Abstract
Using response surface method to determine the optimal technological conditions of biomass-based precursor preparation, magnetic Fe3O4 particles were loaded on the surface and internal channel of biomass-based precursor to prepare a magnetic biomass-based solid acid catalyst using the sol-gel method. To investigate the performance of the magnetic catalyst, it was used to hydrolyze cellulose into reducing sugar, whose structure was characterized by infrared spectrum analysis. The optimum process conditions of biomass-based precursor preparation was obtained by quadratic regression model as a carbonization temperature of 549 °C, carbonization time of 13 h, sulfonating temperature of 121 °C, and sulfonating time of 6 h. Using the biomass-based solid acid catalyst to hydrolyze cellulose, a reducing sugar yield of 57.36% was obtained. Compared with the traditional solid acid catalysts, the total reducing sugar yield was increased by 65%. The infrared spectrum analysis showed that magnetic Fe3O4 particles were combined successfully with biomass-based precursor. This magnetic biomass-based solid acid catalyst has a carbon structure layer of vermicular disorder and possesses high stability.
Download PDF
Full Article
Preparation of Magnetic Biomass-based Solid Acid Catalyst and Effective Catalytic Conversion of Cellulose into High Yields of Reducing Sugar
Xueqin Li,a Xiangyu Li,a,* Wei Qi,b Junyou Shi,a Jiping Zhang,aYanhong Xu,a and Jiuyin Pang a
Using response surface method to determine the optimal technological conditions of biomass-based precursor preparation, magnetic Fe3O4 particles were loaded on the surface and internal channel of biomass-based precursor to prepare a magnetic biomass-based solid acid catalyst using the sol-gel method. To investigate the performance of the magnetic catalyst, it was used to hydrolyze cellulose into reducing sugar, whose structure was characterized by infrared spectrum analysis. The optimum process conditions of biomass-based precursor preparation was obtained by quadratic regression model as a carbonization temperature of 549 °C, carbonization time of 13 h, sulfonating temperature of 121 °C, and sulfonating time of 6 h. Using the biomass-based solid acid catalyst to hydrolyze cellulose, a reducing sugar yield of 57.36% was obtained. Compared with the traditional solid acid catalysts, the total reducing sugar yield was increased by 65%. The infrared spectrum analysis showed that magnetic Fe3O4 particles were combined successfully with biomass-based precursor. This magnetic biomass-based solid acid catalyst has a carbon structure layer of vermicular disorder and possesses high stability.
Keywords: Biomass; Magnetic solid acid catalyst; Hydrolysis; Cellulose; Reducing sugar
Contact information: a: Wood Material Science and Engineering Key Laboratory, Beihua University, Jilin Province, Jilin, China, 132013; b: Guangzhou Institute of Energy Conversion, CAS Key Laboratory of Renewable Energy, Guangdong Province, Guangzhou, China, 510640;
* Corresponding author: lixyv@126.com
INTRODUCTION
Biomass can be regarded as a primary form of fixed carbon, which can be used as renewable energy in power plants and as the raw material to produce liquid fuels. Biomass provides an alternative source of energy, ensuring its steady supply, and reducing environmental pollution. Lignocellulose, which can be regarded as a combination of hemicellulose, cellulose and lignin, accounts for most of the world’s biomass and can be considered as an alternative to fossil resources for sustainable production of chemicals and fuels (Shimizu and Satsuma 2011). The three components of lignocellulose are bound together through hydrogen bonds and other linkages, forming a relatively dense structure, which can be compared to reinforced concrete. As shown in Fig. 1, the reaction of any one component will be restricted by other components.
The hydrolytic saccharification of lignocellulose is the first step to making bioethanol. Among biofuels, ethanol can be regarded as the most direct and the most convenient alternative to petroleum-based liquid fuel. Accordingly it has received widespread attention and the development and utilization on a large scale (Basha et al. 2009). At present, the biomass fuels and chemicals industry is working to develop a variety of catalysts to accommodate the processing requirements of different raw materials (Tomishige et al. 2004). Using biomass corn stalk as carbon material to prepare solid acid catalyst has become a cutting-edge technology in the catalyst field (Dora et al. 2012). In 2014, Bai et al. (2014) prepared a novel sulfonated biomass-based catalyst by efficient hydrolyzation of cellulose in ionic liquid. Xu et al. (2011) studied the catalytic performances of glucose char sulfonic acids. However, these two kinds of carbon-based solid acid catalysts showed better catalytic activity and stability only in the esterification reaction. But the separation and recycling of the catalyst were not easy.
Based on the previous analysis of the lignocellulose hydrolysis mechanism, this paper puts forward a way to employ biomass from corn straw after its transformation to a carbonized form. This will be used as the substrate for adsorption of magnetic Fe3O4 particles to the surface and internal channels of the carbonized materials, to prepare magnetic biomass-based solid acid catalyst. In this work the catalyst was used to hydrolyze cellulose into high yields of reducing sugar to estimate the performance of the catalyst. The overall reaction for the magnetic biomass-based solid acid catalyst in the conversion of cellulose to reducing sugars is shown in Fig. 2.
Fig. 1. Lignocellulose structure diagram
Fig. 2. Mechanism of hydrolysis of cellulose with magnetic biomass-based solid acid catalyst
The interaction between magnetic catalyst and cellulose particles (Berry and Curtis 2003) is shown in Fig. 3. Magnetic Fe3O4 particles were well combined with the biomass-based precursor. The magnetic biomass-based solid acid catalyst has a carbon structure layer of vermicular disorder and high stability. Compared with conventional solid acid catalyst, the magnetic biomass-based solid acid catalyst has higher stability, and its molecular structure and properties were similar with cellulose molecules. Compared with conventional carbon solid acid catalyst, although the performance of this catalyst is similar with cellulose hydrolysis residue, the magnetic particle is very easy to be separated from cellulose hydrolysis residue. Therefore, the catalyst can complete the hydrolysis reaction of cellulose effectively, with high selectivity under mild reaction conditions.
Fig. 3. Interaction of between magnetic catalyst and cellulose particles
EXPERIMENTAL
Preparation of Solid Acid Catalyst
Corn stalk obtained from Jilin City suburbs was subjected to crushing and drying. It was then carbonized (Fig. 4) to black powder in a tube furnace under the protection of N2. The carbide products were sulfonated using concentrated H2SO4 as solvent (solid and liquid of ratio for 1:10) in a constant temperature oil bath. After cooling, deionized water was added (1:25, v/v), allowed to stand for solid-liquid separation, and the black solid was filtered. The solid was washed repeatedly with boiling water until the solution is neutral and then dried at 80 °C to obtain the biomass-based precursor.
Fig. 4. Scheme of carbonization reaction device
Preparation of Magnetic Biomass-based Solid Acid Catalyst by Sol-Gel Method
3.50 g of FeSO4·7H2O and 5.5 g of Fe2(SO4)3·9H2O were dissolved in 250 mL deionized water with mechanical stirring and heated up to 55 °C. Then, 2 M of NaOH solution was added to adjust pH to 11 with continued heating at 60 °C for another 6 h. Using a permanent magnet for magnetic settling, the supernatant fluid was separated, and the sediment was washed using deionized water until the aqueous solution was neutral. Vacuum drying the solid at 60 °C for 24 h, afforded the magnetic Fe3O4 particles.
Magnetic Fe3O4 particles and biomass-based solid acid precursor were mixed according to the certain proportion, soaked in 1 M H2SO4 solution for 24 h, and filtered. Drying the solid in muffle furnace to a high temperature calcination for 3 h gave the magnetic biomass-based solid acid catalyst (Fig. 5).
Fig. 5. Flow chart of magnetic biomass-based solid acid catalyst preparation
Response Surface Design of Experiment
Carbonization temperature, carbonization time, sulfonating temperature, and sulfonating time were the independent variables (Liu et al. 2010), and reducing sugar yield from catalytic hydrolysis of cellulose was the response value. These factors were were used for designing the response surface analysis. Factor and level code values are shown in Table 1.
The Design-Expert 8.0.6 software was used to simulate multivariate data. This software uses multiple quadratic regression equations to estimate a function tool, usually used in a variety of process parameters of optimization process; the analysis of regression equation, optimization of process parameters, and predicting the statistical method of the corresponding value.
Table 1. Coded Factors and Levels of Response Surface Analysis
Evaluation of Catalyst Performance
The magnetic catalyst (1.0 g) and 0.5 g of cellulose were mixed in a high pressure reaction kettle, with 50 mL distilled water and allowed to react for 6 h at 150 °C. The total reducing sugar yield was determined by the phenol-sulfuric acid method, which has been used for measuring neutral sugars in oligosaccharides, proteoglycans, glycoproteins, and glycolipids (Masuko et al. 2005). This method is used widely because of its sensitivity and simplicity. In its original form, it required only 10 to 80 nmol of sugars, as reported previously (Lee et al. 1969). But subsequently this method has been modified and optimized by many researchers, and it allows a longer linear range (1 to 150 nmol) to gain greater sensitivity.
Fourier Transform Infrared Spectroscopy
The presence of functional groups on the surface of magnetic biomass-based solid acid catalyst was evaluated by infrared spectroscopy (by means of a model EQUINOX55, Bruker, Germany), using the KBr tablet method. Results were recorded as an average of 16 scans in the wavelength range of 4000 to 400 cm-1.
RESULTS AND DISCUSSION
Analysis of Response Surface Method
Figure 6(a) shows that carbonization time had a greater effect than carbonization temperature on the catalyst. Parts (b) and (c) of the figure show that the interaction items had a great effect on the catalytic activity. Part (d) shows that at constant carbonization time, with increasing carbonization temperature, the reducing sugar yield increased gradually until it became stable, whereas (e) shows that the effect of carbonization temperature was greater than sulfonating time. Part (f) confirms that the interaction of sulfonating time and sulfonating temperature had little influence on the catalytic activity. The results show that the best process conditions were as follows: carbonization temperature 549 °C, carbonization time 13 h, sulfonating temperature 121 °C, and sulfonating time 6 h.
Using the Design-Expert 8.0.6 software to simulate multivariate data, the following quadratic multivariate regression equation was obtained:
R1=1.85+0.26A-0.83B-0.37C+0.41D+0.65AB-1.33AC-1.73AD-0.33BC-0.79BD+0.10CD+1.93A2+1.17B2+0.65C2+1.00D2 (1)
The P value of the model was 0.0096 < 0.01, so the response surface regression model reaches a significant level; the lack of fit P = 0.0001 < 0.01 reached an extremely significant level. The coefficient of determination was high (R2 = 0.9981). The results show that the model was very suitable. The order of four factors on effect of catalyst activity was as follows: B > A > D > C.
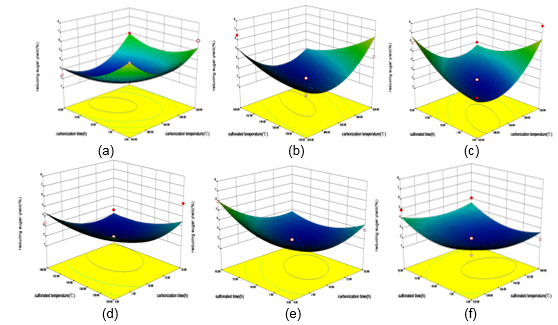
Fig. 6. Effect of different factors on the reducing sugar yield
Influence of Calcination Temperature on the Catalytic Activity
A series of catalysts was calcined for 3 h at 300, 400, 500, 600, and 700 °C. The results are shown in Fig. 7. When the calcination temperature was 500 °C, the reducing sugar yield was highest. The most appropriate calcination temperature was judged to be 500 °C.
Fig. 7. Effect of calcination temperature
Fig. 8. Effect of magnetic Fe3O4 content
Effect of Amount of Magnetic Fe3O4 on Catalyst Activity
The effect of the amount of magnetic Fe3O4 on catalyst activity is shown in Fig. 8. The most favorable calcination temperature was 500 °C. When Fe3O4 content gradually increased, the catalyst activity increased gradually until the doping ratio of Fe3O4 was 2%, at which point the catalyst activity reached the highest and the reducing sugar yield was 55%. Therefore, the optimal proportion of Fe3O4 is 2%.
Effect of Reaction Time on the Cellulose Conversion
The influence of different reaction time on magnetic biomass-based solid acid catalyst catalytic hydrolysis of cellulose is shown in Fig. 9. The magnetic catalyst (1.0 g) and 0.5 g of cellulose were mixed in a high pressure reaction kettle, with 50 mL distilled water, at 150 °C to react for 1, 2, 3, 4, 5, 6, 7, 8, 9 h. When reaction time gradually increased, the reducing sugar yield also gradually increased until reaction time was 6 h, at which point the cellulose conversion is the largest; the cellulose had basically been transformed and the amount of magnetic biomass-based solid acid catalyst was reduced. The reducing sugar yield reached 55%.
Fig. 9. Effect of reaction time on the cellulose conversion
Result of Infrared Spectrum Analysis
Figure 10 shows a very strong and obvious absorption peak at 598, 1029, 1205, 1029 to 1205, 1600 to 1900, 1900, 3500 to 2500 cm-1, and 3500 cm-1.
Fig. 10. FT-IR spectra of magnetic biomass-based solid acid catalyst
According to the literature (Yamaguchi et al. 2009), the absorption peak at 598 cm-1 belongs to the bending vibration of the -OH group in -SO3H, the peak at 1025 cm-1 belongs to the O=S=O bond in -SO3H (symmetric and anti-symmetric stretching vibration), the peak at 1500 to 1675 cm-1 belongs to the stretching vibration of C-C, the peak at 1600 to 1900 cm-1 belongs to the stretching vibration of C-O, and the peak at 2375 to 2500 cm-1 belongs to the stretching vibration of S-H. Although the infrared spectrum analysis showed that the catalyst had a weak -SO3H group, the surface was repeatedly washed above 80 °C with deionized water, and magnetic Fe3O4 particles were loaded successfully on the biomass-based precursor at 500 °C. As a result, these experimental results confirmed that -SO3H groups and magnetic Fe3O4 particles had been successfully grafted onto the surface and internal channels of the carbon-based solid acid catalyst.
Validation Test
Based on the response surface optimization design, using corn straw to prepare magnetic biomass-based solid acid catalyst for catalytic conversion of cellulose into high yields of reducing sugar, it is concluded that the best conditions for magnetic biomass-based solid acid catalyst preparation are as follows: carbonization temperature 549 °C, carbonization time 13 h, sulfonation temperature of 121 °C, and sulfonating time 6 h. When using the optimum technology condition three times as a verification test, the optimal proportion of Fe3O4 was 2% and hydrolysis reaction time was 6 h; the reducing sugar yield was 54.95%, 54.75%, and 55.17%, with an average value of 54.95%. The determined values were close to the predicted values, the repeatability was very good, the cellulose conversion rate was at a maximum, and the amount of magnetic biomass-based solid acid catalyst was reduced. Compared with the traditional solid acid catalysts, the total reducing sugar yield was increased by 65%.
CONCLUSIONS
- Using biomass corn stalk as a raw material, an optimal technology of biomass-based precursor preparation by RSM was achieved, using a carbonization temperature of 549 °C, carbonization time of 13 h, sulfonation temperature of 121 °C, and sulfonating time of 6 h.
- The variance analysis of the response surfaces showed that every factor influenced catalyst performance. The sequence of the factors was carbonization temperature > carbonization time > sulfonating temperature > sulfonating time.
- The optimized condition for magnetic biomass-based solid acid catalyst preparation was as follows: amount of magnetic Fe3O42%, calcination temperature 500 °C, hydrolysis reaction time 6 h; the reducing sugar yield from catalytic hydrolysis of cellulose was 55%. Compared with the traditional solid acid catalysts, the total reducing sugar yield was increased by 65%. This shows that using RSM to optimize the conditions of biomass-based precursor preparation is feasible, and magnetic Fe3O4 particles were well combined with the biomass-based precursor. The verification experiment showed that, at the end of the cellulose hydrolysis experiments, the cellulose conversion rate was at a maximum and the amount of magnetic biomass-based solid acid catalyst was reduced.
- The infrared spectral analysis showed that magnetic Fe3O4particles were loaded successfully on the surface and internal channels of biomass-based precursor, and the magnetic biomass-based solid acid catalyst had a carbon structure layer of vermicular disorder and possessed high stability. This study provides an efficient method for the development process of biomass-based solid acid catalyst.
ACKNOWLEDGEMENTS
The authors are grateful for the support of the China 12th Five-Year-Plan Project: Using oak starch and straw biomass to refine biodiesel and its comprehensive utilization industrialization demonstration: 2014BAD02B02; National Natural Fund Project: Mechanism study of cellulose hydrolysis by magnetic mimetic enzyme solid acid catalyst in water phase: 21376241; the Introducing Foreign Advanced Forestry Technology, “948” Project: import of corn straw biomass directional liquefaction technology: 2014-4-28; The Scientific Research Fund of Jilin Province: Study on the key techniques of using agriculture and forestry biomass waste to prepare solid forming of fuel: 20130206054NY; National spark plan project: the key technology of ultra low formaldehyde release of man-made board manufacturing application demonstration: 2014GA660006; and the Special funds support of Jilin province of straw comprehensive utilization technology innovation platform.
REFERENCES CITED
Bai, Y. Y., Xiao, L. P., and Sun, R. C. (2014). “Efficient hydrolyzation of cellulose in ionic liquid by novel sulfonated biomass-based catalysts,” Cellulose 21(4), 2327-2336. DOI: 10.1007/S10570-014-0287-2
Basha, S. A., Gopal, K. R., and Jebaraj, S. (2009). “A review on biodiesel production, combustion, emissions and performance,” Renewable and Sustainable Energy Reviews 13(6), 1628-1634. DOI: 10.1016/j.rser.2008.09.031
Berry, C. C., and Curtis, A. S. G. (2003). “Functionalisation of magnetic nanoparticles for applications in biomedicine,” Journal of Physics D Applied Physics 36(13), R198-R206(9). DOI: 10.1088/0022-3727/36/13/203
Dora, S., Bhaskar, T., Singh, R., Naik, D. V., and Adhikari, D. K. (2012). “Effective catalytic conversion of cellulose into high yields of methyl glucosides over sulfonated carbon based catalyst,” Bioresource Technology 120, 318-321. DOI: 10.1016/j.biortech.2012.06.036
Lee, Y. C., Scocca, J. R., and Muir, L. (1969). “Rapid automatic analysis of sugar components in glycoproteins: I. Hexosamines,” Analytical Biochemistry 27(3), 559-566. DOI: 10.1016/0003-2697(69)90070-0
Liu, X. Y., Huang, M., Ma, H. L., Zhang, Z. Q., Gao, J. M., Zhu, Y. L., Han X. J., and Guo, X. Y. (2010). “Preparation of a carbon-based solid acid catalyst by sulfonating activated carbon in a chemical reduction process,” Molecules 15(10), 7188-7196. DOI: 10.3390/molecules15107188
Masuko, T., Minami, A., Iwasaki, N., Majima, T., Nishimure, S. I., and Lee, Y. C. (2005). “Carbohydrate analysis by a phenol-sulfuric acid method in microplate format,” Analytical Biochemistry 339(1), 69-72. DOI: 10.1016/j.ab.2004.12.001
Shimizu, K., and Satsuma, A. (2011). “Toward a rational control of solid acid catalysis for green synthesis and biomass conversion,” Energy and Environmental Science 4(9), 3140-3153. DOI: 10.1039/C1EE01458G
Tomishige, K., Yasuda, H., Yoshida, Y., Nurunnabi, M., Li, B., and Kunimori, K. (2004). “Catalytic performance and properties of ceria based catalysts for cyclic carbonate synthesis from glycol and carbon dioxide,” Green Chemistry 6(4), 206-214. DOI: 10.1039/B401215A
Xu, Q., Yin, D. L., and Xia, Y. (2011). “Preparation and catalytic performances of glucose char sulfonic acids,” Advanced Materials Research 3(236), 112-115. DOI: 10.4028/www.scientific.net/AMR.236-238.112
Yamaguchi, D., Kitano, M., Suganuma, S., Nakajima, K., Kato, H., and Hara, M. (2009). “Hydrolysis of cellulose by a solid acid catalyst under optimal reaction conditions,” Journal of Physical Chemistry C113(8), 3181-3188. DOI: 10.1021/jp808676d
Article submitted: April 7, 2015; Peer review completed: July 24, 2015; Revised version received: August 11, 2015; Accepted: August 12, 2015; Published: August 24, 2015.
DOI: 10.15376/biores.10.4.6720-6729
