Abstract
Furans are high value-added biomass-derived chemicals that can be used to replace petrochemicals. In this study, sulfated solid acid catalysts were prepared by precipitation and impregnation and were used for the conversion of a cellulosic pulp sheet into furans. The physicochemical properties of the prepared sulfated solid acid with different calcination temperatures and different mol ratios of Ti-Al were characterized using XRD, elemental analysis, TG, and NH3-TPD. Furthermore, the effects of various processing parameters such as temperature, time, and catalyst dosage on the reaction performance were studied. The combined yield of 5-hydroxymethyl-furfural and furfural reached 8.9% and 4.5% of pulp sheet mass with a 5% dosage of SO42-/TiO2-Al2O3 catalyst at 220 °C for 30 min. The activity for recovered catalyst was also investigated in this study.
Download PDF
Full Article
Production of furans from pulp sheet over sulfated solid acid catalysts
Hongdan Zhang, Shubin Wu,* Jun Zhang, and Bo Li
Furans are high value-added biomass-derived chemicals that can be used to replace petrochemicals. In this study, sulfated solid acid catalysts were prepared by precipitation and impregnation and were used for the conversion of a cellulosic pulp sheet into furans. The physicochemical properties of the prepared sulfated solid acid with different calcination temperatures and different mol ratios of Ti-Al were characterized using XRD, elemental analysis, TG, and NH3-TPD. Furthermore, the effects of various processing parameters such as temperature, time, and catalyst dosage on the reaction performance were studied. The combined yield of 5-hydroxymethyl-furfural and furfural reached 8.9% and 4.5% of pulp sheet mass with a 5% dosage of SO42-/TiO2-Al2O3 catalyst at 220 oC for 30 min. The activity for recovered catalyst was also investigated in this study.
Keywords: Pulp sheet; Sulfated solid acid; 5-Hydroxymethylfurfural; Furfural
Contact information: State Key Laboratory of Pulp and Paper Engineering, South China University of Technology, Guangzhou, Guangdong, 510640, China; * Corresponding author: shubinwu@scut.edu.cn
INTRODUCTION
Nowadays, with the gradual depletion of fossil resources, abundant renewable biomass is regarded as a promising alternative to non-renewable natural resources for sustainable production of biofuels and biochemicals in the future (Huber et al. 2006; Christensen et al. 2008; Takagaki et al. 2008; Almeida et al. 2010). It is well known that furan compounds are valuable platform chemicals due to their extensive applications (Huber et al. 2005; Chheda et al. 2007). Presently, researchers are attempting to investigate various pathways for the production of 5-hydroxymethylfurfural (HMF) and furfural with different raw materials and efficient catalysts.
Typically, the materials for the production of HMF and furfural mainly are sugar-based (fructose, glucose, sucrose, and xylose) or carbohydrate-based (cellulose, xylan, and lignocellulosic) feedstocks. For glucose, an HMF yield of 22% was achieved in 10 min at 160 oC under microwave heating (Yang et al. 2012). The production of HMF and furfural with monosaccharides as reactants under biphasic reaction systems was studied, leading to selectivity of 89%, 91%, and 53% for the dehydration of fructose, xylose, and glucose, respectively (Chheda et al.2007). Cassava waste with a sulfonated carbon-based catalyst at a temperature of 250 oC was investigated, reaching 10.8% HMF and 2.1% furfural (Daengprasert et al. 2011). The conversion of biomass resources to HMF and furfural can be accomplished in two main steps: (1) hydrolysis of biomass resources into xylose and glucose; (2) dehydration of the xylose into furfural and the glucose into HMF, including isomerization to fructose during the conversion of glucose to HMF. Both the hydrolysis and dehydration reactions could be catalyzed by acid; as a result many acid catalysts have been used for the conversion of biomass resources into HMF and furfural.
In earlier studies, homogeneous acids such as H2SO4 and HCl were used for biomass conversion, and it is clear that the addition of these acid-catalysts can promote the dehydration reaction (Tuercke et al. 2009). With the development of green chemical processes, however, the utilization of heterogeneous catalysts, especially solid acid, is one of the effective approaches for both biomass conversion and maintaining green chemical processing conditions (Beach et al. 2009; Anastas and Eghbali 2010; Gupta et al. 2010; Gu et al. 2010). Solid acid catalyst has been widely reported to be easily separated and recovered from the process. Recently, a few studies have proposed the use of solid acid catalysts for the dehydration reaction. For instance, it was demonstrated that the production of HMF and furfural from monomeric sugars in the HCW process can be promoted by the use of TiO2, ZrO2, and mixed-oxide TiO2–ZrO2 catalysts under hot compressed water (HCW) conditions (Chareonlimkul et al. 2010). A series of Al–Zr mixed oxides with different molar ratios with glucose that were reacted in hot compressed water at 180 oC showed that the glucose conversion increased from 46.6 to 85% (Zeng et al. 2009).
Though there have been many studies on the production of HMF and furfural, few studies have focused on the use of a sulfated metal oxides solid catalyst, one kind of heterogeneous catalyst, for lignocellulosic biomass conversion. In this study, a SO42-/TiO2-Al2O3 catalyst prepared by precipitation and impregnation was employed for the production of HMF and furfural from a pulp sheet, which was selected due to its low price and broad source. For the SO42-/TiO2-Al2O3 catalyst, different mol ratios of Ti-Al and different calcination temperatures for the production of HMF and furfural were studied. The suitable temperature, reaction time, and the dosage of catalyst for the production of HMF, furfural, and sugar yield were also determined. Furthermore, the activity for recovered catalyst was also investigated in this study.
EXPERIMENTAL
Materials
The raw material (a pulp sheet made from a sulfate process of lobular acacia wood) used in this study was provided by a paper mill in Guangzhou, Guangdong, China. The dry pulp sheet was immersed in deionized water for 24 h, where it was stirred with a stirring rod to disperse the fiber bundles, then stored in a refrigerator until further use. The pulp sheet was composed of glucan 80.56% and xylan 17.63% (dry weight basis).
Preparation of Catalysts
SO42-/TiO2-Al2O3 catalysts were prepared by precipitation and impregnation. The main preparation process of the SO42-/TiO2 catalyst is presented below. TiCl4 was dissolved in icy deionized water, and ammonia solution with 25% mass fraction was added to adjust the pH value of solution to between 9 and 10. The mixed solution was then aged for 24 h to form Ti(OH)4. The obtained precipitate was thoroughly washed with deionized water until chloride was not detected by AgNO3, and then the precipitate was dried at 110 oC for 24 h. The dried precipitate was ground to below 60 mesh followed by impregnation in a 0.5 mol/L H2SO4 solution and stirred at 500 rpm for 1 h. The obtained precipitated solid was filtered, subsequently dried at 110 oC for 12 h, and finally calcined at a specified temperature for 3 h to obtain SO42-/TiO2. For the preparation of the SO42-/ TiO2-Al2O3 catalyst, the co-precipitates of Ti(OH)4– Al(OH)3 with different Ti to Al mol ratios were obtained by adding ammonia solution with 25% mass fraction to a mixed aqueous solution of TiCl4 and Al2(SO4)3 with stirring until the pH value was between 9 and 10. The subsequent procedures were the same as for the preparation of SO42-/TiO2.
Characterization of Catalyst
X-ray powder diffraction (XRD) was performed in a Bruker D8 Advance diffract-tometer with Cu K radiation. The operating voltage and current were 40 kV and 40 mA, respectively. The step length was 0.02 degrees with a scanning rate of 2°min-1. TG analysis was performed in a TG 209 F1 (made by NETZSCH) with N2 used as the carrier gas, a temperature range of room temperature to 850 oC, and a temperature rise rate of 10 oC/min. The sulfur content of the catalyst was detected by an Elementar Vario EL according to the general method of element analysis (JY/T017-1996). The measurements of acidity of the samples were carried out in a Micromeritics AutochemII 2920 chemi-sorption analyzer following an NH3 temperature-programmed desorption (TPD) method. The sample was heated up to 600 oC and kept for 30 min in a flow of He gas to remove adsorbed species on the surface. Then the sample was cooled down to 100 oC in He flow, followed by adsorption of NH3 in 10% NH3 gas flow for 1 h. After flushing with He for 1 h to remove physically adsorbed NH3, the TPD data were measured from 100 oC to 600 oC with a ramp of 15 oC/min.
Methods
The conversion of the pulp sheet was carried out using an apparatus consisting of a 1L SS 316 stainless steel reactor, an electric heater, an agitator, and a temperature controller. The temperature and agitation speed during the hydrolysis was controlled and monitored by a modular controller (Cara et al. 2008). The amount of dry feedstock loaded was 18 g, and water was added at a solid/liquid ratio of 3/100 (w/v). Agitation was set at 500 rpm. Firstly, a comparison of catalyst activity of SO42-/TiO2-Al2O3 with different quantities of Al was made to determine the optimum addition of Al. Based on this approach, a suitable calcination temperature (400, 500, 600, and 700 oC) of SO42-/TiO2-Al2O3 was selected. Then the optimum conditions for pulp sheet conversion were pursued. The effects of reaction temperature (180, 200, 220, and 240 oC), reaction time (0, 15, 30, 45, and 60 min), and the dosage of catalyst (0, 5%, 10%, 20%, and 30%) were determined. Lastly, the activity for recovered SO42-/TiO2-Al2O3 catalyst was also studied. It should be noted that the reaction time of 0 min refers to the time at which the system reached the desired temperature after placing the reactor into the electric heater. When the hydrolysis was finished, the reactor was immediately removed from the heating jacket and then cooled with cooling water to stop the reaction. The residue was then separated by filtration. The liquid fraction was analyzed by high pressure liquid chromatography (HPLC) and ion chromatography (IC) to determine the concentration of HMF, furfural, glucose, xylose, and fructose.
Product Analysis
All liquid products from the hydrolysis tests were filtered using a 0.22 µm filter and then diluted appropriately with deionized water. The concentrations of glucose, xylose, and fructose were quantitatively analyzed at 30 oC by ion chromatography (Dionex ICS-3000) with a CarboPacTM PA1 column. The eluents were NaOH and CH3COONa with a flow rate of 0.25 mL/min. The sample loop had a volume of 50 L, and the column temperature was 30 oC. The quantification of HMF and furfural in the liquid product was analyzed at 30 oC using a high performance liquid chromatography (HPLC) system with a C18 column. Methanol and water (30/70 v/v) were used as the eluents at a flow rate of 1.0 mL/min. The concentrations of HMF and furfural in the liquid reaction product (20 μL of injection volume) were analyzed based on a photodiode array detector (DAD) at 284 nm and 277 nm, respectively.
The product yield on a dry weight basis (DWB) was calculated using the following equation:
YieldDWB(%) = (weight of product formed / dry weight of substrate utilized)*100% (1)
RESULTS AND DISCUSSION
Catalyst Characterization
XRD test
Phase identification of the prepared catalysts was performed by XRD, as shown in Fig. 1a. For the SO42-/TiO2 samples, several peaks appeared at 2 = 25.4, 38.0, 48.1, 55.0, and 62.6, which indicated the formation of an anatase phase. The catalyst SO42-/TiO2-Al2O3 with a high concentration of TiO2 (Ti/Al = 9/1 or 7/3) had similar peaks as pure SO42-/TiO2, but the intensity of the related peaks decreased with decreasing titanium concentration; at the same time, the widths of the peaks increased.
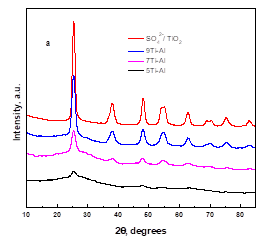
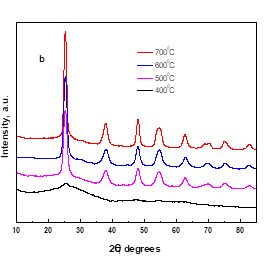
Fig. 1. a. XRD patterns of SO42-/TiO2-Al2O3 catalysts with different Ti / Al molar ratios, b. Effect of calcination temperature on the XRD patterns of SO42-/TiO2-Al2O3 catalyst
The SO42-/TiO2-Al2O3 catalysts with a Ti/Al mol ratio of 5/5 were amorphous as can be seen from the XRD patterns. This was probably due to the presence of Al2O3 in the samples, which could effectively suppress the growth of crystallites.
XRD studies of the SO42-/TiO2-Al2O3 catalysts (9Ti/Al) with different calcination temperatures were undertaken. As the XRD lines in Fig. 1b showed, when the calcination temperature was 400 oC, the SO42-/TiO2-Al2O3 catalyst was almost amorphous. When the calcination temperature reached 500 oC, the polymorphs of SO42-/TiO2-Al2O3 formed with characteristic peaks appearing at 2 values of 25.4, 38.0, 48.1, 55.0, and 62.6. Then with the increase in calcination temperature, the polymorphs gradually became perfect. Calcination temperature therefore played an important part in the formation of the crystalline phase in the SO42-/TiO2-Al2O3 catalyst. However, the results obtained in the catalytic experiment demonstrated that the furans product yields were not affected by the crystallinity of these catalysts.
Sulfur content test
The sulfur contents of catalysts prepared under different conditions were detected using an Elementar Vario EL. The results are shown in Table 1. A small amount of S was detected in all samples. The S mass fraction of the SO42-/TiO2-Al2O3 catalysts at 500 oC calcination temperature was higher than that of the SO42-/TiO2 catalyst. Especially when the Ti/Al mol ratio was 9/1, the S mass fraction reached 4.0%. This phenomenon could be explained by the addition of Al, which could stabilize the S mass of the catalyst. For the SO42-/TiO2-Al2O3 catalyst(Ti/Al mol ratio of 9/1), when the calcination temperature was 400 oC, the S mass fraction reached 6.4%. With the increase in calcination temperature, the S mass fraction decreased gradually. When the calcination temperature reached 700 oC, the S mass fraction had decreased to 1.3%. It is concluded that the S mass fraction decreased because of its decomposition at higher calcination temperatures. The S content in catalyst was a reflection of the surface acidity of the catalyst (Samantaray and Parida 2001; Yadav and Nair 1999). The addition of Al could stabilize the S content in catalyst at high temperature, so the thermostability of catalyst SO42-/TiO2 was increased with the addition of Al. It is important to note that the results obtained in the catalytic experiment demonstrated that the furans product yields were higher with catalyst SO42-/TiO2-Al2O3 than the catalyst SO42-/TiO2.
Table 1. Sulfur Content of Catalysts with Different Calcination Temperatures and Different Mol Ratios of Ti/Al
TG analysis
The TG analyses of the SO42-/TiO2 catalysts and SO42-/TiO2-Al2O3 (9Ti/Al) catalysts are shown in Fig. 2. Both of the lines showed lost weight under 250 oC due to the evaporation of physically adsorbed water; the weight loss between 500 and 850 oC was caused by the decomposition of SO42-. Catalyst SO42-/TiO2 began to decompose at 539 oC, and then it stopped at 700 oC with a weight loss of 6.0%. Compared to catalyst SO42-/TiO2, catalyst SO42-/TiO2-Al2O3 decomposed from 553 oC to 850 oC, resulting in a weight loss of 7.5%. This phenomenon could be explained by the fact that with the addition of Al, the S of the catalyst became more stable at high temperature and the decomposition temperature was higher. From the weight loss of S, it could be concluded that the S mass fraction of catalyst SO42-/TiO2-Al2O3 was higher than that of catalyst SO42-/TiO2, which agreed with the sulfur content test mentioned above.
Fig. 2. TG analysis of the SO42-/TiO2 catalysts and SO42-/TiO2-Al2O3 catalysts
NH3-TPD measurement
Fig. 3. NH3-TPD profiles and the corresponding results of acid amount for catalyst
The acidic properties and catalytic activity of sulphated oxides are dependent on sulfate groups and their intrinsic properties. The TPD profiles of desorbed ammonia (NH3) on samples of catalyst and the corresponding results of acid amount as well as peak temperatures are listed in Fig. 3. As is well known, the desorption temperature indicated the acid strength of the catalyst; that is to say, the higher the temperature of desorption, the stronger the acid strength. The peak temperature of catalyst SO42-/TiO2-Al2O3 was higher than catalyst SO42-/TiO2. And it was also found that the acidity of catalyst SO42-/TiO2-Al2O3 calcinated at 500 oC was more than catalyst SO42-/TiO2. On this basis it can be concluded that acidic activity of catalyst SO42-/TiO2-Al2O3 was more effective for the conversion of pulp sheet to furans, which was in agreement with further conversion results. When the catalyst was calcinated at 700 oC, the acid amount decreased to 0.3 mmol/g because of the loss of sulfur. As shown in the sulfur content test, the sulfur content of catalyst SO42-/TiO2-Al2O3 (9Ti-Al) calcinated at 700 oC decreased from 6.4% (calcinated at 400 oC) to 1.3%, which verified the possible loss of the sulfur in the catalyst.
Effect of Different Ti/Al Mol Ratio of SO42-/TiO2-Al2O3 Catalyst
This research was carried out to determine the activity of SO42-/TiO2 and SO42-/ TiO2-Al2O3 catalysts with different mol ratios of Ti/Al. Firstly, the SO42-/TiO2 catalyst and the 9/1, 7/3, and 5/5 Ti/Al mol ratios in the SO42-/TiO2-Al2O3 catalysts were made and calcinated at 500 oC for 3h. The reactions were then carried out at 220 oC for 30 min with a 10% dosage of the catalyst. As clearly illustrated in Fig. 4, the yields of HMF and furfural increased with catalyst SO42-/TiO2-Al2O3. That is to say that catalyst SO42-/TiO2-Al2O3 was more effective than catalyst SO42-/TiO2 during the conversion of the pulp sheet biomass.
Fig. 4. Effect of SO42-/TiO2-Al2O3 catalysts with different mol ratios of Ti/Al on 5-HMF yield and furfural yield
With the addition of Al, the sulfur content improved (Guan et al. 2005). When the mole ratio of Ti/Al in the SO42-/TiO2-Al2O3 catalyst was 9/1, the product reached the highest with 9.0 % HMF yield and 4.0 % furfural yield. When the Al loading increased, the yield of furans decreased gradually. Therefore, the SO42-/TiO2-Al2O3 catalyst with a Ti/Al mol ratio of 9/1 was used for further study.
Effect of Calcination Temperature of Catalyst SO42-/TiO2-Al2O3
For solid acid catalysts, the raw material form, calcination time, calcination temperature, acid type, and acid density are all influencing factors on the catalysts’ performance. The most important factor, however, is calcination temperature, so its effect on the solid acid catalyst in the range of 400 oC to 700 oC was studied for the conversion of the pulp sheet with a 9/1 mol ratio of Ti/Al in the SO42-/TiO2-Al2O3 catalyst at 220 oC and 30 min. The results in Fig. 5 indicated that in the process with catalyst SO42-/TiO2-Al2O3 calcinated at 500 oC, the HMF and furfural yields reached the highest values, and then they decreased gradually when the calcination temperature reached 600 oC and above. This phenomenon implies that at a certain calcination temperature, TiO2-Al2O3 achieves a structure and composition favorable for the hydrolysis of biomass conversion, and the catalyst loses activity rapidly with further increase of the calcination temperature.
Fig. 5. Effect of SO42-/TiO2– Al2O3 catalysts with different calcination temperatures on 5-HMF and furfural yields
Determination of Suitable Conditions for Pulp Sheet Conversion
Effect of temperature
The effect of reaction temperature was investigated in the range of 180 to 240 oC for the conversion of the pulp sheet in hot compressed water with a 9/1 mol ratio of Ti/Al in the SO42-/TiO2-Al2O3 catalyst (at 30 min and with a catalyst loading of 0.1 g/1.0 g material). As shown in Table 2, the main reaction products were HMF, furfural, glucose, xylose, and a little fructose. When considering the sugar products (glucose, xylose, and fructose), the yield of glucose increased with the increasing temperature, reaching 9.5% on DWB at 220 oC, while on the other hand, the xylose yield in the reaction product was found to decrease with increasing temperature; that is to say, more xylose was transformed to furfural. Fructose, the isomer of glucose, first reached the highest yield at 200 oC, and then it decreased with the increasing temperature due to its conversion to HMF at 220 oC. The total products also reached the highest yield of 22.9% on DWB at 220 oC, and then decreased with increasing temperature. All sugar products could not be observed at the reaction temperature of 240 oC. Figure 6 indicates that the conversion of material increased rapidly with increase in temperature. For HMF and furfural, the yields increased with the increasing reaction temperature from 180 to 220 oC, reaching the highest yields of 9.0% and 4.0% on DWB, respectively. When the reaction time reached 240 oC, the yield of levulinic acid was 5.4% on DWB. At higher temperature, however, the HMF and furfural yields decreased because of further decomposition to other byproducts such as LA, formic acid, gas products, or condensation products (Asghari and Yoshida 2006;Daengprasert et al. 2011).
Table 2. The Conversion and Yields of HMF, Furfural, and Sugars at Different Reaction Temperatures
nd: not detected; Conversion(%) = (dry weight of substrate utilized – dry weight of residue) / ( dry weight of substrate utilized)*100 (%)
Fig. 6. Effect of temperature on conversion, HMF, and furfural yield at 30 min, with the dose of 10% SO42-/ TiO2-Al2O3 catalyst
Effect of Reaction Time
The effect of reaction time between 0 and 60 min on the production yields was determined for the reaction with 0.1 g catalyst/1.0 g material at 220 oC. The results shown in Table 3 indicate that the glucose yield reached the highest level of 10.1% on DWB and then decreased with time. Further increase in reaction time brought about a slight decrease in the xylose and fructose yields. Figure 7 shows the conversion of the pulp sheet and the yields of HMF and furfural at different reaction times at 220 oC. It can be seen that the conversion of material could amount to 82.3% only after 30 min of reacting, indicating that the catalyst is highly active for the dehydration of the pulp sheet. A gradual increase was observed between 0 and 30 min for HMF yield obtained from the pulp sheet. After 30 min, the HMF obtained from the pulp sheet reached its highest yield of 9.0% on DWB.
Table 3. The Conversion and Yields of HMF, Furfural, and Sugars at Different Reaction Temperatures
Fig. 7. Effect of reaction time on conversion and HMF and furfural yields at a temperature of 220 oC with a 10% dosage of SO42-/ TiO2– Al2O3 catalyst
The furfural reached its highest yield of 4.4% on DWB at 0 min and remained around 4.0% between 15 min and 30 min of reaction time, then decreased gradually at 45 min and beyond, possibly due to the decomposition of HMF and furfural to other products (Watanabe et. al 2005). Of the reaction times studied, 30 min was found to be the optimal time for the formation of HMF and furfural; a further increase in reaction time to 45 min and above resulted in a decrease in the furans and sugars yields obtained from the pulp sheet.
Effect of dose of SO42-/TiO2-Al2O3 solid catalyst.
The suitable quantity of SO42-/TiO2-Al2O3 catalyst was determined from the yield of HMF, furfural, and sugar products, in which the reaction of the pulp (at 3 wt % of material) was carried out at 220 oC for 30 min with various catalyst amounts (no catalyst, 5%, 10%, 20%, and 30% of material). The influence of the catalyst dose is shown in Table 4 and Fig. 8. The data show that changes in the dose of the catalysts strongly affected the furans and sugars yields obtained from the pulp. Without the catalyst, the HMF and furfural yields were only 4.4% and 3.8% on DWB, respectively. When the quantity of catalyst was 5%, the HMF and furfural yields increased from 4.4% to 8.9% and from 3.8% to 4.5% on DWB, respectively. Thus, for the process with a catalyst, the production yields were considerably higher than those obtained without the catalyst. When the dose of catalyst was increased from 5% to 10%, the conversion of the pulp increased from 70.7% to 82.3%, but the yields of furfural, glucose, xylose, and fructose decreased. That is to say, the selectivity of the HMF, furfural, and sugar products are higher with a 5% than with a 10% dose of catalyst. For HMF, though, the yield (8.9% on DWB) with 5% dose was a little lower than the yield (9.0% on DWB) with a dose of 10%; the furfural and sugar products achieved the highest yields with a dosage of 5%. The product yields then decreased with an increase in the mass of the catalyst. A further increase in the dosage of the catalyst up to 30% of material showed no positive effect. Above all, a catalyst dosage of 5% was chosen as the most suitable condition.
Table 4. The Conversion and HMF, Furfural, and Sugars Yields with Different Dosages of Catalyst
The Activity of Recovered Catalyst
Recyclability and stability are advantages of solid catalysts, and many particular processes rely on catalysts based on them. After the first reaction was finished, the SO42-/ TiO2– Al2O3catalyst was separated from other residue and then calcinated at 500 oC for 3h to regenerate the activity (Sun et al. 2010). Then it was reused in the next experiments under the same reaction conditions.
Fig. 8. Effect of catalyst dosage on conversion and HMF and furfural yields at 220 oC, 30 min with SO42-/ TiO2-Al2O3 catalyst
The results in Table 5 show the four times repeated reactions of SO42-/TiO2-Al2O3 catalyst. It indicated that the secondary use of catalyst decreased more than half of the HMF yield, from 8.9% to 3.9% on DWB, then decreased gradually for the next cycles. For furfural, the yield decreased slowly with each cycle. Some explanations for the decrease of product yield was that the sulfur in the catalyst was lost gradually by solvation or the acid sites became covered by products during the hydrolysis reaction (Peng et al. 2011). Catalytic activity can be restored to a certain extent by calcination. Accordingly, how to improve the long-term stability and high activity of the solid acid catalyst need future exploration.
Table 5. The Activity for Recovered Catalyst
CONCLUSIONS
- SO42-/TiO2-Al2O3 catalysts were prepared by precipitation and impregnation with different Ti/Al mol ratios. The products were characterized and applied to catalyze pulp sheet hydrolysis and dehydration.
- SO42-/TiO2-Al2O3 catalysts with a 9/1 mol ratio of Ti/Al with different calcination temperatures were characterized and applied to catalyze pulp sheet hydrolysis and dehydration.
- Characterization and activities of the SO42-/ TiO2-Al2O3 catalyst with a 9/1 mol ratio of Ti/Al at a 500 oC calcination temperature were examined. The SO42-/TiO2-Al2O3 catalyst was determined as a feasible alternative for pulp sheet biomass conversion to HMF and furfural.
- The total yield of the product reached 26.59% on DWB and the yields of HMF and furfural were 8.9% and 4.5% on DWB, respectively, at 220 oC for 30 min with a 5% dosage of catalyst.
- Studies on recovered catalyst indicated that the catalytic activity of SO42-/TiO2-Al2O3 catalysts still needed to be enhanced.
ACKNOWLEDGMENTS
This work was supported by the National High Technology Research and Development Program of China (No. 2012AA101806), the Science and Technology Department of Guangdong Province, China (No. 2010y1-C071), the National Natural Science Foundation of China (No. 21176095), and the Major Research Projects of Guangdong Province, China (No. 2011A090200006).
REFERENCES CITED
Almeida, R. M., Li, J., Nederlof, C., Makkee, M., and Moulijn, J. (2010). “Cellulose conversion to isosorbide in molten salt hydrate media,” ChemSusChem 3(3), 325-328.
Anastas, P., and Eghbali, N. (2010). “Green chemistry: Principles and practice,” Chem. Soc. Rev. 39(1), 301-312.
Asghari, F. S., Yoshida, H. (2006). “Acid-catalyzed production of 5-hydroxymethyl-fur-fural from D-fructose in subcritical water,” Ind. Eng. Chem. Res. 45(7), 2163-2173.
Beach, E. S., Cui, A., and Anastas, P. T. (2009). “Green chemistry: A design framework for sustainability,” Energy Environ. Sci. 2(10), 1038-1049.
Cara,C., Ruiz, E., Oliva, J. M., Saez, F., and Castro, E. (2008). “Conversion of olive tree biomass into fermentable sugars by dilute acid pretreatment and enzymatic saccharification,” Bioresour. Technol. 99(6), 1869-1876.
Chareonlimkul, A., Champreda, V., Shotipruk, A., and Laosiripojana, N. (2010). “Catalytic conversion of sugarcane bagasse, rice husk and corncob in the presence of TiO2, ZrO2 and mixed-oxide TiO2-ZrO2 under hotcompressed water (HCW) condition,” Bioresour. Technol. 101(11), 4179-4186.
Chheda, J. N., and Dumesic, J. A. (2007). “An overview of dehydration, aldol-conden-sation and hydrogenation processes for production of liquid alkanes from biomass-derived carbohydrates,” Catal. Today 123(1-4), 59-70.
Chheda, J. N., Roman-Leshkov, Y., and Dumesic, J. A. (2007). “Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono- and poly-saccharides,” Green Chem. 9(4), 342-350.
Christensen, C. H., Rass-Hansen, J., Marsden, C. C., Taarning, E., and Egeblad, K. (2008). “The Renewable Chemicals Industry,” ChemSusChem 1(4), 283-289.
Daengprasert, W., Boonnoun, P., Laosiripojana, N., Goto, M., and Shotipruk A. (2011). “Application of sulfonated carbon-based catalyst for solvothermal conversion of cassava waste to hydroxymethylfurfural and furfural,” Ind. Eng. Chem. Res 50(13), 7903-7910.
Guan, G. F., Tan, Q., and Wan, H. (2005). “Characterization of SO42-/ TiO2-Al2O3 solid acid catalyst and its catalytic activity in tetraoctyl pyromelliate,” Synthesis Petro. Chemi. Technol.34(7), 643-647.
Gupta, M., Paul, S., and Gupta, R. (2010). “General aspects of 12 basic principles of green chemistry with applications,” Curr. Sci. 99(10), 1341-1360.
Gu, Y. L., and Jerome, F. (2010). “Glycerol as a sustainable solvent for green chemistry,” Green Chem. 12(7), 1127-1138.
Huber, G. W., Iborra, S., and Corma, A. (2006). “Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering,” Chem. Rev. 106(9), 4044-4098.
Huber, G. W., Chheda, J. N., Barrett, C. J., and Dumestic, J. A. (2005). “Production of liquid alkanes by aqueous-phase processing of biomass-derived carbohydrates,” Science 308(5727), 1446-1450.
Peng, L., Lin, L., Li, H., and Yang, Q. L. (2011), “Conversion of carbohydrates biomass into levulinate esters using heterogeneous catalysts,” Appl. Energ. 88(12), 4590-4596.
Samantaray, S. K., and Parida, K. M. (2001), “SO42 −/TiO2-SiO2 mixed oxide catalyst. 2. Effect of the fluoride ion and calcination temperature on esterification of acetic acid,” Appl. Catal. A – Gen. 211(2), 175-187.
Sun, H., Ding, Y. Q., and Duan, J. Z. (2010), “Transesterification of sunflower oil to biodiesel on ZrO2 supported La2O3 catalyst,” Bioresour. Technol. 101(3), 953-958.
Takagaki, A., Tagusagawa, C., and Domen, K. (2008). “Glucose production from saccharides using layered transition metal oxide and exfoliated nanosheets as a water-tolerant solid acid catalyst,” Chem. Commun. (42), 5363-5365.
Tuercke, T., Panic, S., and Loebbecke, S. (2009). “Microreactor process for the optim-ized synthesis of 5-hydroxymethylfurfural: A promising building block obtained by catalytic dehydration of fructose,” Chem. Eng. Technol. 32(11), 1815-1822.
Watanabe, M., Aizawa, Y., Iida, T., Aida, T. M., Levy, C., Sue, K., and Inomata, H. (2005). “Glucose reactions with acid and base catalysts in hot compressed water at 473 K,” Carbohydr. Res. 340(12), 1925-1930.
Yadav, G. D., and Nair, J. J. (1999), “Sulfated zirconia and its modified versions as promising catalysts for industrial processes,” Micropor. Mesopor. Mat. 33(1-3), 1-48.
Yang, Y., Hu, C., and AbuOmar, M. M. ( 2012). “Conversion of carbohydrates and lignocellulosic biomass into 5-hydroxymethylfurfural using AlCl3·6H2O catalyst in a biphasic solvent system,” Green Chem. 14(2), 509-513.
Zeng, W., Cheng, D., Chen, F., and Zhan, X. (2009), “Catalytic conversion of glucose on Al–Zr mixed oxides in hot compressed water,” Catal. Lett. 133(1-2), 221-226.
Article submitted: May 19, 2012; Peer review completed: July 11, 2012; Revised version received and accepted: July 23, 2012; Published: August 6, 2012.
