Abstract
Acetylation of cellulose fiber extracted by methylbenzene/ethanol (2/1), sodium chlorite solution, and sodium hydroxide from raw wheat straw (RWS) was studied to examine its potential as an oil-spill adsorbent. Wheat straw cellulosic sorbent was produced by using acetic anhydride as an acetylating reagent and N-bromosuccinimide (NBS) as a catalyst. Effects of the volume ratio of acetic anhydride (from 6.25% to 57.5%), catalyst concentration (from 10 to 60 mM NBS), reaction temperature (from 50 to 120 °C), and reaction time (from 0.5 to 3 h) on oil-sorption properties were evaluated. The best oil absorbencies for diesel fuel, diesel oil slick, corn oil, and corn oil slick treatments were 24.21 ± 0.76, 22.39 ± 0.77, 25.61 ± 2.13, and 24.73 ± 1.19 g/g, respectively. Chemical composition and morphologic structure of RWS before and after acetylation were investigated and compared. Oil-absorption capacity, oil-retention ability, recyclability, and selectivity of RWS, pretreated wheat straw, and acetylated wheat straw were also discussed. The acetylated wheat straw demonstrated good potential for the utilization of agricultural residues as natural sorbents in oil cleanup.
Download PDF
Full Article
Preparation of an Efficient Oil-Spill Adsorbent Based on Wheat Straw
Ermeng Lv, Wuyang Xia, Mingxiao Tang, and Yuewu Pu *
Acetylation of cellulose fiber extracted by methylbenzene/ethanol (2/1), sodium chlorite solution, and sodium hydroxide from raw wheat straw (RWS) was studied to examine its potential as an oil-spill adsorbent. Wheat straw cellulosic sorbent was produced by using acetic anhydride as an acetylating reagent and N-bromosuccinimide (NBS) as a catalyst. Effects of the volume ratio of acetic anhydride (from 6.25% to 57.5%), catalyst concentration (from 10 to 60 mM NBS), reaction temperature (from 50 to 120 °C), and reaction time (from 0.5 to 3 h) on oil-sorption properties were evaluated. The best oil absorbencies for diesel fuel, diesel oil slick, corn oil, and corn oil slick treatments were 24.21 ± 0.76, 22.39 ± 0.77, 25.61 ± 2.13, and 24.73 ± 1.19 g/g, respectively. Chemical composition and morphologic structure of RWS before and after acetylation were investigated and compared. Oil-absorption capacity, oil-retention ability, recyclability, and selectivity of RWS, pretreated wheat straw, and acetylated wheat straw were also discussed. The acetylated wheat straw demonstrated good potential for the utilization of agricultural residues as natural sorbents in oil cleanup.
Keywords: Wheat straw; N-bromosuccinimide; Acetic anhydride; Acetylation; Adsorbent
Contact information: School of Bioscience and Bioengineering, South China University of Technology, Guangzhou 510006, PR China; *Corresponding author: g96123@scut.edu.cn
INTRODUCTION
With increasing rates of energy consumption, the harmful impacts of petroleum products and their derivatives are becoming more pronounced. Refinement, extraction, transfer, and storage of crude oil, especially during ocean transportation, can lead to leakages and other issues, which seriously affect the ocean ecology and the natural environment (Aguilera et al. 2010).
Development of environmentally friendly and cost-effective technologies to minimize petroleum-related pollution is urgently required. Oil absorbents are very promising materials to deal with oil spills, as they typically have high cleanup efficiencies. Oil sorbents can concentrate and transform liquid oil into a semi-solid or solid phase, which can then be removed from water and handled in a convenient manner without the oil draining out. In recent years, natural fibers have been shown to have many advantageous properties for oil remediation work; in particular, such materials are biodegradable, renewable, and low in cost (Alemdar and Sain 2008). Natural fibers have attracted a broad interest in current research on hydrophobic treatments, and exemplary studies have focused on oil-adsorption materials such as kapok filters (Huang and Lim 2006), corn stalk (Husseien et al. 2009), cotton (Wang et al. 2015), pomelo peel (Zou et al. 2015), rice husk (Kumagai et al. 2007), sugarcane bagasse (Boni et al. 2016), and sisal (Robinson et al. 2002). Natural fibers show commendable oil-absorbing performance, with potentially wide applications.
Wheat straw is one of the most important agricultural residues, and it is an annually renewable fiber resource available in abundant quantities in many regions around the world. About 54 million ton of dry wheat straw is produced annually worldwide (Robinson et al. 2002). However, large amounts of wheat straw residues are disposed of as waste. Wheat straw represents an abundant, inexpensive, and readily available resource of renewable cellulosic biomass, which is composed of cellulose, hemicellulose, and lignin (about 41%, 32.51%, and 15.4%, respectively, on a dry weight basis).
Cellulose can be an effective sorbent for oil sorption, but the hydroxyl groups located on its fibers have hydrophilic properties that may affect its efficiency and oil-uptake capacity in aqueous systems (Deschamps et al. 2003). Adsorbents with hydrophobic and oleophilic properties can be readily used to solve problems in regard to oil pollution. Replacing the hydroxyl groups can make the hydrophilic surface of cellulose more hydrophobic (Jonoobi et al. 2010). Hydroxyl groups can be replaced by hydrophobic acetyl groups though acetylation (Chen et al. 2016). After acetylation, the more hydrophobic cellulosic sorbent traps oil more quickly, thus making recovery efforts easier.
To date, few works have reported on the preparation of a high-efficiency oil-spill adsorbent by isolation and acetylation of wheat straw cellulose fiber. In this study, natural fiber and wheat straw were selected as the source of cellulose, N-bromosuccinimide (NBS) as a catalyst, acetic anhydride as the acetylating reagent, and dimethylacetamide (DMAC) as the reaction solvent. These reagents were used to execute an acetylation reaction and obtain high-efficiency oil adsorbents. Diesel oil and corn oil, which are very common types of oils, have different densities and viscosities, and these properties may influence the adsorption characteristics of adsorbents through different mechanisms. Therefore, diesel oil and corn oil were used in this study to investigate the adsorption performance of the adsorbents. The oil-adsorption analysis, oil-retention ability, and recyclability of raw wheat straw (RWS), pretreated wheat straw (PWS), and acetylated wheat straw (AWS) were discussed to illustrate the oil-absorbing potential of the wheat straw adsorbent. The oil-sorption capacities of RWS, PWS, and AWS for diesel oil, diesel oil slick, corn oil, and corn oil slick treatments were measured. In addition, Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM), and contact angle analyses were performed to characterize the RWS, PWS, and AWS.
EXPERIMENTAL
Materials and Reagents
Wheat straw was obtained from a farm in Henan, China. Corn oil was purchased from the Shibo Supermarket (Guangzhou, China). Diesel oil was received from the Hongji Gas Station (Guangzhou, China). The NBS, sodium hydroxide, and sodium chlorite were obtained from the Aladdin Chemical Company (Shanghai, China). Ethanol (absolute) and DMAC were obtained from the Damao Chemical Company (Tianjin, China). Acetic anhydride and acetone were provided by the Kaixin Chemical Company (Hunan, China). All chemicals were of analytical reagent grade and directly used as they were received, without further purification.
Pretreatment
The wheat straw samples were washed twice with running water, soaked in boiling water overnight, washed three times with deionized water, and oven-dried at 60 °C for 24 h. The wheat straw samples were then cut into small pieces (1 to 3 cm), extracted with toluene–ethanol (2:1, v/v) in a Soxhlet for 4 h to remove the waxy layer (Hori et al. 2000), washed with ethanol twice, and again oven-dried at 60 °C for 24 h. Next, the dewaxed wheat straw was treated with an aqueous solution of 1.3% NaClO2 (at a liquid ratio of 1:30, with the solution pH adjusted from 3.5 to 4.0 by using glacial acetic acid) at 75 °C for 2 h to remove the lignin (Sidiras and Konstantinou 2012), washed with deionized water, rinsed until neutral, and soaked in ethanol for 15 min. Subsequently, sodium hydroxide (NaOH) at a concentration of 10% (w/w) was used to treat the samples at 20 °C for 10 h to remove the hemicellulose and swell the cellulose (Li et al. 2013). The samples were then washed with deionized water, rinsed until neutral, soaked in ethanol for 15 min, and washed with acetone. Finally, the PWS was oven-dried at 60 °C for 24 h and stored in a sealed bag.
Acetylation
Samples were acetylated under mild conditions in DMAC by using acetic anhydride as an acetylation reagent and NBS as a catalyst. Samples (0.5 g) were suspended in DMAC (15 mL) and NBS (10 to 60 mM) in a 50 mL vial. The mixture was placed at room temperature for 15 min to allow for the dispersion and swelling of the cellulose. The volume ratio of acetic anhydride (6.25% to 57.5%, Va:Vt, where Va is the volume of acetic anhydride and Vt is the total volume of acetic anhydride and DMAC) was added to the vial before it was placed in an oil bath at 50 to 120 °C for cellulose acetylation with magnetic agitation for 0.5 to 3 h. Once the reaction ended, the resulting mixture was poured into 750 mL of a mixture of distilled water and ethanol (2:1, v/v). The precipitates that formed were filtered out and thoroughly washed with 750 mL of ethanol solution (three times). The residues were oven-dried overnight at 60 °C.
Oil-sorption Studies
Two types of oil, namely diesel oil and corn oil, were used to study the adsorption capacity of AWS. Diesel oil is one of the most widely studied oil products in the literature. Thus, in order to make this study relevant to such research, diesel oil was chosen to investigate the oil-sorption mechanism. All tests were carried out at 30 °C. Figure 1 shows the small steel mesh basket (200 mesh, 3.5 cm × 3.5 cm, weight M1) that was used for oil-sorption experiments. The basket was put into a glass dish with a diameter of 6 cm; the weight of this dish was designated as M2. A sample of weight M3 (0.1000 to 0.1050 g, accurate to 0.0001 g) was placed into the basket and suspended in a 250 mL glass beaker, which contained 150 mL of diesel oil. The small basket was immersed in the oil for 30 min, suspended in a blank 250 mL glass beaker for 10 min so that oil could drip-off, and then put back into the glass dish, weighed, and labeled as M4. The oil absorption of the blank basket (to perform the experiment with a blank basket, the difference of the basket quality before and after the oil absorption step was assessed) was designated as M5. The final sorption capacity of the sorbent could be calculated by Eq. 1 as follows,
Q = (M4 – M1 – M2 – M3 – M5)/M3 (1)
where Q is the sorption capacity of the sample (g/g), M1 is the weight of the small steel mesh basket (g), M2 is the weight of the glass dish (g), M3 is the dry weight of the sample used as the sorbent (g), M4 is the total measured weight of the basket, glass dish, and sorbent after sorption (g), and M5 is the sorption capacity of the blank basket (g). Three parallel samples were measured.

Fig. 1. Structure of the small steel mesh basket used for oil-adsorption measurements
Characterization of the Wheat Straw
FTIR spectra
The RWS, PWS, and AWS samples were prepared by using KBr pellets. The samples (2 mg) were mixed with 200 mg of KBr and ground into powders to form a transparent sheet. The FTIR spectra of each sample were obtained in the range from 4000 to 400 cm−1. Spectra were obtained on a VERTEX 33 Fourier transform infrared spectrometer (Bruker, Karlsruhe, Germany).
XRD
The crystallinity of the cellulose fiber was measured on a D8 ADVANCE X-ray diffractometer (Bruker, Karlsruhe, Germany). Cellulose fiber was ground to a powder by using a pestle and compressed on the test plate. The sweep was carried out from 5° to 40° at an operating voltage of 40 kV and an operating current of 40 mA.
SEM
The samples were examined with scanning electron microscopy (Merlin, Germany). Before the SEM observations, the samples were fixed on aluminum stubs and sputter-coated with gold. The SEM images were examined by using an accelerating voltage set to 20 kV.
Contact angle
Contact angle measurements were carried out by using an OCA-25 optical contact angle measuring device (Dataphysics, Stuttgart, Germany) at ambient temperature. Droplets of distilled water or diesel oil (5 μL) were dropped carefully onto the surface. The average value was obtained from three measurements at different positions on the sample.
Oil Slick Test
A 250 mL beaker filled with 140 mL water, and 10 mL diesel oil or corn oil was placed in an incubator at 30 °C and preheated for 30 min. The M3 (0.1000 to 0.1050 g) sample was placed in the basket (weight M1), which was then suspended in the 250 mL beaker. After 30 min, the basket was suspended in a blank 250 mL glass beaker for 10 min to allow for the oil to drip off, and then, it was placed in a glass dish (weight M2). Next, the sample was heated at 60 °C for 2 h to evaporate the water adsorbed by the sample, weighed, and marked as M4. The final sorption capacity of the sorbent was calculated by Eq. 2 as follows:
Q = (M4 – M1 – M2 – M3 – M5)/M3 (2)
where Q is the sorption capacity of the sample (g/g), M1 is the weight of the small steel mesh basket (g), M2 is the weight of the glass (g), M3 is the dry weight of the sample used as the sorbent (g), M4 is the total measured weight of the basket, glass dish, and sorbent after evaporating the water (g), and M5 is the sorption capacity of the blank basket (g). Three parallel samples were measured.
Degree of Hydrophobicity
The degree of hydrophobicity of the sorbents was determined by the method proposed by Asadpour et al. (2016).
Recyclability
A 250 mL beaker containing 150 mL of diesel oil was placed in an incubator at 30 °C and preheated for 30 min. A total of 0.1 g AWS was placed in the basket, which was immersed in the oil for 30 min, taken out, allowed to drip for 10 min, and weighed. The AWS was vacuum-filtered for 20 min with a G2 frit funnel to remove the oil, and then, the oil adsorbent was used to carry out the recyclability oil-sorption experiment. The final sorption capacity of the sorbent was calculated by using Eq. 1. These steps were repeated five times using three parallel samples.
RESULTS AND DISCUSSION
Characterization of the Wheat Straw
FTIR spectra
To test whether the hydroxyl groups were substituted by acetyl groups, the cellulose fiber was analyzed by FTIR spectroscopy (Fig. 2 and Table 1). The absorption peaks of RWS at 3436, 2925, 1751, 1373, 1236, and 1049 cm−1 were characteristic of wheat straw (Wan et al. 2013). Compared with the RWS spectrum, there were obvious increases in the intensity of the peaks at 3436 and 2925 cm–1 in the PWS spectrum, which can be attributed to the stretching vibration of OH- groups and -CH2 or -CH3 (Chai et al. 2015). This occurs because the wax layer, pectin, and other substances were removed from the wheat straw fiber surface during pretreatment; thus, some hydrogen bonds in the fiber wall were broken. The carbonyl (C=O) stretching absorbance at 1752 cm–1 in RWS, which was not observed in the PWS spectrum, was attributed to the ester linkage of carboxylic groups of the ferulic and p-coumaric acids of lignin and/or hemicellulose (Ding et al. 2011), which indicates that a large amount of hemicellulose and lignin was removed during pretreatment. An obvious increase in the intensity of the absorption peaks was observed at 1751 cm–1, 1373 cm–1, and 1236 cm–1 in the AWS spectrum compared to the PWS spectrum, which confirms that the acetyl groups were introduced into the modified product. As expected, there was no absorption in the range of 1840 to 1760 cm−1 in the AWS spectrum, which indicates that the product was free of unreacted acetic anhydride.
Table 1. Fourier Transform Infrared Spectroscopy (FTIR) Peaks, Corresponding Functional Groups, and Absorbance
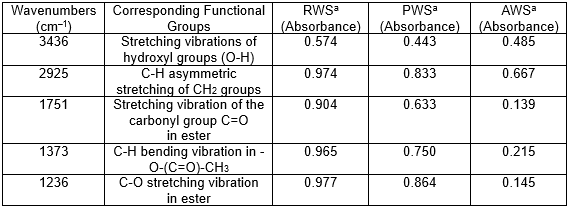
aRWS, raw wheat straw; PWS, pretreated wheat straw; AWS, acetylated wheat straw
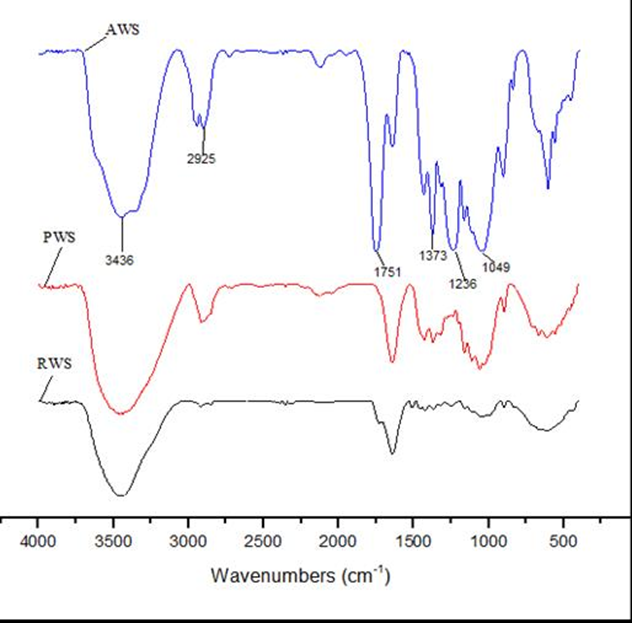
Fig. 2. Fourier transform infrared spectroscopy (FTIR) spectrum of raw wheat straw (RWS), pretreated wheat straw (PWS), and acetylated wheat straw (AWS)
XRD
The roughness and fold of the fiber surface is related to its adsorption properties, mainly crystallinity, which can be measured with XRD. The XRD data for RWS, PWS, and AWS are shown in Fig. 3. The diffraction peaks were indicative of relatively high degrees of crystallinity in the structure of the fibers. Specifically, the crystallinity values were 51.39%, 60.51%, and 40.25% for RWS, PWS, and AWS, respectively. The amount of crystalline cellulose was higher in PWS than in RWS because of the partial removal of the hemicelluloses and lignin during chemical pretreatment. Higher numbers of crystallinity regions are associated with increases in the rigidity of cellulose (Alemdar and Sain 2008). The diffraction peak of AWS at 22.25° was much lower than that of PWS; thus, the crystallinity of AWS was lower. This was probably due to the substitution of hydroxyl groups on the cellulose by acetyl groups, which caused the cellulose content to decrease and partially destroyed the crystalline area.
The decrement in the crystallinity also indicates that the percentage of the disordered regions and fraction voids on the surface of the fibers increased, which was propitious for oil sorption and storage. In addition, a new diffraction peak in the diffraction spectra of AWS was detected at 8.68°, which shows that the cellulose was acetylated (Glegg et al. 1968; Fan et al. 2013).
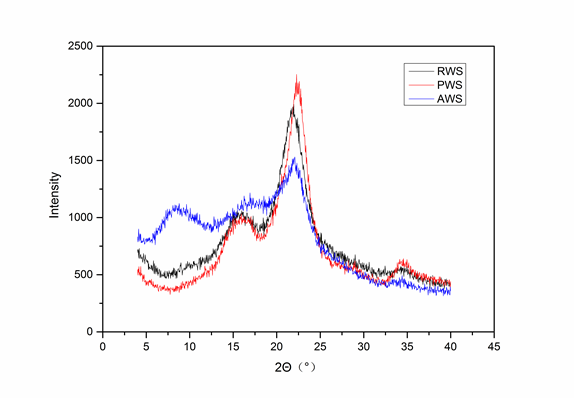
Fig. 3. X-ray diffraction (XRD) patterns of raw wheat straw (RWS), pretreated wheat straw (PWS), and acetylated wheat straw (AWS)
SEM
In order to observe the changes in morphology and microstructure, SEM data on RWS, PWS, and AWS were collected. The SEM microphotographs of the samples are shown in Fig. 4. As shown in Figs. 4a and 4b, the structure of RWS was compact and its storage space was very limited. Figure 4c shows that the structure of PWS was very loose and fibrous, and its storage space was greatly improved compared to that of RWS. From the comparison of Figs. 4b and 4e, it can be concluded that the roughness and fold of the fibers’ surface increased sharply after treatment by acetic anhydride, thus providing more space for oil storage.
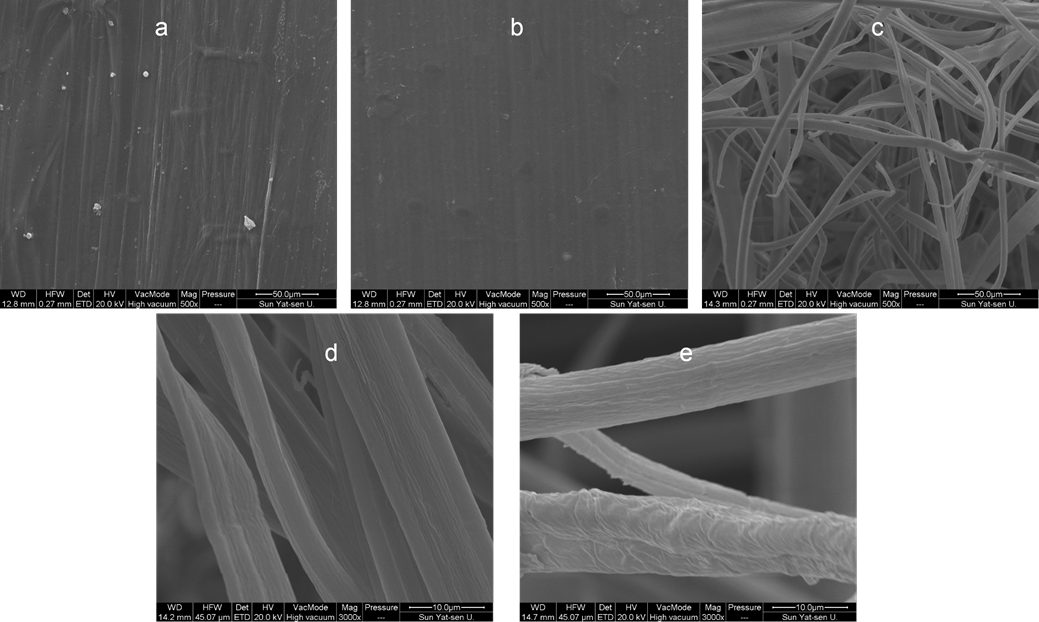
Fig. 4. Scanning electron microscopy (SEM) images of (a, b) raw wheat straw (RWS), (c, d) pretreated wheat straw (PWS), and (e) acetylated wheat straw (AWS)
Contact angle
A substance is regarded as hydrophobic if the contact angle of a water droplet on its surface is greater than 90°. Figure 5 shows photographs of water and diesel oil droplets on the surface of RWS, PWS, and AWS. The RWS displayed a low contact angle for water (44.5°) and oil (44.9°) (Figs. 5a and 5d, respectively), while PWS displayed a contact angle for both water and oil of 0°; these data demonstrated that RWS and PWS were hydrophilic and lipophilic, respectively.
Wheat straw modified by acetylation displayed a water contact angle of about 129.5° and an oil contact angle of 0°, thus demonstrating both hydrophobic and lipophilic properties in the usual sense (Figs. 5c and 5f). This result is in line with improvements in the hydrophobicity degree of wheat straw cellulose from 12.3% to 81.7% after the treatment. Alongside the effects of the cellulose fiber structure and morphology, surface tension also influences the oil-sorption rate and capacity. Generally, roughness and low surface energy contribute to the hydrophobicity (Chai et al. 2015). Here, the surface hydrophobicity of PWS increased with acetylation.
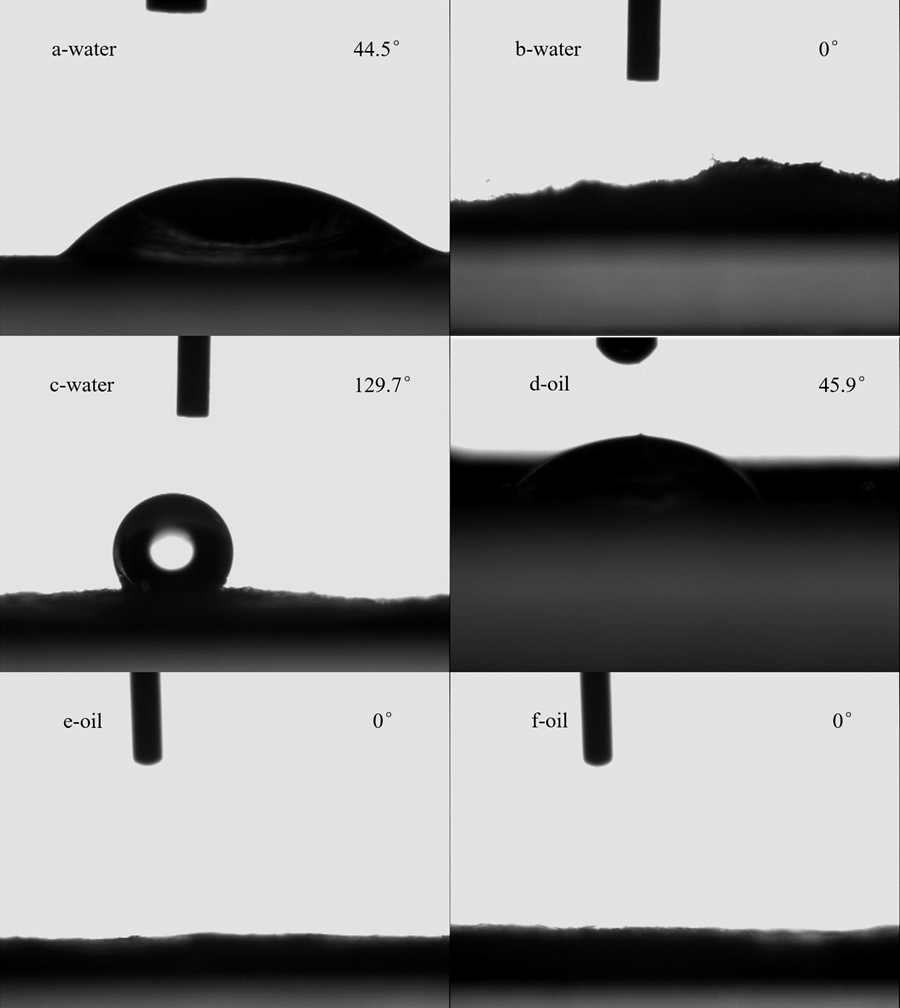
Fig. 5. Contact angle measurements of a water droplet on the surface of (a) raw wheat straw (RWS), (b) pretreated wheat straw (PWS), and (c) acetylated wheat straw (AWS), and an oil droplet on the surface of (d) RWS, (e) PWS, and (f) AWS
Effect of Preparation Conditions on the Suction Magnification
In this study, the effects of the reaction temperature (50 to 120 °C), reaction time (0.5 to 3 h), catalyst concentration (10 to 60 mM), and acetic anhydride dosage (1 to 20 mL) on the optimum preparation conditions for AWS were discussed. Single factor experiments and orthogonal experiments were used to determine the optimum conditions of acetylation.
Figure 6a shows that from 50 to 100 °C, the oil absorbency of AWS increased conspicuously, and it reached a maximum value at 100 °C. The significant rise in oil-absorption rate indicates that the increasing temperature favored the compatibility of the reaction ingredients, expansibility of cellulose, and molecular diffusion (Sun et al. 2004). As the temperature rose, the oil absorption decreased, possibly due to the strong penetration of the solvent and high temperature. This caused the excessive swelling of cellulose, thereby destroying the hydrogen bonds between molecules; dramatic degradation of fiber polymers and damage to the original loose structure followed (Li et al. 2013). Therefore, the optimal acetylation temperature was 100 °C.
As shown in Fig. 6b, prolonging the reaction time from 0.5 to 1.5 h increased the AWS oil absorbency from 18.87 g/g to 23.85 g/g. However, extending the reaction time beyond this led to a rapid decline in the oil-absorption capacity of AWS. The decrease in the oil-absorption capacity with extended reaction time was due to the production of acetic acid by-products (Li et al. 2013). The decrease in oil-sorption capacity might have been due to two phenomena. First, the acetic acid that formed corresponded to a reduction in the concentration of acetic anhydride, which reduced the reaction rate (Sun et al. 2002). Moreover, acetic acid by-products can corrode the fiber surface and damage the structure of PWS, thus resulting in a decrease in oil-sorption capacity (Wang and Wang 2013). Hence, the optimal reaction time was 1.5 h.
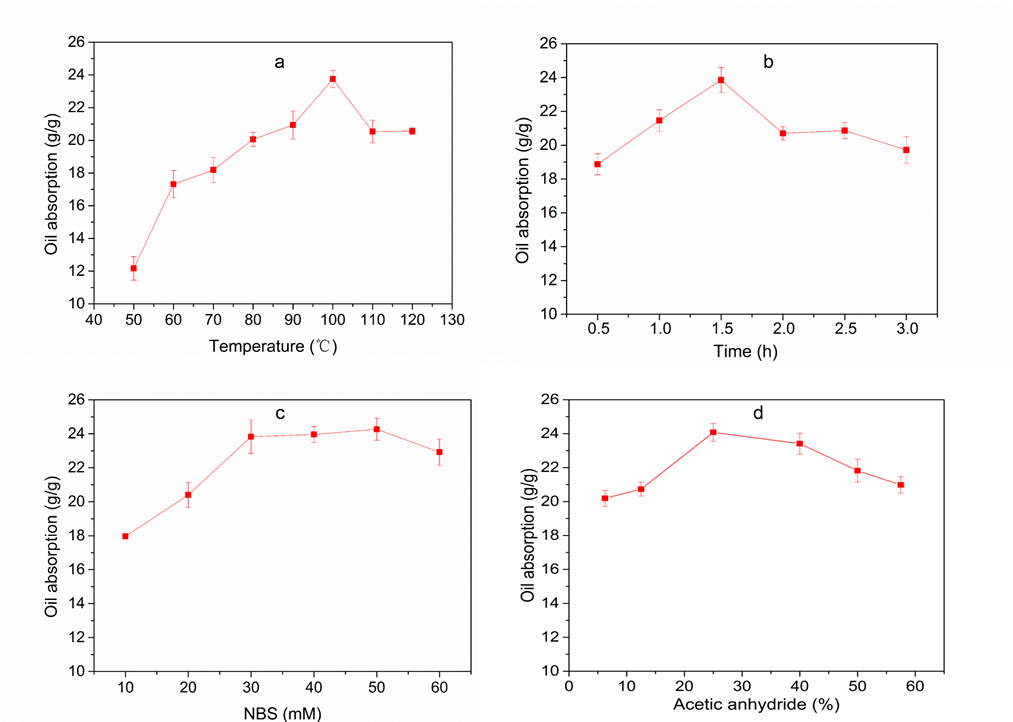
Fig. 6. Effect of reaction temperature, reaction time, N-bromosuccinimide (NBS) concentration, and amount of acetic anhydride. (a) Acetylation conditions: NBS: 30 mM; volume ratio of acetic anhydride: 25%; reaction temperature: 50, 60, 70, 80, 90, 100, 110, and 120 °C; reaction time: 1.5 h. (b) Reaction conditions: reaction temperature: 100 °C; reaction time: 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 h; other conditions as in (a). (c) Acetylation conditions: NBS: 10, 20, 30, 40, 50, and 60 mM; reaction time: 1.5 h: other conditions as in (a). (d) Acetylation conditions: NBS: 50 mM; volume ratio of acetic anhydride: 6.25%, 12.5%, 25%, 40%, 50%, and 57.5%; other conditions as in (a)
When the catalyst was added at a concentration of 10 to 50 mM, the oil-absorption rate of AWS increased with the rising NBS concentration, which was probably due to the rise in catalytic activity (Fig. 6c). When the concentration of NBS was more than 50 mM, the oil-absorption capacity decreased gradually. The role of NBS was not clear, but a plausible explanation is that it might act as a resource for Br–, which in turn activates the carbonyl groups of acetic anhydride to produce a highly reactive acetyl agent (CH3-CO-N-(OCCH2CH2CO-). The acetyl agent reacts with the hydroxyl groups of PWS, which upon elimination of NBS, produces acetylated PWS (PWS-O-CO-CH3) (Karimi and Seradj 2001; Sun et al. 2004). However, when the concentration of NBS is too high, a large amount of undecomposed NBS is adsorbed on the active sites of PWS, thus leading to a corresponding reduction in the contact area between acetic anhydride and hydroxyl, thereby effectively inhibiting the acetylation reaction and resulting in a low degree of acetylation. As a result, the best concentration of NBS was 50 mM.
Figure 6d shows that when 6.25% to 57.5% of acetic anhydride was added, the oil-absorption rate of AWS increased. However, when the volume ratio of acetic anhydride added was more than 25%, the oil-absorption capacity of AWS decreased rapidly. For the acetylation of PWS, the acetylation reaction rate was proportional to the concentration of acetic anhydride in any amount, and more hydroxyl groups were acetylated with an increase in the accessibility of acetic anhydride. With increased additions of acetic anhydride, the unreacted acetic anhydride and acetic acid by-product began to accumulate, which caused strong acid activity and resulted in the degradation of the fiber polymer and destruction of the hollow fiber structure (Zhang et al. 2012). Therefore, the optimum amount of acetic anhydride was 5 mL.
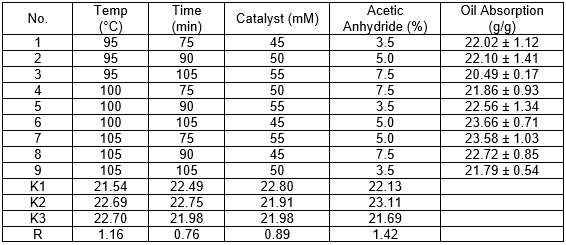
Based on the single factor experiments, four factors (reaction temperature, reaction time, NBS concentration, and acetic anhydride) and three levels of orthogonal experiments were evaluated to determine the optimum acetylation reaction conditions (Table 2). The primary and secondary factors of the acetylation reaction affected the oil-absorbing capacity of AWS in the following order: acetic anhydride > reaction temperature > catalyst > reaction time. In summary, the following optimum conditions were confirmed by single factor experiments and orthogonal experiments: NBS concentration, 55 mM; the volume ratio of acetic anhydride, 25%; reaction temperature, 100 °C; and reaction time, 1.5 h. The best oil absorbency for diesel oil was 24.21 ± 0.76 g/g.
Table 2. Orthogonal Experiments
Absorption Kinetic Analysis
Using pure diesel oil, the effects of different adsorption times on the oil-adsorption capacity of RWS, PWS, and AWS at 30 °C were investigated. Figure 7 shows the changes in the oil-absorption capacity of each sorbent with time. The RWS, PWS, and AWS showed a fast absorption rate during the initial 10 s. The equilibrium adsorption capacity was reached within 10 min, and the equilibrium adsorption capacity was 8.19, 15.67, and 24.21 g/g for RWS, PWS, and AWS, respectively. Compared with RWS, PWS showed a much greater capacity for diesel oil adsorption, probably owing to its pretreatment by NaClO2 and NaOH, which made the spatial structure looser, increased its specific surface area, and led to the formation of more numerous oil-absorbing sites and storage space. The equilibrium adsorption capacity of AWS was clearly the highest, it was 3 times that of RWS and 1.6 times that of PWS. The sorption increase might suggest that acetylation enhanced the hydrophobicity and roughness of the cellulose surface, which improved the stability of the capillary bridging between fiber bundles and thus effectively prevented oil from escaping from the fiber assembly (Dong et al. 2014). Another possible explanation was that acetylation increased the pore sizes of AWS, which provided more space for the oil to be adsorbed. This finding implies that acetylation is very useful for preparing oil sorbents with excellent oil absorption capacities.
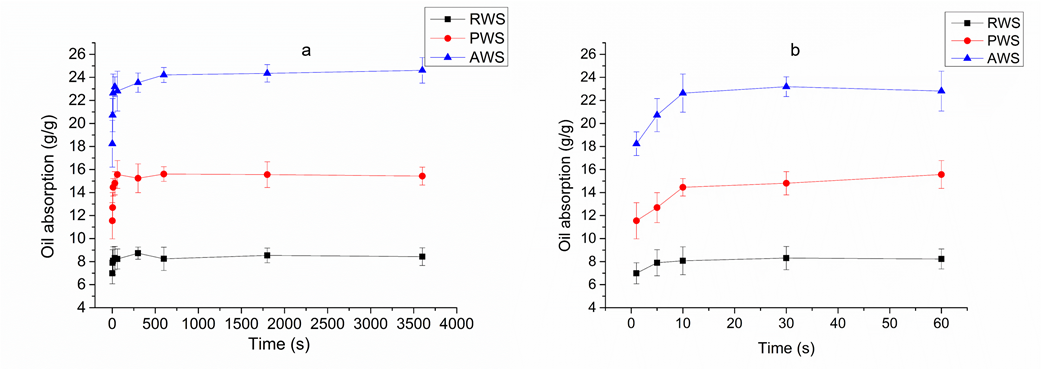
Fig. 7. Adsorption capacity curves of raw wheat straw (RWS), pretreated wheat straw (PWS), and acetylated wheat straw (AWS). (a) Entire process of change in oil absorption with time; (b) change in oil absorption with time during the first stage
Oil-retention Capacity
In this study, the oil-retention capacities of the absorbing materials were studied in a pure oil system. The dynamic oil-retention curves of RWS, PWS, and AWS are shown in Figs. 8a and 8b. The loss of diesel oil resolved on the adsorbent surface could be roughly divided into three stages. The first stage was the rapid resolution phase (1 to 30 s). During this stage, the oil absorbency of RWS, PWS, and AWS decreased from the original 15.50, 31.15, and 39.01 g/g sharply down to 9.17, 18.75, and 28.23 g/g, respectively, because of the escape of excess oil, which had adhered superficially to the surface of fiber bundles (Wang et al. 2015). The second stage was the slow resolution phase (30–600 s). During this stage, the oil absorbency of RWS, PWS, and AWS fell from 9.17, 18.57, and 28.23 g/g slowly down to 8.19, 15.56, and 24.37 g/g, respectively. The oil contained in the fiber lumen and the inter-fiber interstices drained out slowly, as the capillary pressure and the van der Waals forces were insufficient to hold the weight of the oil. The third stage was the equilibrium stage after 600 s. The oil absorbency above the adsorbent remained in basic balance. During this stage, the diesel molecules and fibers were stabilized through a stabilization force that was imparted by the capillary pressure inside the tubular structure. For increasing the reliability of the determination, the measurement of oil retention ability was initiated 30 s after the rapid resolution phase (stage 1). After this 30 s period, the absorbed oil showed 13.67%, 19.98%, and 15.67% losses compared to the initial oil-saturated AWS, PWS, and RWS materials, respectively. The loss of oil could be explained by the reduction of capillary retention pressure between the fiber assembly due to the filled oil in the fiber lumen and the inter-fiber interstices (Abdullah et al. 2010; Singh et al. 2013). In addition, it was obvious that AWS exhibited the highest oil retention ability compared to RWS and PWS, which was related to the draining process of the oil. The draining of oil in the extra-lumen occurs when the capillary pressure is insufficient to hold the weight of the oil (Singh et al. 2013). The oil bridges formed among the bundles of PWS and RWS would have been easier to destabilize (Wang et al. 2015). After acetylation, the sliding of absorbed oil on the single fiber surface was prevented by the hydrophobicity of the fiber surface, which enhanced the stability of the capillary pressure within the AWS material, and thus, a drastic improvement in the oil-retention capacity was achieved.
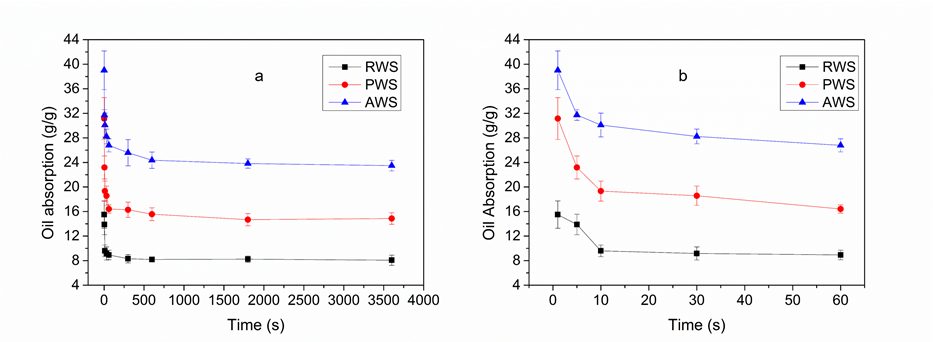
Fig. 8. Dynamic oil-retention curves of raw wheat straw (RWS), pretreated wheat straw (PWS), and acetylated wheat straw (AWS). (a) Entire duration of oil absorption; (b) the first stage of oil absorption
Recyclability
The recyclability of AWS will play an important role in reducing oil cleanup costs, and high quality adsorbents should be recyclable. Absorbed oil can be removed from oil-absorbent materials by mechanical pressing, high-speed centrifugation, or filtration. Then, the oil and oil-absorbent materials can be recycled and reused. The relationship between the number of times AWS was reused and its oil-absorption performance are shown in Fig. 9.
The adsorption capacity of AWS decreased with repetitive use (Fig. 9). Specifically, after the first use, the oil-absorption capacity was reduced by 25.92% (the initial oil absorption was 24.365 g/g). The main reason for the decline in the oil-absorption capacity of AWS after recycling was the oil that was still retained in the AWS after filtration; specifically, this retained oil occupied some of the oil-absorption sites and used up a portion of the storage space. There was about 3.0 to 4.5 g/g of residual oil left after each filtration. Despite this residue, from the second to the fifth reuse, the oil-absorption capacity of AWS decreased slowly, and the findings showed that the material could maintain absorption of more than 15.5 g/g, which was equivalent to 63.6% of the initial amount of oil absorption. These findings are consistent with the results of Zhu et al. (2011). A suitable method for separating the residual oil that will not destroy the space structure and the void ratio of the adsorbent would greatly improve the recyclability of AWS. Despite this disadvantage, the results imply that AWS could be reused and recycled several times with the help of vacuum filtration. This property of recyclability consequently makes AWS attractive for large-scale cleanups of oil spills in water.
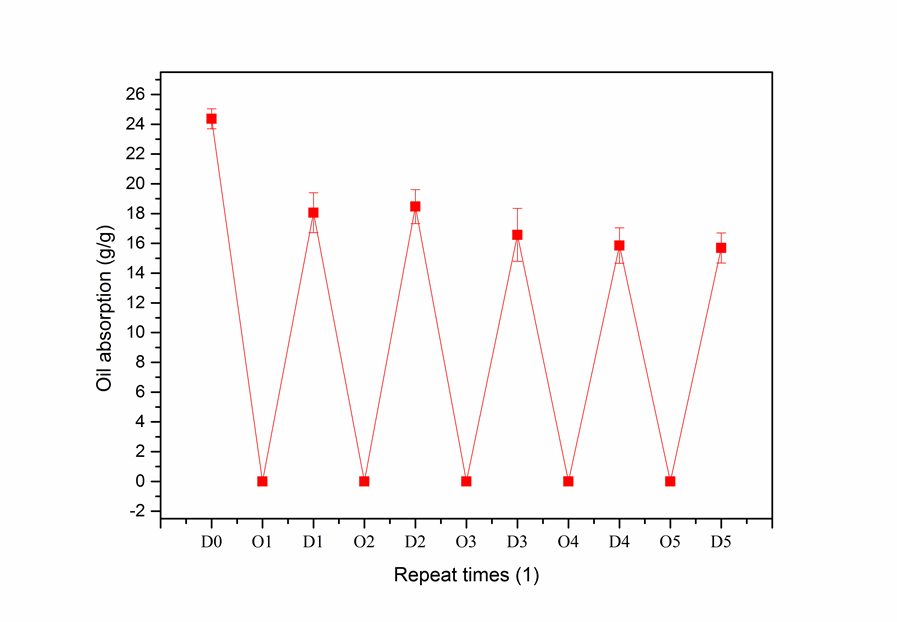
Fig. 9. Recyclability and number of reuses
Acetylation of Different Pretreated Wheat Straw
The acetylation of RWS, dewaxed wheat straw (DWS), wheat straw pretreated by NaClO2 (NWS), and PWS were investigated (Fig. 10). After modification by acetylation, the oil absorption of RWS and DWS decreased compared with non-acetylated RWS and DWS, while that of NWS and PWS increased. The RWS was wrapped in a waxy layer and lignin, and DWS was wrapped with lignin, which formed a compact protective layer that made it difficult for the acetylating agent to reach the cellulose interior and bond with the hydroxyl groups of cellulose.
The original tubular structure of straw was destroyed because of the high temperature and strong permeability of the solvent, and this decreased the oil-absorption capacity. The increased rate of oil absorption for NWS and PWS before and after acetylation amounted to 56.56% and 32.03%. For NWS, the outside lignin was removed, so more cellulose was exposed to the outside and the structure of NWS had not changed, which resulted in a small increase in the degree of acetylation and oil-absorption capacity. For PWS, the lignin and hemicellulose was removed, which resulted in much more cellulose being exposed to the outside and a much looser structure, which increased the rate of oil absorption of PWS, i.e., it was much higher than that of NWS. This suggests that a loose spatial structure and exposed cellulose hydroxyl groups were necessary for acetylation.
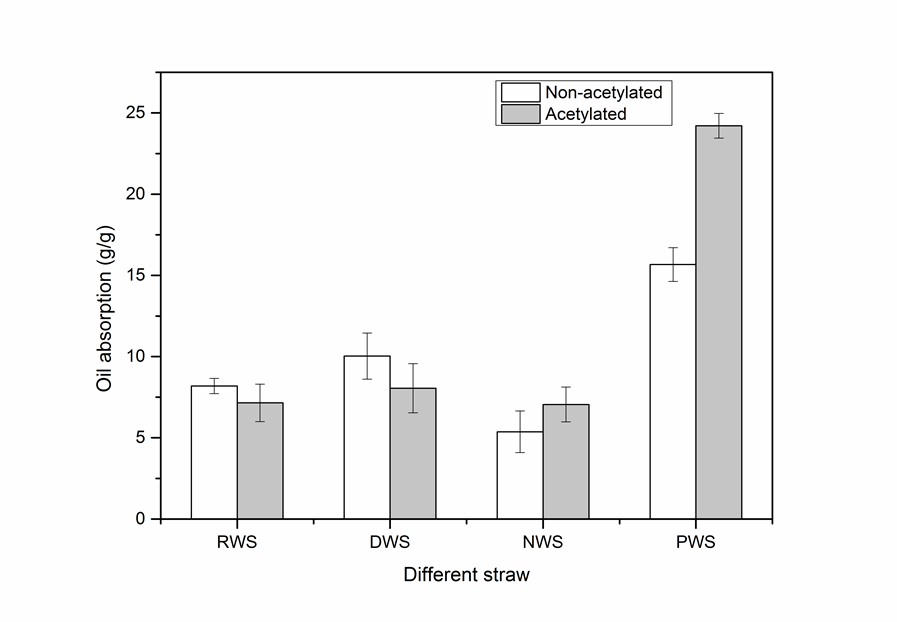
Fig. 10. Oil absorption of different types of wheat straw before and after acetylation; RWS, raw wheat straw; DWS, dewaxed wheat straw; NWS, NaClO2-treated wheat straw; PWS, pretreated wheat straw
Oil-absorption Characteristics for Different Types of Oil
The oil-absorption values of RWS, PWS, and AWS are shown in Fig. 11. The oil-absorption values of RWS for diesel oil, diesel oil slick, corn oil, and corn oil slick treatments were 8.19 ± 0.47, 7.83 ± 1.14, 9.23 ± 1.34, and 8.68 ± 1.23 g/g, respectively. Surprisingly, the oil absorption of AWS for the above four organic treatments were 24.21 ± 0.76, 22.39 ± 0.77, 25.61 ± 2.13, and 24.73 ± 1.19 g/g, respectively. This was about three times the absorption capacity of RWS. The above result implies that acetylation was a very effective preparation method to achieve oil adsorbents with a high oil-absorption capacity. The oil-absorption values of AWS, RWS and PWS were all greater for corn oil than diesel oil. This was probably because of the higher viscosity of corn oil compared to diesel oil. However, high oil viscosity can have two opposing effects, namely, it can increase sorption by improving the adherence of oil onto the fiber surface, but it may also decrease sorption by inhibiting oil penetration into the interior of the sorbent (Zhang et al. 2012). There was only a slight decline in the oil absorption of AWS for the oil slick compared with that for pure oil, while there was a great decrease in the absorption of PWS. This shows that the surface of AWS had a strong hydrophobicity. The acetylation reaction enhanced the hydrophobic and oleophilic properties of AWS, and these hydrophobic and oleophilic properties were important to create an effective oil sorbent. Acetylation not only changed the surface characteristics of wheat straw, but also created more oil-absorbing sites and storage space. Hence, acetylation played a crucial role in the preparation of an efficient oil-spill adsorbent based on wheat straw, and the prepared AWS showed great promise for applications in oil-spill remediation.
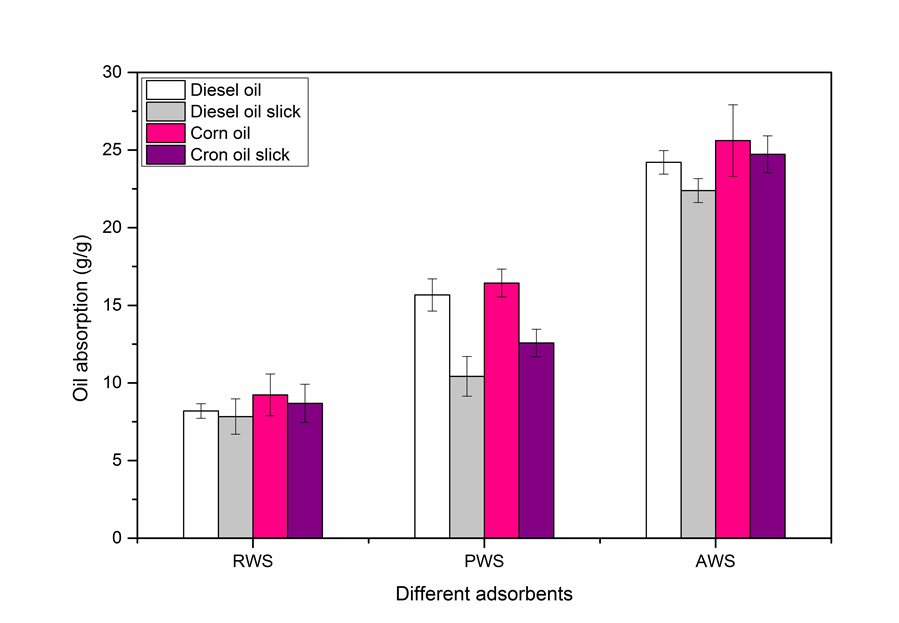
Fig. 11. Oil-absorption values of raw wheat straw (RWS), pretreated wheat straw (PWS), and acetylated wheat straw (AWS) for the diesel oil, diesel oil slick, corn oil, and corn oil slick treatments
CONCLUSIONS
- The optimum preparation conditions for AWS were confirmed with single factor experiments and orthogonal experiments. The optimum conditions were as follows: NBS concentration, 55 mM; acetic anhydride content, 5 mL; reaction temperature, 100 °C; and reaction time, 1.5 h.
- Acetylated wheat straw was shown to be an effective oil adsorbent through the analysis of its absorption capacity, oil-retention capacity, and recyclability.
- This work also demonstrated that AWS was equipped with a hydrophobic and oleophilic surface and contained abundant oil-absorbing sites and storage space, which makes it promising for environmental remediation applications such as during the large-scale removal of spilled oil from water.
ACKNOWLEDGEMENTS
This work was supported by The National Key Technology R&D Program during the 12th Five-Year Plan period (2014BAL06B02).
REFERENCES CITED
Abdullah, A., Rahmah, A., and Man, Z. (2010). “Physicochemical and sorption characteristics of Malaysian Ceiba pentandra (L.) Gaertn. as a natural oil sorbent,” Journal of Hazardous Materials 177(1-3), 683-691. DOI: 10.1016/j.jhazmat.2009.12.085
Aguilera, F., Méndez, J., Pásaro, E., and Laffon, B. (2010). “Review on the effects of exposure to spilled oils on human health,” Journal of Applied Toxicology 30(4), 291-301. DOI: 210.1002/jat.1521/
Alemdar, A., and Sain, M. (2008). “Isolation and characterization of nanofibers from agricultural residues – Wheat straw and soy hulls,” Bioresource Technology 99(6), 1664-1671. DOI: 10.1016/j.biortech.2007.04.029
Asadpour, R., Sapari, N. B., Isa, M. H., and Kakooei, S. (2016). “Acetylation of oil palm empty fruit bunch fiber as an adsorbent for removal of crude oil,” Environmental Science and Pollution Research 12(23), 11740-11750. DOI: 10.1007/s11356-016-6349-2
Boni, H., de Oliveira, D., Ulson de Souza, A., and Ulson de Souza, S. (2016). “Bioadsorption by sugarcane bagasse for the reduction in oil and grease content in aqueous effluent,” International Journal of Environmental Science and Technology 13(4), 1169-1176. DOI: 10.1007/s13762-016-0962-y
Chai, W., Liu, X., Zou, J., Zhang, X., Li, B., and Yin, T. (2015). “Pomelo peel modified with acetic anhydride and styrene as new sorbents for removal of oil pollution,” Carbohydrate Polymers 132, 245-251. DOI: 10.1016/j.carbpol.2015.06.060/
Chen, J., Xu, J., Wang, K., Cao, X., and Sun, R. (2016). “Cellulose acetate fibers prepared from different raw materials with rapid synthesis method,” Carbohydrate Polymers 137, 685-692. DOI: 10.1016/j.carbpol.2015.11.034
Deschamps, G., Caruel, H., Borredon, M., Albasi, C., Riba, J., Bonnin, C., and Vignoles, C. (2003). “Oil removal from water by sorption on hydrophobic cotton fibers. Study of sorption properties in dynamic mode,” Environmental Science and Technology 37(21), 5034-5039. DOI: 10.1021/es020249b
Ding, B., Lin, J., Wang, X., Yu, J., Yang, J., and Cai, Y. (2011). “Investigation of silica nanoparticle distribution in nanoporous polystyrene fibers,” Soft Matter 7(18), 8376-8383. DOI: 10.1039/C1SM05791J
Dong, T., Wang, F., and Xu, G. (2014). “Theoretical and experimental study on the oil sorption behavior of kapok assemblies,” Industrial Crops and Products 61, 325-330. DOI: 10.1016/j.indcrop.2014.07.020
Glegg, R., Ingerick, D., Parmerter, R., Salzer, J., and Warburton, R. (1968). “Acetylation of cellulose I and II studied by limiting viscosity and X-ray diffraction,” Journal of Polymer Science Part B: Polymer Physics 6(4), 745-773. DOI: 10.1002/pol.1968.160060410
Hori, K., Flavier, M., Kuga, S., Lam, T., and Ilyama, K. (2000). “Excellent oil absorbent kapok [Ceiba pentandra (L.) Gaertn.] fiber: Fiber structure, chemical characteristics, and application,” Journal of Wood Science 46, 401-404. DOI: 10.1007/BF00776404
Huang, X., and Lim, T. (2006). “Performance and mechanism of a hydrophobic–oleophilic kapok filter for oil/water separation,” Desalination 190(1-3), 295-307. DOI: 10.1016/j.desal.2005.09.009
Husseien, M., Amer, A., El-Maghraby, A., and Hamedallah, N. (2009). “A comprehensive characterization of corn stalk and study of carbonized corn stalk in dye and gas oil sorption,” Journal of Analytical and Applied Pyrolysis 86(2), 360-363. DOI: 10.1016/j.jaap.2009.08.003
Jonoobi, M., Harun, J., Mathew, A., Hussein, M., and Oksman, K. (2010). “Preparation of cellulose nanofibers with hydrophobic surface characteristics,” Cellulose 17(2), 299-307. DOI: 10.1007/s10570-009-9387-9
Karimi, B., and Seradj, H. (2001). “N-Bromosuccinimide (NBS), a novel and highly effective catalyst for acetylation of alcohols under mild reaction conditions,” Cheminform 32(29), 74-74. DOI: 10.1002/chin.200129074
Kumagai, S., Noguchi, Y., Kurimoto, Y., and Takeda, K. (2007). “Oil adsorbent produced by the carbonization of rice husks,” Waste Management 27(4), 554-561. DOI: 10.1016/j.wasman.2006.04.006
Li, D., Zhu, F., Li, J., Na, P., and Wang, N. (2013). “Preparation and characterization of cellulose fibers from corn straw as natural oil sorbents,” Industrial & Engineering Chemistry Research 52(1), 516-524. DOI: 10.1021/ie302288k/.
Robinson, T., Chandran, B., and Nigam, P. (2002). “Removal of dyes from a synthetic textile dye effluent by biosorption on apple pomace and wheat straw,” Water Research 11(36), 2824-2830. DOI: 10.1016/S0043-1354(01)00521-8
Sidiras, D., and Konstantinou, I. (2012). “A new oil spill adsorbent from sulfuric acid modified wheat straw,” Latest Trends in Environmental and Manufacturing Engineering, Quality and Production Systems 132(6), 132-137. DOI: 10.5071/19thEUBCE2011-VP2.7.19
Singh, V., Kendall, R., Hake, K., and Ramkumar, S. (2013). “Crude oil sorption by raw cotton,” Industrial & Engineering Chemistry Research 52(18), 6277-6281. DOI: 10.1021/ie4005942/
Sun, X., Cang, R., Cang, A., and Sun, J. (2002). “Acetylation of rice straw with or without catalysts and its characterization as a natural sorbent in oil spill cleanup,” Journal of Agricultural and Food Chemistry 50(22), 6428-6433. DOI: 10.1021/jf020392o
Sun, X., Sun, R., and Sun, J. (2004). “Acetylation of sugarcane bagasse using NBS as a catalyst under mild reaction conditions for the production of oil sorption-active materials,” Bioresource Technology 95(3), 343-350. DOI: 10.1016/j.biortech.2004.02.025
Wan, T., Huang, R., Zhao, Q., Xiong, L., Qin, L., Tan, X., and Cai, G. (2013). “Synthesis of wheat straw composite superabsorbent,” Journal of Applied Polymer Science 130(5), 3404-3410. DOI: 10.1002/app.39573
Wang, J., and Wang, A. (2013). “Acetylated modification of kapok fiber and application for oil absorption,” Fibers and Polymers 14(11), 1834-1840. DOI: 10.1007/s12221-013-1834-4/
Wang, J., Geng, G., Wang, A., Liu, X., Du, J., Zou, Z., Zhang, S., and Han, F. (2015). “Double biomimetic fabrication of robustly superhydrophobic cotton fiber and its application in oil spill cleanup,” Industrial Crops and Products 77, 36-43. DOI: 10.1016/j.indcrop.2015.08.044
Zhang, A., Liu, C., Sun, R., Xie, J., and Chen, X. (2012). “Homogeneous acylation of eucalyptus wood at room temperature in dimethyl sulfoxide/N-methylimidazole,” Bioresource Technology 125, 328-331. DOI: 10.1016/j.biortech.2012.08.131/
Zhu, H., Qiu, S., Jiang, W., Daxiong, W., and Canying, Z. (2011). “Evaluation of electrospun polyvinyl chloride/polystyrene fibers as sorbent materials for oil spill cleanup,” Environmental Science & Technology 45(10), 4527-4531. DOI: 10.1021/es2002343/
Zou, J., Chai, W., Liu, X., Li, B., Zhang, X., and Yin, T. (2015). “Magnetic pomelo peel as a new absorption material for oil-polluted water,” Desalination and Water Treatment 57(27), 12536-12545. DOI: 10.1080/19443994.2015.1049958
Article submitted: June 7, 2016; Peer review completed: July 30, 2016; Revised version received and accepted: October 25, 2016; Published: November 14, 2016.
DOI: 10.15376/biores.12.1.296-315
