Some of the non-biodegradable plastic debris from packaging ends up in the ocean, where it can harm fish and other organisms (Gregory 2009). According to Lindström and Aulin (2014), less than 4% of the consumption of fossil gas and oil resources goes into the production of plastics. It follows that even a complete switching to fully bio-based packaging would only slightly affect the overall consumption of fossil resources. Therefore, the cited authors proposed that emphasis be placed on biodegradability and various adverse effects of litter.
Possible adverse effects of nanomaterials have received much attention from regulatory agencies, and there is much uncertainty regarding future regulations (Hannon et al. 2015; Shatkin and Kim 2015; Gomez et al. 2016). Shatkin and Kim (2015) expressed greatest concern regarding respiratory issues. The small size of nanomaterials, especially during manufacturing operations, makes it likely that they can remain airborne and pass into people’s lungs. Future studies will be needed to quantify any tendency of the nanocellulose to later become released into the air during usage or final deconstruction of the composite materials. Also, there has been concern about food contact safety. Hannon et al. (2015) considered gaps in knowledge about risks and considered ways of carrying out risk assessments when nanomaterials are employed in food-contact applications. Future studies are needed in order to ensure that nanocellulose-containing films live up to their potential as non-toxic materials, especially when pure.
Recyclability
Individually, just about every material that has been mentioned in this article can be recycled. Problems arise, however, when materials of different types are so intimately or finely mixed that they are no longer practical to separate. Many packaging structures involve laminations or composite mixtures that are quite well bonded together. Efforts to recycle the components materials in packages have been reviewed (Arvanitoyannis and Bosnea 2001; Cimpan et al. 2015). Also, the value of used packaging as a fuel source has been considered (Arvanitoyannis and Bosnea 2001). These are challenging issues that will require much research.
Figure 13 envisions a situation likely to occur with increasing frequency if or when it becomes common to add nanocellulose as a reinforcement in the production of petroleum-derived plastic materials. Part A of the figure represents the current situation in which plastic material, after their automated separation according to type, can be used in the manufacture of secondary plastic material and products. Part B represent a hypothetical future situation in which nanocellulose present in a fraction of the collected waste material has potential to interfere with unit operations in the recycling and remanufacturing processes. Due to the likelihood of increased operational problems during separation and reprocessing of the waste material, as well as the degraded character of the final product, it becomes less likely that anyone will choose to continue recycling this waste stream.
Fig. 13. Nanocellulose reinforcement and the recycling of used plastics and paper. A and C: Representation of the current situation, in which waste plastic and paper are recycled into usable products; B and D: Hypothetical future situation in which there is enough nanocellulose-containing respective material in the waste streams to that it is no longer practical to try to run reprocessing operations, due to operational problems in the attributes or variability of the final product but at the same time it can be help in improving strength properties in the case of paper.
Biodegradability
Several authors cited already in this article have used “biodegradability” as one of their motivations for selecting nanocellulose as a candidate for the reinforcement of films or layers in packages (da Silva et al. 2012; Abdollahi et al. 2013b). Azeredo et al. (2010) had a further requirement that the materials all be suitable for eating. The US Food and Drug Administration has a category of substances that, based on their long history of usage in foodstuffs, are “generally recognized as safe” (FDA 2006). According to Ludwicka et al. (2016), bacterial cellulose is included on that list.
Other authors have shown that their packaging structures conformed to biodegradability standards (Avella et al. 2005). Kibedi-Szabo et al. (2012), on the other hand, demonstrated the biodegradability of composite materials comprised of poly-(vinyl alcohol), bacterial cellulose, and chitosan. Biodegradation was observed to take place within the composites, not just at their surface. Luzi et al. (2015) likewise demonstrated the biodegradability of composites comprising CNC and poly-(lactic acid). Machado et al. (2012) designed a package to be biodegradable, using starch as the matrix; during storage it was found that the packaging materials became oxidized, thus serving as a sacrificial material to minimize oxidation of the contents of the package. The study of related issues can be expected to expand in the coming years, as policy-makers seek to implement practical, integrated systems for collection, selective recycling, composting, and other forms of minimizing the adverse environmental effects of disposal.
SUMMARY COMMENTS
In their review article of 2014, Lindström and Aulin identified some key challenges that might block or delay industrial implementation of nanocellulose in packaging applications. While progress has been made in addressing some of the challenges considered in that article, some important issues seem to pose intractable problems. Table 4 lists some such challenges and suggests possible kinds of research that could be carried out.
As is apparent from Table 4, some serious and perhaps insurmountable challenges face those who wish to implement various aspects of nanocellulose usage in packaging. However, as shown in many articles cited in this review, important progress is being made. Nanocellulose therefore can be expected to play an increasingly important role in future packaging systems.
In addition to the continuing challenges just discussed, there are practical issues that need more attention with respect to scale-up and implementation of nanocellulose technologies for packaging. Future studies and articles are needed that allow better estimates of the likely future costs and available amounts of nanocellulose. Also, given the expectation that nanocellulose costs will tend to remain high and supplies will remain constrained in the near future, some analysis is needed to identify the high-added-value applications of nanocellulose that are most likely to become implemented first. These analyses should include consideration of competing technologies, including competing nanomaterials that might be used for packaging films. Projections of market share, based on various assumptions, could be helpful. And finally, continuing work is needed with respect to life cycle analysis of nanocellulose-based packaging film technologies.
Table 4. Some Key Challenges Noted by Lindström and Aulin (2014)
ACKNOWLEDGMENTS
The authors are grateful for the following sources of support for this work: The Buckman endowment, which supports the work of Dr. Hubbe; the Department of Forest Biomaterials and College of Natural Resources, which supports Dr. Pal, The NCSU Provost’s Fellowship Award, which supports the work of Preeti Tyagi, the Hallburton project, which supported the work of Ana Ferrer, and the Graduate Student Innovation Plan of Jiangsu Province of China (KYLX16_0790), which supports Yuanyuan Yin. Dr. Rojas acknowledges the Academy of Finland’s Centers of Excellence program (project 264677, HYBER) for their support. The authors also wish to thank Justin Zoppe and Miha Humar for their comments, which helped to improve the quality of the work.
REFERENCES CITED
Abdollahi, M., Alboofetileh, M., Behrooz, R., Rezaei, M., and Miraki, R. (2013a). “Reducing water sensitivity of alginate bio-nanocomposite film using cellulose nanoparticles,” International Journal of Biological Macromolecules 54, 166-173. DOI: 10.1016/j.ijbiomac.2012.12.016
Abdollahi, M., Alboofetileh, M., Rezaei, M., and Behrooz, R. (2013b). “Comparing physico-mechanical and thermal properties of alginate nanocomposite films reinforced with organic and/or inorganic nanofillers,” Food Hydrocolloids 32(2), 416-424. DOI: 10.1016/j.foodhyd.2013.02.006
Ago, M., Ferrer, A., and Rojas, O. J. (2016). “Starch-based biofoams reinforced with lignocellulosic nanofibrils from residual palm empty fruit bunches: Water sorption and mechanical strength,” ACS Sustainable Chem. Eng. 4, 5546-5552.
Ahola, S., Österberg, M., and Laine, J. (2008a). “Cellulose nanofibrils – Adsorption with poly(amideamine) epichlorohydrin studied by QCM-D and application as a paper strength additive,” Cellulose 15(2), 303-314. DOI: 10.1007/s10570-007-9167-3
Ahola, S., Salmi, J., Johansson, L. S., Laine, J., and Österberg, M. (2008b). “Model films from native cellulose nanofibrils. Preparation, swelling, and surface interactions,” Biomacromolecules 9(4), 1273-1282. DOI: 10.1021/bm701317k
Algar, I., Garcia-Astrain, C., Gonzalez, A., Martin, L., Gabilondo, N., Retegi, A., and Eceiza, A. (2016). “Improved permeability properties for bacterial cellulose/ montmorillonite hybrid bionanocomposite membranes by in-situ assembling,” J. Renewable Mater. 4(1), 57-65. DOI: 10.7569/JRM.2015.634124
Alves, J. S., dos Reis, K. C., Menezes, E. G. T., Pereira, F. V., and Pereira, J. (2015). “Effect of cellulose nanocrystals and gelatin in corn starch plasticized films,” Carbohyd. Polym. 115, 215-222. DOI: 10.1016/j.carbpol.2014.08.057
Ambrosio-Martin, J., Fabra, M. J., Lopez-Rubio, A., and Lagaron, J. M. (2015a). “Melt polycondensation to improve the dispersion of bacterial cellulose into polylactide via melt compounding: Enhanced barrier and mechanical properties,” Cellulose 22(2), 1201-1226. DOI: 10.1007/s10570-014-0523-9
Ambrosio-Martin, J., Lopez-Rubio, A., Fabra, M. J., Gorrasi, G., Pantani, R., and Lagaron, J. M. (2015b). “Assessment of ball milling methodology to develop polylactide-bacterial cellulose nanocrystals nanocomposites,” J. Appl. Polym. Sci. 132(10), Article no. 41605. DOI: 10.1002/app.41605
Amini, E., Azadfallah, M., Layeghi, M., and Talaei-Hassanloui, R. (2016). “Silver-nanoparticle-impregnated cellulose nanofiber coating for packaging paper,” Cellulose 23(1), 557-570. DOI: 10.1007/s10570-015-0846-1
Andresen, M., Stenstad, P., Moretro, T., Langsrud, S., Syverud, K., Johansson, L. S., and Stenius, P. (2007). “Nonleaching antimicrobial films prepared from surface-modified microfibrillated cellulose,” Biomacromolecules 8(7), 2149-2155. DOI: 10.1021/bm070304e
Angles, M. N., and Dufresne, A. (2000). “Plasticized starch/tunicin whiskers nanocom-posites. 1. Structural analysis,” Macromolecules 33, 8344-8353. DOI: 10.1021/ma0008701
Angles, M. N., and Dufresne, A. (2001). “Plasticized starch/tunicin whiskers nanocomposite materials. 2. Mechanical behavior,” Macromol. 34(9), 2921-2931. DOI: 10.1021/ma001555h
Ankerfors, M., Lindstrom, T., and Glad Nordmark, G. (2016). “Multilayer assembly onto pulp fibres using oppositely charged microfibrillated celluloses, starches, and wet-strength resins – Effect on mechanical properties of CTMP-sheets,” Nordic Pulp Paper Res. J. 31(1), 135-141. DOI: 10.3183/NPPRJ-2016-31-01-p135-141
Ankerfors, M., Lindström, T., and Söderberg, D. (2014). “The use of microfibrillated cellulose in fine paper manufacturing – Results from a pilot scale papermaking trial,” Nordic Pulp Paper Res. J. 29(3), 476-483. DOI: 10.3183/NPPRJ-2014-29-03-p476-483
Arrieta, M. P., Fortunati, E., Dominici, F., Lopez, J., and Kenny, J. M. (2015). “Bionanocomposite films based on plasticized PLA-PHB/cellulose nanocrystal blends,” Carbohydr. Polym. 121, 265-275. DOI: 10.1016/j.carbpol.2014.12.056
Arrieta, M. P., Fortunati, E., Dominici, F., Rayon, E., Lopez, J., and Kenny, J. M. (2014a). “Multifunctional PLA-PHB/cellulose nanocrystal films: Processing, structural and thermal properties,” Carbohyd. Polym. 107, 16-24. DOI: 10.1016/j.carbpol.2014.02.044
Arrieta, M. P., Fortunati, E., Dominici, F., Rayon, E., Lopez, J., and Kenny, J. M. (2014b). “PLA-PHB/cellulose based films: Mechanical, barrier and disintegration properties,” Polym. Degrad. Stabil. 107, 139-149. DOI: 10.1016/j.polymdegradstab.2014.05.010
Arvanitoyannis, I. S., and Bosnea, L. A. (2001). “Recycling of polymeric materials used for food packaging: Current status and perspectives,” Food Rev. Intl. 17(3), 291-346. DOI: 10.1081/FRI-100104703
Aulin, C., Ahola, S., Josefsson, P., Nishino, T., Hirose, Y., Österberg, M., and Wågberg, L. (2009a). “Nanoscale cellulose films with different crystallinities and mesostructures – Their surface properties and interaction with water,” Langmuir 25(13), 7675-7685. DOI: 10.1021/la900323n
Aulin, C., Gallstedt, M., and Lindström, T. (2010a). “Oxygen and oil barrier properties of microfibrillated cellulose films and coatings,” Cellulose 17(3), 559-574. DOI: 10.1007/s10570-009-9393-y
Aulin, C., Johansson, E., Wågberg, L., and Lindström, T. (2010b). “Self-organized films from cellulose I nanofibrils using the layer-by-layer technique,” Biomacromolecules 11(4), 872-882. DOI: 10.1021/Bm100075e
Aulin, C., Karabulut, E., Tran, A., Wågberg, L., and Lindström, T. (2013). “Transparent nanocellulosic multilayer thin films on polylactic acid with tunable gas barrier properties,” ACS Appl. Mater. Interfaces 5(15), 7352-7359. DOI: 10.1021/am401700n
Aulin, C., Salazar-Alvarez, G., and Lindström, T. (2012). “High strength, flexible and transparent nanofibrillated cellulose-nanoclay biohybrid films with tunable oxygen and water vapor permeability,” Nanoscale 4(20), 6622-6628. DOI: 10.1039/c2nr31726e
Aulin, C., and Ström, G. (2013). “Multilayered alkyd resin/nanocellulose coatings for use in renewable packaging solutions with a high level of moisture resistance,” Indust. Eng. Chem. Res. 52(7), 2582-2589. DOI: 10.1021/ie301785a
Aulin, C., Yun, S. H., Wågberg, L., and Lindström, T. (2009b). “Design of highly oleophobic cellulose surfaces from structured silicon templates,” ACS Appl. Mater. Interfaces 1(11), 2443-2452. DOI: 10.1021/am900394y
Avella, M., Vlieger, J. J. D., Errico, M. E., Fischer, S., Vacca, P., and Volpe, M. G. (2005). “Biodegradable starch/clay nanocomposite films for food packaging applications,” Food Chem. 93, 467-474. DOI: 10.1016/j.foodchem.2004.10.024
Ayuk, J. E., Mathew, A. P., and Oksman, K. (2009). “The effect of plasticizer and cellulose nanowhisker content on the dispersion properties of cellulose acetate butyrate nanocomposites,” J. Appl. Polym. Sci. 114(5), 2723-2730. DOI: 10.1002/app.30583
Azeredo, H. M. C. (2009). “Nanocomposites for packaging applications,” Food Res. Int. 42, 1240-1253. DOI: 10.1016/j.foodres.2009.03.019
Azeredo, H. M., Mattoso, L. H., Avena-Bustillos, R. J., Filho, G. C., Munford, M. L., Wood, D., and McHugh, T. H. (2010). “Nanocellulose reinforced chitosan composite films as affected by nanofiller loading and plasticizer content,” J. Food Sci. 75, N1-N7. DOI: 10.1111/j.1750-3841.2009.01386.x
Azeredo, H. M., Mattoso, L. H., Wood, D., Williams, T. G., Avena-Bustillos, R. J., and McHugh, T. H. (2009). “Nanocomposite edible films from mango puree reinforced with cellulose nanofibers,” J. Food Sci. 74, 31-35. DOI: 10.1111/j.1750-3841.2009.01186.x
Azizi Samir, M. A. S., Alloin, F., Paillet, M., and Dufresne, A. (2004a). “Tangling effect in fibrillated cellulose reinforced nanocomposites,” Macromolecules 37, 4313-4316. DOI: 10.1021/ma035939u
Azizi Samir, M. A. S., Alloin, F., Sanchez, J. Y., and Dufresne, A. (2004b). “Cellulose nanocrystals reinforced poly(oxyethylene),” Polymer 45(12), 4149-4157. DOI: 10.1016/j.polymer.2004.03.094
Azizi Samir, M. A. S., Alloin, F., and Dufresne, A. (2015). “Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field,” Biomacromolecules 6(2), 612-626. DOI: 10.1021/bm0493685
Baez, C., Considine, J., and Rowlands, R. (2014). “Influence of drying restraint on physical and mechanical properties of nanofibrillated cellulose films,” Cellulose 21, 347-356. DOI: 10.1007/s10570-013-0159-1
Baheti, V., and Militky, J. (2013). “Reinforcement of wet milled jute nano/micro particles in polyvinyl alcohol films,” Fibers Polymers 14(1), 133-137. DOI: 10.1007/s12221-013-0133-4
Bai, H. Y., Li, Y. F., Wang, W., Chen, G. L., Rojas, O. J., Dong, W. F., and Liu, X. Y. (2015a). “Interpenetrated polymer networks in composites with poly(vinyl alcohol), micro- and nano-fibrillated cellulose (M/NFC) and polyHEMA to develop packaging materials,” Cellulose 22(6), 3877-3894. DOI: 10.1007/s10570-015-0748-2
Bai, H., Li, Y., Liu, X., and Lv, W. (2015b). “Preparation of composite film used in packaging material, involves mixing polyvinyl alcohol with microfibrillated cellulose suspension, heating, cooling, adding photoinitiator, stirring, defoaming, providing on glass plate and drying,” China Patent, CN104558996-A.
Bajpai, M., Bajpai, S. K., and Jyotishi, P. (2016). “Water absorption and moisture permeation properties of chitosan/poly(acrylamide-co-itaconic acid) IPC films,” Intl. J. Biol. Macromol. 84, 1-9. DOI: 10.1016/j.ijbiomac.2015.11.088
Balan, T., Guezennec, C., Nicu, R., Ciolacu, F., and Bobu, E. (2015). “Improving barrier and strength properties of paper by multi-layer coating with bio-based additives,” Cellulose Chem. Technol. 49(7-8), 607-615.
Bardet, R., Roussel, F., Coindeau, S., Belgacem, N., and Bras, J. (2015). “Engineered pigments based on iridescent cellulose nanocrystal films,” Carbohydrate Polymers 122, 367-375. DOI: 10.1016/j.carbpol.2014.10.020
Barud, H. S., Ribeiro, S. J. L., Carone, C. L. P., Ligabue, R., Einloft, S., Queiroz, P. V. S., Borges, A. P. B., and Jahno, V. D. (2013). “Optically transparent membrane based on bacterial cellulose/polycaprolactone,” Polimeros-Ciencia e Technologia 23(1), 135-138. DOI: 10.1590/S0104-14282013005000018
Bayati, F., Boluk, Y., and Choi, P. (2014). “Diffusion behavior of water at infinite dilution in hydroxypropyl xylan films with sorbitol and cellulose nanocrystals,” ACS Sustain. Chem. Eng. 2(5), 1305-1311. DOI: 10.1021/sc500133p
Belbekhouche, S., Bras, J., Siqueira, G., Chappey, C., Lebrun, L., Khelifi, B., Marais, S., and Dufresne, A. (2011). “Water sorption behavior and gas barrier properties of cellulose whiskers and microfibrils films,” Carbohyd. Polym. 83, 1740-1748. DOI: 10.1016/j.carbpol.2010.10.036
Bhardwaj, U., Dhar, P., Kumar, A., and Katiyar, V. (2014). “Polyhyroxyalkanoates (PHA)-cellulose based nanobiocomposites for food packaging applications,” in: Food Additives and Packaging, Komolprasert, V., and Turowski, P. (eds.), ACS Symposium Series, Vol. 1162, pp. 275-314.
Bilbao-Sáinz, C., Avena-Bustillos, R. J., Wood, D. F., Williams, T. G., and McHugh, T. H. (2010). “Composite edible films based on hydroxypropyl methylcellulose reinforced with microcrystalline cellulose nanoparticles,” J. Agri. Food Chem. 58(6), 3753-3760. DOI: 10.1021/jf9033128
Bilbao-Sáinz, C., Bras, J., Williams, T., Sénechal, T., and Orts, W. (2011). “HPMC reinforced with different cellulose nano-particles,” Carbohydrate Polymers 86(4), 1549-1557. DOI: 10.1016/j.carbpol.2011.06.060
Boumail, A., Salmieri, S., Klimas, E., Tawema, P. O., Bouchard, J., and Lacroix, M. (2013a). “Physico-chemical properties of antimicrobial film based on polycaprolactone and nanocellulose and their capacity to inhibit salmonella typhimurium on vegetables,” J-For – J. Sci. Technol. Forest Prod. Proc. 3(1), 45-49.
Boumail, A., Salmieri, S., Klimas, E., Tawema, P. O., Bouchard, J., and Lacroix, M. (2013b). “Characterization of trilayer antimicrobial diffusion films (ADFs) based on methylcellulose-polycaprolactone composites,” J. Agric. Food Chem. 61(4), 811-821. DOI: 10.1021/jf304439s
Bras, J., Hassan, M. L., Bruzesse, C., Hassan, E. A., El-Wakil, N. A., and Dufresne, A. (2010). “Mechanical, barrier, and biodegradability properties of bagasse cellulose whiskers reinforced natural rubber nanocomposites,” Industrial Crops and Products 32, 627-633. DOI: 10.1016/j.indcrop.2010.07.018
Bras, J., Viet, D., Bruzzese, C., and Dufresne, A. (2011). “Correlation between stiffness of sheets prepared from cellulose whiskers and nanoparticles dimensions,” Carbohyd. Polym. 84(1), 211-215. DOI: 10.1016/j.carbpol.2010.11.022
Bulota, M., Kreitsmann, K., Hughes, M., and Paltakari, J. (2012). “Acetylated microfibrillated cellulose as a toughening agent in poly(lactic acid),” Journal of Applied Polymer Science 126, E448-E457. DOI: 10.1002/App.36787.
Cai, Z. J., and Yang, G. (2011). “Optical nanocomposites prepared by incorporating bacterial cellulose nanofibrils into poly(3-hydroxybutyrate),” Mater. Lett. 65(2), 182-184. DOI: 10.1016/j.matlet.2010.09.055
Camarero-Espinosa, S., Boday, D. J., Weder, C., and Foster, E. J. (2015). “Cellulose nanocrystal driven crystallization of poly(D,L-lactide) and improvement of the thermomechanical properties,” J. Appl. Polymer Sci. 132(10), article no. 41607. DOI: 10.1002/app.41607
Cao, X., Chen, Y., Chang, P. R., Muir, A. D., and Falk, G. (2008). “Starch based nanocomposites reinforced with flax cellulose nanocrystals,” Express Polymer Letters 2(7), 502-510. DOI: 10.3144/expresspolymlett.2008.60
Capadona, J. R., Shanmuganathan, K., Triftschuh, S., Seidel, S., Rowan, S. J., and Weder, C. (2009). “Polymer nanocomposites with nanowhiskers isolated from microcrystalline cellulose,” Biomacromolecules 10(4), 712-716. DOI: 10.1021/bm8010903
Carrillo, C. A., Nypelö, T., and Rojas, O. J. (2016). “Double emulsions for the compatibilization of hydrophilic nanocellulose with non-polar polymers and validation in the synthesis of composite fibers,” Soft Matter 12, 2721-2728.
Chakraborty, A., Sain, M., and Kortschot, M. (2006). “Reinforcing potential of wood pulp derived microfibres in a PVA matrix,” Holzforschung 60(1), 53-58. DOI: 10.1515/HF.2006.010
Chang, C. P., Wang, I. C., and Perng, Y. S. (2013). “Enhanced thermal behavior, mechanical properties and UV shielding of polylactic acid (PLA) composites reinforced with nanocrystalline cellulose and filled with nanosericite,” Cellulose Chem. Technol. 47(1-2), 111-123.
Charani, P. R., Dehghani-Firouzabadi, M., Afra, E., Blademo, A., Naderi, A., and Lindstrom, T. (2013). “Production of microfibrillated cellulose from unbleached kraft pulp of Kenaf and Scotch pine and its effect on the properties of hardwood kraft: microfibrillated cellulose paper,” Cellulose 20(5), 2559-2567. DOI: 10.1007/s10570-013-9998-z
Chen, L. H., Zhu, J. Y., Baez, C., Kitin, P., and Elder, T. (2016a). “Highly thermal-stable and functional cellulose nanocrystals and nanofibrils produced using fully recyclable organic acids,” Green Chem. 18(13), 3835-3843. DOI: 10.1039/c6gc00687f
Chen, Y. W., Lee, H. V., Juan, J. C., and Phang, S. M. (2016b). “Production of new cellulose nanomaterial from red algae marine biomass Gelidium elegans,” Carbohydr. Polym. 151, 1210-1219. DOI: 10.1016/j.carbpol.2016.06.083
Cheng, S. L., Zhang, Y. P., Cha, R. T., Yang, J. L., and Jiang, X. Y. (2016). “Water-soluble nanocrystalline cellulose films with highly transparent and oxygen barrier properties,” Nanoscale 8(2), 973-978. DOI: 10.1039/c5nr07647a
Cheng, Q., Wang, S., and Rials, T. G. (2009). “Poly(vinyl alcohol) nanocomposites reinforced with cellulose fibrils isolated by high intensity ultrasonication,” Composites Part A: Applied Science and Manufacturing 40(2), 218-224. DOI: 10.1016/j.compositesa.2008.11.009
Chiellini, E. (ed) (2008). Environmentally Compatible Food Packaging, CRC Press, Boca Raton.
Chinga-Carrasco, G., Averianova, N., Gibadullin, M., Petrov, V., Leirset, I., and Syverud, K. (2013). “Micro-structural characterisation of homogeneous and layered MFC nano-composites,” Micron 44, 331-338. DOI: 10.1016/j.micron.2012.08.005
Chinga-Carrasco, G., Kuznetsova, N., Garaeva, M., Leirset, I., Galiullina, G., Kostochko, A., and Syverud, K. (2012). “Bleached and unbleached MFC nanobarriers: Properties and hydrophobisation with hexamethyldisilazane,” J. Nanopart. Res. 14(12), Article no. 1280. DOI: 10.1007/s11051-012-1280-z
Chinga-Carrasco, G., and Syverud, K. (2010). “Computer-assisted quantification of the multi-scale structure of films made of nanofibrillated cellulose,” J. Nanoparticle Res. 12(3), 841-851. DOI: 10.1007/s11051-009-9710-2
Chinga-Carrasco, G., and Syverud, K. (2012). “On the structure and oxygen transmission rate of biodegradable cellulose nanobarriers,” Nanoscale Res. Let. 7, article no. 192. DOI: 10.1186/1556-276X-7-192
Choi, Y., and Simonsen, J. (2006). “Cellulose nanocrystal-filled carboxymethyl cellulose nanocomposites,” J. Nanosci. Nanotech. 6(3), 633-639. DOI: 10.1166/jnn.2006.132
Cimpan, C., Maul, A., Jansen, M., Pretz, T., and Wenzel, H. (2015). “Central sorting and recovery of MSW recyclable materials: A review of technological state-of-the-art, cases, practice and implications for materials recycling,” J. Environ. Manag. 156, 181-199. DOI: 10.1016/j.jenvman.2015.03.025
Costa, S. S., Druzian, J. I., Machado, B. A. S., de Souza, C. O., and Guimaraes, A. G. (2014). “Bi-functional biobased packing of the cassava starch, glycerol, licuri nanocellulose and red propolis,” Plos One 9(11), Article no. e112554. DOI: 10.1371/journal.pone.0112554
Cowie, J., Bilek, E. M., Wegner, T. H., and Shatkin, J. A. (2014). “Market projections of cellulose nanomaterial-enabled products – Part 2: Volume estimates,” TAPPI J. 13(6), 57-69.
Cozzolino, C. A., Campanella, G., Ture, H., Olsson, R. T., and Farris, S. (2016). “Microfibrillated cellulose and borax as mechanical, O2-barrier, and surface-modulating agents of pullulan biocomposite coatings on BOPP,” Carbohydr. Polym. 143, 179-187. DOI: 10.1016/j.carbpol.2016.01.068
Cozzolino, C. A., Cerri, G., Brundu, A., and Farris, S. (2014). “Microfibrillated cellulose (MFC): Pullulan bionanocomposite films,” Cellulose 21(6), 4323-4335. DOI: 10.1007/s10570-014-0433-x
Cozzolino, C. A., Nilsson, F., Iotti, M., Sacchi, B., Piga, A., and Farris, S. (2013). “Exploiting the nano-sized features of microfibrillated cellulose (MFC) for the development of controlled-release packaging,” Colloids Surf. B – Biointerfaces 110, 208-216. DOI: 10.1016/j.colsurfb.2013.04.046
Criaclo, P., Fraschini, C., Salmieri, S., Becher, D., Safrany, A., and Lacroix, M. (2016). “Free radical grafting of gallic acid (GA) on cellulose nanocrystals (CNCS) and evaluation of antioxidant reinforced gellan gum films,” Radiation Phys. Chem. 118, 61-69. DOI: 10.1016/j.radphyschem.2015.05.030
Dai, L., Wang, B., Long, Z., Chen, L., Zhang, D., and Guo, S. (2015). “Properties of hydroxypropyl guar/TEMPO-oxidized cellulose nanofibrils composite films,” Cellulose 22(5), 3117-3126. DOI: 10.1007/s10570-015-0691-2
da Silva, J. B. A., Pereira, F. V., and Druzian, J. I. (2012). “Cassava starch-based films plasticized with sucrose and inverted sugar and reinforced with cellulose nanocrystals,” Journal of Food Science 77(6), N14–N19. DOI: 10.1111/j.1750-3841.2012.02710.x
Davison, R. W. (1980). “Theory of dry strength development,” in W. F. Reynolds (ed.), Dry Strength Additives, TAPPI Press, Atlanta, Ch. 1, 1-31.
Decher, G. (1997). “Fuzzy nanoassemblies: Toward layered polymeric multicomposites,” Science 277(5330), 1232-1237. DOI: 10.1126/science.277.5330.1232
Dehnad, D., Emarn-Djomeh, Z., Mirzaei, H., Jafari, S. M., and Dadashi, S. (2014a). “Optimization of physical and mechanical properties for chitosan-nanocellulose biocomposites,” Carbohydrate Polymers 105, 222-228. DOI: 10.1016/j.carbpol.2014.01.094
Dehnad, D., Mirzaei, H., Emam-Djomeh, Z., Jafari, S. M., and Dadashi, S. (2014b). “Thermal and antimicrobial properties of chitosan-nanocellulose films for extending shelf life of ground meat,” Carbohydrate Polymers 109, 148-154. DOI: 10.1016/j.carbpol.2014.03.063
de Mesquita, J. P., Donnici, C. L., and Pereira, F. V. (2010). “Biobased nanocomposites from layer-by-layer assembly of cellulose nanowhiskers with chitosan,” Biomacromol. 11(2), 473-480. DOI: 10.1021/bm9011985
Despond, S., Espuche, N., Cartier, N., and Domard, A. (2005). “Barrier properties of paper–chitosan and paper–chitosan–carnauba wax films,” Journal of Applied Polymer Science 98(2), 704-710. DOI: 10.1002/app.21754
Dhar, P., Bhardwaj, U., Kumar, A., and Katiyar, V. (2015). “Poly (3-hydroxybutyrate)/cellulose nanocrystal films for food packaging applications: Barrier and migration studies,” Polymer Eng. Sci. 55(10), 2388-2395. DOI: 10.1002/pen.24127
Dimic-Misic, K., Rantanen, J., Maloney, T. C., and Gane, P. A. C. (2016). “Gel structure phase behavior in micro nanofibrillated cellulose containing in situ precipitated calcium carbonate,” J. Appl. Polymer Sci. 133(22), article no. 43486. DOI: 10.1002/app.43486
Dimic-Misic, K., Salo, T., Paltakari, J., and Gane, P. (2014). “Comparing the rheological properties of novel nanofibrillar cellulose-formulated pigment coating colours with those using traditional thickener,” Nordic Pulp Paper Res. J. 29(2), 253-270. DOI: 10.3183/NPPRJ-2014-29-02-p253-270
Dobre, L. M., Stoica-Guzun, A., Stroescu, M., Jipa, I. M., Dobre, T., Ferdes, M., and Ciumpiliac, S. (2012). “Modelling of sorbic acid diffusion through bacterial cellulose-based antimicrobial films,” Chem. Papers 66(2), 144-151. DOI: 10.2478/s11696-011-0086-2
Dogan, N., and McHugh, T. H. (2007). “Effects of microcrystalline cellulose on functional properties of hydroxypropylmethyl cellulose microcomposite films,” J. Food Sci. 72(1), 16-22. DOI: 10.1111/j.1750-3841.2006.00237.x
Dong, F., Li, S. J., Yan, M. L., and Li, C. J. (2014). “Preparation and properties of chitosan/nanocrystalline cellulose composite films for food packaging,” Asian J. Chem. 26(17), 5895-5898.
Dufresne, A. (2008). “Polysaccharide nanocrystals reinforced nanocomposites,” Canadian Journal of Chemistry 86(6), 484-494. DOI: 10.1139/v07-152
Dufresne, A. (2012). Nanocellulose: From Nature to High Performance Tailored Materials, De Gruyter, Germany. DOI: 10.1515/9783110254600
Dufresne, A., Cavaille, J. Y., and Vignon, M. R. (1997). “Mechanical behavior of sheets prepared from sugar beet cellulose microfibrils,” Journal of Applied Polymer Science 64(6), 1185-1194. DOI: 10.1002/(SICI)1097-4628(19970509)64:6<1185::AID-APP19>3.0.CO;2-V
Dufresne, A., Dupeyre, D., and Vignon, M. R. (2000). “Cellulose microfibrils from potato tuber cells: Processing and characterization of starch-cellulose microfibril composites,” Journal of Applied Polymer Science 76(14), 2080-2092. DOI: 10.1002/(SICI)1097-4628(20000628)76:14<2080::AID-APP12>3.0.CO;2-U
Dufresne, A., and Vignon, M. R. (1998). “Improvement of starch film performances using cellulose microfibrils,” Macromolecules 31(8), 2693-2696. DOI: 10.1021/ma971532b
Edlund, U., Ryberg, Y. Z., and Albertsson, A. C. (2010). “Barrier films from renewable forestry waste,” Biomacromol. 11(9), 2532-2538. DOI: 10.1021/bm100767g
Eichhorn, S. J. (2011). “Cellulose nanowhiskers: Promising materials for advanced applications,” Soft Matter 7, 303-315. DOI: 10.1039/C0SM00142B
Eichhorn, S. J., Dufresne, A., Aranguren, M., Marcovich, N. E., Capadona, J. R., Rowan, S. J., Weder, C., Thielemans, W., Roman, M., Rennecker, S., Gindl, W., Veigel, S., Keckes, J., Yano, H., Abe, K., Nogi, M., Nakagaito, A. N., Mangalam, A., Simonsen, J., Benight, A. S., Bismarck, A., Berglund, L. A., and Peijs, T. (2009). “Review: Current international research into cellulose nanofibers and nanocomposites,” J. Mater. Sci. 45, 1-33. DOI: 10.1007/s10853-009-3874-0
Elazzouzi-Hafraoui, S., Nishiyama, Y., Putaux, J. L., Heux, L., Dubreuil, F., and Rochas, C. (2008). “The shape and size distribution of crystalline nanoparticles prepared by acid hydrolysis of native cellulose,” Biomacromolecules 9, 57-65. DOI: 10.1021/bm700769p
El Miri, N., Abdelouandi, K., Barakat, A., Zahouily, M., Fihri, A., Solhy, A., and El Achaby, M. (2015). “Bio-nanocomposite films reinforced with cellulose nanocrystals: Rheology of film-forming solutions, transparency, water vapor barrier and tensile properties of films,” Carbohydr. Polym. 129, 156-167. DOI: 10.1016/j.carbpol.2015.04.051
El-Wakil, N. A., Hassan, E. A., Abou-Zeid, R. E., and Dufresne, A. (2015). “Development of wheat gluten/nanocellulose/titanium dioxide nanocomposites for active food packaging,” Carbohydrate Polymers 124, 337-346. DOI: 10.1016/j.carbpol.2015.01.076
Eriksen, Ö., Syverud, K., and Gregerson, Ö. (2008). “The use of microfibrillated cellulose produced from kraft pulp as strength enhancer in TMP paper,” Nordic Pulp Pap. Res. J. 23(3), 299-304. DOI: 10.3183/NPPRJ-2008-23-03-p299-304
Espino-Pérez, E., Bras, J., Ducruet, V., Guinault, A., Dufresne, A., and Domenek, S. (2013). “Influence of chemical surface modification of cellulose nanowhiskers on thermal, mechanical, and barrier properties of poly(lactide) based bionanocomposites,” Eur. Polymer J. 49(10), 3144-3154. DOI: 10.1016/j.eurpolymj.2013.07.017
Faruk, O., Bledzki, A. K., Fink, H. P., and Sain, M. (2012). “Biocomposites reinforced with natural fibers: 2000–2010,” Prog. Polym. Sci. 37, 1552-1596. DOI: 10.1016/j.progpolymsci.2012.04.003
Faruk, O., Bledzki, A. K., Fink, H. P., and Sain, M. (2014). “Progress report on natural fiber reinforced composites,” Macromol. Mater. Eng. 299(1), 9-26. DOI: 10.1002/mame.20130000
FDA (2006). “FDA’s approach to the GRAS provision: A history of processes,” The US Food and Drug Administration, http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/ucm094040.htm
Feng, H., He, W., Jiang, B., Jiang, S., and Liu, W. (2015a). “Preparation of multi-moisture biodegradable chitosan film used for food packaging, involves dispersing nanocellulose in ethanol, adding acetic acid solution, adding silane coupling agent, lyophilizing, and performing alkylation process,” China Patent, CN104371127-A.
Feng, X., Meng, X. H., Zhao, J. P., Miao, M., Shi, L. Y., Zhang, S. P., and Fang, J. H. (2015b). “Extraction and preparation of cellulose nanocrystals from dealginate kelp residue: Structures and morphological characterization,” Cellulose 22(3), 1763-1772. DOI: 10.1007/s10570-015-0617-z
Feng, X. C., Ullah, N., Wang, X. J., Sun, X. C., Li, C. Y., Bai, Y., Chen, L., and Li, Z. X. (2015c). “Characterization of bacterial cellulose by Gluconacetobacter hansenii CGMCC 3917,” J. Food Sci. 80(10), E2217-E2227. DOI: 10.1111/1750-3841.13010
Fernandes, S. C. M., Freire, C. S. R., Silvestre, A. J. D., Pascoal Neto, C., Gandini, A., Berglund, L. A., and Salmén, L. (2010). “Transparent chitosan films reinforced with a high content of nanofibrillated cellulose,” Carbohydrate Polymers 81, 394-401. DOI: 10.1016/j.carbpol.2010.02.037
Fernandes, S. C. M., Oliveira, L., Freire, C. S. R., Silve Fernandez, A., Sanchez, M. D., Ankerfors, M., and Lagaron, J. M., (2008). “Effects of ionizing radiation in ethylene-vinyl alcohol copolymers and in composites containing microfibrillated cellulose,” J. Appl. Polymer Sci. 109(1), 126-134. DOI: 10.1002/app.27709
Fernandes, S. C. M., Oliveira, L., Freire, C. S. R., Silvestre, A. J. D., Neto, C. P., Gandini, A., and Desbrieres, J. (2009). “Novel transparent nanocomposite films based on chitosan and bacterial cellulose,” Green Chemistry 11(12), 2023-2029. DOI: 10.1039/b919112g
Ferrer, A., Filpponen, I., Rodríguez, A., Laine, J., and Rojas, O. J. (2012a). “Valorization of residual empty palm fruit bunch fibers (EPFBF) by microfluidization: Production of nanofibrillated cellulose and EPFBF nanopaper,” Bioresource Technology 125, 249-255 (2012). DOI: 10.1016/j.biortech.2012.08.108
Ferrer, A., Hoeger, I. C., Lu, X., and Rojas, O. J. (2016a). “Reinforcement of polypropylene with lignocellulose nanofibrils and compatibilization with biobased polymers,” Journal of Applied Polymer Science 133, 1097-4628. DOI: 10.1002/app.43854
Ferrer, A., Quintana, E., Filpponen, I., Solala, I., Vidal, V., Rodríguez, R., Laine, J., and Rojas, O. J. (2012b). “Effect of residual lignin and heteropolysaccharides in nanofibrillar cellulose and nanopaper,” Cellulose 19, 2179-2193. DOI: 10.1007/s10570-012-9788-z
Ferrer, A., Salas, C., and Rojas, O. J. (2015). “Dewatering of MNFC containing microfibrils and microparticles from soybean hulls: Mechanical and transport properties of hybrid films,” Cellulose 22, 3919-3928. DOI: 10.1007/s10570-015-0768-y
Ferrer, A., Salas, C., and Rojas, O. J. (2016b). “Physical, thermal, chemical and rheological characterization of cellulosic microfibrils and microparticles produced from soybean hulls,” Industrial Crops and Products 84, 337-343 (2016). DOI:10.1016/j.indcrop.2016.02.014
Figueiredo, A. R. P., Silvestre, A. J. D., Neto, C. P., and Freire, C. S. R. (2015). “In situ synthesis of bacterial cellulose/polycaprolactone blends for hot pressing nanocomposite films production,” Carbohydrate Polymers 132, 400-408. DOI: 10.1016/j.carbpol.2015.06.001
Follain, N., Belbekhouche, S., Bras, J., Siqueira, G., Marais, S., and Dufresne, A. (2013). “Water transport properties of bio-nanocomposites reinforced by Luffa cylindrica cellulose nanocrystals,” J. Membr. Sci. 427, 218-229. DOI: 10.1016/j.memsci.2012.09.048
Fortunati, E., Armentano, I., Zhou, Q., Iannoni, A., Saino, E., Visai, L., Berglund, L. A., and Kenny, J. M. (2012a). “Multifunctional bionanocomposite films of poly(lactic acid), cellulose nanocrystals and silver nanoparticles,” Carbohyd. Polym. 87(2), 1596-1605. DOI: 10.1016/j.carbpol.2011.09.066
Fortunati, E., Luzi, F., Puglia, D., Dominici, F., Santulli, C., Kenny, J. M., and Torre, L. (2014). “Investigation of thermo-mechanical, chemical and degradative properties of PLA-limonene films reinforced with cellulose nanocrystals extracted from Phormium tenax leaves,” European Polymer J. 56, 77-91. DOI: 10.1016/j.eurpolymj.2014.03.030
Fortunati, E., Luzi, F., Puglia, D., Petrucci, R., Kenny, J. M., and Torre, L. (2015). “Processing of PLA nanocomposites with cellulose nanocrystals extracted from Posidonia oceanica waste: Innovative reuse of coastal plant,” Indust. Crops Prod. 67, 439-447. DOI: 10.1016/j.indcrop.2015.01.075
Fortunati, E., Peltzer, M., Armentano, I., Torre, L., Jiménez, A., and Kenny, J. M. (2012b). “Effects of modified cellulose nanocrystals on the barrier and migration properties of PLA nano-biocomposites,” Carbohydr. Polym. 90(2), 948-956. DOI: 10.1016/j.carbpol.2012.06.025
Fortunati, E., Peltzer, M., Armentano, I., Jimenez, A., and Kenny, J. M. (2013). “Combined effects of cellulose nanocrystals and silver nanoparticles on the barrier and migration properties of PLA nano-biocomposites,” J. Food Eng. 118(1), 117-124. DOI: 10.1016/j.jfoodeng.2013.03.025
Freire, C. S. R., Fernandes, S. C. M., Silvestre, A. J. D., and Neto, C. P. (2013). “Novel cellulose-based composites based on nanofibrillated plant and bacterial cellulose: Recent advances at the University of Aveiro – A review,” Holzforschung 67(6), 603-612. DOI: 10.1515/hf-2012-0127
Fukuzumi, H., Saito, T., Iwata, T., Kumamoto, Y., and Isogai, A. (2009). “Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation,” Biomacromolecules 10, 162-165. DOI: 10.1021/bm801065u
Gamelas, J. A. F., and Ferraz, E. (2015). “Composite films based on nanocellulose and nanoclay minerals as high strength materials with gas barrier capabilities: Key points and challenges,” BioResources 10(4), 6310-6313. DOI: 10.15376/biores.10.4.6310-6313
Gao, W.-H., Chen, K.-F., Yang, R.-D., Yang, F., and Han, W.-J. (2011). “Properties of bacterial cellulose and its influence on the physical properties of paper,” BioResources 6(1), 144-153. DOI: 10.15376/biores.6.1.144-153
George, J., Bawa, A. S., and Siddaramaiah (2010). “Synthesis and characterization of bacterial cellulose nanocrystals and their PVA nanocomposites,” in: Multi-Functional Materials and Structures III, J. H. Lee (ed.), Book Series: Advanced Materials Research, Vol. 123-125, pp. 383-386. DOI: 10.4028/www.scientific.net/AMR.123-125.383
George, J., Kumar, R., Sajeevkumar, V. A., Ramana, K. V., Rajamanickam, R., Abhishek, V., Nadanasabapathy, S., and Siddaramaiah (2014). “Hybrid HPMC nanocomposites containing bacterial cellulose nanocrystals and silver nanoparticles,” Carbohyd. Polym. 105, 285-292. DOI: 10.1016/j.carbpol.2014.01.057
George, J., Ramana, K. V., Sabapathy, S. N., and Bawa, A. S. (2005). “Physico-mechan-ical properties of chemically treated bacterial (Acetobacter xylinum) cellulose mem-brane,” World J. Microb. Biot. 21, 1323-1327. DOI: 10.1007/s11274-005-3574-0
George, J., Sabapathy, S. N., and Siddaramaiah (2016). “Edible nanocomposite films based on hydroxypropyl methyl cellulose reinforced with bacterial cellulose nanocrystals,” in: Micro- and Nanostructured Polymer Systems: From Synthesis to Applications, S. Thomas, R. A. Shanks, and J. Joy (eds.), pp. 93-100.
George, J., Sajeevkumar, V. A., Ramana, K. V., Sabapathy, S. N., and Siddaramaiah. (2012). “Augmented properties of PVA hybrid nanocomposites containing cellulose nanocrystals and silver nanoparticles,” J. Mater. Chem. 22(42), 22433-22439. DOI: 10.1039/c2jm35235d
George, J., and Siddaramaiah. (2012). “High performance edible nanocomposite films containing bacterial cellulose nanocrystals,” Carbohydrate Polymers 87(3), 2031-2037. DOI: 10.1016/j.carbpol.2011.10.019
Ghaderi, M., Mousavi, M., Yousefi, H., and Labbafi, M. (2014). “All-cellulose nanocomposite film made from bagasse cellulose nanofibers for food packaging application,” Carbohydrate Polymers 104, 59-65. DOI: 10.1016/j.carbpol.2014.01.013
Gomez, C., Serpa, A., Velasquez-Cock, J., Ganan, P., Castro, C., Velez, L., and Zuluaga, R. (2016). “Vegetable nanocellulose in food science: A review,” Food Hydrocolloids 57, 178-186. DOI: 10.1016/j.foodhyd.2016.01.023
González, I., Boufi, S., Pèlach, M. A., Alcalà, M., Vilaseca, F., and Mutjé, P. (2012). “Nanofibrillated cellulose as paper additive in Eucalyptus pulps,” BioResources 7(4), 5167-5180. DOI: 10.15376/biores.7.4.5167-5180
Gregory, M. R. (2009). “Environmental implications of plastic debris in marine settings – Entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions,” Phil. Trans. Royal Soc. B – Biol. Sci. 364(1526), 2013-2025. DOI: 10.1098/rstb.2008.0265
Grunert, M., and Winter, W. T. (2002). “Nanocomposites of cellulose acetate butyrate reinforced with cellulose nanocrystals,” J. Polym. Environ. 10, 27-30. DOI: 10.1023/A:1021065905986
Guimond, R., Chabot, B., Law, K. N., and Daneault, C. (2010). “The use of cellulose nanofibres in papermaking,” J. Pulp Paper Sci. 36(1-2), 55-61.
Guo, J., Fang, W., Welle, A., Feng, W., Filpponen, I., Rojas, O. J., and Levkin, P. (2016). “Superhydrophobic and slippery lubricant-infused flexible transparent nanocellulose films by photo-induced thiol-ene functionalization,” ACS Appl. Mater. Interfaces 8(49), 34115-34122. DOI: 10.1021/acsami.6b11741
Habibi, Y., Lucia, L. A., and Rojas, O. J. (2010). “Cellulose nanocrystals: Chemistry, self-assembly, and applications,” Chem. Rev. 110(6), 3479-3500. DOI: 10.1021/cr900339w
Hai, L. V., Son, H. N., and Seo, Y. B. (2015). “Physical and bio-composite properties of nanocrystalline cellulose from wood, cotton linters, cattail, and red algae,” Cellulose 22(3), 1789-1798. DOI: 10.1007/s10570-015-0633-z
Hamada, H., Beckvermit, J., and Bousfield, W. D. (2010). “Nanofibrillated cellulose with fine clay as a coating agent to improve print quality,” in: PaperCon 2010 Conference (Vol. session 20.1), Atlanta, USA, p. 11.
Hambardzumyan, A., Foulon, L., Bercu, N. B., Pernes, M., Maigret, J. E., Molinari, M., Chabbert, B., and Aguie-Beghin, V. (2015). “Organosolv lignin as natural grafting additive to improve the water resistance of films using cellulose nanocrystals,” Chem. Eng. J. 264, 780-788. DOI: 10.1016/j.cej.2014.12.004
Hannon, J. C., Kerry, J., Cruz-Romero, M., Morris, M., and Cummins, E. (2015). “Advances and challenges for the use of engineered nanoparticles in food contact materials,” Trends Food Sci. Technol. 43(1), 43-62. DOI: 10.1016/j.tifs.2015.01.008
Hansen, N. M. L., Blomfeldt, T. O. J., Hedenqvist, M. S., and Plackett, D. V. (2012). “Properties of plasticized composite films prepared from nanofibrillated cellulose and birch wood xylan,” Cellulose 19(6), 2015-2031. DOI: 10.1007/s10570-012-9764-7
Hassan, M. L., Bras, J., Hassan, E. A., Fadel, S. M., and Dufresne, A. (2012). “Polycaprolactone/modified bagasse whisker nanocomposites with improved moisture-barrier and biodegradability properties,” J. Appl. Polym. Sci. 125(Suppl. 2), E10-E19. DOI:10.1002/app.36373
Hassan, M. L., Hassan, E. A., and Oksman, K. N. (2011). “Effect of pretreatment of bagasse fibers on the properties of chitosan/microfibrillated cellulose nanocomposites,” Journal of Materials Science 46(6), 1732-1740. DOI: 10.1007/s10853-010-4992-4
Henriksson, M., Berglund, L. A., Isaksson, P., Lindström, T., and Nishino, T. (2008). “Cellulose nanopaper structures of high toughness,” Biomacromolecules 9(6), 1579-1585. DOI: 10.1021/bm800038n
Herrera, N., Salaberria, A. M., Mathew, A. P., and Oksman, K. (2016). “Plasticized polylactic acid nanocomposite films with cellulose and chitin nanocrystals prepared using extrusion and compression molding with two cooling rates: Effects on mechanical, thermal and optical properties,” Composites Part A – Appl Sci. Manufac. 83, 89-97. DOI: 10.1016/j.compositesa.2015.05.024
Honorato, C., Kumar, V., Liu, J., Koivula, H., Xu, C. L., and Toivakka, M. (2015). “Transparent nanocellulose-pigment composite films,” J. Mater. Sci. 50(22), 7343-7352. DOI: 10.1007/s10853-015-9291-7
Hossain, K. M. Z., Ahmed, I., Parsons, A. J., Scotchford, C. A., Walker, G. S., Thielemans, W., and Rudd, C. D. (2011). “Physico-chemical and mechanical properties of nanocomposites prepared using cellulose nanowhiskers and poly(lactic acid),” J. Mater. Sci. 47(6), 2675-2686. DOI: 10.1007/s10853-011-6093-4
Hu, D. Y., and Wang, L. J. (2016). “Physical and antibacterial properties of polyvinyl alcohol films reinforced with quaternized cellulose,” J. Appl. Polymer Sci. 133(25), article no. 43552. DOI: 10.1002/app.43552
Hu, L., Zheng, G., Yao, J., Liu, N., Weil, B., Eskilsson, M., Karabulut, E., Ruan, Z., Fan, S., Bloking, J. T., McGehee, M. D., Wagberg, L., and Cui, Y. (2013). “Transparent and conductive paper from nanocellulose fibers,” Energy Environ. Sci. 6, 513-518. DOI: 10.1039/c2ee23635d
Hubbe, M. A., Gardner, D. J., and Shen, W. (2015a). “Contact angles and wettability of cellulosic surfaces: A review of proposed mechanisms and test strategies,” BioResources 10(4), 8657-8749. DOI: 10.15376/biores.10.4.Hubbe_Gardner_Shen
Hubbe, M. A., and Rojas, O. J. (2008). “Colloidal stability and aggregation of lignocellulosic materials in aqueous suspension: A review,” BioResources 3(4), 1419-1491. DOI: 10.15376/biores.3.4.1419-1491
Hubbe, M. A., Rojas, O. J., and Lucia, L. A. (2015b). “Green modification of surface characteristics of cellulosic materials at the molecular or nano scale: A review,” BioResources 10(3), 6095-6229. DOI: 10.15376/biores.10.3.Hubbe
Hubbe, M. A., Rojas, O. J., Lucia, L. A., and Sain, M. (2008). “Cellulosic nanocomposites: A review,” BioResources 3, 929-980. DOI: 10.15376/biores.3.3.929-980
Huber, T., Müssig, J., Curnow, O., Pang, S., Bickerton, S., and Staiger, M. P. (2012). “A critical review of all-cellulose composites,” Journal of Materials Science 47, 1171-1186. DOI: 10.1007/s10853-011-5774-3
Hult, E.-L., Iotti, M., and Lenes, M. (2010). “Efficient approach to high barrier packaging using microfibrillar cellulose and shellac,” Cellulose 17, 575-586. DOI: 10.1007/s10570-010-9408-8
Husband, J. C., Svending, P., Skuse, D. R., Motsi, T., Likitalo, M., and Coles, A. (2011). “Paper filler method,” US Patent 8,231,764.
Ibn Yaich, A., Edlund, U., and Albertsson, A. C. (2015). “Barriers from wood hydrolysate/quaternized cellulose polyelectrolyte complexes,” Cellulose 22(3), 1977-1991. DOI: 10.1007/s10570-015-0621-3
Iotti, M. (2014). “Aqueous coating composition useful as a coating layer, and as an oxygen barrier or smoothing layer, comprises nanocellulose, and at least one cationic surfactant,” US Pat. US2015225590-A1.
Iwamoto, S., Abe, K., and Yano, H. (2008). “The effect of hemicelluloses on wood pulp nanofibrillation and nanofiber network characteristics,” Biomacromolecules 9, 1022-1026. DOI: 10.1021/bm701157n
Jebel, F. S., and Almasi, H. (2016). “Morphological, physical, antimicrobial and release properties of ZnO nanoparticles-loaded bacterial cellulose films,” Carbohydr. Polym. 149, 8-19. DOI: 10.1016/j.carbpol.2016.04.089
Jiang, F., Kondo, T., and Hsieh, Y. L. (2016). “Rice straw cellulose nanofibrils via aqueous counter collision and differential centrifugation and their self-assembled structures,” ACS Sustainable Chem. Eng. 4(3), 1697-1706. DOI: 10.1021/acssuschemeng.5b01653
Johansson, C., Bras, J., Mondragon, I., Nechita, P., Plackett, D., Simon, P., Svetec, D. G., Virtanen, S., Baschetti, M. G., Breen, C., et al. (2012). “Renewable fibers and bio-based materials for packaging applications – A Review of recent developments,” BioResources 7(2), 2505-2552. DOI: 10.15376/biores.7.2.2506-2552
Johnson, R. K., Zink-Sharp, A., Renneckar, S. H., and Glasser, W. G. (2009). “A new bio-based nanocomposite: Fibrillated TEMPO-oxidized celluloses in hydroxypropylcellulose matrix,” Cellulose 16(2), 227-238. DOI: 10.1007/s10570-008-9269-6
Johnsy, G., and Siddaramaiah, H. (2012). “High performance edible nanocomposite films containing bacterial cellulose nanocrystals,” Carbohydrate Polymers 87(3), 2031-2037. DOI: 10.1016/j.carbpol.2011.10.019
Kangas, H., Lahtinen, P., Sneck, A., Saariaho, A. M., Laitinen, O., and Hellen, E. (2014). “Characterization of fibrillated celluloses. A short review and evaluation of characteristics with a combination of methods,” Nordic Pulp Paper Res. J. 29(1), 129-143. DOI: 10.3183/NPPRJ-2014-29-01-p129-143
Keshavarzi, N., Rad, F. M., Mace, A., Ansari, F., Akhtar, F., Nilsson, U., Berglund, L., and Bergstrom, L. (2015). “Nanocellulose-zeolite composite films for odor elimination,” ACS Appl. Mater. Interfaces 7(26), 14254-14262. DOI: 10.1021/acsami.5b02252
Khalil, H. P. S. A., Bhat, A. H., and Yusra, A. F. I. (2012). “Green composites from sustainable cellulose nanofibrils: A review,” Carbohydrate Polymers 87(2), 963-979. DOI: 10.1016/j.carbpol.2011.08.078
Khalil, H. P. S. A., Davoudpour, Y., Islam, M. N., Mustapha, A., Sudesh, K., Dungani, R., and Jawaid, M. (2014). “Production and modification of nanofibrillated cellulose using various mechanical processes: A review,” Carbohydrate Polymers 99, 649-665. DOI: 10.1016/j.carbpol.2013.08.069
Khalil, H. P. S. A., Saurabh, C. K., Adnan, A. S., Fazita, M. R. N., Syakir, M. I., Davoudpour, Y., Rafatullah, M., Abdullah, C. K., Haafiz, M. K. M., and Dungani, R. (2016). “A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications,” Carbohydrate Polym. 150, 216-226. DOI: 10.1016/j.carbpol.2016.05.028
Khan, A., Huq, T., Khan, R. A., Riedl, B., and Lacroix, M. (2014a). “Nanocellulose-based composites and bioactive agents for food packaging,” Crit. Rev. Food Sci. Nutrition 54(2), 163-174. DOI: 10.1080/10408398.2011.578765
Khan, A., Khan, R. A., Salmieri, S., Le Tien, C., Riedl, B., Bouchard, J., Chauve, G., Tan, V., Kamal, M. R., and Lacroix, M. (2012). “Mechanical and barrier properties of nanocrystalline cellulose reinforced chitosan based nanocomposite films,” Carbohydrate Polymers 90(4), 1601-1608. DOI: 10.1016/j.carbpol.2012.07.037
Khan, R. A., Beck, S., Dussault, D., Salmieri, S., Bouchard, J., and Lacroix, M. (2013). “Mechanical and barrier properties of nanocrystalline cellulose reinforced poly(caprolactone) composites: Effect of gamma radiation,” J. Appl. Polym. Sci. 129(5), 3038-3046. DOI: 10.1002/app.38896
Khan, R. A., Korehei, R., Salem, H. J., Darychuk, N., Martinez, D. M., and Olson, J. A. (2014b). “Fabrication and characterization of microfibrillated cellulose reinforced sodium alginate-based biodegradable films for packaging applications,” J-For-J. Sci. Technol. Forest Prod. Proc. 4(1), 58-64.
Khan, R. A., Salmieri, S., Dussault, D., Uribe-Calderon, J., Kamal, M. R., Safrany, A., and Lacroix, M. (2010). “Production and properties of nanocellulose-reinforced methylcellulose-based biodegradable films,” J. Agric. Food Chem. 58(13), 7878-7885. DOI: 10.1021/jf1006853
Kibedi-Szabo, C. Z., Stroescu, M., Stoica-Guzun, A., Jinga, S. I., Szilveszter, S., Jipa, I. and Dobre, T. (2012). “Biodegradation behavior of composite films with poly (vinyl alcohol) matrix,” J. Polym Environ. 20(2), 422-430. DOI: 10.1007/s10924-011-0391-4
Kim, Y., Jung, R., Kim, H. S., and Jin, H. J. (2009). “Transparent nanocomposites prepared by incorporating microbial nanofibrils into poly(L-lactic acid),” Curr. Appl. Phys. 9(1 SUPPL.), S69-S71. DOI: 10.1016/j.cap.2008.08.010
Kisonen, V., Prakobna, K., Xu, C. L., Salminen, A., Mikkonen, K. S., Valtakari, D., Eklund, P., Seppala, J., Tenkanen, M., and Willfor, S. (2015). “Composite films of nanofibrillated cellulose and O-acetyl galactoglucomannan (GGM) coated with succinic esters of GGM showing potential as barrier material in food packaging,” J. Mater. Sci. 50(8), 3189-3199. DOI: 10.1007/s10853-015-8882-7
Kiviranta, A. (2000). “Paper and board grades,” in: Forest Productions Chemistry, of Papermaking Science and Technology series, Vol. 18, J. Gullichsen and H. Paulapuro (eds.), Finnish Paper Engineers’ Assoc. and TAPPI.
Kiziltas, E. E., Kiziltasa, A., Bollin, S. C., and Gardner, D. J. (2015). “Preparation and characterization of transparent PMMA-cellulose-based nanocomposites,” Carbohyd. Polym. 127, 381-389. DOI: 10.1016/j.carbpol.2015.03.029
Klemm, D., Kramer, F., Moritz, S., Lindström, T., Ankerfors, M., Gray, D., and Dorris, A. (2011). “Nanocelluloses: A new family of nature-based materials,” Angew. Chem. Intl. Ed. 50(24), 5438-5466. DOI: 10.1002/anie.201001273
Kolakovic, R., Peltonen, L., Laukkanen, A., Hirvonen, J., and Laaksonen, T. (2012). “Nanofibrillar cellulose films for controlled drug delivery,” European Journal of Pharmaceutics and Biopharmaceutics 82(2), 308-315. DOI: 10.1016/j.ejpb.2012.06.011
Krochta, J. M., and DeMulderJohnston, C. (1997). “Edible and biodegradable polymer films: Challenges and opportunities,” Food Technol. 51(2), 61-74.
Kumar, V., Bollstrom, R., Yang, A., Chen, Q. X., Chen, G., Salminen, P., Bousfield, D., and Toivakka, M. (2014). “Comparison of nano- and microfibrillated cellulose films,” Cellulose 21(5), 3443-3456. DOI: 10.1007/s10570-014-0357-5
Kumar, V., Elfving, A., Koivula, H., Bousfield, D., and Toivakka, M. (2016). “Roll-to-roll processed cellulose nanofiber coatings,” Indust. Eng. Chem. Res. 55(12), 3603-3613. DOI: 10.1021/acs.iecr.6b00417
Kurihara, T., and Isogai, A. (2014). “Properties of poly(acrylamide)/TEMPO-oxidized cellulose nanofibril composite films,” Cellulose 21, 291-299. DOI: 10.1007/s10570-013-0124-z
Lagaron, J. M., Catala, R., and Gavara, R. (2004). “Structural characteristics defining high barrier properties in polymeric materials,” Mater. Sci. Technol. 20, 1-7. DOI: 10.1179/026708304225010442
Larsson, K., Berglund, L. A., Ankerfors, M., and Lindström, T. (2012). “Polylactide latex/nanofibrillated cellulose bionanocomposites of high nanofibrillated cellulose content and nanopaper network structure prepared by a papermaking route,” Journal of Applied Polymer Science 125, 2460-2466. DOI: 10.1002/app.36413
Lavoine, N., Bras, J., and Desloges, I. (2014a). “Mechanical and barrier properties of cardboard and 3D packaging coated with microfibrillated cellulose,” J. Applied Polymer Sci. 131(8), article no. 40106. DOI: 10.1002/app.40106
Lavoine, N., Desloges, I., and Bras, J. (2011). “Impact of different coating processes of MFC on barrier and mechanical properties,” in: TAPPI International Conference on Nanotechnology for Renewable Materials, Arlington, VA, USA, (p. 38).
Lavoine, N., Desloges, I., and Bras, J. (2014b). “Microfibrillated cellulose coatings as new release systems for active packaging,” Carbohydrate Polymers 103, 528-537. DOI: 10.1016/j.carbpol.2013.12.035
Lavoine, N., Desloges, I., Dufresne, A., and Bras, J. (2012). “Microfibrillated cellulose: Its barrier properties and applications in cellulosic materials: A review,” Carbohyd. Polym. 90, 735-764. DOI: 10.1016/j.carbpol.2012.05.026
Lavoine, N., Desloges, I., Khelifi, B., and Bras, J. (2014c). “Impact of different coating processes of microfibrillated cellulose on the mechanical and barrier properties of paper,” J. Mater. Sci. 49(7), 2879-2893. DOI: 10.1007/s10853-013-7995-0
Lavoine, N., Desloges, I., Manship, B., and Bras, J. (2015). “Antibacterial paperboard packaging using microfibrillated cellulose,” J. Food Sci. Technol. – Mysore 52(9), 5590-5600. DOI: 10.1007/s13197-014-1675-1
Lavoine, N., Guillard, V., Desloges, I., Gontard, N., and Bras, J. (2016). “Active bio-based food-packaging: Diffusion and release of active substances through and from cellulose nanofiber coating toward food-packaging design,” Carbohydrate Polym. 149, 40-50. DOI: 10.1016/j.carbpol.2016.04.048
Lee, K.-Y., Tammelin, T., Schulfter, K., Kiiskinen, H., Samela, J., and Bismarck, A. (2012). “High performance cellulose nanocomposites: Comparing the reinforcing ability of bacterial cellulose and nanofibrillated cellulose,” Appl. Mater. Interfaces 4(8), 4078-4086. DOI: 10.1021/am300852a
Lee, S. Y., Mohan, D. J., Kang, I. A., Doh, G. H., Lee, S., and Han, S. O. (2009). “Nanocellulose reinforced PVA composite films: Effects of acid treatment and filler loading,” Fibers. Polym. 10(1), 77-82. DOI: 10.1007/s12221-009-0077-x
Lendvai, L., Karger-Kocsis, J., Kmetty, A., and Drakopoulos, S. X. (2016). “Production and characterization of microfibrillated cellulose-reinforced thermoplastic starch composites,” J. Appl. Polymer Sci. 133(2), article no. 42397. DOI: 10.1002/app.42397
Le Normand, M., Moriana, R., and Ek, M. (2014). “The bark biorefinery: A side-stream of the forest industry converted into nanocomposites with high oxygen-barrier properties,” Cellulose 21(6), 4583-4594. DOI: 10.1007/s10570-014-0423-z
Li, F., Biagioni, P., Bollani, M., Maccagnan, A., and Piergiovanni, L. (2013a). “Multi-functional coating of cellulose nanocrystals for flexible packaging applications,” Cellulose 20, 2491-2504. DOI: 10.1007/s10570-013-0015-3
Li, F., Biagioni, P., Finazzi, M., Tavazzi, S., and Piergiovanni, L. (2013b). “Tunable green oxygen barrier through layer-by-layer self-assembly of chitosan and cellulose,” Carbohydr. Polym. 92(2), 2128-2134. DOI: 10.1016/j.carbpol.2012.11.091
Li, F., Mascheroni, E., and Piergiovanni, L. (2015a). “The potential of nanocellulose in the packaging field: A review,” Packaging Technol. Sci. 28(6), 475-508. DOI: 10.1002/pts.2121
Li, H. Z., Chen, S. C., and Wang, Y. Z. (2015b). “Preparation and characterization of nanocomposites of polyvinyl alcohol/cellulose nanowhiskers/chitosan,” Composites Sci. Technol. 115, 60-65. DOI: 10.1016/j.compscitech.2015.05.004
Li, Q., Zhou, J., and Zhang, L. (2009). “Structure and properties of the nanocomposite films of chitosan reinforced with cellulose whiskers,” Journal of Polymer Science B: Polymer Physics 47, 1069-1077. DOI: 10.1002/polb.21711
Liimatainen, H., Ezekiel, N., Sliz, R., Ohenoja, K., Sirvio, J. A., Berglund, L., Hormi, O., and Niinimaki, J. (2013). “High-strength nanocellulose-talc hybrid barrier films,” ACS Appl. Mater. Interfaces 5(24), 13412-13418. DOI: 10.1021/am4043273
Lindström, S. B., Karabulut, E., Kulachenko, A., Sehaqui, H., and Wagberg, L. (2012). “Mechanosorptive creep in nanocellulose materials,” Cellulose 19(3), 809-819. DOI: 10.1007/s10570-012-9665-9
Lindström, T., and Aulin, C. (2014). “Market and technical challenges and opportunities in the area of innovative new materials and composites based on nanocellulosics,” Scan. J. Forest Res. 29(4), 345-351. DOI: 10.1080/02827581.2014.928365
Liu, A. D., and Berglund, L. A. (2012). “Clay nanopaper composites of nacre-like structure based on montmorrilonite and cellulose nanofibers-Improvements due to chitosan addition,” Carbohydrate Polymers 87(1), 53-60. DOI: 10.1016/j.carbpol.2011.07.019
Liu, K., Lin, X. X., Chen, L. H., Huang, L. L., Cao, S. L., and Wang, H. W. (2013). “Preparation of microfibrillated cellulose/chitosan-benzalkonium chloride biocomposite for enhancing antibacterium and strength of sodium alginate films,” J. Agr. Food Chem. 61(26), 6562-6567. DOI: 10.1021/jf4010065
Liu, K., Nasrallah, J., Chen, L. H., Huang, L. L., and Ni, Y. H. (2015). “Preparation of CNC-dispersed Fe3O4 nanoparticles and their application in conductive paper,” Carbohydrate Polymers 126, 175-178. DOI: 10.1016/j.carbpol.2015.03.009
Liu, L., Chen, Y. Z., and Zhang, Z. J. (2014). “Preparation of the microfibrillated cellulose and its application in the food packaging paper,” in: Research on Food Packaging Technology, Yun, O., Min, X., Li, Y. T., and Ting, X. L. (eds.), Book Series: Applied Mechanics and Materials, Vol. 469, pp. 87-90. DOI: 10.4028/www.scientific.net/AMM.469.87
Lopacka, J. (2013). “Nanoparticles used to improve physical properties of polymer composites for food packaging materials,” Polimery 58(11-12), 864-868. DOI: 10.14314/polimery.2013.864
López-Rubio, A., Lagaron, J. M., Ankerfors, M., Lindström, T., Nordqvist, D., Mattozzi, A., and Hedenqvist, M. S. (2007). “Enhanced film forming and film properties of amylopectin using micro-fibrillated cellulose,” Carbohydr. Polym. 68, 718-727. DOI: 10.1016/j.carbpol.2006.08.008
Lu, F. F., Yu, H. Y., Yan, C. F., and Yao, J. M. (2016). “Polylactic acid nanocomposite films with spherical nanocelluloses as efficient nucleation agents: Effects on crystallization, mechanical and thermal properties,” RSC Advances 6(51), 46008-46018. DOI: 10.1039/c6ra02768g
Lu, J., Wang, T., and Drzal, L. T. (2008). “Preparation and properties of microfibrillated cellulose polyvinyl alcohol composite materials,” Compos. A 39(5), 738-746. DOI: 10.1016/j.compositesa.2008.02.003
Lu, P., Xiao, H. N., and Pan, Y. F. (2015). “Improving water vapor barrier of green-based nanocellulose film via hydrophobic coating,” Proceedings of the 2014 International Conference on Materials Science and Energy Engineering (CMSEE 2014), Chung, S. L., and Li, X. (eds.), pp. 148-153. DOI: 10.1142/9789814678971_0023
Lu, P., Xiao, H. N., Zhang, W. W., and Gong, G. (2014). “Reactive coating of soybean oil-based polymer on nanofibrillated cellulose film for water vapor barrier packaging,” Carbohydrate Polymers 111, 524-529. DOI: 10.1016/j.carbpol.2014.04.071
Ludwicka, K., Jedrzejczak-Krzepkowska, M., Kubiak, K., Kolodzeijczyck, M., Penkiewicz, T., and Bielecki, S. (2016). “Medical and cosmetic applications of bacterial nanocellulose,” in: Bacterial Nanocellulose: From Biotechnology to Bio-Economy, M. Gama, F. Dourado, and S. Bielecki (eds.), Elsevier, Amsterdam, pp. 145-165. DOI: 10.1016/b978-0-444-63458-0.00009-3
Lundahl, M. J., Cunha, A. G., Rojo, E., Papageorgiou, A. C., Rautkari. L., Arboleda, J. C., and Rojas, O. J. (2016). “Strength and water interactions of cellulose I filaments wet-spun from cellulose nanofibril hydrogels,” Scientific Reports 6, 30695. DOI: 10.1038/srep30695
Luzi, F., Fortunati, E., Puglia, D., Petrucci, R., Kenny, J. M., and Torre, L. (2015). “Study of disintegrability in compost and enzymatic degradation of PLA and PLA nanocomposites reinforced with cellulose nanocrystals extracted from Posidonia oceanica,” Polym. Degrad. Stabil. 121,105-115. DOI: 10.1016/j.polymdegradstab.2015.08.016
Ma, X. F., Chang, P. R., and Yu, J. G. (2008). “Properties of biodegradable thermoplastic pea starch/carboxymethyl cellulose and pea starch/microcrystalline cellulose composites,” Carbohydrate Polymers 72(3), 369-375. DOI: 10.1016/j.carbpol.2007.09.002
Machado, B. A. S., Nunes, I. L., Pereira, F. V., and Druzian, J. I. (2012). “Development and evaluation of the effectiveness of biodegradable films of cassava starch with nanocellulose as reinforcement and yerba mate extract as an additive antioxidant,” Ciencia Rural 42(11), 2085-2091. DOI: 10.1590/S0103-84782012001100028
Mandal, A., and Chakrabarty, D. (2015). “Characterization of nanocellulose reinforced semi-interpenetrating polymer network of poly(vinyl alcohol) & polyacrylamide composite films,” Carbohydr. Polym. 134, 240-250. DOI: 10.1016/j.carbpol.2015.07.093
Marais, A., Utsel, S., Gustafsson, E., and Wågberg, L. (2014). “Towards a super-strainable paper using the layer-by-layer technique,” Carbohydrate Polymers 100, 218-224. DOI: 10.1016/j.carbpol.2013.03.049
Mariano, M., El Kissi, N., and Dufresne, A. (2014). “Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges,” J. Polym. Sci. Part B – Polym. Phys. 52(12), 791-806. DOI: 10.1002/polb.23490
Mariano, M., El Kissi, N., and Dufresne, A. (2016). “Cellulose nanocrystal reinforced oxidized natural rubber nanocomposites,” Carbohydrate Polym. 137, 174-183. DOI: 10.1016/j.carbpol.2015.10.027
Martínez-Sanz, M., Lopez-Rubio, A., and Lagaron, J. M. (2012). “Optimization of the dispersion of unmodified bacterial cellulose nanowhiskers into polylactide via melt compounding to significantly enhance barrier and mechanical properties,” Biomacromolecules 13(11), 3887-3899. DOI:10.1021/bm301430j
Martínez-Sanz, M., Abdelwahab, M. A., Lopez-Rubio, A., Lagaron, J. M., Chiellini, E., Williams, T. G., Wood, D. F., Orts, W. J., and Imam, S. H. (2013a). “Incorporation of poly(glycidylmethacrylate) grafted bacterial cellulose nanowhiskers in poly(lactic acid) nanocomposites: Improved barrier and mechanical properties,” Eur. Polymer J. 49(8), 2062-2072. DOI: 10.1016/j.eurpolymj.2013.04.035
Martínez-Sanz, M., Lopez-Rubio, A., and Lagaron, J. M. (2013b). “Nanocomposites of ethylene vinyl alcohol copolymer with thermally resistant cellulose nanowhiskers by melt compounding (I): Morphology and thermal properties,” J. Appl. Polym. Sci. 128(5), 2666-2678. DOI: 10.1002/app.38433
Martínez-Sanz, M., Lopez-Rubio, A., and Lagaron, J. M. (2013c). “Nanocomposites of ethylene vinyl alcohol copolymer with thermally resistant cellulose nanowhiskers by melt compounding (II): Water barrier and mechanical properties,” J. Appl. Polym. Sci. 128(3), 2197-2207. DOI:10.1002/app.38432
Martins, I. M. G., Magina, S. P., Oliveira, L., Freire, C. S. R., Silvestre, A. J. D., Neto, C. P., and Gandini, A. (2009). “New biocomposites based on thermoplastic starch and bacterial cellulose,” Compos. Sci. Technol. 69(13), 2163-2168. DOI: 10.1016/j.compscitech.2009.05.012
Mascheroni, E., Rampazzo, R., Ortenzi, M. A., Piva, G., Bonetti, S., and Piergiovanni, L. (2016). “Comparison of cellulose nanocrystals obtained by sulfuric acid hydrolysis and ammonium persulfate, to be used as coating on flexible food-packaging materials,” Cellulose 23(1), 779-793. DOI: 10.1007/s10570-015-0853-2
Mathew, A. P., Thielemans, W., and Dufresne, A. (2008). “Mechanical properties of nanocomposites from sorbitol plasticized starch and tunicin whiskers,” Journal of Applied Polymer Science 109, 4065-4074. DOI: 10.1002/app.28623
Merindol, R., Diabang, S., Felix, O., Roland, T., Gauthier, C., and Decher, G. (2015). “Bio-inspired multiproperty materials: Strong, self-healing, and transparent artificial wood nanostructures,” ACS Nano 9(2), 1127-1136. DOI: 10.1021/nn504334u
Miettinen, A., Chinga-Carrasco, G., and Kataja, M. (2014). “Three-dimensional microstructural properties of nanofibrillated cellulose films,” Internat. J. Molec. Sci. 15(4), 6423-6440. DOI: 10.3390/ijms15046423
Mihindukulasuriya, S. D. F., and Lim, L. T. (2014). “Nanotechnology development in food packaging: A review,” Trends Food Sci. Technol. 40(2), 149-167. DOI: 10.1016/j.tifs.2014.09.009
Mikkonen, K. S., Stevanic, J. S., Joly, C., Dole, P., Pirkkalainen, K., Serimaa, R., Salmén, L., and Tenkanen, M. (2011). “Composite films from spruce galactoglucomannans with microfibrillated spruce wood cellulose,” Cellulose 18, 713-726. DOI:10.1007/s10570-011-9524-0
Minelli, M., Baschetti, M. G., Doghieri, F., Ankerfors, M., Lindström, T., Siró, I., and Plackett, D. (2010). “Investigation of mass transport properties of microfibrillated cellulose (MFC) films,” J. Membrane Sci. 358, 67-75. DOI: 10.1016/j.memsci.2010.04.030
Miranda, C. S., Ferreira, M. S., Magalhaes, M. T., Bispo, A. P. G., Oliveira, J. C., Silva, J. B. A., and Jose, N. M. (2015). “Starch-based films plasticized with glycerol and lignin from piassava fiber reinforced with nanocrystals from eucalyptus,” Mater. Today – Proc. 2(1), 134-140. DOI: 10.1016/j.matpr.2015.04.038
Moon, R. J., Martini, A., Nairn, J., Simonsen, J., and Youngblood, J. (2011). “Cellulose nanomaterials review: Structure, properties and nanocomposites,” Chem. Soc. Rev. 40, 3941-3994. DOI: 10.1039/c0cs00108b
Naderi, A., Lindstrom, T., Weise, C. F., Flodberg, G., Sundstrom, J., Junel, K., Erlandsson, J., and Runebjork, A. (2016). “Phosphorylated nanofibrillated cellulose: Production and properties,” Nordic Pulp Paper Res. J. 31(1), 20-29. DOI: 10.3183/NPPRJ-2016-31-01-p020-029
Nakagaito, A. N., Iwamoto, S., and Yano, H. (2005). “Bacterial cellulose: The ultimate nano-scalar cellulose morphology for the production of high-strength composites,” Appl. Phys. A Mater. 80, 93-97. DOI: 10.1007/s00339-004-2932-3
Nakagaito, A. N., and Yano, H. (2004). “The effect of morphological changes from pulp fiber towards nano-scale fibrillated cellulose on the mechanical properties of high-strength plant fiber based composites,” Appl. Phys. A Mater. 78, 547-552. DOI: 10.1007/s00339-003-2453-5
Nakagaito, A. N., and Yano, H. (2005). “Novel high-strength biocomposites based on microfibrillated cellulose having nano-order-unit web-like network structure,” Applied Physics A: Materials Science & Processing 80(1), 155-159. DOI: 10.1007/s00339-003-2225-2
Nakagaito, A., and Yano, H. (2008a). “The effect of fiber content on the mechanical and thermal expansion properties of biocomposites based on microfibrillated cellulose,” Cellulose 15(4), 555-559. DOI: 10.1007/s10570-008-9212-x
Nakagaito, A., and Yano, H. (2008b). “Toughness enhancement of cellulose nanocomposites by alkali treatment of the reinforcing cellulose nanofibers,” Cellulose 15(2), 323-331. DOI: 10.1007/s10570-007-9168-2
Nelson, K., Retsina, T., Iakovlev, M., van Heiningen, A., Deng, Y. L., Shatkin, J. A., and Mulyadi, A. (2016). “American process: Production of low cost nanocellulose for renewable, advanced materials applications,” in: Materials Research for Manufacturing: An Industrial Perspective of Turning Materials into New Products, L. D. Madsen and E. B. Svedberg (eds.), Springer Ser. Mater. Sci., DOI: 10.1007/978-3-319-23419-9_9
Nogi, M., Iwamoto, S., Nakagaito, A. N., and Yano, H. (2009). “Optically transparent nanofiber paper,” Adv. Mater. 21, 1595-1598. DOI: 10.1002/adma.200803174
Nordqvist, D., Idermark, J., Hedenqvist, M., Gällstedt, M., Ankerfors, M., and Lindström, T. (2007). “Enhancement of the wet properties of transparent chitosan-acetic acid-salt films using microfibrillated cellulose,” Biomacromolecules 8, 2398-2403. DOI: 10.1021/bm070246x
Noshirvani, N., Ghanbarzadeh, B., Fasihi, H., and Almasi, H. (2016). “Starch-PVA nanocomposite film incorporated with cellulose nanocrystals and MMT: A comparative study,” Intl. J. Food Eng. 12(1), 37-48. DOI: 10.1515/ijfe-2015-0145
Nygårds, S., Aulin, C., and Ström, G. (2011). “Nanocellulose in pigment coatings – Aspects of barrier properties and printability in offset,” Department of Physics, Chemistry and Biology (Master’s thesis), Linköping University and Invenntia AB, Sweden.
Oksman, K., Mathew, A. P., and Sain, M. (2009). “Novel bionanocomposites: Processing, properties and potential applications,” Plastics Rubber Composites 38(9-10), 396-405. DOI: 10.1179/146580109X12540995045723
Ollier, R. P., Perez, C. J., and Alvarez, V. A. (2013). “Preparation and characterization of micro and nanocomposites based on poly(vinyl alcohol) for packaging applications,” J. Mater. Sci. 48(20), 7088-7096. DOI: 10.1007/s10853-013-7521-4
Olsson, R. T., Fogelstrom, L., Martinez-Sanz, M., and Henriksson, M. (2011). “Cellulose nanofillers for food packaging,” in: Multifunctional and Nanoreinforced Polymers for Food Packaging, Lagaron, J. M. (ed.), Book Series: Woodhead Publishing in Materials, pp. 86-107. DOI: 10.1533/9780857092786.1.86
Osorio, M. A., Restrepo, D., Velasquez-Cock, J. A., Zuluaga, R. O., Montoya, U., Rojas, O., Ganan, P. F., Marin, D., and Castro, C. I. (2014). “Synthesis of thermoplastic starch-bacterial cellulose nanocomposites via in situ fermentation,” J. Braz. Chem. Soc. 25(9), 1607-1613. DOI: 10.5935/0103-5053.20140146
Österberg, M., Vartiainen, J., Lucenius, J., Hippi, U., Seppala, J., Serimaa, R., and Laine, J. (2013). “A fast method to produce strong NFC films as a platform for barrier and functional materials,” ACS Appl. Mater. Interfaces 5, 4640-4647. DOI:10.1021/am401046x
Oun, A. A., and Rhim, J. W. (2015). “Preparation and characterization of sodium carboxymethyl cellulose/cotton linter cellulose nanofibril composite films,” Carbohydrate Polymers 127, 101-109. DOI: 10.1016/j.carbpol.2015.03.073
Pääkkö, M., Ankerfors, M., Kosonen, H., Nykanen, A., Ahola, S., Österberg, M., Ruokolainen, J., Laine, J., Larsson, P. T., Ikkala, O., et al. (2007). “Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels,” Biomacromolecules 8(6), 1934-1941. DOI: 10.1021/bm061215p
Pääkkö, M., Vapaavuori, J., Silvennoinen, R., Kosonen, H., Ankerfors, M., Lindström, T., Berglund, L. A., and Ikkala, O. (2008). “Long and entangled native cellulose I nanofibers allow flexible aerogels and hierarchically porous templates for functionalities,” Soft Matter 4(12), 2492-2499. DOI: 10.1039/b810371b
Padrao, J., Goncalves, S., Silva, J. P., Sencadas, V., Lanceros-Mendez, S., Pinheiro, A. C., Vicente, A. A., Rodrigues, L. R., and Dourado, F. (2016). “Bacterial cellulose-lactoferrin as an antimicrobial edible packaging,” Food Hydrocolloids 58, 126-140. DOI: 10.1016/j.foodhyd.2016.02.019
Palaninathan, V., Chauhan, N., Poulose, A. C., Raveendran, S., Mizuki, T., Hasumura, T., Fukuda, T., Morimoto, H., Yoshida, Y., Maekawa, T., et al. (2014). “Acetosulfation of bacterial cellulose: An unexplored promising incipient candidate for highly transparent thin film,” Mater. Express 4(5), 415-421. DOI: 10.1166/mex.2014.1191
Panaitescu, D. M., Frone, A. N., Ghiurea, M., and Chiulan, I. (2015). “Influence of storage conditions on starch/PVA films containing cellulose nanofibers,” Indust. Crops Prod. 70, 170-177. DOI: 10.1016/j.indcrop.2015.03.028
Paunonen, S. (2013a). “Strength and barrier enhancements of cellophane and cellulose derivative films: A Review,” BioResources 8(2), 3098-3121. DOI: 10.15376/biores.8.2.3098-3121
Paunonen, S. (2013b). “Strength and barrier enhancements of composites and packaging boards by nanocelluloses – A literature review,” Nordic Pulp Paper Res. J. 28(2), 165-181. DOI: 10.3183/NPPRJ-2013-28-02-p165-181
Peng, X. W., Ren, J. L., Zhong, L. X., and Sun, R. C. (2011). “Nanocomposite films based on xylan-rich hemicelluloses and cellulose nanofibers with enhanced mechanical properties,” Biomacromolecules 12, 3321-3329. DOI: 10.1021/bm2008795
Pereda, M., Amica, G., Rácz, I., and Marcovich, N. E. (2011). “Structure and properties of nanocomposite films based on sodium caseinate and nanocellulose fibers,” J. Food Eng. 103, 76-83. DOI: 10.1016/j.jfoodeng.2010.10.001
Pereda, M., Dufresne, A., Aranguren, M. I., and Marcovich, N. E. (2014). “Polyelectrolyte films based on chitosan/olive oil and reinforced with cellulose nanocrystals,” Carbohydrate Polymers 101, 1018-1026. DOI: 10.1016/j.carbpol.2013.10.046
Pereira, A. L. S., do Nascirnento, D. M., Souza, M. D. M., Moraisd, J. P. S., Vasconcelos, N. F., Feitosa, J. P. A., Brigida, A. I. S., and Rosa, M. D. (2014). “Improvement of polyvinyl alcohol properties by adding nanocrystalline cellulose isolated from banana pseudostems,” Carbohydrate Polymers 112, 165-172. DOI: 10.1016/j.carbpol.2014.05.090
Peresin, M. S., Vesterinen, A.-H., Habibi, Y., Johansson, L.-S., Pawlak, J. J., Nevzorov, A. A., and Rojas, O. J. (2014). “Crosslinked PVA nanofibers reinforced with cellulose nanocrystals: Water interactions and thermomechanical properties,” Journal Applied Polymer Science, article 40334, 12 pp. DOI: 10.1002/ app.40334.
Petersen, N., and Gatenholm, P. (2011). “Bacterial cellulose-based materials and medical devices: Current state and perspectives,” Appl. Microbiol. Biotechnol. 91, 1277-1286. DOI: 10.1007/s00253-011-3432-y
Petersson, L., Mathew, A. P., and Oksman, K. (2009). “Dispersion and properties of cellulose nanowhiskers and layered silicates in cellulose acetate butyrate nanocomposites,” J. Appl. Polym. Sci. 112(4), 2001-2009. DOI: 10.1002/app.29661
Petersson, L., and Oksman, K. (2006). “Biopolymer based nanocomposites: Comparing layered silicates and microcrystalline cellulose as nanoreinforcement,” Composite Science and Technology 66, 2187-2196. DOI: 10.1016/j.compscitech.2005.12.010
Piao, G. Z., and Zhang, D. W. (2016). “Development in tunicate cellulose,” in: Handbook of Sustainable Polymers: Processing and Applications, V. K. Thakur and M. K. Thakur (eds.), Pan Stanford Publ., Singapore.
Plackett, D., Anturi, H., Hedenqvist, M., Ankerfors, M., Gallstedt, M., Lindstrom, T., and Siro, I. (2010). “Physical properties and morphology of films prepared from microfibrillated cellulose and microfibrillated cellulose in combination with amylopectin,” J. Appl. Polymer Sci. 117(6), 3601-3609. DOI: 10.1002/app.32254
Pönni, R., Vuorinen, T., and Kontturi, E. (2012). “Proposed nano-scale coalescence of cellulose in chemical pulp fibers during technical treatments,” BioResources 7(4), 6077-6108. DOI: 10.15376/biores.7.4.6077-6108
Prakobna, K., Terenzi, C., Zhou, Q., Furo, I., and Berglund, L. A. (2015). “Core-shell cellulose nanofibers for biocomposites – Nanostructural effects in hydrated state,” Carbohydrate Polymers 125, 92-102. DOI: 10.1016/j.carbpol.2015.02.059
Qing, Y., Sabo, R., Wu, Y., and Cai, Z. (2012). “High-performance cellulose nanofibril composite films,” BioResources 7(3), 3064-3075. DOI: 10.15376/biores.7.3.3064-3075
Rabinovich, M. L., Melnik, M. S., and Boloboba, A. V. (2002). “Microbial cellulases (Review),” Appl. Biochem. Microbiol. 38(4), 305-321. DOI: 10.1023/A:1016264219885
Rafieian, F., Shahedi, M., Keramat, J., and Simonsen, J. (2014). “Mechanical, thermal and barrier properties of nano-biocomposite based on gluten and carboxylated cellulose nanocrystals,” Industrial Crops Prod. 53, 282-288. DOI: 10.1016/j.indcrop.2013.12.016
Rafieian, F., and Simonsen, J. (2014). “Fabrication and characterization of carboxylated cellulose nanocrystals reinforced glutenin nanocomposite,” Cellulose 21(6), 4167-4180. DOI: 10.1007/s10570-014-0305-4
Raghu, S. (2015). “Formation of nanocellulose composite used for forming packaging material, involves blending copolymer which is reaction product of hydrophilic monomer, cellulose-reactive monomer and amphiphobic monomer, with nanocellulose,” US Pat., US2015072581-A1.
Rastogi, V. K., and Samyn, P. (2015). “Bio-based coatings for paper applications,” Coatings 5(4), 887-930. DOI: 10.3390/coatings5040887
Rautkoski, H., Pajari, H., Koskela, H., Sneck, A., and Moilanen, P. (2015). “Use of cellulose nanofibrils (CNF) in coating colors,” Nordic Pulp Paper Res. J. 30(3), 511-518. DOI: 10.3183/NPPRJ-2015-30-03-p511-518
Reddy, J. P., and Rhim, J. W. (2014). “Characterization of bionanocomposite films prepared with agar and paper-mulberry pulp nanocellulose,” Carbohydrate Polymers 110, 480-488. DOI: 10.1016/j.carbpol.2014.04.056
Rhim, J. W. (2007). “Potential use of biopolymer-based nanocomposite films in food packaging applications,” Food Science and Biotechnology 16, 691-709.
Rhim, J. W., and Ng, P. K. W. (2007). “Natural biopolymer-based nanocomposite films for packaging applications,” Critical Reviews in Food Science and Nutrition 47, 411-433. DOI: 10.1080/10408390600846366
Rhim, J. W., Park, H. M., and Ha, C. S. (2013). “Bio-nanocomposites for food packaging applications,” Prog. Polymer Sci. 38(10-11), 1629-1652. DOI: 10.1016/j.progpolymsci.2013.05.008
Ridgway, C. J., and Gane, P. A. C. (2012). “Constructing NFC-pigment composite surface treatment for enhanced paper stiffness and surface properties,” Cellulose 19(2), 547-560. DOI: 10.1007/s10570-011-9634-8
Ridgway, C. J., and Gane, P. A. C. (2013). “Size-selective absorption and adsorption in anionic pigmented porous coating structures: Case study cationic starch polymer versus nanofibrillated cellulose,” Cellulose 20(2), 933-951. DOI: 10.1007/s10570-013-9878-6
Rodionova, G., Lenes, M., Eriksen, O., and Gregersen, O. (2011). “Surface chemical modification of microfibrillated cellulose: Improvement of barrier properties for packaging applications,” Cellulose 18(1), 127-134. DOI: 10.1007/s10570-010-9474-y
Rodionova, G., Roudot, S., Eriksen, O., Mannle, F., and Gregersen, O. (2012a). “The formation and characterization of sustainable layered films incorporating microfibrillated cellulose (MFC),” BioResources 7(3), 3690-3700. DOI: 10.15376/biores.7.3.3690-3700
Rodionova, G., Saito, T., Lenes, M., Eriksen, O., Gregersen, O., Fukuzumi, H., and Isogai, A. (2012b). “Mechanical and oxygen barrier properties of films prepared from fibrillated dispersions of TEMPO-oxidized Norway spruce and Eucalyptus pulps,” Cellulose 19(3), 705-711. DOI: 10.1007/s10570-012-9664-x
Rojo, E., Peresin, M. S., Sampson, W. W., Hoeger, I. C., Vartiainen, J., Laine, J., and Rojas, O. J. (2015). “Comprehensive elucidation of the effect of residual lignin on the physical, barrier, mechanical and surface properties of nanocellulose films,” Green Chemistry 17, 1853-1866. DOI: 10.1039/c4gc02398f
Saastamoinen, P., Mattinen, M. L., Hippi, U., Nousiainen, P., Sipila, J., Lille, M., Suurnakki, A., and Pere, J. (2012). “Laccase aided modification of nanofibrillated cellulose with dodecyl gallate,” BioResources 7(4), 5749-5770. DOI: 10.15376/biores.7.4.5749-5770
Saini, S., Falco, C. Y., Belgacem, M. N., and Bras, J. (2016a). “Surface cationized cellulose nanofibrils for the production of contact active antimicrobial surfaces,” Carbohyd. Polym. 135, 239-247. DOI: 10.1016/j.carbpol.2015.09.002
Saini, S., Belgacem, M. N., Missoum, K., and Bras, J. 2015). “Natural active molecule chemical grafting on the surface of microfibrillated cellulose for fabrication of contact active antimicrobial surfaces,” Indust. Crops Prod. 78, 82-90. DOI: 10.1016/j.indcrop.2015.10.022
Saini, S., Sillard, C., Belgacem, M. N., and Bras, J. (2016b). “Nisin anchored cellulose nanofibers for long term antimicrobial active food packaging,” RSC Advances 6(15), 12437-12445. DOI: 10.1039/c5ra22748h
Salehudin, M. H., Salleh, E., Muhamad, I. I., and Mamat, S. N. H. (2014). “Starch-based biofilm reinforced with empty fruit bunch cellulose nanofiber,” Mater. Res. Innov. 18(suppl. S6), 322-325. DOI: 10.1179/1432891714Z.000000000977
Salmieri, S., Islam, F., Khan, R. A., Hossain, F. M., Ibrahim, H. M. M., Miao, C. W., Hamad, W. Y., and Lacroix, M. (2014a). “Antimicrobial nanocomposite films made of poly(lactic acid)-cellulose nanocrystals (PLA-CNC) in food applications: Part A – Effect of nisin release on the inactivation of Listeria monocytogenes in ham,” Cellulose 21(3), 1837-1850. DOI: 10.1007/s10570-014-0230-6
Salmieri, S., Islam, F., Khan, R. A., Hossain, F. M., Ibrahim, H. M. M., Miao, C. W., Hamad, W. Y., and Lacroix, M. (2014b). “Antimicrobial nanocomposite films made of poly(lactic acid)-cellulose nanocrystals (PLA-CNC) in food applications – Part B: Effect of oregano essential oil release on the inactivation of Listeria monocytogenes in mixed vegetables,” Cellulose 21(6), 4271-4285. DOI: 10.1007/s10570-014-0406-0
Salo, T., Dimic-Misic, K., Gane, P., and Paltakari, J. (2015). “Application of pigmented coating colours containing MFC/NFC: Coating properties and link to rheology,” Nordic Pulp Paper Res. J. 30(1), 165-178. DOI: 10.3183/NPPRJ-2015-30-01-p165-178
Sanchez-Garcia, M. D., and Lagaron, J. M. (2010). “On the use of plant cellulose nanowhiskers to enhance the barrier properties of polylactic acid,” Cellulose 17(5), 987-1004. DOI: 10.1007/s10570-010-9430-x
Sanchez-Garcia, M. D., Gimenez, E., and Lagaron, J. M. (2008). “Morphology and barrier properties of solvent cast composites of thermoplastic biopolymers and purified cellulose fibers,” Carbohydr. Polym. 71(2), 235-244. DOI: 10.1016/j.carbpol.2007.05.041
Sanchez-Garcia, M. D., Hilliou, L., and Lagaron, J. M. (2010). “Morphology and water barrier properties of nanobiocomposites of j/l-hybrid carrageenan and cellulose nanowhiskers,” J. Agric. Food. Chem. 58(24), 12847-12857. DOI: 10.1021/jf102764e
Sandquist, D. (2013). “New horizons for microfibrillated cellulose,” APPITA J. 66(2), 156-162.
Santos, T. M., Souza, M. D. M., Caceres, C. A., Rosa, M. F., Morais, J. P. S., Pinto, A. M. B., and Azeredo, H. M. C. (2014). “Fish gelatin films as affected by cellulose whiskers and sonication,” Food Hydrocolloids 41, 113-118. DOI: 10.1016/j.foodhyd.2014.04.001
Savadekar, N. R., Karande, V. S., Vigneshwaran, N., Bharimalla, A. K., and Mhaske, S. T. (2012). “Preparation of nano cellulose fibers and its application in kappa-carrageenan based film,” Intl. J. Bio. Macromol. 51(5), 1008-1013. DOI: 10.1016/j.ijbiomac.2012.08.014
Saxena, A., and Ragauskas, A. (2009). “Water transmission barrier properties of biodegradable films based on cellulosic whiskers and xylan,” Carbohydrate Polymers 78, 357-360. DOI: 10.1016/j.carbpol.2009.03.039
Schade, M., Weinkoetz, S., and Assmann, J. (2015). “Lignocellulosic material useful e.g. for producing articles e.g. packaging materials, comprises lignocellulose-containing material, microfibrillated cellulose, binder optionally with curing agent, expanded plastic particles, and additives,” Patent number: WO2015052028-A1
Schlosser, H. (2008). “Nano disperse Cellulose und nano fibrillierte Cellulose – neue Produkte fuer die Herstellung und Veredelung von Papier und Karton,” [Nano disperse cellulose and nano-fibrillated cellulose – new products for the manufacturing and refining of paper and cardboard]. Wochenbl. Papierfabr. 6, 1-11. [in German]
Sehaqui, H., Liu, A., Zhou, Q., and Berglund, L. A. (2010). “Fast preparation procedure for large, flat cellulose and cellulose/inorganic nanopaper structures,” Biomacromolecules 11, 2195-2198. DOI: 10.1021/bm100490s
Sehaqui, H., Zhou, Q., Ikkala, O., and Berglund, L. A. (2011). “Strong and tough cellulose nanopaper with high specific surface area and porosity,” Biomacromolecules 12, 3638-3644. DOI: 10.1021/bm2008907
Seydibeyoglu, M. O., and Oksman, K. (2008). “Novel nanocomposites based on polyurethane and micro fibrillated cellulose,” Compos. Sci. Technol. 68, 908-914. DOI: 10.1016/j.compscitech.2007.08.008
Shakeri, A., and Radmanesh, S. (2014). “Preparation of cellulose nanofibrils by high-pressure homogenizer and zein composite films,” in: Ultrafine Grained and Nano-Structured Materials IV, M. H. Parsa (ed.), Book Series: Advanced Materials Research 829, 534-538. DOI: 10.4028/www.scientific.net/AMR.829.534
Shankar, S., and Rhim, J.-W. (2016). “Preparation of nanocellulose from micro-crystalline cellulose: The effect on the performance and properties of agar-based composite films,” Carbohydrate Polymers 135, 18-26. DOI:10.1016/j.carbpol.2015.08.082
Sharma, S., Zhang, X., Nair, S. S., Ragauskas, A. J., Zhu, J. Y., and Deng, Y. (2014). “Thermally enhanced high performance cellulose nano fibril barrier membranes,” RSC Advances 4, 45136-45142. DOI: 10.1039/C4RA07469F
Sharma, S. K., Shukla, S. K., and Vaid, D. N. (1983). “Shellac – Structure, characteristics, and modifications,” Def. Sci. 33(3), 261-271. DOI: 10.14429/dsj.33.6181
Sharmin, N., Khan, R. A., Salmieri, S., Dussault, D., Bouchard, J., and Lacroix, M. (2012). “Modification and characterization of biodegradable methylcellulose films with trimethylolpropane trimethacrylate (TMPTMA) by gamma radiation: Effect of nanocrystalline cellulose,” J. Agric. Food Chem. 60(2), 623-629. DOI: 10.1021/jf203500s
Shatkin, J. A., and Kim, B. (2015). “Cellulose nanomaterials: Life cycle risk assessment, and environmental health and safety roadmap,” Environ. Sci. – Nano 2(5), 477-499. DOI: 10.1039/c5en00059a
Shatkin, J. A., Wegner, T. H., Bilek, E. M., and Cowie, J. (2014). “Market projections of cellulose nanomaterial-enabled products – Part 1: Applications,” Tappi J. 13(5), 9-16.
Shimazaki, Y., Miyazaki, Y., Takezawa, Y., Nogi, M., Abe, K., Ifuku, S., and Yano, H. (2007). “Excellent thermal conductivity of transparent cellulose nanofiber/epoxy resin nanocomposites,” Biomacromolecules 8, 2976-2978. DOI: 10.1021/bm7004998
Shimizu, M., Saito, T., and Isogai, A. (2016). “Water-resistant and high oxygen-barrier nanocellulose films with interfibrillar cross-linkages formed through multivalent metal ions,” J. Membrane Sci. 500, 1-7. DOI: 10.1016/j.memsci.2015.11.002
Simao, C. D., Reparaz, J. S., Wagner, M. R., Graczykowski, B., Kreuzer, M., Ruiz-Blanco, Y. B., Garcia, Y., Malho, J. M., Goni, A. R., Ahopelto, J., et al. (2015). “Optical and mechanical properties of nanofibrillated cellulose: Toward a robust platform for next-generation green technologies,” Carbohydrate Polymers 126, 40-46. DOI: 10.1016/j.carbpol.2015.03.032
Siqueira, G., Bras, J., and Dufresne, A. (2010). “Cellulosic bionanocomposites: A review of preparation, properties and applications,” Polymers 2(4), 728-765. DOI: 10.3390/polym2040728
Siro, I., and Plackett, D. (2010). “Microfibrillated cellulose and new nanocomposite materials: A review,” Cellulose 17(3), 459-494. DOI: 10.1007/s10570-010-9405-y
Siro, I., Plackett, D., Hedenqvist, M., Ankerfors, M., and Lindstrom, T. (2011). “Highly transparent films from carboxymethylated microfibrillated cellulose: The effect of multiple homogenization steps on key properties,” J. Appl. Polymer Sci. 119(5), 2652-2660. DOI: 10.1002/app.32831
Sirviö, J. A., Kolehmainen, A., Liimatainen, H., Niinimaki, J., and Hormi, O. E. O. (2014). “Biocomposite cellulose-alginate films: Promising packaging materials,” Food Chem. 151, 343-351. DOI: 10.1016/j.foodchem.2013.11.037
Song, H., Ankerfors, M., Hoc, M., and Lindström, T. (2010). “Reduction of the linting and dusting propensity of newspaper using starch and microfibrillated cellulose,” Nordic Pulp & Paper Res. J. 25(4), 495-504. DOI: 10.3183/NPPRJ-2010-25-04-p495-504
Song, J. L., and Rojas, O. J. (2013). “Approaching super-hydrophobicity from cellulosic materials: A Review,” Nordic Pulp Paper Res. J. 28(2), 216-238. DOI: 10.3183/NPPRJ-2013-28-02-p216-238
Song, Z. P., Xiao, H. N., and Zhao, Y. (2014). “Hydrophobic-modified nano-cellulose fiber/PLA biodegradable composites for lowering water vapor transmission rate (WVTR) of paper,” Carbohydrate Polymers 111, 442-448. DOI: 10.1016/j.carbpol.2014.04.049
Souza, S. F., Leao, A. L., Cai, J. H., Wu, C., Sain, M., and Cherian, B. M. (2010). “Nanocellulose from curava fibers and their nanocomposites,” Molecular Crystals Liquid Crystals 522, 342-352. DOI: 10.1080/15421401003722955
Spence, K., Venditti, R. A., Habibi, Y., Rojas, O. J., and Pawlak, J. J. (2010a). “The effect of chemical composition on microfibrillar cellulose films from wood pulps: Mechanical processing and physical properties,” Bioresour. Technol. 101, 5961-5968. DOI: 10.1016/j.biortech.2010.02.104
Spence, K. L., Venditti, R. A., Rojas, O. J., Habibi, Y., Pawlak, J. J. (2010b). “The effect of chemical composition on microfibrillar cellulose films from wood pulps: Water interactions and physical properties for packaging applications,” Cellulose 17(4), 835-848. DOI: 10.1007/s10570-010-9424-8
Spence, K. L., Venditti, R. A., Rojas, O. J., Habibi, Y., and Pawlak, J. J. (2011a). “A comparative study of energy consumption and physical properties of microfibrillated cellulose produced by different processing methods,” Cellulose 18, 1097-1111. DOI: 10.1007/s10570-011-9533-z
Spence, K. L., Venditti, R. A., Rojas, O. J., Pawlak, J. J., and Hubbe, M. A. (2011b). “Water vapor barrier properties of coated and filled microfibrillated cellulose composite films,” BioResources 6(4), 4370-4388.
Srinivasa, P., Kulachenko, A., and Aulin, C. (2015). “Experimental characterisation of nanofibrillated cellulose foams,” Cellulose 22(6), 3739-3753. DOI: 10.1007/s10570-015-0753-5
Stevanic, J. S., Bergstrom, E. M., Gatenholm, P., Berglund, L., and Salmen, L. (2012). “Arabinoxylan/nanofibrillated cellulose composite films,” J. Mater. Sci. 47(18), 6724-6732. DOI: 10.1007/s10853-012-6615-8
Stevanic, J. S., Joly, C., Mikkonen, K. S., Pirkkalainen, K., Serimaa, R., Remond, C., Toriz, G., Gatenholm, P., Tenkanen, M., and Salmen, L. (2011). “Bacterial nanocellulose-reinforced arabinoxylan films,” J. Appl. Polym. Sci. 122(2), 1030-1039. DOI: 10.1002/app.34217
Stoica-Guzun, A., Stroescu, M., Jipa, I., Dobre, L., and Zaharescu, T. (2013). “Effect of gamma irradiation on poly(vinyl alcohol) and bacterial cellulose composites used as packaging materials,” Radiation Phys. Chem. 84, 200-204. DOI: 10.1016/j.radphyschem.2012.06.017
Sukumaran, R. K., Singhania, R. R., and Pandey, A. (2005). “Microbial cellulases – Production, applications and challenges,” J. Sci. Indust. Res. 64(11), 832-844.
Suzuki, K., Okumura, H., Kitagawa, K., Sato, S., Nakagaito, A. N., and Yano, H. (2013). “Development of continuous process enabling nanofibrillation of pulp and melt compounding,” Cellulose 20, 201-210. DOI: 10.1007/s10570-012-9843-9
Suzuki, K., Sato, S., Okumura, H., Hahimoto, T., Nakagaito, A. N., and Yano, H. (2014). “Novel high-strength, micro fibrillated cellulose-reinforced polypropylene composites using a cationic polymer as compatibilizer,” Cellulose 21, 507-518. DOI: 10.1007/s10570-013-0143-9
Svagan, A. J., Azizi Samir, M. A., and Berglund, L. A. (2007). “Biomimetic polysaccharide nanocomposites of high cellulose content and high toughness,” Biomacromolecules 8(8), 2556-2563. DOI: 10.1021/bm0703160
Syverud, K., Gregersen, O., Chinga-Carrasco, G., and Eriksen, O. (2009). “The influence of microfibrillated cellulose, MFC, on paper strength and surface properties,” in: Advances in Pulp and Paper Research, Oxford 2009, Ianson, S. J. (ed.), Conference: 14th Fundamental Research Symposium on Advances in Pulp and Paper Research, St Annes Coll, Oxford, UK, pp. 899-930.
Syverud, K., and Stenius, P. (2009). “Strength and barrier properties of MFC films,” Cellulose 16(1), 75-85. DOI: 10.1007/s10570-008-9244-2
Taipale, T., Österberg, M., Nykänen, A., Ruokolainen, J., and Laine, J. (2010). “Effect of microfibrillated cellulose and fines on the drainage of kraft pulp suspension and paper strength,” Cellulose 17, 1005-1020. DOI: 10.1007/s10570-010-9431-9
Tammelin, T., Hippi, U., Salminen, A., and Salminen, T. (2013). “Preparing a film of nanofibrillated cellulose on surface of support material, which is useful for producing e.g. food packaging material, comprises applying and spreading suspension of cellulose onto plastic support material,” US Pat. US2014255688-A1.
Tammelin, T., and Vartiainen, J. (2014). “Nanocellulose films and barriers,” in: Handbook of Green Materials, Vol. 3: Self – and Direct – Assembling of Bionanomaterials, Oksman, K., Mathew, A. P., Bismarck, A., Rojas, O., Sain, M., and Qvintus, P. (eds.), Book Series: Materials and Energy, Vol. 5, pp. 213-229. DOI: 10.1142/9789814566469_0044
Tang, X. Z., Kumar, P., Alavi, S., and Sandeep, K. P. (2012). “Recent advances in biopolymers and biopolymer-based nanocomposites for food packaging materials,” Crit. Rev. Food. Sci. Nutr. 52(5), 426-442. DOI: 10.1080/10408398.2010.500508
Tchang Cervin, N., Bergstroem, L., and Wagberg, L. (2014). “Hydrophobized nanofibrillated cellulose (NFC) foam used for insulation and/or packaging material and used as barrier to gases or liquids, comprises charged hydrophobic amine,” US Pat. US2015158995-A1.
Toivonen, M. S., Kaskela, A., Rojas, O. J., Kauppinen, E. I., and Ikkala, O. (2015a). “Ambient-dried cellulose nanofibril aerogel membranes with high tensile strength and their use for aerosol collection and templates for transparent, flexible devices,” Advanced Functional Materials 25(42), 6618-6626. DOI: 10.1002/adfm.201502566
Toivonen, M. S., Kurki-Suonio, S., Schacher, F. H., Hietala, S., Rojas, O. J., and Ikkala, O. (2015b). “Water-resistant, transparent hybrid nanopaper by physical cross-linking with chitosan,” Biomacromol. 16(3), 1062-1071. DOI: 10.1021/acs.biomac.5b00145
Tome, L. C., Brandao, L., Mendes, A. M., Silvestre, A. J. D., Neto, C. P, Gandini, A., Freire, C. S. R., and Marrucho, I. M. (2010). “Preparation and characterization of bacterial cellulose membranes with tailored surface and barrier properties,” Cellulose 17(6), 1203-1211. DOI: 10.1007/s10570-010-9457-z
Tome, L. C., Fernandes, S. C. M., Perez, D. S., Sadocco, P., Silvestre, A. J. D., Neto, C.P., Marrucho, I. M., and Freire, C. S. R. (2013). “The role of nanocellulose fibers, starch and chitosan on multi-polysaccharide based films,” Cellulose 20(4), 1807-1818. DOI: 10.1007/s10570-013-9959-6
Trifol, J., Plackett, D., Sillard, C., Hassager, O., Daugaard, A. E., Bras, J., and Szabo, P. (2016a). “A comparison of partially acetylated nanocellulose, nanocrystalline cellulose, and nanoclay as fillers for high-performance polylactide nanocomposites,” J. Appl. Polym. Sci. 133(14). DOI: 10.1002/app.43257
Trifol, J., Plackett, D., Sillard, C., Szabo, P., Bras, J., and Daugaard, A. E. (2016b). “Hybrid poly(lactic acid)/nanocellulose/nanoclay composites with synergistically enhanced barrier properties and improved thermomechanical resistance,” Polymer Intl. 65(8), 988-995. DOI: 10.1002/pi.5154
Trovatti, E., Fernandes, S. C. M., Rubatat, L., Freire, C. S. R., Silvestre, A. J. D., and Neto, C. P. (2012a). “Sustainable nanocomposite films based on bacterial cellulose and pullulan,” Cellulose 19(3), 729-737. DOI: 10.1007/s10570-012-9673-9
Trovatti, E., Fernandes, S. C. M., Rubatat, L., Perez, D. S., Freire, C. S. R., Silvestre, A. J. D., and Neto, C. P. (2012b). “Pullulan-nanofibrillated cellulose composite films with improved thermal and mechanical properties,” Compos. Sci. Technol. 72, 1556-1561. 10.1016/j.compscitech.2012.06.003
Turbak, A. F., Snyder, F. W., and Sandberg, K. R. (1983). “Microfibrillated cellulose, a new cellulose product: Properties, uses and commercial potential,” J. Appl. Polym. Sci. Appl. Polym. Symp. 37, 815-827.
Urbina, L., Algar, I., Garcia-Astrain, C., Gabilondo, N., Gonzalez, A., Corcuera, M. A., Eceiza, A., and Retegi, A. (2016). “Biodegradable composites with improved barrier properties and transparency from the impregnation of PLA to bacterial cellulose membranes,” J. Appl. Polymer Sci. 133(28), article no. 43669. DOI: 10.1002/app.43669
Vartiainen, J., Shen, Y. F., Kaljunen, T., Malm, T., Vaha-Nissi, M., Putkonen, M., and Harlin, A. (2016). “Bio-based multilayer barrier films by extrusion, dispersion coating and atomic layer deposition,” J. Appl. Polym. Sci. 133(2), article no. 42260. DOI: 10.1002/app.42260
Velasquez-Cock, J., Ramirez, E., Betancourt, S., Putaux, J. L., Osorio, M., Castro, C., Ganan, P., and Zuluaga, R. (2014). “Influence of the acid type in the production of chitosan films reinforced with bacterial nanocellulose,” International Journal of Biological Macromolecules 69, 208-213. DOI: 10.1016/j.ijbiomac.2014.05.040
Visanko, M., Liimatainen, H., Sirvio, J. A., Mikkonen, K. S., Tenkanen, M., Sliz, R., Hormi, O., and Niinimaki, J. (2015). “Butylamino-functionalized cellulose nanocrystal films: Barrier properties and mechanical strength,” RSC Advances 5(20), 15140-15146. DOI: 10.1039/c4ra15445b
Wågberg, L., Decher, G., Norgren, M., Lindström, T., Ankerfors, M., and Axnäs, K. (2008). “The build-up of polyelectrolyte multilayers of microfibrillated cellulose and cationic polyelectrolytes,” Langmuir 24(3), 784-795. DOI: 10.1021/la702481v
Wan, Y. Z., Luo, H. L., He, F., Liang, H., Huang, Y., and Li, X. L. (2009). “Mechanical, moisture absorption, and biodegradation behaviors of bacterial cellulose fibre-reinforced starch biocomposites,” Compos. Sci. Technol. 69, 1212-1217. DOI: 10.1016/j.compscitech.2009.02.024
Wu, C.-N., Saito, T., Fujisawa, S., Fukuzumi, H., and Isogai, A. (2012). “Ultrastrong and high gas-barrier nanocellulose/clay-layered composites,” Biomacromolecules 13, 1927-1932. DOI: 10.1021/bm300465d
Xu, F., Shi, Y. C., and Wang, D. H. (2013). “X-ray scattering studies of lignocellulosic biomass: A review,” Carbohydr. Polym. 94(2), 904-917. DOI: 10.1016/j.carbpol.2013.02.008
Yan, J., Abdelgawad, A. M., El-Naggar, M. E., and Rojas, O. J. (2016). “Antibacterial activity of silver nanoparticles synthesized in-situ cellulose polymers by solution spraying,” Carbohydrate Polymers 147, 500-508 (2016). DOI: 10.1016/j.carbpol.2016.03.029
Yang, H., Tejado, A., Alam, N., Antal, M., and van de Ven, T. G. M. (2012). “Films prepared from electrosterically stabilized nanocrystalline cellulose,” Langmuir 28(20), 7834-7842. DOI: 10.1021/la2049663
Yang, Q., Fukuzumi, H., Saito, T., Isogai, A., and Zhang, L. (2011). “Transparent cellulose films with high gas barrier properties fabricated from aqueous alkali/urea solutions,” Biomacromolecules 12, 2766−2771. DOI: 10.1021/bm200766v
Yang, S. J., Tang, Y. J., Wang, J. M., Kong, F. G., and Zhang, J. H. (2014). “Surface treatment of cellulosic paper with starch-based composites reinforced with nanocrystalline cellulose,” Indust. Eng. Chem. Res. 53(36), 13980-13988. DOI: 10.1021/ie502125s
Yang, W. J., Dominici, F., Fortunati, E., Kenny, J. M., and Puglia, D. (2015). “Melt free radical grafting of glycidyl methacrylate (GMA) onto fully biodegradable poly(lactic) acid films: Effect of cellulose nanocrystals and a masterbatch process,” RSC Advances 5(41), 32350-32357. DOI: 10.1039/c5ra00894h
Yang, W., Fortunati, E., Dominici, F., Giovanale, G., Mazzaglia, A., Balestra, G. M., Kenny, J. M., and Puglia, D. (2016). “Synergic effect of cellulose and lignin nanostructures in PLA based systems for food antibacterial packaging,” European Polymer J. 79, 1-12. DOI: 10.1016/j.eurpolymj.2016.04.003
Yano, H., and Nakahara, S. (2004). “Bio-composites produced from plant microfiber bundles with a nanometer unit web-like network,” Journal of Materials Science 39(5), 1635-1638. DOI: 10.1023/B:JMSC.0000016162.43897.0a
Yano, H., Sugiyama, J., Nakagaito, A. N., Nogi, M., Matsuura, T., Hikita M., and Handa, K. (2005). “Optically transparent composites reinforced with networks of bacterial nanofibers,” Adv. Mater. 17, 153-155. DOI: 10.1002/adma.200400597
Yildirim, N., Shaler, S. M., Gardner, D. J., Rice, R., and Bousfield, D. W. (2014). “Cellulose nanofibril (CNF) reinforced starch insulating foams,” Cellulose 21(6), 4337-4347. DOI: 10.1007/s10570-014-0450-9
Youssef, B., Soumia, A., Mounir, E., Omar, C., Abdelaziz, L., Mehdi, E., and Mohamed, Z. (2015). “Preparation and properties of bionanocomposite films reinforced with nanocellulose isolated from Moroccan alfa fibres,” Autex Res. J. 15(3), 164-172. DOI: 10.1515/aut-2015-0011
Yu, H. Y., Sun, B., Zhang, D. Z., Chen, G. Y., Yang, X. Y., and Yao, J. M. (2014a). “Reinforcement of biodegradable poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with cellulose nanocrystal/silver nanohybrids as bifunctional nanofillers,” J. Mater. Chem. B 2(48), 8479-8489. DOI: 10.1039/c4tb01372g
Yu, H. Y., Yan, C. F., and Yao, J. M. (2014b). “Fully biodegradable food packaging materials based on functionalized cellulose nanocrystals/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanocomposites,” RSC Advances 4(104), 59792-59802. DOI: 10.1039/c4ra12691b
Yu, H. Y., Yang, X. Y., Lu, F. F., Chen, G. Y., and Yao, J. M. (2016). “Fabrication of multifunctional cellulose nanocrystals/poly(lactic acid) nanocomposites with silver nanoparticles by spraying method,” Carbohydr. Polym. 140, 209-219. DOI: 10.1016/j.carbpol.2015.12.030
Zimmermann, T., Pöhler, E., and Geiger, T. (2004). “Cellulose fibrils for polymer reinforcement,” Adv. Eng. Mater. 6, 754-761. DOI: 10.1002/adem.200400097
DOI: 10.15376/biores.12.1.2143-2233
APPENDIX
Table A. Publications Describing Nanocellulose-containing Barrier Layers, Films, or Coatings
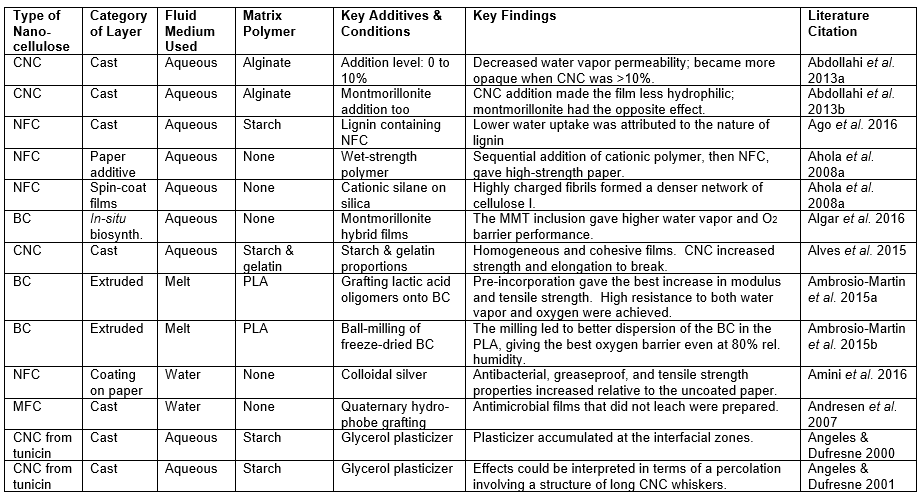
Table B. Compilation of Reported Oxygen and Water Vapor Barrier Performance of Nanocellulose Films