Abstract
As a renewable agricultural solid waste, Camellia oleifera nut shell (CONS) is often discarded or burned, causing adverse environmental impact and a waste of resources. The purpose of this work was to develop a CONS-based bioadsorbent for the removal of heavy metals. Both CONS and ethanol/NaOH-modified CONS (MCONS) were prepared. The specimens were characterized using physiochemical composition, Fourier transform infrared spectroscopy (FT-IR), and scanning electron microscopy (SEM) coupled with energy dispersive X-ray analysis (EDX). The effects of pH, initial metal concentration, adsorbent dosage, adsorption time, and temperature on the Cr(VI) and Cu(II) removal were evaluated. The adsorption kinetics, isotherms, and thermodynamics were determined. The MCONS sample had a higher carboxyl group content and surface area than the CONS sample, which helped explain its enhanced adsorption performance of heavy metals. The maximum uptake capacity of Cr(VI) and Cu(II) was 16.39 mg/g and 27.26 mg/g for MCONS, compared with 6.34 mg/g and 9.89 mg/g for CONS. The adsorption kinetics for CONS and MCONS fit well with the pseudo-second-order kinetic model. The adsorption isotherms fit well to the Langmuir model. The thermodynamic analyses revealed that the adsorption process was spontaneous and exothermic.
Download PDF
Full Article
Preparation and Characterization of Camellia oleifera Nut Shell-based Bioadsorbent and its Application for Heavy Metals Removal
Yanxin Liu,a,b,d Xiangzhou Li,a,c,* Yulong Wang,b,d Jun Zhou,a and Wanting He b
As a renewable agricultural solid waste, Camellia oleifera nut shell (CONS) is often discarded or burned, causing adverse environmental impact and a waste of resources. The purpose of this work was to develop a CONS-based bioadsorbent for the removal of heavy metals. Both CONS and ethanol/NaOH-modified CONS (MCONS) were prepared. The specimens were characterized using physiochemical composition, Fourier transform infrared spectroscopy (FT-IR), and scanning electron microscopy (SEM) coupled with energy dispersive X-ray analysis (EDX). The effects of pH, initial metal concentration, adsorbent dosage, adsorption time, and temperature on the Cr(VI) and Cu(II) removal were evaluated. The adsorption kinetics, isotherms, and thermodynamics were determined. The MCONS sample had a higher carboxyl group content and surface area than the CONS sample, which helped explain its enhanced adsorption performance of heavy metals. The maximum uptake capacity of Cr(VI) and Cu(II) was 16.39 mg/g and 27.26 mg/g for MCONS, compared with 6.34 mg/g and 9.89 mg/g for CONS. The adsorption kinetics for CONS and MCONS fit well with the pseudo-second-order kinetic model. The adsorption isotherms fit well to the Langmuir model. The thermodynamic analyses revealed that the adsorption process was spontaneous and exothermic.
Keywords: Bioadsorbent; Camellia oleifera nut shell; Heavy metal; Kinetics; Thermodynamics
Contact information: a: School of Materials and Engineering, Central South University of Forestry and Technology, Changsha 410004, PR China; b: School of Chemistry and Bioengineering, Changsha University of Science and Technology, Changsha 410004, PR China; c: State Key Laboratory of Ecological Applied Technology in Forest Area of South China, Changsha 410004, PR China; d: Limerick Pulp and Paper Centre, Department of Chemical Engineering, University of New Brunswick, Fredericton, New Brunswick E3B 5A3, Canada; *Corresponding author: rlxz@163.com
INTRODUCTION
Heavy metals in discharged industrial water are a major environmental problem because they are not biodegradable and tend to accumulate in living organisms (Khabibi et al. 2016). Hexavalent chromium (Cr(VI)) and copper (Cu(II)) are some of the most prevalent examples of heavy metal pollution. The toxicity of Cr depends on its oxidation states (Kong and Ni 2009). Cr(III) is an essential element for living organisms when it is present at a low concentration, but it is toxic at high concentrations (Altun and Pehlivan 2012). Cr(VI) is highly toxic even at a low concentrations for humans, animals, and plants (Srivastava et al. 2015). Cu(II) is an essential micronutrient and beneficial to organisms at a lower concentration (Chen and Wang 2011). However, excessive intake of copper can cause encephalopathy and lasting damage to human kidneys and the reproductive, nervous, and circulatory systems (Ofomaja et al. 2010). The efficient removal of these harmful pollutants poses a significant challenge worldwide.
Among the numerous technologies reported for heavy metal removal, the bioadsorption process is the most promising, with significant advantages including high efficiency, low cost, simple operation, easy regeneration, reduction of chemical sludge, and the possibility of metal recovery (Li et al. 2012; Velazquez-Jimenez et al. 2013). Many agricultural wastes and by-products have been used as eco-friendly bioadsorbents for removing Cr(VI) and Cu(II) in water, for example persimmon leaf (Lee and Choi 2018), Lagerstroemia speciosa bark (Srivastava et al. 2015), rape straw powders (Liu et al. 2018), corn stalk (Cao et al. 2018), rapeseed waste (Tofan et al. 2011), wheat straw (Dang et al. 2009), pine cone powder (Ofomaja et al. 2010), mushroom Pleurotus eryngii (Kan et al. 2015), etc. These bioadsorbents have polar functional groups, such as hydroxyl groups, carboxyl groups, etc., which are believed to be the active sites for the attachment of metal ions.
To improve the adsorption capacity, much effort has been dedicated to reinforcing the functional groups and increasing the number of active sites by chemical or physical pre-treatment methods. Chemical modification is usually performed with organic acids (citric acid, formic acid, acetic acid, acrylic acid), mineral acids (HCl, HNO3, H2SO4), bases and basic solutions (NaOH, KOH), oxidizing agents (H2O2, KMnO4), and many other agents (formaldehyde, CH3OH) (Shukla et al. 2006; Bansal et al. 2009; Boota et al. 2009; Ofomaja et al. 2010; Tan et al. 2010; Liu et al. 2011; Pehlivan et al. 2012; Velazquez-Jimenez et al. 2013; Kong et al. 2014), while physical pre-treatment focuses on the preparation of biochar or activated carbon by heating agricultural wastes with the aid of chemicals (Liu et al. 2011; Kundu et al. 2014; Komkiene 2016).
Camellia oleifera C. Abel (Theaceae) is the leading oil crop cultivated in the south and east of Asia, especially in China, for the production of edible Camellia oleifera oil, which is also called “Eastern olive oil”. The production of Camellia oleifera oil yields about 260,000 tons per year in China. According to the Camellia oleifera industry development planning of China (2009-2020) (State Forestry Administration of the People’s Republic of China 2009), it is predicted that production of C. oleifera will grow unceasingly in the future. However, many residues are generated accordingly, including C. oleifera nut shell (CONS), C. oleifera seed shell (COS), and C. oleifera cake (COC). Most of the residues are discarded or burned in the countryside, causing a waste of resources and serious environmental impact. Among these residues, the CONS represent over 60% of the total weight of C. oleifera fruits. Therefore, utilizing the CONS has attracted the attention of researchers in recent years. It is well known that CONS is often used to prepare biochar or activated carbon. However, there have been few reports of using non-activated CONS for heavy metals removal. Only Guo et al. (2016) and Lu et al. (2013) have reported to use CONS remove Pb(II) and Cr(VI).
The objective of this study was to assess the potential of using CONS as an alternative bioadsorbent for removing Cr(VI) and Cu(II) from aqueous solutions. Both CONS and ethanol/NaOH-modified CONS (marked as MCONS) bioadsorbents were prepared. Their characterizations were performed based on the physiochemical composition, Fourier transform infrared spectroscopy (FT-IR), and scanning electron microscopy (SEM) coupled with energy dispersive X-ray analysis (EDX). The effect of solution pH, initial metal concentration, adsorbent dosage, adsorption time, and adsorption temperature were investigated, and the adsorption kinetics, isotherms, and thermodynamics were also determined.
EXPERIMENTAL
Chemicals
The chemicals used in this study were of analytical grade. Stock solutions (1000 mg/L) of Cr(VI) and Cu(II) were prepared by dissolving 2.828g of K2Cr2O7 in 1 L of distilled water and dissolving 3.9291 g of Cu(SO4)2•5H2O in 1 L of distilled water, respectively. The solutions of different concentrations were prepared by diluting the stock solution with distilled water. The pH of the solution was adjusted with 0.1 M HCl or 0.1 M NaOH by pH meter.
Preparation of Bioadsorbents
Camellia oleifera nut shell (CONS) used in this research was collected in Nov. 2016 in Changsha, Hunan Province, China. Before use, CONS was washed thoroughly with distilled water and dried in an oven at 60 °C for 12 h. The CONS was then ground in a plant crusher (FZ102, Taisite Instrument Co., LTD, Tianjin, China). The CONS particles with diameters from 0.250 to 0.425 mm were collected using sieves (BZS-200, TongQi Instrument Co., LTD, Hangzhou, China) and stored in polyethylene sealed bags for further use.
The collected samples were modified as follows: 24.99 g of oven dried CONS were first rinsed with ethanol for 2 h, then filtered by vacuum filter and air dried. The dried samples were modified with 300 mL of 0.5 M NaOH for 3 h by 200 rpm stirring rate at room temperature. The samples were filtrated and washed with distilled water until they reached neutral pH. Finally, the samples (marked as MCONS) were dried in an oven at 60 °C for 12 h and stored in polyethylene sealed bags. The weight of the oven dried MCONS was 18.43 g.
Characterization of Bioadsorbents
The chemical composition of the CONS and MCONS were determined according to standard methods. The lignin content was carried out according to TAPPI T222 cm-88 (2006). The 1% NaOH solubility and ethanol-toluence solubility were determined by TAPPI T212 om-02 (2002) and TAPPI T204 cm-97 (2007), respectively, and the ash content was determined by TAPPI T211 om-02 (2002). The content of cellulose was obtained by nitric acid-ethanol method (Shi and He 2009). Holocellulose was determined by sodium chlorite treatment according to the Chinese standards of GB/T2677.10 (1995), and the hemicellulose was calculated by subtracting the cellulose from holocellulose.
The FT-IR spectra were obtained on a Nicolet iS5 FT-IR Spectrometer (Montreal, Canada), accumulating 36 scans from 500 to 4000 cm-1 with a resolution of 4 cm-1. Scanning electron microscopy coupled with energy dispersive X-ray analysis (SEM-EDX) was performed on a JEOL 6400 scanning electron microscope (JEOL, Tokyo, Japan) using an accelerating voltage of 15 kV. Samples were attached to mounting stubs with carbon tape and coated with gold for conductivity. Surface area and porosity were determined by mercury intrusion porosimetry on a PoreMaster 33 (Quantachrome, Boynton Beach, FL, USA).
Batch Adsorption Experiments
Each batch biosorption experiment was carried out in 250 mL Erlenmeyer flasks in water bath oscillator (SHA-C model, China) to study the effects of solution pH (1 to 8), initial metal concentration (20 to 160 mg/L), adsorbent dosage (2 to 20 g/L), adsorption time (0.08 to 180 min), and adsorption temperature (288 K to 338 K) on Cr(VI) and Cu(II) removal. After adsorption, the samples were filtered, and the Cr(VI) concentration in the filtrate was measured using the standard colorimetric method using 1,5-diphenylcarbazide reagent (Clesceri et al. 1998) at 540 nm with a UV-Visible spectrophotometer (752 model, Hong Ji Instrument Co., LTD, Shanghai, China). The concentration of Cu(II) was determined with flame atomic adsorption spectroscopy (FAAS; Z-5000, Hitachi, Tokyo, Japan). The removal efficiency of heavy metals and uptake capacity (qe) were calculated according to Eq. 1 and Eq. 2,
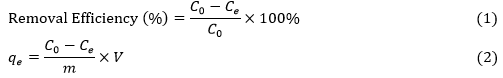
where C0 is the initial metals concentration (mg/L), Ce is the metals concentration (mg/L) at absorption equilibrium, qe is the weight of adsorbed metals per unit mass of adsorbent (mg/g), m is the weight of adsorbent (g), and V is the volume of metals solution (L).
Each experiment was repeated five times, and the mean values were used as experimental data. The differences between the results were smaller than 5%.
RESULTS AND DISCUSSION
Characteristics of CONS and MCONS
Physiochemical properties of CONS and MCONS
The chemical compositions of the CONS and MCONS are shown in Table 1. CONS is mainly composed of cellulose (21.83%), hemicellulose (39.81%), and lignin (28.91%). The three components include hydroxyl, carboxyl, and phenolic groups, which make the CONS capable of binding heavy metals by changing their hydrogen ions to metal ions or giving an electron pair to form complexes with the metal ions (Kumar et al. 2011).

Fig. 1. Solution color of the CONS (a) and MCONS (b) in water
However, CONS also has some water-soluble components, such as tannin, brown pigment, etc. (Qiu et al. 2009). The dissolution of these components in water leads to a brown color of the CONS aqueous solution (Fig. 1a). The dissolved components would bring secondary pollutants to the water and could affect the biosorption process. When the CONS was modified by ethanol/NaOH, as described in the experimental section, the water color of the MCONS was much clearer (Fig. 1b). The chemical composition of the MCONS is also presented in Table 1. It is evident that there was a decrease in the percentage of hemicellulose (27.7%), 1% NaOH solubility (37.0%), and lignin (27.3%), as well as an increase in cellulose (32.2%) and ash (5.3%). Compared with the CONS, the weight of the MCONS lost almost 26.3%. Similar results were obtained by Šoštarić et al. (2018) and Sghaier et al. (2012), who treated apricot shells and agava fiber (Agava americana L.) in NaOH solutions, respectively. The results in this paper showed that tannin, brown pigment, etc. in the CONS was dissolved by ethanol and NaOH, and their removal decreased the color of the MCONS suspension in water (Fig. 1b).
Table 1. Physiochemical Properties of CONS and MCONS
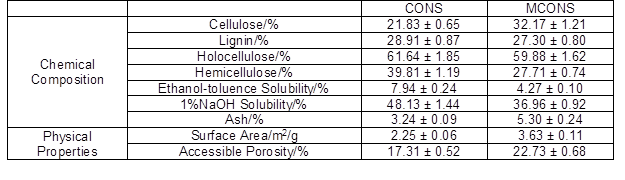
Mercury porosimetry was chosen to determine the physical property changes of the CONS and MCONS. Table 1 shows that the surface area and accessible porosity of the MCONS were higher than those of the CONS, indicating the chemical treatment had partially dissolved some components (such as hemicellulose, tannin, brown pigment, etc.) in the CONS, and thus the surface area and accessible porosity increased. This phenomenon was observed via SEM of the CONS and MCONS, which is discussed later.
FT-IR analysis
The FT-IR spectra of the CONS and MCONS are shown in Fig. 2. For both the CONS and MCONS, the broad peak at 3330 cm-1 is due to the O-H stretching vibrations of cellulose, hemicellulose, and lignin. The peak at around 2925 cm-1 is attributed to the C-H stretching vibrations of methyl, methylene, and methoxy groups (Feng et al. 2011). The band observed at 1604 cm-1 is attributed to asymmetric stretching of the lignin aromatic ring. The peak at 1025 cm-1 is associated with the stretching of alcoholic groups (-OH) (Velazquez-Jimenez et al. 2013).
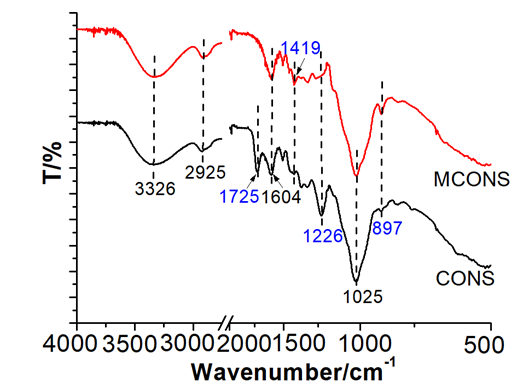
Fig. 2. FT-IR spectra of the CONS and MCONS
Compared with the CONS sample, there were some obvious changes in the MCONS FT-IR spectrum. One is the disappearance of the peak at 1725 cm-1, which is due to the stretching vibration of C=O in the ester groups. The same result was obtained by Šoštarić et al. (2018), who found that the peak at 1732 cm-1 disappeared after the apricot shells were treated with alkali. Another difference is the decreased intensity at 1226 cm-1, which is the C-O-C stretching from aryl-alkyl ether linkage (Yang et al. 2007). This was attributed to the partial cleavage of C-O-C in alkali solutions. Besides, the intensified peaks at 1419 cm-1 and 897 cm-1 of the MCONS sample were indicative of the increased COO- content (Šoštarić et al. 2018) and the increased O-H contents, respectively, implying that the MCONS sample will have a more enhanced adsorption capacity for heavy metals than the CONS.
SEM-EDX analysis
Figure 3 shows the SEM-EDX results of the CONS, MCONS, MCONS-Cu, and MCONS-Cr samples. Significant differences of surface morphology between the CONS and MCONS were evident (Fig. 3a vs. Fig. 3b). The CONS showed smooth and/or even surfaces, while after the modification, the MCONS surfaces were irregular and rough, with some newly formed pores. Table 1 exhibits that the accessible porosity of the CONS and MCONS was 17.3% and 22.7%, respectively. The increase in the accessible pores allowed the MCONS to have increased adsorption capacity for heavy metals.
The EDX spectra of the CONS and MCONS are shown in Figs. 3a and 3b, respectively. The spectra reveal the presence of potassium ions on the surface of the CONS while sodium ions appear on the surface of the MCONS, which was introduced by the ethanol/NaOH pretreatment.
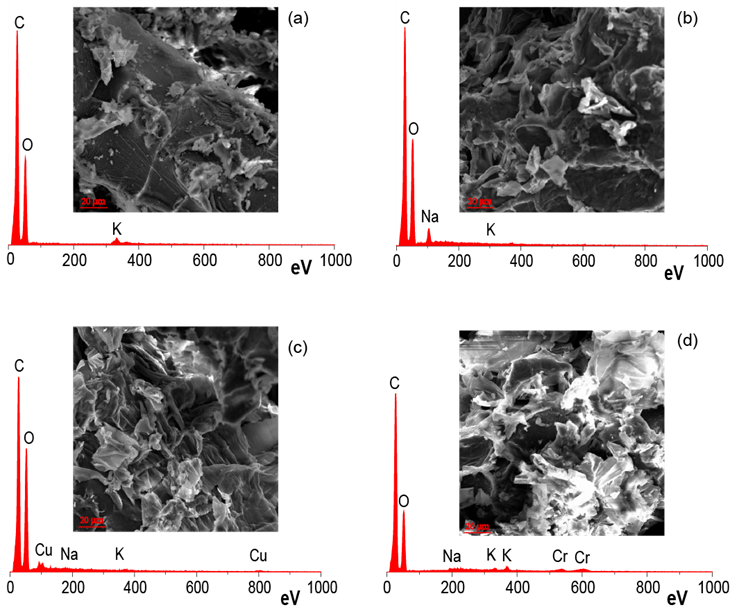
Fig. 3. SEM-EDX of the CONS(a), MCONS(b), MCONS-Cu(c), and MCONS-Cr(d)
Figures 3c and 3d illustrate the surface morphology of the MCONS after the adsorption of Cu(II) and Cr(VI). Compared with Fig. 3b, there were no obvious changes on the surface of the MCONS-Cu and MCONS-Cr. The EDX spectra (Fig. 3c and 3d) confirmed the presence of Cu and Cr on the MCONS, supporting the conclusion that transition metal ions were successfully adsorbed onto the MCONS.
Effect of Operating Parameters
Effect of pH Value
The solution pH has a significant impact on the removal of heavy metals because it determines not only the surface charge of the adsorbent but also the degree of ionization and speciation of the metal ions in the solution (Kołodyńska et al. 2012). The effects of the pH on Cr(VI) and Cu(II) removal were performed with varying pH from 1.0 to 8.0 under the conditions of initial metal concentration of 100 mg/mL, adsorption dosage of 10 g/L, adsorption temperature of 298 K, and adsorption time of 60 min.
Figure 4a shows that the removal efficiency of Cr(VI) by the MCONS and CONS decreased while the removal efficiency of Cu(II) by the MCONS and CONS increased with the solution pH increasing from 1.0 to 8.0. This is due to the different chemical properties of Cr(VI) and Cu(II). As for the Cr(VI), the dominant species of Cr(VI) is HCrO4- at lower pH, and when the pH increases, HCrO4- is converted to CrO42- and Cr2O72- (Hyder et al. 2015). At lower pH (1.0 to 5.0), the surface of the CONS and MCONS are positive charged with H+ ion, which can cause higher removal efficiency due to the electrostatic attraction between positive surface and HCrO4-. At higher pH (5.0 to 8.0), the surface of the CONS and MCONS become negatively charged. The rapid decrease of removal efficiency may be due to the decreased electrostatic attraction between the Cr anionic species (CrO42- and Cr2O72-) and anionic surface of the CONS and MCONS. As for the Cu(II), H+ competes with Cu(II) at lower pH, causing a decrease in removal efficiency. When pH increases, the surface of the CONS and MCONS are negatively charged, which favored Cu(II) adsorption due to electrostatic interaction, thus leading to the increase of removal efficiency. According to the FT-IR analysis of the CONS and MCONS, there is significant carboxyl and hydroxyl groups content in the MCONS, so the adsorption efficiency of Cr(VI) and Cu(II) by the MCONS is much higher than that by the CONS.
As the initial pH of these two metals solution with the CONS and MCONS is around 5.0, and at these two pH ranges, higher biosorption efficiency can also be achieved, so there was no need to adjust the initial pH value. Thus, the optimum pH value of the following experiments in this research was set at around 5.0.
Effect of adsorbent dosage
The optimal choice of adsorbent dosage is based on the adsorption of more heavy metals with a small amount of adsorbent. In order to achieve this result, the effect of adsorbent dosage on Cr(VI) and Cu(II) removal was evaluated under the conditions of initial metal concentration of 100 mg/mL, pH of 5.0, adsorption temperature of 298 K, and adsorption time of 60 min. The removal efficiency of Cr(VI) and Cu(II) is shown in Fig. 4b. It can be clearly seen that more dosage of the adsorbent can get more Cr(VI) and Cu(II) removal efficiency, and when the amount of the adsorbent reaches a certain value (more than 10 g/L), the adsorption efficiency tends to be stabilized. The main reason is that the increase in adsorbent dosage leads to an increase in the specific surface area, which also increases the probability of contact of the adsorbent with heavy metals, leading to the increase of removal efficiency. But when the dosage of metal ions increases to an extent, the adsorption sites tends to saturation, leading to a decrease in the availability of active sites on the adsorbent. As a consequent, the removal efficiency declined. Thus, the dosage of 10g/L was considered as optimum dose and used in our study.
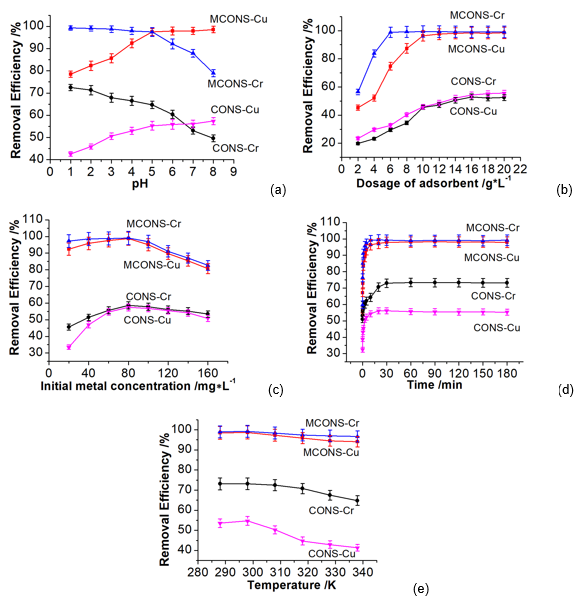
Fig. 4. Effect of operating parameters on Cr(VI) and Cu(II) removal by the CONS and MCONS
Effect of initial metal concentration
The effect of initial metal concentration on Cr(VI) and Cu(II) removal was evaluated under the conditions of pH of 5.0, adsorbent dosage of 10 g/L, adsorption temperature of 298 K, and adsorption time of 60 min. Figure 4c shows that the removal efficiency of Cr(VI) and Cu(II) by the CONS and MCONS first increased with increasing initial metal concentration from 20 mg/L to 80 mg/L, and then it decreased with higher initial metal concentration. The highest removal efficiency can be achieved at 80 mg/L. Sahmoune et al. (2011) have reported that heavy metal ions can transfer from the solution to the surface of adsorbent owing to a driving force made by the initial metal concentration. So at lower initial concentration, the driving force increases with increasing initial metal concentration, and more metals can be adsorbed. The removal efficiency increases accordingly. On the other hand, higher initial metal concentration can saturate adsorption sites of the adsorbent surface and then decrease the removal efficiency. The best initial metal concentration for this research was judged to be 80 mg/L.
Effect of adsorption time
The effects of adsorption time on the removal of Cr(VI) and Cu(II) were evaluated under the conditions of pH of 5.0, adsorbent dosage of 10 g/L, adsorption temperature of 298 K, and initial metal concentration of 80 mg/L. Figure 4d shows that the MCONS had very high Cr(VI) and Cu(II) removal efficiency, 91.2% and 83.7% for Cr(VI) and Cu(II), respectively, within 1 min, while the CONS had only 59.4% of Cr(VI) and 45.5% of Cu(II) removal within the same time. Both Cr(VI) and Cu(II) removal by the CONS and MCONS increased rapidly in the first 20 to 30 min to the maximum value and then remained almost unchanged. The results show that the removal of Cr(VI) and Cu(II) by the CONS and MCONS were fast adsorption processes. To get better removal efficiency, 60 min was considered to be sufficient for the adsorption equilibrium.
Effect of adsorption temperature
The effect of temperature on Cr(VI) and Cu(II) removal was studied with the conditions of pH of 5.0, adsorbent dosage of 10 g/L, adsorption time of 60 min, and initial metal concentration of 80 mg/L. Figure 4e shows that the removal of both Cr(VI) and Cu(II) decreased with the temperature rising from 298 K to 338 K, which indicates that the adsorption of Cr(VI) and Cu(II) by the CONS and MCONS was exothermic. Similar trends were obtained with the biosorption of Cd(II) onto cashew nut shells (Kumar et al. 2012) as well as Cu(II) and Zn(II) onto Citrus reticulate (Boota et al. 2009). According to the results, 298 K was chosen for this study.
Adsorption Kinetics
The adsorption kinetics are always used to examine the controlling mechanism of the adsorption process. In this study, the pseudo-first-order kinetic model and pseudo-second-order kinetic model were chosen to test the experimental data. The pseudo-first-order kinetic model assumes that the uptake rate of heavy metals with time is directly proportional to the amount of available active sites on the adsorption surface (Elhafez et al. 2016). The pseudo-second-order kinetic model predicts the behavior over the whole adsorption time and is in agreement with the adsorption mechanism being the rate-controlling step (Wu et al. 2013). Mathematically, the models are given in Eqs. 3 and 4.
Pseudo-first-order kinetic model:
![]()
Pseudo-second-order kinetic model:
![]()
where qt(mg/g) and qe(mg/g) are the amount of metals adsorbed onto the adsorbent at a fixed time and at equilibrium, respectively, k1(min-1) is the adsorption rate constant for the pseudo-first-order and can be calculated from the slope of the linear plot of ln (qe-qt) vs. t, and k2(g/mg•min-1) is the adsorption rate constant for the pseudo-second-order and can be calculated from the intercept and slope of the linear plot of t/qe vs. t.
The comparison of the experimental data for the pseudo-first-order and pseudo-second-order are shown in Fig. 5a and Fig. 5b, and the corresponding parameters of these two models are presented in Table 2.
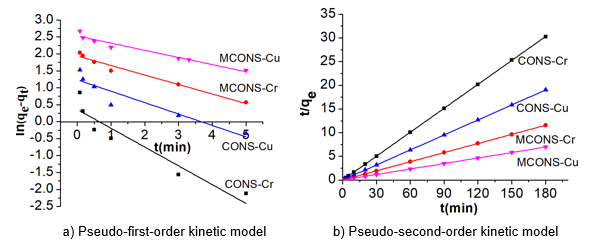
Fig. 5. Adsorption kinetics of Cr(VI) and Cu(II) by the CONS and MCONS
Table 2. Fitting Parameters of the Kinetics Models
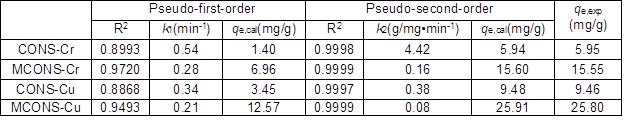
Figure 5 and Table 2 show that the coefficients of determination R2 of the pseudo-first-order model for the CONS and MCONS adsorbing Cr(VI) and Cu(II) were 0.8993, 0.8868, 0.9720, and 0.9493, respectively, with the calculated qe of 1.40 mg/g, 3.45 mg/g, 6.96 mg/g, and 12.57 mg/g, respectively. The coefficients of determination R2 of pseudo-second-order model for the CONS and MCONS adsorbing Cr(VI) and Cu(II) were 0.9998, 0.9997, 0.9999, and 0.9999 with the calculated qe of 5.94 mg/g, 9.48 mg/g, 15.60 mg/g, and 25.91 mg/g, respectively.
The qe calculated by the pseudo-second-order model were close to the experimental data (Table 2). Thus, these results show that the adsorption of Cr(VI) and Cu(II) by the CONS and MCONS fit better to the pseudo-second-order model than to the pseudo-first-order model.
Adsorption Isotherms
Adsorption isotherms can describe the relationship between the mass of the adsorbed heavy metals per sorbent mass and the concentration of the component in the solution (Witek-Krowiak et al. 2011). Two widely-used adsorption isotherms were chosen in this study, the monolayer adsorption model developed by Langmuir and the multilayer adsorption model by Freundlich.
The Langmuir adsorption model fits the following equation:

The Freundlich adsorption model is valid for heterogeneous surfaces, fitting the following equation,
![]()
where Ce is the metals concentration in the solution at equilibrium (mg/L), qe is the weight adsorbed metals per unit mass of adsorbent (mg/g), qm is the maximum monolayer adsorption capacity (mg/g), b is the Langmuir constant. The parameter n is the Freundlich constant. For n values in the range 0.1 < 1/n < 1, adsorption is favorable. KF is the constant of Freundlich adsorption.
The adsorption isotherms were obtained experimentally by using several initial concentrations of metals. The corresponding Langmuir and Freundlich isotherms parameters are presented in Table 3.
Table 3. Langmuir and Freundlich parameters for Cr(VI) and Cu(II)
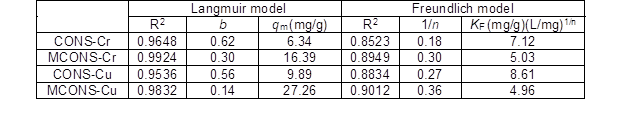
Table 3 shows that the values of the coefficients of determination, R2, for the Langmuir adsorption model were all higher than those for the Freundlich adsorption model, indicating that the Langmuir isotherm gave a better fit. This result suggested that the biosorption of Cr(VI) and Cu(II) by the CONS and MCONS was dominated by mono-layer adsorption. According to Table 3, the maximum monolayer adsorption capacities, qm, calculated according to the Langmuir adsorption model are 6.3 mg/g and 16.4 mg/g for Cr(VI) by the CONS and MOCNS, 9.9 mg/g and 27.3 mg/g for Cu(II) by the CONS and MOCNS, respectively. The adsorption capacity of heavy metals is caused not only the difference in properties of heavy metals (such as the molecular size, electronegativity, and affinity), but also the heavy metals selectivity of the functional groups of the adsorbents. Table 4 shows the comparison of Cr(VI) and Cu(II) maximum capacity by other different bioadsorbents reported in the literature. It is apparent that the CONS and MCONS had favorable adsorption results for Cr(VI) and Cu(II) in comparison to some other bioadsorbents, supporting the conclusion that the CONS is a very promising potential adsorbent to remove heavy metals from aqueous solutions.
Table 4. Comparison of Maximum Adsorption Capacity of Cr(VI) and Cu(II) by Different Bioadsorbents
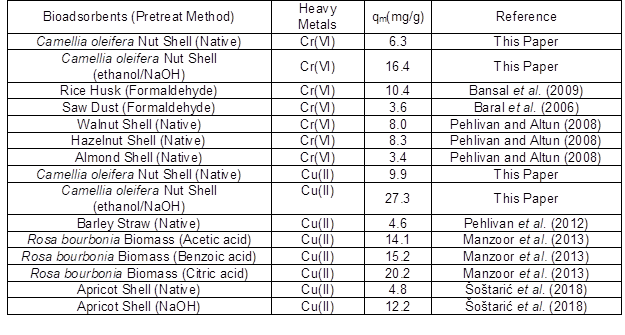
Although the Freundlich adsorption model, according to the R2 value, did not fit the adsorption data perfectly, the values of 1/n (seen in Table 3) were in the range of 0.1 to 1.0, indicating relatively strong adsorption of Cr(VI) and Cu(II) by the CONS and MCONS.
Adsorption Thermodynamics
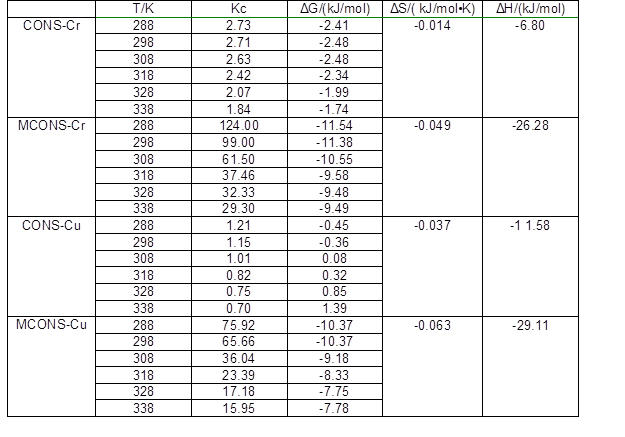
To further elucidate the biosorption process, the thermodynamic parameters, free energy (ΔG), enthalpy (ΔH), and entropy (ΔS) are often used. The values of ΔH and ΔS were calculated according to the intercepts and slopes of the van’t Hoff plots of lnKc vs. 1/T (Eq. 7), which were found to be nearly linear (Fig. 6). The values of ΔG at different temperatures were obtained according to Eq. 8; the parameters calculated are shown in Table 5.

where Kc is distribution coefficient and is calculated from the following equation: Kc=CAe/Ce, and where CAe (mg/L) and Ce (mg/L) are the equilibrium concentrations for solute on the sorbent and in the solution, respectively. R is the gas constant (8.314 J/mol K), and T is the temperature of the adsorption process (K).
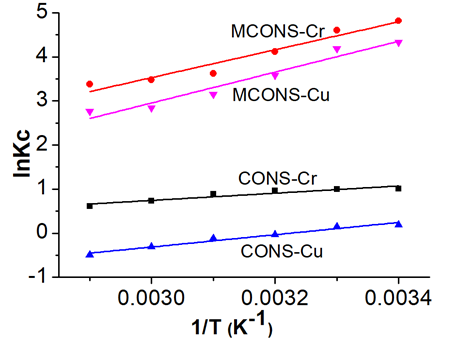
Fig. 6. The van’t Hoff plots for Cr(VI) and Cu(II) removal by the CONS and MCONS
As can be seen in Table 5, the values of ΔG were all negative, indicating the spontaneous nature for removal of Cr(VI) and Cu(II) by the CONS and MCONS. Moreover, the negative value of ΔH suggests that the sorption was exothermic, which is in accordance with the results drawn from the temperature effect on absorption. The negative value of ΔS confirmed the decreases in randomness at the adsorbent and solution interface, indicating the high probability of adsorption. These analyses have shown that removal of Cr(VI) and Cu(II) by both CONS and MCONS is spontaneous and exothermic, which means that the removal process occurs easily.
Table 5. Comparison of Thermodynamic Parameters
CONCLUSIONS
Compared with the Camellia oleifera nut shell (CONS), the ethanol/NaOH modified CONS (MCONS) had less content of hemicellulose, lignin, ethanol-toluence solubility, 1%NaOH solubility, and more carboxyl group content and higher surface area, which can explain why the MCONS sample had higher Cu(II) and Cr(VI) uptake than the CONS sample.
The adsorption kinetics of Cr(VI) and Cu(II) by the CONS and MCONS fit well with the pseudo-second-order model. The adsorption isotherms suggested the biosorption process was mainly dominated by monolayer adsorption. The thermodynamic results demonstrated that the adsorption process was spontaneous and exothermic.
Camellia oleifera nut shell was effective for Cr(VI) and Cu(II) removal in aqueous solutions. The best adsorption conditions for CONS and MOCNS to remove Cu(II) and Cr(VI) in aqueous solution are pH of 5.0, an initial metal concentration of 80 mg/L, adsorbent dosage of 10g/L, adsorption temperature of 298 K, and adsorption time of 60 min.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Yonghao Ni (Professor and Chair, Department of Chemical Engineering, University of New Brunswick) for his insightful technical advice and help in polishing this manuscript and for the support of the forestry science and technology plan projects of Hunan Province, China, Grant No. 20131028.
REFERENCES CITED
Altun, T., and Pehlivan, E., (2012). “Removal of Cr (VI) from aqueous solutions by modified walnut shells,” Food Chem. 132(2), 693-700. DOI: 10.1016/j.foodchem.2011.10.099
Bansal, M., Garg, U., Singh, D., and Garg, V. K. (2009). “Removal of Cr(vi) from aqueous solutions using pre-consumer processing agricultural waste: A case study of rice husk,” J. Hazard. Mater. 162(1), 312-320. DOI: 10.1016/j.jhazmat.2008.05.037
Baral, S. S., Das, S. N., and Rath, P. (2006). “Hexavalent chromium removal from aqueous solution by adsorption on treated sawdust,” Biochem. Eng. J. 31(3), 216-222. DOI: 10.1016/j.bej.2006.08.003
Boota, R., Bhatti, H. N., and Hanif, M. A. (2009). “Removal of Cu(II) and Zn(II) using lignocellulosic fiber derived from Citrus reticulata (Kinnow) waste biomass,” Sep. Sci. Technol. 44, 4000-4022. DOI: 10.1080/01496390903183196
Cao, W., Wang, Z., Ao, H., and Yuan, B. (2018). “Removal of Cr (VI) by corn stalk based anion exchanger: The extent and rate of Cr (VI) reduction as side reaction,” Colloids and Surfaces A: Physicochemical and Engineering Aspects 539, 424-432. DOI: 10.1016/j.colsurfa.2017.12.049
Chen, Y., and Wang, J. (2011). “Preparation and characterization of magnetic chitosan nanoparticles and its application for Cu (II) removal,” Chem. Eng. J. 168(1), 286-292. DOI: 10.1016/j.cej.2011.01.006
Clesceri, L. S., Greenberg, A. E., and Eaton (Eds.), A.D. (1998). “Standard Methods for the Examination of Water and Wastewater,” 20th Ed., American Public Health Association, Washington, DC.
Dang, V. B. H., Doan, H. D., Dang-Vu, T., and Lohi, A. (2009). “Equilibrium and kinetics of biosorption of cadmium (II)) and copper (II) ions by wheat straw,” Bioresource Technol. 100, 211-219. DOI: 10.1016/j.biortech.2008.05.031
Elhafez, S. E. A., Hamad, H. A., Zaatout, A. A., and Malash, G. F. (2016). “Management of agricultural waste for removal of heavy metals from aqueous solution: Adsorption behaviors, adsorption mechanisms, environmental protection, and techno-economic analysis,” Environ. Sci. Pollut. R. 1-19. DOI: 10.1007/s11356-016-7891-7
Feng, N., Guo, X., Liang, S., Zhu, Y., and Liu, J. (2011). “Biosorption of heavy metals from aqueous solutions by chemically modified orange peel,” J. Hazard. Mater. 185(1), 49-54. DOI: 10.1016/j.jhazmat.2010.08.114
GB/T 2677.20 (1995). “Fibrous material – Determination of holocellulose,” Standardization Administration of China, Beijing, China.
Guo, H. Q., Yan, L. S., Song, D. Y., and Li, K. X. (2016). “Citric acid modified Camellia oleifera shell for removal of crystal violet and Pb(II): Parameters study and kinetic and thermodynamic profile,” Desalin. Water Treat. 57(33), 15373-15383, DOI: 10.1080/19443994.2015.1072057
Hyder, A. H. M. G., Begum, S. A., and Egiebor, N. O. (2015). “Adsorption isotherm and kinetic studies of hexavalent chromium removal from aqueous solution onto bone char,” J. Environ. Chem. Eng. 3(2), 1329-1336. DOI: 10.1016/j.jece.2014.12.005
Kan, S. H., Sun, B. Y., Xu, F., Song, Q. X., and Zhang, S. F. (2015). “Biosorption of aquatic copper (II) by mushroom biomass Pleurotus eryngii: Kinetic and isotherm studies,” Water Sci. Technol. 71(2), 283-288. DOI: 10.2166/wst.2014.511
Khabibi, J., Syafii, W., and Sari, R. K. (2016). “Reducing hazardous heavy metal ions using mangium bark waste,” Environ. Sci. Pollut. R. 23(16), 16631-16640. DOI: 10.1007/s11356-016-6776-0
Kołodyńska, D., Wnętrzak, R., Leahy, J. J., Hayes, M. H. B., Kwapiński, W., and Hubicki, Z. (2012). “Kinetic and adsorptive characterization of biochar in metal ions removal,” Chem. Eng. J. 197, 295-305. DOI: 10.1016/j.cej.2012.05.025
Komkiene, J. (2016). “Biochar as adsorbent for removal of heavy metal ions cadmium(ii), copper(ii), lead(ii), zinc(ii)] from aqueous phase,” Int. J. Environ. Sci. Technol. 13(2), 471-482. DOI: 10.1007/s13762-015-0873-3
Kong, F., and Ni, Y. (2009). “Development of cellulosic paper-based test strips for Cr(VI) determination,” BioResources 4(3), 1088-1097. DOI: 10.15376/biores.4.3.1088-1097
Kong, W., Ren, J., Wang, S., and Chen, Q. (2014). “Removal of heavy metals from aqueous solutions using acrylic-modified sugarcane bagasse-based adsorbents: Equilibrium and kinetic studies,” BioResources 9(2), 3184-3196. DOI: 10.15376/biores.9.2.3184-3196
Kumar, J., Balomajumder, C., and Mondal, P. (2011). “Application of agro-based biomasses for zinc removal from wastewater-a review,” Clean-Soil Air Water 39, 641-652. DOI: 10.1002/clen.201000100
Kumar, P. S., Ramalingam, S., Sathyaselvabala, V., Kirupha, S. D., Murugesan, A., and Sivanesan, S. (2012). “Removal of Cd(II) from aqueous solution by agricultural waste cashew nut shell,” Korean J. Chem. Eng. 29, 756-768. DOI: 10.1007/s11814-011-0259-2
Kundu, A., Redzwan, G., Sahu, J. N., Mukherjee, S., Gupta, B. S., and Hashim, M. A. (2014). “Hexavalent chromium adsorption by a novel activated carbon prepared by microwave activation,” BioResources 9(1), 1498-1518. DOI: 10.15376/biores.9.1.1498-1518
Lee, S. Y., and Choi, H. J. (2018). “Persimmon leaf bio-waste for adsorptive removal of heavy metals from aqueous solution,” J. Environ. Manage. 209, 382-392. DOI: 10.1016/j.jenvman.2017.12.080
Li, W., Gong, X., Li, X., Zhang, D., and Gong, H. (2012). “Removal of Cr(VI) from low-temperature micro-polluted surface water by tannic acid immobilized powdered activated carbon,” Bioresource Technol. 113, 106-113. DOI: 10.1016/j.biortech.2011.12.037
Liu, X., Chen, Z. Q., Han, B., Su, C. L., Han, Q., and Chen, W. Z. (2018). “Biosorption of copper ions from aqueous solution using rape straw powders: Optimization, equilibrium and kinetic studies,” Ecotox. Environ. Safe. 150, 251-259. DOI: 10.1016/j.ecoenv.2017.12.042
Liu, X., Fatehi, P., and Ni, Y. (2011). “Adsorption of lignocelluloses dissolved in prehydrolysis liquor of kraft-based dissolving pulp process on oxidized activated carbons,” Ind. Eng. Chem. Res. 50(20), 11706-11711. DOI: 10.1021/ie201036q
Lu, Y. D., Wu, W.L., Lin, S., and Wu, Z. H. (2013). “Removal of Cr(VI) from aqueous solution using Camellia oleifera Abel shells,” Materials Science Forum. Vols. 743-744, 463-468. DOI: 10.4028/www.scientific.net/MSF.743-744.463
Manzoor, Q., Nadeem, R., Iqbal, M., Saeed, R., and Ansari, T. M. (2013). “Organic acids pretreatment effect on Rosa bourbonia phyto-biomass for removal of Pb (II) and Cu (II) from aqueous media,” Bioresource Technol. 132, 446-452. DOI: 10.1016/j.biortech.2013.01.156
Ofomaja, A. E., Naidoo, E. B., and Modise, S. J. (2010). “Biosorption of copper(II) and lead(II) onto potassium hydroxide treated pine cone powder,” J. Environ. Manage. 91(8), 1674-1685. DOI: 10.1016/j.jenvman.2010.03.005
Pehlivan, E., and Altun, T. (2008). “Biosorption of chromium (VI) ion from aqueous solutions using walnut, hazelnut and almond shell,” J. Hazard. Mater. 155(1-2), 378-384. DOI: 10.1016/j.jhazmat.2007.11.071
Pehlivan, E., Altun, T., and Parlayici, Ş. (2012). “Modified barley straw as a potential biosorbent for removal of copper ions from aqueous solution,” Food Chem. 135(4), 2229-2234. DOI: 10.1016/j.foodchem.2012.07.017
Qiu, H., Chen J., and He J. (2009). “Microwave extraction and stability of brown pigment from Camellia oleifera shells,” Food Science 30(16), 198-202.
Sahmoune, M. N., Louhab, K., and Boukhiar, A. (2011). “Advanced biosorbents materials for removal of chromium from water and wastewaters,” Environ Prog Sustain 30(3), 284-293. DOI: 10.1002/ep.10473
Sghaier, A. E. O. B., Chaabouni, Y., Msahli, S., and Sakli, F. (2012). “Morphological and crystalline characterization of NaOH and NaOCl treated Agave americana L. fiber,” Ind. Crop. Prod. 36(1), 257-266. DOI: 10.1016/j.indcrop.2011.09.012
Shi, S., and He, F. (2009). Analysis and Detection of Pulping and Papermaking, Chinese Light Industry Press, Beijing.
Shukla, S. R., Pai, R. S., and Shendarkar, A. D. (2006). “Adsorption of Ni(II), Zn(II) and Fe(II) on modified coir fibres,” Sep. Purif. Technol. 47(3), 141-147. DOI: 10.1016/j.seppur.2005.06.014
Šoštarić, T. D., Petrović, M. S., Pastor, F. T., Lončarević, D. R., Petrović, J. T., Milojković, J. V., and Stojanović, M. D. (2018). “Study of heavy metals biosorption on native and alkali-treated apricot shells and its application in wastewater treatment,” J. Mol. Liq. 259, 340-349. DOI: 10.1016/j.molliq.2018.03.055
Srivastava, S., Agrawal, S. B., and Mondal, M. K. (2015). “Biosorption isotherms and kinetics on removal of Cr(VI) using native and chemically modified Lagerstroemia speciosa bark,” Ecol. Eng. 85, 56-66. DOI: 10.1016/j.ecoleng.2015.10.011
State Forestry Administration (2009). Camellia oleifera Industry Development Planning of P.R. China (2009-2020), Beijing, China.
Tan, G., Yuan, H., Liu, Y., and Xiao, D. (2010). “Removal of lead from aqueous solution with native and chemically modified corncobs,” J. Hazard. Mater. 174(1-3), 740-745. DOI: 10.1016/j.jhazmat.2009.09.114
TAPPI T212 om-02 (2002). “One percent sodium hydroxide solubility of wood and pulp,” TAPPI Press, Atlanta, GA.
TAPPI T222 cm-88 (2006). “Acid-insoluble lignin in wood and pulp,” TAPPI Press, Atlanta, GA.
TAPPI T204 cm-97 (2007). “Solvent extractives of wood and pulp,” TAPPI Press, Atlanta, GA.
TAPPI T211 om-02 (2002). “Ash in wood, pulp, paper and paperboard,” TAPPI Press, Atlanta, GA.
Tofan, L., Paduraru, C., Volf, I., and Toma, O. (2011). “Waste of rapeseed from biodiesel production as a potential biosorbent for heavy metal ions,” BioResources 6(4), 3727-3741. DOI: 10.15376/biores.6.4.3727-3741
Velazquez-Jimenez, L. H., Pavlick, A., and Rangel-Mendez, J. R. (2013). “Chemical characterization of raw and treated agave bagasse and its potential as adsorbent of metal cations from water,” Ind. Crop. Prod. 43, 200-206. DOI: 10.1016/j.indcrop.2012.06.049
Witek-Krowiak, A., Szafran, R. G., and Modelski, S. (2011). “Biosorption of heavy metals from aqueous solutions onto peanut shell as a low-cost biosorbent,” Desalination 265, 126-134. DOI: 10.1016/j.desal.2010.07.042
Wu, Y., Wen, Y., Zhou, J., Cao, J., Jin, Y., and Wu, Y. (2013). “Comparative and competitive adsorption of Cr(VI), As(III), and Ni(II) onto coconut charcoal,” Environ. Sci. Pollu. R. 20(4), 2210-2219. DOI: 10.1007/s11356-012-1066-y
Yang, H., Yan, R., Chen, H., Lee, D. H., and Zheng, C. (2007). “Characteristics of hemicellulose, cellulose and lignin pyrolysis,” Fuel 86(12-13), 1781-1788. DOI: 10.1016/j.fuel.2006.12.013
Article submitted: July 25, 2018; Peer review completed: October 20, 2018; Revised version received and accepted: November 8, 2018; Published: November 14, 2018.
DOI: 10.15376/biores.14.1.234-250
