Abstract
Bagasse pulp waste (BPW) is a material generated from the depithing of sugarcane bagasse stems prior to pulping. It was subjected to a modified oxygen delignification or ammonia catalytic steam explosion (AE) pretreatment for delignification and retaining carbohydrates of raw materials, followed by simultaneous saccharification and co-fermentation (SSCF) to ethanol. Based on this process, an environmentally sustainable two-stage process for lignin purification was employed to obtain nontoxic “green lignin”. The results indicated that both pretreatment methods, particularly AE, had outstanding performance in the SSCF process. The highest ethanol yield (based on dry matter of BPW), 67.5% for the AE pretreatment, was obtained from the SSCF procedure at 8% (w/v) solids loading. Furthermore, the obtained lignin products from this process possessed better structural integrity.
Download PDF
Full Article
Preliminary Study on Integrated Production of Ethanol and Lignin from Bagasse Pulp Waste
Huanlei Yang,a,b,* Jinhua Yan,a and Jun Li b
Bagasse pulp waste (BPW) is a material generated from the depithing of sugarcane bagasse stems prior to pulping. It was subjected to a modified oxygen delignification or ammonia catalytic steam explosion (AE) pretreatment for delignification and retaining carbohydrates of raw materials, followed by simultaneous saccharification and co-fermentation (SSCF) to ethanol. Based on this process, an environmentally sustainable two-stage process for lignin purification was employed to obtain nontoxic “green lignin”. The results indicated that both pretreatment methods, particularly AE, had outstanding performance in the SSCF process. The highest ethanol yield (based on dry matter of BPW), 67.5% for the AE pretreatment, was obtained from the SSCF procedure at 8% (w/v) solids loading. Furthermore, the obtained lignin products from this process possessed better structural integrity.
Keywords: Bagasse pith; Bioethanol; Pretreatment; Simultaneous saccharification and co-fermentation (SSCF); Lignin
Contact information: a: School of Chemical Technology, Guangdong Industry Polytechnic, Guangzhou 510300, China; b: State Key Laboratory of Pulp and Paper Engineering, South China University of Technology, Guangzhou 510640, China; *Corresponding author: yhl07031219@163.com
GRAPHICAL ABSTRACT
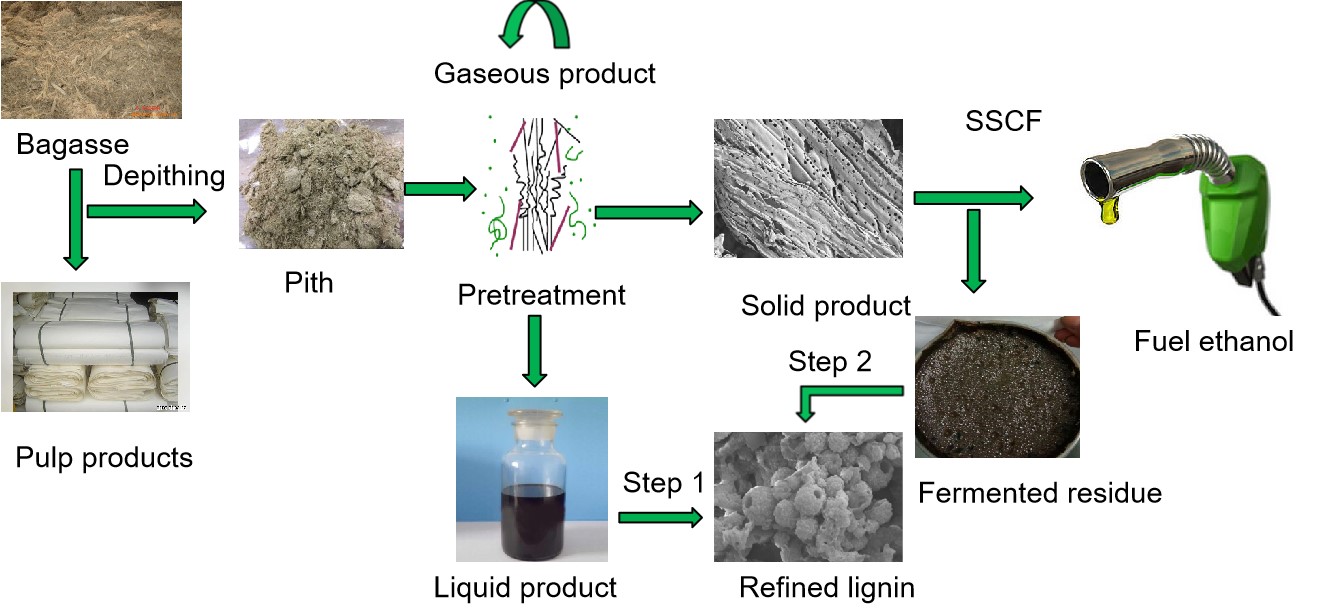
INTRODUCTION
Sugarcane bagasse is the most abundant natural lignocellulosic resource that is obtained from sugar milling in Latin America and South Asia. The sugarcane bagasse stalk is composed of hard rind fibers and soft pith; the pith contains most of the sucrose within the stalk (Brienzo et al. 2016). During sugar processing, the stalk is crushed to obtain the sugar juice that is used for ethanol production (Chandel et al. 2012); as a result, abundant longer and finer fibers in the outer rind are collected from the crushed bagasse. Hence, sugarcane bagasse is widely used for papermaking in many areas, such as in Guangxi, China, where sugar and papermaking productions have been established in recent years. According to Brienzo et al. (2016), bagasse pith is rich in sucrose-storing parenchyma cells and vascular tissues. These thin-walled cells preferentially absorb chemicals, and are completely dissolved during the chemical pulping processes; this results in high chemical consumption and low yields during pulping. In addition, after pulping, these residual parenchyma cells significantly reduce the drainage performance of the accepted pulps and the strength index of paper in sheet-making processes due to their short length, viscidity, and poor cohesion property. Therefore, a depithing step is necessary to reduce the number of parenchyma cells in bagasse pulp. This procedure produces large volumes of residue, which is mostly bagasse pith that is composed of carbohydrates. Therefore, the bioconversion of bagasse pith into ethanol may have sustainable economic and strategic benefits.
Substantial progress, which has been made in the development of various technologies for converting lignocellulosic biomasses, offers the promise of lower cost fermentable sugars for the production of ethanol and feedstock chemicals (Lynd 1989; Cotana et al. 2015). However, prior to biomass hydrolysis, pretreatment is necessary to overcome the recalcitrant structure of the cell walls of the material to enzymatic and microbial treatments (McCann and Carpita 2015). Of the pretreatment methods, the steam explosion process has been reported to be the most effective method for pretreating hardwood and agricultural residues, which contain a high content of acetyl groups in the hemicelluloses (Wooley et al. 1999). During pretreatment, O-acetyl and uronic acid substituents on the hemicelluloses are cleaved to afford organic acids, which autocatalyze the hydrolysis of hemicelluloses to monosaccharides by breaking the glycosidic bonds (Wang et al. 2016). However, only some lignin is removed from this process due to the occurrence of lignin condensation reaction against an acidic medium. The result is that the lignin is deposited onto the cellulose surface, which inhibits the cellulose hydrolysis by cellulase by binding (Kumar et al. 2012). Hence, alkali types, such as NaOH, Ca(OH)2, or ammonia water (NH3H2O), have been proposed as catalysts to improve pretreatment efficiency, due in part to their high effectiveness of lignin solubilization and partly because lower enzyme loadings are required for enzymatic hydrolysis ( Singh et al. 2015). Some studies report the addition of oxidants, such as O2, H2O2, and peracids, to alkali pretreatment can improve its efficiency by enhancing lignin removal (Geng et al. 2014); in such cases, the lignin fragments are retained in alkali liquor of the pretreatment, as well as in the solid residue after the subsequent fermentation process. The lignin, after being purified, can be further utilized for high value-added products, such as the production of aromatic chemicals (Lee et al. 2015), chemical feedstock intermediates (Wang et al. 2013), and polymeric fillers (Mousavioun et al. 2010).
In this study, bagasse pulp waste (BPW) from bagasse depithing was used as feedstock for production of ethanol and lignin. Oxygen delignification (O2-NaOH) and catalytic ammonia steam explosion (AE) pretreatments were examined to improve the enzymatic hydrolysis performance of BPW to fermentable sugars. Subsequently, the pretreated BPW samples are ultimately fermented to ethanol during the simultaneous saccharification and co-fermentation (SSCF) experiments. The refined lignin products are isolated from the alkali pretreatment liquor and from the fermented solid residues by employing environmentally sustainable recovery procedures. Finally, productivity is calculated based on the overall mass balances of ethanol conversion and lignin recovery from the BPW.
EXPERIMENTAL
Materials
The BPW used was supplied by a paper mill located in Hechi, Guangxi, China. The BPW was air-dried to a constant mass. A commercial cellulase product (Celluclast 1.5) with a filter paper activity of 127 FPU/mL, β-glucosidase product (Novozyme 188) with an enzyme activity of 285 CBU/mL, and xylanase product with an enzyme activity of 495 U/g were all obtained from Novozymes Biotechnology Co., Ltd. (Tianjin, China). Saccharomyces cerevisiae (CICC1416) and Candida shehatae (GIM2.175) were supplied by BeNa Culture Collection Co., Ltd. (Beijing, China). All other chemical reagents used in this study were of analytical reagent grade and were purchased from Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China).
Pretreatment
Oxygen delignification with alkali (OA) was completed in a 2.0-L FYX autoclave (Tongchan Autoclave Manufacturing Co., Ltd., Dalian, China) using 80 g oven-dry BPW. The BPW samples were first impregnated with sodium hydroxide at specified dosages, which varied from 4 to 8% (w/w), at 60 °C for 30 min prior to oxygen delignification. Then, the alkali BPW slurry was further treated by an oxygen delignification stage using an oxygen pressure of 0.5 MPa.
The AE pretreatment was conducted with liquid ammonia impregnation and subsequent steam explosion. The aqueous ammonia impregnation was performed by mixing 200 g of oven-dry BPW with a 30% ammonia solution; the slurry was then incubated at room temperature for 6 h. Then, steam explosion pretreatments of the slurries were completed in a removable BL-08 steam-explosion device (Prosperous Technology Co., Ltd., Beijing, China). The pretreated AE solids were separated from the liquor using a Büchner funnel with a 120-mesh cloth filter. Subsequently, the collected solids were washed with distilled water until the filtrate pH was neutral. Finally, the washed and pretreated solids were air-dried to a constant mass.
Simultaneous Saccharification and Co-fermentation
The SSCF experiments were performed in several 250-mL sterile bottles. The bottles contained a fermentation medium volume of 100 mL and the bottles were agitated with an incubator shaker. The sterilized bottles were equipped with two air valves and a sampling valve. The fermentation medium consisted of the following items: 1 g/L (NH4)2SO4, 2.5 g/L KH2PO4, 0.4 g/L MgSO4, and 0.1 g/L CaCl2. The substrate was mixed with the liquid medium at 8% (w/v) and then sterilized at 121 °C for 30 min. Subsequently, cellulase, β-glucosidase, and xylanase were added to the mixture at 30 FPU/g, 60 CBU/g, and 15 U/g, respectively, to the substrates for enzymatic hydrolysis at 50 °C and 250 rpm for 24 h, followed by inoculation of 5 g/L (w/v) yeast extracts of Saccharomyces cerevisiae and Candida shehatae. The SSCF process began at 40 °C and 150 rpm for 24 h under a nitrogen atmosphere. This was followed by incubation at 30 °C and 150 rpm for 60 h under an oxygen atmosphere. Samples were taken from the bottles at regular time internals, centrifuged, and stored at 4 °C for fermentation sugar (glucose and xylose) and ethanol analyses.
Recovery of Residual Lignin
Two types of lignin, obtained from the pretreatment aqueous product and the fermented residue, were isolated and further purified by the following step1 and step2,respectively (Fig. 1).
Methods
Determination of the composition of samples
The composition of the BWP and the refined lignin samples were determined by the NREL standard method (Sluiter et al. 2011). The hydrolysate obtained from this method was retained for monosaccharides and acid-soluble lignin analyses. Acid-soluble lignin measurements were performed using an S-3100 ultraviolet-visible (UV-vis) spectrometer (Scinco Co., Ltd., Seoul, South Korea), which measured absorptions at 205 nm wavelength. Concentrations of monosaccharides were analyzed using a Dionex ICS-5000 ion chromatography system (Thermo Fisher Scientific, Waltham, MA, USA) with an electrochemical detector. The monosaccharides were separated by a Dionex CarboPac PA20 column (Thermo Fisher Scientific) using a 2 mmol/L NaOH solution at a flow rate of 0.5 mL/min at 30 °C. The eluents were 200 mmol/L NaOH and 1 mol/L sodium acetate.
Fig. 1. Scheme for recovery of lignin samples from the pretreatment aqueous product and the fermented residue
Determination of ethanol concentration
The ethanol concentration was determined by full evaporation headspace gas chromatography using a TriPlus 300 automatic headspace sampler (Thermo Fisher Scientific, Waltham, MA, USA); the sampler was connected to an Agilent 7890A gas chromatograph (GC) (Agilent Technologies Inc., Santa Clara, CA, USA) that had a flame ionization detector (FID). For the full evaporation method, sealed 21.6 mL vials that contained 50 μL of the sample solution were placed into the headspace sampler and kept at 105 °C for 30 min. The GC was equipped with an HP-5 capillary column. The GC injection temperature was set at 250 °C, and the oven temperature was set at 35 °C for 2 min. Helium was used as the carrier gas at a flow rate of 1 mL/min. The FID was performed at 250 °C under hydrogen and air at flow rates of 40 and 450 mL/min, respectively. The formula for calculating the yield of ethanol is described by Eq. 1,
(1)
where [EtOH] is the concentration of ethanol in the fermented mash (g/L), and [C6] and [C5] are the concentrations of C6 sugars and C5 sugars in the pretreated BPW, respectively.
13C NMR spectroscopy
Solid-state carbon-13 nuclear magnetic resonance (13C NMR) spectroscopy was performed with an AVANCE III HD 400 MHz spectrometer (Brüker, Ettlingen, Germany). The 13C NMR spectra with cross-polarization/magic angle spinning (CP/MAS) were recorded at a resonance frequency of 100 MHz at 25 °C. Other parameters applied to the CP/MAS 13C NMR experiments were a 7-mm magic angle probe, a 90° pulse angle, 4 μs cross-polarization time, 800 μs contact duration, 2.5 s relaxation delay, and 3000 scans. For 13C NMR spectroscopy, 120 mg of the isolated lignin was dissolved in 0.6 mL of DMSO-d6 (99.9%). The 13C NMR spectra were recorded in a Fourier transform (FT) mode at 100.6 Hz, 90° pulse angle, 2 s relaxation delay, and 60,000 scans.
Field emission-scanning electron microscopy analysis
Field emission-scanning electron microscopy (FE-SEM) measurements of samples were performed on a MERLIN FE-SEM instrument (Carl Zeiss Microscopy, Oberkochen, Germany). Samples were Au-coated by a sputtering method using a JFC-1600 sputter coater (JEOL, Tokyo, Japan) prior to FE-SEM.
RESULTS AND DISCUSSION
Composition of BPW
The composition of the BPW used in this study is shown in Table 1. The glucose of cellulose is a principal component of the plant’s cell wall; it comprises 43.7% of the dry matter of the BPW. Xylose, as the major monosaccharide of the hemicelluloses, comprises 27.2%. Sugarcane pith is comprised of up to 30% of parenchyma cells, which had a lower content of glucose and a higher level of xylose than are observed in the BPW versus the reported values for sugarcane bagasse of 45 to 55% glucose and 18 to 25% xylose. The results found for the natural BPW consisted of a certain proportion of hard rind fibers, besides the pith.
Table 1. Composition of Untreated BPW
Impact of the Various Pretreatments on the Chemical Characteristics of BPW
The composition of the BPW samples after O2-NaOH or AE pretreatments are now discussed. As shown in Table 2, the glucan content was nearly constant after all the pretreatments when compared to the original BPW. In particular, the recovery of glucan was 91.6% and 99.2% for the OA1, and AE1 samples, respectively. Although there were slight decreases in recoveries of glucose when the pretreatment conditions were more severe, the values were more than 80.6% and 87.9%, respectively.
Table 2. Recovery Rates of Major Components of BPW After O2-NaOH or Catalytic Ammonia Steam Explosion Pretreatment (Percentages Based on the Initial BPW)
The objectives of this study were to recover the hemicelluloses to decompose them into fermentable monosaccharides for ethanol production, which increases the utilization ratio of the lignocellulosic BWP. Under alkali conditions, the degradation of the hemicelluloses is mainly due to the cleavage of the glycosidic bonds and to alkali peeling reactions occurring at the reducing ends of the hemicelluloses. In any case, the larger amount of hemicelluloses after the alkali pretreatments was reserved for the pretreated solid fractions of BPW compared to that of prehydrolysis or dilute acid catalyzed pretreatments.
Figure 2 shows the surface morphologies of the original BPW and the O2-NaOH- and AE-pretreated samples. As observed, the raw BPW exhibited a flat fiber structure and abundant pores all around the surfaces, but the pores were filled with lignin and carbohydrates. After oxygen delignification pretreatments, a fragmented surface morphology was observed for the pretreated solid, which was probably due to the removal of the lignin. The fiber structure of BPW simultaneously underwent fragmentation and swelling by oxygen delignification pretreatment and released small fiber components consequently enlarging the specific surface area of lignocelluloses. A previous report on the peroxide pretreatment provoked morphological alteration of both the node and internode in sugarcane bagasse, thereby reducing the recalcitrance of biomass (Brienzo et al. 2016). The electron microscope image shows a collapse surface morphology with abundant cracks and holes for AE-pretreated solid (AE3). In addition, the expanding and dredging pores were observed on the fiber surface. This was probably due to the ammonia causing lignin removal by polymer fragmentation and by shearing the fiber structure during the steam explosion process.
As observed in a previous report, it is also noted in this study that the vascular bundles in the bagasse are highly lignified (Sant’Anna et al. 2013); the AE pretreatment resulted in the removal of lignin that lie in the interior of the vascular bundles, which increased the internal specific surface area and surface porosity of the pretreated BWP. This assertion is supported by the 13C NMR spectra of the pretreated BPW samples.
Fig. 2. The surface morphologies of typical BPW samples by FE-SEM analyses, and their corresponding CP/MAS 13C NMR analyses
In Fig. 2, the disappearance of the acetyl group of the hemicelluloses at δ 21 ppm indicated the degradation of hemicelluloses in the two different pretreatments. The signals at δ 63 ppm and δ 84 ppm are assigned to the C-6 and C-4 of amorphous cellulose, respectively. The signals at δ 89 ppm and δ 105 ppm are assigned to C-1 and C-4 of crystalline cellulose, respectively. Moreover, a predominant signal at δ 72 ppm represents the resonances of C-2, C-3, and C-5 of cellulose (Fransen et al. 2000). The relative increase of the cellulose signal intensity at δ 63 ppm and δ 105 ppm in the pretreated substrates suggested the content of cellulose increased after both pretreatments. Additionally, there was an increase in the intensity of the signals at δ 89 ppm, which indicated an increase in the crystallinity index of the cellulose in pretreated BPW. Signals assigned to the aromatic rings and side chains of lignin are mainly in the region of δ 110 to 160 ppm. In particular, the signals located at δ 136 ppm, 148 ppm, and 154 ppm corresponded to C-1, C-4, and C-5 of the aromatic rings of lignin, as well as the aryl methoxyl carbons of lignin (at δ 56 ppm) (Alesiani et al. 2005). The relative reduction of signal intensity at these chemical shifts was observed in the pretreated lignocellulosic materials, which indicated the degradation of syringyl (S) and guaiacyl (G) units of lignin during the alkaline pretreatments.
Production of Ethanol from BPW by SSCF
The conversion of hemicelluloses to monosaccharides, particularly to xylose, is to increase the ethanol yield during fermentation. It has been reported that xylans can irreversibly absorb on the surface of cellulose so as to shield the cellulose from enzymatic deconstruction (Zhang et al. 2012). In addition, xylooligomers are powerful inhibitors of cellulose hydrolysis by enzymes (Qing et al. 2010). Hence, by supplementing the enzyme mixture with additional hemicellulases or xylanases, it would be possible to increase the enzyme hydrolysis efficiency of substrates that contain high levels of hemicelluloses. In the SSCF experiments, the raw and pretreated BPW samples were used as the substrates for pre-enzymatic hydrolysis and the subsequent consumption to ethanol were investigated.
The SSCF experiments were conducted on the dry BPW at high substrate loading (8%, w/v); the time profiles of the monosaccharides and ethanol concentrations are shown in Fig. 3. It was observed that the glucose concentration gradually increased for the pretreated BPW samples when treated with the enzyme mixture (before 24 h), and then it sharply declined when the co-fermentation was started at 24 h. The corresponding ethanol concentration rapidly increased at 24 h, while the xylose concentration did not sharply decline after inoculation. This observation might be explained by the fact that Saccharomyces cerevisiae could not convert the xylose to ethanol, whereas Candida shehatae preferred glucose to xylose when the levels of glucose are high in the co-fermentation process (Huang et al. 2015). This is observed in Fig. 2, where the xylose concentration rapidly decreased after the glucose was consumed simultaneously by Saccharomyces cerevisiae and Candida shehatae; in this phase, the corresponding ethanol concentration slightly increased.
Fig. 3. SSCF of the original BPW, and the O2-NaOH- or AE-pretreated samples with an 8% (w/v) substrate loading
For comparison, the relatively higher ethanol concentrations were achieved for both pretreated samples versus the original sample. The highest ethanol concentrations reached 20.8 and 22.2 g/L at 8% (w/v) substrate loading after 108 h co-fermentation for OA2 and AE3, respectively, while only 6.6 g/L of ethanol concentration was achieved for the original sample. This could be explained by more monosaccharides being produced by enzymatic hydrolysis from the pretreated substrates, which led to higher ethanol yield.
Recovery of Lignin from BPW
Table 3. Composition and Recovery Yields (to Total Lignin) of Lignin Samples Obtained from Step-1 and Step-2
The composition of the recovered lignin samples were analyzed, and their recovery yields to total lignin are shown in Table 3. As expected, all of the obtained lignin samples had relatively low contaminants from monosaccharides. This was primarily because the wet ball milling treatment further reduced the recalcitrance structure of the biomass by deconstructing the lignin-carbohydrate complexes (LCCs), which consequently led to the effective removal of carbohydrates by enzymatic hydrolysis. Further, the degraded monosaccharides and residual inorganic salts were both effectively removed by the dialysis process. Even more remarkable, there was no organic solvent used in the lignin purification process, which resulted in recovery yields of “green lignin” that were approximately 46.9% and 52.3% for the OA and AE pretreatments, respectively.
Fig. 4. The 13C NMR spectra of refined lignin and their surface morphologies as revealed by FE-SEM: (a) OA2-S1 and (b) AE3-S1
Figure 4 displays the 13C NMR spectra of the typical lignin from OA2-S1 and AE3-S1 samples. The signals of the β–O-4’ linkages of lignin are identified in the region of δ 72.0 to 84.3 ppm. The lignin from the AE3-S1 sample showed signals with stronger intensities at δ 72.0 ppm, 77.8 ppm, and 84.3 ppm versus the lignin from the OA2-S1 sample; this observation indicated that more β–O-4’ linkages were broken in the lignin during the OA pretreatment. Furthermore, the principal signals at δ 130.4 ppm and 144.7 ppm are assigned to p-coumarate (PCA) ester linkages. The relatively lower signal intensities at these chemical shifts were also observed in the OA2-S1 sample, which indicated that the phenylcoumaran structures of lignin were largely degraded during the OA pretreatment.
In the oxygen delignification, lignin can react with the superoxide anion radicals (O2-•) that are formed from oxygen under alkaline conditions, which can open the aromatic rings of lignin to form organic acid groups that assist with the dissolution of the lignin. Moreover, the decomposition products from hydrogen peroxide, such as hydroxyl radicals (HO•) and superoxide anion radicals (O2-•) can further oxidize lignin structures by the introduction of carboxyl groups and the cleavage of β–O-4’ ether bonds, thereby leading to lignin dissolution (Palamae et al. 2014). Hence, there was relatively less fragmentation that occurred in the lignin structure by catalytic ammonia hydroxide steam explosion pretreatment because of its lower erosivity in comparison to that of sodium hydroxide. As expected, the lignin from the AE3-S1 sample exhibited spherical and round-shaped surface morphology.
Table 4. Functional Group Analyses of 13C NMR Spectra for Representative Lignin Samples
Based on the results presented in Table 4, both alkali lignin samples contained higher amounts of guaiacyl (G) units of lignin. Moreover, after the OA pretreatment, there were relatively large increases in the syringyl-to-guaiacyl (S/G) ratio values, which indicated a preferential removal of guaiacyl units when compared to syringyl (S) and p-hydroxyphenyl (H) units of lignin during the OA pretreatment.
Overall Balance of Converting BPW to Ethanol and Lignin
Figure 5 presents the overall mass balance of converting the pretreated BPW to ethanol and refined lignin, which is based on the SSCF and lignin recovery processes. The figure clearly illustrates the release of monomeric sugars from the pretreated solid fraction during enzymatic hydrolysis as well as subsequent digestion during fermentation to produce ethanol. For the O2-NaOH runs, the loss of the pretreated solid (27.2%) was mainly due to the removal of lignin, and the corresponding recovery of glucose and xylose in the pretreated solids were 85.6% and 70.6%, respectively. Certainly, the ultimate ethanol yield was 207 kg based on 1000 kg dry BPW, which was equivalent to 66.3% of the total theoretical yield based on the total amounts of glucose and xylose in untreated BPW. Meanwhile, the recovery of lignin was as high as 121 kg, which was equivalent to 46.9% of the total lignin (theoretically). For the AE pretreatment runs, the highest ethanol yield reached 224 kg based on 1000 kg dry matter of BPW, which was equivalent to 67.5% total theoretical yield, and the corresponding recovered lignin yield was 52.3% of the total lignin (theoretically).
Fig. 5. Mass balance of converting pretreated BPW to ethanol and refined lignin based on the SSCF and lignin recovery processes
The higher solid loading (8% (w/v)) of the pretreated solid during SSCF limited the hydrolysis of the carbohydrates to glucose and xylose by the enzymes, which affected the subsequent fermentation of these sugars to ethanol. The ethanol yield, in general, could be increased by decreasing the solids loading in the SSCF procedure; however, this will produce a lower concentration of alcoholic fermentation and increase the volume of wastewater produced. In addition, the cost of pretreatment is critical to evaluate the feasibility of pretreatment methods. For the O2-NaOH pretreatment, a large amount of sodium hydroxide is required, which suggests that a supporting alkali recovery procedure is required to lower the consumption of chemicals. Of this concern, AE-pretreated BPW has more advantages as demonstrated in this study; hence, this pretreatment may be a promising alternative to be employed for ethanol production from BPW.
CONCLUSION
- Both O2-NaOH (OA) and ammonia catalytic steam explosion (AE) pretreatment methods were effective for the delignification of bagasse pulp waste (BPW) without the degradation of the carbohydrates.
- The simultaneous saccharification and co-fermentation (SSCF) experiments indicated that the pretreated samples were easier to ferment to ethanol; the highest ethanol yield approached 207 and 224 kg based on 1000 kg raw BPW for the O2-NaOH and AE pretreatment, respectively.
- Two lignin products were obtained from the process of waste through environmentally sustainable recovery procedures. The recovery yield of lignin was approximately 52.3% for the AE pretreatment.
- The 13C NMR spectra indicated that the recovered lignin from the AE pretreatment underwent less fragmentation to the lignin structure when compared to the lignin from the OA pretreatment.
ACKNOWLEDGEMENTS
This work was made possible by the financial support provided by the Special Support Plan for High-Level Talent Cultivation of Guangdong Industry Polytechnic (No. KYRC2018-020), and Guangdong University Youth Innovation Fund Project (No. 2018GKQNCX032).
REFERENCES CITED
Alesiani, M., Proietti, F., Capuani, S., Paci, M., Fioravanti, M., and Maraviglia, B. (2005). “13C CPMAS NMR spectroscopic analysis applied to wood characterization,” Applied Magnetic Resonance 29(2), 177-184. DOI: 10.1007/BF03167005
Brienzo, M., Abud, Y., Ferreira, S., Corrales, R. C., Ferreira-Leitão, V. S., Souza, W. D., and Sant’Anna, C. (2016). “Characterization of anatomy, lignin distribution, and response to pretreatments of sugarcane culm node and internode,” Industrial Crops and Products 84, 305-313. DOI: 10.1016/j.indcrop.2016.01.039
Chandel, A. K., Da Silva, S. S., Carvalho, W., and Singh, O. V. (2012). “Sugarcane bagasse and leaves: Foreseeable biomass of biofuel and bio-products,” Journal of Chemical Technology and Biotechnology 87(1), 11-20. DOI: 10.1002/jctb.2742
Cotana, F., Cavalaglio, G., Pisello, A. L., Gelosia, M., Ingles, D., and Pompili, E. (2015). “Sustainable ethanol production from common reed (Phragmites australis) through simultaneous saccharification and fermentation,” Sustainability 7(9), 12149-12163. DOI: 10.3390/su70912149
Fransen, C. T. M., Van Laar, H., Kamerling, J. P., and Vliegenthart, J. F. G. (2000). “CP-MAS NMR analysis of carbohydrate fractions of soybean hulls and endosperm,” Carbohydrate Reseach 328(4), 549-559. DOI: 10.1016/S0008-6215(00)00138-5
Geng, W., Huang, T., Jin, Y., Song, J., Chang, H.-M., and Jameel, H. (2014). “Comparison of sodium carbonate-oxygen and sodium hydroxide-oxygen pretreatments on the chemical composition and enzymatic saccharification of wheat straw,” Bioresource Technology 161, 63-68. DOI: 10.1016/j.biortech.2014.03.024
GB/T 2677.3 (1993). “Fibrous raw material – Determination of ash,” Standardization Administration of China, Beijing, China.
Huang, Y. P., Qin, X. L., Luo, X. M., Nong, Q. D., Yang, Q., Zhang, Z., Gao, Y., Lu, F. X., Chen, Y., Yu, Z. W., et al. (2015). “Efficient enzymatic hydrolysis and simultaneous saccharification and fermentation of sugarcane bagasse pulp for ethanol production by cellulase from Penicillium oxalicum EU2106 and thermotolerant Saccharomyces cerevisiae ZM1-5,” Biomass and Bioenergy 77, 53-63. DOI: 10.1016/j.biombioe.2015.03.020
Jacquet, N., Quievy, N., Vanderghem, C., Janas, S., Blecker, C., Devaux, J., Wathelet, B., and Paquot, M. (2011). “Influence of steam explosion on the thermal stability of cellulose fibers,” Polymer Degradation and Stability 96(9), 1582-1588. DOI: 10.1016/j.polymdegradstab.2011.05.021
Kumar, L., Arantes, V., Chandra, R., and Saddler, J. (2012). “The lignin present in steam pretreated softwood binds enzymes and limits cellulose accessibility,” Bioresource Technology 103(1), 201-208. DOI: 10.1016/j.biortech.2011.09.091
Lee, H., Lee, Y. M., Heo, Y. M., Lee, H., Hong, J. H., Jang, S., Min, M., Lee, J., Kim, J. S., Kim, G. H., et al. (2015). “Optimization of endoglucanase production by Trichoderma harzianum KUC1716 and enzymatic hydrolysis of lignocellulosic biomass,” BioResources 10(4), 7466-7476. DOI: 10.15376/biores.10.4.7466-7476
Lynd, L. R. (1989). “Production of ethanol from lignocellulosic materials using thermophilic bacteria: Critical evaluation of potential and review,” in: Lignocellulosic Materials. Advances in Biochemical Engineering/Biotechnology, A. Fiechter (ed.), Vol. 38, Springer-Verlag GmbH, Heidelberg, Germany, pp. 1-52. DOI: 10.1007/BFb0007858
McCann, M. C., and Carpita, N. C. (2015). “Biomass recalcitrance: A multi-scale, multi-factor, and conversion-specific property,” Journal of Experimental Botany 66(14), 4109-4118. DOI: 10.1093/jxb/erv267
Mousavioun, P., Doherty, W. O. S., and George, G. (2010). “Thermal stability and miscibility of poly(hydroxybutyrate) and soda lignin blends,” Industrial Crops and Products 32(3), 656-661. DOI: 10.1016/j.indcrop.2010.08.001
Palamae, S., Palachum, W., Chisti, Y., and Choorit, W. (2014). “Retention of hemicellulose during delignification of oil palm empty fruit bunch (EFB) fiber with peracetic acid and alkaline peroxide,” Biomass and Bioenergy 66, 240-248. DOI: 10.1016/j.biombioe.2014.03.045
Qing, Q., Yang, B., and Wyman, C. E. (2010). “Xylooligomers are strong inhibitors of cellulose hydrolysis by enzymes,” Bioresource Technology 101(24), 9624-9630. DOI: 10.1016/j.biortech.2010.06.137
Sant’Anna, C., Costa, L. T., Abud, Y., Biancatto, L., Miguens, F. C., and De Souza, W. (2013). “Sugarcane cell wall structure and lignin distribution investigated by confocal and electron microscopy,” Microscopy Research and Technique 76(8), 829-834. DOI: 10.1002/jemt.22235
Singh, J., Suhag, M., and Dhaka, A. (2015). “Augmented digestion of lignocellulose by steam explosion, acid, and alkaline pretreatment methods: A review,” Carbohydrate Polymers 117, 624-631. DOI: 10.1016/j.carbpol.2014.10.012
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluitter, J., Templeton, D., and Crocker, D. (2011). Determination of Structural Carbohydrates and Lignin in Biomass, Laboratory Analytical Procedure (LAP) (NREL/TP-510-42618), National Renewable Energy Laboratory (NREL), U.S. Dept. of Energy, Golden, CO, USA.
Wang, P., Fua, Y., Shao, Z., Zhang, F., and Qin, M. (2016). “Structural changes to aspen wood lignin during autohydrolysis pretreatment,” BioResources 11(2), 4086-4103. DOI: 10.15376/biores.11.2.4086-4103
Wang, W. H., Hou, Y. C., Wu, W. Z., and Niu, M. G. (2013). “Simultaneous production of small-molecule fatty acids and benzene polycarboxylic acids from lignite by alkali-oxygen oxidation,” Fuel Processing Technology 112, 7-11. DOI: 10.1016/j.fuproc.2013.02.008
Wooley, R., Ruth, M., Glassner, D., and Sheehan, J. (1999). “Process design and costing of bioethanol technology: A tool for determining the status and direction of research and development,” Biotechnology Progress 15(5), 794-803. DOI: 10.1021/bp990107u
Zhang, J. H., Tang, M., and Viikari, L. (2012). “Xylans inhibit enzymatic hydrolysis of lignocellulosic materials by cellulases,” Bioresource Technology 121, 8-12. DOI: 10.1016/j.biortech.2012.07.010
Article submitted: October 28, 2019; Peer review completed: December 21, 2019; Revised version received and accepted: September 2, 2020; Published: September 10, 2020.
DOI: 10.15376/biores.15.4.8161-8174
