Abstract
The integration of combinatorial green chemistry (CGC), a more benign approach to combinatorial chemistry, with environmental life cycle assessment (LCA) methodologies as an improved process development methodology is discussed. It is expected that the CGC approach will require less labor and result in more globally optimized assay results, leading to more optimized unit process design. The technique utilizes chemical assay stage information to rapidly predict globally optimized process conditions based on techno-economic and LCA indicators. A simplified kraft pulping case study of the application of CGC-LCA is demonstrated herein, but CGC analyses could be applied to virtually any chemical-based project development and implementation project.
Download PDF
Full Article
Novel Screening Technique: Integrated Combinatorial Green Chemistry & Life Cycle Analysis (CGC-LCA)
Carter W. Reeb,a Lucian A. Lucia,a,b,* and Richard A. Vendittia
The integration of combinatorial green chemistry (CGC), a more benign approach to combinatorial chemistry, with environmental life cycle assessment (LCA) methodologies as an improved process development methodology is discussed. It is expected that the CGC approach will require less labor and result in more globally optimized assay results, leading to more optimized unit process design. The technique utilizes chemical assay stage information to rapidly predict globally optimized process conditions based on techno-economic and LCA indicators. A simplified kraft pulping case study of the application of CGC-LCA is demonstrated herein, but CGC analyses could be applied to virtually any chemical-based project development and implementation project.
Keywords: Combinatorial chemistry; Green chemistry; Life cycle analysis; Novel screening protocol; Assay evaluation
Contact information: a: Department of Forest Biomaterials (Wood & Paper Science), 2820 Faucette Drive, Raleigh, North Carolina 27695-8005 USA; b: Department of Chemistry, 2620 Yarbrough Drive, Raleigh, North Carolina 27695-8204 mail to: lalucia@ncsu.edu
Introduction
Currently, process development for chemically based systems relies on bench-scale experiments followed by modeling, followed by pilot and semi-commercial trials with extensive one variable-at-a-time experiments. These studies usually provide the more slowly developing techno-economic and environmental information late in the development process. Issues in this methodology include not exploring significant condition map areas or falling into locally, but not globally optimized points of sets of conditions. Also, financial, technical, and environmental implications are explored after only a limited set of experiments is performed, and often too late in the development process, leading to inaccurate conclusions and non-optimized results. There is clearly a need for a better approach to chemical systems process development.
Combinatorial Chemistry (CC) is a parallel reaction assay methodology that expressly allows for small variations in reaction chemistry between spatially discrete and independent reaction wells to ideally obtain an optimized reaction/process output (Ghose et al. 1999; Lehn 1999; Lehn and Eliseev 2001; Kappe et al. 2012; Moulin et al. 2012). By virtue of the fact that the reaction wells are typically executed simultaneously, the possibility exists that data concerning kinetics, production efficiency/yields, or scaled-up financial implications can be obtained (the hypothetical optimized reaction pathway). In the pharmaceuticals industry, robotics, materials science, pulp & paper industry, and related chemical and biochemical industries, it is often necessary to use CC to evaluate many different chemical reactions simultaneously instead of using the typical linear chemical analysis method, an approach that tends to be fraught with waste in terms of manpower and time.
Green chemistry (GC), a novel chemistry discipline embracing the concepts of efficiency, safety, low waste, low toxicity, and atom economy, explicitly dictates that the efficiency and cost effectiveness of chemical reactions is key, but not at the expense of increased environmental burden due to the reaction, system, or product. An overall reduction in the total amount of chemicals necessary (optimization of the chemical reactions) is often the simplest and best primary method for reducing net environmental burden. Optimization of the reaction using a yield metric, however, does not appreciably take into account the environmental burdens or benefits associated with each chemical used and therefore cannot compare financial feasibility of a proposed reaction with the scaled-up environmental feasibility (Ragauskas et al. 2006; Decker et al. 2009). Therefore, because CC does not always take into account the principles of green chemistry that espouses choosing specific “friendly” chemicals, low/no solvents, and high safety/low environmental impact, the melding of CC with GC, termed combinatorial green chemistry CGC, produces a synergistic approach, which has at its core the elimination of reagents or process conditions that are not green.
Life cycle analysis (LCA) is often used to quantify the inputs/outputs to a system or product and the associated environmental impacts. LCA data could be collated for each chemical used in a unique CGC (Combinatorial Green Chemistry) array, and the net environmental burden for the scaled-up version of each reaction well could be ranked or graphed by environmental burdens to reflect environmental feasibility (Mann and Spath 1997; Ozata et al. 2009). This approach is termed CGC-LCA.
The CGC-LCA approach incorporates the optimization of the chemical reaction for financial, environmental, or yield parameters by graphing the assay yield data, scaled up financial implications, and modeled environmental impacts from the LCA. Multiple wells could be determined to be “points of optimization” based upon the prioritization of the proposed processes’ operator for financial, environmental, or yield optimization.
A case study has been completed that provides an example of how CGC and LCA can be effectively combined and is discussed here. This case study investigates varia-tions in sulfidity of white liquor and the percent reduction of lignin in the resulting pulp for the kraft pulping process. Increases in chemical use will affect environmental impact, process chemical costs, and the quality of the pulp product at the commercial-scale.
The associated global warming potential (GWP) for each chemical analyzed in the CGC assay was multiplied against the chemical usage values to determine net GWP (in kg CO2 eq./Short Ton of de-lignified pulp) for each reaction well if scaled up to a commercial process. All values were graphed (Fig. 1) to show the result of the integrated analysis. The maximum lignin removal was predicted between wells 11 and 17. If any of these wells met the product requirements in lignin removal, then it follows that for both cost (which significantly changes with well number) and environmental burden (which is less sensitive to well number) considerations, that well 11 would be the preferred set of operating conditions. This simplified case study demonstrates the utility and effort savings of the use of the CGC-LCA approach.
Other environmental impact categories (such as eutrophication, acidification, respiratory impacts, photochemical smog, carcinogens, non-carcinogens, and eco-toxicity) could be calculated for each reaction well scenario, but have been omitted here to facilitate communication of the methodology. By mapping environmental burdens on the basis of operating conditions explored in the reaction wells, process conditions that have significant environmental effects can be identified and addressed. The relative importance of different variables can be explored simultaneously. This information should lead to identification of unit operations in the process that can be considered environmental “hot spots”, or activities contributing most to increased net environmental burden. Future work in this area could explore the use of integrated CGC and LCA analysis for other industries and methods for normalization/standardization in the interpretation of assay results.
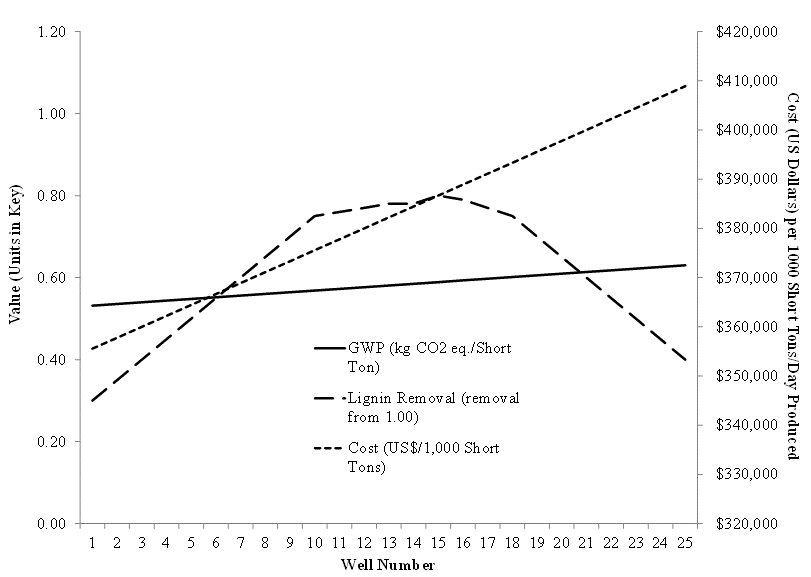
Fig. 1. Scaled-up cost estimates, lignin removal, and global warming impact for the 25 scenarios analyzed using integrated CGC and LCA. For a 5×5 well CGC assay, the percent sulfidity of white liquor ranged from 16% to 40% with a 2.5:4.5 white liquor to pulp ratio and a 1 gram pulp sample used per reaction well. The scaled-up hypothetical kraft pulping process was assumed to be 1,000 short tons per day with a range for NaOH of 333 kg to 466 kg and for Na2S of 222 kg to 88 kg. Chemical costs for each reaction well if scaled up were calculated also. An LCA was completed for the production and disposal of NaOH and Na2S using the openLCA calculation framework, EcoInvent and NREL U.S. LCI data, and the TRACI impact assessment method (NREL 2003; Curran 2006; Sharaai et al. 2010; SCLCI 2012).
Bibliography
Curran, M. A. 2006. Life Cycle Assessment: Principles and Practice, EPA/600/R-06/060. National Risk Management Research Laboratory. Cincinnati, OH. 1-88.
Decker, S. R., Brunecky, R., Tucker, M. P., Himmel, M. E., and Selig, M. J. (2009). “High-throughput screening techniques for biomass conversion,” BioEnergy Research 2(4), 179-192.
Ghose, A. K., Viswanadhan, V. N., and Wendoloski, J. J. (1999). “A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases,” Journal of Combinational Chemistry 1(1), 55-68.
Kappe, C. O., Stadler, A., and Dallinger, D. (2012). “Literature survey part D: Combinatorial chemistry and high-throughput organic synthesis,” Chapter 8. In: Microwaves in Organic and Medicinal Chemistry, Wiley-VCH Verlag GmbH & Co. 2, 543-648.
Lehn, J.-M. (1999). “Dynamic combinatorial chemistry and virtual combinatorial libraries,” Chemical Engineering Journal 5(9), 2455-2463.
Lehn, J.-M. and Eliseev, A. V. (2001). “Dynamic combinatorial chemistry,” Science 291(5512), 2331-2332.
Mann, M. K., and Spath, P. L. (1997). Life Cycle Assessment of a Biomass Gasification Combined-Cycle Power System, NREL. Golden, CO, 1-87.
Moulin, E., Cormos, G., and Giuseppone, N. (2012). “Dynamic combinatorial chemistry as a tool for the design of functional materials and devices,” Chem. Soc. Rev. 41(3), 1031-1049.
NREL. (2003). United States Life Cycle Inventory Database, N. R. E. Laboratory. Denver, CO.
Ozata, I., Ciliz, N., Mammadov, A., Buyukbay, B., and Ekinci, E. (2009). Comparative Life Cycle Assessment Approach for Sustainable Transport Fuel Production from Waste Cooking Oil and Rapeseed, Istanbul Technical University, Bogazici University, Isik University, 1-16.
Ragauskas, A., Williams, C., Davison, B., Britovsek, G., Cairney, J., Eckert, C. A., Frederick Jr., W. J., Hallett, J. P., Leak, D. J., and Liotta, C. L. (2006). “The path forward for biofuels and biomaterials,” Science 311(5760), 484.
SCLCI. (2012). Ecoinvent Database v2.2, Switzerland, Swiss Centre for Life Cycle Inventories.
Sharaai, A. H., Mahmood, N. Z., and Sulaiman, A. H. (2010). “Life cycle impact assessment (LCIA) using TRACI methodology: An analysis of potential impact on potable water production,” Australian Journal of Basic and Applied Sciences 4(9), 4313-4322.
