Abstract
Wood is an environmentally friendly material for the construction of buildings, and it possesses great physical and mechanical properties. However, under certain circumstances, it needs to be protected from degradation. This can be achieved either by proper design or treatment. In this study, eastern white pine (Pinus strobus L.) and white spruce (Picea glauca (Moench) Voss) were impregnated with propiconazole and 3-iodo-2-propynyl butylcarbamate, which are two organic fungicides. Unlike most impregnation techniques, no pressure treatment was needed. Instead, an aqueous buffered amine oxide system was used to allow the fungicides to diffuse rapidly into the wood. Many combinations of fungicides and amine oxides, as well as different diffusion times were tested to study the effect of the treatment on the dimensional stability and resistance to decay fungi. It was found that only the amine oxide affected the dimensional stability of the treated wood, with anti-swelling and anti-shrinking efficiencies values up to 30%. Amine oxides and fungicides both had an impact on the weight loss caused by the brown rot fungi. The weight loss after 10 weeks of exposure to Rhodonia placenta was reduced by half when using amine oxides or fungicides, and it was completely inhibited when they were combined.
Download PDF
Full Article
Performances of White Pine and White Spruce Treated with Organic Fungicides Using an Aqueous Buffered Amine Oxide Preservation System
Simon Pepin,* Pierre Blanchet, and Véronic Landry
Wood is an environmentally friendly material for the construction of buildings, and it possesses great physical and mechanical properties. However, under certain circumstances, it needs to be protected from degradation. This can be achieved either by proper design or treatment. In this study, eastern white pine (Pinus strobus L.) and white spruce (Picea glauca (Moench) Voss) were impregnated with propiconazole and 3-iodo-2-propynyl butylcarbamate, which are two organic fungicides. Unlike most impregnation techniques, no pressure treatment was needed. Instead, an aqueous buffered amine oxide system was used to allow the fungicides to diffuse rapidly into the wood. Many combinations of fungicides and amine oxides, as well as different diffusion times were tested to study the effect of the treatment on the dimensional stability and resistance to decay fungi. It was found that only the amine oxide affected the dimensional stability of the treated wood, with anti-swelling and anti-shrinking efficiencies values up to 30%. Amine oxides and fungicides both had an impact on the weight loss caused by the brown rot fungi. The weight loss after 10 weeks of exposure to Rhodonia placenta was reduced by half when using amine oxides or fungicides, and it was completely inhibited when they were combined.
Keywords: Wood preservation; Amine oxide; Propiconazole; 3-Iodo-2-propynyl N-butylcarbamate, Brown rot; Dimensional stability; White pine; White spruce
Contact information: Natural Sciences and Engineering Research Council of Canada (NSERC) Industrial Chair on Eco-responsible Wood Construction, Université Laval, G1V 0A6, 2425 Rue de l’Université, Québec, Qc, Canada; *Corresponding author: Simon.pepin.1@ulaval.ca
INTRODUCTION
Wood is one of the most versatile materials known to man. A whole variety of goods can be made with it, such as boxes, bridges, tools, and houses. Because of its relatively low density and high mechanical properties, it is a material of great interest in construction. However, any material used in this field is expected to last for years, even decades. Because it can crack under the effect of water and be deteriorated by fungi, insects, ultraviolet rays, and more (Hill 2006), it is a great challenge to maintain the good structural and aesthetic conditions of wood for long periods. Such long-term performance is required to ensure wood’s competitiveness with other materials, such as steel, concrete, polymers and composites. Its price, appearance, properties, low environmental impact (Lippke et al. 2004; Nath 2017), and capacity to sequester CO2 contribute to making this challenge worthwhile.
If suitable conditions are met, decay fungi can degrade wood polymer components, which affects its mechanical and chemical properties, as well as its appearance. The optimal conditions for fungal growth usually include a temperature between 21 °C and 32 °C, appropriate levels of O2 (1% to 4%), CO2 (up to 20%), and nitrogen (over 0.3%) into the wood, a moisture content (MC) slightly over the fiber saturation point (30%), and more (Carll and Highley 1999; Tudor et al. 2012; Reinprecht 2016). However, both the extreme and optimal conditions depend on the species and can include a temperature between 10 °C and 45 °C, and a MC from 12.3% to 210% (Huckfeldt and Schmidt 2006; Stienen et al. 2014; Meyer and Brischke 2015). Brown rot fungi feed on cellulose and hemicelluloses (Hill 2006). Using Fenton’s oxidation (H2O2/Fe2+/(COOH)2), a chemical reaction using ferrous ions and hydrogen peroxide to create hydroxyl radicals, these fungi can easily break down crystalline cellulose and resist copper treatments (Goldstein et al. 1993; Reinprecht 2016). They can also modify lignin but will not consume it. Brown rot fungi cause wood to become darkened, cracked, and fragile. White rot fungi can feed on all wood components, but primarily they feed on lignin (Blanchette et al. 1990). These fungi have two different decay patterns. Some species, called “simultaneous white rot”, will break down all three wood polymers at the same time, while the “selective white rot” will degrade the lignin and hemicelluloses to a greater extent than cellulose (Goodell et al. 2008). They make the wood pale (native cellulose color) and brittle.
The hygroscopicity of wood causes its MC to change according to ambient conditions (temperature and relative humidity). Its dimensions change accordingly because of water molecules entering/leaving the amorphous zones of cellulose, which causes the separation/combination of microfibrils and the swelling/shrinking of cell walls (Siau 1995). Dimensional changes are not the same in each direction and are approximately twice as important in the tangential axis compared to the radial axis (Panshin et al. 1964). Disproportionate swelling and shrinking cause important stresses in the wood structure, which eventually warps and cracks (Glass and Zelinka 2010). Moreover, the wood MC at equilibrium is influenced not only by ambient conditions, but also by whether the wood is gaining or losing moisture (Siau 1995). This phenomenon, known as hysteresis, is caused by the free energy difference between water adsorption and desorption and is mostly influenced by compressive stresses in the wood during the adsorption of water (Navi and Heger 2005). This phenomenon can be illustrated in a sorption isotherm graph (Siau 1995).
Wood can be protected against biodegradation through impregnation. Impregnation is usually performed in an autoclave to help deliver the treatment solution to the wood cells using cycles of vacuum and pressure (Leightley 2003; Freeman 2008). Pressure has been used for treatments including borates, triazoles, quaternary ammoniums, synthetic pyrethroids, carbamates, copper oxides, and copper carbonates (with or without tebuconazole) (Schultz and Nicholas 2003; Laks 2008; Ross 2008). These substances can be dissolved or diluted in water or organic solvents such as white spirit.
Impregnation can also be used to increase the dimensional stability of wood. This can be achieved by bulking the lumen or by modifying and crosslinking the hydroxyl groups of the cell wall polymers (Navi and Heger 2005). These methods include bulking with silane, phenol, or amino resins and modification with formaldehyde, anhydrides, epoxides, and 1,3-dimethylol-4,5-dihydroxyethyleneurea (Wang and Piao 2011; Yuan et al. 2013; Kocaefe et al. 2015; Reinprecht 2016). Acetylation is the most important chemical modification for wood that is commercially available.
Recently, an aqueous wood treatment has been developed to make wood more resistant to biodegradation. It uses the ability of tertiary amine N-oxides, non-toxic surfactants, to dissolve organic pesticides in water and diffuse into wood to allow for impregnation without using a pressure treatment (Walker and Shen 2002). The amine oxides (AO), having antiseptic properties themselves, can increase the resistance to biodegradation even more (Tseng et al. 2002). An AO with a long aliphatic chain can also increase the dimensional stability of the treated wood (Tseng and Walker 2000). The contact of the AO with the acids of the hemicelluloses allows their fixation into the wood (Jiang 2008). To ensure maximum penetration, the pH needs to be controlled with a buffer, preferably made of borates (Ross and Cutler 2015). Because their toxicity is very low, the replacement of a physical mean of impregnation (vacuum/pressure) for a chemical one does not make the treatment and treated wood any more harmful (Sanderson et al. 2006; Sanderson et al. 2009). Although such treatment is already commercialized in a few countries, little has been published on the subject.
The aim of this study is to develop a wood treatment that uses an aqueous buffered AO delivery system to impregnate wood with organic fungicides through diffusion. The objective is twofold. First, the study will use a factorial approach to understand the influence of different factors, as well as their interactions, on two important aspects of the service performances, namely the fungal decay resistance and dimensional stability. The factors are the AOs, fungicides, and diffusion time. Second, the study will evaluate the potential of such a treatment in terms of the performances, within the limits of the combinations being tested. The performances will be assessed by the mass loss caused by brown rot and white rot fungi degradation, as well as the decrease in the swelling and shrinkage.
EXPERIMENTAL
Materials
The wood samples for this study were cut from boards of eastern white pine (Pinus strobus L.), which were obtained from Bois Delta (Quebec City, Canada), and white spruce (Picea glauca (Moench) Voss) from Maibec (St-Pamphile, Canada), which were initially brought to a MC of approximately 12% in a conditioning room at 20 °C ± 2 °C and 65% ± 5% relative humidity (RH). These species were selected because they are of great importance for the construction market in Canada, while also having important differences in permeability to impregnation treatments (Alberta Canada 2013; Morris 2017). They were then sawn into 25 mm × 25 mm × 10 mm (longitudinal × tangential × radial) blocks for the biodegradation tests and 20 mm × 20 mm × 20 mm cubes with ring growth angles of less than 10° along the tangential direction for the dimensional stability tests. All of the samples were free of knots and visible stains. All the samples for the biodegradation tests were made of sapwood. Some heartwood (about 5%) could be found in the dimensional stability samples, but they were distributed in order to limit their impact on the statistical analysis.
The N,N-dimethyldodecylamine N-oxide (approximately 30% aqueous solution; DDAO), N,N-dimethylhexadecylamine (95%), 3-iodo-2-propynyl N-butylcarbamate (97%; IPBC), and 50% hydrogen peroxide (H2O2) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). The American Chemical Society (ACS)-grade ortho-boric acid was obtained from Anachemia (Mississauga, Canada). Sodium tetraborate (98%) was purchased from Thermo Fisher Scientific (Ottawa, Canada). Propiconazole (Tilt 250E, 25% aqueous solution) was generously supplied by Syngenta (Plattsville, Canada). Bacteriological grade agar was obtained from Hardy Diagnostics (Santa Maria, CA, USA). The malt extract was a product of Becton, Dickinson & Co. (Mississauga, Canada). Fungi for the biodegradation tests came from the Canadian fungi collection and were offered by FPInnovations (Quebec City, Canada) a few years ago and pricked out regularly by our department.
Methods
Wood treatment
The method to treat the wood samples was inspired by the methods of Ward and Scott (2009) and Morris et al. (2014). Samples of white pine and white spruce were individually dipped for 15 s in various treatment solutions maintained at 65 °C. After removing the excess solution, the samples were sealed in plastic wrap for 6 h to prevent evaporation. The plastic wrap was then disposed of, and the samples were placed in a conditioning chamber at 85 °C ± 1 °C and 85% ± 3% RH for different periods of time to allow for diffusion. The samples were then left for two weeks in the conditioning room at 20 °C ± 2 °C and 65% ± 5% RH to bring them back to a 12% MC. At this step, hysteresis was observed, which kept the MC at 14% to 15%. Therefore, the samples were stored in the laboratory for a week, where the ambient conditions were drier, before spending another week in the conditioning chamber. The samples were weighed before treatment to monitor the target MC (12%), immediately after dipping, after removal of the plastic wrap, and at the end of the process (12% MC).
Synthesis of N,N-dimethylhexadecylamine N-oxide
The N,N-dimethylhexadecylamine N-oxide (DHAO) used in this study could not be provided and was therefore prepared following the method by Prabhu (1999). In a 500-mL round-bottom flask, 69.95 g (0.260 mol) of N,N-dimethylhexadecylamine, 70.10 g of propan-2-ol, and a few drops of acetic acid were mixed under mild stirring. The mixture was heated to 55 °C and 21.20 g (0.294 mol) of 50% H2O2 were added over 60 min. The temperature was then increased to 65 °C for 18 h. The final product was isolated using a rotary evaporator at 45 °C and 5 kPa for 4 h and characterized by hydrogen nuclear magnetic resonance (H1 NMR, 8 scans, CDCl3) spectroscopy (500 MHz; Direct Drive Model, Agilent, Santa Clara, CA, USA). Before being used, the DHAO was dissolved in deionized water to obtain a 30% w/w solution.
Treatment solutions
The treatments were prepared using a factorial method. The solutions used in this study were prepared using a borate buffer with different combinations of AOs and fungicides, which were paired with different diffusion times. All of the treatment combinations are shown in Table 1. The AOs used were DDAO and DHAO. The solutions containing no AO were not buffered. The fungicides chosen were propiconazole and IPBC, which are two organic fungicides broadly used for wood protection.
Preparation of the solutions
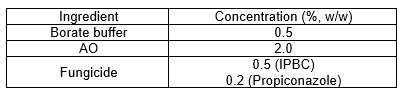
The concentration of each compound is shown in Table 2. Depending on their formulation, the treatment solutions could have contained 5.00 g of borate buffer (2.50 g of both ortho-boric acid and sodium tetraborate) combined with a total of 66.66 g of AO solution (20.00 g of AO) or no borate and AO. They could have contained 5.00 g of IPBC, 2.00 g of propiconazole, or no fungicide. Water was added to bring the solution to a total weight of 1000 g. The mixture was heated to 65 °C under stirring for 30 min to ensure complete solubilization of the fungicides.
Table 1. Conditions of the Different Treatments and Their ID
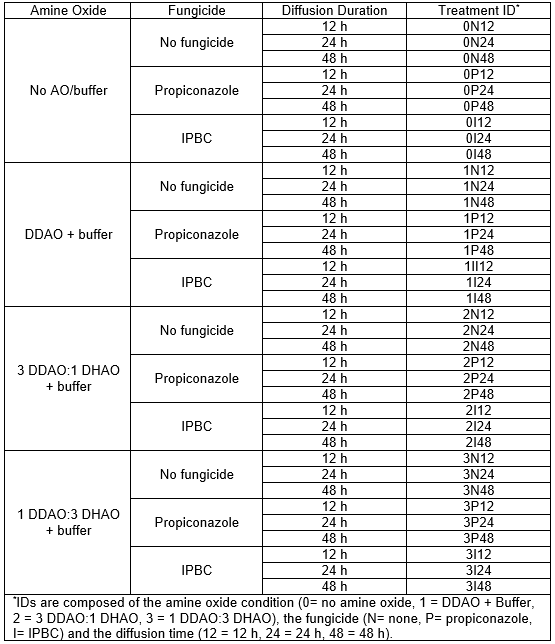
Table 2. Concentration of the Active Ingredients in the Treatment Solutions
Products retention
To calculate the products retention (PR) of the samples following treatment, the samples were weighed before the treatment (w1, kg). After being treated, they were brought back to their initial MC and weighed again (w2, kg). The PR was expressed as the mass of products (sum of the borates, amine oxides and fungicides) absorbed by the samples, divided by its volume (V, m3), and was calculated with Eq. 1:
PR (kg/m3) = [(w2 – w1)/V] (1)
Biodegradation
The biodegradation tests were performed following a method based on AWPA E10-12 (2012), and took place in mason jars lying on their side. The culture medium used was a malt-agar gel with 20 g/L malt as a food source and 15 g/L agar to form the gel. The hot malt-agar mixture (20 mL) was added to the mason jars and allowed to cool until the gel formed. V-shaped glass rods were heated with a flame and deposited on the gel. An inoculum of the brown rot fungus Rhodonia placenta or white rot fungus Irpex lacteus was added to the mason jars and was given a two-week incubation period.
Meanwhile, the samples were oven-dried for 24 h at 60 °C, which was followed by further drying at 103 °C for 24 h. They were then weighed to determine their initial dry mass and placed in aluminum cups. The cups were layered in a large plastic bin, with each layer separated by cheese cloth. The bottom of the bin was filled with 5 cm of deionized water, which was separated from the samples by a fiberglass mesh. The bin was sterilized in an autoclave and sealed for two weeks. This allowed the samples to reach a MC of 22% to 25%.
The samples were positioned on the V-shaped glass rods in the inoculated mason jars. The jars were placed in a conditioning chamber (26.7 °C ± 0.1 °C, 70% ± 3% RH) for a 10-week incubation period. The caps were perforated and lined with a cloth filter (Filtration Québec Ltée, Bedford, Canada), which allowed air and moisture exchange. After 10 weeks, the fungal growth was carefully brushed from the samples, which were then oven-dried (60 °C for 24 h, and then 103 °C for 24 h) and weighed to determine their final dry mass.
The degradation was measured by the mass difference between the oven-dried samples before (m1, g) and after (m2, g) exposure to the fungi and was expressed as a percentage. It was calculated with Eq. 2:
Mass loss (%) = [(m2 – m1)/m1] × 100 (2)
Because of equipment and space limitations, this test used three samples for each treatment and three untreated standards. To increase the anatomical variability, each of these three samples were cut from different boards.
Dimensional stability
The dimensional stability tests were performed following two different methods. To measure the swelling, the samples were oven-dried at 103 °C ± 2 °C for 24 h, after which they would either (1) be placed in a conditioning chamber at 20 °C ± 1 °C and 95% ± 3% RH for 90 h or (2) be completely immersed in water for 72 h (20 °C ± 2 °C). During these periods, the samples would be weighed every 6 h to ensure that either (1) their mass was stable for at least 24 h before measurement, which shows that a moisture equilibrium has been attained, or (2) that the MC was beyond the fiber saturation point (MC = 30%), which is the point after which swelling no longer occurs (Siau 1995). The swollen samples were then oven-dried to measure their shrinkage, thereby completing a cycle. Because wood treatment components are likely to leach and become less effective over time, three full cycles were performed for each method (Hill 2006).
Both the swelling and shrinkage were measured in the radial (R) and tangential (T) axes using a digital micrometer with a 0.0001-mm precision. According to literature, longitudinal dimensional changes are small (approximately 0.2%) and can be ignored (Panshin et al. 1964; Glass and Zelinka 2010). The calculations were based on ISO 4469 (1981) and ISO 4859 (1982). For the swelling, the samples were measured after oven-drying (R1 and T1) and at equilibrium (R2 and T2). The volumetric swelling (α) was calculated with the following equations,
αR (%) = [(R2 – R1)/R1] × 100 (3)
αT (%) = [(T2 – T1)/T1] × 100 (4)
α (%) = αR + αT (5)
where αR and αT are the radial and tangential swelling, respectively.
Similarly, the shrinkage was measured after swelling (R2 and T2, mm) and drying (R3 and T3, mm). The volumetric shrinkage (β) was calculated with the following equations,
βR (%) = [(R2 – R3)/R2] × 100 (6)
βT (%) = [(T2 – T3)/T2] × 100 (7)
β (%) = βR + βT (8)
where βR and βT are the radial and tangential shrinkage, respectively.
Improvement in the dimensional stability was defined by the anti-swelling/ shrinking efficiency (ASE) (Hill 2006), which can be calculated by comparing the untreated and treated samples with the following equations,
ASE (%) = [(αu – αt)/αu] × 100 (9)
ASE (%) = [(βu – βt)/βu] × 100 (10)
where αu and βu are the swelling and shrinkage of the untreated samples, respectively, and αt and βt are the swelling and shrinkage of the treated samples, respectively.
For each wood species, both methods used 10 samples for each treatment and 10 untreated standards. The high RH tests were further characterized by the equilibrium moisture content (EMC) of the samples and sorption isotherms. The EMC is described by Hill (2006) as the constant MC wood will attain when placed in an environment where the RH is stable. It was calculated with Eq. 11,
EMC (%) = [(w2 – w1)/w1] × 100 (11)
where w1 and w2 are the weight (g) of the samples when oven-dried and after 90 h in the conditioning chamber (20 °C and 95% RH), respectively.
The sorption isotherms were obtained with a VTI-SA+ vapor sorption analyzer (TA Instruments, New Castle, DE, USA). Samples with the dimensions 5 mm × 5 mm × 5 mm were oven-dried and exposed to increasing RH levels (5%, 20%, 40%, 60%, 80%, and 95% ± 1%) for the adsorption curve and then decreasing RH levels (80%, 60%, 40%, 20%, and 5% ± 1%) for the desorption curve. For each level, the EMC was determined when the mass of the samples increased less than 0.003% within 5 min. The temperature was 25.0 °C ± 0.1 °C for the duration of the experiment.
Statistical analysis
The factorial design observations were analysed with an analysis of variance (ANOVA) using the mixed procedure in the SAS University software (SAS, Cary, NC, USA) at an α of 0.01.
RESULTS AND DISCUSSION
Synthesis of N,N-Dimethylhexadecylamine N-Oxide
From the 69.95 g (0.260 mol) of N,N-dimethylhexadecylamine used, 77.45 g (0.268 mol) of the DHAO were obtained, which resulted in a yield of 103%. After evaporation of the reaction medium, the product obtained was a vitreous, white solid. The H1 NMR spectra (Fig. 1) displayed shifting of the C1 (from δ = 2.20 ppm to 3.23 ppm), C2 (δ = 2.25 ppm to 3.29 ppm), and C3 peaks (δ = 1.45 ppm to 1.85 ppm), which showed an increase in polarity on the nitrogen atom. The introduction of a small singlet (integration 0.64) at a δ of 1.99 ppm demonstrated the presence of oxygen atoms under an O-/OH equilibrium. The surprising absence of the singlet at a δ of 2.20 ppm on the AO spectrum indicated the complete conversion of the amine, even though the amine was not distilled before the reaction. Synthesis performed directly on commercially available tertiary amines usually yields an 85% to 87% conversion (Singh et al. 2006).
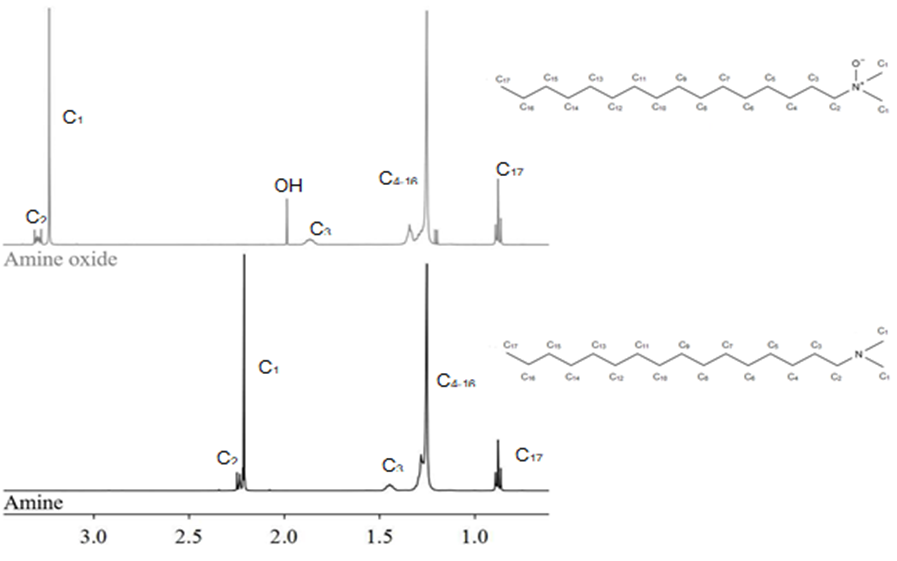
Fig. 1. H1 NMR spectra of the N,N-dimethylhexadecylamine and DHAO
Because the solid was too hard to be scraped from the round-bottom flask, it was dissolved under stirring in 180 mL of water to make a 30% solution. The solution was white, translucent, and viscous.
Products Retention
Despite their different shapes, the PR values of the samples used for the biodegradation and dimensional stability tests were similar (Figs. 2 and 3). The dimensional stability samples even had higher PR values in many instances, although their surface/volume ratio was notably lower. The mass of the white spruce samples increased more than that of the white pine, which even had negative values in some cases. This was first explained by the loss of water-soluble extractives in the case of the white pine, which was expected because of a rather quick yellowing of the solutions during treatments. The period in the conditioning room (85 °C and 85% RH) could also have promoted the loss of volatile compounds from the samples. However, a test using untreated samples of both species submitted to, either a 15-s dipping in water or 24 h in the conditioning room, and brought back to a their initial MC, showed that neither of these steps caused the loss of any material in such amounts that affected the final weight of the samples. In contrast, while the white pine samples did come back to their initial mass in all of the cases, the white spruce samples, which were placed in the conditioning room, had a final mass approximately 2% higher than their initial mass, probably caused by a higher MC. This difference in MC, which could originate from a difference between the desorption curves of the two wood species, was probably the real reason behind their different PR values. This could have been avoided by comparing the oven-dried mass of the samples, but the PR values were expected to be so low that the loss of volatile compounds during drying was predicted to affect the results more than hysteresis. Furthermore, the loss of some of these compounds before the treatments could also have affected impregnation.
These PR values were lower than the PR required to obtain good performances with acetylation, typically 20% of the mass of the dried wood (80 kg/m3 for a wood weighting 400 kg/m3), and quaternary ammonia compounds, approximately 7.7 to 18.5 kg/m3 (Kartal et al. 2006; Terzi et al. 2011), which allows for keeping the advantages of a low wood density. It is however higher than the typical values of PR from pressure impregnation of IPBC (0.04-0.06 kg/m3) and propiconazole (0.04-0.12 kg/m3) (EC Directive 98/8/EC 2007; EC Directive 98/8/EC 2008), since amine oxides and borates are also impregnated.
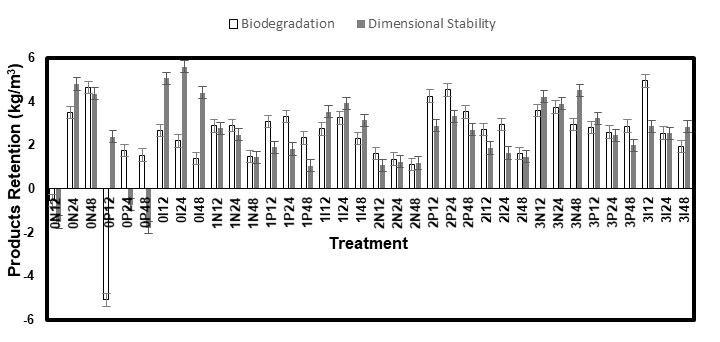
Fig. 2. Products retention of the eastern white pine samples following treatment
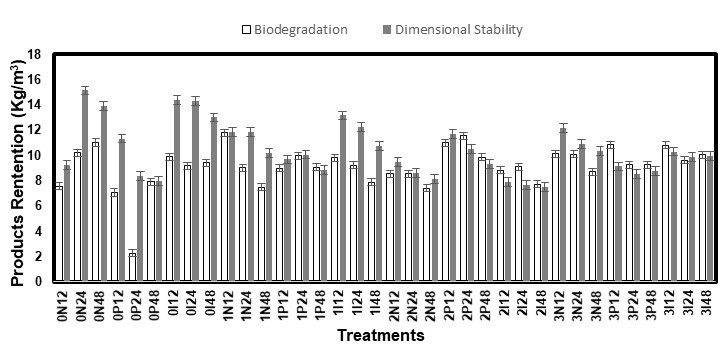
Fig. 3. Products retention of the white spruce samples following treatment
The ANOVA of the PR results showed a significant (p < 0.0001) AO*fungicides*time relationship for both species and sample sizes (Table 3). Because AOs are polar, their attraction to water may have caused them to be slowly washed from the surface during the diffusion period when condensation droplets formed in the conditioning chamber and fell onto the samples. Likewise, a slight odor during this period suggested they may also have been carried into the air by the high air moisture content (85% RH). Both of these elements suggested that a longer diffusion period could lead to more AO losses.
Additionally, because the fungicides have a higher affinity with AOs than water, they were likely washed off to a greater extent in their presence. Because the fungicides were affected by the AOs and the AOs were affected by the time, it was logical that these three factors were connected. A higher PR means more active ingredients in the wood. Therefore, it was indicated that shorter diffusion times will likely lead to better performances of the treated wood, which also fits the needs of the industry. The ANOVA also showed, with high F values, that the AO*fungicide interaction was important. It was established that the presence of AOs enhanced the penetration of the fungicides into the wood and increased the PR.
The solutions with IPBC usually had the highest PR, which was explained by its higher concentration (0.5% IPBC vs. 0.2% propiconazole). Surprisingly, most of the time the solutions with propiconazole led to the smallest PR, even lower than that of the solutions without a fungicide. Likewise, the solutions with AO condition 2 (3 DDAO:1 DHAO) had the smallest PR of all of the AO conditions. However, the highest PR was obtained for these two conditions when they were combined (Fig. 4). While this apparent synergy between propiconazole and AO condition 2 was hard to explain, it is a useful piece of information if propiconazole is the fungicide used with this treatment. Its higher affinity with wood, lower leaching, and higher stability in wood are incentives to use it (Schlutz and Nicholas 2003; Mazela and Perdoch 2012; Schiopu and Tiruta-Barna 2012; Kukowski et al. 2017).
Table 3. Statistical Analysis of the PR of White Pine and White Spruce Samples for the Biodegradation and Dimensional Stability Tests Following Treatment
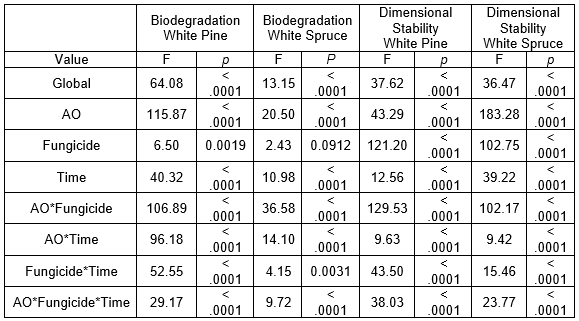
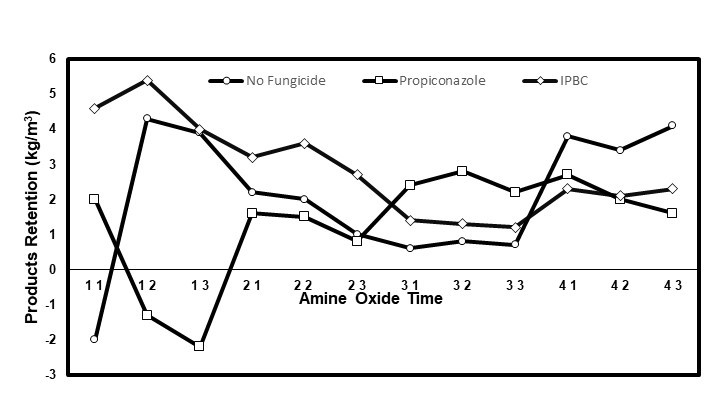
Fig. 4. Interaction plot of the AO*fungicide*time relationship for the PR of the eastern white pine dimensional stability samples; AO: 1 = No AO, 2 = DDAO, 3 = 3 DDAO:1 DHAO, and 4 = 1 DDAO:3 DHAO; time: 1 = 12 h, 2 = 24 h, and 3 = 48 h
Biodegradation
After incubation, the untreated samples of both species that were exposed to the brown rot fungus R. placenta were covered with a thick layer of fungal growth. This was also the case for the samples treated without AO or fungicide (solution 0N). After removal of the fungal growth, these samples were bent and showed visible cracks on their top surface. The samples treated with the other solutions were all sound and free of fungal growth, with only a few exceptions that showed a small amount of fungal growth.
Figures 5 and 6 show that, for both species, the treatments using only a fungicide (0I and 0P) or AO (1N, 2N, and 3N) led to a lower mass loss compared with the untreated specimens. For the white pine, there was only slightly less decay than for the untreated specimens, while the white spruce showed an almost complete inhibition of mass loss, except with IPBC. The poor performance of the IPBC might have been because of its low solubility in water (156 mg/L at 20 °C) (Juergensen et al. 2000), which led to an insufficient accumulation in the wood. In contrast, because propiconazole was used as a ready-to-use commercial solution, its solubility might have been increased by the presence of emulsifiers and solvents. These conclusions seemed to contradict those from the PR but could also have been the result of the propiconazole’s efficiency against this fungus, even at lower concentrations.
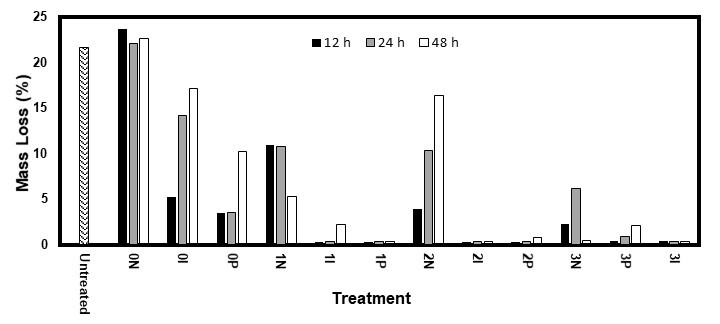
Fig. 5. Mass loss of the eastern white pine samples after 10 weeks of exposure to the brown rot fungus R. placenta
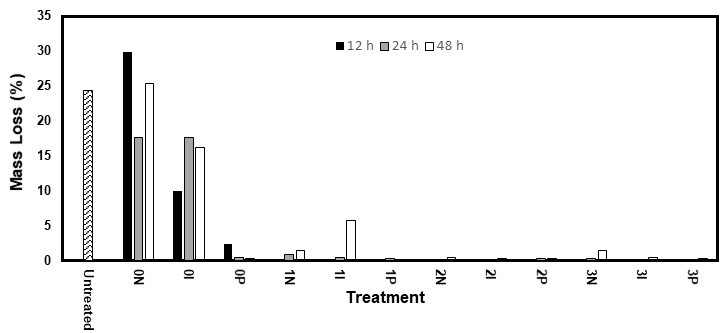
Fig. 6. Mass loss of the white spruce samples after 10 weeks of exposure to the brown rot fungus R. placenta
Amine oxides also have antifungal properties (Tseng et al. 2002). While they are probably less potent than propiconazole and IPBC, their high solubility in water and ease of impregnating wood might have allowed them to reach a concentration high enough to display similar performances. The treated white spruce showed less mass loss than the white pine. Its lower permeability allowed for a higher concentration of the fungicides and AOs near the surface, which granted better protection. While this might be seen as an advantage, it could make it easier for treatments kept near the surface to leach out of the wood and compromise its durability.
When fungicides and AOs were used together, the mass loss was nearly nullified for both species, with values typically between 0.2% and 0.9%. This indicated synergy between the AOs and fungicides. Only three samples (one 3P48 for pine and one 1I48 for both pine and spruce) showed high mass losses. This was explained by the greater solubility of fungicides and their improved capacity to penetrate wood in the presence of AOs, which markedly increased their accumulation in the wood. Although similar performances were obtained for the white spruce while using only propiconazole, the use of AOs could also improve the depth of the impregnation of the fungicides and increase their resistance to leaching. De Vetter et al. (2009) vacuum treated Scots pine with IPBC (1.5-2 kg/m3) and a mix of IPBC (1.5-2 kg/m3) and propiconazole (2.5–4 kg/m3). They found nearly no mass loss after 16 weeks of exposure to C. puteana, which isn’t surprising as this retention is way higher than the suggested retention for these products (EC Directive 98/8/EC 2007; EC Directive 98/8/EC 2008). Terzi et al. (2011) studied the resistance of Scots pine samples that were pressure-treated with didecyl dimethyl ammonium chloride and didecyl dimethyl ammonium tetrafluoroborate. These products, which possess a chemical structure similar to that of AOs, are often used as co-biocides with IPBC (Wallace et al. 2008). After 12 weeks of exposure to another brown rot fungus, Tyromyces palustris, the samples showed 5% to 8% and 1% to 4% mass loss, depending on the fungicide concentration.
While the number of samples used may have been too low to define trends, the results did not seem to be influenced by the nature of the fungicide and AO. However, the mass loss seemed to increase with the diffusion period. This confirmed that a longer diffusion time of the treatment washed the ingredients from the surface of the samples. Because the nature of the AOs and fungicides seemed to have a minor impact on the performances, other considerations, such as their toxicity, price, and availability, could facilitate their selection.
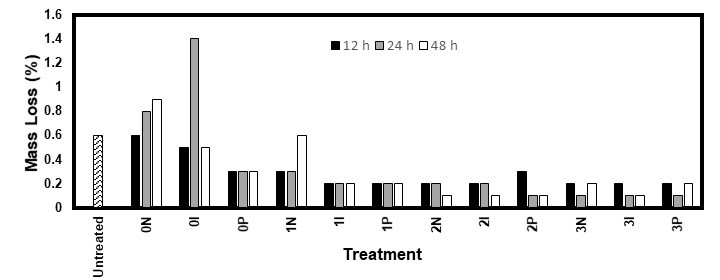
Fig. 7. Mass loss of the eastern white pine samples after 10 weeks of exposure to the white rot fungus I. lacteus
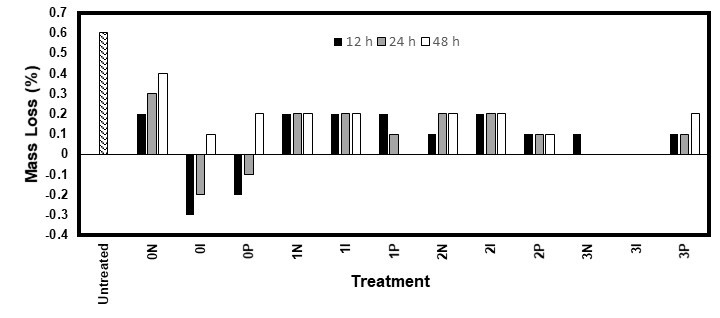
Fig. 8. Mass loss of the white spruce samples after 10 weeks of exposure to the white rot fungus I. lacteus
Unlike R. placenta, the white rot fungus I. lacteus did not cover any wood sample, even the untreated ones. Figures 7 and 8 show that the fungus was barely able to deteriorate any sample, even though the MC was sufficient (22% to 25%). This might have been because of the structure of the softwood lignin, which is formed almost exclusively of coniferyl alcohol (Stevanovic 2016). It is harder for white rot fungi to degrade this type of lignin. Furthermore, white rot fungi cannot degrade the polysaccharides in the wood without first breaking down the lignin (Blanchette et al. 1990). Thus, I. lacteus would not have been able to degrade the wood samples within the allotted time due to its chemical structure.
Dimensional Stability
Both the high RH and immersion methods used for the dimensional stability tests had comparable results, though the immersion test had slightly lower ASE values. Figures 9 through 12 show the ASE values for both species and methods. For the white pine, the statistical analysis for both the ASE values showed a significant (p < 0.0001) effect from the AOs, while the other factors were not significant. However, the relationship between the AO used and ASE was not clear because the samples treated without AOs had quite high ASE values. Additionally, AO conditions 2 and 3 (3 DDAO:1 DHAO and 1 DDAO:3 DHAO, respectively) led to similar results. In contrast, the solutions containing only DDAO (condition 1) exhibited clearly lower ASE values. For the white spruce, both the AOs and fungicides, as well as their interaction, were significant factors. In this case, the samples treated without AOs did not show any improvements in the dimensional stability. The ASE improved when the samples were treated with AOs and increased with the concentration of DHAO. Therefore, it was clear for both species that the presence of DHAO imbued the wood with a greater dimensional stability than the DDAO, which could have been caused by the bulking effect of its longer aliphatic chain (Tseng and Walker 2000). In contrast, the effect of the fungicides varied with each test, which made the conclusions unclear. The diffusion time was statistically not significant, which could have meant that a period shorter than 12 h could be used to achieve a similar dimensional stability performance. This would make the treatment even more practical and economical.
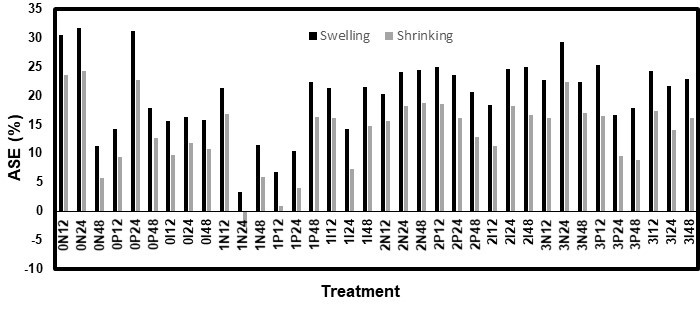
Fig. 9. ASE values for the high RH test on the eastern white pine
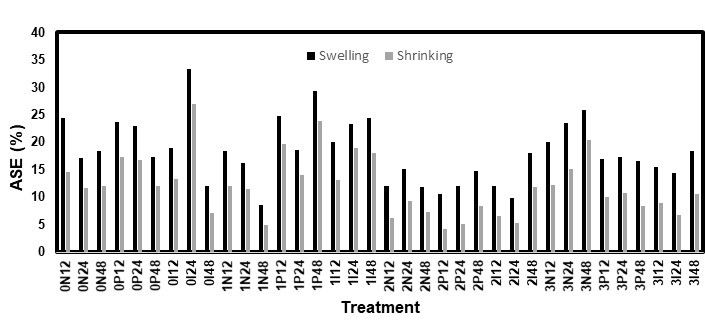
Fig. 10. ASE values for the immersion test on the eastern white pine
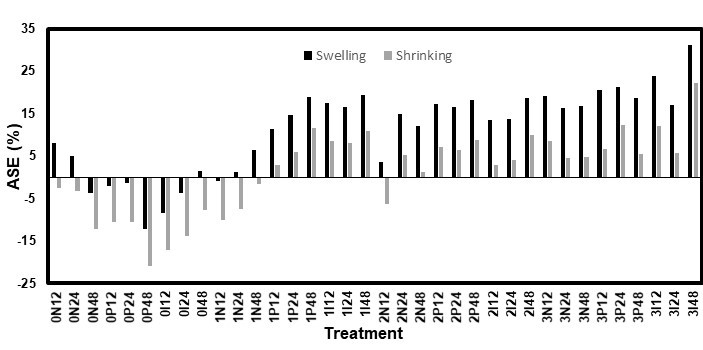
Fig. 11. ASE values for the high RH test on the white spruce
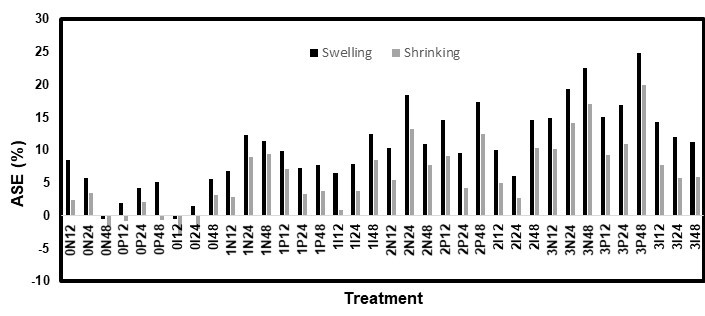
Fig. 12. ASE values for the immersion test on the white spruce
However, further investigation is needed because it is possible that other important aspects of wood impregnation, like the penetration depth and leaching resistance, would be affected by such a change.
The EMCs for the high RH test were statistically analysed and showed no significant difference (p < 0.01) for any factor. However, the white pine samples treated without AOs (0N, 0P, and 0I) showed fairly low EMC values (Fig. 13) compared with that of the untreated samples. This might have explained why their ASE values were so high when nothing was used to improve their dimensional stability.
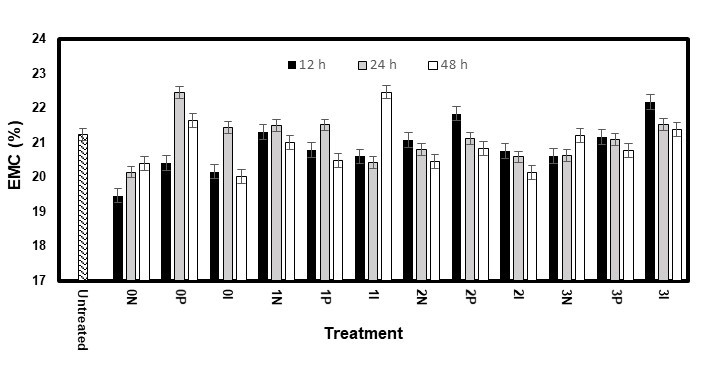
Fig. 13. EMC of the eastern white pine samples during the first cycle of the high RH test
However, the presence of AOs did not seem to have any impact on the EMC. Furthermore, the sorption isotherms (Fig. 15) show that, for both species, the samples treated with AOs adsorbed moisture at a higher rate and had a higher EMC. This was explained by the ability of AOs to form hydrogen bonds with water, where one AO molecule is able to interact with eight water molecules (Kocherbitov et al. 2007). This would add sites for many water molecules to be bound in the wood, thereby increasing its EMC. However, only water bound within the cell wall polymers has an impact on the swelling and shrinkage of wood (Rowell 2014). Consequently, while the EMC was similar between the untreated samples and samples treated with AOs, the amount of moisture affecting the dimensional changes was reduced.
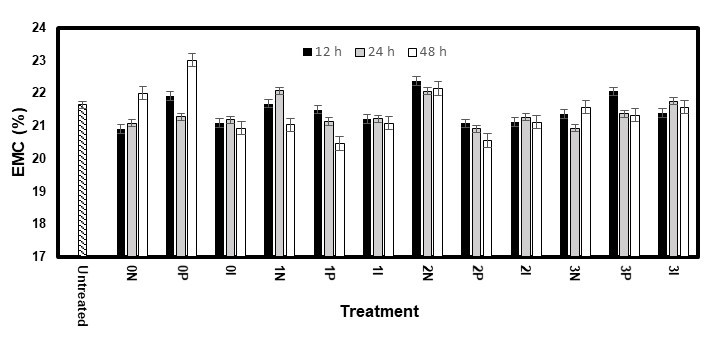
Fig. 14. EMC of the white spruce samples during the first cycle of the high RH test
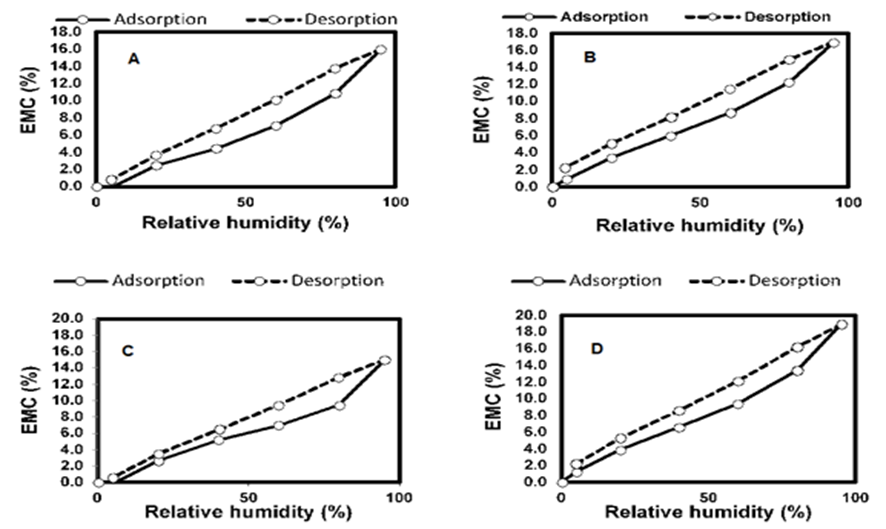
Fig. 15. Sorption isotherms for the (A) untreated eastern white pine, (B) treated eastern white pine, (C) untreated white spruce, and (D) treated white spruce
When doing three cycles of swelling and shrinking, the ASE values tended to decrease (Tables 4 and 5). This was particularly obvious when comparing the first and second cycles. However, the swelling and shrinkage of the treated samples were found to be nearly the same for each cycle in most cases, which revealed that the performances were not impacted.
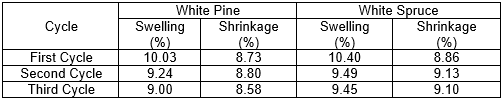
Table 4. Anti-swelling and Anti-shrinking Efficiencies for the Three Successive Cycles of the High RH Test on the White Pine
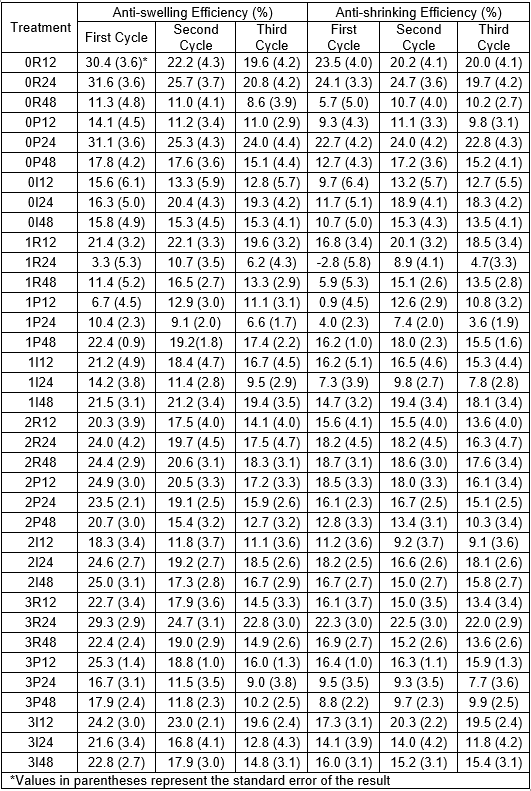
Table 5. Anti-swelling and Anti-shrinking Efficiencies for the Three Successive Cycles of the High RH Test on the White Spruce
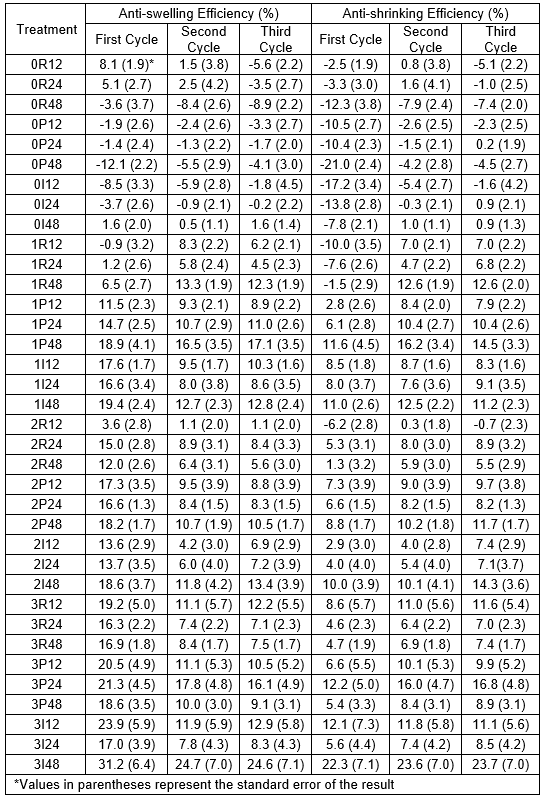
In contrast, the dimensional changes of the untreated samples were often smaller after each cycle, particularly the swelling (Table 6). This was caused by the shrinkage being considerably smaller than the previous swelling, which led to larger dry dimensions of the samples after each cycle. Thus, it was logical that if the samples did not completely shrink back to their original size, the following swelling was smaller. This phenomenon was more important in the radial axis than the tangential axis. Because the ASE compared the treated and untreated samples (see Eqs. 8 and 9), it was obvious that a decrease in the dimensional changes of the untreated samples led to lower ASE values, which gave the false impression that the performances of the treatment were worsening. However, as the dimensions of the treated samples were stable from cycle to cycle, it was concluded that this was not the case.
These performances did not match what can be achieved with acetylation, which can be as high as 70% for white pine as the wood is permanently swollen by the treatment (Navi and Heger 2005; Kocaefe et al. 2015). However, the density of the wood is much more affected, increasing by about 22% (88 kg/m3 for a piece of wood weighting 400 kg/m3) in the case of white pine. Furthermore, these results were fairly satisfying when bearing in mind that dimensional stability caused by the AOs was the not the purpose of this treatment, but a secondary advantage. The real purpose was to dissolve and carry the fungicides into the wood. Many impregnation products, like waxes and resins, only slow down the uptake of liquid water (Rowell and Banks 1985; Wang and Piao 2011). These will not reduce the dimensional changes or affect the uptake of air and moisture.
Table 6. Dimensional Changes of the Untreated Eastern White Pine and White Spruce
CONCLUSIONS
Impregnating wood with fungicides through diffusion by using an aqueous buffered amine oxides (AO) delivery system was possible and improved the service performances. All of the factors tested had an impact on some of the properties of the treated wood. The resistance to decay fungi was affected by both the AO and fungicide, while the dimensional stability was affected solely by the AO. A longer treatment time usually led to reduced performances.
The mass loss because of fungal activity did not seem to be affected by the AO or fungicide chosen, but both of them helped prevent degradation. While the fungicides alone did not seem soluble enough and AOs may not be potent enough by themselves at this concentration, they still showed some efficiency at preventing decay. Using the two classes of products together led to a strong synergistic effect and almost completely inhibited decay.
The contribution of the treatment to the dimensional stability of the treated white pine was not perfectly clear, as even the samples treated without AOs were substantially more stable. However, the samples treated with N,N-dimethyldodecylamine N-oxide (DHAO) showed significantly higher anti-swelling/ shrinking efficiency (ASE) values than those treated only with DDAO. In the case of white spruce, only the samples treated with AOs showed an improved dimensional stability, which increased with the DHAO concentration. The dimensional stability enhancement might have been because of a reduced moisture content (MC) in the cell wall polymers, which would be concealed by the hygroscopicity of the AOs. The dimensional changes of the samples did not increase after three full cycles of swelling and shrinking, which showed the good stability of the treatment. While the ASE values obtained were fairly low compared with those of some wood modification treatments, they were still rather satisfying considering that they were not the main aim of this treatment, but only a secondary effect of the use of AOs to carry fungicides into the wood.
While other treatments, such as acetylation and pressure impregnation of fungicides, offer comparable or better performances than this treatment, the impregnation of fungicides through diffusion with AOs offers interesting performances, while using water as the carrier and needing little equipment. It also introduced a low amount of water into the wood during impregnation, which limited the drying costs, and had a negligible impact on the weight of the wood. This makes it an interesting treatment overall. Because of the high hygroscopicity of the amine oxides, it would however be advised to avoid ground contact and to use a water-proof topcoat in order to limit water absorption.
ACKNOWLEDGMENTS
This work is part of the research program of the Natural Sciences and Engineering Research Council of Canada (NSERC) Industrial Research Chair on Eco-Construction in Wood (CIRCERB) through programs IRC (IRCPJ 461745-12) and CRD (RDCPJ 445200-12). The authors are also grateful to the industrial partners of the NSERC Industrial Chair on Eco-Responsible Wood Construction (CIRCERB).
REFERENCES CITED
Alberta Canada (2013). “White Spruce,” Alberta – Facts on Wood Series, https://www.albertacanada.com/files/albertacanada/AIS-BP_WhiteSpruce.pdf
AWPA E10-12 (2012). “Standard method of testing wood preservatives by laboratory soil-block cultures,” American Wood Protection Association, Birmingham, AL.
Blanchette, R. A., Nilsson, T., Daniel, G., and Abad, A. (1990). “Biological degradation of wood,” in: Archaeological Wood, R. M. Rowell and R. J. Barbour (eds.), American Chemical Society, Washington, D.C., pp. 141-174.
Carll, C. G., and Highley, T. L. (1999). “Decay of wood and wood-based products above ground in buildings,” Journal of Testing and Evaluation 27(2), 150-158.
De Vetter, L., Depraetere, G., Stevens, M., Janssen, C., and Van Acker, J. (2009). “Potential contribution of organosilicon compounds to reduced leaching of biocides in wood protection,” Annals of Forest Science 66(2), 209p1-209p7. DOI: 10.1051/forest/2008091
European Commission (EC) Directive 98/8/EC (2007). “Directive 98/8/EC concerning the placing of biocidal products on the market – Inclusion of active substances in Annex I to directive 98/8/EC – Propiconazole,” European Union, Finland.
European Commission (EC) Directive 98/8/EC (2008). “Directive 98/8/EC concerning the placing of biocidal products on the market – Inclusion of active substances in Annex I to directive 98/8/EC – IPBC,” European Union, Denmark.
Freeman, M. H. (2008). “Wood preservative formulation development and systems: Organic- and inorganic-based systems,” in: Development of Commercial Wood Preservatives, T. P. Schulz, H. Militz, M. H. Freeman, B. Goodell, and D. D. Nicholas (eds.), American Chemical Society, Washington, D.C., pp. 408-426.
Glass, S. V., and Zelinka, S. L. (2010). “Moisture relations and physical properties of wood,” in: Wood Handbook: Wood as an Engineering Material (FPL-GTR-190), R. J. Ross (ed.), U.S. Department of Agriculture Forest Products Laboratory, Madison, WI.
Goldstein, S., Meyerstein, D., and Czapski, G. (1993). “The Fenton reagents,” Free Radical Biology & Medicine 15(4), 435-445. DOI: 10.1016/0891-5849(93)90043-T
Goodell, B., Qian, Y., and Jellison, J. (2008). “Fungal decay of wood: Soft rot – brown rot – white rot,” in: Development of Commercial Wood Preservatives, T. P. Schulz, H. Militz, M. H. Freeman, B. Goodell, and D. D. Nicholas (eds.), American Chemical Society, Washington, D.C., pp. 9-31
Hill, C. A. S. (2006). Wood Modification: Chemical, Thermal and Other Processes, John Wiley & Sons, Chichester, UK.
Huckfeldt, T., and Schmidt, O. (2006). “Identification key for European strand-forming house-rot fungi,” Mycologist 20(2), 42-56. DOI: 10.1016/j.mycol.2006.03.012
ISO 4469 (1981). “Wood – Determination of radial and tangential shrinkage,” International Organization for Standardization, Geneva, Switzerland.
ISO 4859 (1982). “Wood – Determination of radial and tangential swelling,” International Organization for Standardization, Geneva, Switzerland.
Jiang, X. (2008). Amine Oxides for Use in Wood Protection III. Penetration Aids for Wood (IRG/WP 08-30461), International Research Group on Wood Protection, Istanbul, Turkey.
Juergensen, L., Busnarda, J., Caux, P.-Y., and Kent, R. (2000). “Fate, behavior, and aquatic toxicity of the fungicide IPBC in the Canadian environment,” Environ. Toxicol. 15(3), 201-213. DOI: 10.1002/1522-7278(2000)15:3<201::AID-TOX5>3.0.CO;2-Z
Kartal, S. N., Brischke, C., Rapp, A. O., and Imamura, Y. (2006). “Biological effectiveness of didecyl dimethyl ammonium tetrafluoroborate (DBF) against basidiomycetes following preconditioning in soil bed tests,” Wood Sci. Technol. 40(1), 63-71. DOI: 10.1007/s00226-005-0048-3
Kocaefe, D., Huang, X., and Kocaefe, Y. (2015). “Dimensional stabilization of wood,” Current Forestry Reports 1(3), 151-161. DOI: 10.1007/s40725-015-0017-5
Kocherbitov, V., Veryazov, V., and Söderman, O. (2007). “Hydration of trimethylamine-N-oxide and of dimethyldodecylamine-N-oxide: An ab initio study,” J. Mol. Struc.-THEOCHEM 808(1-3), 111-118. DOI: 10.1016/j.theochem.2006.12.043
Kukowski, K., Martinská, V., Sedgeman, C. A., Kuplic, P., Kozliak, E. I., Fisher, S., and Kubátová, A. (2017). “Fate of triazoles in softwood upon environmental exposure,” Chemosphere 184, 261-268. DOI: 10.1016/j.chemosphere.2017.05.168
Laks, P. E. (2008). “Wood preservative fungicides and the American wood preservers’ association use category system,” in: Development of Commercial Wood Preservatives, T. P. Schulz, H. Militz, M. H. Freeman, B. Goodell, and D. D. Nicholas (eds.), American Chemical Society, Washington, D.C., pp. 228-240.
Leightley, L. E. (2003). “Protection of wood using combinations of biocides,” in: Wood Deterioration and Preservation: Advances in Our Changing World, B. Goodell, D. D. Nicholas, and T. P. Schultz (eds.), American Chemical Society, Washington, D.C., pp. 390-398.
Lippke, B., Wilson, J., Perez-Garcia, J., Bowyer, J., and Meil, J. (2004). “CORRIM: Life-cycle environmental performance of renewable building materials,” Forest Products Journal 54(6), 7-19.
Mazela, B., and Perdoch, W. (2012). Stabilization of IPBC in Wood Through the Use of Organosilicon Compounds (IRG/WP 12-30597), International Research Group on Wood Protection, Stockholm, Sweden.
Meyer, L., and Brischke, C. (2015). “Fungal decay at different moisture levels of selected European-grown wood species,” International Biodeterioration and Biodegradation 103, 23-29. DOI: 10.1016/j.ibiod.2015.04.009
Morris, P. I., Grace, J. K., Yoshimura, T, and Tsunoda, K. (2014). “An international termite field test of wood treated with insecticides in a buffered amine oxide carrier,” Forest Products Journal 64(5), 156-160. DOI: 10.13073/FPJ-D-13-00095
Morris, L. (2017). “Woods to know – White pine,” (https://www.canadianwoodworking.com/get-more/white-pine), accessed November 1st 2018.
Nath, S. K. (2017). “Use wood – Combat climate change,” in: Wood is Good: Current Trends and Future Prospects in Wood Utilization, Pandey, K. K., Ramakantha, V., Chauhan, S. S., and Kumar, A. N. A. (eds.), Springer, Singapore. DOI: 10.1007/978-981-10-3115-1
Navi, P., and Heger, F. (2005). Comportement Thermo-hydromécanique du Bois: Applications Technologiques et dans les Structures [Thermo-hydromecanical Behavior of Wood: Technological and Structure Applications], Presses Polytechniques et Universitaires Romandes [Romand Polytechnic and University Presses], Lausanne, Switzerland.
Panshin, A. J., De Zeeuw, C., and Brown, H. P. (1964). Textbook of Wood Technology: Structure, Identification, Uses, and Properties of the Commercial Woods of the United States, McGraw-Hill, New York, NY.
Prabhu, V. S. (1999). “Synthesis of tertiary amine oxides,” U.S. Patent No. 5866718A.
Reinprecht, L. (2016). Wood Deterioration, Protection and Maintenance, John Wiley & Sons, Chichester, UK.
Ross, A. S. (2008). “Organic preservative systems for the protection of wood windows and doors,” in: Development of Commercial Wood Preservatives, T. P. Schulz, H. Militz, M. H. Freeman, B. Goodell, and D. D. Nicholas (eds.), American Chemical Society, Washington, D.C., pp. 470-479.
Ross, A. S., and Cutler, K. A. (2015). “Method of employing enhanced penetration of wood preservatives to protect wood and a related solution,” U.S. Patent No. 9125399B2.
Rowell, R. M. (2014). “Acetylation of wood – A review,” International Journal of Lignocellulosic Products 1(1), 1-27. DOI: 10.22069/IJLP.2014.1920
Rowell, R. M., and Banks, W. B. (1985). Water Repellency and Dimensional Stability of Wood (GTR-FPL-50), U.S. Department of Agriculture Forest Products Laboratory, Madison, WI.
Sanderson, H., Counts, J. L., Stanton, K. L., and Sedlak, R. I. (2006). “Exposure and prioritization – Human screening data and methods for high production volume chemicals in consumer products: Amine oxides a case study,” Risk Analysis 26(6), 1637-1657. DOI: 10.1111/j1539-6924.2006.00829.x
Sanderson, H., Tabazarwa, C., Greggs, W., Versteeg, D. J., Kasai, Y., Stanton, K., and Sedlak, R. I. (2009). “High production volume chemical amine oxide [C8–C20] category environmental risk assessment,” Risk Analysis 29(6), 857- 867. DOI: 10.1111/j.1539-6924.2009.01208.x
Schiopu, N., and Tiruta-Barna, L. (2012). “6 – Wood preservatives,” in: Toxicity of Building Materials, F. Pacheco-Torgal, S. Jalali, and A. Fucic (eds.), Woodhead Publishing, Cambridge, UK, pp. 138-165.
Schultz, T. P., and Nicholas, D. D. (2003). “A brief overview of non-arsenical wood preservative systems,” in: Wood Deterioration and Preservation: Advances in Our Changing World, B. Goodell, D. D. Nicholas, and T. P. Schultz (eds.), American Chemical Society, Washington, D.C., pp. 420-432.
Siau, J. F. (1995). Wood: Influence of Moisture on Physical Properties, Department of Wood Science and Forest Product Virginia Polytechnic Institute and State University, Blacksburg, VA.
Singh, S. K., Bajpai, M., and Tyagi, V. K. (2006). “Amine oxides: A review,” J. Oleo Sci. 55(3), 99-119. DOI: 10.5650/jos.55.99
Stevanovic, T. (2016). “Chemical composition and properties of wood,” in: Lignocellulosic Fibers and Wood Handbook: Renewable Materials for Today’s Environment, N. Belgacem and A. Pizzi (eds.), Scrivener Publishing, Beverly, MA.
Stienen, T., Schimidt, O., and Huckfeldt, T. (2014). “Wood decay by indoor Basidiomycetes at different moisture and temperature,” Holzforschung 68(1), 9-15. DOI: 10.1515/hf-2013-0065
Terzi, E., Kartal, S. N., White, R. H., Shinoda, K., and Imamura, Y. (2011). “Fire performance and decay resistance of solid wood and plywood treated with quaternary ammonia compounds and common fire retardants,” Eur. J. Wood Wood Prod. 69(1), 41-51. DOI: 10.1007/s00107-009-0395-0
Tseng, C.-I., and Walker, L. E. (2000). “Azole/amine oxide wood preservatives,” W.O. Patent No. 2000071314A1.
Tseng, C.-I., Walker, L. E., and Kempinska, C. (2002). “Compositions comprising a boron compound and an amine oxide,” W.O. Patent No. 2002001958A2.
Tudor, D., Robinson, S. C., and Copper, P. A. (2012). “The influence of moisture content variation on fungal pigment formation in spalted wood,” AMB Express 2(69), 1-10. DOI: 10.1186/2191-0855-2-69
Wallace, D. F., Cook, S. R., and Dickinson, D. J. (2008). “The role of non-decay microorganisms in the degradation of organic wood preservatives,” in: Development of Commercial Wood Preservatives, T. P. Schulz, H. Militz, M. H. Freeman, B. Goodell, and D. D. Nichols (eds.), American Chemical Society, Washington, D.C., pp. 312-322.
Walker, L. E., and Shen, S. (2002). “Methods for enhancing penetration of wood preservatives,” U.S. Patent No. 20020061366A1.
Wang, C., and Piao, C. (2011). “From hydrophilicity to hydrophobicity: A critical review – Part II: Hydrophobic conversion,” Wood Fiber Sci. 43(1), 41-56.
Ward, H. A., and Scott, C. (2009). “Method of protecting wood through enhanced penetration of wood preservatives and a related solution,” U.S Patent No. 20090143334
Yuan, J., Hu, Y., Li, L., and Cheng, F. (2013). “The mechanical strength change of wood modified with DMDHEU,” BioResources 8(1), 1076-1088. DOI: 10.15376/biores.8.1.1076-1088
Article submitted: August 17, 2018; Peer review completed: October 20, 2018; Revised version received: November 5, 2018; Accepted: November 7, 2018; Published: November 16, 2018.
DOI: 10.15376/biores.14.1.264-288
