Abstract
Sesame cake and meal, byproducts of the sesame oil process industry and mainly used as feed and fertilizer, are often not optimally utilized and are wasted when the material could be used as a high-quality protein source. This research primarily emphasizes the preparation of a sesame protein-based adhesive with urea and glyoxal modification to use as a wood adhesive. The performance and characterization of the urea and glyoxal modified sesame protein adhesive (USP and GUSP, respectively) were measured precisely. After glyoxal was added, the water resistance of the GUSP adhesive was significantly enhanced, reaching the standard for Type II plywood. The formaldehyde emission test showed that the GUSP adhesive could be utilized as a formaldehyde-free wood adhesive, having a significantly lower than the demand of the E0 level (i.e., 0.5 mg/L). Furthermore, increasing the glyoxal content in the adhesives enhanced the thermal stability but not significantly. A substance with a crosslinking structure was formed from the reaction between the sesame protein and glyoxal, which enhanced the water resistance. Meanwhile, the fractured structure of the GUSP adhesive having a compact surface also was propitious to enhance the water resistance. Thus, the GUSP adhesive could be used as a novel adhesive in plywood fabrication.
Download PDF
Full Article
Performance of a Formaldehyde-Free Sesame Protein Adhesive Modified by Urea in the Presence and Absence of Glyoxal
Xiaobo Wei,a,b Yuxiang Ma,a,* and Xuede Wang a,*
Sesame cake and meal, byproducts of the sesame oil process industry and mainly used as feed and fertilizer, are often not optimally utilized and are wasted when the material could be used as a high-quality protein source. This research primarily emphasizes the preparation of a sesame protein-based adhesive with urea and glyoxal modification to use as a wood adhesive. The performance and characterization of the urea and glyoxal modified sesame protein adhesive (USP and GUSP, respectively) were measured precisely. After glyoxal was added, the water resistance of the GUSP adhesive was significantly enhanced, reaching the standard for Type II plywood. The formaldehyde emission test showed that the GUSP adhesive could be utilized as a formaldehyde-free wood adhesive, having a significantly lower than the demand of the E0 level (i.e., 0.5 mg/L). Furthermore, increasing the glyoxal content in the adhesives enhanced the thermal stability but not significantly. A substance with a crosslinking structure was formed from the reaction between the sesame protein and glyoxal, which enhanced the water resistance. Meanwhile, the fractured structure of the GUSP adhesive having a compact surface also was propitious to enhance the water resistance. Thus, the GUSP adhesive could be used as a novel adhesive in plywood fabrication.
Keywords: Sesame protein adhesive; Urea; Glyoxal; Water resistance
Contact information: a: College of Food Science and Technology, Henan University of Technology, Zhengzhou 450001, P. R. China; b: College of Biosystems Engineering and Food Science, Zhejiang Key Laboratory of Agro-Food Processing, Key Laboratory of Agro-Products Postharvest Handling of Ministry of Agriculture and Rural Affairs, Zhejiang University, Hangzhou, 310058, P. R. China;
* Corresponding author: wangxuede1962@126.com; 153120363@qq.com
GRAPHICAL ABSTRACT
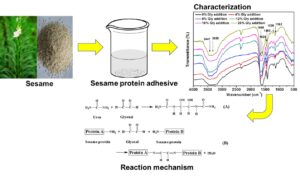
INTRODUCTION
Wood adhesives play an important role in the plywood fabrication industry. Formaldehyde-based adhesives, including urea- (UF), phenol- (PF), and melamine- (MF) formaldehyde resins, serve as the predominant types of adhesives in the wood adhesive field (Luo et al. 2015a). These formaldehyde-based adhesives have some advantages, including low cost, long shelf life, and excellent water resistance, which allow them to meet the application requirement for adhesives in the plywood industry (Li et al. 2014). However, the formaldehyde emission of these formaldehyde-based adhesives is an important threat to health. Moreover, many plant proteins have been used for preparing wood adhesive, such as soy protein, cotton protein, peanut protein, canola protein, and wheat (Li et al. 2012; Nikvash et al. 2012; Nordqvist et al. 2012; Wang et al. 2014; Li et al. 2015; Cheng et al. 2016; Eslah et al. 2016; He et al. 2016; Xin et al. 2016). Plant proteins have several advantages, including being renewable, abundant, readily biodegradable, and non-toxic, such that they can be considered an ideal natural material for a wood adhesive (Huang and Li 2008). However, it is essential to obtain more in-depth research on how to improve the bonding properties of plant-based adhesives.
Sesame (Sesamum indicum L.) is primarily cultivated in Asian and African countries as a traditional oilseed crop, while China has been the first and greatest sesame production and consumption industry in the world (Pathak et al. 2014; Wei et al. 2017). Sesame seeds have the highest protein (25%) and oil content (55%) among all oilseed crops (Raja et al. 2007a,b; Wei et al. 2016). The sesame meal byproduct obtained after sesame oil extraction is mainly used as a protein source for livestock feed and crop fertilizer; therefore, it is not fully utilized. Sesame meal is mainly comprised of protein (50.1%), carbohydrates (28.1%), fiber (6.2%), and ash (15.3%) (Inyang and Iduh 1996; Onsaard et al. 2010). Hence, developing new uses for this protein obtained by sesame meal is an important issue for the sesame industry worldwide. Sesame protein primarily consist of globulins (67.3%), glutelins (6.9%), albumins (8.6%), and prolamins (1.4%) (Nilo et al. 1981). Two major storage sesame proteins are α-globulin and β-globulin, which account for 60 to 70% and 25%, of total globulins in sesame seeds, respectively (Tai et al. 2001). Sesame protein-based adhesive can be prepared using chemical modification based on the structure and functional groups, which include carboxyl (-COOH), amino (-NH2), hydroxyl (-OH), and thiol (-SH) groups, of the sesame protein. Thus, the sesame protein could be used as a new potential source to prepare a protein-based adhesive.
In order to improve the properties of protein adhesive, such as shelf life and water resistance, chemical modification processes for producing the plant protein-based adhesive need to be investigated. These three processes are classified as: plant protein denaturation method (Hettiarachchy et al. 1995; Huang and Sun 2000), plant protein molecular modification method (Qi et al. 2013; Luo et al. 2015b), and plant protein and crosslinkers or resins blend modification method (Zhong and Sun 2007; Luo et al. 2016b). In earlier studies, urea has been applied to prepare the protein-based adhesive to be a protein denaturing agent (Sun and Ke 1999; Zhang and Hua 2007). The highly ordered globular structure of the plant protein can be unfolded by using urea modification, and the inside polar groups, such as -COOH, -NH2, and -OH, of the plant protein are exposed, which can avoid water penetration into the cured adhesive, thus enhancing its water resistance. However, the efforts to improve the water resistance of protein-based adhesives by using urea modification are limited.
Some investigators have blended the plant protein-based adhesive with synthetic resins, including melamine-urea-formaldehyde resin (Qiang et al. 2012) and phenol-formaldehyde resin (Zhong and Sun 2007) to enhance their water resistance. Nevertheless, the plywood products that bonded with these adhesives led to harmful formaldehyde emissions, and it was difficult to meet the E0 level (0.5 mg/L) requirement. Thus, it is essential to find a new substance to replace formaldehyde in the production of protein-based adhesives. As a favorable environment-friendly agent, glyoxal is a non-toxic (LD50 rat ≥ 2960 mg/kg; LD50 mouse ≥ 1280 mg/kg) and non-volatile aldehyde (Deng et al. 2014). Compared with formaldehyde, glyoxal has two aldehyde groups with high reactivity, which can react with the protein and urea via a complex condensation reaction mechanism. Currently, glyoxal has been widely applied in paper and textile manufacturing because of its developed processing, low cost, and easy biodegradation (Samanta et al. 2008; Yuan and Hu 2012). Glyoxal can react with the groups that are broken by urea from the sesame protein to achieve a high-water resistance in the sesame protein-based adhesive. Therefore, urea and glyoxal modification can be used as an effective way to prepare a sesame protein-based adhesive with excellent performance.
To the authors’ knowledge, little attention has been devoted to the preparation of sesame protein-based adhesives. The purpose of this study was to prepare a high-water resistance and zero-formaldehyde emission protein-based adhesive based on the expanding the application of sesame cake and meal. In this research, sesame protein was extracted from defatted sesame cake to produce the sesame protein-based adhesive with urea and glyoxal modification. The solid content, pH value, and apparent viscosity of the sesame protein-based adhesive were measured. The Fourier transform infrared (FTIR) spectroscopy, thermogravimetric (TGA), and scanning electron microscopy (SEM) of the cured sesame protein-based adhesive were measured. Herein, the water resistance and formaldehyde emission of the plywood panels bonded by the sesame protein-based adhesive were determined.
EXPERIMENTAL
Materials
Peeled sesame seeds were provided by PingYu compro hui xin Oil Company (Henan, China). Urea and glyoxal (40% w/w) were purchased from Shanghai Macklin Biochemical Co. Ltd (Shanghai, China), which came from analytical-grade reagents. Poplar veneers (300 mm × 300 mm × 2.0 mm) were supplied by Bio Biologic Materials Co. (Henan, China).
Preparation of Sesame Protein
Degreasing of peeled sesame
Peeled sesame seeds were milled into powder (≥ 60 mesh) after being crushed by a screw press at a pressure of 30 MPa for 40 min in a room temperature environment. The peeled sesame powder was degreased by solvent extraction at a temperature of 50 °C for 8 h and then stored in a drying cabinet at 45 °C for 2 h.
Extraction of Sesame Protein
The sesame protein extraction method was based on the process reported by Onsaard et al. (2010) with some improvements. The sesame protein that resulted from degreased sesame protein powders were extracted using deionized water with a ratio of 1:20 (w/v). The mixture was constantly stirred at 50 °C for 3 h using 1 M NaOH to adjust the pH value to 10, and the supernatant was obtained using centrifugation at 4000 rpm for 10 min. The sesame protein was precipitated from the supernatants using 1 M HCl to adjust the pH value to 4.2. The precipitate proteins were washed twice with deionized water, centrifuged at 4000 rpm for 10 min, and then freeze-dried using a freeze dryer. The sesame protein was then ground into powder using a ball grinder. Lastly, the components of sesame protein were measured (88.7% protein, 3.5% moisture, 3.4% ash, 0.6% fat, 0.1% fiber, and 2.8% polysaccharides).
Adhesive Preparation
Sesame protein was gradually dispersed in a 3 mol/L urea solution (80 mL). The slurry was added to a 40% w/v glyoxal solution (0, 4, 8, 12, 16, and 20% based on the weight of the sesame protein) after being stirred for 20 min at room temperature, and the admixture was stirred for 220 min at room temperature to develop the urea-modified sesame protein adhesive (USP) and glyoxal-modified sesame protein adhesive including urea (GUSP).
Solid Content and pH Test
The pH value of different USP/GUSP adhesives was tested using a pH meter and reported by taking the average of three replicates.
The solid content of USP/GUSP adhesives were measured using the method described by Li et al. (2014). Approximately 3 g (weight β) of the adhesive sample was dried in a drying cabinet at a temperature of 105 °C for several hours until an invariable weight (weight α) was achieved. The solids content was calculated using Eq. 1,
(1)
The measurement of the solids content of the USP/GUSP adhesives was done three times, and the mean values were recorded.
Rheology Properties Measurement
The apparent viscosity of USP/GUSP adhesives were measured using a RS-6000 Rheometer (Thermo Fisher Scientific Corporation, Waltham, MA) with a cone and plate fixture (10 mm semidiameter). The space between the cone and plate was set to 1 mm for all adhesive samples. All tests were carried out under steady shear flow at 25 °C. The shear rates ranged from 0.01 to 100 s-1 with a rate of 10 s-1. Three replicates were done for all adhesive samples, and the mean values were recorded.
Preparation of Three-ply Plywood
The poplar veneer with dimensions of 300 mm x 300 mm x 2.0 mm was glued using USP/GUSP adhesives to prepare three-ply plywood samples for evaluating the performance of the developed adhesives. The poplar veneer was smeared at 240 g/m2 using a single spreading method. The smeared veneer was assembled with another veneer for the texture direction of the adjoining veneer to be orthogonal to the other. The assembled veneers were hot-pressed at 130 °C and 1.3 MPa for 10 min. The three-ply plywood panels were placed under room temperature for 12 h after hot pressing.
Bonding Strength Test
Twelve plywood specimens with dimensions of 100 mm × 25 mm (length × width) were cut from two plywood panels for evaluating the bonding strength. The planform and profile of the plywood specimens are shown in Fig. 1A and B. The wet shear strength of specimens was measured in accordance with the method described in China National Standard GB/T 17657-2013 for Type II plywood (≥ 0.70 MPa). The plywood specimens were submerged in water at 63 ± 2 °C for 3 h and then stored in ambient conditions for 10 min. The wet shear strength of the specimens was tested using a CMT 6203 Universal Testing Machine (Sans Material Test Instrument Company, Shenzhen, China) operating at a crosshead speed of 5 mm/min. The maximum force (N) needed to break the glue area was recorded. The stripping and tearing of the plywood specimen are shown in Fig. 1C and D. The wet shear strength of specimens was calculated from Eq. 2,
(2)
The reported wet shear strength data were the mean values of twelve repetitions for each plywood.
Formaldehyde Emissions Test
The formaldehyde emission of plywood was measured using a desiccator method reported by Deng et al. (2014). After storing at the ordinary temperature for 24 h, the plywood specimens with dimensions of 150 mm × 50 mm (length × width) were made. Ten plywood specimens were stored in a 11-liter closed desiccator at 20 ± 2 °C for 24 h. The emitted formaldehyde was absorbed with a separate container containing 300 mL of distilled water. The formaldehyde concentration in the sample solution was measured using a visible spectrophotometer (UV-6000PC, Metash Instruments Company, Shanghai China) to obtain the absorbance with colorimetric detection at 412 nm. The formaldehyde concentration was the average value of three replications for each plywood specimen.
Fig. 1. Schematic diagram of plywood panels before and after being destroyed
FTIR Spectroscopy Analysis
The USP/GUSP adhesives were dried using a drying cabinet (120 ± 2 °C) until a constant weight was obtained and then crushed into powder. The FTIR spectra of the adhesive samples was tested by a Nicolet 6700 Spectrometer (Thermo Nicolet Corporation, Madison, WI) under the following conditions: 32 scans per sample, scan region: 4000 to 500 cm-1, and scan resolution: 8 cm-1.
Thermogravimetric (TG) Analysis
The USP/GUSP adhesives were dried using a drying cabinet (120 ± 2 °C) until a constant weight was obtained and then was ground into powder. The thermal stability of the adhesive samples was measured with a TGA instrument (TA Q50, Waters Company, Milford, MA, USA). Approximately 5 mg of the cured USP/GUSP adhesive samples were put into the pan and heated at a rate of 10 °C/min over a temperature range of 40 to 600 °C in a nitrogen atmosphere.
Scanning Electron Microscope (SEM) Imaging
The USP/GUSP adhesives were dried using a drying cabinet (120 ± 2 °C) until a constant weight was obtained. A QUANTA FEG 250 (FEI Company, Hillsboro, OR, USA) scanning electron microscope was used to observe the fractured structure of the cured USP/GUSP adhesive samples. The surface of the cured adhesive samples was measured before being coated with gold using a sputter coater.
RESULTS AND DISCUSSION
Solid Content and pH of the USP/GUSP Adhesives
Solid content is an important property in plywood manufacturing because it can influence the gluing properties of the adhesive during the hot-pressing process. The necessity of a low solid content of an adhesive show that the high moisture content needs to be removed from the adhesive during the hot-pressing process, which reduces the bonding strength of the adhesive in plywood manufacturing (Gao et al. 2012). However, an adhesive with a high solids content cannot be well-distributed in the process of coating, which influences the quality of plywood products. The mean value of the solids content of the USP/GUSP adhesives calculated by triplicate is shown in Fig. 2. The solids content of USP adhesive was 24.21%. After glyoxal was introduced, there was no significant change in the solids content of the GUSP adhesives, which changed from 24.12% to 23.71% when the glyoxal addition increased from 4% to 20%. This result indicated that the cross-linking reaction between the sesame protein and glyoxal was limited to some extent and led to the molecular weight of the resultant was low.
The pH value of an adhesive is a basic parameter that influences the color of the adhesive layer and plywood products. The mean pH values of the USP/GUSP adhesives were calculated in triplicate and is presented in Fig. 2. The pH value of the USP adhesive was 7.18 without glyoxal addition. The pH value of the GUSP adhesives decreased from 6.67 to 6.20 when the glyoxal addition increased from 4 to 20%. This behavior suggested that the glyoxal, as an acidic material, evidently decreased the pH value of the GUSP adhesives.
Fig. 2. The solid content and pH of the different USP/GUSP adhesives
Rheology Properties Analysis
The apparent viscosity plays a predominant role in plywood manufacturing and influences the process of coating. A high viscosity of an adhesive results in poor wettability and makes it hard to uniformly spread on the wood surface. However, a low viscosity leads to poor water resistance because of the over-penetration into the wood. The apparent viscosity of different USP/GUSP adhesives is presented in Fig. 3, and the initial viscosity of the different USP/GUSP adhesives is shown in Table 1. The viscosity of the USP/GUSP adhesives gradually decreased with increasing shear rate. This phenomenon showed that the sesame protein adhesives were shear-thinning fluids, and this result was consistent with Wei et al. (2017). The initial viscosity of the USP adhesive without glyoxal addition was 71560 mPa·s. This was attributed to by the globular structure of the sesame protein being degraded to form smaller polypeptide segments by using urea modification and caused the USP adhesive to have a high viscosity. After glyoxal was introduced, the initial viscosity of the GUSP adhesive decreased remarkably from 33330 to 22670 mPa·s with glyoxal addition increasing from 4 to 8% compared to the USP adhesive. This behavior was attributed to the orderly structure of the adhesive molecule being formed from the reaction between glyoxal and the sesame protein, which was observed by SEM (Fig. 8B and C). By further increasing the glyoxal addition from 12 to 20%, the initial viscosity of the GUSP adhesive clearly increased from 31200 to 60630 mPa·s. This phenomenon was due to the resultant of the chelate reaction between glyoxal and the sesame protein being increased with increasing glyoxal addition (Fig. 6B). A more ordered structure of the molecule in GUSP adhesives was also formed and increased the initial viscosity, which was confirmed by SEM (Fig. 8D, E, and F).
Fig. 3. Apparent viscosity of the different USP/GUSP adhesives
Bonding Strength and Formaldehyde Emission of Panels Boned by USP/GUSP Adhesive
The wet shear strength of plywood panels bonded by different USP/GUSP adhesives is illustrated in Fig. 4. Compared with soy and peanut protein, sesame protein has a higher percentage of hydrophobic amino acids, such as methionine and cysteine, which is difficult to dissolve in water and prepare a protein-based adhesive from. Without glyoxal addition, the wet shear strength of plywood panels bonded by the USP adhesive was 0.45 MPa, which did not meet the requirement of Type II plywood according to China National Standard GB/T 17567-2013. Thus, a poor water resistance was ascribed to the fact that the low molecular weight of sesame protein that was degraded by urea led to a good water dissolution and low bonding strength. Meanwhile, the SEM micrograph (Fig. 8A) indicated that the cured USP adhesive had a loose and porous morphology, which can help to explain the low water resistance.
After glyoxal was introduced, there was a significant increase in wet shear strength when the glyoxal addition increased from 4 to 12%. The wet shear strength of plywood panels bonded by GUSP adhesive with 12% glyoxal addition improved by 115.5% to a maximum value of 0.97 MPa compared to the USP adhesive. Some factors that were considered to contribute to the enhanced wet shear strength for the GUSP adhesives, such as the degree of chelate reaction.
The crosslinking from the reaction between sesame protein and glyoxal, which combined with the polar groups of wood, improved the water resistance, as illustrated by Fig. 6B. In addition, the resultant from the reaction between urea and glyoxal also improved the adhesion properties of the GUSP adhesives, as illustrated by Fig. 6A. By further increasing the glyoxal addition, the wet shear strength of the plywood panels bonded by GUSP adhesive decreased from 0.88 to 0.80 MPa as glyoxal addition increased from 16 to 20%, but still met the demand of Type II plywood requirements. This behavior might be attributed to by the following reason. The high viscosity of GUSP adhesive was due to the overfull crosslinking reaction between sesame protein and glyoxal, which resulted in more difficulty in coating the wood surface uniformly and led to a low water resistance, as shown in Table 1.
The formaldehyde emission of plywood panels bonded by different USP/GUSP adhesives is shown in Table 2. In general, there are two ways for formaldehyde to be emitted from plywood, which are freeing formaldehyde in the adhesive layer or the destruction of chemical bonds (Luo et al. 2017). As shown in Table 2, the formaldehyde emission of the plywood panels bonded by the USP adhesive without glyoxal addition was 0.043 mg/L, which was much lower than the requirement for the E0 level (0.5 mg/L) according to China National Standard GB/T 9846-2004.
After glyoxal was introduced, there was no significant change in the formaldehyde emission of plywood panels bonded by the GUSP adhesives with increasing glyoxal addition compared with the USP adhesive. The results were consistent with that of the literature (Ballerini et al. 2005; Despres et al. 2010; Deng et al. 2014). This phenomenon showed that the formaldehyde emission of plywood panels may be emitted from the wood itself and not from the adhesive layer. Therefore, the plywood products bonded by the GUSP adhesive could be applied straight to the interior decoration as a non-formaldehyde emission material.
Fig. 4. Wet shear strength of plywood panels bonded by the different USP/GUSP adhesives
FTIR Spectroscopy Analysis
The FTIR spectra of different USP/GUSP adhesives and sesame protein is illustrated in Fig. 5. The broad peaks observed at 3447 cm-1 and 3358 cm-1 in the spectra of all USP/GUSP adhesive were associated with the stretching vibration of N-H and O-H groups, respectively, which could react with other groups and form hydrogen bonds, as shown in Fig. 5A. The absorption peaks observed at 1656, 1535, and 1237 cm-1 was attributed to the C=O stretching vibration of amide I, N-H bending vibration of amide II, and C-N stretching vibration of amide III, respectively, and were the main characteristic absorption peaks of sesame protein, as shown in Fig. 5B. After the sesame protein was modified by urea, the absorption peak of amide II at 1535 cm-1 disappeared and the absorption at 1624 cm-1 appeared, as shown in Fig. 5A. This behavior may have been attributed to the position of the amide II changing because of the modification with a high concentration of urea (Sara et al. 2010). The absorption peaks of the amide I and amide III were shifted to 1668 cm-1 and 1237 cm-1, respectively. The absorption peaks at 1453 cm-1 and 1162 cm-1 were associated with the -C-H bending and -C-O-C- stretching, respectively (Wei et al. 2017).
There were no absorption peaks observed at 2850 and 2720 cm-1 in the spectra of the GUSP adhesive with different glyoxal addition concentrations, which were attributed to the C-H stretching vibration of the aldehyde group, as shown in Fig. 5A. This occurrence effectively supported the result described in Table 2. This phenomenon also indicated that the free glyoxal was not present in the GUSP adhesives and may have reacted with the urea and sesame protein with the following two approaches, while the possible reaction process is shown in Fig. 6. The main condensation reaction between the amino group of the sesame protein and the aldehyde group of the glyoxal occurred and formed the substance with a crosslinked structure, which enhanced the water resistance of the GUSP adhesive.
Fig. 5. FTIR spectra of USP/GUSP adhesives and sesame protein
Fig. 6. The possible reaction mechanism of urea and sesame protein with glyoxal
THERMAL STABILITY ANALYSIS
The thermogravimetric (TG) and derivative thermogravimetric (DTG) curves of the cured different USP/GUSP adhesives are presented in Fig. 7. The thermal degradation curves of the cured USP/GUSP adhesives could be considered a gradual process with three main stages.
Fig. 7. The thermogravimetric (TG) and derivative thermogravimetric (DTG) curves of the USP/GUSP adhesives
The first stage (I) accounted for about 4% of weight loss in the temperature region from 40 to 130 °C, which is associated with the evaporation of free and bound water in the cured USP/GUSP adhesives (Wang et al. 2014). The second stage (II), which occurred in the temperature region from 130 to 250 °C, was the decomposition of the substance that was broken by the sesame protein with urea modification, including small molecules and unstable chemical bonds. The final stage (III) occurred in the temperature region from 250 to 600 °C, which was considered as the skeleton structure decomposition stage. At this stage, the skeleton structure of the adhesive was completely decomposed to form the different gases, such as CO, CO2, and NH3 (Das et al. 2008).
The weight loss of different USP/GUSP adhesives in three degradation stages is shown in Table 3. The weight loss of USP/GUSP adhesives in the second stage (small molecules degradation stage) decreased holistically with increasing glyoxal addition. This behavior showed that the possible reaction between USP and glyoxal occurred and formed the crosslinking structure to enhance the gluing properties of the GUSP adhesive. After heating to 600 °C, the residual weights of the different USP/ZUSP adhesives were 15.2, 14.2, 15.6, 16.2, 16.7, and 16.8%, respectively. This finding indicated that the thermal stability of the GUSP adhesives was improved but not significantly with the glyoxal addition.
SEM Analysis
Many lumen walls in wood are mainly composed of lignin, cellulose, and hemicelluloses, which are used as the glue area of the wood surface (Wang et al. 2014). The combining ability of the adhesive and wood extremely influences the bonding strength of plywood. The morphological properties of the USP/GUSP adhesives are shown in Fig. 8 as SEM micrographs. A large number of holes and cracks in the USP adhesive were observed in Fig. 8A, which were formed by the transpiration of water in the cured process. These holes and cracks could act as the channel of moisture in the water resistance test and could have led to a low water resistance (Luo et al. 2015b). After glyoxal addition, there were no holes and cracks in the GUSP adhesives and the fractured structure of the GUSP adhesives became smoother and more compact compared to the USP adhesive lacking glyoxal, as illustrated by Fig. 8B and C. This behavior indicated that the resultant from the reaction between the sesame protein and glyoxal filled the holes properly and improved the water resistance. By further increasing the glyoxal addition from 12 to 16%, the function of granules acting as an adhesive joint was observed in the GUSP adhesive, as illustrated by Fig. 8D and E. Because of the adhesive joint, the rough wood surface could be combined with the GUSP adhesives commendably, and the plywood panels bonded by these GUSP adhesives would have better water resistance. However, when the glyoxal addition was in excess of 20%, the reaction between the sesame protein and glyoxal may have been saturated, and the complete reaction between urea and glyoxal was finished. Thus, a multiphase adhesive system was formed, as illustrated by Fig. 8F, which could adversely influence the properties of the GUSP adhesive (Luo et al. 2016a).
Fig. 8. SEM micrograph of the fracture surface of the cured USP/GUSP adhesives. USP adhesive (A); 4% GUSP adhesive (B); 8% GUSP adhesive (C); 12% GUSP adhesive (D); 16% GUSP adhesive (E), and 20% GUSP adhesive (F)
CONCLUSIONS
- After glyoxal was introduced, the resultant from the condensation reaction between the sesame protein and glyoxal was generated, as illustrated by FTIR spectra. Thus, the structure of the GUSP adhesive, as shown by examination of fractured surfaces, became more compact and prevented the moisture invasion in the water resistance test and the formation of the adhesive joint improved the bonding ability of the wood and adhesive, as illustrated by SEM micrographs.
- The wet shear strength of the plywood panel bonded by the GUSP adhesive with 12% glyoxal addition reached a maximum value of 0.97 MPa, with a growth rate of 115.5% compared with the USP adhesive, which met the requirement of Type II plywood (≥ 0.70 MPa), according to the China National Standard GB/T 17657-2013. The formaldehyde emission of the GUSP adhesive varied little with increasing glyoxal addition, which was much lower than the requirement of the E0 level (≤ 0.5 mg/L) according to the China National Standard GB/T 9846-2004.
- Both urea degradation and glyoxal modification were found to be effective methods to prepare the sesame protein-based adhesive with a high-water resistance and non-formaldehyde emission.
ACKNOWLEDGEMENTS
The authors express gratitude for the financial support by earmarked fund for the Key Project of Science and Technology of Henan Province (201300110600), the Key Scientific Research Projects in Colleges and Universities of Henan Province (21B550001), and Modern Agro-industry Technology Research System (CARS14-1-29).
REFERENCES CITED
Ballerini, A., Despres, A., and Pizzi, A. (2005). “Non-toxic, zero emission tannin-glyoxal adhesives for wood panels,” Holz Roh. Werkst. 63(6), 477-478. DOI: 10.1007/s00107-005-0048-x
Cheng, H. N., Ford, C., Dowd, M. K., and He, Z. (2016). “Use of additives to enhance the properties of cottonseed protein as wood adhesives,” Int. J. Adhes. Adhes. 68, 156-160. DOI: 10.1016/j.ijadhadh.2016.02.012
Das, S. N., Routray, M., Nayak, and P. L. (2008). “Spectral, thermal, and mechanical properties of furfural and formaldehyde cross-linked soy protein concentrate: A comparative study,” Polym. Plast. Technol. 47(6), 576-582. DOI: 10.1080/03602550701866634
Deng, S., Du, G., Li, X., and Pizzi, A. (2014). “Performance and reaction mechanism of zero formaldehyde-emission urea-glyoxal (UG) resin,” J. Taiwan Inst. Chem. E. 45(4), 2029-2038. DOI: 10.1016/j.jtice.2014.02.007
Despres, A., Pizzi, A., Vu, C., and Pasch, H. (2010). “Formaldehyde-free aminoresin wood adhesives based on dimethoxyethanal,” J. Appl. Polym. Sci. 110(6), 3908-3916. DOI: 10.1002/app.28936
Eslah, F., Jonoobi, M., Faezipour, M., Afsharpour, M., and Enayati, A.A. (2016). “Preparation and development of a chemically modified bio-adhesive derived from soybean flour protein,” Int. J. Adhes. Adhes. 71, 48-54. DOI: 10.1016/j.ijadhadh.2016.08.011
Gao, Q., Shi, S. Q., Li, J., Liang, K., and Zhang, X. (2012). “Soybean meal-based wood adhesives enhanced by modified polyacrylic acid solution,” BioResources 7(1), 946-956. DOI: 10.15376/biores.7.1.0946-0956
He, Z., Chapital, D. C., and Cheng, H. N. (2016). “Effects of pH and storage time on the adhesive and rheological properties of cottonseed meal-based products,” J. Appl. Polym. Sci. 133(27), 43637-43643. DOI: 10.1002/app.43637
Hettiarachchy, N. S., Kalapathy, U., and Myers, D. J. (1995). “Alkali-modified soy protein with improved adhesive and hydrophobic properties,” J. Am. Oil Chem. Soc. 72(12), 1461-1464. DOI: 10.1007/BF02577838
Huang, J., and Li, K. (2008). “A new soy flour-based adhesive for making interior type II plywood,” J. Am. Oil Chem. Soc. 85(1), 63-70. DOI: 10.1007/s11746-007-1162-1
Huang, W., and Sun, X. (2000). “Adhesive properties of soy proteins modified by sodium dodecyl sulfate and sodium dodecylbenzene sulfonate,” J. Am. Oil Chem. Soc. 77(7), 705-708. DOI: 10.1007/s11746-000-0113-6
Inyang, U. E., and Iduh, A. O. (1996). “Influence of pH and salt concentration on protein solubility, emulsifying and foaming properties of sesame protein concentrate,” J. Am. Oil Chem. Soc. 73(12), 1663-1667. DOI: 10.1007/BF02517969
Li, H., Li, C., Gao, Q., Zhang, S., and Li, J. (2014). “Properties of soybean-flour-based adhesives enhanced by attapulgite and glycerol polyglycidyl ether,” Ind. Crops Prod. 59(8), 35-40. DOI: 10.1016/j.indcrop.2014.04.041
Li, J., Li, X., and Gao, Q. (2015). “Investigating the use of peanut meal: A potential new resource for wood adhesives,” Rsc Adv. 5(98), 80136-80141. DOI: 10.1039/c5ra10003h
Li, N., Qi, G., Sun, X. S., Stamm, M. J., and Wang, D. (2012). “Physicochemical properties and adhesion performance of canola protein modified with sodium bisulfite,” J. Am. Oil Chem. Soc. 89(5), 897-908. DOI: 10.1007/s11746-011-1977-7
Luo, J., Luo, J., Gao, Q., and Li, J. (2015a). “Effects of heat treatment on wet shear strength of plywood bonded with soybean meal-based adhesive,” Ind. Crops Prod. 63(1), 281-286. DOI: 10.1016/j.indcrop.2014.09.054
Luo, J., Luo, J., Yuan, C., Zhang, W., Li, J., Gao, Q., and Chen, H. (2015b). “An eco-friendly wood adhesive from soy protein and lignin: performance properties,” RSC Adv. 5(122), 100849-100855. DOI: 10.1039/c5ra19232c
Luo, J., Li, L., Luo, J., Li, X., Li, K., and Gao, Q. (2016a). “A high solid content bioadhesive derived from soybean meal and egg white: Preparation and properties,” J. Polym. Environ. 25(3), 1-12. DOI: 10.1007/s10924-016-0875-3
Luo, J., Li, X., Zhang, H., Gao, Q., and Li, J. (2016b). “Properties of a soybean meal-based plywood adhesive modified by a commercial epoxy resin,” Int. J. Adhes. Adhes. 71, 99-104. DOI: 10.1016/j.ijadhadh.2016.09.002
Luo, J., Li, X., Shi, R., Gao, Q., Li, J., and Li, L. (2017). “Determination of formaldehyde and TVOC emission behavior from interior use plywood using various post heat treatment processes,” J. Appl. Polym. Sci. 134(22), 44909. DOI: 10.1002/app.44909
Nikvash, N., Kharazipour, A., and Euring, M. (2012). “Effects of wheat protein as a biological binder in the manufacture of particleboards using a mixture of canola, hemp, bagasse, and commercial wood,” Forest Prod. J. 62(1), 49-57. DOI: 10.13073/FPJ-D-11-00102.1
Nilo, R.R., Dench, J.E., and Caygill, J. C. (1981). “Nitrogen extractability of sesame (Sesamum indicum L.) seed and the preparation of two protein isolates,” J. Sci. Food Agr. 32(6), 565-571. DOI: 10.1002/jsfa.2740320607
Nordqvist, P., Thedjil, D., Khosravi, S., Lawther, M., Malmström, E., and Khabbaz, F. (2012). “Wheat gluten fractions as wood adhesives – Glutenins versus Gliadins,” J. Appl. Polym. Sci. 123(3), 1530-1538. DOI: 10.1002/app.34312
Onsaard, E., Pomsamud, P., and Audtum, P. (2010). “Functional properties of sesame protein concentrates from sesame meal,” As. J. Food Ag-Ind. 3(4), 420-431.
Pathak, N., Rai, A. K., Kumari, R., Thapa, A., and Bhat, K. V. (2014). “Sesame crop: An underexploited oilseed holds tremendous potential for enhanced food value,” Agr. Sci. 5(5), 519-529. DOI: 10.4236/as.2014.56054
Qi, G., Li, N., Wang, D., and Sun, X. S. (2013). “Physicochemical properties of soy protein adhesives modified by 2-octen-1-ylsuccinic anhydride,” Ind. Crops Prod. 46(4), 165-172. DOI: 10.1016/j.indcrop.2013.01.024
Qiang, G., Shi, S. Q., Zhang, S., Li, J., Wang, X., Ding, W., Liang, K., and Wang, J. (2012). “Soybean meal-based adhesive enhanced by MUF resin,” J. Appl. Polym. Sci. 125(5), 3676-3681. DOI: 10.1002/app.36700
Raja, A., Hattab, K. O., Gurusamy, L., and Suganya, S. (2007a). “Sulphur levels on nutrient uptake and yield of sesame varieties and nutrient availability,” J. Soil Sci. 2, 278-285. DOI: 10.3923/ijss.2007.278.285
Raja, A., Hattab, K. O., Gurusamy, L., Vembu, G., and Suganya, S. (2007b). “Sulphur application on growth and yield and quality of sesame varieties,” J. Agr. Res. 2(7), 599-606. DOI: 10.3923/ijar.2007.599.606
Samanta, A. K., Biswas, S. K., Mitra, S., Basu, G., and Mahalanabis, K. K. (2008). “Chemical modification of jute fabric with ethylene glycol and its mixtures with glyoxal and aminosilicone compound under different catalyst systems for improving its textile related properties and thermal behaviour,” J. Polym. Mater. 25(2), 159-183.
Sun, X., and Ke, B. (1999). “Shear strength and water resistance of modified soy protein adhesives,” J. Am. Oil Chem. Soc. 76(8), 977-980. DOI: 10.1007/s11746-999-0115-2
Tai, S. S. K., Lee, T. T. T., Tsai, C. C. Y., Yiu, T. J., and Tzen, J. T. C. (2001). “Expression pattern and deposition of three storage proteins, 11S globulin, 2S albumin and 7S globulin in maturing sesame seeds,” Plant Physiol. Bioch. 39(11), 981-992. DOI: 10.1016/S0981-9428(01)01314-6
Wang, C., Wu, J., and Bernard, G. M. (2014). “Preparation and characterization of canola protein isolate-poly (glycidyl methacrylate) conjugates: A bio-based adhesive,” Ind. Crops Prod. 57(2), 124-131. DOI: 10.1016/j.indcrop.2014.03.024
Wei, X., Liu, K., Zhang, Y., Feng, Q., Wang, L., Zhao, Y., Li, D., Zhao, Q., Zhu, X., and Zhu, X. (2016). “Genetic discovery for oil production and quality in sesame,” Nat. Commun. 33, 8609. DOI: 10.1038/ncomms9609
Wei, X., Wang, X., Li, Y., and Ma, Y. (2017). “Properties of a new renewable sesame protein adhesive modified by urea in the absence and presence of zinc oxide,” RSC Adv. 7(73), 46388-46394. DOI: 10.1039/c7ra07578b
Xin, J., Zhang, P., Wolcott, M. P., Zhang, J., Hiscox, W. C., and Zhang, X. (2016). “A novel and formaldehyde-free preparation method for lignin amine and its enhancement for soy protein adhesive,” J. Polym. Environ. 25(3), 1-7. DOI: 10.1007/s10924-016-0844-x
Yuan, Z., and Hu, H. (2012). “Preparation and characterization of crosslinked glyoxalated polyacrylamide paper-strengthening agent,” J. Appl. Polym. Sci. 126(s1), 459-469. DOI: 10.1002/app.36779
Zhang, Z., and Hu, Y. F. (2007). “Urea-modified soy globulin proteins (7S and 11S): Effect of wettability and secondary structure on adhesion,” J. Am. Oil Chem. Soc. 84(9), 853-857. DOI: 10.1007/s11746-007-1108-7
Zhong, Z., and Sun, X. S. (2007). “Plywood adhesives by blending soy protein polymer with phenol-formaldehyde resin,” J. Biobased Mater. Bioenergy 1(3), 380-387. DOI: 10.1166/jbmb.2007.014
Article submitted: November 6, 2018; Peer review completed: January 13, 2021; Revised version received and accepted: February 5, 2021; Published: March 9, 2021.
DOI: 10.15376/biores.16.2.3121-3136
