Abstract
A novel two-step process for pretreatment of corn stover was investigated with the goal of increasing sugar recovery and decreasing the capital cost. In the process, corn stover was first treated with dilute hydrochloric acid to maximize xylan recovery, and then the residue was treated with aqueous ammonia to alter the lignin structure and swell the cellulose surface. The optimal conditions were 110 °C and 40 min for 1% dilute hydrochloric acid pretreatment with a liquid to solid ratio of 10:1 followed by aqueous ammonia pretreatment at 37% NH3, 130 °C, 30 min, and liquid to solid ratio of 6:1. Under these pretreatment conditions, the glucan and xylan recoveries were 83.2% and 97.3%, respectively, with cellulase dosage at 15 FPU/g of substrate. When the cellulase dosage was decreased from 15 FPU/g to 10 FPU/g of substrate, the glucan recovery only dropped to 70%, while the xylan recovery remained high at 95.1%. The results show that this two-step pretreatment was effective for achieving high sugar recovery from corn stover by enzymatic hydrolysis.
Download PDF
Full Article
Pretreatment of Corn Stover for Sugar Production by a Two-Step Process using Dilute Hydrochloric Acid Followed by Aqueous Ammonia
Zihong Li,a Wen-zhi Li,a*Hanping Hu,a* Shuai Zu,a Ziyu Wang,bHasan Jameel,c and Hou-min Chang c
A novel two-step process for pretreatment of corn stover was investigated with the goal of increasing sugar recovery and decreasing the capital cost. In the process, corn stover was first treated with dilute hydrochloric acid to maximize xylan recovery, and then the residue was treated with aqueous ammonia to alter the lignin structure and swell the cellulose surface. The optimal conditions were 110 °C and 40 min for 1% dilute hydrochloric acid pretreatment with a liquid to solid ratio of 10:1 followed by aqueous ammonia pretreatment at 37% NH3, 130 °C, 30 min, and liquid to solid ratio of 6:1. Under these pretreatment conditions, the glucan and xylan recoveries were 83.2% and 97.3%, respectively, with cellulase dosage at 15 FPU/g of substrate. When the cellulase dosage was decreased from 15 FPU/g to 10 FPU/g of substrate, the glucan recovery only dropped to 70%, while the xylan recovery remained high at 95.1%. The results show that this two-step pretreatment was effective for achieving high sugar recovery from corn stover by enzymatic hydrolysis.
Keywords: Pretreatment; Enzymatic hydrolysis; Dilute acid; Ammonia; Corn stover
Contact information: a: Department of Thermal Science and Energy Engineering, University of Science and Technology of China, Hefei 230026, People’s Republic of China; b: American Process Inc. 750 Piedmont Ave NE Atlanta, GA 30308, USA; c: Department of Forest Biomaterials, North Carolina State University, Raleigh, NC 27695-8005, USA; *Corresponding author: Tel: +86 0551 63600786, E-mail address: liwenzhi@ustc.edu.cn (Wen-zhi Li)
INTRODUCTION
Lignocellulose is a promising alternative energy source due to its availability and renewability, and as such it has attracted much attention all around the world. Lignocellulose biomass (Warsamem and Danielsson 2012) such as agricultural waste, woods, and herbage, can be used to produce monosaccharides by hydrolysis. On this basis, a sugar platform can be established to produce a variety of liquid fuels or other high-valued chemicals. In general, it is difficult for enzymes to degrade cellulose within lignocellulose biomass without any pretreatment due to the recalcitrance formed by the cross-linked lignin and the rigid cellulose structure (da Costa Sousa et al. 2009). In order to improve the enzymatic hydrolysis efficiency, biomass must be pretreated before hydrolysis (Yu et al. 2011). Extensive research studies on biomass pretreatment have been done during the past several decades (Nlewem and Thrash 2010; Agbor et al. 2011), such as steam explosion pretreatment (Öhgren et al. 2007; Chang et al.2012), liquid hot water pretreatment (Mosier et al. 2005; Lee et al.2009), dilute acid pretreatment (Weiss et al. 2010; Redding et al.2011), alkaline pretreatment (McIntosh and Vancov 2011; Wang et al.2010; Sills and Gossett 2011; Jin et al. 2010; Wu et al. 2010), and ionic liquid pretreatment (Dadi et al. 2006; Shill et al. 2011).
Different pretreatment methods have different effects on the properties of the substrate. Among various pretreatments, dilute acid and alkaline pretreatments are considered more efficient than others and have been extensively studied (McIntosh and Vancov 2011; Chen et al. 2012). Dilute acid, including sulfuric acid, hydrochloric acid, and other acids (Qin et al. 2012; Wei et al. 2012), works by mainly hydrolyzing the hemicelluloses and effectively recovering sugars from the hemicelluloses. The porosity of biomass can be increased after the removal of hemicelluloses, which makes it easier for the enzyme to access the remaining cellulose. However, acid pretreatment has little effect on lignin removal, which normally has a negative impact on enzyme hydrolysis. Alkalis, such as sodium hydroxide, calcium hydroxide, and ammonia, are effective for delignification, which makes cellulose more accessible to enzymes as well. The ammonia recycle percolation (ARP) process (Kim et al.2003), as an alkaline pretreatment, is considered an effective method and has already been further investigated. Ammonia can swell cellulosic materials, remove lignin, and has little interaction with carbohydrates, which can be easily separated (Kim et al. 2003). Like all other alkaline pretreatment processes, the ARP process can improve glucan recovery, but hemicelluloses are also partially solubilized and their utilization requires further precipitation and separation of lignin (McIntosh and Vancov 2010). In order to improve total sugar recovery, a two-step biomass pretreatment method (Kim 2011) using sulfuric acid and ammonium hydroxide has been explored. In this method, sugars from hemicellulose are recovered with high efficiency by acid hydrolysis, and glucan is recovered as glucose in the subsequent enzymatic hydrolysis.
A number of pretreatment approaches have been studied over a wide range of raw materials. However, production of liquid fuels or high-valued chemicals from lignocellulose has not become an industrial success because of the high cost of enzyme. Any new process that can increase sugar recovery with low enzyme loading will be a promising alternative. In this study, a novel two-step process was investigated as to its effects on sugar recovery from corn stover. The raw material was pretreated by dilute hydrochloric acid in the first step with an attempt to maximize xylan recovery. Compared with dilute sulfuric acid, when dilute hydrochloric acid is used for hydrolysis, a relatively low pretreatment temperature is needed (Bustos et al. 2003; Li et al. 2008), resulting in lower contents of some inhibitory products such as HMF and furfural (Lee et al. 2013). The Cl- could be recycled in this way: xylose in hydrolysate could be converted to furfural in biphasic system; thus dilute hydrochloric acid could be reused after the organic phase was removed.
Aqueous ammonia was used in the second step to further alter lignin structure and swell the internal surface of cellulose. Although aqueous ammonia is considered a weak base, sugar recovery could be significantly improved under appropriate conditions. Meanwhile, ammonia can be easily separated by evaporation. The enzymatic hydrolysis was carried out to test the impact of the two-step pretreatment on sugar recovery of pretreated corn stover. Different reaction temperatures, residence times, and enzyme dosages were investigated. SEM images, crystal face indices, and degree of polymerization were used to characterize the features of corn stover in the two-step pretreatment. To make a comparison, four different pretreatment methods with diverse chemical reagents (ammonia aqueous, dilute hydrochloric acid, mixture of ammonia aqueous and ethanol, and dilute hydrochloric acid followed by aqueous ammonia) were investigated.
METHODS
Materials
Corn stover was obtained from the northern Anhui Province of China. The biomass was smashed and put through a 40-mesh screen. After cleaning the surface clay by washing with deionized water, the solids were air dried and then stored in sealed plastic bucket at room temperature.
Two-step Pretreatment of Corn Stover
In the first step, 7 g of dried biomass and 70 mL of 1.0% dilute hydrochloric acid were mixed and reacted in a 100 mL batch reactorwith agitation. The reaction temperature (T) (110 oC) and the liquid to solid ratio (L: S) (10:1) were fixed, whereas different reaction times (20, 30, 40, 50, and 60 min) were investigated. It took about 30 min to reach 110 oC, and then the reaction was kept at this temperature for the different reaction times. After pretreatment, the reactor was separated from the heating jacket and cooled down with circulating water immediately. The pretreated biomass slurry was then filtered to separate liquid from solids. The hydrolysate was collected to determine the amount of various sugars by HPLC. The optimal conditions were selected based on xylan recovery and temporal content of products. The solid phase from the optimal conditions was used for the second step treatment with aqueous ammonia.
In the second step, the biomass pretreated by dilute hydrochloric acid was washed with distilled water to rinse away the residual acid, and then the wet biomass was further pretreated with aqueous ammonia in a 100 mL batch reactor with agitation. The liquid to solid ratio (6:1) and reaction time (30 min) were kept unchanged in the aqueous ammonia pretreatment process. Different reaction temperatures (110 oC, 120 oC, 130 oC, 140 oC, and 150 oC) were utilized to explore the effect of the second step pretreatment temperature on the subsequent enzyme hydrolysis. After pretreatment, the solid residue was recovered by filtration under vacuum and washed with 500 mL of distilled water to remove excess ammonia, soluble lignin, and other byproducts.
Enzymatic Hydrolysis
Celluclast 1.5 L (cellulase complex, produced by Trichoderma reesei) and Novozyme 188 (-glucosidase, produced by Aspergillus niger) were kindly donated by Novozymes (China) Investment Co, Ltd. The enzyme activities were determined to be 74.5 filter paper units (FPU)/mL (expressed as micromoles of glucose produced per minute, with filter paper as substrate) and 261.4 cellobiose units (CBU)/mL (expressed as micromoles of cellobiose that is converted to glucose per minute, with cellobiose as a substrate) for Celluclast 1.5 L and Novozyme 188, respectively (Ghose 1987).
Wet pretreated sample (0.2 g dry biomass) was mixed with a specific amount of 0.05 M sodium acetate buffer (pH 4.8), and the mass concentration of substrate was 5%. Cellulase (5, 10, and 15 FPU per gram substrate) and -glucosidase (20 CBU per gram substrate) were added into the mixture. The enzymatic hydrolysis was performed in a shaking incubator at 50 oC and 120 rpm. After 72 h hydrolysis, the hydrolysate was collected by centrifugation and analyzed for sugar concentration using HPLC. Each enzymatic hydrolysis was carried out in duplicate.
Analysis Methods
Composition analysis of raw and pretreated biomass
The solid samples were analyzed for total solids, ash, carbohydrates, and acid-insoluble lignin in the raw and the pretreated biomass according to the Laboratory Analytical Procedures (LAP) established by National Renewable Energy Laboratory (NREL) (Sluiter et al.2005, 2008).
Sugar analysis
Sugars were determined by a high-performance liquid chromatography (HPLC) system equipped with SHODEX SP 0810 column (8×300 mm; Shodex, Tokyo) and differential refractive index detector (Waters 2414, USA). The sugars used for calculating the final sugar recovery included two parts (from both dilute acid hydrolysis and enzymatic hydrolysis). The recoveries of glucan and xylan were calculated as follows:
Glucan recovery = [(grams of glucose produced in the hydrolyzate) × 0.9]/ (grams of glucan in raw biomass) (1)
Xylan recovery = [(grams of xylose produced in the hydrolyzate) × 0.88]/ (grams of xylan in raw biomass) (2)
Scanning electron microscopy (SEM)
SEM pictures of untreated and two-step pretreated corn stover were taken at a magnification of 1000 times and 20 kV. In order to maintain their original structures, untreated and two-step pretreated corn stover samples were freeze-dried before observation through SEM. All samples were sputter-coated with a thin layer of gold before pictures were taken.
Indices of crystal face
Wide angle X-ray diffraction (WAXD) was used to measure the crystal face indices of untreated, dilute hydrochloric acid pretreated, and two-stage pretreated corn stover (after freeze-dried). Samples were scanned in a range from 5°to 40°using an X′pert diffractometer (Philips). The crystal face indices of all samples were analyzed by using X-ray diffraction technique and Rietveld refinement method (Daymond et al. 1997; Wilson et al. 1999).
Porosity
The porosity of untreated, dilute hydrochloric acid pretreated, and two-step pretreated corn stover was measured. Specific surface area (SSA) was calculated by N2 adsorption/desorption isotherms using the Brunauer–Emmett–Teller (BET) method on a Tristar II 3020M instrument from Micrometritics. Total volume of pores was determined by a single point adsorption total pore volume of pores with a pressure ratio (P/P0) at 0.974.
Degree of polymerization
Due to the presence of lignin in untreated and pretreated corn stover, the solids could not be completely dissolved in 0.5 M copper diethylene amine solution. On the other hand, further delignification could result in undesired effect on the average degree of polymerization (DPv) of cellulose. The method reported by Kumar et al. (2009) was used to determine the cellulose DPv by assuming that both soluble and insoluble fractions had similar compositions. A small amount of dried and pulverized powder of untreated and pretreated corn stover was used to minimize the lignin effect on viscosity. After a mixing time of about 30 min, the insoluble fraction was filtered out from the solution and weighed. The solution was then used to determine the intrinsic viscosity (dl/g) according to ASTM standard D 1795 (ASTM 1986).
The DPv can be calculated according to a relation after the intrinsic viscosity was determined,
(3)
where η is the intrinsic viscosity (cm3/g), and H and G are the mass fractions of hemicellulose (mainly xylan) and glucan, respectively.
RESULTS AND DISCUSSION
Sugar Recovery for Different Pretreatment Methods
The initial compositions of raw biomass were as follows: 39.6% glucan, 19.5% xylan, 2.0% arabinan, 1.2% galactan, 19.1% acid-insoluble lignin, 3.1% ash, and 15.5% other components. Figure 1 shows the sugar recovery (the recovery of glucan and xylan) using different pretreatments. In these five-group experiments, the same enzymatic hydrolysis conditions were adopted: 15 FPU cellulase and 20 CBU -glucosidase per gram of substrate, 50 oC, and 72 h. The experimental data (Fig. 1) suggest that the sugar recovery from corn stover with aqueous ammonia pretreatment was much higher than that from the untreated sample. Compared with aqueous ammonia pretreatment, the addition of ethanol had no effect on improving sugar recovery. In the aqueous ammonia pretreatment process, the xylan recovery was only about 33.7%. It is generally believed that much of the sugars from hemicelluloses are damaged by aqueous ammonia and could not be recovered. The low content of hemicelluloses in the pretreated materials leads to the low xylan recovery. The xylan recovery using dilute hydrochloric acid pretreatment was high (the xylan recovery was 92.3%), but the glucan recovery was comparatively low (the glucan recovery was 70.5%). After a two-step pretreatment by dilute hydrochloric acid followed by aqueous ammonia, the sugar recovery increased significantly (the glucan recovery was 80.6%, the xylan recovery was 92.7%). With respect to the dilute hydrochloric acid pretreatment, a slight increase of sugar recovery was observed by the two-step pretreatment. The two-step pretreatment method was shown to be a more effective method than single-step pretreatment using either chemical (dilute hydrochloric acid or aqueous ammonia) for improving sugar recovery.
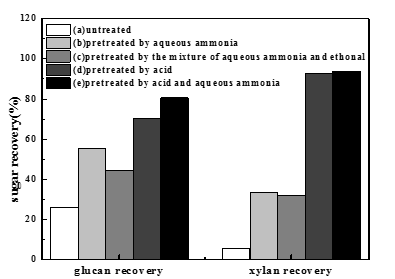
Fig. 1. Sugar recovery (glucan and xylan) by different pretreatment methods: (a)Untreated; (b) Pretreated by aqueous ammonia: T-110 oC, t-30 min, L:S-6:1; (c) Pretreated by aqueous ammonia and ethanol: T-110 oC, t-30 min, L: S-6:1,Vaqueous ammonia :Vethonal-5:1; (d) Pretreated by dilute hydrochloric acid: T-110 oC, t-30 min, L:S-10:1; (e) Pretreated by acid and ammonia aqueous: HCl treatment: T-110 oC, t-30min, L:S-10:1; Alkali treatment:T-110 oC, t-30 min, L:S-6:1.
Effect of Acid Hydrolysis Time on Sugar Recovery
Sugar recovery results for pretreated corn stover at different acid hydrolysis times are shown in Table 1. The experiments were operated at these conditions: HCl treatment step (1.0 % dilute hydrochloric acid based on the weight of acid solution, T of 110 oC, L:S of 10:1, reaction time of 20, 30, 40, 50, and 60 min); Alkali treatment (37.0% aqueous ammonia, T of 110 oC, time of 30 min, L:S of 6:1); Enzymatic hydrolysis conditions were 15 FPU/g cellulaseand 20 CBU β-glucosidase per gram of substrate, 50 oC,72 h.
As can be seen from Table 1, the xylan recovery increase depended on the acid hydrolysis time, and it reached 94.0% and 97.1% at 40 min and 60 min, respectively. Most of the xylan was recovered as xylose in the acid hydrolysate, and only a little was obtained from the enzymatic hydrolysate. It was shown that much of the hemicelluloses was hydrolyzed by dilute hydrochloric acid, which made it easier for cellulase to absorb on cellulose. However, very little glucan was recovered from the first step pretreatment (dilute acid hydrolysis), which indicated that cellulose was not significantly hydrolyzed by dilute hydrochloric acid under these conditions. Table 1 shows that the glucan recovery amounts derived from enzymatic hydrolysate at different acidolysis time were not significantly different, and the total glucan recovery at different time also showed no big difference (the maximum discrepancy was only 4.4% difference from the 78.7% to 74.3%). It is not beneficial to increase the glucan recovery by changing the acid pretreatment condition in this two-step pretreatment method. In order to improve glucan recovery, the aqueous ammonia pretreatment step deserved to be studied. Based on the recovery of both glucan and xylan, 40 min was chosen as the best acid hydrolysis time. The substrate pretreated by dilute hydrochloric acid was washed using distilled water and used for the next step.
Table 1. Recovery of Sugar from Corn Stover Pretreated at Different Acidolysis Times
Effect of Aqueous Ammonia Pretreatment Temperature on Sugar Recovery
The corn stover pretreated by diluted hydrochloric acid at the optimal condition was used in the aqueous ammonia pretreatment step. The effect of aqueous ammonia pretreatment temperature (110, 120, 130, 140, and 150 oC) on sugar recovery was studied, and results are shown in Fig. 2.
Fig. 2. Effect of aqueous ammonia pretreatment temperature (110, 120, 130, 140, and 150 oC) on sugar recovery from corn stover after two-step pretreated: treated with dilute hydrochloric acid at 110 °C for 40 min and followed by aqueous ammonia at different temperatures for 30 min. Enzymatic hydrolysis condition: (15 FPU cellulose and 20 CBU ß-glucosidase per gram of substrate, 50 oC, 72 h)
Initially the glucan recovery increased slightly from 78.7% to 83.2% with the increase of temperature from 110 oC to 130 oC. However, there was a big decline (about 18% from 83.2% to 65.7%) when the temperature reached 150 oC. A possible reason was that more cellulose was damaged due to the peeling reaction. The same trend was also observed for xylan. However its effect on xylan recovery was very small because most of the xylan was recovered in the first dilute acid pretreatment step. The optimal conditions of aqueous ammonia pretreatment were determined to be 130 °C and 30 min in terms of glucan recovery.
Effect of Enzyme Dosage on Sugar Recovery
The effect of enzyme dosage on sugar recovery (cellulases loading of 5, 10, and 15 FPU/g of substrate supplemented with -glucosidase 20 CBU per gram of substrate) was investigated. The results in Fig. 3 suggest that, as opposed to a slight increase of xylan recovery due to little xylan left in the substrate after two-step pretreatments, the glucan recovery increased significantly when the cellulase dosage was increased from 5 FPU/g to 15 FPU/g.
Fig. 3. Effect of cellulase dosage (cellulase loading of 5, 10, and 15 FPU/g of substrate supplemented with -glucosidase 20 CBU/g of substrate) on sugar recovery from corn stover after two-step pretreatment: treated with dilute hydrochloric acid at 110 °C for 40 min and followed by aqueous ammonia at 130 °C for 30 min.
The glucan recovery after the two-step treatment was 54.3%, 70.0%, and 83.2% with an enzyme loading 5 FPU/g of substrate, 10 FPU/g of substrate, and 15 FPU/g of substrate, respectively. The glucan recovery of 54.3% was relatively low with an enzyme loading of 5 FPU/g of substrate. However, when the cellulase dosage was increased 1.5 times from 10 FPU/g of substrate to 15 FPU/g of substrate, the glucan recovery was only improved by 13.2% from 70% to 83.2%. Overall a glucan recovery of 70% may be acceptable. Compared with dilute sulfuric acid, the dilute hydrochloric acid pretreatment gave less condensed lignin (Freudenberg 1971; Sharma and Goldstein 1990), which may make it easy for aqueous ammonia to remove the lignin in the subsequent step, resulting in the reduced dosage of enzyme in this two-step pretreatment method.
Characterization of Raw and Pretreated Corn Stover
SEM pictures of untreated and two-step pretreated corn stover at a magnification of 2000 are shown in Fig. 4. The pictures show that the structure of untreated corn stover had a neat and tight structure, which might make it difficult for enzyme to absorb onto the surface and attack the cellulose. After the two-step pretreatment, the tight structure of lignocellulose was damaged, the microfibrils were separated from the initial connected structure and exposed, and the surface of corn stover was swollen. This suggested that a lot of hemicellulose and lignin were removed in the two-step pretreatment process.
Fig. 4. SEM images of (a) untreated corn stover, (b) corn stover pretreated with dilute hydrochloric acid followed by aqueous ammonia (two-stage pretreated)
The indices of crystal face of untreated, dilute hydrochloric acid pretreated, and two-stage pretreated corn stover are shown in Fig. 5. The crystal indices were estimated in different space groups (Nishiyama et al. 2003; Wada et al. 2006). Each peak in the diagram was composed by several crystal faces. Before and after pretreatment, some crystal faces and the space groups disappeared and some new crystal faces formed. In particular, the main crystal faces and space groups were all changed after aqueous ammonia pretreatment. This demonstrates that the crystal structure was changed after pretreatment, which was advantageous for hydrolysis.
Fig. 5. The crystal face indices of untreated, dilute hydrochloric acid pretreated and two-step pretreated corn stover
Table 2. Porosity of Untreated, HCl-pretreated, and Two-step Pretreated Corn Stover
Porosity is one of the important factors that govern the efficiency of enzymatic hydrolysis. With high porosity, the corn stover sample is easily attacked by enzymes. The specific surface area (SSA) (measured by BET absorption) and pore volume of untreated, HCl-pretreated and two-step pretreated corn stover are shown in Table 2. After two-step pretreatment, the specific surface area was increased by nearly two times from 2.66 to 5.02(m2/g), and the total pore volume was also increased enormously from 0.0037 to 0.0207(cm3/g). This may be caused by the deconstruction of lignocellulose and the removal of hemicellulose and lignin. When lignin was removed, the corn fibre became swollen. The swelling of cellulose can increase the specific surface and pore volume. In another words, the pretreatment method using dilute acid followed by aqueous ammonia improved the porosity of the biomass, resulting in improved accessibility of enzymes to cellulose, which resulted in improved enzymatic hydrolysis.
CONCLUSIONS
A novel two-step pretreatment of corn stover using dilute hydrochloric acid followed by aqueous ammonia could effectively recover xylan and glucan as sugar after enzymatic hydrolysis. At the optimal pretreatment conditions (110 oC and 40 min for dilute hydrochloric acid pretreatment, 130 oC and 30 min for aqueous ammonia pretreatment), the glucan and xylan recoveries were 83.2% and 97.3%, respectively, with cellulase dosage of 15 FPU/g of substrate and cellobiase dosage of 20 CBU/g of substrate. When the cellulose dosage was decreased from 15 FPU/g of substrate to 10 FPU/g of substrate, the glucan recovery was reduced to 70%, while the xylan recovery remained high at 95.1%. The two-step pretreatment process resulted in a substrate with a rougher deconstructed surface, a higher porosity, and a changed crystal structure, which improved the overall enzymatic hydrolysis. Compared with dilute hydrochloric acid, a slight increase was observed in the two-step pretreatment. Even though the two-step process is more complicated, the savings in expensive enzymes may justify this modification, and the scale effect of industrialization can cut down the overall cost.
ACKNOWLEDGMENTS
This study was financially supported by the National Basic Research Program of China (No. 2012CB215302), the Key Programs of the Chinese Academy of Sciences (No. KGZD-EW-304-2), and Key Laboratory of Renewable Energy and Gas Hydrate, Chinese Academy of Sciences (No. CX2090130020).The authors acknowledge Novozymes (China) Investment Co, Ltd for kindly providing enzymes.
REFERENCES CITED
Agbor, V. B., Cicek, N., Sparling, R., Berlin, A., and Levin, D. B. (2011). “Biomass pretreatment: Fundamentals toward application,” Biotechnology Advances 29(6), 675 -685.
ASTM, (1986). “Standard test methods for intrinsic viscosity of cellulose (D 1795),” American Society for Testing Materials 15(4), 360-366.
Bustos, G., Ramírez, J. A., Garrote, G., and Vázquez, M. (2003). “Modeling of the hydrolysis of sugar cane bagasse with hydrochloric acid,” Applied Biochemistry and Biotechnology 104(1), 51-68.
Chang, J., Cheng, W., Yin, Q., Zuo, R., Song, A., Zheng, Q., Wang, P., Wang, X., and Liu, J. (2012). “Effect of steam explosion and microbial fermentation on cellulose and lignin degradation of corn stover,” Bioresource Technology 104, 587-592.
Chen, Y., Stevens, M. A., Zhu, Y., Holmes, J., Moxley, G., and Xu, H. (2012). “Reducing acid in dilute acid pretreatment and the impact on enzymatic saccharification,” Journal of Industrial Microbiology and Biotechnology 39(5), 691-700.
Dadi, A. P., Varanasi, S., and Schall, C. A. (2006). “Enhancement of cellulose saccharification kinetics using an ionic liquid pretreatment step,” Biotechnology and Bioengineering 95(5), 904-910.
da Costa Sousa, L., Chundawat, SPS., Balan, V., and Dale, B. E.(2009).“‘Cradle-to-grave’ assessment of existing lignocellulose pretreatment technologies,” Current Opinion in Biotechnology 20(3), 339-347.
Daymond, M. R., Bourke, M. A. M., VonDreele, R. B., Clausen, B., and Lorentzen, T. (1997). “Use of Rietveld refinement for elastic macrostrain determination and for evaluation of plastic strain history from diffraction spectra,” Journal of Applied Physics 82, 1554-1562.
Freudenberg, K. (1971), Textbook of Lignin, K. V. Sarkanen, and C. H. Ludwig, (eds.), USA.
Ghose, T. K. (1987). “Measurement of cellulase activities,” Pure and Applied Chemistry59, 257-268.
Jin, Y., Jameel, H., Chang, H-m., and Phillips, R. B., (2010). “Green liquor pretreatment of mixed hardwood for production of bioethanol in a repurpose kraft mill,” J. Wood Chem. Technol. 30, 86-104.
Kim, T. H., Kim, J. S., Sunwoo, C., and Lee, Y. Y. (2003). “Pretreatment of corn stover by aqueous ammonia,” Bioresource Technology 90(1), 39-47.
Kim, T. H. (2011). “Sequential hydrolysis of hemicellulose and lignin in lignocellulosic biomass by two-stage percolation process using dilute sulfuric acid and ammonium hydroxide,” Korean Journal of Chemical Engineering 28(11), 2156-2162.
Kumar, R., Mago, G., Balan, V., and Wyman, C.E. (2009). “Physical and chemical characterizations of corn stover and poplar solids resulting from leading pretreatment technologies,” Bioresource Technology 100(17), 3948-3962.
Lee, J. M., Shi, J., Venditti, R. A., and Jameel, H. (2009). “Autohydrolysis pretreatment of Coastal Bermuda grass for increased enzyme hydrolysis,” Bioresource Technology 100(24), 6434-6441.
Lee, J. Y., Ryu, H. J., and Oh, K. K. (2013). “Acid-catalyzed hydrothermal severity on the fractionation of agricultural residues for xylose-rich hydrolyzates,” Bioresource Technology 132, 84-90.
Li, W., Xu, J., Wang, J., Yan, Y., Zhu, X., Chen, M., and Tan, Z. (2008). “Studies of monosaccharide production through lignocellulosic waste hydrolysis using double acids,” Energy & Fuels22, 2015-2021.
McIntosh, S., and Vancov, T. (2011). “Optimisation of dilute alkaline pretreatment for enzymatic saccharification of wheat straw,” Biomass and Bioenergy 35(7), 3094-3103.
Mosier, N., Hendrickson, R., Ho, N., Sedlak, M., and Ladisch, M. R. (2005). “Optimization of pH controlled liquid hot water pretreatment of corn stover,” Bioresource Technology 96(18), 1986-1993.
Nishiyama, Y., Sugiyama, J., Chanzy, H., and Langan, P. (2003). “Crystal structure and hydrogen-bonding system in cellulose Iα from synchrotron X-ray and neutron fiber diffraction,” J. American Chemical Society 125(47), 14300-14306.
Nlewem, K. C., and Thrash, M. E. (2010). “Comparison of different pretreatment methods based on residual lignin effect on the enzymatic hydrolysis of switch grass,” Bioresource Technology 101(14), 5426-5430.
Öhgren, K., Bura, R., Saddler, J., and Zacchi, G. (2007). “Effect of hemicellulose and lignin removal on enzymatic hydrolysis of steam pretreated corn stover,” Bioresource Technology 98(13), 2503-2510.
Qin, L., Liu, Zh., Li, B., Dale, B. E., and Yuan, Y.(2012). “Mass balance and transformation of corn stover by pretreatment with different dilute organic acids,” Bioresource Technology 112, 319-326.
Redding, A.P., Wang, Z., Keshwani, D.R., and Cheng, J. J. (2011). “High temperature dilute acid pretreatment of coastal Bermuda grass for enzymatic hydrolysis,” Bioresource Technology 102, 1415-1424.
Sharma, D. K. and Goldstein, I. S., (1990). “Reactivity toward phenol of lignin from the hydrolysis of sweet gum wood with sulfuric acid,” J. Wood Chem. Technol. 10(3), 379-386.
Shill, K., Padmanabhan, S., Xin, Q., Prausnitz, J. M., Clark, D. S., and Blanch, H. W. (2011). “Ionic liquid pretreatment of cellulosic biomass: Enzymatic hydrolysis and ionic liquid recycle,” Biotechnology and Bioengineering 108(3), 511-520.
Sills, D. L., and Gossett, J. M. (2011). “Assessment of commercial hemicellulases for saccharification of alkaline pretreated perennial biomass,” Bioresource Technology 102(2), 1389-1398.
Sluiter, A., Hames, B., Hyman, D., Payne, C., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., and Wolfe, J. (2005a). “Determination of total solids in biomass and total dissolved solids in liquid process samples. Laboratory analytical procedure (LAP),”National Renewable Energy Laboratory, Golden, Colorado. NREL/TP-510–42621.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., and Templeton, D. (2005b). “Determination of ash in biomass. Laboratory analytical procedure (LAP),”National Renewable Energy Laboratory, Golden, Colorado. NREL/TP-510–42622.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., and Crocker, D. (2008). “Determination of structural carbohydrates and lignin in biomass. Laboratory analytical procedure (LAP),” National Renewable Energy Laboratory, Golden, Colorado. NREL/TP-510–42618.
Wada, M., Nishiyama, Y., and Langan, P. (2006). “X-ray structure of ammonia-cellulose I: new insights into the conversion of cellulose I to cellulose III1,” Macromolecules 39(8), 2947-2952.
Wang, Z., Keshwani, D.R., Redding, A.P., and Cheng, J. J. (2010). “Sodium hydroxide pretreatment and enzymatic hydrolysis of coastal Bermuda grass,” Bioresource Technology101, 3583-3585.
Warsamem, M., and Danielsson, B. (2012). “Saccharification of lignocellulose,” Malmö University.
Wei, W., Wu, S., and Liu, L. (2012). “Enzymatic saccharification of dilute acid pretreated eucalyptus chips for fermentable sugar production,” Bioresource Technology. 110, 302-307.
Weiss, N. D., Farmer, J. D., and Schell, D. J. (2010). “Impact of corn stover composition on hemicellulose conversion during dilute acid pretreatment and enzymatic cellulose digestibility of the pretreated solids,” Bioresource Technology 101(2), 674-678.
Wilson, R. M., Elliott, J. C., and Dowker, S. E. P. (1999). “Rietveld refinement of the crystallographic structure of human dental enamel apatites,”American Mineralogist 84, 1406-1414.
Wu, S., Chang, H-m., Jameel, H., and Phillips, R. B. (2010). “Novel green liquor pretreatment of loblolly pine chips to facilitate enzyme hydrolysis into fermentable sugars for ethanol production,” J. Wood Chem. Technol. 30, 205-218.
Yu, Z., Jameel, H., Chang, H., and Park, S. (2011). “The effect of delignification of forest biomass on enzymatic hydrolysis,” Bioresource Technology 102(19), 9083-9089.
Article submitted: April 19, 2014; Peer review completed: May 31, 2014; Revised version received and accepted: June 3, 2014; Published: June 18, 2014.
