Abstract
Lignin/polyaniline composites were prepared by adding kraft lignin for the synthesis of polyaniline (PANI), a typical conductive polymer. The composites were utilized as an adsorbent for the removal of hexavalent chromium (Cr(VI)). When lignin alone was used as an adsorbent, the removal efficiency of Cr was low. However, when the lignin/PANI composite was used, lignin and PANI adsorbed Cr(III) together. The PANI reduced Cr(VI), which resulted in the efficient removal of Cr. In addition, as the dosage of the lignin/PANI composite decreased as an adsorbent, the Cr removal efficiency of the composite decreased considerably compared with pure PANI. However, the composite with a lignin-to-PANI ratio of 1:1 showed a Cr removal efficiency similar to that of pure PANI. The morphology of the lignin/PANI composite was observed to synthesize PANI around the lignin surface. Both Fourier transform infrared spectroscopy and X-ray photoelectron spectroscopy analyses showed that an interaction between the carbonyl groups of lignin and the amine groups of PANI occurred. This study is expected to provide an opportunity to increase the utilization of lignin in the field of environmental science and provide several benefits.
Download PDF
Full Article
Preparation of a Lignin/Polyaniline Composite and Its Application in Cr(VI) Removal from Aqueous Solutions
Jin Ho Seo,a,c Cheol Soon Choi,b Jin Ho Bae,b Hanseob Jeong,c Seung-Hwan Lee,a,b and Yong Sik Kim a,b,*
Lignin/polyaniline composites were prepared by adding kraft lignin for the synthesis of polyaniline (PANI), a typical conductive polymer. The composites were utilized as an adsorbent for the removal of hexavalent chromium (Cr(VI)). When lignin alone was used as an adsorbent, the removal efficiency of Cr was low. However, when the lignin/PANI composite was used, lignin and PANI adsorbed Cr(III) together. The PANI reduced Cr(VI), which resulted in the efficient removal of Cr. In addition, as the dosage of the lignin/PANI composite decreased as an adsorbent, the Cr removal efficiency of the composite decreased considerably compared with pure PANI. However, the composite with a lignin-to-PANI ratio of 1:1 showed a Cr removal efficiency similar to that of pure PANI. The morphology of the lignin/PANI composite was observed to synthesize PANI around the lignin surface. Both Fourier transform infrared spectroscopy and X-ray photoelectron spectroscopy analyses showed that an interaction between the carbonyl groups of lignin and the amine groups of PANI occurred. This study is expected to provide an opportunity to increase the utilization of lignin in the field of environmental science and provide several benefits.
Keywords: Lignin; Polyaniline; Composite; Characterization; Hexavalent chromium adsorption
Contact information: a: The Institute of Forest Science, College of Forest and Environmental Sciences, Kangwon National University, Chuncheon, 24341 Republic of Korea; b: Division of Forest Material Science & Engineering, College of Forest and Environmental Sciences, Kangwon National University, Chuncheon, 24341 Republic of Korea; c: Wood Chemistry Division, National Institute of Forest Science, Seoul, 02455 Republic of Korea; *Corresponding author: yongsikk@kangwon.ac.kr
INTRODUCTION
Lignin is a sustainable natural polymer. An enormous amount of lignin is generated from kraft pulp production each year, but most of it is landfilled or burned for energy production (Bruijnincx and Weckhuysen 2014; Kim and Sung 2018; Seo et al. 2018). Therefore, a variety of efforts are needed for higher utilization of lignin. The utilization of lignin has several possible benefits, including economic and environmental benefits, and it is generally accomplished through depolymerization, derivatization, and production of composites with other polymers (Norgren and Edlund 2014; Ľudmila et al. 2015). In particular, the use of lignin composites is being attempted in various fields such as medicine, electricity, construction, and environmental science (Naseem et al. 2016). To make lignin composites, a wide variety of polymers are used, among which polyaniline (PANI) is the most representative conductive polymer (Sapurina et al. 2003; Goto and Yokoo 2013).
The PANI has many advantages, such as a good adsorption for toxic ions, low cost, high electrical conductivity, and good environmental stability, and it is known to be suitable for the production of a composite (Sapurina et al. 2003). Therefore, various PANI composites with synthetic polymers, inorganic substances, and natural compounds have been studied (Anand et al. 1998; Malinauskas 2001). Recently it was shown that PANI can be synthesized in the presence of kenaf fibers to enhance interfacial interaction and electronic properties (Razak et al. 2013).
Salem et al. (2016) reported that PANI/silver nanocomposites were effective at removing brilliant green dye under highly alkaline conditions. Li et al. (2014) utilized PANI synthesized on filter paper with a porous structure to remove metal ions or low molecular weight materials, and Cr(Ⅵ) removal by this composite was effective under high temperature and low pH conditions. Mansour et al. (2011) and Phan et al. (2010) used a PANI coating on sawdust to remove Cd(Ⅱ) and Cr(Ⅵ). Phan et al. (2010) reported that the Cr(Ⅵ) adsorption of the composite was affected by pH, and the adsorption rate was fastest during the first half hour. The functional groups in PANI have been reported to have a strong affinity for heavy metals such as Cr(Ⅵ) (Eisazadeh 2007; Zhang et al. 2010; Bhaumik et al. 2012; Krishnani et al. 2013). However, because PANI itself has recalcitrant processability, blending it with other materials, such as cellulose, polyvinyl alcohol (PVA), humic acid, and polypyrrole, results in better processability and more efficient adsorption (Yavuz et al. 2011; Bhaumik et al. 2012). In addition, it was known that lignin itself can efficiently adsorb Cr(Ⅲ) via an ion-exchange mechanism and efficiently removes Cr(Ⅲ) from wastewater (Wu et al. 2008). Therefore, it may be expected to have good characteristics in Cr removal because both PANI and lignin were effective for the removal of Cr if lignin/PANI composites are prepared in the presence of lignin instead of kenaf fibers.
In this study, kraft lignin without derivatization was used in the synthesis of lignin/PANI composites. The lignin/PANI composites were prepared by the simple polymerization of PANI in the presence of lignin. Furthermore, the morphology of the composites was analyzed using a scanning electron microscope (SEM) and a field emission transmission electron microscope with energy dispersive X-ray spectroscopy (FE-TEM-EDS). Fourier transform infrared (FT-IR) and X-ray photoelectron spectroscopy (XPS) were performed to analyze the structural properties of the composites. Finally, the Cr(Ⅵ) adsorption of the lignin/PANI composites was measured, and the possibility of their use as an adsorbent for wastewater treatment was investigated.
EXPERIMENTAL
Materials
Kraft lignin, which is a hardwood lignin produced by the kraft pulping of a mixture of various Southeast Asia hardwood chips, was kindly provided by Moorim Pulp & Paper Co. (Ulsan, South Korea). Aniline (99%, Sigma-Aldrich, Darmstadt, Germany) and deionized water (Fisher Scientific, Hampton, NH, USA) were used as received. Sulphuric acid (70%, Daejung Chemical, Siheung, South Korea), ammonium persulfate (98%, Sigma-Aldrich, Darmstadt, Germany), potassium dichromate (Kanto Chemical, Tokyo, Japan), and 1,5-diphenylcarbazide (98%, Sigma-Aldrich, Darmstadt, Germany) were used after dilution.
Methods
Preparation of lignin/PANI composites
Lignin/PANI composites were prepared by the polymerization of aniline in the presence of lignin. As shown in Table 1, specific amounts of aniline, lignin, and sulfuric acid were added to distilled water and stirred for 4 h. Then, the mixture was ultrasonicated for 10 min. Ammonium persulfate dissolved in distilled water was slowly added to the mixture as an initiator of oxidative polymerization, and the reaction mixture was ultrasonicated for 2 h. The mixture was then polymerized at room temperature by stirring for 16 h, and the reactant was filtered with an excess amount of water. The final reaction product was collected by filtration and dried at 60 ℃ for 72 h in a drying oven. A dark green powder was obtained. The pure PANI was prepared by above procedure without lignin and the yield of pure PANI was recovered to 105.43 mg.
Table 1. Preparation Conditions of Lignin/PANI Composites
Characterization of lignin/PANI composites
The morphology of the lignin/PANI composites was analyzed using an ultra-high-resolution SEM (UHR-SEM, S-4800; Hitachi, Tokyo, Japan) and a FE-TEM-EDS (JEM-2100F; JEOL Ltd., Akishima, Japan). The lignin/PANI composites were coated with iridium in preparation for SEM analysis. The FT-IR spectra were obtained with a PerkinElmer Frontier™ (Waltham, MA, USA) instrument with an attenuated total reflectance (ATR) attachment. A total of 128 scans per sample was completed from 4000 to 500 cm−1 at a resolution of 4.0 cm−1. The XPS analysis was performed using the K Alpha+ model from Thermo VG (Waltham, MA, USA) with an ultimate vacuum of 5 × 10-8 mbar. The source gun type was Al Kα, and the calibration of the energy scale was conducted using the C1s peak at 284.8 eV.
Hexavalent chromium adsorption of lignin/PANI composites
A Cr(Ⅵ) solution (50 mg L-1) was prepared for the adsorption study. A total of 0.05 to 0.20 g of lignin/PANI composite was added to an Erlenmeyer flask containing 100 mL of the Cr(Ⅵ) solution. The pH of the solution was adjusted to 4, and then the flask was shaken at 130 rpm for 24 h in a shaker (SSeriker Ⅱ; VISION Scientific, Seoul, South Korea). The 1,5-diphenylcarbazide (DPC) method was used for Cr(Ⅵ) determination, based on the guidelines of the NFT90-043 standard (France). A DPC solution was prepared by dissolving 0.02 g of DPC in 10 mL of ethanol and 40 mL of 1.8 M sulfuric acid. After the solution was filtrated, 0.1 mL of concentrated nitric acid and 1.2 mL of the DPC solution were added to 20 mL of the filtrates. The residual Cr(Ⅵ) of the filtrate was analyzed using a ultraviolet (UV)-visible spectrophotometer (X-ma 3000; Human Corporation, Seoul, South Korea) at 560 nm. Standard solutions of known concentration were measured for the calibration curve, and all experiments were conducted in triplicate. The adsorbed amounts (q) and the adsorption efficiency (E) were calculated using Eq. 1 and Eq. 2, respectively,
q (mg/g) = (Ci – Cf) × V / M (1)
E (%) = [(Ci – Cf) / Ci] × 100 (2)
where Ci and Cf are the initial and final Cr(Ⅵ) concentrations (mg L-1) of the solution, respectively, V is the volume (L) of the Cr(Ⅵ) solution, and M is the weight (g) of the adsorbent.
RESULTS AND DISCUSSION
Morphology of Lignin/PANI Composites
The SEM analyses were performed to observe the morphology of the lignin/PANI composites. The results of the SEM analyses are shown in Fig. 1. The lignin was amorphous, and its size was distributed across various ranges. Compared with pure PANI, the size of the lignin particles was much larger. For lignin/PANI composites, it was confirmed that the area of lignin exposed on the surface of the composite decreased as the PANI ratio increased. In particular, the shape of the PANI_L4 sample showed that a layer of PANI formed on the surface of the lignin.
Fig. 1. SEM images of lignin/PANI composites (a: lignin, b: pure PANI, c: PANI_L1, d: PANI_L2, e: PANI_L3, and f: PANI_L4)
The TEM analyses were performed to confirm the morphological composition of the composites. The results of the TEM analyses are shown in Fig. 2. The TEM analyses reaffirmed that the PANI particles were smaller than the lignin particles. Under all conditions, PANI was observed surrounding the surface of the lignin, but there was no morphological change with an increase in the PANI ratio. Therefore, it was concluded that PANI successfully formed around the surface of the lignin. See supplementary information for further details.
Fig. 2. TEM images of lignin/PANI composites (a: lignin, b: pure PANI, c: PANI_L1, d: PANI_L2, e: PANI_L3, and f: PANI_L4)
Analysis of the Chemical Structure of Lignin/PANI Composites
The FT-IR spectra of the lignin/PANI composites are presented in Fig. 3. Analysis of the spectra was based on the assignments given by Rodrigues et al. (2002), Ibrahim (2017), and Kaitsuka et al. (2016). Hydroxyl groups at 3600 to 3000 cm-1, CH stretching at 2850 to 2970 cm-1, conjugated carbonyl groups at 1700 cm-1, and CO stretching (phenolic hydroxyl groups) at 1215 cm-1 were observed. The intensity of all of these peaks decreased as the dosage of aniline increased. The peak area at 1485 to 1510 cm-1, attributed to C=C stretching in the benzenoid ring, increased as the PANI ratio increased. An aromatic ring band around 1500 cm-1 was covered by this peak. Furthermore, absorption peaks at 1570 to 1580 cm-1 (quinonoid (Q) C=C), 1485 to 1510 cm-1 (benzenoid (B) C=C), and 1120 to 1150 cm-1 (C=N) of the composites were shifted compared with the pure PANI. These shifts could have been the result of an interaction between lignin and PANI. The FT-IR spectra of all composites showed peaks from PANI at 1300 cm-1 (C-N stretching in a quinonoid-benzenoid-quinonoid (QBQ) structure) and 1240 cm-1 (C-N stretching in a benzenoid-benzenoid-benzenoid (BBB) structure).
The results of XPS analysis for pure PANI and PANI_L4 are shown in Table 2. The curve deconvolution results for nitrogen are shown in Fig. 4. In the total atomic percentage, PANI_L4 had a lower nitrogen content and a higher carbon content than pure PANI. It was possible that the content of nitrogen decreased due to the increase in carbon content as the lignin ratio increased. At the S2p peak, 2.88% of pure PANI and 2.32% of PANI_L4 were detected, which was due to the sulfonation of emeraldine salts and -SH groups present in the kraft lignin.
Fig. 3. FT-IR spectra of the lignin/PANI composites
As a result of the curve deconvolution of N1s, two peaks due to Namine and N+ were observed. The nitrogen atoms of pure PANI were mainly found to have the structure of amines in the benzenoid amine group of 399.5 eV. However, PANI_L4 showed lower Namine content and relatively higher N+ content than pure PANI. This is probably due to the interaction between the lignin hydroxyl group and the PANI amine group through the formation of hydrogen bonds (Rodrigues et al. 2001).
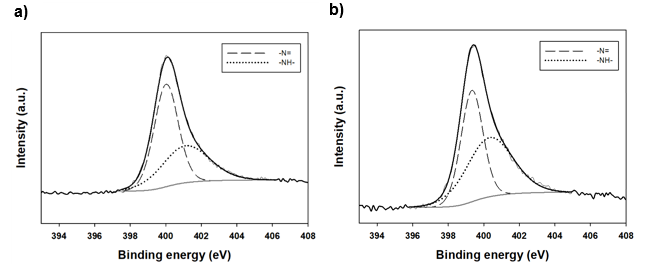
Fig. 4. XPS spectra of the lignin/PANI composites (a: lignin, b: PANI_L4)
Table 2. XPS Results of Lignin/PANI Composites
Cr (VI) Adsorption of Lignin/PANI Composites
The effect of pH on the concentrations of Cr(VI) was an important factor affecting Cr(VI) removal. Namely, the efficient removal of Cr(VI) rapidly increased with pH decreasing from 8.0 to 2.0 (Bhaumik et al. 2012). However, Cr(VI) adsorption experiment was carried out at pH 4 due to the possibility of lignin degradation if the acid conditions were too high. Figure 5 shows the effect of lignin/PANI composites on Cr(VI) removal. The Cr(Ⅵ) removal efficiency of PANI was approximately 90% at 1 h after adsorption and reached a slightly increased state after 2 h. Lignin/PANI composites took a longer time to reach this state as the PANI ratio was lowered, and PANI_L4 showed a Cr(Ⅵ) removal efficiency similar to pure PANI after 2 h. After 24 h, pure PANI showed a Cr(Ⅵ) removal efficiency of 99.9%. The Cr(Ⅵ) removal efficiency of lignin/PANI composites, except for PANI_L2, slightly increased with an increased ratio of PANI in the composites. Although lignin had a very low Cr(Ⅵ) removal efficiency of 32.2%, the lignin/PANI composites showed a Cr(Ⅵ) removal efficiency greater than 99%. In particular, the final Cr(Ⅵ) adsorbed amounts for PANI_L4 were 24.98 mg g-1, and the Cr(Ⅵ) concentration of its filtrate was 0.03 mg L-1. This value was lower than the final Cr(Ⅵ) concentration in the filtrate of pure PANI (0.05 mg L-1), and it was in line with water emission standards of various countries (National Institute of Environmental Research 2010). In addition, Sharma and Forster (1994a, 1994b) report that when biomass is used as an adsorbent of Cr(Ⅵ) in an acidic condition, a reduction reaction and an adsorption reaction occur. In this present study, the Cr(Ⅵ) removal efficiency of the lignin/PANI composites was high due to both the adsorption reaction and the reduction reaction. This was because a composite containing lignin was used, and lignin is a typical biomass adsorbent.
Fig. 5. Cr(VI) removal efficiency of the lignin/PANI composites (dosage: 0.2 g)
Figure 6 shows the effects of the dosage of the lignin/PANI composites on Cr(VI) removal efficiency. The Cr(VI) removal efficiencies of the pure PANI and the lignin/PANI composites were similar at a loading of 0.2 g. However, the gap of the Cr(VI) removal efficiency between the pure PANI and the lignin/PANI composites gradually increased as the adsorbent dose decreased. In particular, the Cr(VI) removal efficiencies of the composites, excluding PANI_L4, were less than 90% at 0.05 g. However, the Cr(VI) removal efficiency of PANI_L4 was 91.5%, which was slightly different from that of pure PANI. Therefore, it is believed that the ratio of PANI is important for the adsorption of Cr(VI), but it appears that PANI_L4 can be used sufficiently as a chromium adsorbent. This suggests that the use of lignin can allow a decrease in the use of PANI.
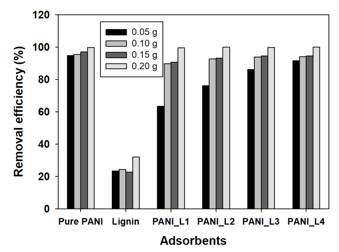
Fig. 6. The effects of dosage of the lignin/PANI composites on Cr(VI) removal efficiency
XPS analyses were performed to investigate the changes in chromium structure after the Cr(Ⅵ) adsorption test with the lignin/PANI composites. The results of the XPS analyses are shown in Fig. 7. The Cr2p peaks were located at 577.0 to 578.0 eV and 586.0 to 588.0 eV, indicating that the adsorbed chromium on the pure PANI and the lignin/PANI composite were present in Cr(Ⅲ) form (Park et al. 2008). The first peak was attributed to the Cr2p3/2 orbital, and the second peak was attributed to the Cr2p1/2 orbital (Park et al. 2008). There was no Cr(Ⅵ) on the pure PANI or on the lignin/PANI composites according to the XPS Cr2p spectra. This suggests that the Cr(Ⅵ) adsorbed on the surface of the sample was totally reduced to Cr(Ⅲ). Qiu et al. (2015) reported that the Cr(Ⅵ) adsorbed on the PANI composite by electrostatic attraction was reduced to Cr(Ⅲ) by the PANI amine groups. Then, Cr(Ⅲ) was adsorbed on the PANI composite by the precipitation of Cr(Ⅲ) and the attraction of amine groups (Qiu et al. 2015). In this present study, it is speculated that the adsorption reaction occurred by the same mechanism. However, Cr(Ⅲ) was not detected in the XPS analysis of lignin after the Cr(Ⅵ) adsorption. Even though lignin efficiently adsorbs Cr(Ⅲ) by the ion-exchange mechanism (Wu et al. 2008), it was assumed that Cr(Ⅲ) was not detected due to the Cr(Ⅵ) reduction ability of lignin, which is relatively low compared with that of PANI. However, for lignin/PANI composites, it is suggested that Cr(Ⅵ) was reduced to Cr(Ⅲ) by the PANI amine groups. Then, this was followed by the adsorption of Cr(Ⅲ) by lignin as well as PANI, which resulted in the efficient removal of Cr.
Fig. 7. The XPS Cr2p spectra of pure PANI and lignin/PANI composites
CONCLUSIONS
- Lignin/polyaniline (lignin/PANI) composites were synthesized using simple PANI polymerization in the presence of lignin, which showed the successful formation of PANI on the lignin surface.
- As the PANI ratio in the lignin/PANI composites increased, the amine structures in the composites decreased due to the formation of hydrogen bonds between the lignin hydroxyl groups and the PANI amine groups. The FT-IR spectra also showed a decrease in OH stretching intensity as the PANI ratio increased.
- The removal efficiency of Cr(Ⅵ) in all of the lignin/PANI composites was over 99%, and PANI_L4 showed higher Cr(Ⅵ) removal efficiency than pure PANI.
- Although the Cr removal efficiency of lignin itself was low, the lignin/PANI composites showed an excellent Cr removal efficiency similar to that of pure PANI due to the adsorption of both lignin and PANI.
- The resulting lignin/PANI composites exhibited excellent Cr removal efficiency with a reduced amount of PANI.
ACKNOWLEDGMENTS
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) and funded by the Ministry of Education (Grant No. 2018R1A6A1A0325582). The authors also acknowledge The Central Laboratory of Kangwon National University for the XPS analyses.
REFERENCES CITED
Anand, J., Palaniappan, S., and Sathyanarayana, D. N. (1998). “Conducting polyaniline blends and composites,” Prog. Polym. Sci. 23(6), 993-1018. DOI: 10.1016/S0079-6700(97)00040-3
Bhaumik, M., Maity, A., Srinivasu, V. V., and Onyango, M. S. (2012). “Removal of hexavalent chromium from aqueous solution using polypyrrole-polyaniline nanofibers,” Chem. Eng. J. 181-182, 323-333. DOI: 10.1016/j.cej.2011.11.088
Bruijnincx, P. C., and Weckhuysen, B. M. (2014). “Biomass conversion: Lignin up for break-down,” Nat. Chem. 6(12), 1035-1036. DOI: 10.1038/nchem.2120
Goto, H., and Yokoo, A. (2013). “Polyaniline nanospheres synthesized in the presence of polyvinyl alcohol followed by preparation of carbon nanobeads structures,” J. Disper. Sci. Technol. 34(3), 406-410. DOI: 10.1080/01932691.2012.662435
Ibrahim, K. A. (2017). “Synthesis and characterization of polyaniline and poly (aniline-co-o-nitroaniline) using vibrational spectroscopy,” Arab. J. Chem. 10(Supplement 2), S2668-S2674. DOI: 10.1016/j.arabjc.2013.10.010
Kaitsuka, Y., Hayashi, N., Shimokawa, T., Togawa, E., and Goto, H. (2016). “Synthesis of polyaniline (PANI) in nano-reaction field of cellulose nanofiber (CNF), and carbonization,” Polymers 8(2), Article Number 40. DOI: 10.3390/polym8020040
Kim, D. S., and Sung, Y. J. (2018). “Preparation of lignin-silica hybrid composite by using the alkaline digestion liquor of rice husk,” Pulp and Paper Technology 50(3), 86-93. DOI: 10.7584/JKTAPPI.2018.06.50.3.86
Krishnani, K. K., Srinives, S., Mohapatra, B. C., Boddu, V. M., Hao, J., Meng, X., and Mulchandani, A. (2013). “Hexavalent chromium removal mechanism using conducting polymers,” J. Hazard. Mater. 252-253, 99-106. DOI: 10.1016/j.jhazmat.2013.01.079
Li, X., Liu, W., Li, M., Li, Y., and Ge, M. (2014). “Characterizations and Cr (VI) adsorption properties of polyaniline/filter‐paper composite,” Polym. Composite. 35(5), 993-998. DOI: 10.1002/pc.22745
Ľudmila, H., Michal, J., Andrea, Š., and Aleš, H. (2015). “Lignin, potential products and their market value,” Wood Res. 60(6), 973-986.
Malinauskas, A. (2001). “Chemical deposition of conducting polymers,” Polymer 42(9), 3957-3972. DOI: 10.1016/S0032-3861(00)00800-4
Mansour, M. S., Ossman, M. E., and Farag, H. A. (2011). “Removal of Cd (II) ion from waste water by adsorption onto polyaniline coated on sawdust,” Desalination 272(1-3), 301-305. DOI: 10.1016/j.desal.2011.01.037
Naseem, A., Tabasum, S., Zia, K. M., Zuber, M., Ali, M., and Noreen, A. (2016). “Lignin-derivatives based polymers, blends and composites: A review,” Int. J. Biol. Macromol. 93(Part A), 296-313. DOI: 10.1016/j.ijbiomac.2016.08.030
Norgren, M., and Edlund, H. (2014). “Lignin: Recent advances and emerging applications,” Curr. Opin. Colloid In. 19(5), 409-416. DOI: 10.1016/j.cocis.2014.08.004
Park, D., Yun, Y. S., and Park, J. M. (2008). “XAS and XPS studies on chromium-binding groups of biomaterial during Cr (VI) biosorption,” J. Colloid Interf. Sci. 317(1), 54-61. DOI: 10.1016/j.jcis.2007.09.049
Phan, T. B., Do, N. Q., and Mai, T. T. T. (2010). “The adsorption ability of Cr(VI) on sawdust–polyaniline nanocomposite,” Adv. Nat. Sci.-Nanosci. 1(3), Article ID 035006. DOI: 10.1088/2043-6262/1/3/035006
Qiu, B., Xu, C., Sun, D., Wang, Q., Gu, H., Zhang, X., Weeks, B. L., Hopper, J., Ho, T. C., Guo, Z., et al. (2015). “Polyaniline coating with various substrates for hexavalent chromium removal,” Appl. Surf. Sci. 334, 7-14. DOI: 10.1016/j.apsusc.2014.07.039
Razak, S.I.A., Rahman, W.A.W.A., Hashim, S., and Yahya, M.Y. (2013). “Enhanced interfacial interaction and electronic properties of novel conducting kenaf/polyaniline biofibers,” Polym-Plast Technol 52(1), 51-57. DOI:10.1080/03602559.2012.718401
Rodrigues, P. C., Cantão, M. P., Janissek, P., Scarpa, P. C., Mathias, A. L., Ramos, L. P., and Gomes, M. A. (2002). “Polyaniline/lignin blends: FTIR, MEV and electrochemical characterization,” Eur. Polym. J. 38(11), 2213-2217. DOI: 10.1016/S0014-3057(02)00114-3
Rodrigues, P. C., Muraro, M., Garcia, C. M., Souza, G. P., Abbate, M., Schreiner, W. H., and Gomes, M. A. (2001). “Polyaniline/lignin blends: Thermal analysis and XPS,” Eur. Polym. J. 37(11), 2217-2223. DOI: 10.1016/S0014-3057(01)00104-5
Salem, M. A., Elsharkawy, R. G., and Hablas, M. F. (2016). “Adsorption of brilliant green dye by polyaniline/silver nanocomposite: Kinetic, equilibrium, and thermodynamic studies,” Eur. Polym. J. 75, 577-590. DOI: 10.1016/j.eurpolymj.2015.12.027
Sapurina, I. Y., Frolov, V. I., and Stejskal, J. (2003). “A conducting composite of polyaniline and wood,” Russ. J. Appl. Chem. 76(5), 835-839. DOI: 10.1023/A:1026050428908
Seo, J. H., Bae, J. H., Choi, C. S., Lee, H. W., Jeong, H., and Kim, Y. S. (2018). “Studies on the physical and chemical properties of lignins extracted from various pretreatment methods,” Pulp and Paper Technology 50(5), 76-85. DOI: 10.7584/JKTAPPI.2018.10.50.5.76
Sharma, D. C., and Forster, C. F. (1994a). “A preliminary examination into the adsorption of hexavalent chromium using low-cost adsorbents,” Bioresource Technol. 47(3), 257-264. DOI: 10.1016/0960-8524(94)90189-9
Sharma, D. C., and Forster, C. F. (1994b). “The treatment of chromium wastewaters using the sorptive potential of leaf mould,” Bioresource Technol. 49(1), 31-40. DOI: 10.1016/0960-8524(94)90170-8
Wu, Y., Zhang, S., Guo, X., and Huang, H. (2008). “Adsorption of chromium (III) on lignin,” Bioresource Technol. 99(16), 7709-7715. DOI: 10.1016/j.biortech.2008.01.069
Yavuz, A. G., Dincturk-Atalay, E., Uygun, A., Gode, F., and Aslan, E. (2011). “A comparison study of adsorption of Cr (VI) from aqueous solutions onto alkyl-substituted polyaniline/chitosan composites,” Desalination 279(1-3), 325-331. DOI: 10.1016/j.desal.2011.06.034
Zhang, R., Ma, H., and Wang, B. (2010). “Removal of chromium(VI) from aqueous solutions using polyaniline doped with sulfuric acid,” Ind. Eng. Chem. Res. 49(20), 9998-10004. DOI: 10.1021/ie1008794
Article submitted: June 10, 2019; Peer review completed: August 25, 2019; Revised version received and accepted: September 29, 2019; Published: October 4, 2019.
DOI: 10.15376/biores.14.4.9169-9182
APPENDIX: SUPPLEMENTARY INFORMATION
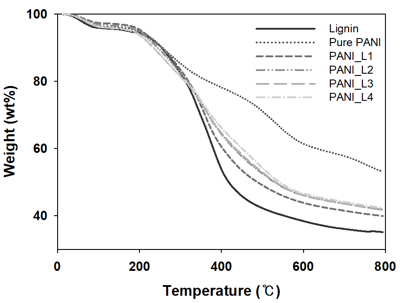
Fig. S1. Thermogravimetric analysis thermograms of the lignin/PANI composites
Table S1. TEM-EDS Results of the Lignin/PANI Composites
Table S2. Weight Loss and Char Residues of the Lignin/PANI Composites
