Abstract
Pretreatment of sawdust using a combination of sodium hydroxide (NaOH) and monochloroacetic acid (MCA) was investigated for the formation of reducing sugars. Optimum conditions for the pretreatment process were determined by the amount of reducing sugars formed during the enzymatic hydrolysis of the pretreated substrate. It was found that mercerization by NaOH played an important role in increasing the degree of substitution (DS) and that the maximum solubility was achieved during the etherification by MCA. A maximum amount of 34.2% lignin was removed in the process. As the DS of the substrate was increased, the efficiency of the hydrolysis process increased, leading to the higher yield of reducing sugars. The optimum operating conditions for the pretreatment process were determined to be 75 ºC at 90 rpm for 4 hours (2 hours for mercerization plus 2 hours for etherification). Under these operating conditions, with 1% (w/v) NaOH and 2% (w/w) MCA loading, a maximum DS of 0.2 and a solubility of 10.3% was attained. At 75 ºC and after 48 hours of the hydrolysis process, cellulases from Aspergillus niger resulted in the production of 2.88 g/L of glucose with a yield of 62.72% reducing sugars. X-ray diffraction (XRD) revealed reduced crystallinity of the sawdust and Scanning Electron Microscopy (SEM) showed distortion of the structure after pretreatment.
Download PDF
Full Article
PRETREATMENT OF CELLULOSIC WASTE SAWDUST INTO REDUCING SUGARS USING MERCERIZATION AND ETHERIFICATION
Beomsoo Kim,a Ishan Gulati,a Jinwon Park,a,* and Jong-Shik Shinb
Pretreatment of sawdust using a combination of sodium hydroxide (NaOH) and monochloroacetic acid (MCA) was investigated for the formation of reducing sugars. Optimum conditions for the pretreatment process were determined by the amount of reducing sugars formed during the enzymatic hydrolysis of the pretreated substrate. It was found that mercerization by NaOH played an important role in increasing the degree of substitution (DS) and that the maximum solubility was achieved during the etherification by MCA. A maximum amount of 34.2% lignin was removed in the process. As the DS of the substrate was increased, the efficiency of the hydrolysis process increased, leading to the higher yield of reducing sugars. The optimum operating conditions for the pretreatment process were determined to be 75 oC at 90 rpm for 4 hours (2 hours for mercerization plus 2 hours for etherification). Under these operating conditions, with 1% (w/v) NaOH and 2% (w/w) MCA loading, a maximum DS of 0.2 and a solubility of 10.3% was attained. At 75 oC and after 48 hours of the hydrolysis process, cellulases from Aspergillus niger resulted in the production of 2.88 g/L of glucose with a yield of 62.72% reducing sugars. X-ray diffraction (XRD) revealed reduced crystallinity of the sawdust and Scanning Electron Microscopy (SEM) showed distortion of the structure after pretreatment.
Keywords: Lignocellulosic biomass; Mercerization; Etherification; Lignin removal; Enzymatic hydrolysis
Contact information: a: Department of Chemical and Bio-molecular Engineering, Yonsei University, 262 Seongsanno, Seodaemun-gu, Seoul 120-749, South Korea; b: Department of Biotechnology, Yonsei University, 262 Seongsanno, Seodaemun-gu, Seoul 120-749, South Korea; *Corresponding author: jwpark@yonsei.ac.kr
INTRODUCTION
The production of bioethanol from lignocellulosic biomass is anticipated to be the most likely solution for progressively diminishing energy resources. South Korea in particular, which is the most rapidly developing economy in the world, is investing a lot of money into the research and development of clean energy. Due to its scarce energy resources, all of its petroleum is imported from the other countries (Kim et al. 2010). Approximately 41% of the Korea’s total energy comes from oil and around 20% from coal. The burning of fossil fuels such as coal, petroleum, and natural gas releases carbon dioxide and other greenhouse gases (GHGs) into the environment. Due to its high standards of living, Korea stands 9th in amount of CO2emissions (Lee et al. 2011). To mitigate the detrimental effects caused by GHGs and to meet the targets specified in the Kyoto Protocol, many countries have set a goal of reducing greenhouse gases in an effort to resolve global warming and to lessen their dependence on fossil fuels. In order to achieve such goals, they have been promoting renewable energy as part of their national policy.
Addition of ethanol to gasoline (i.e. to prepare gasohol) results in a value-added product with higher octane number due to the presence of an oxygen atom in the ethanol molecule (Jeon and Yeom 2010). In principle, the ethanol can be produced from biomass, cellulosic bioethanol. Upon combustion, the released CO2 emissions are absorbed back into plants, thus maintaining a healthy CO2 balance in the atmosphere (Zheng et al. 1998). The utilization of non-food plant waste biomass such as agricultural and forest residue offers considerable potential for the conversion of this biomass feedstock into ethanol. Of the total waste biomass production in Korea, forest residues contribute 66.08%, animal waste 16.12%, municipal wastes 10.27%, agricultural residues 5.58%, and food waste 1.66%. Korea produced 2.4 million tons of furniture waste in three recent years (Heo et al. 2010). Therefore, lots of opportunities exist to convert lignocellulosic waste into reducing sugars. Bioethanol developed from lignocellulosic biomass avoids competition with the food supply and helps in the conservation of natural resources. However, the goal to perfectly hydrolyze lignocellulosic biomass has some technical barriers that need to be addressed.
Lignocellulosic biomass consists of three major components: cellulose, hemicellulose, and lignin. Lignin holds an integral part in the cells walls and is embedded together with cellulose and hemicellulose in a complex matrix. These walls impart rigidity to the structure and provide resistance against enzymatic attack. Therefore, a pretreatment process prior to enzymatic hydrolysis is necessary to: 1) break down the lignin structures, 2) increase the surface area available for robust enzymatic attacks by increasing the pore size, and 3) reduce the cellulose crystallinity, all in order to increase the enzymatic digestibility (Mosier et al. 2005). These structural changes after the pretreatment directly affect the yield of reducing sugars from cellulose. There are several pretreatment methods for the facilitation of enzymatic hydrolysis. Some of these methods and their optimum operating conditions are listed in Table 1. The favorable conditions directly depend on the physical nature and the chemical composition of the substrate used. Chemical pretreatments include the usage of dilute acids (H2SO4, H3PO4, etc.), alkaline conditions (using NaOH, Urea, CaO, NH3, H2O2, etc.), and gases (carbon dioxide, sulphur dioxide, and ozone), whereas thermal pretreatments involve the utilization of high temperature steam or liquid hot water treatment. Physical pretreatments comprise milling or grinding unit operations and microwave irradiation treatments. These physical pretreatments can also be combined with the other pretreatments. Every pretreatment process has its own advantages and disadvantages (Sun and Cheng 2002).
To date, alkaline and dilute acidic pretreatments are considered comparatively advantageous for reasons of process sustainability. Recent studies (Wang et al. 2010; Mohsenzadeh et al.2012; Xu et al. 2010) have reported the details of alkaline pretreatments of different substrates that resulted in higher sugar yields. Alkaline treatments can be done over a wide range of temperatures at the expense of increased reaction time. NaOH causes the mercerization of cellulose and helps in the easy penetration of the enzymes (Mohsenzadeh et al. 2012). Moreover, it easily permeates into the amorphous portion of the cellulose and depolymerizes the crystalline part, thus resulting in reduced crystallinity. It has the advantage of not only altering the lignin structure but of also helping in the hydrolysis of the hemicellulose to xylose. When combined with other alkaline reagents such as Ca(OH)2, urea, and thiourea, NaOH has resulted in higher digestibility with reduced cost (Xu et al. 2010; Choi et al. 2007).
Dilute acid pretreatment, known for its delignification properties, increases the accessible surface area of the substrate. A high corrosion rate and the formation of inhibitory compounds such as acetic acid and furfural limit its use. Saha et al. (2005) investigated the dilute acid pretreatment of wheat straw for purposes of ethanol production and found that 92% of the hemicellulose was converted into sugars, with no measurable amounts of hydroxymethylfurfural (HMF) or furfural on treatment with 0.75% sulphuric acid at 121 oC for 1 hour. Yang et al.(2009) estimated the complete hydrolysis of cellulose on switchgrass with 1.5% sulphuric acid at 121 oC for 1 hour.
Organosolv pretreatment has been viewed as another effective pretreatment method, but solvent recovery and high capital investment have hurdled its development. Steam pretreatment and hydrothermal pretreatment use no chemicals during the process. However, high reaction temperatures and cost have constrained their use. So far, some level of success has been achieved; however, issues such as incomplete lignin removal and low cellulose conversion into sugar are yet to be resolved.
The objective of this study was to test the feasibility of this carboxymethylation pretreatment process. Mercerization by NaOH and etherification by MCA were considered as pretreatment steps. The efficiency of the pretreatment process was determined on the basis of lignin removal and the quantity of total reducing sugars formed.
Table 1. Substrate Pretreatment Techniques and Conditions for Enzymatic Hydrolysis
EXPERIMENTAL
Materials and Reagents
The sawdust was collected from a local furniture factory in Seoul, South Korea. Sodium hydroxide and monochloroacetic acid were purchased from the Duksan Pure Chemical Co, Ltd. The cellulase Onozuka Y-NC (Aspergillus niger) was purchased from the Yakult Pharmaceutical Industry Co., Ltd.
Substrate Pretreatment
To avoid uncertainty of size, the sawdust was screened through a 1 mm aperture mesh and was stored at room temperature. Before every experiment, extractive-free sawdust was dried at 70 oC for 1 hour. The pretreatment method mainly consisted of two unit processes: mercerization and etherification. Exactly 4 g of dried sawdust was impregnated with 40 mL of NaOH in a horizontally shaking water bath at 90 rpm. The reaction was carried out in 250 mL kurex bottles. To ensure that no leakage occurred, all bottles were tightly sealed with Teflon tape. The alkali cellulose that formed after 2 hours was etherified with 40 mL of 2% (w/w) MCA solution. The solid/liquid ratio was changed from 0.1 g/mL during mercerization to 0.05 g/mL upon etherification. The experimental conditions of the process are listed in Table 2.
Table 2. Pretreatment Conditions used in Carboxymethylation Process Technique for Sawdust
The chemically pretreated pulp was filtered and neutralized to pH 7. The pretreated substrate was dried at 70 oC for 2 hours and stored in a desiccator. The mechanism of the process is shown in Fig. 1.
Fig. 1. Mechanism of the carboxymethylcellulose process
Saccharification (enzymatic hydrolysis)
Enzyme obtained from Aspergillus niger was used to hydrolyze the polysac-charides. The characteristics of the enzyme are listed in Table 3. The temperature and pH values were selected according to the optimum working range of the enzymes. Regarding the action of the enzymes, the polysaccharides were converted to cellobiose and other disaccharides. Finally, the disaccharides were converted to monomeric sugar units by the actions of β-glucosidase. The enzymatic hydrolysis reactions were performed in 50 mL falcon tubes in a horizontally shaking water bath at 50 oC and 90 rpm for 48 hours. Then 0.05 g of pretreated sawdust was mixed with 2 mL of an enzyme solution made in 0.05 M of sodium acetate and an acetic acid buffer solution at a pH of 4.8. After incubation, 3 mL of alkaline 3,5-dinitrosalicylic acid (DNS) was added to stop the reaction. To ensure proper mixing, the tubes were inverted and shaken. The solution was then cooled in an ice water bath and filtered with 0.2 μm Whatman nylon filters. All of the experiments were conducted in triplicate so as to attain reliable results, and the curves were best fit to demonstrate trends. A complete flow diagram of the process for reducing sugar from sawdust is shown in Fig. 2. Two primary steps, pretreatment and enzymatic hydrolysis, were employed to complete the processing of the cellulosic waste materials. XRD and FESEM analysis were carried out to study the effects of the pretreatment process.
Table 3. Characteristics of Cellulase Used for Enzymatic Hydrolysis
a Y-NC were purchased from Yakult Co., Japan.
Fig. 2. Experimental process: Classified pretreatment and enzymatic hydrolysis
Analytical Methods
The total extractives were determined by way of treatment with 2:1 (v/v) benzene/ethanol in a soxhlet apparatus and were then dried at 70 oC. Solubility, DS, weight loss, amount of lignin, and glucose concentration were the parameters that were studied in order to validate the pretreatment and enzymatic hydrolysis processes. To determine the amount of substrate that was solubilized after the pretreatment, 0.5 g of dried pretreated sawdust was mixed with 100 mL of distilled water for 10 hours at 60 rpm at room temperature. The solubility was defined by the following expression,
Solubility (%) = ((M (g)-R (g)) / M (g)) × 100% (1)
where M is the mass (g) of the manufactured sample, and R is the mass (g) of the insoluble sample.
The degree of substitution (DS) is defined as the average number of substitution of carboxymethyl groups per Anhydro Glucose Unit (AGU) and was determined by the ASTM standard (Latif et al. 2007; Xu et al. 2010). Exactly 2 g of chemically pretreated sawdust was weighed in a 250 mL Erlenmeyer flask and mixed with 15 mL of 70% methanol. The mixture was allowed to stand for 15 to 20 min, upon which time it was agitated with a mixture of 200 mL of water and 50 mL of 0.5 N NaOH for 3 to 5 hours so as to achieve maximum solubility. Using the potentiometric back titration method, the amount of NaOH substituted was measured via titration with 0.4 N HCl, using phenolphthalein as an indicator. The calculation was done according to Eq. 2,
DS = (0.162×A) / (1-0.058×A) (2)
where A = ((mL of NaOH × normality)-(mL of HCl × normality)) / grams of sample, which corresponds to the milliequivalents of total carboxyl groups per gram of sample.
The Klason lignin was determined according to NREL Laboratory Analytical Procedures (Sluiter et al. 2008). The total reducing sugars were analyzed by the dinitrosalicylic acid (DNS) method (Miller 1959). Filter paper activity (FPase, units of mg-1 mL-1) was assayed at a pH of 4.8 and at 50 oC. A unit activity is defined as the amount of enzyme that produces 1.0 μmol of reducing sugar from the substrate per minute.
X-ray diffraction (XRD) of the samples was measured using an XRD-6000 (Shimadzu, Japan) with Cu Kα radiation of λ = 1.541 Ǻ at 40.0 kV and 30.0 mA with a scanning speed of 4.0 (deg/min). The diffraction angle was varied from 10° to 40°. The morphological characterization of the untreated and pretreated sawdust was observed using the JSM-6701F FESEM (JEOL, Japan). All the samples were platinum-coated before the microscopy.
RESULTS AND DISCUSSION
Optimum Time
Determining the optimum parameters for pretreatment is an important step that allows for the sustainability of the process. Having an optimum time for the process helps in reducing the formation of inhibitory components and, consequently, determines the yield of reducing sugars. The time for both the mercerization and etherification processes was systematically varied in order to explore its effects on the DS and solubility. At a particular concentration (0.5% (w/v) NaOH and 2% (w/w) MCA) and at a particular temperature of 75 oC, an increase in both the DS and solubility was observed (see Fig. 3). After 2 hours of mercerization, the DS (substitution of H by Na) and the solubility reached constant levels.
Fig. 3. Effect of reaction time on the DS and solubility at 75 oC [with 0.5% (w/v) NaOH and 2% (w/w) MCA]
The introduction of MCA further increased solubility, while the DS remained nearly constant. The effect on the DS was more significant during the initiation of the mercerization process. An increase in the DS from 0.079 to 0.115 was obtained. According to Varshney et al. (2006), this process yields water-soluble polymers via the derivatization of cellulose during the etherification step. The best optimum time resulted in 2 hours of mercerization and nearly 2 hours of etherification. A maximum DS was achieved during the mercerization process, while maximum solubility was completed through etherification.
Degree of Substitution and Solubility
The relationship between the DS and solubility was studied at 0.5%, 1%, and 2% (w/v) NaOH loadings for samples that had been pretreated at different temperatures. The DS and solubility varied linearly with the increase in NaOH concentration and temperature. Increasing the solubility directly improves the rate of hydrolysis and increases the quantity of reducing sugars. During enzymatic hydrolysis, more solubility helps to obtain a high concentration of reducing sugars and also leads to a reduction in the effective time of the hydrolysis.
As shown in Fig. 4, an increase in the DS from 0.083 to 0.198 and an increase in solubility from 3.84% to 10.23% was detected with the increase in temperature from 50 to 75 oC. The decrease in DS and solubility upon further increases in temperature may be attributed to the inception of the side reaction or the degradation of the pretreated product at higher severity. The degradation of the cellulosic structure occurs by way of the elimination of water, which reduces the availability of hydroxyl groups for the reaction. The main visual change observed after the pretreatment was a color change (from yellowish brown to dark brown) of the substrate. These color changes can be attributed to the solubilization of more lignin in the pretreatment solution, or they may also be the result of the degradation of the sugars, which are transformed to brownish colors at high temperatures.
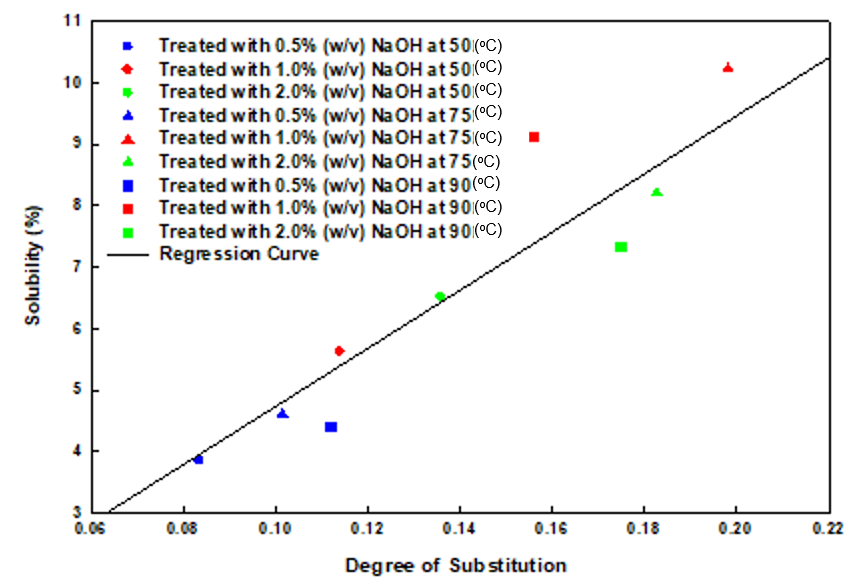
Fig. 4. The relationship between the DS and solubility at varying concentrations and temperatures with 2% (w/w) MCA
Weight Loss
Figure 5 presents the substrate’s weight loss with the increase in the process severity, which includes elevation in temperature and increase in alkalinity. Weight loss and removal of lignin from the substrate during pretreatment were the two important characteristics that were studied in order to determine the effects of the pretreatment conditions on the amount of reducing sugars. Weight loss varied from 8.8% to 21.6% under different pretreatment conditions. This characteristic is an indicator of the amount of available feedstock that can be enzymatically hydrolyzed. Under the alkaline or dilute-acid pretreatment, it is sometimes difficult to prevent the loss of carbohydrates that get dissolved from the sawdust in the pretreatment mixture.
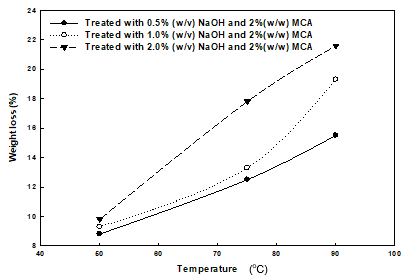
Fig. 5. Effect of increasing temperature and NaOH loading on weight loss of sawdust
The amount of carbohydrates dissolved in the liquid mixture was not studied in this work. Zabihi et al. (2010) studied the weight loss of wheat straw using steam pretreatment; after 30 min of the treatment, the weight loss increased from nearly 10% at 180 oC to 40% at 225 oC. At a constant temperature of 50 oC, no significant rise in weight loss was observed with increases in NaOH loading. The influence of NaOH concentration on weight loss was more evident at higher temperatures. At 75 oC, the weight losses were 9.3%, 13.3%, and 19.3% at NaOH loadings of 0.5%, 1.0%, and 2.0%, respectively. The weight loss was mainly affected by temperature, but at higher temperatures, the alkalinity of the process also influenced weight loss.
Lignin Removal
Lignin is a three-dimensional cross-linked polymer made up of phenylpropane monomer units. Of the main constituents of the plant cell wall, lignin is the component that offers structural support to the cell and acts as a protective sheath for enzymatic digestion. The main goal of the pretreatment process is the debottlenecking of the structure by removing the lignin and making the carbohydrates more easily accessible for saccharification. The sawdust used in this study contained 28% Klason lignin. The pretreatment showed good efficacy in removing lignin, even at lower temperatures.
Figure 6(a) shows the percentages of the lignin remaining after pretreatment. At 50 oC, when the NaOH loading was increased, the lignin decreased to 27.1%, 25.5%, and 24.6% in the substrates with NaOH loadings of 0.5%, 1.0%, and 2.0%, respectively. This shows the reduction of 11.7%, 20.3%, and 25.7%, respectively (see Fig. 6(b)). The lignin reduction increased with increasing alkalinity because the alkalinity broke the ether bonds and changed the hydrophobic nature such that solubility increased. A maximum lignin reduction of 34.2% was achieved at a loading of 2% NaOH and 2% MCA at 90 oC. According to Ko et al. (2009), a delignification between 20 and 65% is considered satisfactory for a smooth enzymatic attack.
Fig. 6. The pretreatment effects of temperature and NaOH concentration on (a) the amount of lignin remaining in the sawdust and (b) the percentage of reduction in lignin obtained after the pretreatment process
XRD Analysis
The effect of pretreatment on the crystallinity of the substrate was examined with XRD analysis. Reductions in the cellulose crystallinity are expected to increase the efficiency of the enzymatic hydrolysis. XRD analysis was performed on the untreated extractive free sample (a) and the pretreated substrate (b). Three crystalline peaks in the untreated sample, two of them broad peaks (002, 101), clearly demonstrate the crystallinity of the cellulose (Figure 7). The peak intensity after pretreatment was lower, which signifies that a decrease in the cellulose crystallinity occurred. The decrease in the crystallinity was due to the rupture of the hydrogen bonding, and the peak shifting was thought to be due to the increase in the distance between the cellulosic units.
Fig. 7. X-ray diffraction of (a) untreated extractive free sawdust and (b) pretreated substrate
Structural Changes
Morphological information on the substrate before and after the pretreatment was obtained and studied using SEM microscopy (Fig. 8). SEM pictures show the surfaces of the extracted free sawdust (Fig. 8(a)), the sawdust after mercerization (Fig. 8(b)), and the sawdust after etherification (Fig. 8(c)). Significant changes were observed after each process. The mercerized sawdust showed swelling and rupturing of the outer layer in contrast to the untreated sawdust. This was due to the alkaline environment, which caused the breakage of the 1-4 glycosidic linkages. Compared to the mercerized treatment, the etherification completely distorted the structure with the formation of holes that made the structure suitable for the enzymatic hydrolysis process. The reduction in the crystallinity and the distortion of the structure with voids was consistent with an expectation that the process would be suitable for the production of reducing sugars.
Enzymatic Hydrolysis
Substrate samples pretreated with 0.5%, 1.0%, and 2.0% NaOH with 2% MCA loadings corresponding to 50 oC, 75 oC, and 90 oC were subjected to enzymatic hydrolysis. Samples were withdrawn at different time intervals (1, 3, 6, 12, 24, 48, and 72 hours), and the quantities of total reducing sugars were analyzed in order to check the efficiency of the pretreatment method used. The results, shown in Fig. 9, indicate that the enzymatic hydrolysis was very sensitive to temperature. Most of the carbohydrates were converted into reducing sugars after 24 hours of hydrolysis, after which the amount of reducing sugars started to reach at constant value.
Fig. 8. SEM images of (a) extractive free sawdust, (b) pretreated with 1% (w/v) NaOH, and (c) pretreated with 1% (w/v) NaOH and 2% (w/w) MCA
After 48 hours, at 90 oC and with 2% NaOH and 2% MCA, a maximum reducing sugar yield of 64.82% was obtained for the 3.42 g/L glucose concentration. The 2% NaOH showed no significant increase in yield from the 1% NaOH, although a notable increase in the lignin reduction was seen. Therefore, after a certain amount of lignin reduction, which is essential to increase the efficiency of the enzymatic hydrolysis process, the pretreatment process does not necessarily lead to a higher yield of reducing sugars. A maximum for concentrated sugars was obtained after a period of 48 hours. The yield of reducing sugars for the substrate pretreated at 90 oC was not significantly higher in comparison to the yield for the substrate pretreated at 75 oC. At 75 oC, the yield of reducing sugar reached 63.18%, while at 90 oC the yield only increased to 64.82%. The decreases in DS and solubility for the 90 oC pretreatment were found to be associated with the reducing sugars yield.
Fig. 9. Effects of NaOH loading to the cellulose on the reducing sugars yield for the substrates pretreated at (a) 50 oC, (b) 75 oC, and (c) 90 oC with 0.5%, 1.0%, and 2.0% (w/v) NaOH and 2% (w/w) MCA, 0.5 g/L enzyme solution
CONCLUSIONS
- This study showed positive results for the NaOH and MCA pretreatment of sawdust from furniture manufacture. The process was shown to be economical for the production of reducing sugars.
- Although the process proved effective in the removal of lignin, enzymatic hydrolysis showed no dependency on the lignin removal after a particular delignification process.
- The addition of MCA to the mercerized sawdust increased the solubility of the sawdust, which signified better performance of the pretreatment process compared to the pretreatment with NaOH alone (only mercerization).
- The pretreatment with 1% NaOH and 2% MCA for a total of 4 hours at 75 oC and a hydrolysis period of 48 hours was determined to be the optimum conditions for the carboxymethylation technique applied to sawdust.
ACKNOWLEDGMENTS
This work was supported by the Advanced Biomass R&D Center (2010-8-2411) and the Korea Grant funded by the Ministry of Education, Science, and Technology.
REFERENCES CITED
Cara, C., Moya, M., Ballesteros, I., Negro, M. J., Gonzalez, A., and Ruiz, E. (2007). “Influence of solid loading on enzymatic hydrolysis of steam exploded or liquid hot water pretreated olive tree biomass,” Process Biochemistry 42, 1003-1009.
Choi, Y. M., Maken, S., Lee, S. M., Chung, E. H., Park, J. W., and Min, B. R. (2007). “Characteristics of water-soluble fiber manufactured from carboxymethylcellulose synthesis,” Korean Journal of Chemical Engineering 24, 288-293.
Chosdu, R., Hilmy, N., Erizal., T. B. E., and Abbas, B. (1993). “Radiation and chemical pretreatment of cellulosic waste,” Radiation Physics and Chemistry 42, 695-698.
Garía-Aparicio, M., Trollope, K., Tyhoda, M., Diedericks, D., and Görgens, J. (2011). “Evaluation of triticale bran as raw material for bioethanol production,” Fuel 90, 1638-1644.
Han, M. H., Kim, Y. L., Kim, Y. R., Chung, B. W., and Choi, G. W. (2011). “Bioethanol production from optimized pretreatment of cassava stem,” Korean Journal of Chemical Engineering 28, 119-125.
Heo, H. S., Park, H. J., Park, Y. K., Ryu, C., Suh, D. J., Suh, Y. W., Yim, J. H., and Kim, S. S. (2010). “Bio-oil production from fast pyrolysis of waste furniture sawdust in a fluidized bed,” Bioresource Technology 101, S91-S96.
Herrera, A., Téllez-Luis, S. J., Ramírez, J. A., and Vázquez, M. (2003). “Production of xylose from sorghum straw using hydrochloric acid,” Journal of Cereal Science 37, 267-274.
Jeon, D. J., and Yeom, S. H. (2010). “Two-step bioprocess employing whole cell and enzyme for economical biodiesel production,” Korean Journal of Chemical Engineering 27, 1555-1559.
Kim, J. S., Park, S. C., Kim, J. W., Park, J. C., Park, S. M., and Lee, J. S. (2010). “Production of bioethanol from lignocellulose: Status and perspectives in Korea,” Bioresource Technology101, 4801-4805.
Ko, J. K., Bak, J. S., Jung, M. W., Lee, H. J., Choi, I. G., Kim, T. H., and Kim, K. H. (2009). “Ethanol production from rice straw using optimized aqueous-ammonia soaking pretreatment and simultaneous saccharification and fermentation processes,” Bioresource Technology 100, 4374-4380.
Latif, A., Anwar, T., and Noor, S. (2007). “Two- step synthesis and characterization of carboxymethylcellulose from rayon grade wood pulp and cotton linters,” Journal of the Chemical Society of Pakistan 29, 143-150.
Lee, J. S., Lee, J. P., Park, J. Y., Lee, J. H., and Park, S. C. (2011). “Status and perspectives on bioenergy in Korea,” Renewable and Sustainable Energy Reviews 15, 4884-4890.
Linde, M., Jakobsson, E. L., Galbe, M., and Zacchi, G. (2008). “Steam pretreatment of dilute H2SO4-impregnated wheat straw and SSF with low yeast and enzyme loadings for bioethanol production,” Biomass and Bioenergy 32, 326-332.
Miller, G. L. (1959). “Use of dinitrosalicylic acid reagent for determination of reducing sugar,” Analytical Chemistry 31, 426-428.
Mohsenzadeh, A., Jeihanipour, A., Karimia, K., and Taherzadeha, M. J. (2012). “Alkali pretreatment of softwood spruce and hardwood birch by NaOH/thiourea, NaOH/urea, NaOH/urea/thiourea, and NaOH/PEG to improve ethanol and biogas production,” Journal of Chemical Technology and Biotechnology, DOI: 10. 1002/jctb.3695.
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y. Y., Holtzapple, M., and Ladisch, M. (2005). “Features of promising technologies for pretreatment of lignocellulosic biomass,” Bioresource Technology 96, 673-686.
Saha, B. C., and Cotta, M. A. (2007). “Enzymatic saccharification and fermentation of alkaline peroxide pretreated rice hulls to ethanol,” Enzyme and Microbial Technology 41, 528-532.
Saha, B. C., Iten, L. B., Cotta, M. A., and Wu, Y. V. (2005). “Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol,” Process Biochemistry 40, 3693-3700.
Sassner, P., Galbe, M., and Zacchi, G. (2008). “Techno-economic evaluation of bioethanol production from three different lignocellulosic materials,” Biomass and Bioenergy 32, 422-430.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., and Crocker, D. (2008). “Determination of structural carbohydrates and lignin in biomass,” NREL: Golden, CO.
Sun, Y., and Cheng, J. (2002). “Hydrolysis of lignocellulosic materials for ethanol production: A review,” Bioresource Technology 83, 1-11.
Varshney, V. K., Gupta, P. K., Naithani, S., Khullar, R., Bhatt, A., and Soni, P. L. (2006). “Carboxymethylation of α-cellulose isolated from Lantana camara with respect to degree of substitution and rheological behavior,” Carbohydrate Polymers 63, 40-45.
Wang, Z., Keshwani, D. R., Redding, A. P., and Cheng, J. J. (2010). “Sodium hydroxide pretreatment and enzymatic hydrolysis of coastal Bermuda grass,” Bioresource Technology 101, 3583-3585.
Xu, J., Cheng, J. J., Sharma-Shivappa, R. R., and Burns, J. C. (2010). “Sodium hydroxide pretreatment of switchgrass for ethanol production,” Energy Fuels 24, 2113-2119.
Yang, Y., Sharma-Shivappa, R., Burns, J. C., and Cheng, J. J. (2009). “Dilute acid pretreatment of oven-dried switchgrass germplasms for bioethanol production,” Energy & Fuels 23, 3759-3766.
Zabihi, S., Alinia, R., Esmaeilzadeh, F., and Kalajahi, J. F. (2010). “Pretreatment of wheat straw using steam, steam/acetic acid and steam/ethanol and its enzymatic hydrolysis for sugar production,” Biosystems Engineering 105, 288-297.
Zheng, Y., Lin, H. M., and Tsao, G. T. (1998). “Pretreatment for cellulose hydrolysis by carbon dioxide explosion,” Biotechnology Progress 14, 890-896.
Zhu, J. Y., Pan, X. J., Wang, G. S., and Gleisner, R. (2009). “Sulfite pretreatment (SPORL) for robust enzymatic saccharification of spruce and red pine,” Bioresource Technology 100, 2411-2418.
Article submitted: May 16, 2012; Peer review completed: August 16, 2012; Revised version received and accepted: August 24, 2012; Published: September 5, 2012.
