Abstract
Non-crystal microporous starch (NCMS) containing microporous and amorphous structures was prepared from native corn starch by heat treatment and solvent exchange. NCMS can be used as fillers, coatings, and raw materials in the preparation of various denatured starch because of its specific surface area and amorphous region. However, the hydrophilicity of NCMS limits its applications in papermaking. Thus, in this study, NCMS was reacted with alkyl ketene dimer (AKD) to prepare hydrophobic NCMS (H–NCMS), which is more stable, and convenient for storage and use in surface sizing. The optimal preparation conditions were selected using single-factor tests. The product, which was prepared at 55 °C for 3 h with an AKD dosage of 80%, had a sizing degree of 67 s. Characterization by X-ray diffraction (XRD), scanning electron microscopy (SEM), and Fourier transform infrared (FT-IR) spectroscopy confirmed that NCMS, H–NCMS, and sized paper were obtained. The thermal stability and hydrophobicity of the paper were measured using thermogravimetric analysis (TGA) and water contact angles, respectively. The results indicated that the sized paper has excellent thermal stability and hydrophobicity after surface sizing with 0.9% H–NCMS. Food wrapping paper with excellent strength and hydrophobicity was successfully prepared using H–NCMS and ultrasonic-assisted wheat straw pulp (UWP).
Download PDF
Full Article
Preparation and Characterization of Hydrophobic Non-Crystal Microporous Starch (NCMS) and its Application in Food Wrapper Paper as a Sizing Agent
Chao Dang,a Ming Xu,a,b Yihui Yin,a and Junwen Pu a,*
Non-crystal microporous starch (NCMS) containing microporous and amorphous structures was prepared from native corn starch by heat treatment and solvent exchange. NCMS can be used as fillers, coatings, and raw materials in the preparation of various denatured starch because of its specific surface area and amorphous region. However, the hydrophilicity of NCMS limits its applications in papermaking. Thus, in this study, NCMS was reacted with alkyl ketene dimer (AKD) to prepare hydrophobic NCMS (H–NCMS), which is more stable, and convenient for storage and use in surface sizing. The optimal preparation conditions were selected using single-factor tests. The product, which was prepared at 55 °C for 3 h with an AKD dosage of 80%, had a sizing degree of 67 s. Characterization by X-ray diffraction (XRD), scanning electron microscopy (SEM), and Fourier transform infrared (FT-IR) spectroscopy confirmed that NCMS, H–NCMS, and sized paper were obtained. The thermal stability and hydrophobicity of the paper were measured using thermogravimetric analysis (TGA) and water contact angles, respectively. The results indicated that the sized paper has excellent thermal stability and hydrophobicity after surface sizing with 0.9% H–NCMS. Food wrapping paper with excellent strength and hydrophobicity was successfully prepared using H–NCMS and ultrasonic-assisted wheat straw pulp (UWP).
Keywords: Non-crystal microporous starch; Alkyl ketene dimer; Hydrophobic; Food wrapper paper; Ultrasonic-assisted wheat straw pulp
Contact information: a: MOE Engineering Research Center of Forestry Biomass Materials and Bioenergy, Beijing Forestry University, Beijing, 100083, P R China; b: Beijing Zhongke Audible and Ultrasound Technology Institute, 100083, P R China; *Corresponding author: jwpu@bjfu.edu.cn
INTRODUCTION
Starch, a biopolymer from cereal and tuber crops (Glenn et al. 2008), has very wide applications and has been used into many kinds of products such as various food and paper, textiles, cosmetics, and other industrial materials due to its biodegradability, availability, and low cost. However, the application of pure starch in these industries has been challenging because of certain limitations, including poor processing, fragility, and incompatibility with several hydrophobic polymers. To address these shortcomings, numerous studies have been carried out to obtain several starch derivatives through physical or chemical modification. Examples of starch that have undergone physical modification include non-crystalline granular starch and microporous starch. Non-crystalline granular starch has a granular shape without a crystalline area. Several strategies have been applied to prepare such materials, among them: high pressure (Stute et al. 1996), rotary-type mill (Tamaki et al. 1998), and heating (Garcia et al. 1997; Zhang et al. 2012). This type of starch has many advantages, and its physical and chemical reactions can be accelerated (Liu et al. 2010).
Because of its unique properties such as non-toxicity, biodegradability, high specific surface area, and high brightness, starch with a porous structure has board ranges of present and potential applications including effective opacifying material, filler (Saari et al. 2005; Wang and Yang 2011), coating (El-Tahlawy et al. 2007), and wood adhesive (Liu and Li 2007). Several authors, have demonstrated the possibility to convert native starch on porous structure by means of extrusion (Zhang and Sun 2007; Willett 2009), baking (Glenn et al. 2001; Shey et al. 2006; Vercelheze et al. 2012; Mello and Mali 2014), microwave heating (Lopez-Gil et al. 2015), and solvent exchange (Patel et al. 2010). In particular, solvent exchange can be employed to obtain a porous structure in starch at a low cost. By this method, the solvent (water) exhibiting a relatively high surface tension is replaced with the solvent exhibiting low surface tension (ethanol), which in turn precipitates the starch (El-Tahlawy et al. 2007).
In this study, corn starch was physically modified by heat treatment and solvent exchange to produce non-crystal microporous starch (NCMS). NCMS can be used as a papermaking additive (e.g., filler) and sizing agent to enhance the optical properties and mechanical strength of paper. However, the hydrophilicity of this new starch limits it from use in surface sizing for improving water resistance. Considering that NCMS has exhibited increased chemical reactivity (Liu et al. 2010), it could be further chemically modified with other hydrophobic polymers to enhance the water resistance.
In recent years, several studies have been conducted on the synthesis of hydrophobic starch. Kosan et al. (2006) used ricinoleic oxazoline maleate to graft three different types (thermoplastic, native, and cationic) of starch to prepare a hydrophobic starch. The grafting was carried out in high grafting yields with grafting contents ranging from 1 to 30 wt.% (Kosan et al. 2006). Cova et al. (2010) obtained hydrophobic starch by modifying cassava starch with octenyl succinic anhydride via microwave radiation. The hydrophobicity of the modified starch increased as the degree of substitution increased (Cova et al. 2010). Wei et al. (2016) silylated starch nanocrystals using hexadecyltri-methoxysilane to improve its hydrophobicity. First, hexadecyltrimethoxysilane was hydrolyzed in ethanol/water, and it was then absorbed onto the starch nanocrystal via hydrogen bonding. Long-chain hydrocarbons were covalently linked to the surface of the SNC. The study results suggested that increased hydrophobicity and hydrophobic stabilities are required as the hexadecyltrimethoxysilane content increases. The contact angle of the modified starch nanocrystal increased from 43° to 119° as the applied hexadecyltrimethoxysilane increased from 0% to 0.3% (V/V) (Wei et al. 2016).
Alkyl ketene dimer (AKD), the most commonly used paper-sizing agent, can be added to fiber suspensions to enhance the hydrophobicity and optical properties of paper (Minami et al. 2008; Nonomura et al. 2010). In the sizing mechanism of AKD, the lactone ring of the AKD reacts with the hydroxyl groups on the surface of the cellulose and generated β-keto esters. The improved water resistance of the paper was attributed to the β-keto esters fixed on the surface of the fiber (Garnier et al. 1998). However, AKD also reacts with water molecules to produce unstable β-keto acid, which then decarboxylates to form a ketone. The reaction rate of AKD with water is faster than with cellulose (Bottorff and Sullivan 1993). To overcome this problem, NCMS, which shares a similar chemical structural unit with cellulose, could also be reacted with AKD to generate β-keto esters that are fixed on the surface. On the basis of the above idea, we produced hydrophobic NCMS (H–NCMS) for use as a surface sizing agent to prevent AKD hydrolyzation and reduce NCMS hydrophilicity during sizing. Figure 1 presents the schematic of this modification.
Ultrasonic-assisted wheat straw pulp (UWP) was produced by ultrasonic pulping, which integrates pulping and bleaching within a reactor to eliminate soda recovery, bleaching, bleaching solution preparation, and a coal-fired steam supply system. In a previous study, our group conducted a series of experiments on UWP, where UWP exhibited superior strength and optical properties compared with those of traditional wheat straw pulp and aspen alkaline peroxide mechanical pulp (Xing et al. 2017). Pursuing our interest in this material, UWP and H–NCMS were used to make food wrapper paper with the aim of achieving economical and environment-friendly results.
In the present study, NCMS was prepared by heat treatment and solvent exchange, followed by the modification of NCMS with AKD. Finally, food wrapper paper was produced using H–NCMS and UWP. The structures of the corn starch, NCMS, H–NCMS, and sized paper were characterized by XRD, scanning electron microscopy (SEM), Fourier transform infrared (FT-IR) spectroscopy, thermogravimetric analysis (TGA) and water droplet contact angles. Moreover, the process parameters of the prepared food wrapper were also examined.
Fig. 1. Modification schematic of the H–NCMS preparation
EXPERIMENTAL
Materials
Corn starch, whose amylose content analyzed by the supplier was 26.3%, was supplied by Kangpuhuiwei Technology Co., Ltd. Cationic polyacrylamide (CPAM) (molecular weight of 8 million g/mol, charge density of 2.47 mmol/g), anhydrous ethanol, AKD, FeCl3, and NH4CNS were all provided by Kebaiao Co., Ltd. (Beijing, China). UWP was supplied by Huasen Paper Co., Ltd. (Anyan, China). Softwood kraft pulp (SKP) was supplied by Sun Paper Co., Ltd. (Yanzhou, China). All chemicals were analytical grade and used without further purification.
Methods
Preparation of the non-crystal microporous starch (NCMS)
Ten grams of corn starch were suspended in 300 mL of deionized water. The solution was then heated to 80 °C with continuous stirring and maintained for 20 min. Then the suspension was filtered, and the solid was oven-dried to collect the non-crystal starch.
NCMS was produced by the solvent exchange technique. Briefly, 100 mL of anhydrous ethanol was added to 5 g of non-crystal starch, which was stirred for 30 min at room temperature, and then the precipitated starches were filtered out via suction. After the precipitate was transferred to a glass beaker and subsequently 100 mL of anhydrous ethanol were added, and the resultant mixture was stirred for 60 min, which was then filtered again. The precipitated NCMS was dried at ambient conditions overnight before further characterization.
Preparation of the hydrophobic NCMS (H–NCMS)
A certain amount of AKD was dissolved completely in 50 mL of anhydrous ethanol with continuous stirring, and then 12 g of NCMS was added to the solution. The reaction mixture was allowed to sit at the desired temperature with continuous stirring for a certain period of time. After the reaction, the resultant mixture was filtered, and the solid was freeze-dried to collect the H–NCMS after it was washed with anhydrous ethanol five times. Figure 2 shows the preparation process of H-NCMS.
Fig. 2. Preparation process of H-NCMS
Preparation of the sized paper
The base paper (50 g/m2) was produced from softwood bleached kraft pulp (40° SR) on a KRK semi-auto sheet machine (Kyoto, Japan). The surface sizing agent was prepared at 0.1%, 0.3%, 0.5%, 0.7%, 0.9%, and 1.1% H–NCMS concentrations, kept in a constant temperature water bath at 30 °C, and was then used for surface sizing. The surface sizing was performed using drawdown rods on the surface of the base paper with a sizing volume 3 g/m2. The sized paper was oven-dried at 105 °C for 30 min and stored in a conditioned environment (23 °C and 50% relative humidity (RH)) for 24 h until further analysis.
Measurement of the sizing degree
The sizing degree was measured according to GB/T 5405 (2002). A 1% FeCl3 solution was dripped on the inside of the paper, and then the paper was put in a 2% NH4CNS solution at the same time a stopwatch was started. The time it took for FeCl3 to react with NH4CNS to produce a red was recorded as the sizing degree, and the average was taken of ten measurements for each sized paper.
Preparation of the food wrapper paper
The furnish of the base paper consisted of 60% UWP (40° SR) and 40% SKP (40° SR). Then, 0.2% CPAM ( mCPAM/mPulp) was added in a mixer and the base paper of basis weight 50 g/m2 was formed using a KRK semi-auto sheet machine. Surface sizing with 0.9% H–NCMS was performed using drawdown rods on the surface of the base paper with a sizing volume of 1, 2, 3, and 4 g/m2, respectively. The sized paper was oven-dried at 105 °C and then stored in a conditioned environment (23 °C and 50% RH) for 24 h.
The properties of the paper were tested by the following ISO standard methods: GB/T 1504 (2002) (water absorption), GB/T 7974 (2002) (whiteness), GB/T 454 (2002) (burst strength), and GB/T 453 (2002) (tensile strength).
X-ray diffraction (XRD) analysis
The crystallinity of the corn starch and NCMS was evaluated by XRD, using a Shimadzu diffractometer (XRD 6000X). The measurement conditions were as follows: Cu Kα radiation with a graphite monochromator, 30 kV, and 40 mA. The patterns were obtained within a 5° to 40° angular interval (2θ) range with a 0.05° step and scanning speed of 2°/min.
Scanning electron microscopy (SEM) analysis
SEM was used to investigate the surface morphology of the corn starch, NCMS, pristine paper, and sized paper. Pieces were cut from the films, coated with gold, and observed with a scanning electron microscope (S-3000N) at an acceleration voltage of 10 kV.
Fourier transform infrared (FT-IR) spectroscopy analysis
The FT-IR spectra of the corn starch, NCMS, H–NCMS, pristine paper, and sized paper were recorded on a Tensor 27 spectrometer (Bruker) with KBr pellets in the range of 4000 to 400 cm-1.
Thermal Analysis
The thermogravimetric analysis (TGA) of corn starch, NCMS, H-NCMS, pristine paper, and sized paper was performed using a TGA Q5000 V3.15 Build 263 thermogravimetric analyzer. The samples weighted around 4 mg were heated from 40° to 600° at heating rate of 10 °C/min under an inert atmosphere of N2.
Contact angle measurement
Five microliters of deionized water were dropped onto the surface of the paper, and the water contact angle for the sized paper was measured with an optical contact angles apparatus (OCA 20) equipped with a video measuring system and high-resolution CCD camera.
RESULTS AND DISCUSSION
XRD Analysis of the Corn Starch and NCMS
XRD determines the degree of crystallinity of a wide range of materials (Poletto et al. 2011). Starch is a semi-crystalline plant polymer composed of amylose and amylopectin (Glenn et al. 2008). Amylose is localized in the amorphous regions of the starch granule, while the crystalline regions are largely comprised of amylopectin (Shamekh et al. 1999). The XRD spectra of the corn starch and NCMS are shown in Fig. 3. It was shown that the XRD profiles of corn starch exhibited typical diffraction angles (2θ) around at 15°, 17°, 18°, and 23°, while, the diffraction angle’s intensity of NCMS was weakened obviously, which illustrated that the crystalline structure was destroyed entirely and the crystallinity decreased during heating treatment.
Fig. 3. XRD spectra of the corn starch (a) and NCMS (b)
SEM Analysis of the Corn starch and NCMS
SEM pictures of corn starch and NCMS are presented in Fig. 4. As can be seen from the picture of NCMS, the integrated and smooth surface of corn starch was converted to a microporous surface by heating and solvent exchange, suggesting that the starch granules were completely modified and prepared.
Fig. 4. SEM images of the corn starch (a) and NCMS (b)
Single-Factor Experiments of H-NCMS Preparation
The AKD dosage, reaction temperature, reaction time, and H–NCMS content were selected to evaluate the effect these conditions have on the sizing performance of the H–NCMS preparation. Figure 5 shows the effect of the AKD dosage on the sizing performance of the H–NCMS. The sizing degree increased with an increase in the AKD dosage and then decreased. This behavior was attributed to the reaction of higher AKD dosages with the hydroxyl groups of the NCMS, which formed more hydrophobic alkyl groups. Paper surfaces are porous as well as chemically and physically heterogeneous (Sun et al. 2014). Consequently, the H–NCMS infiltrated the paper and the hydrophobic alkyl groups were distributed on the fiber surface, which contributed to the water resistance of the material. When the AKD dosage was extremely high, an excess of hydrophobic alkyl groups in the H–NCMS could have led to poor solubility and diffusion for surface sizing. Thus, the optimal AKD dosage was 80%.
Fig. 5. Effect of the AKD dosage on the sizing performance of the H–NCMS (paper grammage: 50 g/m2; reaction temperature: 55 °C; reaction time: 3 h; H–NCMS content: 1.1%; sizing volume: 3 g/m2)
Figure 6 shows the effect of the reaction time on the sizing performance of the H–NCMS. The sizing degree increased rapidly as the reaction time increased until 3 h, indicating there were plenty of hydroxyl groups readily available for esterification with AKD. However, when the reaction time exceeded 3 h, the esterification between the AKD and NCMS remained the same. Consequently, the sizing degree rose initially, and then it remained constant.
Fig. 6. Effect of the reaction time on the sizing performance of the H–NCMS (paper grammage: 50 g/m2; AKD dosage: 80%; reaction temperature: 55 °C; H–NCMS content: 1.1%; sizing volume: 3 g/m2)
Figure 7 shows the effect of the reaction temperature on the sizing performance of the H–NCMS. The sizing degree was enhanced as the reaction temperature increased to 55 °C, and then the sizing performance did not show noticeable changes even after the reaction temperature was raised to 70 °C. This result suggested that the reactivity of esterification between the AKD and NCMS was improved with an increase of reaction temperature within 55 °C. When the temperature exceeded 55 °C, there was a limit to the degree of esterification that could be achieved. Thus, the optimal reaction temperature was 55 °C.
Fig. 7. Effect of reaction temperature on sizing performance of H–NCMS (paper grammage: 50 g/m2; AKD dosage: 80%; reaction time: 3 h; H–NCMS content: 1.1%; sizing volume: 3 g/m2)
Figure 8 shows the effect of the H–NCMS content on the sizing performance of the H–NCMS. The highest sizing degree of 67.4 s occurred at a content of 0.9%. The sizing agent with an increasing H–NCMS content exhibited higher liquidity, which led to a more comprehensive layer on the paper surface. Thus, the water resistance was higher. When the H–NCMS content was more than 0.9%, the water resistance of the sized paper was noticeably decreased.
Fig. 8. Effect of H–NCMS content on sizing performance of H–NCMS (paper grammage: 50 g/m2; AKD dosage: 80%; reaction time: 3 h; reaction temperature: 55 °C; sizing volume: 3 g/m2)
The optimal reaction conditions were as follows: AKD dosage of 80%, reaction time of 3 h, and reaction temperature of 55 °C. Additionally, the sizing agent worked best with a 0.9% H–NCMS content. The H–NCMS obtained under these conditions was chosen for the preparation of the food wrapper paper.
FT-IR Analysis of the Corn Starch, NCMS, H–NCMS, Pristine paper, and Sized Paper
The corn starch, NCMS, and H–NCMS (with optimal reaction conditions) structures were analyzed by FT-IR spectroscopy, as shown in Fig. 9. The FT-IR spectrum of the corn starch (curve a) shows its characteristic peaks at 3427, 1650, and 2926 cm-1, which were attributed to the stretch and vibration of hydroxyl groups, hydrogen bonds, and C-H separately. In the FT-IR spectrum of the NCMS (curve b), the band at 1650 cm-1 corresponding to the hydrogen bond was remarkably decreased, which was in agreement with the XRD analysis above. The FT-IR spectrum of the H–NCMS (curve c), when compared with that of the corn starch and NCMS, showed a decrease in the -OH absorbance at 3436 cm-1, which indicated that esterification occurred between the AKD and NCMS. Also, the peak at 2926 cm-1 (-C-H stretching) showed an increase in the H–NCMS spectrum, and the new peaks at 2848 and 1699 cm-1, which were assigned to the C-H stretching of CH3 of the long-chain alkyl groups and C=O stretching of the AKD, respectively, were observed in the H–NCMS spectrum. These FT-IR spectra suggested that the NCMS structure was changed after modification with AKD. It can also be observed from Fig. 9 that the sized paper (with best sizing efficiency) (e) has a larger band at 3400 cm-1 compared to the pristine paper. This large band can be attributed to the hydrogen bonding interactions arising among the hydroxyl groups (Salam et al. 2013) within the H-NCMS and fibers of paper. The peak at 1650 cm-1 contributing to hydrogen bond in sized paper became stronger, which also could be ascribed to the synergistic effect of hydrogen bonding between H-NCMS and the hydroxyl groups of fiber. Moreover, C-H stretching peaks at 2892 cm-1 in sized paper slightly shifted to a lower wave number, which probably indicated the presence of the interaction between H-NCMS and pulp fiber.
Fig. 9. FT-IR spectra of the corn starch (a), NCMS (b), H–NCMS (c), pristine paper (d) and sized paper (e)
TG Analysis of Corn Starch, NCMS, H-NCMS, Pristine Paper and Sized paper
As can be seen from Figure 10, the TG curves of corn starch (a), NCMS (b), and H-NCMS (with optimal reaction conditions) (c) could be divided into two weight loss stages, corresponding to the dehydration process (50 °C to about 120 °C) and most weight loss ( 120 °C to about 360 °C) stages, respectively. At the first stages, the mass loss was associated with the water evaporation (Salam et al. 2013). The maximum rate of weight loss was observed at the second stage, which was mainly caused by the thermal degradation of glucose of starch/derivatives that decomposed into char residues (Bernabe et al. 2005). Compared with NCMS and H-NCMS, the thermal stability of the corn starch was poorer that of NCMS and H-NCMS since the onset of thermal degradation of NCMS and H-NCMS was about 280 °C and 300 °C, respectively. Meanwhile, two distinct stages exist in the TGA curves of pristine paper (d) and sized paper (with best sizing efficiency) (e). The first stage was a dehydration process (50 °C to about 180 °C), in which free water in the specimens evaporated (Yan et al. 2015). The maximum rate of weight loss was occurred at the second stage, in this stage, a high temperature of thermal degradation (about 290 °C) was observed in the H-NCMS treated paper compared to the pristine paper (about 330 °C), which is probably due to hydrogen bonding between H-NCMS and the hydroxyl groups of fiber. This result is in well accordance with the above FT-IR analysis.
As can be seen from Figure 10, the TG curves of corn starch (a), NCMS (b), and H-NCMS (with optimal reaction conditions) (c) could be divided into two weight loss stages, corresponding to the dehydration process (50 °C to about 120 °C) and most weight loss ( 120 °C to about 360 °C) stages, respectively. At the first stages, the mass loss was associated with the water evaporation (Salam et al. 2013). The maximum rate of weight loss was observed at the second stage, which was mainly caused by the thermal degradation of glucose of starch/derivatives that decomposed into char residues (Bernabe et al. 2005). Compared with NCMS and H-NCMS, the thermal stability of the corn starch was poorer that of NCMS and H-NCMS since the onset of thermal degradation of NCMS and H-NCMS was about 280 °C and 300 °C, respectively. Meanwhile, two distinct stages exist in the TGA curves of pristine paper (d) and sized paper (with best sizing efficiency) (e). The first stage was a dehydration process (50 °C to about 180 °C), in which free water in the specimens evaporated (Yan et al. 2015). The maximum rate of weight loss was occurred at the second stage, in this stage, a high temperature of thermal degradation (about 290 °C) was observed in the H-NCMS treated paper compared to the pristine paper (about 330 °C), which is probably due to hydrogen bonding between H-NCMS and the hydroxyl groups of fiber. This result is in well accordance with the above FT-IR analysis.
Fig. 10. TGA curves of corn starch (a), NCMS (b), H-NCMS (c), pristine paper (d) and sized paper (e)
Contact Angle Measurement and SEM Analysis of the Pristine Paper and Sized Paper
The wettability of the paper surface was measured by the contact angle of water droplets. The pristine paper was a hydrophilic material, as shown in Fig. 11a. In contrast, the sized paper (with best sizing efficiency) exhibited hydrophobicity, as shown in Fig. 11b. By this modification, the lactone ring of the AKD reacted with the hydroxyl groups of the NCMS, and thus the long chain hydrophobic alkyls were successfully introduced onto the NCMS surface. These results were agreement with the FT-IR analysis.
The scanning electron micrographs of the pristine paper and sized paper at the same magnification are shown in Fig. 11. The SEM image of the pristine paper showed that the paper was composed of interlaced fibers, and its surface displayed a porous structure (Zhang et al. 2014). After surface sizing, a large amount of hydrophobic H–NCMS was found to have adhered to the fibers, and the small surface voids of the paper were filled. As a result, the hydrophobicity of the paper was improved.
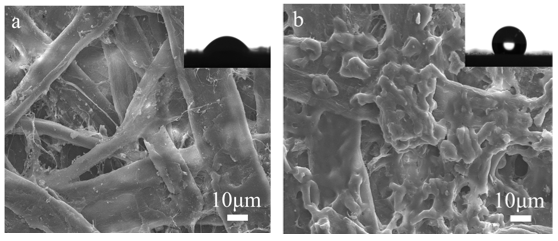
Fig. 11. SEM pictures of pristine paper (a) and sized paper (b). Insets: images of water droplets.
Preparation of the Food Wrapper Paper with UWP, SKP, and H–NCMS
Table 1 shows the effect of the sizing volume on the paper properties, as well as a comparison of the sample with the industry standard. The tensile and burst indexes increased as the sizing volume increased, while the water absorption decreased after surface sizing the paper. This result implied that the sizing agent that penetrated the paper was transformed into an interpenetrating polymer network (Li et al. 2012), in accordance with the above SEM results. Higher sizing volumes resulted in better paper strengths and hydrophobicity. Moreover, the whiteness was also increased after surface sizing, which could be attributed to the increase of H-NCMS with microporous on the surface of paper.
The test value of the paper far exceeded the industry standard QB/T 1014 (2010) for food wrapper paper. Thus, the use of UWP and H–NCMS to make food wrapper paper would be environmentally and economically beneficial.
Table 1. Effect of the Sizing Volume on the Paper Properties
CONCLUSIONS
- The NCMS was produced successfully by heat treatment and solvent exchange.
- The AKD dosage, reaction time, reaction temperature, and H–NCMS content all separately had major effects on the sizing degree. Consequently, the optimal modification conditions were an AKD dosage of 80%, reaction time of 3 h, and reaction temperature of 55 °C. The sized paper with 0.9% H–NCMS presented outstanding hydrophobicity.
- The H–NCMS was characterized by FT-IR, which revealed that the chemical structure changed during modification.
- The food wrapper paper was successfully made using H–NCMS and UWP. Several parameters of the food wrapper paper produced in this study exceeded that of the industry standard.
ACKNOWLEDGMENTS
This work was sponsored by the Special Fund for Beijing Common Construction Project and Beijing Forestry University, Grant No. 2016HXFWCLXYO13.
REFERENCES CITED
Bottorff, K. J., and Sullivan, M. J. (1993). “New insights into the AKD sizing mechanism [alkylketene dimer],” Nord. Pulp Pap. Res. J. 8(1), 86-95.
Cova, A., Sandoval, A. J., Balsamo, V., and Müller, A. J. (2010). “The effect of hydrophobic modifications on the adsorption isotherms of cassava starch,” Carbohyd. Polym. 81(3), 660-667. DOI: 10.1016/j.carbpol.2010.03.028
El-Tahlawy, K., Venditti, R. A., and Pawlak, J. J. (2007). “Aspects of the preparation of starch microcellular foam particles crosslinked with glutaraldehyde using a solvent exchange technique,” Carbohyd. Polym. 67(3), 319-331. DOI: 10.1016/j.carbpol.2006.05.029
Garcia, V., Colonna, P., Bouchet, B., and Gallant, D. J. (1997). “Structural changes of cassava starch granules after heating at intermediate water contents,” Starch-Starke 49(5), 171-179. DOI: 10.1002/star.19970490502
Garnier, G., Wright, J., Godbout, L., and Yu, L. (1998). “Wetting mechanism of alkyl ketene dimers on cellulose films,” Colloid. Surface. A 145(1-3), 153-165. DOI: 10.1016/S0927-7757(98)00668-2
Glenn, G. M., Klamczynski, A., Chiou, B.-S., Orts, W. J., Imam, S. H., and Wood, D. F. (2008). “Temperature related structural changes in wheat and corn starch granules and their effects on gels and dry foam,” Starch-Starke 60(9), 476-484. DOI: 10.1002/star.200800203
Glenn, G. M., Orts, W. J., and Nobes, G. A. R. (2001). “Starch, fiber and CaCO3 effects on the physical properties of foams made by a baking process,” Ind. Crop. Prod. 14(3), 201-212. DOI: 10.1016/S0926-6690(01)00085-1
GB/T 453 (2002). “Paper and board determination of tensile properties – Constant rate of loading methods,” Standardization Administration of China, Beijing, China.
GB/T 454 (2002). “Paper – Determination of burst strength,” Standardization Administration of China, Beijing, China.
GB/T 1504 (2002). “Paper and board determination of water absorption,” Standardization Administration of China, Beijing, China.
GB/T 5405 (2002). “Paper determination of the sizing degree (liquid permeance method),” Standardization Administration of China, Beijing, China.
GB/T 5405 (2002). “Paper and board determination of the brightness (whiteness),” Standardization Administration of China, Beijing, China.
Kosan, B., Meister, F., Liebert, T., and Heinze, T. (2006). “Hydrophobic modification of starch via grafting with an oxazoline-derivative,” Cellulose 13(1), 105-113. DOI: 10.1007/s10570-005-9013-4
Li, Y., Dai, H., Wan, L., and Zhu, Z. (2012). “Surface sizing application of waterborne epoxy resin on low basis weight paper,” BioResources 7(1), 5-14. DOI: 10.15376/biores.7.1.5-14
Liu, P. L., Zhang, B. S., Shen, Q., Hu, X. S., and Li, W. H. (2010). “Preparation and structure analysis of noncrystalline granular starch,” International Journal of Food Engineering 6(4), 61-64. DOI: 10.2202/1556-3758.1900
Liu, Y., and Li, K. (2007). “Development and characterization of adhesives from soy protein for bonding wood,” Int. J. Adhes. Adhes. 27(1), 59-67. DOI: 10.1016/j.ijadhadh.2005.12.004
Lopez-Gil, A., Silva-Bellucci, F., Velasco, D., Ardanuy, M., and Rodriguez-Perez, M. A. (2015). “Cellular structure and mechanical properties of starch-based foamed blocks reinforced with natural fibers and produced by microwave heating,” Ind. Crop. Prod. 66, 194-205. DOI: 10.1016/j.indcrop.2014.12.025
Mello, L. R. P. F., and Mali, S. (2014). “Use of malt bagasse to produce biodegradable baked foams made from cassava starch,” Ind. Crop. Prod. 55, 187-193. DOI: 10.1016/j.indcrop.2014.02.015
Minami, T., Mayama, H., and Tsujii, K. (2008). “Spontaneous formation of super water-repellent fractal surfaces in mixed wax systems,” J. Phys. Chem. B 112(46), 14620-14627. DOI: 10.1021/jp802268j
Nonomura, Y., Morita, Y., Hikima, T., Seino, E., Chida, S., and Mayama, H. (2010). “Spreading behavior of water droplets on fractal agar gel surfaces,” Langmuir 26(20), 16150-16154. DOI: 10.1021/la103123d
Patel, S. V., Venditti, R. A., and Pawlak, J. J. (2010). “Dimensional changes of starch microcellular foam during the exchange of water with ethanol and subsequent drying,” BioResources 5(1), 121-134. DOI: 10.15376/biores.5.1.121-134
Poletto, M., Pistor, V., Zeni, M., and Zattera, A. J. (2011). “Crystalline properties and decomposition kinetics of cellulose fibers in wood pulp obtained by two pulping processes,” Polym. Degrad. Stabil. 96(4), 679-685. DOI: 10.1016/j.polymdegradstab.2010.12.007
QB/T 1014 (2010). “Industry standard of food wrapper paper,” Industry and information Technology Administration of China, Beijing, China.
Shamekh, S., Forssell, P., Suortti, T., Autio, K., and Poutanen, K. (1999). “Fragmentation of oat and barley starch granules during heating,” J. Cereal Sci. 30(2), 173-182. DOI: 10.1006/jcrs.1999.0263
Shey, J., Imam, S. H., Glenn, G. M., and Orts, W. J. (2006). “Properties of baked starch foam with natural rubber latex,” Ind. Crop. Prod. 24(1), 34-40. DOI: 10.1016/j.indcrop.2005.12.001
Stute, R., Heilbronn, Klingler, R. W., Dipl.-Ing, S. B., Dipl.-Ing., M. N. E., and Knorr, D. (1996). “Effects of high pressures treatment on starches,” Starch-Starke 48(11-12), 399-408. DOI: 10.1002/star.19960481104
Salam, A., Lucia, L. A., and Jameel, H. (2013). “Synthesis, characterization, and evaluation of chitosan-complexed starch nanoparticles on the physical properties of recycled paper furnish,” ACS Applied Materials & Interfaces 5(21), 11029. DOI:10.1021/am403261d
Sun, B., Hou, Q., Liu, Z., He, Z., and Ni, Y. (2014). “Stability and efficiency improvement of ASA in internal sizing of cellulosic paper by using cationically modified cellulose nanocrystals,” Cellulose 21(4), 2879-2887. DOI: 10.1007/s10570-014-0283-6
Tamaki, S., Hisamatsu, M., Teranishi, K., Adachi, T., and Yamada, T. (1998). “Structural change of maize starch granules by ball-mill treatment,” Starch-Starke 50(8), 342-348. DOI: 10.1002/(SICI)1521-379X(199808)50:8<342::AID-STAR342>3.0.CO;2-B
Vercelheze, A. E. S., Fakhouri, F. M., Dall’Antônia, L. H., Urbano, A., Youssef, F. Y., Yamashita, F., and Mali, S. (2012). “Properties of baked foams based on cassava starch, sugarcane bagasse fibers and montmorillonite,” Carbohyd. Polym. 87(2), 1302-1310. DOI: 10.1016/j.carbpol.2011.09.016
Wang, B., and Yang, R.-D. (2011). “Preparation and characterization of starch microcellular foam particles crosslinked with glutaraldehyde using a solvent exchange technique,” Gongneng Cailiao/Journal of Functional Materials 42(1), 524-528.
Wei, B., Sun, B., Zhang, B., Long, J., Chen, L., and Tian, Y. (2016). “Synthesis, characterization and hydrophobicity of silylated starch nanocrystal,” Carbohyd. Polym. 136(9), 1203-1208. DOI: 10.1016/j.carbpol.2015.10.025
Willett, J. L. (2009). “Starch in polymer compositions,” in: Starch, J. BeMiller and R. Whistler (eds.), Elsevier, Amsterdam, Netherlands, pp. 715-743.
Xing, L., Xu, M., and Pu, J. (2017). “The properties and application of an ultrasonic-assisted wheat straw pulp having enhanced tendency for ash formation,”
Yan, Y., Dong, Y., Li, J., Zhang, S., Xia, C., Shi, S. Q., and Cai, L. (2015). “Enhancement of mechanical and thermal properties of poplar through the treatment of glyoxal-urea/nano-SiO2,” RSC Advances, 5(67), 54148-54155. DOI: 10.1039/c5ra07294h
Zhang, B., Dhital, S., Haque, E., and Gidley, M. J. (2012). “Preparation and characterization of gelatinized granular starches from aqueous ethanol treatments,” Carbohyd. Polym. 90(4), 1587-1594. DOI: 10.1016/j.carbpol.2012.07.035
Zhang, J.-F., and Sun, X. (2007). “Biodegradable foams of poly(lactic acid)/starch. I. Extrusion condition and cellular size distribution,” J. Appl. Polym. Sci. 106(2), 857-862. DOI: 10.1002/app.26715
Zhang, W., Xiao, H., and Qian, L. (2014). “Enhanced water vapour barrier and grease resistance of paper bilayer-coated with chitosan and beeswax,” Carbohyd. Polym. 101, 401-406. DOI: 10.1016/j.carbpol.2013.09.097
Article submitted: April 3, 2017; Peer review completed: June 10, 2017; Revised version received and accepted: June 22, 2017; Published: June 26, 2017.
DOI: 10.15376/biores.12.3.5775-5789
Erratum: May 19, 2020. Page 5785. The SEM pictures of pristine paper and sized paper in Figure 11 were misassigned and are now corrected. This change does not influence the results of the paper. The authors apologize for this error. December 2, 2024. It has come to our attention that the TGA curves of the pristine paper (d) and sized paper (e) in Figure 10 were misassigned and are now corrected. This error did not affect analysis or interpretation of results in any way.
