Abstract
Bayberry tannin-immobilized collagen/cellulose bio-adsorbent (BT-collagen/cellulose) was prepared via the reaction of bayberry tannin with the amino group of collagen in collagen/cellulose blends. Compared with collagen/cellulose beads without tannin, BT-collagen/cellulose had a more compact structure and higher thermal stability. Furthermore, the crystalline structure of cellulose in BT-collagen/cellulose was preserved. The adsorption properties of BT-collagen/cellulose to Pb(II) in aqueous solution were investigated and compared with those of collagen/cellulose. The adsorption of Pb(II) on both of the two bio-adsorbents reached the maximum at pH near 5.5. Based on the mass content of collagen, the equilibrium adsorption capacity of BT-collagen/cellulose at 25 °C was 1.352 mmol/g, according to Langmuir isotherms, which was higher than that of collagen/cellulose (0.345 mmol/g). In kinetics studies, both of the two bio-adsorbents reached equilibrium within ~240 min, and the experimental data could be well fitted by the pseudo-second-order rate model. Nevertheless, BT-collagen/cellulose had a better reusability after three cycles of adsorption-desorption.
Download PDF
Full Article
Preparation of Tannin-immobilized Collagen/Cellulose Bead for Pb(II) Adsorption in Aqueous Solutions
Min Zhang,a Cuicui Ding,b Lihui Chen,a and Liulian Huang a,*
Bayberry tannin-immobilized collagen/cellulose bio-adsorbent (BT-collagen/cellulose) was prepared via the reaction of bayberry tannin with the amino group of collagen in collagen/cellulose blends. Compared with collagen/cellulose beads without tannin, BT-collagen/cellulose had a more compact structure and higher thermal stability. Furthermore, the crystalline structure of cellulose in BT-collagen/cellulose was preserved. The adsorption properties of BT-collagen/cellulose to Pb(II) in aqueous solution were investigated and compared with those of collagen/cellulose. The adsorption of Pb(II) on both of the two bio-adsorbents reached the maximum at pH near 5.5. Based on the mass content of collagen, the equilibrium adsorption capacity of BT-collagen/cellulose at 25 °C was 1.352 mmol/g, according to Langmuir isotherms, which was higher than that of collagen/cellulose (0.345 mmol/g). In kinetics studies, both of the two bio-adsorbents reached equilibrium within ~240 min, and the experimental data could be well fitted by the pseudo-second-order rate model. Nevertheless, BT-collagen/cellulose had a better reusability after three cycles of adsorption-desorption.
Keywords: Cellulose; Collagen; Ionic liquid; Lead adsorption; Tannin
Contact information: a: College of Materials Engineering, Fujian Agriculture and Forestry University, Fuzhou 350002, PR China; b: The Key Laboratory of Leather Chemistry and Engineering of Ministry of Education, Sichuan University, Chengdu 610065, PR China; *Corresponding author: fafuhll@163.com
INTRODUCTION
Lead (Pb)(II) is a common heavy metal contaminant. In industrial wastewater, Pb(II) concentrations approach as high as 200 to 500 mg/L (Vilar et al. 2005). The presence of Pb(II) in water is a potential threat to human health as it can cause mental deficiency, brain damage, anemia, and behavioral problems (Gyananath and Balhal 2012). The removal of heavy metals, including Pb(II), from wastewater has progressed significantly.
Among the various conventional methods for the removal of heavy metals from wastewater such as ion exchange, flotation, solvent extraction, precipitation, coagulation, membrane filtration, reverse osmosis, and adsorption, adsorption is promising due to its high selectivity, easy handling, lower operating costs, and high efficiency in removing very low levels of heavy metals from aqueous solutions (Fu et al. 2011; Mouni et al. 2011; Xu et al. 2011). Of the various biological adsorbents, tannin was believed to be a potential alternative for the removal of heavy metals from aqueous solution (Liao et al. 2004; Özacar et al. 2008) .
Tannins are widely distributed in the roots, barks, stalks, and fruits of plants. As natural polyphenols, tannins have multiple adjacent phenolic hydroxyls, which exhibit strong affinity to metal ions (Nakajima 2002). However, tannins are water-soluble; thus, they possess the disadvantage of being dissolved by water when used directly as the adsorbent for the recovery of metal from aqueous systems. To overcome this drawback, collagen, the most abundant animal biomass, was employed as the matrix onto which tannins were immobilized via the chemical cross-linking of the aldehyde agent (Liao et al. 2004). Nevertheless, one of the primary limitations in the practical use of collagen is the relatively poor physical properties, wherein they can be easily damaged or disintegrated upon handling (Catalina et al. 2012). One of the primary efforts devoted to improving the properties of collagen is the preparation of composites with other biopolymers.
Cellulose is the most abundant natural polymer and has been widely used in many fields due to its biocompatibility, biodegradability, and desirable mechanical properties (Müller et al.2006). Therefore, binary blends of natural polymers, including both cellulose and collagen, are promising systems for creating new polymer materials with improved physical properties. In a previous study (Zhang et al. 2014), collagen/cellulose composite films were prepared by co-dissolving in an environment-friendly solvent, 1-ethyl-3-methylimidazolium acetate ([Emim]Ac), which is an ionic liquid. Strong intermolecular hydrogen bonds between collagen and cellulose were confirmed in the composites, which presented the possibility of preparing a composite of collagen and cellulose to be a more stable matrix for the immobilization of tannins compared with matrices derived from pure collagen. Furthermore, collagen regenerated from ionic liquid is different with collagen fiber, since more groups would be exposed as for collagen molecules than those for collagen fiber, and thus more tannin can be immobilized, which is expected to increase the adsorption capacity of tannin adsorbent.
To the best of our knowledge, the preparation and characterization of tannin-immobilized collagen/cellulose bio-adsorbent has not yet been reported. Thus, BT-collagen/cellulose beads were prepared and then characterized by several methods, such as field-emission scanning electron microscopy (FE-SEM), X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), and thermogravimetric analysis (TGA). After that, the pH effect, adsorption isotherms, adsorption kinetics, and reusability of BT-collagen/cellulose beads to adsorb Pb(II) in aqueous solution were investigated and compared with those of collagen/cellulose beads.
EXPERIMENTAL
Materials
Bayberry tannin (BT), provided by Sichuan University (China), was obtained from the bark of Myrica esculenta via acetone-water (1:1 v/v) extraction. The tannin content of the extract was approximately 78.0%. Microcrystalline cellulose was purchased from Aladdin Co., Ltd. (China) and [Emim]Ac was provided by Lanzhou Institute of Chemical Physics (China).
Methods
Preparation of collagen
Collagen (300 KDa) was prepared from calf skin by a previously described method (Zhang et al. 2006). The delimed and neutralized bovine split skin was cut into smaller pieces and extracted with 0.5 mol/L acetic acid containing 3% pepsin (1:3000) at 4 °C for 3 d. The supernatant was collected by centrifugation (9000 × g) at 4 °C and salted out by the addition of NaCl (final concentration 0.7 mol/L).
The precipitate was dissolved in 0.5 mol/L acetic acid, dialyzed against 0.1 mol/L acetic acid for 3 d, lyophilized in a freeze dryer (Labconco Freeze Dryer FreeZone 6 Liter, USA) at -50 °C for 2 d, and stored at 4 °C.
Preparation of adsorbents
Microcrystalline cellulose (3.0 g) was added to [Emim]Ac (50.0 g, ~45.4 mL) in a dried beaker and dissolved with agitation at 60 °C for 4 h. After the clear solution was cooled to room temperature, the prepared calf collagen (0.5 g) was added. The following dissolving process was controlled at 25 °C for 6 h. In our previous paper (Zhang et al. 2014), it was found that collagen tended to form continuous phase and thus phase-separation between the two polymers occurred as collagen/cellulose ratio = 1/5, therefore, in the present work collagen/cellulose ratio was limited to be 1/6.
The obtained solution was added dropwise with a 1.0 mm diameter syringe needle to a 10% (v/v) ethanol solution to produce the hydrogel beads; the beads were then soaked in deionized water and renewed every 0.5 h, for a total of 4 times. Bayberry tannin (3.0 g) was dissolved in deionized water (80.0 mL), then mixed with the prepared hydrogel beads and reacted for 10 h at 25 °C. The intermediate product, collected by filtration, was added to 200.0 mL of a 2 wt% glutaraldehyde solution. The mixture was initially reacted for 2 h at 25 °C, and then reacted for 2 h at 40 °C. The product was collected by filtration, fully washed with deionized water, and vacuum-dried at 25 °C. The obtained bayberry tannin-immobilized collagen/cellulose bio-adsorbent (BT-collagen/cellulose) was stored at room temperature.
Field-emission scanning electron microscopy (FE-SEM)
The BT-collagen/cellulose and collagen/cellulose adsorbents were mounted on brass stubs using double sided cellotape and sputter coated with a platinum/palladium alloy. Ultrastructural observations were performed with a field-emission scanning electron microscope (FEI Navo NanoSEM 230; USA) operating at an accelerating voltage of 15 kV.
X-ray photoelectron spectroscopy (XPS)
The surface properties of both adsorbents were analyzed by X-ray photoelectron spectroscopy using monochromatized Al Kα X-ray (hν = 1486.6 eV) radiation (ESCALAB 250, Thermo Fisher Scientific Co.; USA). The shift of the binding energy was corrected using the C1s level at 284.6 eV as an internal standard.
X-Ray diffraction (XRD)
The X-ray diffraction patterns of both adsorbents were recorded using Cu Kα radiation (λ=0.154056 nm) on a diffractometer (Panalytical X’pert Pro MPD; the Netherlands) at a scanning rate of 1°/min in the 2θ range from 5 to 55°.
Thermogravimetric analysis (TGA)
Thermogravimetric analysis of the adsorbents was performed with a thermal analyzer (Netzsch TG 209; Germany). Samples (2.0 to 2.5 mg) were heated from 40 to 600 °C (ramp of 10 °C/min) in a high-purity nitrogen atmosphere flowing at 80 cm3/min.
Effect of initial pH on adsorption capacity
Stock Pb(II) solution (1000 mg/L, which was converted to 4.826 mmol/L) was prepared by dissolving analytical grade Pb(NO3)2 in deionized water, and diluting to the appropriate concentrations. The adsorption experiments were performed by suspending 0.20 g of beads (BT-collagen/cellulose or collagen/cellulose) in 60.0 mL of a 0.965 mmol/L Pb(II) solution, and constantly shaking at 25 °C for 24 h. Six Pb(II) solutions with various initial pH values of 2.5, 3.5, 4.5, 5.0, 5.5, and 6.5, respectively, were prepared. The concentration of Pb(II) in solution was analyzed by inductively coupled plasma atomic emission spectrometer (ICP-AES; Perkin-Elmer Optima 2100DV; USA). The adsorption capacity (qe, mmol/g) of both the adsorbents to Pb(II) was computed in Eq. (1),
(1)
where C0 and Ce are the initial and equilibrium solution concentrations (mmol/L), respectively, V is the volume of the solution (L), and W is the weight of collagen in the beads (g). To facilitate comparison, the content of BT in the BT-collagen/cellulose beads was not taken into account. The experiments were performed three times, and the deviations were found to be within 5%.
Adsorption isotherms
Beads (0.20 g) were suspended in 60.0 mL Pb(II) solutions with initial concentrations ranging from 0.1207 to 1.207 mmol/L. The pH of the Pb(II) solutions was adjusted to 5.5. Two adsorption experiments were conducted by constant shaking at different temperatures, one was conducted at 25 °C and the other one was conducted at 45 °C. Experiments were performed three times, and the deviations were found to be within 5%.
Adsorption kinetics
Beads (0.20 g) was suspended in 60.0 mL of a 0.965 mmol/L Pb(II) solution. The pH was adjusted to 5.5, and the adsorption process was conducted at 25 °C with constant stirring. The concentration of Pb(II) was analyzed with ICP-AES during the adsorption process. Adsorption capacities at time t (min) were obtained by mass balance calculations.
Reusability of BT-collagen/cellulose
Batch desorption experiments with the Pb(II)-adsorbed beads were performed using 30.0 mL of a 0.1 mol/L nitric acid solution, with constant stirring at 25 °C for 24 h. The mixture was filtered, the concentration of Pb(II) in the filtrate was determined by ICP-AES, and the desorption extent was calculated.
The desorbed beads were fully rinsed, and then reused for adsorption testing to evaluate reusability. Experiments on the adsorption kinetics of the desorbed beads were conducted using the same procedures as described above. The adsorption-desorption process was performed in triplicate.
RESULTS AND DISCUSSION
Adsorption of Pb(II) onto Collagen/Cellulose Beads or BT-Collagen/Cellulose Beads
The mechanism of the dissolution, regeneration, and blending of collagen and cellulose in [Emim]Ac has been proposed in previous work (Zhang et al. 2014). That is, the respective hydrogen bonds in collagen and cellulose molecules were broken by [Emim]Ac during dissolution. With [Emim]Ac washed away with water, the hydrogen bonding could be rebuilt among the collagen chains or cellulose chains, or between the collagen and cellulose chains, and finally the composite collagen/cellulose bead was formed.
After this step, bayberry tannin (BT) was added to react with collagen in the composite beads via the cross-linking reaction of glutaraldehyde, and then bayberry tannin immobilized collagen/cellulose (BT-collagen/cellulose) beads were obtained. It was known that there was interaction between tannins and collagen matrices due to the formation of hydrogen bonds and hydrophobic bonds, which means that tannin may be leached out in water during the adsorption process (Sun et al. 2010). Therefore, to prevent the leaching of tannins from BT-collagen/cellulose, stable covalent bonds of the C6 and C8 of A-rings of BT, which had high nucleophilic reaction activity (Zhan and Zhao 2003), were formed with the amino groups of collagen molecules through the cross-linking of glutaraldehyde.
Scheme 1. The possible absorption mechanisms of collagen/cellulose beads and BT-collagen/cellulose beads to Pb(II)
The possible absorption mechanisms of collagen/cellulose beads and BT-collagen/cellulose beads to Pb(II) are presented in Scheme 1. As for collagen/cellulose beads (Scheme 1a), it was assumed that the active sites for Pb(II) adsorption are the nitrogen atoms of the amino groups in the collagen as well as the oxygen atoms of part of the hydroxyl groups in both collagen and cellulose (Wang et al. 2013). The adsorption for Pb(II) would be more complex for BT-collagen/cellulose beads (Scheme 1b) because the active sites could be provided not only from collagen and cellulose, but also from BT. Bayberry tannin is a condensed tannin and mostly consists of polymerized flavan-3-ols. The B-ring of BT is a pyrogallol structure, which has high affinity to metal ions due to its high electrophilic reaction activities (Özacar et al. 2006) and the C-ring is partly attached with galloyl groups, suggesting that the reaction ability of BT with metal ions such as Pb(II) could be improved. Therefore, it could be expected that the adsorption ability of BT-collagen/cellulose beads to Pb(II) would be stronger than that of collagen/cellulose beads.
Characterization of Collagen/Cellulose Beads and BT-Collagen/Cellulose Beads
Surface morphologies of collagen/cellulose and BT-collagen/cellulose observed by FE-SEM are shown in Fig. 1. The surface of collagen/cellulose displayed some fibrous regenerated collagen that was uniformly exposed on the surface of the adsorbent (Fig. 1a). As for BT-collagen/cellulose (Fig. 1b), however, it seemed that the fibrous regenerated collagen was overlaid by BT, which reduced the density of collagen fibers in the surface.
Fig. 1. FE-SEM images and XPS diagrams (inserts) of the surface of (a) collagen/cellulose and (b) BT-collagen/cellulose beads
The XPS analysis indicated that the C 1s spectra of BT-collagen/cellulose beads were almost the same to those of collagen/cellulose beads (not shown); however, the binding energy at peaks in the N 1s spectra of BT-collagen/cellulose beads showed some changes. The insert in Fig. 1 is the XPS N 1s spectra of both the adsorbents, which contains two states of nitrogen. For collagen/cellulose beads, peak 2 at 401.9 eV is related to N–H bond. As for BT-collagen/cellulose beads, the binding energy of peak 2 decreased to 401.5 eV. Moreover, the relative fraction of peak 2 was also increased. These observations suggested that the immobilization of BT on collagen fibers may take place at the N sites in collagen molecule, which demonstrated that a stable covalent linkage was formed between BT and the amino groups of collagen molecules through the cross-linking of glutaraldehyde.
The crystalline order of cellulose in the collagen/cellulose beads and BT-collagen/cellulose beads was examined with XRD (Fig. 2a). The diagrams of collagen/cellulose beads mainly showed features of cellulose II, which displays typical diffraction peaks at 2θ = 12.7°, 21.3°, and 35.2°, assigned to peaks at 101, 002, and 040, respectively. However, the diagrams of BT-collagen/cellulose beads exhibit 2θ = 12.2°, 20.3°, 21.7°, and 34.7°, explaining the split of the 002 peak. It was reported that the dissolved collagen may be induced by tannin to assemble into collagen fibers (Sun et al. 2010). Therefore, the changes in the 002 peak might be attributed to the increase in the amount of collagen fibers in BT-collagen/cellulose, because collagen fiber also had an XRD peak located at ~21°, which represents the diffuse scattering. Crystallization index is an important parameter to describe the crystalline structure of materials. It is calculated as the height ratio between the intensity of the crystalline peak (I002 – IAM) (Park et al. 2010). According to this method, the crystallization indices were calculated as ~61.4% and ~59.1% for collagen/cellulose and BT-collagen/cellulose, respectively. These results revealed that the crystalline structure of cellulose was almost unaffected after the immobilization of BT in the collagen/cellulose blend.
Fig. 2. (a) XRD diagrams and (b) thermal decomposition profiles of collagen/cellulose beads and BT-collagen/cellulose beads
The TG thermographs and the DTG curves for collagen/cellulose and BT-collagen/cellulose are shown in Fig. 2b. As can be seen from the DTG curves, the thermal destruction of the samples consisted of two stages. The first peak was related to the evaporation of unbound water and generally was not analyzed; the second thermal event, from which a starting decomposition temperature (T0) and a maximum decomposition temperature (Tm) could be obtained, included the parallel processes of dehydration and demethoxylation. The T0 and Tmvalues of collagen/cellulose beads were 317.7 and 344.2 °C, respectively, while BT-collagen/cellulose beads had T0 and Tm values of 323.1 and 348.1 °C, respectively. Thus it seemed that the thermal stability of BT-collagen/cellulose was improved, probably due to the formation of stable covalent cross-linking between BT and collagen molecules. The higher adsorbent stability was favorable for its practical application at high temperatures.
Effect of Initial pH on the Adsorption of Collagen/Cellulose Beads and
BT-Collagen/Cellulose Beads to Pb(II)
The pH value of a solution is the most important variable affecting metal ions adsorption, possibly because H+ ions themselves are competing with metal ions (Sarı et al. 2007). Figure 3 shows the influence of initial pH on the adsorption capacity of collagen/cellulose beads and BT-collagen/cellulose beads to Pb(II). The adsorption capacity of Pb(II) on both of the two adsorbents was dependent on pH. That is, the adsorption capacity of Pb(II) gradually increased with the increase of solution pH from 2.5 to 5.5, and then reduced as pH was further increased to 6.5. Therefore, it seemed that the maximum adsorption capacity of Pb(II) on both the two adsorbents could be obtained at pH near 5.5.
Lodeiro et al. (2006) examined the speciation of Pb2+ ions by using MINEQL+, which showed that free Pb2+ ions were the predominant species at the pH values below 6. Wang et al.(2013) also suggested that free Pb2+ ions were the predominant species as the pH values below 6, while as pH was further increased, the fraction of Pb(OH)+ would be increased. Therefore, as the pH values below 5.5, Pb2+ ions were mainly the positively charged adsorbate species. The low adsorption at low pH values was due to the increase in positive charge density on the surface sites, and thus, electrostatic repulsion occurred between the metal ions and the groups with positive charge (–OH2+ and –NH3+ of collagen, –OH2+ of cellulose, and Ph–OH2+ of tannin) on the surface of absorbent. As pH value increased, the surface of the absorbent became negatively charged, thus the increasing electrostatic attraction between positively charged adsorbate species (Pb2+) and negative surface sites would lead to increased adsorption of Pb(II) on beads (Özacar et al. 2008). In addition, as for bayberry tannin, the chelating reaction between negative phenolic hydroxyls and positive lead would also be promoted, which increased the adsorption capacity of BT-collagen/cellulose bead.
However, at pH > 5.5, metal precipitation appeared and the adsorbent was deteriorated with the accumulation of metal ions. Additionally, for pH values higher than 6.0, species with lower charge might be formed, such as Pb(OH)+ (Lodeiro et al. 2006), which further depressed the adsorption capacity of Pb(II) on the adsorbents. Moreover, as for BT-collagen/cellulose beads, the phenolic hydroxyl groups of the tannin would more readily be oxidized as pH was increased (Liao et al. 2004), further reducing its adsorption capacity. Therefore, the optimum pH for both the adsorbents was selected as 5.5 for further studies.
Fig. 3. Effect of initial pH on the adsorption capacity of Pb(II) on collagen/cellulose beads and BT-collagen/cellulose beads (initial concentration of Pb(II) = 0.965 mM)
Moreover, from this figure, it could be noted that the adsorption capacity of Pb(II) on BT-collagen/cellulose beads was higher than that on collagen/cellulose beads, in the whole pH range from 2.5 to 6.5. The higher adsorption capacity of BT-collagen/cellulose beads was related to the immobilization of tannin.
Adsorption Isotherms
By plotting solid phase concentration against liquid phase concentration, one obtains an adsorption isotherm. Figure 4 shows the equilibrium adsorption of Pb(II) using collagen/cellulose beads (a1 and a2) and BT-collagen/cellulose beads (b1 and b2).
Adsorption isotherms are important for the description of how molecules or ions of an adsorbate interact with adsorbent surface sites. Moreover, they are critical in the optimization of the use of an adsorbent. Hence, the correlation of equilibrium data using either a theoretical or empirical equation is essential for the adsorption interpretation as well as the prediction of the adsorption extent. Here, two isotherms including Freundlich (Eq. 2) (Freundlich 1906) and Langmuir (Eq. 3) (Langmuir 1916) were used to analyze the equilibrium experimental data,
(2)
(3)
where Ce is the Pb(II) concentration (mmol/L) at equilibrium, qe is the adsorption capacity at equilibrium (mmol/g), KF (L/mol) is the Freundlich constant, and 1/n is the heterogeneity factor. For ‘1/n’ values in the range from 0.1 to 1 (1 < n < 10), adsorption is favorable (Mohapatra et al. 2009); K (L/mol) and qm (mmol/g) are the Langmuir coefficients, representing the adsorption equilibrium constant and the monolayer capacity, respectively.
Fig. 4. Adsorption isotherms of Pb(II) on collagen/cellulose beads (a1, a2) and BT-collagen/cellulose beads (b1, b2) (pH = 5.5)
The Freundlich isotherm assumes that the adsorption occurs on a heterogeneous surface by multilayer adsorption and the amount of adsorbate adsorbed increases infinitely with the increasing of concentration (Jain et al. 2009). The Langmuir model assumes monolayer adsorption on a homogenous surface where the binding sites have equal affinity and energy, and the interaction between the adsorbed species can be neglected (Repo et al. 2010). The constants and correlation coefficients of the Langmuir and Freundlich models are listed in Table 1.
Table 1. Isotherm Model Constants and Correlation Coefficients (R2) for the Adsorption of Collagen/Cellulose Beads and BT-Collagen/Cellulose Beads to Pb(II)
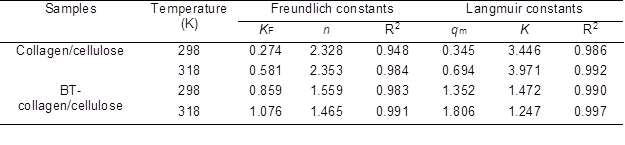
As for collagen/cellulose, the coefficients of determination (R2) of the Freundlich model were obviously lower than those of the Langmuir model. Thus, the adsorption isotherms of Pb(II) on collagen/cellulose beads were more aptly described by using the Langmuir equation. Similarly for BT-collagen/cellulose, the Langmuir model was more suitable to for describing the adsorption isotherms of Pb(II) (R2 > 0.99), although the adsorption isotherms could also be well described by the Freundlich model (R2 > 0.98). The Freundlich empirical coefficient ‘n’ is found to be greater than 1, indicating the high adsorption intensity (Sen et al. 2002).R2: coefficient of determination
The mechanisms accounting for adsorption of Pb(II) ions onto the two adsorbents might be very complex but, as a whole, it seemed that Pb(II) may be adsorbed mainly in the form of a monolayer on the surface of both the adsorbents. Furthermore, the adsorption capacity of collagen/cellulose was more sensitive to the change of temperature, compared with BT-collagen/cellulose. That is, as temperature increased from 298 to 318 K, the qm value of collagen/cellulose increased to ~2 times of the initial value (from 0.345 to 0.694 mM), while that of BT-collagen/cellulose increased by no more than 50% (from 1.352 to 1.806 mM). The higher sensitivity to temperature of collagen/cellulose beads indicated that the activation energy of Pb(II) adsorption was greater than that of BT-collagen/cellulose.
Table 2 compares the qm values of collagen/cellulose beads and BT-collagen/cellulose beads for Pb(II). The BT-collagen/cellulose beads exhibited a qm value nearly 3 times higher than that of collagen/cellulose beads. The results of this comparison confirmed that the adsorption capacity could be improved remarkably by the immobilization of tannin.
The adsorption capacity of various adsorbents for Pb (II) ions taken from some literatures are also presented in Table 2. It could be seen that both of the two beads exhibited a comparatively high adsorption capacity for Pb (II) ions.
Table 2. Comparison of Adsorption Capacity of Collagen/Cellulose Beads and BT-Collagen/Cellulose Beads for Pb(II)
a based on the mass of collagen in bead
Kinetic Studies
Kinetic studies were carried out at 25 °C and the experimental data are illustrated in Fig. 5. It can be observed from the adsorption kinetic curves of collagen/cellulose beads and BT-collagen/cellulose beads that the adsorption rate of Pb(II) was relatively fast during the first 100 min and, finally, the adsorption equilibrium was attained in ~240 min.
Fig. 5. Adsorption kinetics of Pb(II) on collagen/cellulose beads and BT-collagen/cellulose beads (initial concentration of Pb(II) = 0.965 mM; pH = 5.5)
The adsorption kinetic data were further analyzed using a pseudo-first-order rate model (Eq. 4) (Lagergren 1898), a pseudo-second-order rate model (Eq. 5) (Ho and McKay 1999), and an intraparticle diffusion model (Eq. 6) (Chiou and Li 2003),
(4)
(5)
(6)
where qe and qt are the adsorption capacity (mmol/g) of Pb(II) at equilibrium and at contact time t (min), respectively, and k1 (min−1) is the pseudo first-order rate constant, k2 (g/(mmol min)) is the pseudo second-order rate constant, and k3 (mmol/g min−0.5) is the intraparticle diffusion rate constant.
The “pseudo-first” and “pseudo-second” order models are macroscopic kinetic models employed for the description of the adsorption process, which suggests that the adsorption process could be regarded as the “first” or “second” order chemical reaction process, when the adsorption is a rate-controlled step and the intraparticle diffusion resistance could be neglected (Ho and McKay 1999). The intraparticle diffusion model is used to describe the adsorption process where the intraparticle diffusion resistance is a rate-controlled step (Vadivelan and Kumar 2005).
Table 3 lists parameters calculated from these three models. The intraparticle diffusion rate model provides low R2 values for the two adsorbents (R2 value for BT-collagen/cellulose beads was less than zero), which suggested that the Pb(II) adsorption process is not an intraparticle-diffusion-controlled process.
Table 3. Kinetics Model Constants and R2 Values for Adsorption of Collagen/Cellulose Beads and BT-Collagen/Cellulose Beads to Pb(II) at 25 °C
(1): first run; (2): second run; (3): third run; —: curves cannot be fitted
As for collagen/cellulose beads, R2 values for both the pseudo-first-order rate model and the pseudo-second-order rate model were higher than 0.98. The R2 value for the pseudo-first-order rate model of BT-collagen/cellulose was unsatisfactory, so it seemed that the pseudo-second-order rate model was more suitable to describe the adsorption kinetics of Pb(II) on BT-collagen/cellulose. As a whole, these results revealed that the Pb(II) adsorption process on both the absorbents was a rate-controlled process (Ncibi et al. 2008), based on the assumption that the rate limiting step may, especially for BT-collagen/cellulose, include chemical absorption involving valency forces through sharing or exchange of electrons between beads and Pb(II) (Özacar et al. 2008).
Reusability of Collagen/Cellulose Beads and BT-Collagen/Cellulose Beads
Desorption and reusability are important factors for evaluating potential application value of bio-adsorbents (Sun et al. 2011). Desorption experiments showed that more than 95% of Pb(II) removal can be achieved in 24 h. The competition of H+ ions in the adsorption sites promote the release of the adsorbed Pb(II) into the aqueous solution. Therefore, the desorption results confirmed that Pb(II) is mostly adsorbed on the surface by comparatively weak adsorptive interactions and partially by the surface complexes through oxygen bonding (Jaramillo et al. 2009).
Furthermore, the effect of three consecutive adsorption-desorption cycles on the Pb(II) adsorption onto both the adsorbents was studied and the adsorption kinetics curves are shown in Fig. 6.
Fig. 6. Different runs of adsorption kinetics of Pb(II) on collagen/cellulose beads and BT-collagen/cellulose beads (initial concentration of Pb(II) = 0.965 mM; pH = 5.5)
After the adsorption-desorption process, the adsorption kinetics curves could still be fitted by the pseudo-second-order rate model (R2 > 0.97), but the qe,exp. for both of the adsorbents was decreased to a certain extent. The loss in adsorption capacity after three cycles can be calculated from qe,cal. values which are listed in Table 3, as follows (Eq. 7):
(7)
Results showed that there was only a slight loss (3.3%) in adsorption capacity for BT-collagen/cellulose beads after three consecutive cycles of adsorption-desorption process, suggesting a good performance and recyclability. The Pb(II) adsorption capacity of the recycled collagen/cellulose decreased by as much as 20.0%, which was remarkably greater than that of the BT-collagen/cellulose beads. Moreover, the collagen/cellulose beads went slightly moldy after 15 days of soaking in the adsorption solution, while there was no obvious change for the BT-collagen/cellulose beads, confirming that the durability of the adsorbent could be improved by the introduction of BT.
CONCLUSIONS
- The collagen/cellulose beads were prepared via the reconstitution and blending of collagen and cellulose from 1-ethyl-3-methylimidazolium acetate; then bayberry tannin was immobilized to obtain a novel biosorbent that was named bayberry tannin-immobilized collagen/cellulose (BT-collagen/cellulose) beads.
- Compared with the collagen/cellulose beads, the BT-collagen/cellulose beads exhibited a considerably greater adsorption capacity. The BT-collagen/cellulose beads had especially excellent stability and durability as a bio-adsorbent for Pb(II).
- As a whole, BT-collagen/cellulose beads are a promising bio-adsorbent for the adsorption of Pb(II) from wastewater.
ACKNOWLEDGMENTS
This work was financially supported by the National Natural Science Foundation of China (No. 21306024) and the Foundation of Distinguished Young Scholars of Fujian Agriculture and Forestry University (XJQ201212).
REFERENCES CITED
Catalina, M., Cot, J., Balu, A. M., Serrano-Ruiz, J. C., and Luque, R. (2012). “Tailor-made biopolymers from leather waste valorisation,” Green Chem. 14(2), 308-312. DOI: 10.1039/C2GC16330F
Chiou, M., and Li, H. (2003). “Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads,” Chemosphere 50(8), 1095-1105. DOI: 10.1016/S0045-6535(02)00636-7
Chiron, N., Guilet, R., and Deydier, E. (2003). “Adsorption of Cu(II) and Pb(II) onto a grafted silica: Isotherms and kinetic models,” Water Res. 37 (13), 3079–3086. DOI:10.1016/S0043-1354(03)00156-8
Freundlich, H. M. F. (1906). “Über die Adsorption in Lösungen,” Z. Phys. Chem. 57A, 385-470.
Fu, F., and Wang, Q. (2011). “Removal of heavy metal ions from wastewaters: A review,” J. Environ. Manage. 92(3), 407-418. DOI: 10.1016/j.jenvman.2010.11.011
Gyananath, G., and Balhal, D. (2012). “Removal of lead (II) from aqueous solutions by adsorption onto chitosan beads,” Cellul. Chem. Technol. 46(1), 121-124.
Ho, Y. S., and McKay, G. (1999). “Pseudo-second order model for sorption processes,” Process Biochem. 34(5), 451-465. DOI: 10.1016/S0032-9592(98)00112-5
Imamoglu, M., and Tekir, O. (2008). “Removal of copper (II) and lead (II) ions from aqueous solutions by adsorption on activated carbon from a new precursor hazelnut husks,” Desalination. 228 (1-3), 108-113. DOI:10.1016/j.desal.2007.08.011
Jain, M., Garg, V. K., and Kadirvelu, K. (2009). “Chromium(VI) removal from aqueous system using Helianthus annuus (sunflower) stem waste,” J. Hazard. Mater. 162(1), 365-372. DOI: 10.1016/j.jhazmat.2008.05.048
Jaramillo, J., Gómez-Serrano, V., and Álvarez, P. (2009). “Enhanced adsorption of metal ions onto functionalized granular activated carbons prepared from cherry stones,” J. Hazard. Mater. 161(2), 670-676. DOI: 10.1016/j.jhazmat.2008.04.009
Langmuir, I. (1916). “The constitution and fundamental properties of solids and liquids,” J. Am. Chem. Soc. 38(11), 2221-2295. DOI: 10.1021/ja02268a002
Lagergren, S. (1898). “About the theory of so-called adsorption of soluble substances,” Kungliga Svenska Vetenskapsakademiens Handlingar 24(4), 1-39.
Li, Q., Zhai, J., Zhang, W., Wang, M., and Zhou, J. (2007). “Kinetic studies of adsorption of Pb(II), Cr(III) and Cu(II) from aqueous solution by sawdust and modified peanut husk,” J. Hazard. Mater. 141 (1) 163-167. DOI:10.1016/j.jhazmat.2006.06.109
Liao, X., Lu, Z., Zhang, M., Liu, X., and Shi, B. (2004). “Adsorption of Cu (II) from aqueous solutions by tannins immobilized on collagen,” J. Chem. Technol. Biotechnol. 79(4), 335-342. DOI: 10.1002/jctb.974
Lodeiro, P., Barriada, J L., Herrero, R., and Sastre de Vicente, M. E. (2006). “The marine macroalga Cystoseira baccata as biosorbent for cadmium(II) and lead(II) removal: Kinetic and equilibrium studies,” Environ. Pollut. 142 (2), 264-273. doi:10.1016/j.envpol.2005.10.001
Lu, Y., He, J., and Luo, G. (2013). “An improved synthesis of chitosan bead for Pb(II) adsorption,” Chem. Eng. J. 226, 271-278. DOI:10.1016/j.cej.2013.04.078
Müller, F. A., Müller, L., Hofmann, I., Greil, P., Wenzel, M. M., and Staudenmaier, R. (2006). “Cellulose-based scaffold materials for cartilage tissue engineering,” Biomaterials 27(21), 3955-3963. DOI: 10.1016/j.biomaterials.2006.02.031
Mohapatra, M., Khatun, S., and Anand, S. (2009). “Kinetics and thermodynamics of lead(II) adsorption on lateritic nickel ores of Indian origin,” Chem. Eng. J. 155(1), 184-190. DOI: 10.1016/j.cej.2009.07.035
Mouni, L., Merabet, D., Bouzaza, A., and Belkhiri, L. (2011). “Adsorption of Pb(II) from aqueous solutions using activated carbon developed from apricot stone,” Desalination 276(1), 148-153. DOI: 10.1016/j.desal.2011.03.038
Nakajima, A. (2002). “Electron spin resonance study on the vanadium adsorption by persimmon tannin gel,” Talanta 57(3), 537-544. DOI: 10.1016/S0039-9140(02)00068-1
Ncibi, M. C., Mahjoub, B., and Seffen, M. (2008). “Investigation of the sorption mechanisms of metal-complexed dye onto Posidonia oceanica fibres through kinetic modelling analysis,” Bioresour. Technol. 99(13), 5582-5589. DOI: 10.1016/j.biortech.2007.10.040
Özacar, M., Soykan, C., and Şengil, İ. A. (2006). “Studies on synthesis, characterization, and metal adsorption of mimosa and valonia tannin resins,” J. Appl. Polym. Sci. 102(1), 786-797. DOI: 10.1002/app.23944
Özacar, M., Şengil, İ. A., and Türkmenler, H. (2008). “Equilibrium and kinetic data, and adsorption mechanism for adsorption of lead onto valonia tannin resin,” Chem. Eng. J. 143(1), 32-42. DOI: 10.1016/j.cej.2007.12.005
Park, S., Baker, J. O., Himmel, M. E., Parilla, P. A., and Johnson, D. K. (2010). “Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance,” Biotechnol. Biofuels 3, 10. DOI: 10.1186/1754-6834-3-10
Repo, E., Warchol, J. K., Kurniawan, T. A., and Sillanpää, M. E. T. (2010). “Adsorption of Co(II) and Ni(II) by EDTA- and/or DTPA-modified chitosan: Kinetic and equilibrium modeling,” Chem. Eng. J. 161(1-2), 73-82. DOI: 10.1016/j.cej.2010.04.030
Sarı, A., Tuzen, M., Cıtak, D., and Soylak, M. (2007). “Adsorption characteristics of Cu(II) and Pb(II) onto expanded perlite from aqueous solution,” J. Hazard. Mater. 148(1-2), 387-394. DOI: 10.1016/j.jhazmat.2007.02.052
Sen, T. K., Mahajan, S., and Khilar, K. C. (2002). “Adsorption of Cu2+ and Ni2+ on iron oxide and kaolin and its importance on Ni2+ transport in porous media,” Colloid Surf. A-Physicochem. Eng. Asp. 211(1), 91-102. DOI: 10.1016/S0927-7757(02)00235-2
Sun, X., Huang, X., Liao, X.-p., and Shi, B. (2010). “Adsorptive recovery of UO2 2+ from aqueous solutions using collagen-tannin resin,” J. Hazard. Mater. 179(1), 295-302. DOI: 10.1016/j.jhazmat.2010.03.002
Sun, X., Huang, X., Liao, X.-p., and Shi, B. (2011). “Adsorptive removal of Cu(II) from aqueous solutions using collagen-tannin resin,” J. Hazard. Mater. 186(2), 1058-1063. DOI: 10.1016/j.jhazmat.2010.11.098
Vadivelan, V., and Kumar, K. V. (2005). “Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk,” J. Colloid Interf. Sci. 286(1), 90-100. DOI: 10.1016/j.jcis.2005.01.007
Vilar, V. J., Botelho, C., and Boaventura, R. A. (2005). “Influence of pH, ionic strength and temperature on lead biosorption by Gelidium and agar extraction algal waste,” Process Biochem. 40(10), 3267-3275. DOI: 10.1016/j.procbio.2005.03.023
Wang, J., Wei, L., Ma, Y., Li, K., Li, M., Ma, N., Feng, K., and Wang, Y. (2013). “Pb2+ adsorption onto collagen/cellulose hydrogel beads from aqueous solution: Kinetic, isothermal, and thermodynamic analyses,” Desalin. Water. Treat. 2013, 1-12 (published online). DOI: 10.1080/19443994.2013.855886
Wang, T., Liu, W., Xiong, L., Xu, N., and Ni, J. (2013). “Influence of pH, ionic strength and humic acid on competitive adsorption of Pb(II), Cd(II) and Cr(III) onto titanate nanotubes,” Chem. Eng. J. 215–216, 366-374. DOI:10.1016/j.cej.2012.11.029
Xu, J., Song, X. C., Zhang, Q., Pan, H., Liang, Y., Fan, X. W., and Li, Y. Z. (2011). “Characterization of metal removal of immobilized Bacillus strain CR-7 biomass from aqueous solutions,” J. Hazard. Mater. 187(1-3), 450-458. DOI: 10.1016/j.jhazmat.2011.01.047
Zhan, X. M., and Zhao, X. (2003). “Mechanism of lead adsorption from aqueous solutions using an adsorbent synthesized from natural condensed tannin,” Water Res. 37(16), 3905-3912. DOI: 10.1016/S0043-1354(03)00312-9
Zhang, Z., Li, G., and Shi, B. (2006). “Physicochemical properties of collagen, gelatin and collagen hydrolysate derived from bovine limed split wastes,” J. Soc. Leath. Tech. Ch. 90(1), 23.
Zhang, M., Ding, C., Chen, L., and Huang, L. (2014). “The preparation of cellulose/collagen composite films using 1-ethyl-3-methylimidazolium acetate as a solvent,” BioRes. 9(1), 756-771. DOI: 10.15376/biores.9.1.756-771
Zhang, M., Ding, C. C., Chen, L. H., Huang, L. L., and Yang, H. Y. (2014). “Interactions of collagen and cellulose in their blends with 1-ethyl-3-methylimidazolium acetate as solvent,” Cellulose. 21(5), 3311-3322. DOI 10.1007/s10570-014-0372-6
Article submitted: August 10, 2014; Peer review completed: November 29, 2014; Revised version received and accepted: January 19, 2015; Published: January 30, 2015.
