Abstract
A new controlled release fertilizer was developed by coating urea particles with bio-based polyurethane. Coating materials were synthesized from liquefied wheat straw, isocyanate, and castor oil. The effects of liquefied solvents, mass ratio of liquid to solid, size of wheat straw, and reaction time during liquefaction process on the nitrogen release rate of polyurethane-coated urea (PCU) were investigated and optimized. The nitrogen release characteristics of PCU were studied in both water and soil. The structural and chemical characteristics of PCU were examined. The PCU coating materials showed high density and good degradability, as well as superior controlled release properties. This product, with excellent controlled release properties, nontoxicity in soil, environmental friendliness, and low cost, could be especially useful in agricultural and horticultural applications.
Download PDF
Full Article
Polyurethane from Liquefied Wheat Straw as Coating Material for Controlled Release Fertilizers
Panfang Lu,a,* Yanfei Zhang,a Cong Jia,a Chongji Wang,a Xiao Li,a and Min Zhang b,*
A new controlled release fertilizer was developed by coating urea particles with bio-based polyurethane. Coating materials were synthesized from liquefied wheat straw, isocyanate, and castor oil. The effects of liquefied solvents, mass ratio of liquid to solid, size of wheat straw, and reaction time during liquefaction process on the nitrogen release rate of polyurethane-coated urea (PCU) were investigated and optimized. The nitrogen release characteristics of PCU were studied in both water and soil. The structural and chemical characteristics of PCU were examined. The PCU coating materials showed high density and good degradability, as well as superior controlled release properties. This product, with excellent controlled release properties, nontoxicity in soil, environmental friendliness, and low cost, could be especially useful in agricultural and horticultural applications.
Keywords: Polyurethane-coated urea; Wheat straw; Liquefaction; Nitrogen release behavior
Contact information: a: College of Chemistry and Material Science, Shandong Agricultural University, Tai’an, Shandong 271018, P. R. China; b: National Engineering Research Center for Slow/Controlled Release Fertilizers, National Engineering Laboratory for Efficient Utilization of Soil and Fertilizer Resources, College of Resources and Environment, Shandong Agricultural University, Tai’an, Shandong 271018, P. R. China;
* Corresponding authors: xiaolu980216@163.com; minzhang-2002@163.com
INTRODUCTION
Exponential growth has led to a global population of approximately 7.0 billion, and it is expected to approach 9.5 billion by 2050 (United Nations 1998). To meet global food requirements, an enormous quantity of fertilizers must be used in the agricultural and horticultural domain. To date, these fertilizers have had undesirable environmental impacts. It is therefore of considerable importance and necessity to develop new types of fertilizers that can increase crop production and reduce environmental problems.
The use of controlled release fertilizers (CRFs) may be an effective solution. These fertilizers are commonly prepared by coating granules of conventional fertilizers with various materials that reduce their nutrient dissolution rates. The release and dissolution rates of core fertilizers depend on the structure and properties of the coating materials (Jarosiewicz and Tomaszewska 2003). The coating materials applied most frequently are sulfur (McClellan and Schieb 1973; Prasad 1973; McClellan and Schieb 1975), synthetic polymers (Shoji and Kanno 1994; Tomaszewska and Jarosiewicz 2006; Yanget al. 2012), and natural polymers (Krajewska 2005; Han et al. 2009; Zhong et al. 2013; Lubkowski et al. 2015). Sulfur was selected as an initial coating material and can be regarded as one of the best commercial controlled release fertilizers at present, but it is difficult to precisely control the release properties of sulfur-coated fertilizers because sulfur is easily oxidized and very brittle. Synthetic polymer-coated fertilizers were developed because of their excellent controlled release properties. However, increases in petrochemical product costs, and coating materials with non-renewable and non-biodegradable properties would limit the further development of CRFs, especially in China (Briassoulis and Dejean 2010). Recently, renewable and biodegradable natural polymer materials, such as starch (Zhong et al. 2013), poly(butylene succinate) (Lubkowski et al. 2015), chitosan (Krajewska 2005), and polyvinyl alcohol (Han et al. 2009), have been used directly or have been modified as coating materials for fertilizers. These natural polymer coating materials are good water absorbers, and their nutrient release longevities as CRFs are short, normally less than 30 days (Ni et al. 2011). CRFs with a nutrient release longevity of less than 30 days will not meet the nitrogen supply requirements for field crops because their growing period is commonly longer than two or three months (Yang et al.2012). Therefore, the development of low-cost, biodegradable polymer materials with excellent controlled release properties as CRF coating materials is of significant interest.
Agricultural residues such as wheat straw, corn stover, and rice straw are essentially a renewable biomass resource and are readily available, especially in China. However, the overall utilization of agricultural residues is very low. The most of residues are dealt with by landfill disposal or incineration, which is environmentally hazardous and poses a significant waste of resources. Recently, a number of studies have been conducted to convert bioresources, including wood and agricultural residues, to value-added products such as bio-polyols (Yamada and Ono 1999; Jin et al. 2011) and bio-oils (Demirbas 2001). Liquefied polyol products converted by bioresources through liquefaction technology have been used successfully to prepare foams or adhesive products (Kurimoto et al. 2000, 2001; Lee et al. 2002; Wang et al. 2008; Kunaver et al. 2010). For example, Wang et al. (2008) prepared polyurethane foam with liquefied corn stover and discussed the effect of the [NCO]/[OH] ratio on the resultant mechanical properties. Lee et al. (2002) reported the liquefaction of wood in the presence of phenol, and the phenolic foams from liquefied wood showed satisfactory densities and compressive properties. However, little research has been reported on the preparation of controlled release fertilizers from liquefied wheat straw.
Based on this background and our previous studies on polymers (Lu et al. 2013; Zhang and Lu 2013), a series of novel polyurethane-coated urea (PCU) fertilizers was prepared using various types of liquefied wheat straw (LWS) as the main coating material. The utilization of LWS reduced the preparation costs of PCU and enhanced coating material biodegradability in soil after releasing nutrient. To achieve optimal coating materials, the effects of some parameters during liquefaction process on the controlled release behavior of PCU were analyzed for the first time. The major objectives of this research are to provide some guidance for further large-scale application of CRFs with polyurethane coating materials from liquefied biomass.
EXPERIMENTAL
Materials and Chemicals
Agricultural residues were harvested from a farm at the National Engineering Research Center for Slow/Controlled Release Fertilizers, Shandong Agricultural University, Shandong, China. The material was milled and the 60- to 400-mesh fractions were selected for the liquefaction experiments. Material was dried in an oven at 105 °C for 24 h before being used for liquefaction. Commercial urea prills from Shanxi Lanhua Coal Mining Group Co. Ltd (Shanxi, China) with a particle size range of 2 to 5 mm were used. The isocyanates (polymethylene polyphenyl isocyanate (PAPI)) with 30.03 wt% NCO group were obtained from Yantai Wanhua Co. Ltd (Shandong, China). Castor oil (BP) was obtained from Qingdao Castor Kay Chemical Co. Ltd (Shandong, China). Sulfuric acid was obtained from Shanghai Aladdin Chemical Co. Ltd (Shanghai, China). A series of ethylene glycol/ethylene carbonate (EG/EC, w/w) blended solvents was used as liquefied wheat straw solvents during liquefaction. All reagents were of commercial purity or analytical reagent grade and were easily obtained from commercial sources.
Preparation of Liquefied Biomass
A certain amount of solvent and sulfuric acid (3 wt% of the solvent) were added to a three-necked flask equipped with a reflux condenser, a thermometer, and a stirrer. When the flask was heated to 150 °C, agricultural residues powder were added to the flask at a set mass ratio of liquid to solid. The mixture was stirred at 150 °C for 30 to 120 min to achieve liquefaction. The flask was cooled to room temperature, and the resultant liquefied biomass, such as wheat straw, corn stover, and rice straw, was collected for analysis and used as CRF coating material.
Preparation of PCU
LWS, PAPI, and BP were mixed uniformly to obtain the coating liquid. Urea particles were heated to 50 to 70 °C in a rotary drum. The coating liquid was sprayed uniformly onto the surface of urea particle in the rotary drum and cured for approximately 5 min to synthesize the polyurethane coating materials. After spraying a measured amount of coating liquid, the final PCU products were obtained, cooled to room temperature, and stored in bags (Fig. 1). The LWS liquefaction parameters and the coating components of the corresponding PCU are listed in Table 1.
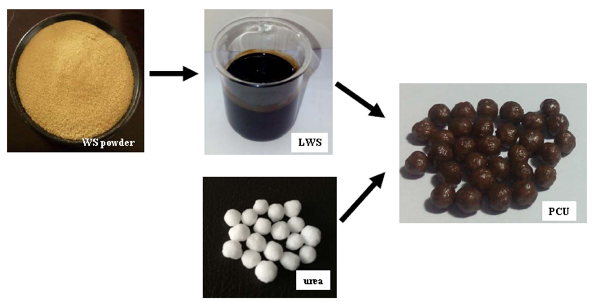
Fig. 1. Schematic illustration of preparation of PCU
Nitrogen Release Behavior of PCU
The nitrogen release behavior of PCU in water at 25 °C was measured according to Chinese National Standard GB/T 23348-2009 (2009). For each sample, 10 g of PCU was sealed in a glass bottle containing 200 mL of deionized water and placed in an incubator at 25 °C. After each incubation period (day 1, 3, 5, 7, 10, 14, 28, 42, 56, 84, and 112), the solution was decanted to measure the nitrogen content. Another 200 mL of deionized water was added to the glass bottle, and incubation at 25 °C was continued. The solution was vibrated at least three times before sampling. The nitrogen concentration was measured using the Kjeldahl method (Bradstreet 1954). All samples were carried out in triplicate, and the average value was taken as the nitrogen concentration of each sample. The nitrogen release longevity of the CRFs is defined as the time when the cumulative nitrogen release reaches 80% of the total nitrogen (Yang et al. 2013). The buried bag method was used to determine the nitrogen release rate of PCU in soil (Hyatt et al. 2010). The buried experiments were conducted according to the method of Yang et al. (2013).
Morphology of PCU
PCU granules were studied using a JSM-5800 scanning electron microscope (SEM). They were split into two halves, and the cross-section of PCU was attached to sample holders with double-sided adhesive tape. Samples were coated by sputtering with gold before observation.
FTIR of PCU coating materials
FTIR spectra were obtained using a Nicolet 380 FTIR spectrometer with 4 cm-1 resolution. The scanning was from 4000 to 500 cm-1. PCU coating materials were mixed with KBr, and disks of sample/KBr mixtures were prepared to obtain the FTIR spectra.
Table 1. Description of the Liquefaction Parameters of LWS and the Composition of the Coating Chemicals for PCU
a polyurethane coated urea; b parameters for the liquefaction process of wheat straw; c LWS contents in the coating materials; d BP contents in the coating materials; e PAPI contents in the coating materials; f total coating materials content in PCU
Degradation of PCU Coating Materials in Soil
Degradation of PCU coating materials was conducted in garden soil at the experimental field at the National Engineering Research Center for Slow/Controlled Release Fertilizers. PCU was buried at least 2 cm apart and 20 cm below the surface of soil. After samples had been buried for 12 months, soil was removed from the PCU coating materials and dried under vacuum (48 h, 40 °C). The PCU coating materials buried for 12 months are termed as PU12, and those that had not been buried in soil are termed as PU0.
RESULTS AND DISCUSSION
Liquefaction Yield of Agricultural Residues
Agricultural residues including wheat straw, corn stover, and rice straw were converted to liquid completely by the liquefaction technology. The liquefaction yields of agricultural residues are shown in Fig. 2. The reaction time and agricultural residues type had an important effect on liquefaction yield. An increased reaction time resulted in a pronounced condensation reaction for various agricultural residues increasing the amount of formed residues. Wheat straw was used in all further experiments because of its high reaction yield and low condensation with time.
Fig. 2. The liquefaction yield of wheat straw (MJ), corn stover (YM), and rice straw (DC)
Effect of Size of Wheat Straw on Nitrogen Release Characteristics of PCU
The nitrogen release characteristics of PCU were affected markedly by the liquefaction parameters of biomass polyols in the PCU coating materials. The influence of size of wheat straw on nitrogen release behavior of PCU was studied. Other liquefaction parameters are displayed in Table 1. As shown in Fig. 3a, the nitrogen release longevities of PCU were 52 d (60 mesh), 110 d (200 mesh), and 25 d (400 mesh), respectively. PCU with the smallest size of wheat straw did not exhibit the highest nitrogen release longevity at 25 °C in water, and the size of wheat straw used in the liquefaction process was not linear with the nitrogen release longevity of PCU. In the early stages of liquefaction, a reduction in size simultaneously promotes wheat straw degradation and the recondensation polymerization of small fragments (Chen and Lu 2009). With the reduction in size of wheat straw, particles are easily degraded to smaller fragments. Solvent more easily permeates the interior of fragments and reacts with active groups on the molecular chains, improving the liquefaction effect and decreasing the residues. When the size of wheat straw reached 200 mesh, the polyurethane coating synthesized from the obtained liquefaction products showed an excellent dense structure, indicating maximum nitrogen release longevity for PCU. However, with a further size increased to 400 mesh, the controlled release property of PCU decreased clearly. Because the particle size was too small, its specific surface area and reactivity increase greatly, resulting in occurrence of agglomeration in the liquefaction process and increasing residues in the liquefaction products.
Coating materials containing the unliquefied fragments of many residues are sensitive to water. When the corresponding PCU are immersed in water, the coating surface adsorbs water molecules easily and swells to accelerate the dissolution rate of fertilizers. It can be concluded that a particle size of 200 mesh is optimal for the liquefaction of wheat straw in the PCU coating materials.
Effect of Mass Ratio of Liquid to Solid on Nitrogen Release Characteristics of PCU
The effect of liquid–solid mass ratio on nitrogen release behavior of PCU is shown in Fig. 3b. The nitrogen release longevity of PCU increased initially and then decreased substantially with decreasing liquid–solid ratio from 5:1 to 2:1. An increase in wheat straw means a decrease in solvent in the liquefaction system. However, the solvent has a dual effect in the liquefaction process. It can react with reactive groups on the molecular chains of wheat straw and simultaneously it can dissolve degradation products and derivatives of wheat straw, which hinder further recondensation of the degraded biomass. Therefore, the relative decrease in solvent causes partial recondensation of the degradation products and the formation of more residues. Increased residues lead to a high viscosity of liquefaction products, which results in poor film-forming on the surface of urea. When the corresponding PCU is immersed in water, the liquid water rapidly accesses the core through micropores and dissolves the urea, resulting in the quick nutrient release rate. However, for a mass ratio of 5:1, the nitrogen release rate decreases to 4:1. This occurs because of a decrease in rigid aromatic structure and lignin derivatives in the liquefaction products. A liquid–solid mass ratio of 4:1 is optimal for the liquefaction of wheat straw used in PCU coating materials.
Fig. 3. Nitrogen release rate of PCU at 25 °C in water: (a) size of wheat straw: 60, 200, and 400 mesh; (b) mass ratio of liquid to solid: 5:1, 4:1, 3:1, and 2:1; (c) EG/EC (w/w) blended liquefied solvents: 2/8, 3/7, 4/6, 5/5, and 8/2; and (d) liquefaction time: 30, 60, 90, 120, and 150 min
Effect of Liquefied Solvents on Nitrogen Release Characteristics of PCU
The series EG/EC blended system was selected as the liquefied solvents in the liquefaction of wheat straw. The effect of liquefied solvents on the nitrogen release longevity of PCU is shown in Fig. 3c. Most of PCU exhibited an “S”-shaped release curve, which coincides with the growth curve of plants. When the mass ratios of EG/EC blended liquefied solvents were 2:8, 3:7, 4:6, and 5:5, the nitrogen release longevity of PCU was in the range of 90 to 110 d. However, for a EG/EC mass ratio of 8:2, the nitrogen release longevity of PCU decreased to 41 d, suggesting that too much EG in the blend solvents was disadvantageous for liquefaction and consequently affected the structure and property of the coating materials. The main components of wheat straw are cellulose (including hemicellulose) and lignin. Cellulose is the most resistant component to liquefaction during the liquefaction of wheat straw. According to the literature (Jin et al. 2011), EC is the best solvent for cellulose that is completely degraded or liquefied in the presence of EC, whereas EG is the worst solvent. When the EG/EC mass ratio was 8:2, many cellulose derivatives were present in the liquefaction products, and a high residue rate decreased the polyurethane crosslinking density during the synthesis of liquefied wheat straw and isocyanates. Coating materials in the final PCU products have a poor water resistance. Thus, 2:8 was chosen as the mass ratio for the EG/EC blended liquefied solvent of wheat straw.
Effect of Reaction Time on Nitrogen Release Characteristics of PCU
The effect of reaction time on the nitrogen release longevity of PCU is shown in Fig. 3d. The nitrogen release longevity of PCU was 42 d (30 min), 96 d (60 min), 150 d (90 min), 48 d (120 min), and 15 d (150 min), and their initial nitrogen release rate was 3.16% (30 min), 0% (60 min), 0% (90 min), 1.33% (120 min), and 10.72% (150 min), respectively. The nitrogen release longevity of PCU was related to the structure and property of the coating materials, and the nitrogen release rate within 24 h for the PCU reflected proportion of uncoating, which was related to the coating technology and the film-forming property of coating materials. For a liquefaction reaction of 30 min, the hydroxyl value in the system was high because of alcoholysis, and the solvent reacted with the degraded products of wheat straw to produce oligomers. When these liquefied products reacted with isocyanate, surplus hydroxyl groups of the molecular chains cannot react with isocyanate to produce urethane and only combined with intermolecular forces, which resulted in the poor strength properties and crosslinking density of coating materials. The coating materials absorbed water easily, swelled, and became hydrolyzed to produce holes and break because of the presence of many hydroxyl and ester groups. Therefore, the nutrient release rate of PCU was high and the nitrogen release longevity of PCU was considerably shorter. However, for a liquefaction reaction of 60 to 90 min, the pyranoid ring in the cellulose was also gradually decomposed because of the further degradation of oligomer during the liquefaction process. This led to a reduction in hydroxyl groups in the liquefied products. The polyurethane resin synthesized from the liquefied products exhibited the highest crosslinking density and water-resistant properties. For a prolonged reaction time, the alcoholysis reaction was reduced gradually, and oxidation and condensation reactions took priority in the system. Oxidization of hydroxyl groups led to the formation of carboxyl and ester groups. The residues also increased because the formed free radicals condensed easily to form residues in acidic media. These components played an important role in the properties of coating materials. When the liquefaction time was 150 min, the nitrogen release longevity of PCU was the lowest. Therefore, the optimal liquefaction time was judged to be 90 min.
FTIR Analysis of Coating Materials
Excellent coating materials were prepared according to the optimal liquefaction parameters of wheat straw, and the structure, morphology, and properties of the coating materials were studied in detail. The FTIR spectrum of the coating materials (Fig. 4) shows a strong and broad band around 3385 cm-1, which can be attributed to N-H stretching vibrations of isocyanates and the OH group of biomass polyols; bands in the range of 2932 to 2800 cm-1 arise from C-H stretching vibrations in methyl, methine, and methylene. Sharp absorption peaks near 1594 and 1632 cm-1 can be attributed to the aromatic compounds of lignin and C=O stretching vibrations of urethane, respectively. The prominent peak at 1347 cm-1 is associated with CH2 twisting, wagging, and scissoring vibrations in biomass polyols. Several weak peaks near 1230 and 1057 cm-1 correspond to C-O stretching vibration from the N-CO-O of isocyanate. These results indicate that polyurethane was synthesized from biomass polyols, isocyanates, and castor oil.
Fig. 4. FTIR spectra of the coating materials of PCU
Morphology of PCU
The microscopic morphology of the surface and cross section of PCU was observed from the SEM images in Fig. 5a–d. It can be seen from the SEM image in Fig. 5a that the surface of PCU coating was very smooth and dense; there were no small pores or gaps on the surface of PCU coating materials at 20,000× magnification (Fig. 5b).
The SEM image of the cross section of PCU (Fig. 5c) showed a two-part structure. The smooth layer is the dense polyurethane coating material of PCU, the thickness of which is 20 to 30 μm and reduces the rate of water diffusion into the core and nutrient diffusion outside the core.
When the cross section of PCU coating material was enlarged 10,000 times, no pinholes or gaps were observed in Fig. 5d. Therefore, when PCU was dipped in water, water molecules entered the core extremely slowly through pin pores to dissolve urea. This resulted in the excellent controlled release property of PCU.
Fig. 5. SEM images of PCU: (a) surface of PCU at 3000× magnification; (b) surface of PCU at 20,000× magnification; (c) cross section of PCU at 1000× magnification; (d) cross section of coating materials at 10,000× magnification.
Degradation of PCU Coating Materials
SEM images of PCU coating materials after being buried in soil for 12 months are shown in Fig. 6. The tendency for polyurethane degradation was obvious compared with the PU0 coating materials in Fig. 5. Many fragments produced during the degradation process were stacked in a disorderly fashion on the surface of PU12 (Fig. 6a) and formed several loose holes of approximately 5 to 10 μm in size. When the surface of the PU12 coating materials was enlarged 3000 times, small fragments became apparent on the PU12 surface (Fig. 6b). The figure shows a network morphology with many different sizes of holes. In this research, polyurethane coating materials were synthesized from liquefied wheat straw, isocyanates and castor oil. However, the natural polymer compounds derived from liquefied wheat straw in the polyurethane were easily hydrolyzed and eroded by microorganisms, resulted in breakage of polymer molecules, producing many small pores and smaller molecular mass chips on the polyurethane coating material surface. Enzymes produced by mobile micro-organisms eroded the natural polymer compounds and easily attacked groups inside the polyurethane utilizing small pores and breakages, which enabled the holes to increase in size and become deeper over time, as evident in the stereovision holes.
Fig. 6. SEM image of surface (a: magnif 500×; b: magnif 3000×) of PCU coating materials after 12 months in soil
Nitrogen Release Characteristics of PCU in Soil
The nitrogen release characteristics of PCU with various coating contents in soil were studied. The nitrogen release rate of PCU is shown in Fig. 7. When the coating material contents were increased from 3% to 5% and 7%, the nitrogen release rate within 24 hours in soil for PCU decreased gradually from 10.72% to 2.95% and 1.28%, respectively. The nitrogen release longevity of the PCU increased from 14 to 53 and 88 d, respectively, and the cumulative nitrogen release curves of PCU changed from an “inverted-L” to an “S” shape. Therefore, changing the coating material contents may be used as a strategy to control the nitrogen release rate of PCU.
Fig. 7. Nitrogen release rate of PCU in soil
CONCLUSIONS
- Bio-based polyurethane was synthesized using liquefied wheat straw, which was used as a coating material for CRF.
- The optimal liquefaction parameters of a mass ratio of EG/EC blended liquefied solvents of 2:8, liquid-solid mass ratio of 4:1, wheat straw size of 200 mesh, and liquefaction time of 90 min were confirmed during the liquefaction process.
- PCU exhibited excellent controlled release behavior in water and soil. Polyurethane coating materials had a good degradation tendency in soil.
- This work provides a technological guide for the design of a high-performance cost ratio of CRF with environment-friendly coating materials from renewable resources.
ACKNOWLEDGMENTS
This project was supported by National Natural Science Foundation of China (Grant No. 31301848), the Science and Technology Development Project of Shandong Province (Grant No. 2014GNC112006), the Natural Science Foundation of Shandong Province (Grant No. ZR2013CQ013), and the China Postdoctoral Science Foundation funded project (Grant No. 2014M560568).
REFERENCES CITED
Briassoulis, D., and Dejean, C. (2010). “Critical review of norms and standards for biodegradable agricultural plastics part 1. Biodegradation in soil,” J. Polym. Environ. 18(3), 384-400. DOI: 10.1007/s10924-010-0168-1
Bradstreet, R. B. (1954). “Kjeldahl method for organic nitrogen,” Anal. Chem. 26(1), 185-187. DOI: 10.1021/ac60085a028
Chen, F., and Lu, Z. (2009). “Liquefaction of wheat straw and preparation of rigid polyurethane foam from the liquefaction products,” J. Appl. Polym. Sci. 111(1), 508-516. DOI: 10.1002/app.29107
Demirbas, D. (2001). “Biomass resource facilities and biomass conversion processing for fuels and chemicals,” Energ. Conver. Manag. 42(7), 1357-1378. DOI: 10.1016/S0196-8904(00)00137-0
GB/T 23348-2009 (2009). “Slow release fertilizer,” China Standards Press, Beijing, China.
Han, X., Chen, S., and Hu, X. (2009). “Controlled-release fertilizer encapsulated by starch/polyvinyl alcohol coating,” Desalination 240(1-3), 21-26. DOI: 10.1016/j.desal.2008.01.047
Hyatt, C. R., Venterea, R. T., Rosen, C. J., McNearney, M., Wilson, M. L., and Dolan, M. S. (2010). “Polymer-coated urea maintains potato yields and reduces nitrous oxide emissions in a Minnesota loamy sand,” Soil Sci. Soc. Am. J. 74(2), 419-428. DOI: 10.2136/sssaj2009.0126
Jarosiewicz, A., and Tomaszewska, M. (2003). “Controlled-release NPK fertilizer encapsulated by polymeric membranes,” J. Agric. Food. Chem. 51(2), 413-417. DOI: 10.1021/jf020800o
Jin, Y., Ruan, X., Cheng, X., and Lü, Q. (2011). “Liquefaction of lignin by polyethyleneglycol and glycerol,” Bioresour. Technol. 102(3), 3581-3583. DOI: 10.1016/j.biortech.2010.10.050
Krajewska, B. (2005). “Membrane-based processes performed with use of chitin/chitosan materials,” Sep. Purif. Technol. 41(3), 305-312. DOI: 10.1016/j.seppur.2004.03.019
Kunaver, M., Medved, S., Čuk, N., Jasiukaitytė, E., Poljanšek, I., and Strnad, T. (2010). “Application of liquefied wood as a new particle board adhesive system,” Bioresour. Technol. 101(4), 1361-1368. DOI: 10.1016/j.biortech.2009.09.066
Kurimoto, Y., Takeda, M., Koizumi, A., Yamauchi, S., Doi, S., and Tamura, Y. (2000). “Mechanical properties of polyurethane films prepared from liquefied wood with polymeric MDI,” Bioresour. Technol. 74(2), 151-157. DOI: 10.1016/s0960-8524(00)00009-2
Kurimoto, Y., Takeda, M., Doi, S., Tamura, Y., and Ono, H. (2001). “Network structures and thermal properties of polyurethane films prepared from liquefied wood,” Bioresour. Technol. 77(1), 33-40.DOI: 10.1016/s0960-8524(00)00136-x
Lee, S.-H., Teramoto, Y., and Shiraishi, N. (2002). “Resol-type phenolic resin from liquefied phenolated wood and its application to phenolic foam,” J. App. Polym. Sci. 84(3), 468-472. DOI: 10.1002/app.10018
Lu, P., Zhang, M., Li, Q., and Xu, Y. (2013). “Structure and properties of controlled release fertilizers coated with thermosetting resin,” Polym-Plast. Technol. Eng. 52(4), 381-386. DOI: 10.1080/03602559.2012.752000
Lubkowski, K., Smorowska, A., Grzmil, B., and Kozłowska, A. (2015). “Controlled-release fertilizer prepared using a biodegradable aliphatic copolyester of poly(butylene succinate) and dimerized fatty acid,” J. Agric. Food. Chem. 63(10), 2597-2605. DOI: 10.1021/acs.jafc.5b00518
McClellan, G. H., and Scheib, R. M. (1973). “Characterization of sulphur coating on urea,” Sulph. Institut. 9, 3-4.
McClellan, G. H., and Scheib R. M. (1975). “Texture of sulfur coatings on urea,” Adv. Chem. Ser. 140,18-32. DOI: 10.1021/ba-1975-0140.ch002
Ni, B., Liu, M., Lü, S., Xie, L., and Wang, Y. (2011). “Environmentally friendly slow-release nitrogen fertilizer,” J. Agric. Food. Chem. 59(18), 10169-10175. DOI: 10.1021/jf202131z
Prasad, M. (1973). “Evaluation of isobutylidenediurea and sulfur-coated urea for grass and lettuce,” J. Agric. Food. Chem. 21(5), 919-922. DOI: 10.1021/jf60189a047
Shoji, S., and Kanno, H. (1994). “Use of polyolefin-coated fertilizers for increasing fertilizer efficiency and reducing nitrate leaching and nitrous oxide emissions,” Fert. Res. 39(2), 147-152. DOI: 10.1007/bf00750913
Tomaszewska, M., and Jarosiewicz, A. (2006). “Encapsulation of mineral fertilizer by polysulfone using a spraying method,” Desalination 198(1-3), 346-352. DOI: 10.1016/j.desal.2006.01.032
United Nations (1998). “World populations projections to 2150,” Department of International Economic and Social Affairs, New York.
Wang, T., Zhang, L., Li, D., Yin, J., Wu, S., and Mao, Z. (2008). “Mechanical properties of polyurethane foams prepared from liquefied corn stover with PAPI,” Bioresour. Technol. 99(7), 2265-2268. DOI: 10.1016/j.biortech.2007.05.003
Yamada, T., and Ono, H. (1999). “Rapid liquefaction of lignocellulosic waste by using ethylene carbonate,” Bioresour. Technol. 70(1), 61-67. DOI: 10.1016/s0960-8524(99)00008-5
Yang, Y.-c., Zhang, M., Li, Y., Fan, X.-h., and Geng, Y.-q. (2012). “Improving the quality of polymer-coated urea with recycled plastic, proper additives, and large tablets,” J. Agric. Food. Chem. 60(45), 11229-11237. DOI: 10.1021/jf302813g
Yang, Y., Tong, Z., Geng, Y., Li, Y., and Zhang, M. (2013). “Biobased polymer composites derived from corn stover and feather meals as double-coating materials for controlled-release and water-retention urea fertilizers,” J. Agric. Food. Chem. 61(34), 8166-8174. DOI: 10.1021/jf402519t
Zhang, M., and Lu, P. (2013). “Crop straw as coating material to make controlled release fertilizers and processing methods,” Chinese Patent #ZL 201210261735.0 (in Chinese).
Zhong, K., Lin, Z.-T., Zheng, X.-L., Jiang, G.-B., Fang, Y.-S., Mao, X.-Y., and Liao, Z.-W. (2013). “Starch derivative-based superabsorbent with integration of water-retaining and controlled-release fertilizers,” Carbohydr. Polym. 92(2), 1367-1376. DOI: 10.1016/j.carbpol.2012.10.030
Article submitted: June 15, 2015; Peer review completed: July 30, 2015; Revised version received: August 6, 2015; Accepted: August 16, 2015; Published: October 6, 2015.
DOI: 10.15376/biores.10.4.7877-7888
