Abstract
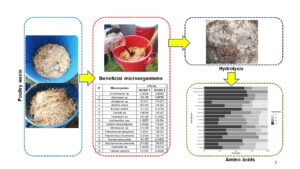
Poultry waste can be hydrolyzed using microorganisms to obtain useful amino acids for agriculture processes. This research treated poultry waste by applying beneficial microbial consortia. Microorganisms were obtained from Brassica oleracea (commonly known as cabbage) and Jungia rugosa (widely known as matico de puna) plants. Each sample was sent to the laboratory for gender, species, and concentration identification. Poultry waste (feathers, offal, blood) and a liquid solution made up of water, molasses, and microorganisms were placed inside plastic tanks. Four treatments were established (T1, T2, T3, and T4). T1 and T3 were composed of 80% water, 10% molasses, and 10% microorganisms; T2 and T4 were composed of 70% water, 20% molasses, and 10% microorganisms. The contents in each tank were periodically stirred for one month. Sixteen microorganisms were identified in each sample. In each treatment, nine essential and nine non-essential amino acids were found in different concentration levels. It is assumed that indigenous microbial consortia benefit the hydrolysis of poultry waste. Furthermore, the type and content of amino acids are related to the microbial activity of each consortium.
Download PDF
Full Article
Poultry Feathers and Offal Treatment by Using Beneficial Microorganisms
Manuel Alvarez-Vera,a,c,* Jacinto Vázquez,b Daniel Fernández,c and Rosalía Reinoso c
Poultry waste can be hydrolyzed using microorganisms to obtain useful amino acids for agriculture processes. This research treated poultry waste by applying beneficial microbial consortia. Microorganisms were obtained from Brassica oleracea (commonly known as cabbage) and Jungia rugosa (widely known as matico de puna) plants. Each sample was sent to the laboratory for gender, species, and concentration identification. Poultry waste (feathers, offal, blood) and a liquid solution made up of water, molasses, and microorganisms were placed inside plastic tanks. Four treatments were established (T1, T2, T3, and T4). T1 and T3 were composed of 80% water, 10% molasses, and 10% microorganisms; T2 and T4 were composed of 70% water, 20% molasses, and 10% microorganisms. The contents in each tank were periodically stirred for one month. Sixteen microorganisms were identified in each sample. In each treatment, nine essential and nine non-essential amino acids were found in different concentration levels. It is assumed that indigenous microbial consortia benefit the hydrolysis of poultry waste. Furthermore, the type and content of amino acids are related to the microbial activity of each consortium.
DOI: 10.15376/biores.17.1.64-74
Keywords: Poultry waste; Beneficial microbial consortia; Amino acids; Phyllosphere; Keratin; Agriculture
Contact information: a: Postgraduate Leadership Office, Catholic University of Cuenca, UCACUE, Cuenca-Ecuador; b: Academic Unit of Agricultural Sciences, Catholic University of Cuenca, UCACUE, Cuenca-Ecuador; c: Career in Environmental Engineering, Catholic University of Cuenca, UCACUE, Cuenca-Ecuador; *Corresponding author: malvarezv@ucacue.edu.ec
GRAPHICAL ABSTRACT

INTRODUCTION
World population growth leads to greater food demand, in which poultry meat is an essential component, leading to the increase of poultry industries. This increase causes a more significant amount of solid waste such as bones, feathers, offal (Brandelli et al. 2015), blood, heads, and other remains, which are considered useless.
The global chicken-for-meat waste is equal to 45.9 million tons a year, considering a 70% yield during processing (Seidavi et al. 2019); this waste is a source of environmental contamination, affecting the quality of life of the population. Poultry slaughtering and processing generate different types of organic waste, which, when not managed properly, may result in economic loss, a risk for the environment, and a health hazard (Ozdemir and Yetilmezsoy 2020). Disposing waste from poultry processing into the environment without proper care can cause water, air, and soil pollution (Do Nascimento et al. 2015).
Indigenous microorganisms-based technology treats and manages poultry waste in an environmentally friendly manner (Kumar and Gopal 2015). Microbial management of organic waste is a promising and eco-friendly approach to manage large amounts of organic waste due to its recycling potential (Patchaye et al. 2018). Indigenous microbial populations can degrade poultry waste. The process can be accelerated by using feather-degrading bacteria (Ichida et al. 2001).
In nature, plants are colonized by different organisms (Gong and Xin 2021), at different levels: rhizosphere, endosphere, and phyllosphere (Pattnaik et al. 2020). The latter houses a diverse variety of microorganisms (Stone et al. 2018), being one of the most prevalent habitats for microorganisms (Bringel and Couée 2015). The microbial population living in cabbage is not clearly known, as it is affected by several factors. Microorganisms are diverse according to the type of plant and climatic conditions prevailing in each place. In the environment where this study was carried out, plants containing hydrolytic enzymes are unknown; thus, a microbial population was obtained from a cabbage plant, which is common in such environments.
Poultry feathers are keratin-rich by-products that are a source of nutrients (amino acids) for animals and plants (Korniłłowicz-Kowalska and Bohacz 2011). Significant amounts of essential amino acids are found in poultry waste (Kazemi-Bonchenari et al. 2017); therefore, it should be considered raw material instead of waste. Keratin is a fibrous and recalcitrant structural protein and is the next most abundant polymer in nature after cellulose, hemicellulose, lignin, and chitin (Lange et al. 2016).
Enzymatic hydrolysis is used to degrade keratin (Holkar et al. 2018). Different microorganisms —actinomycetes, fungi, and bacteria— can degrade keratin by producing the keratinase enzyme (Sharma and Devi 2018). It is likely that beneficial indigenous microorganisms found in the environment fulfill this purpose.
Effective strategies for solid waste management should be considered in developing countries to improve global sustainability (Ferronato et al. 2019). Using beneficial microorganisms is a viable option. Large-scale production, short time of cultivation, and ease of handling are some of the advantages that enhance the application of microorganisms in biotechnology (Da Silva 2017).
Chemical hydrolysis, hydrolysis under pressure, and enzymatic hydrolysis, are known methods for degrading keratin (Holkar et al. 2018) and obtaining amino acids. Amino acids produced by fermentation are important for treating poultry feathers. The production of specialized amino acids is driven by metabolic engineering (Wendisch 2020).
Feather waste, which is high in keratin proteins and amino acids, is a potential renewable source to recover valuable products (Cheong et al. 2017). Microbial keratinases hydrolyze keratins into peptides, soluble proteins, and amino acids (Adelere and Lateef 2019), which can be applied in industrial and agricultural processes.
The growth of poultry processing and egg production industries depends largely on proper waste management, which is generated in different processes. Waste must be efficiently treated to be available for use while reducing environmental pollution and shifting away from a linear economy (Jayathilakan et al. 2012).
By managing and reusing waste, the circular economy (CE) is a path towards more sustainable economic growth (Ranta et al. 2018). Waste is a vital part of the economy as a by-product of economic activity (Halkos and Petrou 2016). Through the application of economic and eco-friendly techniques, waste is leveraged and turned into useful products.
This research treated poultry waste via hydrolysis and applying beneficial indigenous microbial consortia to obtain useful amino acids.
EXPERIMENTAL
Obtaining Beneficial Microbial Consortia
According to Alvarez et al. (2018), beneficial microorganisms were obtained from the phyllosphere of two plant species grown in the environment: Brassica oleracea (commonly known as cabbage) and Jungia rugosa (widely known as matico de puna). To prepare the stock solution of microorganisms, each plant species was divided into small pieces and placed into containers with water and molasses. This solution was left to rest for a 12-day period, at which time the preparation was filtered out, and the resulting liquid was mixed with water and molasses. Samples were collected from each container and sent to the laboratory for microbial load analysis.
Hydrolysis of Poultry Waste
The hydrolysis of poultry waste was carried out in plastic tanks for 30 days. Water, beneficial microorganisms, and molasses were applied to the poultry waste.
Two beneficial microbial consortia were used: M1, microorganisms obtained from the cabbage plant and M2, microorganisms obtained from the matico de puna plant. Molasses was applied in two doses: 10% concentration (D1) and 20% concentration (D2). As a result, four treatments were created: T1 (M1D1), T2 (M1D2), T3 (M2D1), and T4 (M2D2), which were distributed in four repetitions under greenhouse conditions (Fig. 1).
Fig. 1. Treatment arrangements with four repetitions
Poultry waste included feathers, offal, and blood. Homogeneous samples of these components were prepared. Subsequently, their weight (30 pounds) was recorded, and they were placed inside each tank. At room temperature (22 °C), an unpleasant odor emanated from the waste, so it was necessary to use a face mask to carry out the activities.
After placing the waste inside the tanks, a liquid solution of water, microorganisms, and molasses was placed inside each tank. In total, four applications were performed: the first one on the day the research started, and then every three days, as shown in Table 1.
Table 1. Doses Applied in Each Treatment for the Hydrolysis of Poultry Waste
During the applications, a wooden object was used to mix the elements. Finally, each tank was hermetically sealed. After the first week, the unpleasant odor gradually disappeared. Feathers and offal progressively lost their structure, which facilitated homogenization. In the fourth week, the characteristics of the liquid solution changed: the odor became sweet and pleasant; the color turned dark brown. During the evaluation period, pH, temperature, and electrical conductivity data were recorded.
Fig. 2. Hydrolysis process of poultry waste using beneficial microbial consortia
After the mixtures from each tank were filtered, the remaining solids were equivalent to 10% of the initial weight. A total of 3 L of liquid solution were taken from each treatment, totaling 12 L, which were homogenized, and 1 L was used to identify amino acids. The hydrolysis process of poultry waste is presented in Fig. 2.
RESULTS AND DISCUSSION
Sixteen types of microorganisms were found in both plants, but the bacterial load (CFU/mL) varied in each sample (Table 2). There were higher concentrations of Bacillus cereus, Bacillus subtilis, Pseudomonas putida, and Saccharomyces cerevisiae in sample 1 (cabbage plant), while Lactobacillus spp., Pseudomonas aeruginosa, and Pseudomonas fluorescens were higher in sample 2 (matico de puna plant). Microorganisms found in the phyllosphere of plants depend on environmental conditions and the type of plant. Even plants located in the same place can host different microorganisms (Dastogeer et al. 2020).
Table 2. Beneficial Microorganisms Found in Both Selected Plants
The presence of microorganisms on the surface of a plant depends both on environmental conditions and on aspects of each plant species. Plant characteristics and geographical location determine the type of microorganisms present in the phyllosphere (Alvarez-Vera et al. 2018). Both selected plants come from the same geographical location, but their phenology is different. Their physical-chemical conditions, as well as the resistance and surrounding environment, have an impact on the development of microorganisms found on the surface of leaves, as well as on their diversity and dispersal (Sivakumar et al. 2020). The type of host plant, the absence or presence of diseases, phenology, and environmental conditions, are factors that have an impact on plant microbiome structure and function (Rossmann et al. 2017).
In this case, it is assumed that the characteristics of each plant species affect the type of microorganisms and their concentration. Plant biology is related to bacteria that exist on aerial plant surfaces (Massoni et al. 2020). The diversity and composition of the microorganism community existing on the surface of tree leaves (phyllosphere), vary according to each species and along temporal, spatial, and environmental gradients (Laforest-Lapointe I et al. 2016). Phyllosphere microbial load is vulnerable to water stress (Aung et al. 2018), which could condition population success.
Fig. 3. Concentration of amino acids in the poultry waste hydrolyzed solution (ng/mL)
Hydrolysis of poultry waste, using indigenous beneficial microbial consortia obtained from plants, yielded a product with commercial potential. Based on the aminogram presented in Fig. 3, amino acids were produced in all four treatments; the presence of nine essential and nine non-essential amino acids in T4 is important. However, essential amino acids methionine and threonine, and non-essential amino acids alanine, arginine, aspartic acid, glutamic acid, glycine and serine were not present in T1. Moreover, the levels of essential amino acids leucine (33 ng/mL), lysine (25 ng/mL), and tryptophan (88 ng/mL) and non-essential amino acid cysteine (45 ng/mL) were higher in T3 than in the other treatments.
According to nutritional classification, amino acids can be essential or indispensable and non-essential or dispensable. Among the essential amino acids are histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine (Sánchez 2010), and they must be obtained from external sources. Traditionally, it is assumed that non-essential amino acids (endogenous synthesis) such as arginine, alanine, aspartic acid, glutamic acid, glycine, glutamine, cysteine, proline, and tyrosine, are synthesized inside the body (Wu et al. 2013).
Microorganisms are a low-cost, efficient, and eco-friendly method for waste management and production of high biotechnological value products, due to their ability to degrade keratin-rich materials generated in agribusiness (Adelere and Lateef 2019). Poultry waste, especially feathers, is rich in keratin, which is a recalcitrant protein difficult to degrade (Patinvoh et al. 2016). Microbial keratinases are a cheap and environmentally friendly alternative to degrade keratinous waste turning it into useful by-products. They are secreted by many microorganisms that can be isolated from different habitats and ecological niches. They are promising biocatalysts in various industries (Sharma and Devi 2018). For example, Bacillus subtilis S14 produces KerS14, a keratinase with a potential application in different biotechnological processes (Dedavid e Silva et al. 2014); Bacillus subtilis CH-1 is an effective and environmentally benign microorganism that degrades feathers under moderate reaction conditions (Liu et al. 2014). A previous study provided a basis for keratinase production and for the conversion of feathers into soluble protein through a fermentation process by Bacillus licheniformis ZJUEL31410 (Ni et al. 2011). Thus, keratin biodegradation by the keratinase enzyme has been demonstrated by Bacillus licheniformis and Bacillus subtilis, which is useful in different processes such as feather degradation (Liu et al. 2013). Likewise, keratinolytic bacterium Bacillus pumilus strain A1 was used for the hydrolysis of feather protein; the medium contained up to 50 g/L of raw feathers, achieving complete degradation (Fakhfakh et al. 2011).
Other microorganisms can also hydrolyze bird feathers. For instance, Pseudomonas sp P5 isolates is highly efficient and shows high keratinolytic activity in the hydrolysis of raw feathers. The hydrolysates contained up to 301 mg/L of free amino acids (Stiborova et al. 2016). Similarly, Streptomyces sp. SCUT-3 efficiently degrades feathers yielding an amino acid-rich product that can be an important nutrition source for animals, plants, and microorganisms (Li et al. 2020). Actinomycetes of the genus Streptomyces produce keratinases that have been used in feather degradation (Allure et al. 2015). Thermoactinomyces, known for its resistance to harsh environmental conditions and its ability to digest a wide range of hard-to-degrade compounds, completely degrades chicken feathers (Wang et al. 2015). Rhodococcus erythropolis, with a maximum enzymatic activity of 33.39 U/mL, also degrades feathers (Alahyaribeik et al. 2020). At lab-scale, a model is being proposed to determine levels of keratin consumption and protein production by hydrolysis, using a keratinolytic enzymatic cocktail with filamentous bacterium Amycolatopsis keratiniphila D2 (DSM 44409) (Rodman et al. 2018).
CONCLUSIONS
- Microbial consortia were obtained from B. oleracea and J. rugosa plants. Sixteen types of microorganisms were reported in both samples; their concentration depended on the species from which they were extracted.
- The hydrolysis of poultry waste, using indigenous beneficial microbial consortia, yields a product that contains amino acids with commercial potential.
- The presence and concentration of amino acids depend on microbial consortium and concentration of molasses used in the hydrolytic process.
- Treatments with the microbial consortium of the matico de puna plant show better results. T4 contains all the reported amino acids: nine essential amino acids and nine non-essential amino acids; while T3 reports higher levels of essential amino acids Leucine, Lysine and Tryptophan and non-essential amino acid Cysteine compared to the other treatments.
- It is recommended to evaluate the effect of the resulting amino acids on plant species development.
ACKNOWLEDGMENTS
The authors thank the Postgraduate Leadership Office of the Catholic University of Cuenca for providing the required facilities to develop this research project, and also thank Ing. Jorge Castillo for his active collaboration during all research stages.
REFERENCES CITED
Adelere, I. A., and Lateef, A. (2019). “Degradation of keratin biomass by different microorganisms,” in: Keratin as a Protein Biopolymer, S. Sharma and A. Kumar, (eds.), Springer, Cham, Switzerland, pp. 187-200. DOI: 10.1007/978-3-030-02901-2_5
Alahyaribeik, S., Sharifi, S. davood, Tabandeh, F., Honarbakhsh, S., and Ghazanfari, S. (2020). “Bioconversion of chicken feather wastes by keratinolytic bacteria,” Process Safety and Environmental Protection 135, 171-178. DOI: 10.1016/j.psep.2020.01.014
Allure, N., Madhusushan, D. N., and Dayanand, A. (2015). “Enhanced production, purification and characterization of alkaline keratinase from Streptomyces minutiscleroticus DNA38,” International Letters of Natural Sciences 43, 27-37. DOI: 10.18052/www.scipress.com/ilns.43.27
Alvarez-Vera, M., Vázquez, J., Castillo, J., Tucta, F., Quispe, E., and Meza, V. (2018). “Potencial de la flora de la provincia del Azuay (Ecuador) como fuente de microorganismos benéficos,” Scientia Agropecuaria 9(4), 561-568. DOI: 10.17268/sci.agropecu.2018.04.12
Aung, K., Jiang, Y., and He, S. Y. (2018). “The role of water in plant-microbe interactions,” Plant Journal 93(4), 771-780. DOI: 10.1111/tpj.13795
Brandelli, A., Sala, L., and Kalil, S. J. (2015). “Microbial enzymes for bioconversion of poultry waste into added-value products,” Food Research International 73, 3-12. DOI: 10.1016/j.foodres.2015.01.015
Bringel, F., and Couée, I. (2015). “Pivotal roles of phyllosphere microorganisms at the interface between plant functioning and atmospheric trace gas dynamics,” Frontiers in Microbiology 6, 1–14. DOI: 10.3389/fmicb.2015.00486
Cheong, C. W., Ahmad, S. A., Ooi, P. T., and Phang, L. Y. (2017). “Treatment of chicken feather waste,” Pertanika Journal of Scholarly Research Reviews 3(1), 32-41.
Dastogeer, K. M. G., Tumpa, F. H., Sultana, A., Akter, M. A., and Chakraborty, A. (2020). “Plant microbiome-an account of the factors that shape community composition and diversity,” Current Plant Biology 23, article no. 100161. DOI: 10.1016/j.cpb.2020.100161
Dedavid e Silva, L. A., Macedo, A. J., and Termignoni, C. (2014). “Production of keratinase by Bacillus subtilis S14,” Annals of Microbiology 64(4), 1725-1733. DOI: 10.1007/s13213-014-0816-0
Fakhfakh, N., Ktari, N., Haddar, A., Mnif, I. H., Dahmen, I., and Nasri, M. (2011). “Total solubilisation of the chicken feathers by fermentation with a keratinolytic bacterium, Bacillus pumilus A1, and the production of protein hydrolysate with high antioxidative activity,” Process Biochemistry 46(9), 1731-1737. DOI: 10.1016/j.procbio.2011.05.023
Ferronato, N., Rada, E. C., Gorritty Portillo, M. A., Cioca, L. I., Ragazzi, M., and Torretta, V. (2019). “Introduction of the circular economy within developing regions: A comparative analysis of advantages and opportunities for waste valorization,” Journal of Environmental Management 230, 366-378. DOI: 10.1016/j.jenvman.2018.09.095
Halkos, G. E., and Petrou, K. N. (2016). “Moving towards a circular economy: Rethinking waste management practices,” Economic and Social Thought 3(2), 220-240. DOI: 10.1453/jest.v3i2.854
Holkar, C. R., Jain, S. S., Jadhav, A. J., and Pinjari, D. V. (2018). “Valorization of keratin based waste,” Process Safety and Environmental Protection 115, 85-98. DOI: 10.1016/j.psep.2017.08.045
Ichida, J. M., Krizova, L., LeFevre, C. A., Keener, H. M., Elwell, D. L., and Burtt, E. H. E. H. (2001). “Bacterial inoculum enhances keratin degradation and biofilm formation in poultry compost,” Journal of Microbiological Methods 47(2), 199-208. DOI: 10.1016/S0167-7012(01)00302-5
Jayathilakan, K., Sultana, K., Radhakrishna, K., and Bawa, A. S. (2012). “Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review,” Journal of Food Science and Technology 49(3), 278-293. DOI: 10.1007/s13197-011-0290-7
Kazemi-Bonchenari, M., Alizadeh, A. R., Javadi, L., Zohrevand, M., Odongo, N. E., and Salem, A. Z. M. (2017). “Use of poultry pre-cooked slaughterhouse waste as ruminant feed to prevent environmental pollution,” Journal of Cleaner Production 145, 151-156. DOI: 10.1016/j.jclepro.2017.01.066
Korniłłowicz-Kowalska, T., and Bohacz, J. (2011). “Biodegradation of keratin waste: Theory and practical aspects,” Waste Management 31(8), 1689-1701. DOI: 10.1016/j.wasman.2011.03.024
Kumar, B. L., and Gopal, D. V. R. S. (2015). “Effective role of indigenous microorganisms for sustainable environment,” 3 Biotech 5, 867-876. DOI: 10.1007/s13205-015-0293-6
Laforest-Lapointe I, Messier, C., and Kembel, S. (2016). “Tree phyllosphere bacterial communities: Exploring the magnitude of intra- and inter-individual variation among host species,” PeerJ 4, e2367. DOI: 10.7717/peerj.2367
Lange, L., Huang, Y., and Busk, P. K. (2016). “Microbial decomposition of keratin in nature—a new hypothesis of industrial relevance,” Applied Microbiology and Biotechnology 100, 2083-2096. DOI: 10.1007/s00253-015-7262-1
Li, Z.-W., Liang, S., Ke, Y., Deng, J.-J., Zhang, M.-S., Lu, D.-L., Li, J.-Z., and Luo, X.-C. (2020). “The feather degradation mechanisms of a new Streptomyces sp. isolate SCUT-3,” Communications Biology 3(191), 1-13. DOI: 10.1038/s42003-020-0918-0
Liu, B., Zhang, J., Li, B., Liao, X., Du, G., and Chen, J. (2013). “Expression and characterization of extreme alkaline, oxidation-resistant keratinase from Bacillus licheniformis in recombinant Bacillus subtilis WB600 expression system and its application in wool fiber processing,” World Journal of Microbiology and Biotechnology, 29, 825-832. DOI: 10.1007/s11274-012-1237-5
Liu, Q., Zhang, T., Song, N., Li, Q., Wang, Z., Zhang, X., Lu, X., Fang, J., and Chen, J. (2014). “Purification and characterization of four key enzymes from a feather-degrading Bacillus subtilis from the gut of tarantula Chilobrachys guangxiensis,” International Biodeterioration and Biodegradation 96, 26-32. DOI: 10.1016/j.ibiod.2014.08.008
Massoni, J., Bortfeld-Miller, M., Jardillier, L., Salazar, G., Sunagawa, S., and Vorholt, J. A. (2020). “Consistent host and organ occupancy of phyllosphere bacteria in a community of wild herbaceous plant species,” The ISME Journal 14, 245-258. DOI: 10.1038/s41396-019-0531-8
Do Nascimento, C. D. V., Filho, R. A. P., Artur, A. G., and Costa, M. C. G. (2015). “Application of poultry processing industry waste: A strategy for vegetation growth in degraded soil,” Waste Management 36, 316-322. DOI: 10.1016/j.wasman.2014.11.001
Ni, H., Chen, Q. H., Chen, F., Fu, M., Dong, Y., and Cai, H. (2011). “Improved keratinase production for feather degradation by Bacillus licheniformis ZJUEL31410 in submerged cultivation,” African Journal of Biotechnology 10(37), 7236-7244. DOI: 10.5897/AJB11.168
Ozdemir, S., and Yetilmezsoy, K. (2020). “A mini literature review on sustainable management of poultry abattoir wastes,” Journal of Material Cycles and Waste Management 22, 11-21. DOI: 10.1007/s10163-019-00934-1
Patchaye, M., Sundarkrishnan, B., Tamilselvan, S., and Sakthivel, N. (2018). “Microbial management of organic waste in agroecosystem,” in: Microorganisms for Green Revolution, D. G. Panpatte, Y. K. Jhala, H. N. Shelat, and R. V. Vyas (eds.), Springer, Singapore, pp. 45-73. DOI: 10.1007/978-981-10-7146-1_3
Patinvoh, R. J., Feuk-Lagerstedt, E., Lundin, M., Sárvári Horváth, I., and Taherzadeh, M. J. (2016). “Biological pretreatment of chicken feather and biogas production from total broth,” Applied Biochemistry and Biotechnology 180, 1401-1415. DOI: 10.1007/s12010-016-2175-8
Ranta, V., Aarikka-Stenroos, L., and Mäkinen, S. J. (2018). “Creating value in the circular economy: A structured multiple-case analysis of business models,” Journal of Cleaner Production 201, 988-1000. DOI: 10.1016/j.jclepro.2018.08.072
Rodman, A., Falco, F., Gerogiorgis, D. I., and Gernaey, K. V. (2018). “Enzymatic keratin hydrolysis: Dynamic modelling, parameter estimation and validation,” in: 28th European Symposium on Computer Aided Process Engineering, Graz, Austria, pp. 1553-1558. DOI: 10.1016/B978-0-444-64235-6.50271-0
Rossmann, M., Sarango-Flores, S. W., Chiaramonte, J. B., Kmit, M. C. P., and Mendes, R. (2017). “Plant microbiome: Composition and functions in plant compartments,” in: The Brazilian Microbiome, V. Pylro and L. Roesch (eds.), Springer, Cham, Switzerland, pp. 7-20. DOI: 10.1007/978-3-319-59997-7
Sánchez, F. (2010). “Metabolismo de los aminoácidos,” in: Tratado de Nutrición, A. Gil, (ed.), Editorial Médica Panamericana, Madrid, pp. 451-485.
Seidavi, A., Zaker-Esteghamati, H., and Scanes, C. (2019). “Chicken processing: Impact, co-products and potential,” World’s Poultry Science Journal 75(1), 55-68. DOI: 10.1017/S0043933918000764
Sharma, R., and Devi, S. (2018). “Versatility and commercial status of microbial keratinases: A review,” Reviews in Environmental Science and Biotechnology 17, 19-45. DOI: 10.1007/s11157-017-9454-x
Da Silva, R. R. (2017). “Bacterial and fungal proteolytic enzymes: Production, catalysis and potential applications,” Applied Biochemistry and Biotechnology 183, 1-19. DOI: 10.1007/s12010-017-2427-2
Sivakumar, N., Sathishkumar, R., Selvakumar, G., Shyamkumar, R., and Arjunekumar, K. (2020). “Phyllospheric microbiomes: Diversity, ecological significance, and biotechnological applications,” in: Plant Microbiomes for Sustainable Agriculture. Sustainable Development and Biodiversity, A. Yadav, J. Singh, A. Rastegari, and N. Yadav (eds.), Springer, Cham, Udaipur, India, pp. 113-172. DOI: 10.1007/978-3-030-38453-1_5
Stiborova, H., Branska, B., Vesela, T., Lovecka, P., Stranska, M., Hajslova, J., Jiru, M., Patakova, P., and Demnerova, K. (2016). “Transformation of raw feather waste into digestible peptides and amino acids,” Journal of Chemical Technology and Biotechnology 91, 1629-1637. DOI: 10.1002/jctb.4912
Wang, L., Cheng, G., Ren, Y., Dai, Z., Zhao, Z.-S., Liu, F., Li, S., Wei, Y., Xiong, J., Tang, X.-F., and Tang, B. (2015). “Degradation of intact chicken feathers by Thermoactinomyces sp. CDF and characterization of its keratinolytic protease,” Applied Microbiology and Biotechnology 99(9), 3949-3959. DOI: 10.1007/s00253-014-6207-4
Wu, G., Wu, Z., Dai, Z., Yang, Y., Wang, W., Liu, C., Wang, B., Wang, J., and Yin, Y. (2013). “Dietary requirements of ‘nutritionally non-essential amino acids’ by animals and humans,” Amino Acids 44, 1107-1113. DOI: 10.1007/s00726-012-1444-2
Article submitted: June 24, 2021; Peer review completed: September 28, 2021; Revised version received: October 5, 2021; Accepted: October 6, 2021; Published: November 8, 2021.
DOI: 10.15376/biores.17.1.64-74
