Abstract
Luffa was evaluated as a potential energy crop. A considerable amount of luffa sponge biomass can be grown in a vertical direction with approximately 70% polysaccharide content and low lignin content. When concentrated H3PO4 was employed to pretreat luffa sponge, hemicelluloses were the most sensitive component, followed by cellulose and lignin. Hemicellulose solubilization and cellulose loss positively responded to the elevated temperature, time, H3PO4 concentration, and dosage for pretreatment. However, lignin solubilization was not affected greatly. Although the initial hydrolysis rate was accelerated by increasing pretreatment temperature, the final glucose conversion was reduced as temperature was raised higher than 50 °C. Prolonging pretreatment time was positively correlated to enzymatic digestibility. When H3PO4 concentration was lower than 80%, the final glucose conversion was increased with increasing H3PO4 concentration. Increasing H3PO4 concentration to 84% for pretreatment caused the final glucose conversion to decrease slightly, although the hydrolysis rate was initially accelerated. Additionally, an improvement of glucose conversion was obtained with increasing the substrate-to-phosphoric acid ratio. However, the improvement was not cost-effective, as the ratio was lower than 1-to-8.
Download PDF
Full Article
Pretreating Luffa Sponge (Luffa cylindrica L.) with Concentrated Phosphoric Acid and Subsequent Enzymatic Saccharification
Qing Wang,a Fei Shen,a,* Gang Yang,a Yanzong Zhang,a Shihuai Deng,a Yaodong Hu,b Jing Zhang,a Chun Song,a and Yongmei Zeng c
Luffa was evaluated as a potential energy crop. A considerable amount of luffa sponge biomass can be grown in a vertical direction with approximately 70% polysaccharide content and low lignin content. When concentrated H3PO4 was employed to pretreat luffa sponge, hemicelluloses were the most sensitive component, followed by cellulose and lignin. Hemicellulose solubilization and cellulose loss positively responded to the elevated temperature, time, H3PO4 concentration, and dosage for pretreatment. However, lignin solubilization was not affected greatly. Although the initial hydrolysis rate was accelerated by increasing pretreatment temperature, the final glucose conversion was reduced as temperature was raised higher than 50 °C. Prolonging pretreatment time was positively correlated to enzymatic digestibility. When H3PO4 concentration was lower than 80%, the final glucose conversion was increased with increasing H3PO4 concentration. Increasing H3PO4 concentration to 84% for pretreatment caused the final glucose conversion to decrease slightly, although the hydrolysis rate was initially accelerated. Additionally, an improvement of glucose conversion was obtained with increasing the substrate-to-phosphoric acid ratio. However, the improvement was not cost-effective, as the ratio was lower than 1-to-8.
Keywords: Luffa sponge; Concentrated phosphoric acid; Pretreatment; Enzymatic hydrolysis
Contact information: a: Institute of Ecological and Environmental Sciences, Sichuan Agricultural University, Chengdu, Sichuan 611130, P. R. China; b: Institute of Animal Genetics and Breeding, College of Animal Science and Technology, Sichuan Agricultural University, Chengdu, Sichuan 611130, P. R. China; c: Department of Information Consultation and Integration, Library of Sichuan Agricultural University, Chengdu, Sichuan 611130, P.R. China; *Corresponding author: fishensjtu@gmail.com
INTRODUCTION
Increasing concerns of environmental pollution from fossil fuel consumption, depletion of petroleum reserves, and unstable oil prices have focused attention on alternative fuel production from biomass. Refining bioethanol from lignocellulosic biomass has promising potential. However, the conversion of lignocellulosic biomass to ethanol has many technical and economic challenges to overcome to make it feasible (Sindhu et al. 2012). In order to make the biomass conversion more economical, it is necessary to seek some suitable raw materials or to lower the cost of pretreatment and enzymatic hydrolysis.
Lignocellulosic substrates obtained from energy crops, woody biomass, and agricultural residues are extensively regarded to be globally abundant and represent excellent resources for renewable energy in theory (Lin and Tanaka 2006). Most of the woody biomass used for energy currently comes from forest residues and saw mills. However, in terms of available feed stocks for energy conversion, wood biomass is actually restricted by the wood industry and cannot be increased much in the future (Hoffmann and Weih 2005). Agricultural residues can be regarded as an abundant source for bioethanol production; however, their supply will inevitably compete with their utilization as forage and fertilizer, which may negatively affect the stability of feedstock supply for bioethanol production (Banerjee et al. 2012). When energy crops are considered as the biomass sources for biofuel production, excessive unitary cropping patterns may cause serious issues for biodiversity conservation (Zhang et al. 2007; Bals 2011; Archambault-Leger et al. 2012). Moreover, the production of traditional energy crops, such as sweet sorghum and switchgrass, will be seriously restricted by the area of land in the horizontal direction; however, the vertical space for biomass yield could not be greatly improved. Thus, seeking potential plant varieties to diversify energy crops can be a possible solution to the biodiversity conservation. In this context, the vine plants of luffa (Luffa cylindrica L.) were investigated as a lignocellulosic biomass for bioenergy conversion. Moreover, the luffa plant is an annual trailing vine, which can extend the growth space in the vertical direction to achieve higher biomass yield. It can be widely cultivated in China with excellent adaptability to climate and barren soil.
Ethanol production from lignocellulosic feedstocks by biological routes should generally undergo three key steps including pretreatment, enzymatic hydrolysis for fermentable monosaccharides, and fermentation. The recalcitrance of lignocellulose to deconstruction is a primary obstacle to its economic utilization as a source for bioethanol production (Alvira et al. 2010; Banerjee et al. 2012). Hence, pretreatment is an essential step to break down the lignin or hemicelluloses, as well as to disrupt the crystalline structure of cellulose. This action enhances the accessibility of the enzymes to the substrate, which facilitates monosaccharide production by enzymatic hydrolysis and subsequent ethanol fermentation. Currently, a number of pretreatment methods have been intensively investigated on various lignocellulosic feedstocks. For example, alkaline-based pretreatment approaches, including lime, NaOH, and ammonia recycling percolation (ARP), can effectively reduce the lignin content in most agricultural residues, but they are less satisfactory for processing recalcitrant feedstocks, such as softwoods (Bura et al. 2009). Typically, low pH pretreatments (e.g., dilute acid, un-catalyzed and catalyzed steam explosion with either acid or SO2 as a catalyst) can remove most of the hemicelluloses from the lignocellulosic feedstocks with a small portion of lignin (Wei et al. 1996). Also, ammonia fiber explosion, hydrothermal flow-through, and organosolv (OS) pretreatments have been thoroughly investigated (Bals 2011; Mesa et al. 2011; Del Rio 2012; Archambault-Leger et al. 2012). However, all of these pretreatment processes technically suffer from relatively low sugar yields, severe process conditions, and high capital investment and processing costs (Zhang et al. 2007). Therefore, pretreatments with gentle process conditions for lignocellulosic feedstocks are desired and are being investigated.
Recently, cellulose solvent-based pretreatments have been proposed and can greatly improve the cellulose accessibility when compared to conventional pretreatment methods, such as dilute acid, steam explosion, hot water pretreatment (Sathitsuksanoh et al. 2013). The reported cellulose solvents generally includes ionic liquids, concentrated phosphoric acid, N-methylmorpholine-N-oxide (NMMO), urea/NaOH, and N,N-dimethylacetamide (DMAc)/LiCl2. Substrates that are pretreated by concentrated phosphoric acid have been found to exhibit higher glucose conversion (Wang et al. 2011). However, most current works on solvent-based biomass pretreatment by concentrated phosphoric acid have focused on pure cellulose and cotton-based biomass, in which the cellulose could be well dissolved and decrystallized, which results in very high enzymatic hydrolysis yields (Zhang et al. 2009; Shen et al. 2013). Besides, there have been some reports indicating that superior glucose conversion can be achieved on various lignocellulosic feedstocks, including agricultural residues, energy crops, softwoods, and hardwoods, after being pretreated by concentrated phosphoric acid (Zhang et al. 2007; Wang et al. 2011; Takata et al. 2013). However, detailed responses of main lignocellulosic composition and enzymatic hydrolysis to the pretreatment variables have not been investigated yet.
Given this literature review, the luffa sponge (Luffa cylindrica L.) was selected for investigation as a potential feedstock for bioenergy production. The concentrated phosphoric acid pretreatment was attempted on this substrate. The investigated variables were temperature, pretreatment duration, concentration of phosphoric acid, and solid/liquid ratio of substrate phosphoric acid. Their responses to the chemical composition and subsequent enzymatic hydrolysis of the pretreated luffa sponge were clarified.
EXPERIMENTAL
Materials
Luffa sponge
The dried luffa fruits were collected from the farm of the Sichuan Agricultural University-Ya’an Campus. The luffa sponge was hammer-milled and screened with a grinder, and the milled substrates were screened through a 20-mesh screen prior to pretreatment. The main chemical composition of the luffa sponge is listed in Table 1.
Methods
Pretreatment
Pretreatments were carried out in 250 mL serum bottles with solid/liquid ratios of 1:6 to 1:12 (wt./wt.) using a phosphoric acid concentration that ranged from 78% to 84%. The serum bottle was sealed by a rubber stopper and hooped using an aluminum-seal, and shaken at 100 rpm for 1.0 to 7.0 h at a constant temperature of 20 to 50 °C. Substrate pretreatment was terminated by rapid five-fold dilution using distilled water (450 mL). The pretreated substrates were washed and dewatered using a dewatering centrifuge until the pH was higher than 5.5. The washed substrates were stored at -20 °C in a freezer until composition analyses and enzymatic hydrolysis could be performed.
Enzymatic hydrolysis
A commercial cellulase produced by Trichodema reesei (ATCC26924) and augmented with cellobiase (Sigma, Sigma-Aldrich Co., Ltd, USA) was used for the enzymatic hydrolysis. The activities of cellulase and cellobiase were 700 FPU•g-1 and 250 IU•g-1, respectively. The pretreated substrates were enzymatically hydrolyzed in an acetate buffer (0.05 M, pH 5.0) with the solid concentration of 2.0 % (w/v, dry basis). The enzyme loadings of cellulase and cellobiase were 20 FPU•g-1 glucan and 40 IU•g-1 glucan, respectively. All hydrolysis runs were performed in duplicate using 150 mL screw flasks (with 20 mL working volume) that were shaken at 150 rpm at 50 °C for 72 h in an incubator. Then 0.1 mL of tetracycline solution (50 mg•L-1) was added prior to the hydrolysis to inhibit microorganisms that may potentially consume the produced sugar. Periodically, 800 μL aliquots of the hydrolysate were taken during the hydrolysis process. The samples were boiled for 10 min to denature the enzymes and then centrifuged at 1.3×104 rpm, 4 °C for 10 min. The supernatant was stored at -20 °C in a freezer until further sugar analyses.
Analytical methods
Klason insoluble lignin and carbohydrates in the unpretreated luffa sponge were determined using TAPPI Standard T222 om-88 (Kaar et al. 1991). Ash and extractives were determined according to TAPPI Standard T211 om-85 and T264 om-88, respectively (Siqueira et al. 2010). The hydrolysate from the Klason lignin analysis was retained and analyzed for soluble lignin with an UV/vis spectrophotometer operating at 205 nm wavelength (TAPPI 1991). Carbohydrates, including glucose, xylose, galactose, arabinose, and mannose in the hydrolysate were quantified by using high-performance liquid chromatography (HPLC) (Dionex, ICS-3000, Sunyvale, CA). The HPLC had an autosampler (Dionex, AS-50), a gradient pump (Dionex, GP50), an anion exchange column (Dionex, CarboPac PA1), and an electrochemical detector (Dionex, ED50). The detailed protocols for the carbohydrates were based on Shen et al. (2011). The lignin and carbohydrates in the pretreated luffa sponge was similar to those of the raw feedstock. Glucose and xylose content in the enzymatic hydrolysate were measured using another HPLC (Flexar, PerkinElmer, Inc., Waltham, MA, USA). 100 μL of sample was injected into the HPLC with lactose (~0.5 g•L-1, Sigma, Sigma-Aldrich Co., Ltd, USA) as an internal standard. The sugars were separated using a sugar column (SH1011, Shodex, Showa Denko America, Inc., New York, USA) operating at 60 °C using 0.05 M H2SO4 as the mobile phase (flow rate of 0.8 mL•min-1). The separated sugars were detected using a refractive index detector operating at 50 °C. Based on the determined contents of lignin, hemicellulose and cellulose in the pretreated substrates (solid fraction), the lignin recovery, hemicellulose recovery, and glucan recovery from unpretreated luffa sponge were calculated according to the following equations (Li et al. 2013). The recovery of lignin, hemicellulose, or cellulose can be understood as the fraction of the lignin, hemicellulose, or cellulose remaining in the solid phase compared to that in the original biomass,
![]()
where Wp is the weight of pretreated luffa sponge (dry basis) (g), and Wo is the weight of the original luffa sponge used for pretreatment (dry basis) (g),
![]()
where PLignin is the lignin content of the pretreated luffa sponge (%), and OLignin is the lignin content of the original luffa sponge (%),
![]()
where PXylan is the xylan content of the pretreated luffa sponge (%), and OXylan is the xylan content of the original luffa sponge (%),
![]()
where PGlucan is the glucan content of the pretreated luffa sponge (%), and OGlucan is the glucan content of the original luffa sponge (%).
RESULTS AND DISCUSSION
Main Composition of Luffa Sponge
Cellulose, hemicelluloses, lignin, extractives, and ash content of the luffa sponge was measured and are presented in Table 1. Overall, most of these constituents were in the range reported by Satyanarayana et al. (2007) and Siqueira et al. (2010), except for the higher ash content in current work. The glucan content of 51% was the main carbohydrate in luffa sponge, which was typically higher than that of conventional lignocellulosic feedstocks, such as crop residues, softwoods, hardwoods, and bamboo (Yang et al. 2002; Linde et al. 2006; Balan et al. 2009; Bura et al. 2009; Wang et al. 2014). This observation indicated that luffa sponge could be used as a potential source for glucose for fermentation. Xylan was the major monosaccharide derived from the hemicelluloses, which is similar with most of annual agricultural residues. However, the hemicellulose content was lower when compared with the feedstocks of conventional crop residues, softwoods, and hardwoods. Meanwhile, the lignin content in the luffa sponge was 16%, which was also lower than most of the aforementioned lignocellulosic feedstocks. The lower hemicellulose and lignin content may potentially facilitate the pretreatment and subsequent hydrolysis (Ohgren et al. 2007).
In addition, based on the index of chemical composition, biomass yield, ecological adaptability, and calorific value, the evaluation for luffa sponge as a potential energy crop was carried out and compared with other feedstocks (the evaluation process is not shown) (McKendry 2002). The luffa sponge scored 68.1, which was close to some crop residues, such as cotton stalk (64.5), rice straw (65.3), wheat straw (68.4), ryegrass (68.5), Bermuda grass (54.7), and reed canary grass (68.6). The score was lower than that of eight other feedstocks, including switch grass (82.6), hybrid aspen (81.8), elephant grass (76.6), miscanthus (76.3), short-rotation-coppice willow (75.2), alfalfa (73.7), corn stover (70.7) and sugarcane bagasse (70.4). Although the score of luffa sponge was not comparable with some typical energy crops mentioned earlier, it still has potential to be a promising crop by increasing biomass yield through developing new varieties or optimizing planting patterns. After all, it is a climbing vine plant with the advantage of upward growth space to achieve a higher biomass yield.
Table 1. Main Composition of Luffa Sponge
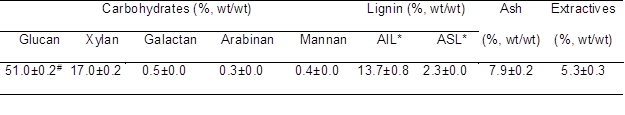
* AIL and ASL referred to acid-insoluble lignin and acid-soluble lignin, receptively
# All data in the table were expressed as mean ± standard deviation (n=2).
Effects of Pretreatment Temperature
The luffa sponge was pretreated at a temperature of 20, 30, 40, or 50 °C for 4 h using a phosphoric acid concentration of 85% and a solid/liquid ratio of 1:10. The main composition and their recoveries after pretreatment are listed in Table 2. Although the glucan content in the pretreated luffa sponge increased significantly (p<0.05) as the pretreatment temperature was varied from 20 to 40 °C, a slight decrease was observed at 50 °C. These results suggested that cellulose degradation may occur at higher pretreatment temperatures. Moreover, the decreased glucan recovery could directly reflect the existing degradation of cellulose, indicating that acid hydrolysis was strengthened at high pretreatment temperature (Zhang et al. 2009). Xylan content in the pretreated luffa sponge was significantly decreased with increasing pretreatment temperature. This hemicellulose could be completely solubilized as the pretreatment temperature was raised above 40 °C. The rapid decrease of xylan recovery with increasing temperature responded well to the decrease of xylan content. According to these results, as the main carbohydrates in the luffa sponge, xylan was more sensitive to pretreatment temperature than glucan, and hemicellulose could be easily solubilized consequently. In addition, a slight decrease was observed with lignin recovery, which indicated that lignin was not sensitive to pretreatment temperature and the increase of lignin content was mainly due to the rapid degradation of hemicelluloses. This result was similar to that of Napier grass pretreated by the concentrated phosphoric acid (Takata et al. 2013). Besides, as the pretreatment temperature was increased from 20 to 50 °C, the solid recovery decreased from 88.4% to 74.1%, again demonstrating that some constituents in the luffa sponge, including hemicellulose, cellulose, and even lignin, were gradually degraded with the increased temperature.
Table 2. Effects of Pretreatment Temperature on Main Chemical Composition and their Corresponding Recovery
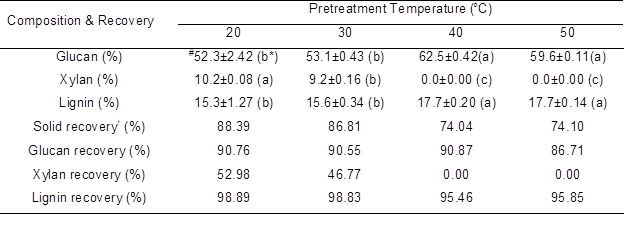
The other pretreatment conditions for this part were controlled as H3PO4 concentration of 85%, solid/liquid ratio of 1:10, and pretreatment time of 4 h;
# The data were expressed as mean ± standard deviation (n=2);
* The letters in the parentheses are to exhibit the results of ANOVA. If the letters are different between any 2 groups; this means that the difference of these 2 groups is significant (p<0.05);
† Recovery can be understood as the remained fraction of glucan, xylan, and lignin in the solid residues after pretreatment.
When the enzymatic hydrolysis was evaluated (Fig. 1) on the pretreated luffa, the glucose conversion at 72 h was significantly improved from 59.5% to 92.5% (p<0.05) with the increased pretreatment temperature. This result could be related to the solubilization of hemicellulose and lignin during pretreatment. Moreover, the conversion of cellulose I to the amorphous state during the pretreatment of concentrated phosphoric acid may be intensified by the increased temperature, which also could improve the enzymatic hydrolysis (Zhang et al. 2009; Takata et al. 2013). In addition, the hydrolysis rate can be accelerated as the pretreatment temperature was increased from 20 to 40 °C; however, the glucose conversion was obviously reduced at 50 °C. As statements in the referred work (Wei et al. 1996), the DP (degree of polymerization) was greatly reduced as the elevated temperature, by which the cellulose chain was shortened greatly at higher temperature (50℃). Thus, the action of hydrogen bonding amongst the depolymerized cellulose was intensified and cellulose crystallites was formed again, which retarded the enzymatic hydrolysis potentially.
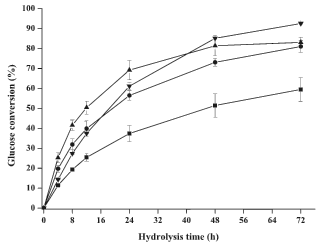
Fig. 1. Effects of pretreatment temperature on glucose yield during the enzymatic hydrolysis (■ 20 °C, ● 30 °C, ▲40 °C and ▼50 °C). All points were plotted as mean ± error bar (n=2);
Effects of Pretreatment Time
As presented in Table 3, the solid recovery decreased from 81.6% to 68.7% as the pretreatment time was prolonged from 1.0 to 7.0 h, which indicated the dissolution of the components in the luffa sponge by phosphoric acid was intensified. Although glucan content appeared to increase as the pretreatment time was prolonged from 1.0 to 5.0 h, it decreased to 60.0% after 7.0 h. The glucan recovery decreased from 93.4% to 81.0% when the pretreatment time was extended from 3.0 to 7.0 h. When the luffa sponge was pretreated for 1.0 h, the xylan content was significantly reduced to 6.9%. This amount was further reduced to 3.0% after 3 h and to 0% after 5 h of pretreatment. The lignin content ranged from 15.8% to 17.8% in the pretreated luffa, and their difference was not significant when compared to the lignin content in the untreated luffa sponge (p<0.05). The lignin recovery indicated that only 14.7% lignin was solubilized when the pretreatment time was extended to 7.0 h. These results indicated that the xylan (representing hemicellulose) was the most sensitive to the concentrated phosphoric acid pretreatment, followed by glucan (cellulose) and then lignin.
When the pretreated luffa sponges were employed for enzymatic hydrolysis, glucose conversion could be significantly increased from 73.7% to 86.3% with prolonging pretreatment time from 1.0 h to 5.0 h. A complete hydrolysis could be achieved when luffa sponge was pretreated at 7.0 h. The xylan solubilization, and lignin solubilization, glucan solubilization (at 1.0, 3.0 and 5.0 h) were correlated with the glucose conversion at 72 h, and the positive linear relationship can be observed with the correlation coefficient (R2) of 0.97, 0.98 and 0.93, respectively. The slopes of linear regression were 2.62, 0.31, and 0.10 which indicated that the enzymatic hydrolysis more depended on the hemicellulose removal (Öhgren et al. 2007). By contrast, the glucan can be completely hydrolyzed as the pretreatment time was prolonged to 7.0 h, although only 14.7% lignin was solubilized. This may be due to the fact that the cellulose was totally regenerated as amorphous cellulose. Approximately 30% of the glucan was lost, implying that part of regenerated cellulose (amorphous cellulose) was acid-hydrolyzed with extra-long time.
Table 3. Effects of Pretreatment Duration on the Main Composition and their Corresponding Recovery
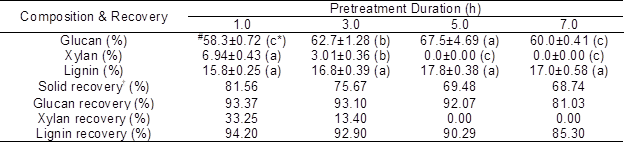
The other pretreatment conditions for this part were controlled as H3PO4 concentration of 85%, solid/liquid ratio of 1:10, and pretreatment temperature of 50 °C;
# The data were expressed as mean ± standard deviation (n=2);
* The letters in the parentheses are to exhibit the results of ANOVA. If the letters are different between any 2 groups, this means that the difference of these 2 groups is significant (p<0.05);
† Recovery can be understood as the remained fraction of glucan, xylan, and lignin in the solid residues after pretreatment.
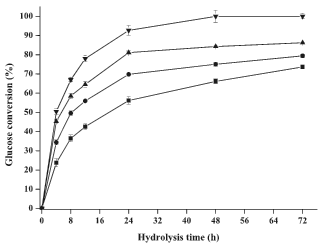
Fig. 2. Effects of pretreatment duration on glucose yield during the enzymatic hydrolysis (■ 1.0 h, ● 3.0 h, ▲ 5.0 h and ▼ 7.0 h). All points were plotted as mean ± error bar (n=2).
Effects of Phosphoric Acid Concentration
When the luffa sponges were pretreated with the phosphoric acid concentration of 78.0 to 84.0%, the solid recovery decreased from 81.4% to 75.1% (Table 4). The xylan was completely solubilized when the temperature was at 50 °C after 4.0 h pretreatment (Table 2), which was similar with the reported work using flax shives as the raw material (Kim and Mazza 2008). Thus, it was clear that hemicelluloses were very sensitive to phosphoric acid. However, the resulting products (i.e. xylose or xylo-oligosaccharides) from xylan after being pretreated by the concentrated phosphoric acid still remains to be clarified. Cellulose and lignin were not sensitive to the increased phosphoric acid concentration in contrast to the hemicellulose (Table 4). The lignin content varied slightly with no significant mass loss, which indicated that increased phosphoric acid concentration had no effect on lignin solubilization. The result on lignin solubilization was different in the reported work of Zhang et al. (2007), in which it was found that 75% of the lignin from corn stover can be removed due to the co-action of concentrated phosphoric acid and acetone.
As the pretreated luffa sponge in this part was hydrolyzed to evaluate enzymatic digestibility (Fig. 3), the final glucose conversion at 72 h was 80.0% and 87.1% as the phosphoric acid concentration was 78% and 80%, respectively. Afterwards, the glucose conversion has a slight decrease to 84.7% and 83.4% as the phosphoric acid concentration was promoted to 82% and 84%. Based on the data in Table 4, total xylan solubilization indicated that the contribution from hemicelluloses to the decrease in digestibility can be excluded (Alvira et al. 2010). The almost equivalent lignin content (Table 4) and adequate enzyme loadings (20 FPU•g-1 glucan for cellulase and 40 IU•g-1 glucan for cellobiase) for hydrolysis suggested that the negative effects on digestibility from the obstacle of lignin also can be excluded (Zhang et al. 2009).
Table 4. Effects of Phosphoric Acid Concentration on the Main Composition and their Corresponding Recovery

The other pretreatment conditions for this part were controlled as H3PO4 concentration of 85%, solid/liquid ratio of 1:10, and pretreatment temperature of 50 °C;
# The data were expressed as mean ± standard deviation (n=2);
* The letters in the parentheses are to exhibit the results of ANOVA. If the letters are different between any 2 groups, this means that the difference of these 2 groups is significant (p<0.05);
† Recovery can be understood as the remained fraction of glucan, xylan, and lignin in the solid residues after pretreatment.
It was reported that a transition from cellulose swelling to dissolution was previously observed at a small phosphoric acid concentration range (Zhang et al. 2006). The crystalline of regenerated cellulose from phosphoric acid pretreatment also became excessively low as the concentration increased. The flocculability of the regenerated cellulose was correspondingly enhanced significantly, which might have a negative influence on the enzymatic hydrolysis (Shen et al. 2013). Besides, it can be observed that the hydrolysis of pretreated luffa sponge in the first 24 h was accelerated by elevating phosphoric acid concentration. It can be easily understood that the enhanced flocculability at the higher phosphoric acid concentration may cause the easier digestibility of substrate surface and the harder accessibility to the inner substrate. Besides, when the pretreated luffa sponge was prepared for composition analysis, the dried substrates pretreated by 78.0% and 80.0% phosphoric acid were of relatively low density and could be pulverized easily. However, the pretreated luffa sponge with 82.0% and 84.0% phosphoric acid were very compact in nature after being dried and harder to be pulverized. Based on these observations, it can be deduced that the elevated phosphoric acid concentration for pretreatment may potentially compact the supermolecular structure of pretreated luffa sponge, causing the slight decrease on final glucose conversion (72 h).
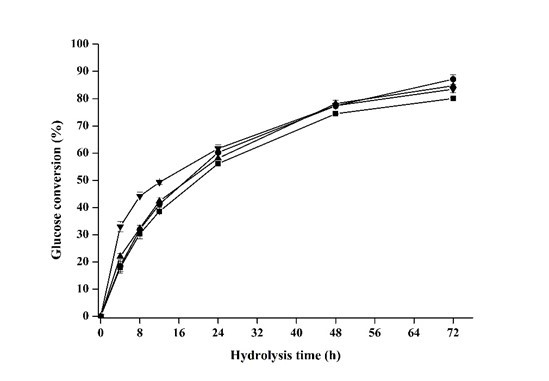
Fig. 3. Effects of phosphoric acid concentration on glucose yield during the enzymatic hydrolysis (■ 78.0%, ● 80.0%, ▲82.0%, and ▼84.0%). All points were plotted as mean ± error bar (n=2).
Effects of the Ratio of Substrate-to-Phosphoric Acid
The ratio of substrate-to-phosphoric acid in the pretreatment related to the usage of phosphoric acid and pretreatment cost, and the luffa sponge thereby was pretreated at various ratios at 30 °C with 85% phosphoric acid for 4.0 h. The glucan, xylan, and lignin content did not vary significantly (p>0.05) (Table 5). As expected, the solid recovery decreased slightly from 88.0% to 85.9% as the ratio decreased from 1:6 to 1:12. Half of the xylan was solubilized when the ratio was between 1:6 and 1:12. Moreover, the xylan solubilization was intensified with the decrease of ratio of substrate-to-phosphoric acid. Similarly, glucan recovery was also decreased as the ratio of substrate-to-phosphoric acid was reduced. In addition, the lignin could not be efficiently solubilized when the pretreatment temperature was 30 °C, even when the substrate-to-phosphoric acid ratio was high. Thus, it can be seen that the solid mass loss during pretreatment is mainly attributable to xylan solubilization.
Although enzymatic digestibility of the pretreated luffa sponge can be improved with more phosphoric acid used in pretreatment (Fig. 4), it was still low (56.2%) when the ratio of 1:6 was employed, which was mainly attributed to the insufficient contact between luffa sponge and phosphoric acid. As the ratio was raised to 1:8 to 1:12, the glucose conversion was not greatly affected, which varied in the range of 81.0 to 87.4% (Fig. 4). According to the xylan, glucan, and lignin recovery (Table 5), their changes were not significant, and this proved the ratio of substrate-to-phosphoric was not a dominator on chemical composition of luff sponge. The glucose conversion in Fig. 4 shows that the digestibility was almost similar except 1:6. It also can be seen that the ratio of 1:8 was enough to satisfy the acid requirement for pretreatment, and the use of more acid would not be cost-effective for improving glucose conversion.
Table 5. Effects of Ratio of Substrate/Phosphoric Acid on the Main Composition and their Corresponding Recovery
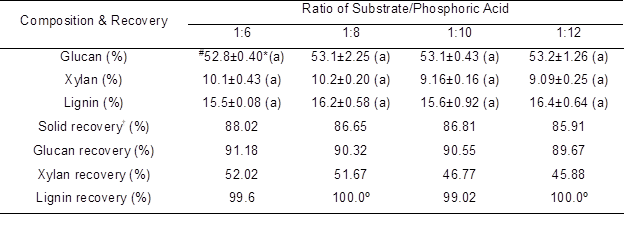
The other pretreatment conditions for this part were controlled as pretreatment time of 4h, solid/liquid ratio of 1:10, and pretreatment temperature of 50 °C;
# The data were expressed as mean ± standard deviation (n=2);
* The letters in the parentheses are to exhibit the results of ANOVA. If the letters are different between any 2 groups, this means that the difference of these 2 groups is significant (p<0.05);
† Recovery can be understood as the remained fraction of glucan, xylan, and lignin in the solid residues after pretreatment;
º The actual calculated lignin recovery at the ratio of 1:8 and 1:10 were 102.3% and 102.6%, respectively, which was attributed to determination errors. Thus, these data were regarded as 100% in the above table.
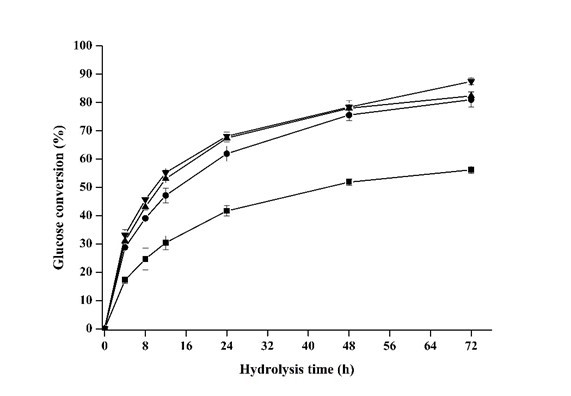
Fig. 4. Effects of ratio of substrate/phosphoric acid on glucose yield during the enzymatic hydrolysis (■ 1:6, ● 1:8, ▲ 1:10, and ▼ 1:12). All points were plotted as mean ± error bar (n=2).
CONCLUSIONS
- Luffa sponge was evaluated as a potential energy crop with relatively high polysaccharide content (70%) and low lignin content (16%).
- Hemicelluloses were the most sensitive fraction to the concentrated phosphoric acid pretreatment, which was followed by cellulose and lignin. Temperature, time, phosphoric acid concentration, and dosage for pretreatment were positive relative to hemicellulose solubilization and negative relative to cellulose recovery. However, lignin solubilization was not greatly affected by these variables.
- Increasing pretreatment temperature can accelerate enzymatic hydrolysis rate, however, extra high temperatures may decrease the glucose conversion. Prolonging the pretreatment time was beneficial to the enzymatic digestibility. The increased phosphoric acid concentration can improve the hydrolysis rate, but resulted in a slight decrease of glucose conversion. Increasing the phosphoric acid dosage (e., substrate-to-acid ratio) was not cost-effective for improving glucose conversion.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (21306120) and the “Program for Changjiang Scholars and Innovative Research Team in University” (IRT13083) from the Ministry and Education of China. The Department of Science and Technology of Sichuan Province is also appreciated for additional funding support (No. 2014JQ0037, 2015NZ0100).
REFERENCES CITED
Alvira, P., Tomás-Pejó, E., Ballesteros, M., and Negro, M. J. (2010). “Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review,” Bioresour. Technol. 101(13), 4851-4861. DOI:10.1016/j.biortech.2009.11.093
Archambault-Leger, V., Shao, X., and Lynd, L. R. (2012). “Integrated analysis of hydrothermal flow through pretreatment,” Biotechnol. Biofuels 5(28), 49. DOI:10.1186/1754-6834-5-49
Balan, V., Sousa, L. D. C., Chundawat, S. P. S., Marshall, D., Sharma, L. N., Chambliss, C. K., and Dale, B. E. (2009). “Enzymatic digestibility and pretreatment degradation products of AFEX-treated hardwoods (Populus nigra),” Biotechnol. Progr. 25(2), 365-375. DOI:10.1002/btpr.160
Bals, B. (2011). “Evaluating the impact of ammonia fiber expansion (AFEX) pretreatment conditions on the cost of ethanol production,” Bioresour. Technol. 102(2), 1277-1283. DOI:10.1016/j.biortech.2010.08.058
Banerjee, G., Car, S., Liu, T., Williams, D. L., Meza, S. L., Walton, J. D., and Hodge, D. B. (2012). “Scale-up and integration of alkaline hydrogen peroxide pretreatment, enzymatic hydrolysis, and ethanolic fermentation,” Biotechnol. Bioeng. 109(4), 922-931. DOI:10.1002/bit.24385
Bura, R., Chandra, R., and Saddler, J. (2009). “Influence of xylan on the enzymatic hydrolysis of steam-preateated corn stover and hybrid poplar,” Biotechnol. Progr. 25(2), 315-322. DOI:10.1002/btpr.98
Del Rio, L. F. (2012). “Fibre size does not appear to influence the ease of enzymatic hydrolysis of organosolv-pretreated softwoods,” Bioresour. Technol. 107(3), 235-242. DOI:10.1016/j.biortech.2011.12.057
Hoffmann, D., and Weih, M. (2005). “Limitations and improvement of the potential utilisation of woody biomass for energy derived from short rotation woody crops in Sweden and Germany,” Biomass Bioenerg. 28(3), 267-279. DOI:10.1016/j.biombioe.2004.08.018
Kaar, W. E., Cool, L. G., Merriman, M. M. and Brink, D. L. (1991). ” The complete analysis of wood polysaccharides using HPLC. ” J. Wood. Chem. Technol. 11(4), 447-463. DOI: 10.1080/02773819108051086
Kim, J., and Mazza, G. (2008). “Optimization of phosphoric acid catalyzed fractionation and enzymatic digestibility of flax shives,” Indust. Crops Prod. 28(3), 346-355. DOI:10.1016/j.indcrop.2008.03.011
Li, Y., Li, X., Shen, F., Wang, Z., Yang, G., Lin, L., Zhang, Y., Zeng, Y., and Deng, S. (2013). “Responses of biomass briquetting and pelleting to water-involved pretreatments and subsequent enzymatic hydrolysis.” Bioresour. Technol. 151c(1) 54-62. DOI:10.1016/j.biortech.2013.10.044
Lin, Y., and Tanaka, S. (2006). “Ethanol fermentation from biomass resources: Current state and prospects.” Appl. Biochem. Biotechnol. 130(1-3), 546-562. DOI:10.1007/s00253-005-0229-x
Linde, M., Galbe, M., and Zacchi, G. (2006). “Steam pretreatment of acid-sprayed and acid-soaked barley straw for production of ethanol,” Appl. Microbiol. Biotechnol., pp. 546-562. DOI:10.1385/ABAB:130:1:546
Mesa, L., González, E., Cara, C., González, M., Castro, E., and Mussatto, S. I. (2011). “The effect of organosolv pretreatment variables on enzymatic hydrolysis of sugarcane bagasse,” Chem. Eng. J. 168(3), 1157-1162. DOI:10.1016/j.cej.2011.02.003
Ohgren, K., Bura, R., Saddler, J., and Zacchi, G. (2007). “Effect of hemicellulose and lignin removal on enzymatic hydrolysis of steam pretreated corn stover,” Bioresour. Technol. 98(13), 2503-2510. DOI:10.1016/j.biortech.2006.09.003
McKendry, P. (2002). “Energy production from biomass (Part 1): Overview of biomass,” Bioresour. Technol. 83(1), 37-46. DOI:10.1016/S0960-8524(01)00118-3
Sathitsuksanoh, N., George, A., and Zhang, Y. H. P. (2013). “New lignocellulose pretreatments using cellulose solvents: A review,” J. Chem. Technol. Biotechnol. 88(2), 169-180. DOI:10.1002/jctb.3959
Satyanarayana, K. G., Guimarães, J .L., and Wypych, F. (2007). “Studies on lignocellulosic fibers of Brazil. Part I: Source, production, morphology, properties and applications,” Compos. Part A – Appl.Sci. 38(7), 1694-1709. DOI:10.1016/j.compositesa.2007.02.006
Shen, F., Yuan, H., Pang, Y., Chen, S., Zhu, B., Zou, D., Liu, Y., Ma, J., Yu, L., and Li, X. (2013). “Performances of anaerobic co-digestion of fruit & vegetable waste (FVW) and food waste (FW): Single-phase vs. two-phase,” Bioresour. Technol. 144, 80-85. DOI: 10.1016/j.biortech.2013.06.099
Shen, F., Zhong, Y., Saddler, J. N., and Liu, R. (2011). “Relatively high-substrate consistency hydrolysis of steam-pretreated sweet sorghum bagasse at relatively low cellulase loading,” Appl. Biochem. Biotechnol. 165(3-4), 1024-1036. DOI:10.1007/s12010-011-9317-9
Sindhu, R., Binod, P., Janu, K. U., Sukumaran, R. K., and Pandey, A. (2012). “Organosolvent pretreatment and enzymatic hydrolysis of rice straw for the production of bioethanol,” World J. Microb. Biotechnol. 28(2), 473-483. DOI:10.1007/s11274-011-0838-8
Siqueira, G., Bras, J., and Dufresne, A. (2010). “Luffa cylindrica as a lignocellulosic source of fiber, microfibrillated cellulose, and cellulose nanocrystals,” BioResources 5(2), 727-740. DOI:10.15376/biores.5.2.727-740
Takata, E., Tsutsumi, K., Tsutsumi, Y., and Tabata, K. (2013). “Production of monosaccharides from napier grass by hydrothermal process with phosphoric acid,” Bioresour. Technol. 143, 53-58. DOI: 10.1016/j.biortech.2013.05.112
TAPPI. (1991). “Acid soluble lignin in wood and pulp,” TAPPI Useful Methods, TAPPI Press, Atlanta.
Wang, K., Yang, H.Y., Xu, F., and Sun, R. (2011). “Structural comparison and enhanced enzymatic hydrolysis of the cellulosic preparation from Populus tomentosa Carr., by different cellulose-soluble solvent systems,” Bioresour. Technol. 102(6), 4524-4529. DOI:10.1016/j.biortech.2010.12.088
Wang, Q., Wang, Z., Shen, F., Hu, J., Sun, F., Lin, L., Yang, G., Zhang, Y., and Deng, S. (2014). “Pretreating lignocellulosic biomass by the concentrated phosphoric acid plus hydrogen peroxide (PHP) for enzymatic hydrolysis: Evaluating the pretreatment flexibility on feedstocks and particle sizes,” Bioresour. Technol. 166, 420-428. DOI:10.1016/j.biortech.2014.05.088
Wei, S., Kumar, V., and Banker, G. (1996). “Phosphoric acid mediated depolymerization and decrystallization of cellulose: preparation of low crystallinity cellulose – A new pharmaceutical excipient,” Int. J. Pharm. 142(2), 175-181. DOI:10.1016/0378-5173(96)04673-X
Yang, B., Boussaid, A., Mansfield, S. D., Gregg, D. J., and Saddler, J. N. (2002). “Fast and efficient alkaline peroxide treatment to enhance the enzymatic digestibility of steam-exploded softwood substrates,” Biotechnol. Bioeng. 77(6), 678-684. DOI:10.1002/bit.10159
Zhang, J., Zhang, J., Lin, L., Chen, T., Zhang, J., Liu, S., Li, Z., and Ouyang, P. (2009). “Dissolution of microcrystalline cellulose in phosphoric acid – Molecular changes and kinetics.” Molecules 14(12), 5027-5041. DOI:10.3390/molecules14125027
Zhang, Y.-H. P., Ding, S.-Y., Mielenz, J. R., Cui, J.-B., Elander, R. T., Laser, M., Himmel, M. E., McMillan, J. R., and Lynd, L. R. (2007). “Fractionating recalcitrant lignocellulose at modest reaction conditions,” Biotechnol. Bioeng. 97(2), 214-223. DOI:10.1002/bit.21386
Zhang, Y. H., Cui, J., Lynd, L. R., and Kuang, L. (2006). “A transition from cellulose swelling to cellulose dissolution by o-phosphoric acid: Evidence from enzymatic hydrolysis and supramolecular structure,” Biomacromolecules 7(2), 644-648. DOI:10.1021/bm050799c
Article submitted: June 23, 2015; Peer review completed: October 4, 2015; Revised version received and accepted: November 11, 2015; Published: November 30, 2015.
DOI: 10.15376/biores.11.1.899-912
