Abstract
A response surface methodology with 2k full factorial design was applied to obtain optimum conditions for bioethanol production using coffee mucilage (CM) as the substrate and Saccharomyces cerevisiae NRRL Y-2034 as the inoculum. CM is an agro-industrial residue mainly composed of simple sugars; the product yield and productivity process were analyzed with respect to the fermentation, pH, temperature, and the initial sugar concentration. Employing the following predicted optimum operational conditions attained the highest bioethanol production: pH 5.1, temperature 32 °C, and initial sugar concentration 61.8 g/L. The estimated bioethanol production was 15.02 g/L, and the experimental production was 16.29 g/L ± 0.39 g/L, with a bioethanol yield of 0.27 g/L and a productivity process of 0.34 g/Lh. Glycerol was the predominant byproduct of the fermentative metabolism of S. cerevisiae. The response surface methodology was successfully employed to optimize CM fermentation. In the fermentative processes with yeast, optimizing the conditions of the culture medium is needed to fully exploit the potential of the strains and maximize the production of bioethanol.
Download PDF
Full Article
Optimization of Bioethanol Production from Coffee Mucilage
Bianca Yadira Pérez-Sariñana,a,* Antonio De León-Rodriguez,b Sergio Saldaña-Trinidad,c and Sebastian Pathiyamattom Joseph a,*
A response surface methodology with 2k full factorial design was applied to obtain optimum conditions for bioethanol production using coffee mucilage (CM) as the substrate and Saccharomyces cerevisiae NRRL Y-2034 as the inoculum. CM is an agro-industrial residue mainly composed of simple sugars; the product yield and productivity process were analyzed with respect to the fermentation, pH, temperature, and the initial sugar concentration. Employing the following predicted optimum operational conditions attained the highest bioethanol production: pH 5.1, temperature 32 °C, and initial sugar concentration 61.8 g/L. The estimated bioethanol production was 15.02 g/L, and the experimental production was 16.29 g/L ± 0.39 g/L, with a bioethanol yield of 0.27 g/L and a productivity process of 0.34 g/Lh. Glycerol was the predominant byproduct of the fermentative metabolism of S. cerevisiae. The response surface methodology was successfully employed to optimize CM fermentation. In the fermentative processes with yeast, optimizing the conditions of the culture medium is needed to fully exploit the potential of the strains and maximize the production of bioethanol.
Keywords: Fermentation; Bioethanol production; Optimization
Contact information: a: Instituto de Energías Renovables-UNAM, Temixco, Morelos, 62580, México; b: Instituto Potosino de Investiación Cientifica y Tecnológica, San Luis Potosí S.L.P, 78216, México; c: Universidad Politécnica de Chiapas, Tuxtla Gutiérrez, Chiapas, 29010, México;
* Corresponding authors: bipes@ier.unam.mx, sjp@ier.unam.mx
INTRODUCTION
Carbon dioxide emissions affect the environment by contributing to global warming; because of this, it is necessary to find alternative sustainable energy sources (Kapdan and Kargi 2006). Bioethanol production by alcoholic fermentation has received a great deal of attention in recent years due to the high demand for fuel and need to supplement gasoline (Balat and Balat 2009; Yan and Lin 2009). One of the main benefits of this change is that biomass fuel is renewable and can potentially provide a sustainable fuel supply in the long term (Mabee and Saddler 2010).
Coffee is one of the most consumed beverages worldwide and is the second most commercialized product, after petroleum products (Mussatto et al. 2011). Due to the high demand for this product, large amounts of waste are generated in the coffee industry, which are toxic and pose serious environmental problems (Mussatto et al. 2012). Chiapas, Mexico, is one of the largest coffee producing states. The extraction process of the coffee bean generates waste as pulp, mucilage, and husk. Recently, investigations have been made using these residues for bioenergy generation (Mussatto et al. 2010; Mussatto et al. 2011; Choi et al. 2012).
By employing the response surface methodology and a 2k full factorial design, the influence of pH, temperature, and initial sugar concentration of coffee mucilage were determined in this study, with the main objective to optimize the production of bioethanol.
EXPERIMENTAL
Materials and Methods
Substrate
Coffee mucilage (CM) was extracted using a mechanical extractor located in the municipality of Las Rosas, Chiapas, México. The composition was 4 kg of coffee cherry per liter of water. This was supplemented with 0.5 g/L ammonium sulphate as a nitrogen source (De Leon-Rodríguez et al. 2008). The CM was centrifuged at 7,000 rpm for 10 min, pasteurized at 65 °C for 25 min, and chilled for 20 min on ice (Alvarado-Cuevas et al. 2013). The initial pH was 4.5. The chemical composition of coffee mucilage is shown in Table 1.
Microorganism cultivation and preparation
Saccharomyces cerevisiae NRRL Y-2034 was obtained from the strain collection center of the Universidad Politécnica de Chiapas. The strain was maintained in YPD agar (1% w/v yeast extract, 2% w/v peptone, 2% w/v glucose, and 2% w/v agar) slants at 4 °C, and fresh cultures (24 to 48 h) in YPD were used as inocula. Strain was cultured in 250 mL shake serological bottles rotated at 200 rpm at 28 °C and using the growth medium and fermentation conditions as previously described. Growth proceeded overnight for 24 h to allow cell growth to reach the exponential phase, after which the broth was centrifuged at 10,000 rpm for 10 min, and the cells were re-suspended in the fermentation medium.
Fermentation
Batch fermentation experiments were carried out in serological bottles of 100 mL with constant shaking at 200 rpm for 48 h, using the initial culture conditions described in the experimental design (Table 2). Cell density was adjusted to an optical density (OD 600 nm) of 0.5. Culture samples of 1 mL were taken every 3 h and centrifuged at 10,000 rpm for 10 min. The supernatant was filtered through a 0.22 m filter (Millipore, Bedford, MA, USA) after it was stored at -20 °C for later analysis. The validation of optimal conditions was assessed in triplicate.
In general Saccharomyces cerevisiae prefers acid pH (pH range of 3 to 7) (Pitt and Hocking 2009), the optimum pH is about 5 to 5.2 (Campbell 2003). S cerevisiae grow optimally between 25 and 30 ºC (Spencer and Spencer 1997) or 30 and 33 ºC (Campbell 2003). Its minimum growth temperature is reported as 4 ºC with a maximum growth temperature of 38 to 39 ºC (Pitt and Hocking 2009).
Based on the previous references, the ranges of pH of 4.5 to 5.5 and temperature of 28 to 38 ºC were selected for the present work. High and low sugar concentrations present in coffee mucilage were selected as 65 g/L and 35 g/L.
Bioethanol production (BP) is calculated as the amount of ethanol produced per liter of culture medium at the end of fermentation. The process yield (product/substrate) (YP/S, Eq. 1) is the amount of ethanol produced per sugar consumed, and the productivity process (PP, Eq. 2) is the amount of ethanol produced per liter and per hour. The process parameters were obtained as follows,
where YP/S is the process yield, Pf is the final concentration of bioethanol (g/L), Pi is the initial concentration of bioethanol (g/L), Sf is the final sugar concentration (g/L), Si is the initial sugar concentration (g/L), PP is the productivity process (g/Lh), BP the bioethanol produced (g/L), and t is the time (h).
Experimental design
A 2k full factorial design with three levels leading to 20 sets of experiments was realized to evaluate the effect of pH (factor X1), temperature (factor X2), and the initial sugar concentration (factor X3) as independent variables of the fermentation. The following equation (Eq. 3) was used to build surface graphs for the model for each response variable and for predicting the optimal value,
where Y is the predicted response corresponding to the bioethanol at the end of the fermentation process, X1, X2, and X3 are independent variables, 0 is an offset term, 1, 2, and 3 are linear effects, 12, 13, and 23 are interaction terms, and 11, 22, and 33 are quadratic coefficients. The model was evaluated with significance, goodness of fit, and the R2 values. The optimal values were obtained by solving the regression equation. The analysis of the response surface, the ANOVA, and the optimal conditions were obtained using the commercial software (Statgraphics Centurion XVI, Manugistics Inc., MD, USA). The adjusted models for BP, YP/S, and PP were evaluated by ANOVA analysis. The significant effects for the dependent variables were determined by a t-test with a probability value smaller than 0.05 (P-value <0.05).
Analysis
The protein concentration was determined by the Lowry method using bovine-serum-albumin (BioRad, CA, USA) as a standard (Lowry et al. 1951). The sugars, ethanol, glycerol, organic acids, and toxic compounds were determined by high-performance liquid chromatography (HPLC), using a column (Phenomenex, inc. USA) eluted at 60 °C with 0.0025 M H2SO4 at a flow rate of 0.5 mL/min and having a refractive-index detector. The redox potential was measured online with an autoclavable redox electrode (Applikon, Schiedam, Netherlands), and the data were registered in a PC interfaced with a potentiometer (B&C Electronics, Italy) using a RS232 port. Minerals were determined by ICP-OES (Inductively coupled plasma – Optic emission spectroscopy, Varian) device. Other compounds of CM were analyzed in a gas chromatograph 7820A, coupled with a mass spectrometer 5977E (Agilent Technologies, United States) using a 5% phenyl-methyl silicon capillary column 30 m long, 250 µm in inner diameter, and 0.25µm-film thickness. The MS detector was operated under electron impact ionization at 70 eV using the Scan mode at 45 to 450 aum. The compounds were identified by comparing their mass spectra to those obtained in the NIST 11 library of the MS database.
RESULTS AND DISCUSSION
Coffee Mucilage Composition
Carbohydrates are the most important constituents in coffee mucilage; for samples analyzed here, the sugar composition in CM was 37.67 g/L galactose, 35.65 g/L glucose, 1.06 g/L lactose, and 0.1193 g/L proteins (Table 1).
Table 1. Chemical Composition of Coffee Mucilage
Also, syringaldehyde, which is produced by lignin hydrolysis, was found in low concentrations. According to the ICP-OES analysis, CM contains several minerals. Potassium was the most abundant element, followed by phosphorus, calcium, sulfur, and magnesium (Table 1). Other compounds were also found, such as glycerine, caffeine, acetic acid, lactic acid, phenol, as well as 2,6 and 3,4-dimethoxyphenol.
Optimization of Fermentation Conditions
Table 2 shows a summary of the results for BP, YP/S, and PP. The BP achieved a maximum value 14.93 g/L and a minimum value of 8.28 g/L. The YP/S values ranged between 0.19 to 0.32, while the PP achieved a maximum value of 0.31 g/Lh, and a minimum value of 0.17 g/Lh.
Table 2. Experimental Design and Summary of Results for Dependent Variables
The statistical significance of the corresponding model equation was checked by F test analysis of variance (ANOVA, Tables 2, 3, and 4).
The adequacy of the models was expressed by the coefficient of correlation R2, which was 0.97, 0.89, and 0.96. These values indicated that 97, 89, and 96% of the variability of response in the bioethanol production, yield process, and productivity process, respectively, were explained by the model. Significant differences were indicated by a probability value less than 0.05 in ANOVA analysis.
The analysis ANOVA showed that BP was significantly affected by X3 and X32 (Table 3). The equation describing BP (Eq. 4), as a function of pH, temperature, and concentration of sugar, is as follows:
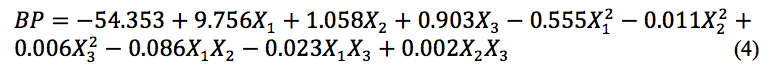
Table 3. Analysis of Variance for the Adjusted Model for Bioethanol Production
The maximum value of BP was 14.92 g/L, and it was obtained when the pH, temperature, and initial sugar concentration were 5.05, 32.5 °C, and 50 g/L (Fig. 1), respectively. A maximum point is observed in the sugar concentration, low pH there is a low bioethanol production. Because of this we can say that with increasing sugar concentration in the medium increases bioethanol production.
The analysis indicated that YP/S was significantly injured by the X3 and X32 (Table 4). The equation 5 describing YP/S, as a function of pH, temperature, and concentration of sugar is as follows:
Fig. 1. Response surfaces described by the models representing the dependence of BP on the pH, temperature, and initial sugar concentration
Table 4. Analysis of Variance for the Adjusted Model for Yield Process
The maximum value of YP/S was 0.32, and it was obtained when the pH, temperature, and initial sugar concentration were 5.7, 28 ºC, and 35 g/L (Fig. 2), respectively. It can be noted that as the sugar concentration increased, there were decreases in the process yield. When the temperature was maintained constant with a low pH, the process yield was low.
S. cerevisiae do not grow and do not produce ethanol at 5 g/L syringaldehyde. In the fermentations containing a variety of toxic compounds, there is no synergetic effect of multiple inhibitory compounds (Lee et al. 2010). In this work the yeast grew, but there was evidence of inhibition in bioethanol production. Therefore there was a low process yield. The inhibition was attribution to the main toxic compounds as syringaldehyde, phenol, acid acetic, and caffeine being present in coffee mucilage.
Fig. 2. Response surfaces described by the models representing the dependence of YP/S on the pH, temperature, and initial sugar concentration
The ANOVA showed that PP was significantly injured by the X3 and X32 terms (Table 5). The equation describing PP (Eq. 6), as a function of pH, temperature, and concentration of sugar, is as follows:
The maximum value of PP was 0.31 g/Lh, and it was obtained when the pH, temperature, and initial sugar concentration were 5.05, 32.5 ºC, and 50 g/L (fig. 3), respectively. The same condition for BP, since PP is a function of time, as defined in equation 6.
Table 5. Analysis of Variance for the Adjusted Model for Productivity Process
Fig. 3. Response surfaces described by the models representing the dependence of PP on the pH, temperature, and initial sugar concentration
Bioethanol is a biofuel; several investigations on the optimization of bioethanol production have been published (Chen 1981; Balusu et al. 2005; Bandaru et al. 2006, Pereira et al. 2010). Criteria such as yields, productivity process, and bioethanol production were used to evaluate the fermentations. For instance, a high bioethanol production and high process yield are not compatible, because the first one requires low substrate concentrations, which cause substrate inhibition leading to a low ethanol productivity process (Balusu et al. 2005).
The optimum conditions for maximizing BP in serological bottles were calculated to be pH 5.1, temperature 32 °C, and a sugar concentration of 61.8 g/L. Under these conditions, the highest BP was estimated as 15.02 g/L.
In Fig. 4, the kinetic behavior of the batch culture for the optimal conditions is shown. In this work, a low concentration of glycerine was found. Most of the studies concerning glycerine formation have been carried out using yeast, S. cerevisiae (Mohammad et al. 2002).
Glycerine is a well-known metabolite formed by many microorganisms including bacteria, yeasts, molds, and algae (Spencer 1968; Vijaikishore and Karanth 1986; Rehm and Redd 2008); it is produced by anabolic reactions during anaerobic conditions by S. cerevisiae (Lagunas and Gancedo 1973; Oura 1977; Taherzadeh et al. 1996; Albers et al. 1998). It is also a predominant byproduct of the fermentative metabolism of S. cerevisiae; in this paper, about 3.1 g/L glycerol was obtained (Bisping and Rehm 1986; Bisping et al. 1989; Andre et al. 1991; Benito et al. 1994).
Fig. 4. (a) Bioethanol concentration (), (b) glycerin concentration () and (c) sugar concentration () over time
CONCLUSIONS
1. Coffee mucilage is an agro-industrial residue mostly composed of carbohydrates, galactose, and glucose that was used as substrate in fermentation process.
2. A response surface methodology was successfully employed to optimize CM fermentation and ammonium sulphate for the efficient production of ethanol by Saccharomyces cerevisiae NRRL Y-2034. Sugar concentration is a variable that significantly affect optimization of bioethanol production from coffee mucilage.
3. During the process there was inhibition of bioethanol production and low yield process, due to the presence of toxic compounds in low concentrations.
4. Optimal conditions for bioethanol production and productivity process were established as: pH 5.1, temperature 32 °C, initial sugar concentration of 61.8 g/L, BP of 15.02 g/L and PP 0.31 g/Lh. Under these conditions experimental bioethanol production was as 16.29 g/L ± 0.39 g/L. In this process, glycerol is a byproduct of the fermentative metabolism, producing 3.1 g/L. The optimizing the conditions of the culture medium is needed to fully exploit the potential of the strains and maximize the production of bioethanol.
ACKNOWLEDGMENTS
We acknowledge the financial support from Consejo Nacional de Ciencia y Tecnología (CONACYT) through the project 224765 and 178988. We would like to thank M. B. Leandro Gabriel Ordoñez Acevedo of the Division of Molecular Biology from Instituto Potosino de Investigación Científica y Tecnológica (IPICyT) for crucial technical support during this work.
REFERENCES CITED
Albers, E., Lidén, G., Larsson, C., and Gustafsson, L. (1998). “Anaerobic redox balance and nitrogen metabolism in Saccharomyces cerevisiae,” Recent Research Developments in Microbiology 2, 253-79. DOI: 10.1002/yea.709
Alvarado-Cuevas, Z. D, Acevedo-Ordoñez L .G., Ornelas-Salas J. T., and De León-Rodríguez A. (2013). “Nitrogen sources impact hydrogen production by Escherichia coli using cheese whey as substrate,” New Biotechnology 30, 585-590. DOI: 10.1016/j.nbt.2013.03.002
André, L., Hemming, A., and Adler, L. (1991). “Osmoregulation in Saccharomyces cerevisiae. Studies on the osmotic induction of glycerol production and glycerol-3-phosphate dehydrogenase (NAD+),” FEBS Letters 286, 13-7. DOI: 10.1111/j.1574-6976.1997.tb00352.x
Balat, M., and Balat, H. (2009). “Recent trends in global production and utilization of bioethanol fuel,” Applied Energy 86, 2273–82. DOI: 10.1016/j.apenergy.2009.03.015
Balusu, R., Paduru. R.R., Kuravi, S.K., Seenayya, G., and Reddy, G. (2005). “Optimization of critical medium components using response surface methodology for ethanol production from cellulosic biomass by Clostridium thermocellum SS19,” Process Biochemistry 40, 3025-3030. DOI: 10.1016/j.procbio.2005.02.003
Bandaru, V. V. R., Somalanka, S. R., Mendu, D. R., Madicherla, N. R., and Chityala, A. (2006). “Optimization of fermentation conditions for the production of ethanol from sago starch by co-immobilized amyloglucosidase and cells of Zymomonas mobilis using response surface methodology,” Enzyme and Microbial Technology 40, 209-214. DOI: 10.1016/j.enzmictec.2005.06.002
Benito, G., Ozores, M., and Peña, M. (1994). “Continuous glycerol production in a packed-bed bioreactor with immobilized cells of Saccharomyces cerevisiae,” Bioresource Technology 49, 209-12. DOI: 10.1016/0960-8524(94)90041-8
Bisping, B., Hecker, D., and Rehm, H. (1989). “Glycerol production by semicontinuous fed-batch fermentation with immobilized cells of Saccharomyces cerevisiae,” Applied Microbiology and Biotechnology 32, 119-123. DOI: 10.1007/BF00165873
Bisping, B., and Rehm, H. (1986). “Glycerol production of cells by Saccharomyces cerevisiaeimmobilized in sintered glass,” Applied Microbiology and Biotechnology 23, 174-9. DOI, 10.1007/BF00261909
Campbell, I. (2003). “Yeast and fermentation,” in: Whisky: Technology, Production and Marketing, G. Stewar, C. Bamforth, and I. Russell (eds.), Elsevier. DOI: 10.1016/B978-012669202-0.50021-X
Chen, S. L. (1981). “Optimization of batch alcoholic fermentation of glucose syrup substrate,” Biotechnology and Bioengineering 23, 1827-1836. DOI: 10.1002/bit.260230810
Choi, I. S., Wi, S. G., Kim, S. B., and Bae, H. J. (2012). “Conversion of coffee residue waste into bioethanol with using popping pretreatment,” Bioresource Technology 125, 132-137. DIO: 10.1016/j.biortech.2012.08.080
De Leon-Rodríguez, A., Escalante-Minakata, P., Barba de la Rosa, A. P, and Blaschek, H. P. (2008). “Optimization of fermentation conditions for the production of the mezcal from Agave salmiana using response surface methodology,” Chemical Engineering Process: Process Intensification 47, 76-82. DOI: 10.1016/j.cep.2007.08.010
Kapdan, I. K., and Kargi, F. (2009). “Bio-hydrogen production from waste materials,” Enzyme and Microbial Technology 38, 569-82. DOI: 10.1016/j.enzmictec.2005.09.015
Lagunas, R., and Gancedo, J. M. (1973). “Reduced pyridine-nucleotides balance in glucose-growing Saccharomyces cerevisiae,” Europena Journal of Biochemistry 37, 90-94. DOI: 10.1111/j.1432-1033.1973.tb02961.x
Lee, H., Kim, Y. H., Lee, S. J., Kim, S. W., Kim, T.-H., and Park, C. (2010). “Ethanol production from lignocellulosic materials by S. cerevisiae and P. stipites,” Journal of Biotechnology 150, 160. DOI:10.1016/j.jbiotec.2010.08.415
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951). “Protein measurement with the Folin phenol reagent,” The Journal of Biological Chemistry,” 193, 265-275.
Mabee, W. E., and Saddler, J. N. (2010). “Bioethanol from lignocellulosics: Status and perspectives in Canada,” Bioresource Technology 101, 4806-4813. DOI: 10.1016/j.biortech.2009.10.098
Mohammad, J. T., Lennart, A., and Gunnar, L. (2002). “Strategies for enhancing fermentative production of glycerol – A review,” Enzyme and Microbial Technology 31, 53-66. DOI: 10.1016/S0141-0229(02)00069-8
Mussatto, S. I., Machado, E. M., Carneiro, L. M., and Teixeira, J. A. (2012). “Sugars metabolism and ethanol production by different yeast strains from coffee industry wastes hydrolysates,” Applied Energy 92, 763-768. DOI: 10.1016/j.apenergy.2011.08.020
Mussatto, S. I., Machado, E. M., Martins, S., and Teixeira, J. A. (2011). “Production, composition, and application of coffee and its industrial residues,” Food and Bioprocess Technology 4, 661-672. DOI: 10.1007/s11947-011-0565-z
Mussatto, S. I., Carneiro, L. M., Silva, J. P., Roberto, I. C., and Teixeira, J. (2010). “A study on chemical constituents and sugars extraction from spent coffee grounds,” Carbohydrate Polymers 83, 368-374. DOI: 10.1016/j.carbpol.2010.07.063
Oura, E. (1977). “Reaction products of yeast fermentations,” Process Biochemistry 12, 19-21.
Pereira, F. B., Guimarães, P. M., Teixeira, J. A., and Domingues, L. (2010). “Optimization of low-cost medium for very high gravity ethanol fermentations by Saccharomyces cerevisiae using statistical experimental designs,” Bioresource Technology 101, 7856-7863. DOI: 10.1016/j.biortech.2010.04.082
Pitt, J. I., and Hocking, A. D. (2009). “Yeast,” in: Fungi and Food Spoilage,” Springer, New York. DOI: 10.1007/978-0-387-92207-2
Rehm, H., and Redd, G. (2008). “Microbial production of glycerol and other polyols,” in: Biotechnology Set, Wiley-VCH Verlag GmbH, Weinheim, Germany. DOI: 10.1002/9783527620999
Spencer, J. F. T. (1968). “Production of polyhydric alcohols by yeasts,” Progress in Industrial Microbiol. 7, 1-42.
Spencer J. F.T., and Spencer. D. M. (1997). “The Yeast: Sex and Nonsex. Life Cycles, Sporlation and Genetics,” in: Yeasts in Natural and Artificial Habitats,” Springer, New York. DOI: 10.1007/978-3-662-03370-8
Taherzadeh, M. J., Lidén, G., Gustafsson, L., and Niklasson, C. (1996). “The effect of pantothenate deficiency and acetate addition on anaerobic batch fermentation of glucose by Saccharomyces cerevisiae,” Applied Microbiology and Biotechnology 46, 176-182. DOI: 10.1007/s002530050801
Vijaikishore, P., and Karanth, N. (1986). “Glycerol production by fermentation – A review,” Process Biochemistry 21, 54-7.
Yan, J., and Lin, T. (2009). “Biofuels in Asia,” Applied Energy 86, S1-S10. DOI: 10.1016/j.apenergy.2009.07.004
Article submitted: January 20, 2015; Peer review completed: March 17, 2015; Revised version revised: May 9, 2015; Accepted: May 10 2015; Published: May 28, 2015.
DOI: 10.15376/biores.10.3.4326-4338
