Abstract
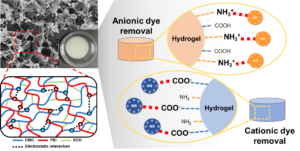
A carboxymethyl cellulose (CMC)/polyethyleneimine (PEI) hybrid hydrogel was successfully prepared under green processing conditions. The CMC and PEI formed a polyelectrolyte complex, and the three-dimensional (3D) network structure was hardened by chemical crosslinking, resulting in suitable hydrogel properties. In the prepared CMC/PEI polyelectrolyte hydrogel, the surface charge was easily switched according to the pH change, and the resulting swelling and contraction was reversible. The pH sensitivity of the CMC/PEI hydrogel was effective in removing ionic dye contaminants, and as a result, it showed an excellent removal capacity of 319 mg/g for anionic acid orange (AO) and 129 mg/g for cationic methylene blue (MB). In addition, the CMC/PEI polyelectrolyte hydrogel maintained structural stability despite repeated changes in surface charge characteristics and shrinkage-swelling due to repeated pH conversion. As a result, in the reuse process through repeated adsorption and desorption, it showed an excellent reuse efficiency of more than 93% even after 10 reuse cycles.
Download PDF
Full Article
pH-responsive Hydrogels of Carboxymethyl Cellulose and Polyethyleneimine for Efficient Removal of Ionic Dye Molecules
Minjung Jung,a Jungkyu Kim,a Seungoh Jung,a YunJin Kim,a Junsik Bang,a Hwanmyeong Yeo,a,b In-Gyu Choi,a,b and Hyo Won Kwak a,b,*
A carboxymethyl cellulose (CMC)/polyethyleneimine (PEI) hybrid hydrogel was successfully prepared under green processing conditions. The CMC and PEI formed a polyelectrolyte complex, and the three-dimensional (3D) network structure was hardened by chemical crosslinking, resulting in suitable hydrogel properties. In the prepared CMC/PEI polyelectrolyte hydrogel, the surface charge was easily switched according to the pH change, and the resulting swelling and contraction was reversible. The pH sensitivity of the CMC/PEI hydrogel was effective in removing ionic dye contaminants, and as a result, it showed an excellent removal capacity of 319 mg/g for anionic acid orange (AO) and 129 mg/g for cationic methylene blue (MB). In addition, the CMC/PEI polyelectrolyte hydrogel maintained structural stability despite repeated changes in surface charge characteristics and shrinkage-swelling due to repeated pH conversion. As a result, in the reuse process through repeated adsorption and desorption, it showed an excellent reuse efficiency of more than 93% even after 10 reuse cycles.
DOI: 10.15376/biores.17.4.5785-5802
Keywords: Carboxymethyl cellulose; CMC; Polyethyleneimine; PEI; Polyelectrolyte; Hydrogel; Dye removal
Contact information: a: Department of Agriculture, Forestry and Bioresources, College of Agriculture and Life Sciences, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul 08826, Republic of Korea; b: Research Institute of Agriculture and Life Sciences, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul 08826, Republic of Korea; *Corresponding author: bk0502@snu.ac.kr
GRAPHICAL ABSTRACT

INTRODUCTION
A dye molecule is a specific compound used as a substance that binds to a selected material to give it a specific color (Faria et al. 2004; Chang et al. 2020). Today, dye molecules are widely used not only in the traditional textile and paper industry but also in other industries, including materials, and the amount used is 50 million tons per year (Hameed 2009; Salleh et al. 2011). However, in the general dyeing process, a large amount of unreacted dyes that do not participate in the color expression of the material are generated. In addition, waste dyes whose characteristic colors are damaged due to changes in the physicochemical properties of dye molecules during the dyeing process are continuously generated (Faria et al. 2004). In general, these unreacted and decomposed dye molecules also exist in a dissolved form in water, and these contaminants are collectively called dye wastewater (Wojnárovits and Takács 2008; Gupta and Suhas 2009).
To purify dye wastewater, various environmental restoration processes have been proposed and used in practice based on physics (Li et al. 2017b), chemistry (Natarajan et al. 2018), and biology (Aksu 2005). Physically, sedimentation, screening, filtration, and membrane separation processes have been tried as aerobic or anaerobic processes, but the slow purification rate has been a disadvantage. Chemically, there are chemical precipitation, agglomeration, ion exchange, solvent substitution, and adsorption processes, which generally boast high purification efficiency and faster speed than physico/biological processes (Roa et al. 2021). In these chemical processes, adsorption is the most popular water treatment process and has advantages such as simple operation, high process efficiency, reusability of adsorbent materials, and non-generation of secondary pollutants (Kim et al. 2021). In general, activated carbon has been used most widely in the practical adsorption process thus far, but the use of petroleum-based carbon precursors and the high energy required during the carbonization process inevitably cause an increase in economic problems (Luo and Zhang 2009; Chong and Tam 2020). In addition, the use of unformulated activated carbon powder makes it difficult to recover the adsorbent from contaminated water after the process, so there is also difficulty in the regeneration and reuse process (Mokhtar et al. 2020). Therefore, recently, there has been an explosive increase in interest in the development of biopolymer-based high-performance adsorption materials away from such activated carbon-based adsorption materials. In this regard, research on the manufacturing process of micro/nano particles, fibers, macro beads, films, and hydro or aerogels to have structural stability in aqueous environments through polymer processing has also been actively conducted (Dassanayake et al. 2021).
A hydrogel is a hydrophilic polymer in which individual polymer chains form a specific three-dimensional (3D) dynamic water-swollen network structure to contain a large amount of water molecules while maintaining the structure (Mahinroosta et al. 2018; Van Tran et al. 2018; Kong et al. 2019). The gelation phenomenon, which means the formation of a 3D network structure of a polymer, can occur in various ways through the physical and chemical crosslinking process of natural and synthetic polymers (Mohammadzadeh Pakdel and Peighambardoust 2018; Kordjazi et al. 2020). In general, because these hydrogels are fabricated based on a solution process, they can be mass-produced and do not require a separate coagulation bath, unlike particle production by wet spinning and dripping processes (Ahmed 2015). In addition, the hydrophilicity of hydrogels has the advantage of being able to easily prevent the diffusion of contaminants in the water treatment process that occurs in other types of solid adsorption materials.
Recently, there has been increasing interest in smart hydrogels that have stimuli sensitivity to various environments, such as temperature, light, electric field, pressure, CO2, and pH, by combining the functionalities of polymers that serve as a skeleton beyond the simple functional properties of hydrogels (Zhu et al. 2018; Liu et al. 2020; Rungsima et al. 2020). In particular, in water treatment processes, pH-sensitive hydrogels can not only provide convenience for desorption and reuse processes but also they can induce high-performance adsorption processes based on strong interactions, including electrostatic attraction with target contaminants, by easily converting the surface charge properties of the adsorbent (Zhu et al. 2018; Monir et al. 2019).
In this study, a cellulose-based pH-sensitive smart hydrogel was prepared for the efficient production of ionic dyes. Here, the pH sensitivity of the hydrogel means a fast surface charge conversion in a specific pH environment. Through this, it is possible to maximize the adsorption efficiency of the ionic dye having an opposite charge to the hydrogel surface. At the same time, it is possible to provide a strong desorption driving force for the dye molecules having the same charge as the hydrogel surface. To this end, a polyelectrolyte complex was prepared using carboxymethyl cellulose (CMC), a representative anionic cellulose derivative, and polyethyleneimine (PEI), which is rich in cationic amine groups (Hossieni-Aghdam et al. 2018; Zhang et al. 2018). CMC is a cellulose derivative in which the hydroxyl group of cellulose is substituted with a carboxymethyl group. Unlike cellulose, it is easily soluble in water and can be used to manufacture hydrogels. Meanwhile, PEI is a representative cationic synthetic polymer most often used in the surface modification process to improve the adsorption capacity of the existing biopolymer-based adsorbent. The gelation behavior according to the ratio of CMC/PEI, the concentration of the crosslinking agent and the water stability of the prepared hydrogel was investigated. The pH sensitivity of the prepared smart hydrogel was confirmed by changing the zeta potential and swelling degree according to each pH environment. Ultimately, the ionic dye removal properties of the prepared smart hydrogel were evaluated by selecting cationic methylene blue (MB) and anionic acid orange (AO) as model dye molecules and performing adsorption experiments.
EXPERIMENTAL
Materials
Carboxymethyl cellulose sodium salt (CMC, Extra Pure) and sodium hydroxide (NaOH) were purchased from Junsei Chemical (Tokyo, Japan). Polyethyleneimine (PEI, branched, Mw ~800 Daltons), epichlorohydrin (ECH, ≥ 99%), methylene blue (MB), and acid orange A (AO) were purchased from Sigma–Aldrich (Yongin, Korea). Hydrochloric acid (HCl, 36.5 to 38.0%) was purchased from Alfa Aesar (Incheon, Korea).
Preparation of the CMC/PEI Hydrogel
First, to determine the optimal CMC/PEI blend ratio for hydrogel preparation, CMC 2 wt% and PEI 2 wt% solutions were mixed in a 20 mL vial at a constant ratio (100:0, 75:25, 50:50, 25:75, or 0:0). Next, 62.5 µL of HCl was added and mixed. Various volumes of ECH solution were used as a crosslinking agent, and hydrogel formation was confirmed depending on the amount of the crosslinking agent (0%, 0.05%, 0.10%, 0.15%, or 0.20%). After all the solvents were mixed, the reaction was completed in an oven at 65 °C for 1 h. After the reaction finished, the hydrogel was washed with distilled water for one day to remove residual acidic and/or unreacted substances.
Characterization of the CMC/PEI Hydrogel
Optical images of CMC/PEI hydrogels were obtained by digital photography (iPhone 12, Apple Inc., Los Altos, CA, USA). The morphologies of the CMC/PEI hydrogel were characterized using field-emission scanning electron microscopy (FE-SEM; SUPRA, Carl Zeiss, Berlin, Germany). The ImageJ software was adopted to measure the pore size distribution of prepared CMC/PEI hydrogels (ImageJ; Wayne Rasband, Bethesda, USA). The chemical structures of the hydrogels were examined by Fourier transform infrared spectroscopy (FTIR; Nicolet Summit FTIR Spectrometer, Thermo Fisher Scientific, Waltham, MA, USA). Attenuated total reflection (ATR) mode was used for 64 scans with 4 cm−1 resolution to obtain a spectral range of 4000 to 700 cm−1. The chemical bonding and elemental contents of the CMC/PEI hydrogels were analyzed with X-ray photoelectron spectroscopy (XPS; Axis Supra, Kratos, UK) and an elemental analyzer (Vision-EA, Isoprime, UK). Using a Zetasizer (Litesizer 500, Malvern, UK), the zeta potential of CMC, PEI, and CMC/PEI hydrogels were investigated according to the pH of the solution.
Swelling Property of the Hydrogel
To measure the swelling ratio, the hydrogels were immersed in 0.1 M HCl, distilled water, and 0.1 M NaOH solution for 1 h. After immersion, the swelling ratio (SR) was calculated by Eq. 1,
SR (g/g) = (Wi – W0) / W0 (1)
where Wi is the weight (g) of the sample include all of the water in the system after immersion and W0 is the dry weight (g) of the sample.
To measure the swelling reversibility, the hydrogels were sequentially immersed in different pH solutions at 1 h intervals (pH 3, 7, and 10). After the swelling behavior of the hydrogel reached a steady state, the swelling ratio was calculated by Eq. 1.
Dye Adsorption Studies
The MB and AO dye removal experiments were conducted through batch adsorption experiments. The adsorption experiment was performed via addition of 50 mg of CMC/PEI hydrogel into a 70 mL vial, and 50 mL of MB and AO solutions with various concentrations (50 to 300 mg/L) were added. Samples were taken at regular time intervals, and the concentration of each dye molecule was measured using an ultraviolet–visible (UV–vis) spectrometer (Optizen Pop, Mecasys, Korea) (MB: 665 nm, AO: 486 nm). The MB and AO removal efficiency (%, qe) were calculated using Eq. 2,
qe = (C0 – Ce) / C0 ×100 (2)
where C0 is the initial concentration (mg/L) and Ce is the equilibrium concentration of the dye solution (mg/L).
To analyze the effect of solution pH on the dye adsorption efficiency of the CMC/PEI hydrogels, adsorption experiments were performed for various pH (3, 7, and 10) conditions, which were controlled using 0.1 M HCl and 0.1 M NaOH solutions.
Adsorption Isotherm Studies
The relationship between CMC/PEI hydrogels and dye molecules was described with Langmuir and Freundlich linear isotherm models, which are described by Eqs. 3 and 4,
Ce / qe =1 / (KL × qm) + Ce / qm (3)
lnqe = 1/n × lnCe + lnKF (4)
where qe is the adsorption capacity of dye molecules at equilibrium (mg/g), qm is the adsorption capacity of dye molecules at maximum (mg/g), Ce is the equilibrium concentration of dye solution (mg/L), KL is the Langmuir adsorption constant (L/mg), and KF is the Freundlich capacity of the adsorbent (mg/g). 1/n is the heterogeneity factor of the Freundlich model (mg/L).
Desorption and Reuse Experiment
Various desorption agents were tested to determine the optimal desorption and regeneration conditions (distilled water, 0.1 M HCl, and 0.1 M NaOH). Prior to the desorption experiments, CMC/PEI hydrogels were stirred in a 200 mg/L MB and AO aqueous solution (150 rpm, room temperature, for 6 h). After the adsorption process, the dye-adsorbed hydrogel was recovered and transferred to a 0.1 M HCl solution for MB and a 0.1 M NaOH solution for AO, followed by a desorption process through stirring. To calculate the desorption efficiency, the concentration of the desorbed dye was analyzed via UV-vis analysis. To study the recycling efficiency, 10 cycles of adsorption-desorption experiments were performed. Adsorption and desorption experiments were performed during a contact time of 6 h and a stirring speed of 180 rpm, respectively. After each experimental cycle, the CMC/PEI hydrogel was washed 3 times with distilled water to ensure conditioning for the next adsorption-desorption cycle.
RESULTS AND DISCUSSION
The formation of a polyelectrolyte complex is an effective way to increase the interaction of polymer chains in a physically given space when forming a hydrogel. In general, when solutions of a pair of polymer materials, one having negative ionic charges and the other having positive charges are combined in the solution phase, a polyelectrolyte complex is formed. Specifically, the distance between each polymer chain decreases due to electrostatic attraction, and ultimately, an interpenetrating structure is formed. In addition, by adding a suitable crosslinking agent to the already formed polymer electrolyte composite, a hydrogel with improved structural stability can be fabricated (Liew and Ramesh 2015; Li et al. 2017a). Figure 1(a). shows the results of the CMC/PEI solution gelation experiment according to the CMC/PEI ratio and the concentration of ECH used as a crosslinking agent. For the separate CMC and PEI solutions, the opacification phenomenon does not occur because polyelectrolyte complexes are not formed. In addition, the gelation phenomenon does not occur despite the increase in the crosslinking agent concentration. In contrast, when CMC and PEI were both present, opacification occurred regardless of the mixing ratio, which is clear evidence of the formation of a polyelectrolyte complex. As the concentration of the crosslinking agent increased, a three-dimensional hydrogel having a dense and stable network structure by covalent interchain bonding was formed (Wang et al. 2021). This means that chemical crosslinking is essential for the gelation of the CMC/PEI polyelectrolyte.
Hydrogels for use in water treatment processes must ensure structural stability against process conditions, particularly external physical stimuli, such as stirring or agitation (Ren et al. 2022). Figure 1(b). shows the shape of the CMC/PEI hydrogel that was gelled, which was supported in distilled water and stirred at 180 rpm for 30 min. As shown in Fig. 1(b), in all hydrogels except CMC:PEI = 50:50, 0.2% ECH, the hydrogel structure collapsed due to physical stimulation. The mechanical properties of the CMC/PEI hydrogel were affected by both the degree of polyelectrolyte formation and the chemical covalent bond. Therefore, considering these results, CMC:PEI = 50:50, 0.2% ECH was selected as the optimal processing condition, a hydrogel was prepared, and additional physicochemical characterization of the hydrogel and dye removal experiments were conducted.
To analyze the chemical properties of the prepared CMC/PEI polyelectrolyte hydrogel, FTIR and XPS analyses were performed, and the results are shown in Figure. 2(a). The PEI exhibited a broad absorption band within the 3500 to 3200 cm-1 region, which can be attributed to the stretching vibrations of N-H. In addition, the characteristics of the PEI chain were revealed through the presence of -CH2 and -CH stretching vibrations and N-H bending characteristic peaks at 2940, 2810, and 1450 cm-1, respectively (Song et al. 2019). In the FTIR spectrum of CMC, a broad peak of 3500 to 3000 cm-1 was also observed, which is attributed to the O-H stretching vibration (Sun et al. 2014; Guo et al. 2019; Kim et al. 2022). Additionally, two strong absorption bands were observed at 1600 and 1420 cm-1, attributed to the asymmetric and symmetric stretching of COO–, respectively (Yang et al. 2011; Choi et al. 2018).
Fig. 1. Optical images of solution gelation experiment according to different CMC/PEI and ECH ratio (a) Gelation behavior according to the CMC/PEI ratio: and crosslinker concentration; (b) Stability evaluation result of the CMC/PEI hydrogel in an aquatic environment
Meanwhile, for the prepared CMC/PEI polyelectrolyte hydrogel, the peak in the 3500 to 3000 cm-1 region was further broadened because the N-H and O-H groups coexist and form numerous hydrogen bonds. Meanwhile, the intensity of peaks corresponding to the most characteristic functional groups of PEI and CMC, N-H, and COO–, decreased. This means that both the amine of PEI and the carboxyl group of CMC participate in the covalent bond formation of the crosslinking agent ECH. The formation of such a covalent bond can also be confirmed through the absence of a general peak of the epoxy group at 1250 or 910 cm-1 (Yang et al. 2011). In general, the easiest way to prove PEI surface modification of polysaccharide-based polymer materials is to detect nitrogen atoms present in PEI. To confirm the PEI present in the CMC/PEI polyelectrolyte hydrogel, C, N, and H elemental analysis using the dynamic flash combustion method was performed, and the results are shown in Fig. 2(b). Because this method cannot measure the oxygen content, CMC consists of 87% carbon and 13% hydrogen. However, the CMC/PEI polyelectrolyte hydrogel showed a nitrogen content of 9%, confirming the successful preparation of the CMC/PEI polyelectrolyte hydrogel. The existence of these nitrogen atoms can also be confirmed through CMC/PEI hydrogel surface analysis through XPS. Figure 2(c) shows the results of the XPS full scan survey, and a signal in the 406 to 394 eV range corresponding to nitrogen can be clearly seen. This was not present in CMC, and it was observed only in CMC/PEI. The functional groups of the CMC/PEI polyelectrolyte hydrogel surface were analyzed through high-resolution C 1s and N 1s spectral interpretation, and the results are shown in Fig. 2(d). It can be seen from the C 1s spectra that the CMC/PEI polyelectrolyte hydrogel is composed of C-C/C-H, C-O/C-N, C=O, and O-C-O groups. Among them, the peak at 285.7 eV corresponding to C-O and C-N is due to the characteristic binding present in the main chains of cellulose and PEI. In addition, the C=O bond at 286.7 eV is due to the carboxyl group of CMC, and through this, the generation of COO– upon pH change is expected. In addition, according to the N 1s spectra, the surface of the CMC/PEI polyelectrolyte hydrogel is composed of amines and imines. In particular, for NH2, which is a primary amine, the ionic species NH3+ is formed by the pH change, thereby exhibiting a cationic surface charge (Choi et al. 2018).
Fig. 2. Chemical properties of the CMC/PEI hydrogel: (a) FTIR spectra; (b) Elemental composition of CMC and CMC/PEI hydrogel; (c) Full scan XPS spectra of CMC and CMC/PEI hydrogel; (d) high-resolution C 1s and N 1s spectra of CMC/PEI hydrogel
To investigate the morphology of the prepared CMC/PEI polyelectrolyte hydrogel, freeze-drying was performed, followed by FE-SEM analysis, and the obtained images are shown in Figs. 3(a, b). The pore size distribution of the CMC/PEI hydrogel is shown in Fig. 3(c). The freeze-dried CMC/PEI polyelectrolyte hydrogel exhibited a 3D network microstructure with macroscale pores ranging from 2 to 10 μm. The high-resolution image shows that open pores and closed pores coexisted. In general, for a hydrogel polymer formed by a single mechanism, such as physical entanglement of polymer chains or formation of a network structure by chemical covalent bonding, it will have a uniform pore structure (Nele et al. 2020). However, in the case of the CMC/PEI hydrogel, pores of a wider range were expressed, which can be attributed to a 3D network structure simultaneously involving the formation of a polyelectrolyte complex by electrostatic attraction and covalent chemical bonding by ECH (Yang et al. 2011). In more detail, because the hydroxyl group of CMC and the amine group of PEI can both become reaction sites with ECH, the formation of such a chemical network structure can help the formation of the structure of the CMC/PEI polyelectrolyte. A formation mechanism of the CMC/PEI hydrogel is proposed in Fig. 3(d).
Fig. 3. (a, b) FE-SEM images of the CMC/PEI hydrogel; (c) Pore size distribution of the CMC/PEI hydrogel; (d) Formation mechanism of the CMC/PEI hydrogel
The pH response and durability of the prepared CMC/PEI polyelectrolyte hydrogel were evaluated through zeta potential, swelling, and continuous pH conversion experiments in Fig. 4. In the zeta potential measurement results shown in Fig. 4(a)., CMC, the raw material, showed a negative charge, and PEI showed a positive charge, respectively, at the pH values of 3, 5, 7, 10, and 12. This is because the carboxy group of CMC and the amine group of PEI can be charged with COO– and NH3+, respectively, by deprotonation and protonation behavior depending on the pH environment (Song et al. 2019). In contrast, the CMC/PEI polyelectrolyte hydrogel showed a positive charge of +32.8 mV at pH 3, which is an acidic condition. Meanwhile, at pH 7, a neutral condition, +1.3 mV was exhibited, converging on the surface net charge of the hydrogel. In addition, at pH 10, which is a basic condition, a negative value of -28.9 mV was shown, confirming the anionic properties of the hydrogel surface. The pH sensitivity of the CMC/PEI polyelectrolyte is because, in a neutral condition, a balance of the net charge of the carboxy group and the amine group is maintained, but when placed in an acidic or basic environment, NH3+ and COO– are predominantly charged, respectively (Song et al. 2019). Figure 4(b). shows the pH-responsive swelling behavior of the CMC/PEI polyelectrolyte hydrogels at various pH values (distilled water, 0.1 M HCl, 0.1 M NaOH). The swelling ratio of CMC/PEI was high in 0.1 M HCl (17.7 g/g) and 0.1 M NaOH (25.9 g/g), while it was low at distilled water conditions (8.6 g/g). This pH-specific swelling behavior is possible because the carboxylic acid and amine groups in the hydrogel structure have different surface charges, depending on the pH environment (Song et al. 2019). When the pH is lower than 7, the carboxyl groups in the CMC units in the CMC/PEI polyelectrolyte are protonated in an acidic environment, thus weakening the electrostatic interaction. However, the amine group included in PEI is protonated to increase the repulsive force between the polymer chains, and this repulsive force dominates the swelling process, expanding the intramolecular space of the hydrogel and allowing more water molecules to penetrate the hydrogel. In contrast, in an environment close to pH 7, both the protonation tendency of the amine group and the deprotonation tendency of the carboxy group decrease so that the average net charge is close to neutral. As the electrostatic repulsive force between each CMC, PEI, and CMC/PEI polymer chain is minimized, it has a dense structure of hydrogel network structure and thus has a low swelling degree. Finally, under basic conditions with a pH value greater than pH 7, the amine groups of PEI mainly are present in the protonated form NH2, and the CMC carboxylic acid groups become negatively charged after deprotonation. At this time, the repulsive force between negative charges (COO–) begins to play a major role in expanding the hydrogel network structure, and as a result, the hydrogel can absorb more water molecules and increase the swelling rate. The expansion and water absorption characteristics of such a pH-sensitive polymer network structure are clearly shown not only in the microenvironment but also in the change in the bulk structure (Song et al. 2019). Figure 4(c). shows the change in the volume expansion degree and images of the CMC/PEI polyelectrolyte under each pH condition. As shown in the Fig. 4(d)., the hydrogel exhibited the smallest volume under the distilled water condition because the dense polyelectrolyte-based 3D network structure is expressed. In contrast, under the conditions of 0.1 M HCl and 0.1 M NaOH, volume expansion of the hydrogel clearly occurred and tended to coincide with the swelling degree value. This volume expansion is a phenomenon caused by the simultaneous expression of water absorption and distance expansion of the polymer 3D network structure.
In terms of the desorption and reuse of contaminants in the water treatment process using the actual stimulus-sensitive hydrogel, it is important to ensure the durability of the response to the constant stimulation of the pH-sensitive hydrogel (Abdel-Halim and Al-Deyab 2011). Considering the pH environment of dye-contaminated wastewater, to study the swelling reversibility of the CMC/PEI polyelectrolyte hydrogel, sequential immersion at pH 3, 7, and 10 was repeated to observe the change in swelling degree according to pH dependence. As shown in Fig. 4(e), pH 7 of the solution showed the lowest degree of swelling, whereas pH 3 and 10 showed excellent swelling behavior of 8 g/g or more. In addition, the degree of swelling continuously and sensitively responded to changes in the pH environment. This means that the carboxylic acid and amine groups of CMC/PEI can be rapidly protonated and deprotonated, and the expansion and contraction of the 3D network structure can be reversible. As a result, it was confirmed that the water absorption behavior of the CMC/PEI hydrogel can also repeatedly maintain pH reactivity.
Fig. 4. (a) Zeta potential results of CMC, PEI, and CMC/PEI hydrogel; (b) Swelling ratio, (c) Swelling volume comparison, and (d) Optical image of hydrogels after swelling in 0.1 M HCl, distilled water, 0.1 M NaOH solution; (e) Swelling reversibility of CMC/PEI hydrogel
To confirm the correlation between the pH-sensitive properties of the CMC/PEI hydrogel and the actual dye removal properties, MB and AO were selected as representative cationic and anionic model dyes, respectively, and the adsorption efficiency for each pH was evaluated (Ghariani et al. 2019; Naresh Yadav et al. 2021). Figure 5 shows the results of the adsorption efficiency for each dye of the CMC/PEI hydrogel at pH 3, 7, and 10. The AO adsorption showed an excellent adsorption efficiency of 99% at pH 3, which is an acidic condition, and the adsorption efficiency tended to decrease as the pH increased to neutrality and basicity. Conversely, cationic MB showed the highest adsorption efficiency of 65% at a basic pH of 10, and the efficiency gradually decreased as the pH decreased. The reason that the adsorption efficiency of the ionic dye varies according to the pH conditions is that the charge characteristics of the hydrogel surface are converted according to the pH conditions. That is, under acidic conditions, cationization by amines is dominant. By contrast under basic conditions, anionization by carboxylic acid groups prevails. Accordingly, the adsorption efficiency of each ionic dye is greatly affected. The reason that the adsorption efficiencies of anionic AO and cationic MB differed under each optimal condition can be attributed to the fact that the amount of amine groups in CMC/PEI was somewhat higher than that of carboxylic acids.
Fig. 5. Effects of pH on the adsorption of MB and AO dyes onto the CMC/PEI hydrogel (C0 = 100 mg/L; contact time: 6 h; mass of CMC/PEI hydrogel = 200 mg; temperature: room temperature)
Figure 6 shows the change in the adsorption capacity of the CMC/PEI polyelectrolyte hydrogel according to the concentration and stirring time of the dye to investigate the adsorption performance of each ionic dye and its mechanism. As shown in the Figs. 6(a,d), regardless of the ionic characteristics of the dye molecules, the adsorption capacity of each dye molecule increased as the initial dye concentration increased. This is because as the concentration of the adsorbate increases, the interaction between the adsorbate and the adsorbent surface could be favored. However, the further increment of increase decreased due to the equilibrium and saturation of the surface adsorption process. When the concentration increases above a certain point, the increase in the adsorption capacity decreases because equilibrium occurs due to the saturation of the surface adsorption site. AO showed an excellent adsorption capacity of 60 to 180 mg/g in the concentration range of 100 to 300 mg/L, whereas MB showed a relatively low adsorption capacity of 16 to 92 mg/g in the range of 20 to 150 mg/L. This is consistent with the previous results of dye adsorption efficiency according to pH, and it is because the CMC/PEI polyelectrolyte complex has an abundance of amine groups that can be relatively protonated (Song et al. 2019). In contrast, the relatively low MB removal efficiency was attributed to the presence of fewer carboxylic acid groups, the main adsorption sites of cationic MB, than the amine groups of PEI in the CMC/PEI polyelectrolyte complex. This has an influence on the low DS (~1.0) of CMC used for hydrogel preparation.
Fig. 6. (a) Equilibrium adsorption capacity, (b) Langmuir linear plot, and (c) Freundlich linear plot of AO adsorption; (d) Equilibrium adsorption capacity, (e) Langmuir linear plot, and (f) Freundlich linear plot of MB adsorption
The fitting of the experimental results using the isothermal adsorption model and the interpretation of the results provide useful information for the relative evaluation of the adsorption performance of the prepared adsorption material and for understanding its adsorption mechanism. The linear fitting process was performed by applying the Langmuir and Freundlich models, which are representative isothermal adsorption models, to the results of the change in adsorption capacity according to the concentration, and the results are shown in Figs. 6(b, c, e, and f). (Kwak et al. 2015; Duman et al. 2020).
As shown in Tables 1 and 2, both AO and MB showed higher R2 values for the Langmuir model than for the Freundlich model.
However, the Freundlich model also showed a high R2 value of 0.9 or more. The qm value in the Langmuir model refers to the maximum monolayer adsorption capacity and is a factor mainly used to evaluate the capacity of the manufactured adsorbent material (Zhai et al. 2019; Beh et al. 2020). The qm value of AO was 319 mg/g, which means that the CMC/PEI hydrogel has excellent adsorption capacity for anionic dyes. In contrast, the qm of cationic MB was lower (129 mg/g). In principle, it is expected that the value would be increased by the additional introduction of an anion functional group or the use of CMC with high DS. In the bioremediation process, in particular with adsorption as the main mechanism, desorption and reuse efficiency are important factors in the design of an efficient contaminant removal process and its long-term operation.
Table 1. Langmuir and Freundlich Isotherm Parameters of AO Adsorption on CMC/PEI Hydrogels
Table 2. Langmuir and Freundlich Isotherm Parameters of MB Adsorption on CMC/PEI Hydrogels
In general, desorption of contaminants can be implemented by using a desorbent that interacts more strongly with contaminants or by blocking the main interaction between the adsorbent and contaminants (Yu et al. 2010). As mentioned above, the main adsorption mechanism of the CMC/PEI polyelectrolyte complex hydrogel and ionic dye was electrostatic attraction, and thus, the pH in the solution had a great effect on the overall adsorption efficiency. Therefore, in this study, desorption was performed under acidic conditions (0.1 M HCl) for cationic MB and basic (0.1 M NaOH) conditions for anionic AO, and the desorption efficiency is shown in Fig. 7(a).
Fig. 7. (a) Desorption efficiency (b) Optical image of CMC/PEI hydrogel after adsorption and desorption (c) Removal efficiency of CMC/PEI hydrogel according to reuse cycle of MB and AO (C0 = 100 mg/L; contact time: 6 h; mass of CMC/PEI hydrogel = 200 mg; temperature: room temperature)
Both MB and AO present on the surface and inside of the CMC/PEI hydrogel showed excellent desorption efficiency of over 93% under each desorption condition, which means that pH shifting can be effectively used for desorption of ionic dyes. As shown in Fig. 7(b), when CMC/PEI adsorbed each ionic dye, the color of the hydrogel appeared to adsorb each dye molecule, and after desorption, the color of the hydrogel returned to that of the original hydrogel. In addition, physical dimension changes or mechanical destruction of the hydrogel were not observed even after adsorption and desorption. This means that the CMC/PEI hydrogel maintains structural stability in the extreme physicochemical environment of the swelling and contraction process according to the pH change and the surface adsorption-diffusion-desorption of the dye pollutant (Song et al. 2019). To evaluate the reuse efficiency of the CMC/PEI hydrogel, repeated adsorption/desorption experiments were performed for each dye of AO and MB, and the results are shown in Fig. 7(c). The reuse efficiency of the CMC/PEI hydrogel gradually decreased as the number of reuses increased. Nevertheless, it showed an excellent reuse efficiency of 95% or more for AO or more than 93% for MB compared to the initial adsorption efficiency even after 10 reuses. This is because the conversion of the surface charge of the CMC/PEI polyelectrolyte according to the pH change and the conversion of the physical hydrogel network structure by the resulting contraction and expansion are stably and reversibly driven. This confirms that the CMC/PEI polyelectrolyte complex hydrogel can serve as an effective biosorbent for the sustainable removal of dye substances, especially ionic dye molecules.
CONCLUSIONS
- In this work, a carboxymethylcellulose/polyethyleneimine (CMC/PEI) polyelectrolyte-complex pH-sensitive smart hydrogel was prepared and applied to the ionic dye removal process. A crosslinking agent (epichlorohydrin) was used to achieve a hydrogel structure capable of maintaining its integrity over a very wide range of pH conditions.
- The carboxylic acid of CMC and the amine of PEI were the main pH-sensitive sites, and protonation and deprotonation of functional groups according to the pH environment could reversibly implement the hydrogel surface charge and consequent swelling-shrinkage of the hydrogel.
- pH-sensitive surface charge conversion behavior of CMC/PEI hydrogel strongly induced electrostatic interactions with ionic dye molecules having opposite charges.
- The adsorbed ionic dyes were easily desorbed through a simple pH conversion process, and as a result, an excellent reuse efficiency of 95% or more was maintained without structural destruction of the hydrogel even after repeated 10 reuse cycles.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2020R1C1C1012623). This study was carried out with the support of the ‘R&D Program for Forest Science Technology (2020215B10-2222-AC01)’ provided by the Korea Forest Service (Korea Forestry Promotion Institute).
REFERENCES CITED
Abdel-Halim, E. S., and Al-Deyab, S. S. (2011). “Hydrogel from crosslinked polyacrylamide/guar gum graft copolymer for sorption of hexavalent chromium ion,” Carbohyd. Polym. 86(3), 1306-1312. DOI: 10.1016/j.carbpol.2011.06.033
Ahmed, E. M. (2015). “Hydrogel: Preparation, characterization, and applications: A review,” J. Adv. Res. 6(2), 105-21. DOI: 10.1016/j.jare.2013.07.006
Aksu, Z. (2005). “Application of biosorption for the removal of organic pollutants: A review,” Process Biochem. 40(3-4), 997-1026. DOI: 10.1016/j.procbio.2004.04.008
Beh, J. H., Lim, T. H., Lew, J. H., and Lai, J. C. (2020). “Cellulose nanofibril-based aerogel derived from sago pith waste and its application on methylene blue removal,” Int. J. Biol. Macromol. 160, 836-845. DOI: 10.1016/j.ijbiomac.2020.05.227
Chang, Y., Dou, N., Liu, M., Jiang, M., Men, J., Cui, Y., Li, R., and Zhu, Y. (2020). “Efficient removal of anionic dyes from aqueous solution using CTAB and β-cyclodextrin-induced dye aggregation,” J. Mol. Liq. 309, article ID 113021. DOI: 10.1016/j.molliq.2020.113021
Choi, K., Lee, S., Park, J. O., Park, J. A., Cho, S. H., Lee, S. Y., Lee, J. H., and Choi, J. W. (2018). “Chromium removal from aqueous solution by a PEI-silica nanocomposite,” Sci. Rep. 8(1), article no. 1438. DOI: 10.1038/s41598-018-20017-9
Chong, M. Y., and Tam, Y. J. (2020). “Bioremediation of dyes using coconut parts via adsorption: A review,” SN Appl. Sci. 2, article no. 187. DOI: 10.1007/s42452-020-1978-y
Dassanayake, R. S., Acharya, S., and Abidi, N. (2021). “Recent advances in biopolymer-based dye removal technologies,” Molecules 26(15), article no. 4697. DOI: 10.3390/molecules26154697
Duman, O., Polat, T. G., Diker, C. O., and Tunc, S. (2020). “Agar/kappa-carrageenan composite hydrogel adsorbent for the removal of Methylene Blue from water,” Int. J. Biol. Macromol. 160, 823-835. DOI: 10.1016/j.ijbiomac.2020.05.191
Faria, P. C., Orfao, J. J., and Pereira, M. F. (2004). “Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries,” Water Res. 38(8), 2043-2052. DOI: 10.1016/j.watres.2004.01.034
Ghariani, B., Messaoud, M., Louati, I., Mtibaa, R., Nasri, M., and Mechichi, T. (2019). “Removal of Acid Orange 51 by micro zero-valent iron under different operational conditions and evaluation of toxicity,” Environ. Sci. Pollut. Res. 26(18), 18392-18402. DOI: 10.1007/s11356-019-04929-1
Guo, T., Song, J., Jin, Y., Sun, Z., and Li, L. (2019). “Thermally stable and green cellulose-based composites strengthened by styrene-co-acrylate latex for lithium-ion battery separators,” Carbohydr. Polym. 206, 801-810. DOI: 10.1016/j.carbpol.2018.11.025
Gupta, V. K., and Suhas (2009). “Application of low-cost adsorbents for dye removal–A review,” J. Environ. Manage. 90(8), 2313-2342. DOI: 10.1016/j.jenvman.2008.11.017
Hameed, B. H. (2009). “Spent tea leaves: A new non-conventional and low-cost adsorbent for removal of basic dye from aqueous solutions,” J. Hazard. Mater. 161(2-3), 753-759. DOI: 10.1016/j.jhazmat.2008.04.019
Hossieni-Aghdam, S. J., Foroughi-Nia, B., Zare-Akbari, Z., Mojarad-Jabali, S., Motasadizadeh, H., and Farhadnejad, H. (2018). “Facile fabrication and characterization of a novel oral pH-sensitive drug delivery system based on CMC hydrogel and HNT-AT nanohybrid,” Int. J. Biol. Macromol. 107(Part B), 2436-2449. DOI: 10.1016/j.ijbiomac.2017.10.128
Kim, J. C., Kim, J., Park, J., Oh, J. K., Choi, I. G., and Kwak, H. W. (2021). “Highly efficient and sustainable alginate/carboxylated lignin hybrid beads as adsorbent for cationic dye removal,” React. Funct. Polym. 161, article ID 104839. DOI: 10.1016/j.reactfunctpolym.2021.104839
Kim, Y., Bang, J., Kim, J., Choi, J. H., Hwang, S. W., Yeo, H., Choi, I. G., Jin, H. J., and Kwak, H. W. (2022). “Cationic surface-modified regenerated nanocellulose hydrogel for efficient Cr(VI) remediation,” Carbohydr Polym. 278, article ID 118930. DOI: 10.1016/j.carbpol.2021.118930
Kong, Y., Zhuang, Y., Han, Z., Yu, J., Shi, B., Han, K., and Hao, H. (2019). “Dye removal by eco-friendly physically cross-linked double network polymer hydrogel beads and their functionalized composites,” J. Environ. Sci. (China) 78, 81-91. DOI: 10.1016/j.jes.2018.07.006
Kordjazi, S., Kamyab, K., and Hemmatinejad, N. (2020). “Super-hydrophilic/oleophobic chitosan/acrylamide hydrogel: An efficient water/oil separation filter,” Adv. Compos. Mater. 3(2), 167-176. DOI: 10.1007/s42114-020-00150-8
Kwak, H. W., Kim, M. K., Lee, J. Y., Yun, H., Kim, M. H., Park, Y. H., and Lee, K. H. (2015). “Preparation of bead-type biosorbent from water-soluble Spirulina platensis extracts for chromium (VI) removal,” Algal Res. 7, 92-99. DOI: 10.1016/j.algal.2014.12.006
Li, J., Liu, H., Wang, C., and Huang, G. (2017a). “A facile method to fabricate hybrid hydrogels with mechanical toughness using a novel multifunctional cross-linker,” RSC Adv. 7(56), 35311-35319. DOI: 10.1039/c7ra05645a
Li, Q., Xue, D. X., Zhang, Y. F., Zhang, Z. H., Wang, Q., Gao, Z., and Bai, J. (2017b). “A copper-organic framework as scavenger towards organic dyes pollutants via physical adsorption and visible-light photodegradation,” Inorg. 85, 78-83. DOI: 10.1016/j.inoche.2017.06.021
Liew, C. W., and Ramesh, S. (2015). “Electrical, structural, thermal and electrochemical properties of corn starch-based biopolymer electrolytes,” Carbohydr. Polym. 124, 222-228. DOI: 10.1016/j.carbpol.2015.02.024
Liu, Q., Liu, M., Li, H., and Lam, K. Y. (2020). “Multiphysics modeling of responsive deformation of dual magnetic-pH-sensitive hydrogel,” Int. J. Solids Struct. 190, 76-92. DOI: 10.1016/j.ijsolstr.2019.11.002
Luo, X., and Zhang, L. (2009). “High effective adsorption of organic dyes on magnetic cellulose beads entrapping activated carbon,” J. Hazard. Mater. 171(1-3), 340-347. DOI: 10.1016/j.jhazmat.2009.06.009
Mahinroosta, M., Jomeh Farsangi, Z., Allahverdi, A., and Shakoori, Z. (2018). “Hydrogels as intelligent materials: A brief review of synthesis, properties and applications,” Mater. Today Chem. 8, 42-55. DOI: 10.1016/j.mtchem.2018.02.004
Mohammadzadeh Pakdel, P., and Peighambardoust, S. J. (2018). “Review on recent progress in chitosan-based hydrogels for wastewater treatment application,” Carbohydr Polym. 201, 264-279. DOI: 10.1016/j.carbpol.2018.08.070
Mokhtar, A., Abdelkrim, S., Djelad, A., Sardi, A., Boukoussa, B., Sassi, M., and Bengueddach, A. (2020). “Adsorption behavior of cationic and anionic dyes on magadiite-chitosan composite beads,” Carbohydr Polym. 229, article ID 115399. DOI: 10.1016/j.carbpol.2019.115399
Monir, T. S. B., Afroz, S., Khan, R. A., Miah, M. Y., Takafuji, M., and Alam, M. A. (2019). “pH-Sensitive hydrogel from polyethylene oxide and acrylic acid by gamma radiation,” J. Compos. Sci. 3(2), article no. 58. DOI: 10.3390/jcs3020058
Naresh Yadav, D., Anand Kishore, K., and Saroj, D. (2021). “A study on removal of Methylene Blue dye by photo catalysis integrated with nanofiltration using statistical and experimental approaches,” Environ. Technol. 42(19), 2968-2981. DOI: 10.1080/09593330.2020.1720303
Natarajan, S., Bajaj, H. C., and Tayade, R. J. (2018). “Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process,” J. Environ. Sci. (China) 65, 201-222. DOI: 10.1016/j.jes.2017.03.011
Nele, V., Wojciechowski, J. P., Armstrong, J. P. K., and Stevens, M. M. (2020). “Tailoring gelation mechanisms for advanced hydrogel applications,” Adv. Funct. Mater. 30(42), article ID 2002759. DOI: 10.1002/adfm.202002759
Ren, J., Li, R., Wang, X., Li, M., and Yang, W. (2022). “A superabsorbent hydrogel for removal of dyes from aqueous solution,” J. Polym. Environ. 30, 3327-3339. DOI: 10.1007/s10924-022-02434-0
Roa, K., Oyarce, E., Boulett, A., Alsamman, M., Oyarzún, D., Pizarro, G. D. C., and Sánchez, J. (2021). “Lignocellulose-based materials and their application in the removal of dyes from water: A review,” Sustain. Mater. Technol. 29, article ID e00320. DOI: 10.1016/j.susmat.2021.e00320
Rungsima, C., Boonyan, N., Klorvan, M., and Kusuktham, B. (2020). “Hydrogel sensors with pH sensitivity,” Polym. Bull. 78(10), 5769-578. DOI: 10.1007/s00289-020-03398-8
Salleh, M. A. M., Mahmoud, D. K., Karim, W. A. W. A., and Idris, A. (2011). “Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review,” Desalination 280(1-3), 1-13. DOI: 10.1016/j.desal.2011.07.019
Song, L., Liu, F., Zhu, C., and Li, A. (2019). “Facile one-step fabrication of carboxymethyl cellulose based hydrogel for highly efficient removal of Cr(VI) under mild acidic condition,” Chem. Eng. J. 369, 641-651. DOI: 10.1016/j.cej.2019.03.126
Sun, X. F., Xie, Y., Shan, S., Li, W., and Sun, L. (2022). “Chemically-crosslinked xylan/graphene oxide composite hydrogel for copper ions removal,” J. Polym. Environ. (Online). DOI: 10.1007/s10924-022-02475-5
Sun, X., Yang, L., Li, Q., Zhao, J., Li, X., Wang, X., and Liu, H. (2014). “Amino-functionalized magnetic cellulose nanocomposite as adsorbent for removal of Cr(VI): Synthesis and adsorption studies,” Chem. Eng. J. 241, 175-183. DOI: 10.1016/j.cej.2013.12.051
Van Tran, V., Park, D., and Lee, Y. C. (2018). “Hydrogel applications for adsorption of contaminants in water and wastewater treatment,” Environ. Sci. Pollut. Res. 25, 24569-24599. DOI: 10.1007/s11356-018-2605-y
Wang, Y., Lin, N., Gong, Y., Wang, R., and Zhang, X. (2021). “Cu-Fe embedded cross-linked 3D hydrogel for enhanced reductive removal of Cr(VI): Characterization, performance, and mechanisms,” Chemosphere 280, article ID 130663. DOI: 10.1016/j.chemosphere.2021.130663
Wojnárovits, L., and Takács, E. (2008). “Irradiation treatment of azo dye containing wastewater: An overview,” Radiat. Phys. Chem. 77(3), 225-244. DOI: 10.1016/j.radphyschem.2007.05.003
Yang, S., Fu, S., Liu, H., Zhou, Y., and Li, X. (2011). “Hydrogel beads based on carboxymethyl cellulose for removal heavy metal ions,” J. Appl. Polym. Sci. 119(2), 1204-1210. DOI: 10.1002/app.32822
Yu, J. X., Chi, R. A., Su, X. Z., He, Z. Y., Qi, Y. F., and Zhang, Y. F. (2010). “Desorption behavior of methylene blue on pyromellitic dianhydride modified biosorbent by a novel eluent: Acid TiO2 hydrosol,” J. Hazard. Mater. 177(1-3), 222-227. DOI: 10.1016/j.jhazmat.2009.12.021
Zhai, S., Li, M., Wang, D., Zhang, L., Yang, Y., and Fu, S. (2019). “In situ loading metal oxide particles on bio-chars: Reusable materials for efficient removal of methylene blue from wastewater,” J. Clean. Prod. 220, 460-474. DOI: 10.1016/j.jclepro.2019.02.152
Zhang, J., Zhu, Y., Song, J., Yang, J., Pan, C., Xu, T., and Zhang, L. (2018). “Novel balanced charged alginate/PEI polyelectrolyte hydrogel that resists foreign-body reaction,” ACS Appl. Mater. Interfaces 10(8), 6879-6886. DOI: 10.1021/acsami.7b17670
Zhu, J., Han, H., Ye, T. T., Li, F. X., Wang, X. L., Yu, J. Y., and Wu, D. Q. (2018). “Biodegradable and pH sensitive peptide based hydrogel as controlled release system for antibacterial wound dressing application,” Molecules 23(12), article no. 3383. DOI: 10.3390/molecules23123383
Article submitted: July 5, 2022; Peer review completed: August 6, 2022; Revised version received and accepted: August 16, 2022; Published: August 19, 2022.
DOI: 10.15376/biores.17.4.5785-5802
