Abstract
Camelina sativa is a cool-season oil seed crop that has been proven to produce various biofuels. The present study investigated the technical possibilities of using whole camelina biomass as a model feedstock in a biorefinery. This investigation examined the possibilities of using camelina seeds as a source of oil for biodiesel, sugars for ethanol, and meal for one-portfolio products. The camelina harvest residues (straw) can serve as the main source for green sugars. This study found that the energy input for the whole biorefinery process was 25.1 MJ/L ethanol, while the energy output was 54.3 MJ/L ethanol. The net energy ratio of 2.16 MJ/L ethanol was found to be competitive with other energy crops. The process was environmentally friendly, and it reduced greenhouse gas emissions by 40% if the produced biodiesel replaced petroleum diesel. The seed meals and glycerin were found to be a good source of revenue as high value-added products and can provide an additional revenue of $1/kg of produced oil.
Download PDF
Full Article
Production of Multiple Biofuels from Whole Camelina Material: A Renewable Energy Crop
Balsam T. Mohammad,a Mohammad Al-Shannag,b Mohammad Alnaief,a Lakhveer Singh,c Eric Singsaas,d and Malek Alkasrawi e,*
Camelina sativa is a cool-season oil seed crop that has been proven to produce various biofuels. The present study investigated the technical possibilities of using whole camelina biomass as a model feedstock in a biorefinery. This investigation examined the possibilities of using camelina seeds as a source of oil for biodiesel, sugars for ethanol, and meal for one-portfolio products. The camelina harvest residues (straw) can serve as the main source for green sugars. This study found that the energy input for the whole biorefinery process was 25.1 MJ/L ethanol, while the energy output was 54.3 MJ/L ethanol. The net energy ratio of 2.16 MJ/L ethanol was found to be competitive with other energy crops. The process was environmentally friendly, and it reduced greenhouse gas emissions by 40% if the produced biodiesel replaced petroleum diesel. The seed meals and glycerin were found to be a good source of revenue as high value-added products and can provide an additional revenue of $1/kg of produced oil.
Keywords: Camelina seed; Meal; Enzymatic hydrolysis; Biodiesel; Net energy ratio; Bioethanol
Contact information: a: Pharmaceutical-Chemical Engineering Department, German Jordanian University, Amman 11180 Jordan; b: Department of Chemical Engineering, The University of Jordan, 11942 Amman, Jordan; c: Faculty of Engineering Technology, Universiti Malaysia Pahang, Pahang, Malaysia; d: Natural Resources Research Institute, University of Minnesota Duluth, Duluth, MN, USA; e: Department of PS & Chemical Engineering, University of Wisconsin Stevens Point, Stevens Point, WI, USA; *Corresponding author: Malek.Alkasrawi@uwsp.edu
INTRODUCTION
Biofuels (mainly ethanol) derived from lignocellulosic materials (Alkasrawi et al. 2013), industrial waste (Gurram et al. 2015; Alkasrawi et al. 2016), agricultural waste (Elum et al. 2017), and energy crops (Pimentel and Patzek 2005) have been receiving a great amount of attention. Energy crops, such as sugar beets, sugar cane, corn, and sweet sorghum, are vital resources for fuel today and in the future. Although there is a debate concerning the use of some crops for food versus for biofuel (Young 2009), energy crops are considered major players in biofuel production. These energy crops include sugar cane in Brazil and corn in the U.S. (Enciso et al. 2016).
Many studies have investigated various types of energy crops as alternatives to petroleum fuel. One interesting and promising crop is Camelina sativa with its rich content of oil and high yield of harvest residues (straw). Keske et al. (2013) modeled the economic feasibility of growing the C. sativa oil seed crop in the Western U.S. to produce a value-added protein feed supplement and biofuel. Moreover, Ciubota-Rosie et al. (2013) performed a detailed study of C. sativa characteristics to fully evaluate its potential as a biofuel source.
Camelina can be grown in a variety of climatic and soil conditions. It has many agronomic advantages, such as a short growing season and a tolerance for cold weather, droughts, semiarid conditions, and low-fertility or saline soils Moser (2012). Additionally, it has lower water, pesticide, and fertilizer needs compared with other oil seed crops (Ciubota-Rosie et al. 2013).
The water-deprived regions of the Western U.S. and colder climates of the upper Midwestern U.S. create a perfect niche for farmers to utilize camelina as a rotation crop. Camelina fits into the Western U.S. crop rotation of wheat, followed by corn and fallow (Keske et al.2013). Currently, the farmers of the Midwest grow extensive amounts of corn and rotate with soybeans that are later marketed for vegetable oil or biodiesel. If utilized as a commodity for both biodiesel and protein meal for livestock, camelina provides farms and communities with a source of economic diversification. Some estimates indicate that the U.S. state of Montana alone could support between 0.8 million hectares and 1.2 million hectares of camelina per year Moser (2012).
The present study investigated the technical possibility of producing ethanol and biodiesel from the whole camelina crops (The straw and the seeds). The study was divided into two different stages. The first stage investigated (lab scale experiments) the optimal conditions for sugar production and evaluated their potential for fermentation into ethanol as well as biodiesel production. In the second stage, a technical and economic evaluation was performed by rigorous process simulation using AspenPLUS software for mass and energy calculation. Ultimately, the whole crop biorefinery concept promoted the first-generation biofuels to be economic competitive with second-generation biofuels pathways with respect to energy yield and GHG reduction.
EXPERIMENTAL
Chemical Composition Analysis
The chemical compositions of both the seeds and straw were analyzed. Montana State University Northern provided the C. sativaseeds and straw substrate (Havre, MT). Raw seeds and straw were stored at room temperature in sealed plastic bags. The moisture contents for both the seeds and straw were determined by a standard gravimetric method by placing the samples in a pre-weighed moisture bottle (pre-dried in an oven at 100 °C for 30 min) (Gurram et al. 2016). Samples were then kept in an oven at 105 °C overnight. After cooling to room temperature, the bottles were weighed again to determine the moisture content.
The sugar and lignin composition of the straw, seed, and meal were estimated according to a method developed by the National Renewable Energy Laboratory (NREL). A dried oven sample of 0.3 g added to 3 mL 72% (w/v) sulfuric acid and diluted using distilled water to 87 mL. The solution was mixed well and kept at 30 °C in a water bath for 1 h. The material was autoclaved at 121 °C for 1 h to hydrolyze all sugars from cellulose and hemicellulose part. The solid residue after hydrolysis was filtered, washed and dried for lignin and ash determination.
The carbohydrate contents of the seeds and straw were determined according to the National Renewable Lab method (Sluiter et al.2010), which was followed by analysis with a Dionex ICS 3000 and 4 mm x 250 mm Carbopac PA1 column ion chromatograph (Thermo Scientific Waltham, MA, USA).
The oil yield was determined after weighing the oil produced after the filter press. The protein content was measured using the Kjeldahl method (Horwitzs 1975) by using a nitrogen-to-protein conversion factor of 6.25.
Sugar Production Platform
Sugar exists in whole camelina crops as free soluble sugars as part of the seed or as a main structural component in the form of cellulose and hemicellulose in the straw.
In the sugar production platform, several lab-testing methods were proposed to produce various sugars from the whole camelina crop. The free sugars in the seeds were obtained by treatment with water and weak acid, while the structural sugars in the straw were extracted via chemical, thermal, and biological methods.
Extraction of the Soluble (Non-structural) Sugars from the Plant Seeds
Soxhlet extraction is an efficient technique for soluble sugar extraction and provides a high yield recovery (Hymowitz et al. 1972).In this study, 2 g of camelina raw seeds or pressed meal were placed in a Soxhlet apparatus (TICH Scientific, Orlando, FL USA) for 4 h using 200 mL of either acetic or formic acid as the main solvent. Various concentrations of the solvents were used to determine the optimum solvent concentration and type. The concentrations of acetic acid were 1%, 5%, 10%, and 15%, while for formic acid they were 1%, 5%, and 10%. The extractions were done as follows. Two grams of camelina seed substrate (or meal) were put into a 30 mm x 100 mm Whatman glass Microfibre filter (Sigma Aldrich, Orion Township, MI, USA), which was then placed into a 45/50 mm Soxhlet chamber (TICH Scientific, Orlando, FL, USA). This chamber was inserted on one end into a round bottom flask, while the other end was put into a 45/50 mm glass condenser. The solvent was set to a moderate boil and each extraction was run for approximately 4 h. All of the analyses were done in triplicate, and the extracted sugars were analyzed with the ionic chromatographic method described later in this paper.
Extraction of the Structural Sugars from the Straw
The structural sugars are the sugars that make up the cellulose and hemicellulose of straw. Sugar recovery from the straw was investigated with thermal pretreatment and enzymatic hydrolysis. Both methods were extensively studied with the goal of achieving high sugar yields and low production costs. The methods for enzymatic hydrolysis and thermal pretreatment were studied separately.
Thermal pretreatment
The straw specimens were ground using a Wiley Mill with a 40” sieve (TICH Scientific, Orlando, FL USA). Ten grams of biomass were run in a Parr reactor (Parr Instruments, Moline, IL, USA) at 150 °C for 16 min. Sulfuric acid was added as a catalyst at different ratios with respect to the solid load, which were 1 w/v%, 2 w/v%, and 3 w/v%. The liquid fraction was separated via filtration with Whatman filter paper (TICH Scientific, Orlando, FL USA) and then frozen for the sugar analysis. The solid residue was collected, stored, and refrigerated for later use in enzymatic hydrolysis.
Enzymatic hydrolysis
The effectiveness of various initial concentrations of the following commercial enzymes was evaluated: Novozyme Cellic(R)CTec2 cellulase, α-amylase, and amyloglucosidase. One gram of dried seed meal was crushed and placed into a 250-mL media bottle, followed with pH adjustment to 4.8 by using 0.05 M citrate buffer. Different enzyme combinations were tested as follows: 20 µL, 50 µL, 80 µL, and 100 µL of cellulase alone; 80 µL of cellulase with 50 µL of each α-amylase and amyloglucosidase in all experiments; α-amylase and 50 µL of each cellulase and amyloglucosidase; and α-amylase alone. Hydrolysis was done in sealed bottles incubated in shaking incubator for 72 h at 50 °C. Samples were withdrawn at regular intervals. Experiments were repeated in triplicate as described by Gurram et al. (2015). All enzymes were generously supplied by Novozymes A/S, Bagsvaerd, Denmark.
Fermentation
The sugar produced (soluble free sugar and structural sugar) was evaluated for ethanol production in a fermentation process. The sugar stream was diluted to the level that the yeast could tolerate (150 g/L). Industrial yeast FermPro™ (Ferm Solutions Inc., Danville, USA) was used for the ethanol fermentation experiments. One milliliter of frozen yeast concentrate was pre-cultured for 16 h at 37 °C in yeast peptone dextrose media that contained 20 g/L glucose, 20 g/L peptone, and 10 g/L yeast extract. The solid-free Pantone Plus Series (PMS) sugar solution was supplemented with the following additional nutrients according to Tanner (2007): 10 g/L KH2PO4, 20 g/L MgSO47H2O, 4 g/L CaCl22H2O, 200 mg/L ZnSO47H2O, 20 mg/L Na2MoO42H2O, 200 mg/L CoCl22H2O, 2 mg/L d-biotin, 5 mg/L p-aminobenzenoic acid, 5 mg/L Nicotinic acid, 5 mg/L calcium pantothenate, 10 mg/L pyridoxine–HCl, 5 mg/L thiamine-HCl, and 10 mg/L lactoside. Fermentation was performed in 500-mL mini bioreactors (BioBundle Applikon Biotechnology, Foster City, USA) in triplicate with a total working volume of 350 mL. The fermenter assembly with the nutrient rich sugar solution was sterilized at 121 °C for 20 min in an autoclave (Tuttnauer/Brinkmann VWR International, Arlington Heights, USA). The fermentation experiments were performed at a pH of 5.0, 37 °C, and 200 rpm with a dissolved oxygen value of 10%. Two-milliliter sample aliquots were drawn from the sample port at different intervals using sterilized syringes.
Sugar and Ethanol Determination
The sugar and ethanol concentrations were monitored with ion chromatography (Dionex ICS 3000, Thermo Scientific, Waltham, USA). The optical density was measured at 595 nm using a Thermo Scientific Evolution 605 UV/Vis Spectrophotometer to determine the cell density over the course of fermentation.
Biodiesel Production
The biodiesel was produced according to a well-established method described by Soriano and Narani (2012). One liter of camelina oil was produced by filter pressing camelina seeds and heating them to 55 °C. The catalyst was prepared with the ratios of methanol and NaOH to oil 1:30 (weight : volume) and allowing the solution to mix gently for 20 min. This led to the formation of sodium methoxide, which is a proven catalyst for biodiesel production. The prepared catalyst was added slowly to the filtered and heated oil at 55 °C and gently stirred for 1 h. The mixture was left overnight until a separation between the glycerol and biodiesel was clearly distinct. The oil was gently decanted from the glycerol, and the yield was calculated. The biodiesel washed gently and subsequently dried before estimating the final yield.
Conceptual Design of the Biorefinery Process
The configuration of the present process (Fig. 1) represents the overall biorefinery concept for the production of multiple biofuels utilizing the whole camelina crop as the main feedstock. The conceptual design was developed from several studies to provide a basis for the mass and energy balances of the process (Gurram et al.2016). The main target was to achieve production of multiple biofuels (ethanol and biodiesel) within the same process configuration.
The conceptual design of the process was based on the daily processing of 30,000 t of whole camelina crop. The assumptions of the biorefinery capacity is based on Inbicon demo commercial facility Inbicon (Larsen 2012). The seeds (10,000 t, 10% dry matter) and straw (20000 t, 50% dry matter) arrive separately, as separation is done in the field. The seeds enter a unit for oil extraction and subsequently utilized for biodiesel production. The meal (pressed seeds) is utilized as animal feed or burned to generate heat for the facility. The extracted oil was used for biodiesel production, which is implemented using a well-established method. The straw was thermally treated at 150 °C using sulfuric acid as a catalyst. After thermal pretreatment, the solid fraction is hydrolyzed enzymatically to produce sugars and mixed with the liquid fraction prior to alcoholic fermentation. Another stream of the sugar production platform (extracted from the seeds) is added to the fermentation step. The residual lignin can be burned to generate steam for use in the proposed process.
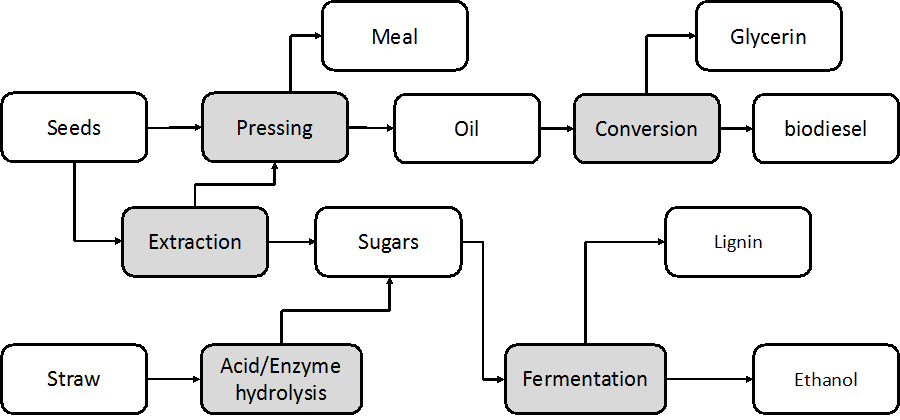
Fig. 1. Overall schematic flow sheet for the conceptual design of the biorefinery for the production of multiple biofuels and high value-added products. The conceptual design was the base case scenario for the process simulation.
Process Simulation
Based on the proposed conceptual design, the whole process was simulated in AspenPLUS v8.4, (AspenTech, Houston, TX, USA) to calculate the overall process efficiency and feasibility. The simulation methods utilized by AspenPLUS were developed by the National Renewable Laboratory (NREL) and used in a similar study by Gurram et al. (2016). The NREL method developed a physical and chemical property data bank for ethanol production from ligoncelluosic feedstock. A flow sheet was first developed in AspenPLUS according to the conceptual design proposed in Fig. 1. All of the process inputs were specficied in AspenPLUS as described in the conceptual design. The basis for the sugar yield, ethanol production, and biodisel yield was specificed in AspenPLUS according to the results obtained in the first part of this study.
RESULTS AND DISCUSSION
Chemical Composition Analysis
The whole camelina crop seed, seed meal, and straw contain a potential amount of stored sugars in the seed and structural sugar in the form of cellulose in the straw (Table 1). The seeds were about 10% (based on dry weight) free soluble sugars, which was determined by the compositional analysis. Soluble sugars are easy to extract and do not require severe chemical or thermal pretreatment. This fact has a major economic impact on process scaling because this type of process requires less energy consumption, and thus it decreases the operational costs compared with thermal and enzymatic hydrolysis. The soluble sugars in the seeds comprised 15% of the available sugar for fermentation from the whole plant (seeds and straw). Assuming that the amount of energy required to recover the soluble sugar from the seed is marginal compared with that required for thermal pretreatment of the lignocellulosic sugar (Schmer et al.2008; Kumar et al. 2009), this suggests that a 15% reduction of the required energy is possible with respect to the energy input per volume of ethanol produced (kJ/L ethanol). Table 1 could be used to determine the main product value of the crop. For example, the seeds contain a substantial amount of (30%) is very competitive when compared with other oil crops, such as rapeseed, soybean, and oil palm (Mattsson et al. 2000). This is a substantial amount of oil provide an economical feedstock to produce biodiesel for transportation.
Table 1. Dry Matter Compositional Analysis of the Whole Camelina Crop
Sugar Production Platform
Soluble sugar recovery from the seeds and meal
The highest possible yield for the sugar recovery was achieved at yield values of 30 mg/g and 34 mg/g for the seeds and meal, respectively. Both the formic and acetic acid proved to be viable and mild catalysts for lignocellulose feedstock fractionation (Schneider et al. 2016a,b; Li et al. 2017). An interesting finding was that more sugar was recovered from the meal than from the seeds. This was likely because the mechanical extraction for the oil provided a higher surface area for the solvent to recover sugars. Additionally, the oil extraction did not negatively affect sugar recovery but enhanced it. This was an interesting finding because sugar extraction requires less energy demand, and thus lowers the production costs compared with lignocellulosic feedstock (Jørgensen et al. 2007; Viikari et al. 2012). This factor will definitely have a major impact on the final production cost of biofuels derived from whole camelina crops.
Sugar production from the straw
The whole plant straw was investigated for sugar production using several scenarios. The sugar recovery from thermal decomposition using H2SO4 resulted in the hydrolysis of the hemicellulose into a liquid fraction. Several studies have also shown sulfuric acid to be a very promising catalyst with a high yield recovery (Söderström et al. 2003). The optimum sugar recovery was obtained at 1% H2SO4, 160 oC, and a total time of 16 min. This could have corresponded to 50% of the theoretical glucose. The aim was about 50% of the theoretical yield to avoid the generation of inhibitors, as the residues would be further hydrolyzed enzymatically. These results were found to be very competitive with other fraction methods reported for similar feedstocks, such as straw (Schneider et al. 2016b, 2017). The residue solid obtained after acid hydrolysis mainly contained the complex cellulose-lignin structure subjected to cellulase hydrolysis for complete cellulose recovery during enzymatic hydrolysis.
Enzymatic Hydrolysis
The highest sugar yield was obtained at an enzyme load of 80 µL after 56 h. These results corresponded to 98% of the theoretical glucose in the residues after acid pretreatment in the Parr reactor. This agreed with several other results from studies that used similar feedstock residues after acid pretreatment (Saha et al. 2005; Chen et al. 2008; Schneider et al. 2017), and it is possible for the residue to be further treated with enzymatic hydrolysis. An interesting finding was that the enzyme load was lower than values reported in previous studies. For the enzymatic hydrolysis of acid-pretreated straw, acid pretreatment in the Parr reactor facilitated the open structure of the cellulose, which led to better enzymatic accessibility. Higher conversion rates of cellulose were obtained with the pretreated residues because a larger surface of the pretreated cellulose was available for enzymatic hydrolysis.
Ethanol Production in the Fermentation Process
All of the sugars produced from the various processes were mixed and underwent alcoholic fermentation. The results of the ethanol fermentation showed that the yield was 93% of the theoretical yield. This was most likely because less inhibitors were generated during sugar production from the straw.
Low inhibitor generation is always a positive for the process because it impacts the theoretical yield of ethanol (Kotarska et al. 2015; Muktham et al. 2016). This yield was very competitive compared with similar studies performed with other energy crops. Although alcoholic fermentation is a well-established process, this test was done as a baseline for the initial parameters in the AspenPLUS simulation.
Oil Recovery and Biodiesel Production
The recovered oil from the seeds was about 30% of the dry mass and provided a substantial amount of oil for biodiesel production (Table 2). A substantial amount of biodiesel was obtained, and the remainder was glycerol, which provided another profitable aspect to the life cycle analysis of this crop. The biodiesel production is a well-established technology, although several researchers have recently discussed a novel catalyst for higher yields and a better fuel quality. Soriano and Narani (2012) characterized the properties of biodiesel from camelina oil using the same seed used in this work. Their work discovered that a few of the biodiesel properties of camelina are similar to those of sunflower biodiesel properties, such as the flash point, cloud point, cold filter plugging point, kinematic viscosity (40 °C), and oil stability index. Camelina biodiesel has been blended with a standard JP-8 jet fuel and successfully used by the U.S. Air Force to test fly F/A-18 Super Hornet and A-10 Thunderbolt II fighter jets (Liu et al. 2013).
Conceptual Design and Process Simulation
The process flow sheet developed in the conceptual design was simulated in AspenPLUS for mass and energy balance calculations based on the initial compositional analysis of both the seeds and straw. Technical and economic analyses of lignocellulosic ethanol based on process flow sheet simulations are an attractive tool for studying the overall efficiency and process profitability (Shemfe et al.2015). For the energy balnce evuation, the net energy value (NEV) and the net energy ratio (NER) were used in the present work. The NEV is the difference between energy input and output, whereas the NER is the ration of net energy value to the energy input.
Tables 2 and 3 shows a summary of the overall energy and mass balances. The feed inputs presented in Table 2 represent the theoretical amount of the original feed feedstock.
Table 2. Simulation Results of Mass Balance for the Feed Flow and the Biofuels Outflow
Table 3. Simulation Results of Energy Balance and the Net Energy Value
Results from a simulation of the daily processing of 20,000 t of straw (50% dry matter content) and 10,000 t seeds (10% dry matter content) showed a total of 4976 L of ethanol produced. There was about 2% to 3% sugar loss because of decomposition in the acid pretreatment of the straw. After fractionation of the lignocellulosic materials, the solid residues contained mainly lignin with a heat value to be used as energy input displacement. Sugar extraction from the seeds provided an additional 1620 (theoretical amount is 1800) t of sugar, which was mainly glucose (Table 2). The combination of sugars recovered from the straw and seeds was fermented to fuel ethanol in a 12000-m3fermentation vessel. In the downstream processing, two distillation columns were connected in series with a high purity ethanol rectifier by shifting the azeotrope point under vacuum distillation to produce 98% ethanol. The mass and energy balance calculations showed that the processes for the conversion of sugar to ethanol (sugar extraction and fermentation) required 3.3 MJ/L ethanol. The downstream processing and ethanol distillation and rectification required 12.1 MJ/L ethanol. An additional 1.5 MJ/L ethanol was depleted by heat and work requirements from the pumps and heat exchangers (Table 3). The overall energy consumption for ethanol production was about 16.9 MJ/L ethanol, whereas biodiesel required less energy input, 8.2 MJ/L ethanol. The overall NEV were 15.4 and 10 ML/L Ethanol for both ethanol and biodiesel production respectively. The All of the energy input results for ethanol production were very similar to the results obtained by Gurram et al. (2016) because the same model was used and testing was done in the same lab. The total energy requirement for the whole biorefinery is 25.1 MJ/L ethanol, which was lower compared with the total energy yield of 54.3 MJ/L ethanol. The total energy yield was based on the heat values of the ethanol, lignin, and biodiesel. This process had a very competitive NER 2.16 compared with the NER of corn grain biomass 1.25 Morales et al(2015) and soybean biodiesel 1.93 (Tilman et al. 2006). Table 4 shows that the NER value of camelina is very competitive compared to other energy crops. This is a truly interesting value that can be expected to encourage biofuel industries to further investigate the commercial benefits of camelina crop.
Interestingly, the present finding agrees with several studies on camelina crop. Krohn and Fripp (2012), calculated that the camelina biodiesel reduced greenhouse gas (GHG) emissions by 40% to 60% compared with petroleum diesel (Table 4). A different study conducted by Miller and Kumar (2013) found that the GHG reduction and net energy ratio (NER) for camelina-based biodiesel ranged from 30 g CO2 eq/MJ to 82 g CO2 eq/MJ and 1.0 MJ/MJ to 2.3 MJ/MJ, respectively. Reducing the GHG emissions by 82 g CO2 eq/MJ was found to be very competitive with petroleum-derived diesel and made the whole crop environmentally friendly as an energy crop. However, Krohn and Fripp (2012) calculated that a seed yield of 403 kg/ac to 807 kg/ac must be maintained to sustain a reduction in the GHG. Chen et al. (2015) reported that tuning the practices of a crop management system would lower the production price if a camelina–wheat rotation was implemented.
Table 4. Net Energy Ratio and GHG for the Whole Camelina Bioprocess in the Biorefinery Platform Compared to Other Competing Crops
In the present study, the commercial benefit of the byproducts from the proposed process, such as meal and glycerin, were also investigated. A higher profit would be obtained with the production of both meal and glycerin. Tisserat et al. (2014) investigated the use of camelina meal with coffee tree woods a composite material. The meal properties included a 5% omega-3 fatty acid content, 40% protein content, and metabolizable energy of approximately 3 KJ/kg. High concentrations of glucosinolates, which have a potential health hazard at high doses, have been found inside camelina seeds. Lawrence et al. (2016) and Koçar and Civaş (2013) stated that camelina meal exhibits a good growth performance in dairy heifers (compared with dry distilled grain) when using it as a protein supply. Currently, the U.S. Food and Drug Administration allows 10% camelina meal (by weight) in beef cattle and broiler chicken feed. Research on the effects of camelina meal on animal livestock is still in progress but has provided reliable evidence that camelina may be used not only as a biofuel, but as a rich protein additive. Camelina seed meal and glycerin byproducts can provide an important source of revenue to improve the overall process economy. Very little information is available on the nutritional quality and economic value of camelina seed meal. Fortunately, soybean meal is very similar to camelina meal with respect to the nutritional quality (protein content). Therefore, the available data on soya meal was used to anticipate the economic revenue of camelina meal and its use as an animal feed (Ryhänen et al. 2007). The estimated price of soy meal and glycerin is $0.28/lb and $0.15/lb, respectively. Neibergs et al. (2016) reported that the price of camelina meals varies between $100 to 350/ton, whereas, the price history presented by Heming (2005) for glycerin in US market ranged between $100 and 120/ton. This would correspond to a monetary value of $1/kg meal of produced oil. Interestingly, both products (meal and glycerin) provided an energy displacement of 42 MJ/lb, according to Krohn and Fripp (2012).
Camelina has been been shown to grow successfully under cooler and arid climates with the end goal of harvesting the seeds as a viable biofuel and protein additive. The complex capabilities of camelina oil and seed meal have proven to be a diverse commodity for Midwestern farmers in the expanding biodiesel market. Because it is so complex, camelina is paving new roads in research and development, and only time will tell its true potential as a biodiesel feedstock and healthy food additive.
CONCLUSIONS
- The process for the production of multiple biofuels from whole camelina crops is considered feasible. The process exhibits improved energy yield and environmental suitability relative to other crop-based biofuel processes.
- The process analysis suggests that this process is profitable and sustainable because of the production of other valuable byproducts, mainly meal and glycerin.
- Both of the aforementioned commodities can be sold directly, without further preprocessing. Lignin was also recovered as a byproduct, and it can be used as a heating fuel in the biorefinery.
ACKNOWLEDGMENTS
This work was funded in part by an Economic Development Incentive Grant (131-5-909511) from the University of Wisconsin System.
REFERENCES CITED
Alkasrawi, M., Jrai, A. A., and Al-Muhtaseb, A. H. (2013). “Simultaneous saccharification and fermentation process for ethanol production from steam-pretreated softwood: Recirculation of condensate streams,” Chem. Eng. J. 225, 574-579. DOI: 10.1016/j.cej.2013.04.014
Alkasrawi, M., Al-Hamamre, Z., Al-Shannag, M., Abedin, M. J., and Singsaas, E. (2016). “Conversion of paper mill residuals to fermentable sugars,” Bioresources 11(1), 2287-2296. DOI: 10.15376/biores.11.1.2287-2296
Horwitzs, W. (1975). Official methods of analysis of the Association of Official Analytical Chemist, AOAC, Washington, DC.
Chen, M., Zhao, J., and Xia, L. (2008). “Enzymatic hydrolysis of maize straw polysaccharides for the production of reducing sugars,” Carbohyd. Polym. 71(3), 411-415. DOI: 10.1016/j.carbpol.2007.06.011
Chen, C., Bekkerman, A., Afshar, R. K., and Neill, K. (2015). “Intensification of dryland cropping systems for bio-feedstock production: Evaluation of agronomic and economic benefits of Camelina sativa,” Ind. Crop. Prod. 71, 114-121. DOI: 10.1016/j.indcrop.2015.02.065
Ciubota-Rosie, C., Ruiz, J. R., Ramos, M. J., and Pérez, Á. (2013). “Biodiesel from Camelina sativa: A comprehensive characterisation,” Fuel 105, 572-577. DOI: 10.1016/j.fuel.2012.09.062
Elum, Z. A., Modise, D. M., and Nhamo, G. (2017). “Climate change mitigation: The potential of agriculture as a renewable energy source in Nigeria,” Environ. Sci. Pollut. R. 24(4), 3260-3273. DOI: 10.1007/s11356-016-8187-7
Enciso, S. R. A., Fellmann, T., Dominguez, I. P., and Santini, F. (2016). “Abolishing biofuel policies: Possible impacts on agricultural price levels, price variability and global food security,” Food Policy61, 9-26. DOI: 10.1016/j.foodpol.2016.01.007
Gurram, R., Al-Shannag, M., Knapp, S., Das, T., Singsaas, E., and Alkasrawi, M. (2016). “Technical possibilities of bioethanol production from coffee pulp: A renewable feedstock,” Clean Technol. Envir. 18(1), 269-278. DOI: 10.1007/s10098-015-1015-9
Gurram, R. N., Al-Shannag, M., Lecher, N. J., Duncan, S. M., Singsaas, E. L., and Alkasrawi, M. (2015). “Bioconversion of paper mill sludge to bioethanol in the presence of accelerants or hydrogen peroxide pretreatment,” Bioresource Technol. 192, 529-539. DOI: 10.1016/j.biortech.2015.06.010
Heming, M. P. D. (December 2005). Glycerine Market Report, no. 71, Oleoline®.
Hymowitz, T., Collins, F. I., Panczner, J., and Walker, W. M. (1972). “Relationship between the content of oil, protein, and sugar in soybean seed,” Agron. J. 64(5), 613-616. DOI: 10.2134/agronj1972.00021962006400050019x
Jørgensen, H., Kristensen, J. B., and Felby, C. (2007). “Enzymatic conversion of lignocellulose into fermentable sugars: Challenges and opportunities,” Biofuel. Bioprod. Bior. 1(2), 119-134. DOI: 10.1002/bbb.4
Keske, C. M. H., Hoag, D. L., Brandess, A., and Johnson, J. J. (2013). “Is it economically feasible for farmers to grow their own fuel? A study of Camelina sativa produced in the western United States as an on-farm biofuel,” Biomass Bioenerg. 54, 89-99. DOI: 10.1016/j.biombioe.2013.03.015
Koçar, G., and Civaş, N. (2013). “An overview of biofuels from energy crops: Current status and future prospects,” Renew. Sust. Energ. Rev. 28, 900-916. DOI: 10.1016/j.rser.2013.08.022
Kotarska, K., Świerczyńska, A., and Dziemianowicz, W. (2015). “Study on the decomposition of lignocellulosic biomass and subjecting it to alcoholic fermentation: Study on the decomposition of lignocellulosic biomass,” Renew. Energ. 75, 389-394. DOI: 10.1016/j.renene.2014.10.018
Kritana, P., and Gheewala, H. S. (2006). “Energy and greenhouse gas implications of biodiesel production from Jatropha curcas L.,” In Proceedings of the 2nd Joint International Conference on Sustainable Energy and Environment, pp. 21-23.
Krohn, B. J., and Fripp, M. (2012). “A life cycle assessment of biodiesel derived from the ‘niche filling’ energy crop camelina in the USA,” Appl. Energ. 92, 92-98. DOI: 10.1016/j.apenergy.2011.10.025
Kumar, P., Barrett, D. M., Delwiche, M. J., and Stroeve, P. (2009). “Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production,” Ind. Eng. Chem. Res. 48(8), 3713-3729. DOI: 10.1021/ie801542g
Lawrence, R. D., Anderson, J. L., and Clapper, J. A. (2016). “Evaluation of Camelina meal as a feedstuff for growing dairy heifers,” J. Dairy Sci. 99(8), 6215-6228. DOI: 10.3168/jds.2016-10876
Li, M.-F., Yu, P., Li, S.-X., Wu, X.-F., Xiao, X., and Bian, J. (2017). “Sequential two-step fractionation of lignocellulose with formic acid organosolv followed by alkaline hydrogen peroxide under mild conditions to prepare easily saccharified cellulose and value-added lignin,” Energ. Convers. Manage. 148, 1426-1437. DOI: 10.1016/j.enconman.2017.07.008
Liu, Y. C., Savas, A. J., and Avedisian, C. T. (2013). “The spherically symmetric droplet burning characteristics of jet-A and biofuels derived from Camelina and tallow,” Fuel 108, 824-832. DOI: 10.1016/j.fuel.2013.02.025
Mattsson, B., Cederberg, C., and Blix, L. (2000). “Agricultural land use in life cycle assessment (LCA): Case studies of three vegetable oil crops,” J. Clean. Prod. 8(4), 283-292. DOI: 10.1016/S0959-6526(00)00027-5
Miller, P., and Kumar, A. (2013). “Development of emission parameters and net energy ratio for renewable diesel from canola and camelina,” Energy 58, 426-437. DOI: 10.1016/j.energy.2013.05.027
Morales, M., Quintero, J., Conejeros, R., and Aroca, G. (2015). “Life cycle assessment of lignocellulosic bioethanol: Environmental impacts and energy balance,” Renew Sus Energy Rev, 42, 1349-1361. DOI: 10.1016/j.rser.2014.10.097.
Moser, B. R. (2010). “Camelina (Camelina stativa L.) oil as a biofuels feedstock: Golden opputunity or false hope?,” Lipid Tech. 22 (120) 270-273. DOI: 10.1002/lite.201000068
Muktham, R., Ball, A. S., Bhargava, S. K., and Bankupalli, S. (2016). “Bioethanol production from non-edible de-oiled Pongamia pinnataseed residue-optimization of acid hydrolysis followed by fermentation,” Ind. Crop. Prod. 94, 490-497. DOI: 10.1016/j.indcrop.2016.09.019
Neibergs, J. S., Driver, J. P., and Llewellyn, D. A. (2016). “Valuing Canola and Camelina Biodiesel Byproduct Meal as a Livestock Protein Supplement,” Washington State University Extension, hdl.handle.net/2376/6271
Pimentel, D., and Patzek, T. W. (2005). “Ethanol production using corn, switchgrass, and wood; Biodiesel production using soybean and sunflower,” Natural Resources Research 14(1), 65-76. DOI: 10.1007/s11053-005-4679-8
Ryhänen, E.-L., Perttilä, S., Tupasela, T., Valaja, J., Eriksson, C., and Larkka, K. (2007). “Effect of Camelina sativa expeller cake on performance and meat quality of broilers,” J. Sci. Food Agr. 87(8), 1489-1494. DOI: 10.1002/jsfa.2864
Saha, B. C., Iten, L. B., Cotta, M. A., and Wu, Y. V. (2005). “Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol,” Process Biochem. 40(12), 3693-3700. DOI: 10.1016/j.procbio.2005.04.006
Schmer, M. R., Vogel, K. P., Mitchell, R. B., and Perrin, R. K. (2008). “Net energy of cellulosic ethanol from switchgrass,” P. Natl. Acad. Sci. USA 105(2), 464-469. DOI: 10.1073/pnas.0704767105
Schneider, L., Dong, Y., Haverinen, J., Jaakkola, M., and Lassi, U. (2016a). “Efficiency of acetic acid and formic acid as a catalyst in catalytical and mechanocatalytical pretreatment of barley straw,” Biomass Bioenerg. 91, 134-142. DOI: 10.1016/j.biombioe.2016.05.015
Schneider, L., Haverinen, J., Jaakkola, M., and Lassi, U. (2016b). “Solid acid-catalyzed depolymerization of barley straw driven by ball milling,” Bioresource Technol. 206, 204-210. DOI: 10.1016/j.biortech.2016.01.095
Schneider, L., Haverinen, J., Jaakkola, M., and Lassi, U. (2017). “Pretreatment and fractionation of lignocellulosic barley straw by mechanocatalysis,” Chem. Eng. J. 327, 898-905. DOI: 10.1016/j.cej.2017.06.175
Shemfe, M. B., Gu, S., and Ranganathan, P. (2015). “Techno-economic performance analysis of biofuel production and miniature electric power generation from biomass fast pyrolysis and bio-oil upgrading,” Fuel 143, 361-372. DOI: 10.1016/j.fuel.2014.11.078
Sluiter, J. B., Ruiz, R. O., Scarlata, C. J., Sluiter, A. D., and Templeton, D. W. (2010). “Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods,” J. Agric. Food Chem. 2010, 58 (16), 9043-9053. DOI: 10.1021/jf1008023
Söderström, J., Pilcher, L., Galbe, M., and Zacchi, G. (2003). “Two-step steam pretreatment of softwood by dilute H2SO4 impregnation for ethanol production,” Biomass Bioenery. 24(6), 475-486. DOI: 10.1016/S0961-9534(02)00148-4
Soriano, N. U., and Narani, A. (2012). “Evaluation of biodiesel derived from Camelina sativa oil,” J. Am. Oil Chem. Soc. 89(5), 917-923. DOI: 10.1007/s11746-011-1970-1
Tanner, R. S. (2007). “Cultivation of bacteria and fungi,” in: Manual of
Environmental Microbiology (3rd Ed.), ASM Press, Washington, DC, pp. 69-78.
Tilman, D., Hill, J., and Lehman, C. (2006). “Carbon-negative biofuels from low-input high-diversity grassland biomass,” Science314(5805), 1598-1600. DOI: 10.1126/science.1133306
Tisserat, B. H., Reifschnedier L., López Núñez, J. C., Hughes, S. R., Selling, G., and Finkenstadt, V. L. (2014). “Evaluation of the mechanical and thermal properties of coffee tree wood flour-polypropylene composites,” BioResources 9(3), 4449-4467. DOI: 10.15376/biores.9.3.4449-4467
Viikari, L., Vehmaanperä, J., and Koivula, A. (2012). “Lignocellulosic ethanol: From science to industry,” Biomass Bioenerg. 46, 13-24. DOI: 10.1016/j.biombioe.2012.05.008
Young, A. L. (2009). “Finding the balance between food and biofuels,” Environ. Sci. Pollut. R. 16(2), 117-119. DOI: 10.1007/s11356-009-0106-8
Larsen, J., Haven, M. O., Thirupa, L. (2012). “Inbicon makes lignocellulosic ethanol a commercial reality,” Biomass Bioener. 46, 36-45. DOI: 10.1016/j.biombioe.2012.03.033
Article submitted: December 24, 2017; Peer review completed: March 11, 2018; Revisions accepted: May 1, 2018; Published: May 9, 2018.
DOI: 10.15376/biores.13.3.4870-4883
