Abstract
Biochar is highly valuable in various applications due to its unique physicochemical properties such as high thermal efficiency, high surface area, surface functional groups, and crystal structure. The goal of this review is to establish a systematic strategy of biochar production for applications in various fields. First, the characteristics of biomass as feedstock for biochar production and their classification are discussed according to the types present in nature. Second, the technology for biochar production and the production yield are examined. In thermochemical conversion for biochar production, five major types of pyrolysis processes are suggested, and the production yield is evaluated according to pyrolysis parameters (feedstock pretreatment, operating temperature, heating rate, residence time, carrier gas). In addition, biochar production from pyrolysis of mixed feedstock has recently been suggested; thus, the evaluation of the production yield from co-pyrolysis is included. Finally, analytical techniques for biochar characterization are investigated and the application of biochar in various fields is considered, such as in adsorbents, energy storage devices, and catalysts.
Download PDF
Full Article
Recent Advancements in Biochar Production According to Feedstock Classification, Pyrolysis Conditions, and Applications: A Review
Youngsang Chun,a,1 Soo Kweon Lee,b,1 Hah Young Yoo,c,* and Seung Wook Kim a,b,*
Biochar is highly valuable in various applications due to its unique physicochemical properties such as high thermal efficiency, high surface area, surface functional groups, and crystal structure. The goal of this review is to establish a systematic strategy of biochar production for applications in various fields. First, the characteristics of biomass as feedstock for biochar production and their classification are discussed according to the types present in nature. Second, the technology for biochar production and the production yield are examined. In thermochemical conversion for biochar production, five major types of pyrolysis processes are suggested, and the production yield is evaluated according to pyrolysis parameters (feedstock pretreatment, operating temperature, heating rate, residence time, carrier gas). In addition, biochar production from pyrolysis of mixed feedstock has recently been suggested; thus, the evaluation of the production yield from co-pyrolysis is included. Finally, analytical techniques for biochar characterization are investigated and the application of biochar in various fields is considered, such as in adsorbents, energy storage devices, and catalysts.
Keywords: Biochar; Biomass; Classification; Pyrolysis; Applications
Contact information: a: Department of Interdisciplinary Bio-Micro System Technology, College of Engineering, Korea University, 145 Anam-Ro 5, Seongbuk-Gu, Seoul 02841, Republic of Korea; b: Department of Chemical and Biological Engineering, Korea University, 145, Anam-Ro, Seongbuk-Gu, Seoul 02841, Republic of Korea; c: Department of Biotechnology, Sangmyung University, 20, Hongjimun 2-Gil, Jongno-Gu, Seoul 03016, Republic of Korea; 1These authors contributed equally to this work;
* Corresponding authors: y2h2000@smu.ac.kr (H.Y.Y.) and kimsw@korea.ac.kr (S.W.K.).
GRAPHICAL ABSTRACT
INTRODUCTION
Global warming, accelerated by the reckless use of fossil fuels after the industrial revolution, has led to various social and economic problems such as rising sea level, climate change, species extinction, and food insecurity. According to the International Panel on Climate Change (IPCC), global warming is significantly affected by intensive greenhouse gas emissions (GHGs), including carbon dioxide, into the atmosphere. The problem is expected to increase as the world’s population grows to about 9 billion by 2050 (Antar et al. 2021). Therefore, it is necessary to replace fossil fuels with renewable energy to prevent global warming.
Biomass, which participates in the fast carbon cycle within the biosphere, has been described in many reports as a renewable energy source that can serve as an alternative to fossil fuels (Amiri et al. 2014). The term biomass (Greek bio meaning life + maza meaning mass) is referred to all organic matter derived from plants, animals, and microorganisms (Demirbas 2010). A biorefinery is a process that converts biomass to energy and other beneficial byproducts such as biochar. The International Biochar Initiative (IBI) defines biochar as a solid material produced during oxygen-limited pyrolysis of biomass (Meyer et al. 2017). Biochar is utilized in a variety of industries due to its physicochemical properties such as high thermal efficiency, high surface area, surface functional groups, and crystalline structure. According to market report, the market size of biochar is expected to reach $3.14 billion by 2025 (Hersh et al. 2019).
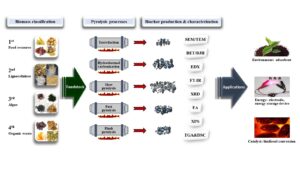
In order to apply biochar to various fields such as environment, energy, and fuel, it is important to characterize biomass as a feedstock and determine various pyrolysis parameters in the biochar production process (Fig. 1). Conventional biomass contains food resources; thus, this review is focused on non-edible resources as feedstock for biochar production. Non-edible biomass can be classified into second, third, and fourth-generations according to its origin and characteristics (Yang et al. 2015b; Yoo and Kim 2021). The second-generation biomass is a plant composed of lignocellulose, and the third-generation includes energy crops, engineering plants, and algae. The fourth-generation biomass, which has recently begun to be classified, refers to organic wastes such as coffee grounds, fruit peels and sewage sludge. The pretreatment including drying, crushing, and sieving is carried out to prepare feedstock in the specific condition required for each process. Biochar is produced through the pyrolysis of the prepared feedstock that is controlled by various parameters.
Fig. 1. Schematic diagram of biomass into biochar conversion process for various applications
Pyrolysis is a well-established method for obtaining high value-added substances such as biochar, bio-oil, and syngas through the thermochemical conversion of biomass. Pyrolysis can be classified into various types such as torrefaction, hydrothermal carbonization (HTC), slow pyrolysis, fast pyrolysis, and flash carbonization with different reaction conditions depending on the target substance (Pandey et al. 2020). Slow pyrolysis is the most reported thermochemical conversion to obtain high-yield biochar as the main product. However, the disadvantage of slow pyrolysis is a long reaction time. In recent years, reports of torrefaction procedures that react at milder operating temperatures than conventional pyrolysis have been increasing, and the method of producing biochar from HTC using wet feedstock is also drawing attention.
Various methods for using the produced biochar have been proposed in recent years. Physicochemical analysis of biochars identifies biochars that exhibit high and stable performance in their applications. Because specific surface area, surface functional groups, and mineral contents of biochar are various, physicochemical properties could be different according to feedstock and pyrolysis process. As analytical techniques to understand the physicochemical properties of biochar have become more advanced, the application of biochar as an adsorbent, energy storage material, and fuel production catalyst has been under considerable research and development.
In biochar research, most reports focus on the characterization and analysis of pyrolysis products based on optimization of process parameters at the laboratory level under their own conditions. In order to produce consistent yield and quality of the biochar, the whole process should be managed carefully. Figure 1 represents the process of biomass conversion into biochar for various applications. Understanding of feedstock is required to convert it into biochar of consistent quality and yield. This is because the pyrolytic product will be affected by the energy conversion efficiency of pyrolysis depending on the type of feedstock composed of various components having a different physicochemical property (Weldekidan et al. 2019). In addition, it is necessary to establish thermal decomposition conditions and control the entire process of biomass conversion.
In this review, fundamental data (Table 1) are provided by the classification of biomass used as a feedstock for biochar production. Table 2 provides various pyrolysis methods for producing biochar, and the effects of feedstock pretreatment (crushing and drying). Pyrolysis conditions (operating temperature, heating rate, residence time, and carrier gas) on the production yield of biochar are examined in Table 3. Table 4 investigates the conversion of biochar through a recently proposed mixed feedstock. Finally, analytical techniques for identifying physicochemical properties of biochar are investigated, and the applications of biochar such as adsorbent, energy storage device, and catalyst, are summarized in Table 5.
CLASSIFICATION OF BIOMASS AS FEEDSTOCK FOR BIOCHAR PRODUCTION
Biomass is the generic term for all organic matter derived from animals and algae as well as plants such as shrubs, trees, and crops (Jacobsson and Johnson 2000). Since the types of biomass are diverse and their composition depends on their origin, systematic classification of biomass is required to utilize as a beneficial feedstock in thermochemical processes. In general, biomass has been classified into starch-based (grain), cellulosic (agricultural by-products), and protein-based (organic waste) resources according to the type. Depending on their origin, they have been classified as agricultural residues, forestry residues, animal residues, algae, and aquatic crops. Recently, wastes generated by human activities, such as food waste, construction waste, and sewage, also have been included in the biomass category (Yuan et al. 2019).
In order to utilize biomass as a feedstock more efficiently, it has been classified into 1st to 3rd generation based on major biotechnology (Dalena et al. 2019; Ibrahim et al. 2018; Naik et al. 2010; Tursi 2019). Recently, organic wastes derived from industry have attracted attention as fourth-generation biomass, which provides a way to prevent environmental pollution (Yang et al. 2015b; Yoo and Kim 2021). Thus in the present review, based on the classification of previous reports (considering both the major biotechnologies and biomass characteristics), biomass will be classified into first, second, third, and fourth-generation. First-generation biomass will be defined to include in food crops such as corn, potato, wheat, sorghum, sugarcane, and rice. The direct usage of edible biomass for energy purposes is recognized as a threat to food security, including rising grain or feed prices and resulting starvation for the marginalized. Therefore, its use as a feedstock for bioindustry should be avoided (Naik et al. 2010). The second-generation biomass feedstock is lignocellulose, which exists in nature as non-edible biomass. As a potential fuel source, lignocellulosic biomass can be an effective alternative to first-generation biomass because of its low cost and high abundance (Wang et al. 2021). Third-generation biomass contains algae, a completely different generation of feedstock. Algae exists as aquatic biomass in nature (Chun et al. 2018). Macroalgae (blue, green, brown, red) and microalgae are representative forms. Unlike lignocellulosic biomass, algal strains have a different growth environment. Besides, it is proposed as an important feedstock for modifying global warming because it could produce renewable energy through the absorption of CO2 (Dalena et al. 2019; Ibrahim et al. 2018). The fourth-generation biomass is organic wastes, which includes organic resources not included in the first, second, and third generations. Unlike other generations, the fourth-generation biomass has the advantage that it does not require cultivation space to obtain the feedstock (Chun et al. 2019; Yoo and Kim 2021). According to the standards, feedstock for biochar can be classified into first to fourth generations, and their compositions are shown in Table 1.
The second-generation lignocellulosic biomass is a plant resource that is mainly composed of cellulose, hemicellulose, and lignin. Cellulose and hemicellulose are polysaccharides, but each has a unique composition and structure. Cellulose is an important structural component of the primary cell wall in plants, and is derived from D-glucose units, which are composed of long-chain linear via β(1, 4)-glycosidic bonds. Hemicellulose can comprise diverse sugar monomers such as xylose, arabinose, rhamnose, mannose, and galactose, and is a short-chain branched polymer with an amorphous structure. In addition, major forms of hemicellulose such as xylan, glucuronoxylan, arabinoxylan, glucomannan, and xyloglucan are well known. Lignin, an aromatic polymer, is a heterogeneous polymer derived from precursor lignols that crosslink in various ways. Hydrophobic lignin plays an important role in plants by covalently binding to hemicellulose. It interferes with the absorption of water into the cell wall, enabling the plant’s vascular tissue to conduct water efficiently, and its other functions are known as mechanical strength and disease resistance (Gundekari et al. 2020). According to Sarip et al. (2016), lignocellulosic biomass, on average, contains 32 to 54% cellulose, 11 to 37% hemicellulose, and 17 to 32% lignin. Similar results were found in a report analyzing wood, leaves, grass, and straw classified as second-generation biomass. Wood’s (birch, pine, willow, spruce) chemical composition has been reported to be 40 to 46% cellulose, 20 to 23% hemicellulose, and 25 to 29% lignin. Platanus leaf, grasses, and straws were reported to have chemical compositions of 18 to 49% cellulose, 8 to 35% hemicellulose, and 10 to 51% lignin
The third-generation biomass, algae, is considered an ideal raw material for biochar production because it could produce a large amount of biomass per hectare. Algae is comprised of organisms, and unlike second-generation biomass, it has been reported to contain a large amount of protein and lipid (Bardhan et al. 2015). Algae can be largely divided into macroalgae and microalgae. The chemical composition of macroalgae (Saccharina japonica, Sargassum fusiforme, Undaria pinnatifida, Capsosiphon fulvescens, Spirulina, Gelidium amansii) has been reported as 2 to 69% cellulose, 0 to 65% hemicellulose, 0.5 to 30% ash, 1 to 58% protein, and 0.04 to 25% lipid. Microalgae (Chlorella vulgaris, Nannochloropsis gaditana, Chlorella pyrenoidosa, Haematococcus) was reported as 5 to 49% cellulose, 0 to 15% hemicellulose, 0 to 8% ash, 21 to 56% protein, and 5 to 23% lipid.
The fourth-generation biomass is organic wastes derived from the bio-industries (Yoo and Kim 2021). Organic wastes from food processing residue (apple residue, soybean curd residue (SCR), instant noodle residue, potato peel residue) was reported as 5 to 84% cellulose, 0 to 34% hemicellulose, 0 to 24% lignin, 0 to 1% ash, 0 to 4% protein, and 0 to 2% lipid.
Table 1. Composition of Feedstock for Biochar
In addition, fruit peel (banana peel, orange peel, grapefruit peel, lemon peel) was reported as 30 to 48% cellulose, 10 to 30% hemicellulose, 0 to 16% lignin, 0 to 1% ash, 4 to 5% protein, and 6 to 8% lipid. The composition of organic wastes from industry (newspapers, waste papers from chemical pulps, oil palm trunks, empty fruit bunch (EFB), and spent coffee grounds (SCG)) is present at 12 to 65% cellulose, 15 to 39% hemicellulose, 8 to 24% lignin, 0 to 1% ash, 0 to 17% protein, and 0 to 2% lipid. The chemical composition of various shells from food processing (soybean shell, peanut shell, coconut shell, ginkgo nut shell, walnut shell, almond shell, pine nut shell, chestnut shell, acorn shell, pumpkin seed shell, sunflower seed shell) is 21 to 47% cellulose, 0 to 32% hemicellulose, 0 to 1% ash, 0 to 17% protein, and 0 to 2% lipid.
Various biomass can be classified into first to fourth generations according to the characteristics and major biotechnologies. However, it was found that several feedstocks have not been analyzed in detail for their chemical composition. Further studies on biomass composition analysis should be performed on the basis of systematized criteria as feedstock for biochar production.
BIOCHAR PRODUCTION
Types of Pyrolysis Processes for Biochar Production
Pyrolysis has been used to produce biochar for thousands of years through traditional earthen, brick, and steel kilns (Laird et al. 2009). The recent thermochemical technologies with different types of reactors for converting biomass into renewable products include torrefaction, hydrothermal carbonization (HTC), gasification, slow pyrolysis, flash pyrolysis, and fast pyrolysis (Garcia-Nunez et al. 2017). These modern pyrolysers are also designed to capture the volatiles for the production of bio-oil and syngas along with biochar. Achievements of higher yield and quality of target product are significantly dependent on operating parameters and the properties of the feedstock. Various thermochemical techniques operating with different reaction conditions (operating temperature, heating rate, residence time, and etc.) for biochar production are summarized in Table 2.
Table 2. Operating Conditions on Different Types of Pyrolysis Process
Torrefaction
Torrefaction, which is often referred to as roasting, is carried out under relatively mild conditions with an inert environment at atmospheric pressure and a temperature range of 200 to 300 °C. In general, the residence time in the reactor is less than 1 hour and the yield of solid product is about 60 to 85%. Under mild conditions, biomass releases a variety of volatile gases from the partially decomposed hemicellulose fraction, resulting in a large amount of solid product suitable for use of bio-coal (Gan et al. 2018). It is assumed that the earliest method originated from France in the 1930s for producing syngas. The torrefaction can be designed for either batch processing or continuous processing equipment, both of which are often performed in laboratory-scale studies. On the pilot-scale, devices such as tray ovens and screw reactors are used. The study of thermochemical conversion of biomass through torrefaction is mainly aimed at the production of syngas or biochar (bio-coal) (Ciolkosz and Wallace 2011).
Hydrothermal carbonization
Hydrothermal carbonization (HTC) is especially used for treating biomass with high moisture content that is generally carried out in a temperature range of 180 to 250 °C under autogenous pressure for over a day. The solid product via HTC is referred to as hydrochar. It is obtained with a high yield of 40 to 70 wt%, depending on the reaction temperature, pressure, residence time, and water-to-biomass ratio (Yan et al. 2010). Since biomass containing moisture is directly used, HTC, which does not require pretreatment for drying, is attracting attention as an economical process compared to other pyrolysis methods. Water molecules act as acid catalysts without the addition of acid under a specific residence time and pressure in the HTC process. This method was firstly reported by Bergius in 1913 to convert cellulose into a coal-like material and is currently referred to as hot compressed water treatment, wet torrefaction, or hydrothermal treatment (Wang et al. 2018).
Slow pyrolysis
In general, the aim of the slow pyrolysis process with a relatively low heating rate (0.1 to 1 °C/s) is the production of high-yield biochar (10 to 90%) from biomass. The reaction is usually carried out in an operating temperature range of 300 to 700 °C and a wide range of residence time (from several hours to days) (Li et al. 2019). In slow pyrolysis, relatively large sizes (~50 mm) of the feedstock can be used, and the process is not significantly dependent on the size of the feedstock. The feedstock is loaded into the reactor in an inert atmosphere from the beginning of the pyrolysis reaction. Recent studies on thermochemical conversion of biomass for various biochar applications have mainly been carried out with slow pyrolysis in fixed-bed reactors (Meyer et al. 2011).
Fast pyrolysis
The fast pyrolysis process is carried out at a temperature range of 450 to 600 °C with a relatively higher heating rate of 10 to 200 °C/s for a short residence time (<30 min). The main purpose of fast pyrolysis is to maximize the conversion of liquid products such as bio-oil and bio-oil-derived products, and the yield of biochar is relatively low, about 10 to 20%. Fast pyrolysis requires the feedstock to be prepared in a size of 1 mm or less for efficient heat transfer during a short residence time, and the feedstock is loaded into the reactor after reaching the operating temperature. The process has been operated in various types of reactors such as conical spouted bed, bubbling fluidized bed, circulating bed, rotating cone, and ablation reactor (Garcia-Nunez et al. 2017).
Flash pyrolysis
Flash carbonization is carried out with a heating rate of at least 1000 °C/s, which is the highest of the pyrolysis methods in the operating range of 700 to 1000 °C for a residence time of less than 30 min. The advantage of this reaction is a relatively short process time, but the production yield of biochar is about 20 to 30%. A dust-like feedstock is prepared to facilitate fluidization, leading to the effective thermochemical conversion of biomass. In this process, the feedstock is first loaded into a packed bed reactor and then a constant pressure of 1 to 2 bar is applied with air. The bottom of the pressurized reactor is heated by a flame, air flows downstream, and the flame rises up, heating the entire packed bed (Meyer et al. 2011).
Biochar Production from Various Feedstock
Biochar production technology is highly dependent on both the pyrolysis process and the characteristics of biomass. In general, pyrolysis has been performed at a high temperature with limited oxygen, but heating conditions such as operating temperature, heating rate, and residence time are inconstant. In particular, the nature of the feedstock is one of the important factors in biochar production because the components and contents are different according to the classification of biomass. The yields of biochar production under various pyrolysis conditions and feedstock are summarized in Table 3.
Masek et al. (2013) performed the slow pyrolysis of pine wood chips (second-generation) and softwood pellets (second-generation) which consists of cellulose, hemicellulose, and lignin to biochar at an operating temperature of 350, 450, and 550 °C under 0.5 L/min N2 flow rate during 1 h. The yield of biochar production based on pinewood chips was measured to be 42% (at 350 °C) and 31% (at 450 °C), respectively, which indicates a decrease in yield as the pyrolysis temperature was increased. This was attributed to the different properties of thermal decomposition of cellulose and hemicellulose.
The yield of biochar production on pyrolysis at 550 °C is 30%, exhibiting an increase in pyrolysis temperature from 450 to 550 °C, did not significantly affect the biochar production in decrease. This is due to the high lignin portion in the feedstock, which is not sensitively responsive to the operating temperature of 450 and 550 °C. The yield of pinewood chips-derived biochar production according to temperature (350 to 550 °C) is related to feedstock composition and the range of their decomposition temperatures. The thermal decomposition temperature and the identification of biomass composition are highly correlated with the yield of biochar. Also, the yields of softwood pellets derived biochar in the pyrolysis temperatures range of 350 to 550 °C showed a similar trend as those of pine wood chips. Thermochemical conversion of second-generation biomass performed at 350 to 550 °C showed similar characteristics due to their composition of the feedstock.
In order to understand the relationship between the pyrolysis temperature and biochar production, several studies have been conducted for biochar production over a wide range of temperatures (200 to 900 °C). Selvarajoo and Oochit (2020) performed various tests at operating temperature in a range of 300 °C, 500 °C, 700 °C, and 900 °C to produce palm fiber (second-generation) derived biochar with an N2 flow rate of 0.03 L/min during 2 h. Biochar production was 54% at 300 °C of pyrolysis temperature. After a temperature (about 500 °C) of lignin decomposition, biochar yield was measured as 29% (at 500 °C), 28% (at 700 °C), and 26% (at 900 °C), resulting in a significant decrease in production at elevating pyrolysis temperature. This is attributed to the fact that the yield of biochars significantly decreased after the temperature at which lignin degradation occurs. Operating temperature, which is responsive to thermal degradation of lignin, influences the biochar production from thermochemical conversion of second-generation biomass. Jung et al. (2016) produced marine macroalage (third-generation) based biochar pyrolyzed with operating temperatures from 200 °C to 800 °C under an N2 atmosphere. Four different biochar yields were 78%, 63%, 37%, and 27% at pyrolysis temperatures of 200 °C, 400 °C, 600 °C, and 800 °C, respectively. As the temperature was increased, thermal degradation of cellulose and hemicellulose occurred in algal biomass, and the biochar yield was significantly decreased. Especially in the third-generation of biomass, which does not contain lignin, the decrease of biochar yield over 500 °C is caused by the complete removal of volatile substances during the pyrolysis process. Therefore, it was observed that the biochar yield between pyrolysis at 400 °C and pyrolysis at 600 °C was significantly reduced by 25.7%, which is a higher gap than that of a temperature range of 600 to 800 °C.
Table 3. Summary of Feedstock and Pyrolysis Conditions for Biochar Production
A yield of biochar from the third-generation biomass was also significantly affected by a temperature around 500 °C, which is similar to the pyrolytic phenomenon of second-generation biomass, because volatile matter was removed during the pyrolysis. The correlation between the production and operating temperature of the second and third-generation biomass-derived biochar was also observed in the 2 h pyrolysis of coconut husk (fourth-generation) biomass. Suman and Gautam (2017) investigated the pyrolysis effect of 400 to 1000 °C on coconut shell biochar during 2 h residence time under an inert atmosphere. The biochar yield appeared to be 43% (400 °C), 36% (600 °C), 34% (800 °C), and 27% (1000 °C), exhibiting a significant decrease in the temperature range of 400 to 600 °C. It is considered that the decomposition of lignin that occurred at about 500 °C affected the reduction of biochar yield. Biochar yield was significantly influenced by the operating temperature around 500 °C, which corresponds to lignin decomposition or elimination of volatile matters in second, third, and fourth-generation biomass.
The yield of biochar varies depending on the pyrolysis conditions and feedstock pretreatment, even in cases where the same feedstock or the same generation is used. He et al. (2018) compared the biochar yield of pyrolysis at 300 to 600 C from various feedstocks (second-generation) of wheat straw, rice straw, corn straw, rape stalk, cotton stalk sieved to under 0.9 mm. At an operating temperature of 300 C, the yield of rice straw-derived biochar exhibited the highest value of 74% compared to 69% of wheat straw, 62% of corn stover, 68% of rape stalk, and 72% of cotton stalk. The various yields of second-generation biomass-derived biochar were observed in the same pyrolysis condition and pretreatment. Yang et al. (2017) reported a low yield (32%) of rice straw-derived biochar, which was obtained from pyrolysis at 350 C with a rice straw sieved under 0.42 mm. According to the above two studies, despite the same feedstock (rice straw), there was a difference in yield of about 32%, which can be attributed to different pyrolysis conditions. It is also suggested that control of biochar production may be highly dependent on the feedstock pretreatment such as mechanical treatment (crushing and sieving) for particle size. Furthermore, since the composition of feedstock differs depending on the part used, such as roots, bark, shells, stems, fruits, etc., the yield of biochar varies despite the use of one plant. A precise collection of feedstock is required to ensure biochar quality because a biochar yield of Chinese fir wood was 43%, and that of Chinese fir bark was 48% at the same pyrolysis temperature of 350 C.
Algal biomass as the third-generation biomass is divided into its origins from fresh and seawater types, which contain different ash contents in the feedstock. Therefore, composition analysis before the thermochemical conversion process is required. Binda et al. (2020) compared the yield of biochar produced from algal in freshwater and seawater pyrolyzed at 350 C for 1 h. The yield of biochars was 34% from Chlorella vulgaris (freshwater) and 36% from Spirulina sp. (freshwater), and 41% from Nannochlorpsis sp. (seawater). It was observed that the highest yield of biochar from seawater algal biomass was due to the high concentration of ash (sodium and potassium), depending on its nature. In addition, different pyrolysis types for biochar production from algal biomass were investigated. Silva et al. (2020) produced biochar with 43% yield by fast pyrolysis (residence time: 1 min) of Spirulina (blue green micro-algae grown in alkaline water) at 280 C. A yield of biochar from fast pyrolysis (43%) was obtained more than that of slow pyrolysis (36%). Biochar yield was also affected by pyrolysis type despite the same feedstock.
Most of all, it is important to determine the various pyrolysis parameters (heating rate, residence time, gas flow rate, etc.) in the biochar production. Yang et al. (2020) investigated the effect of heating rate on biochar production of pruned apple tree branches at a pyrolysis temperature of 500 C. The biochar yield decreased from 34% at 1 C/min to 31% at 6 C/min, assuming that a heating rate is not critical. In addition, similar yields of biochar were produced with different residence time (32% at 0.5 h, 32% at 2 h, 31% at 3 h, 31% at 6 h). It is estimated that the production of biochar may be sufficiently obtained by relatively low energy consumption (slow heating rate and short residence time). Bhattacharjee and Biswas (2019) experimentally investigated the biochar production derived from the pyrolysis of orange bagasse (screened at 425 µm) based on several parameters about the effect of heating rate (25 to 100 °C/min), operating temperature (350 to 600 °C), and N2 gas flow (0.1 to 0.5 L/min). Consequently, the highest yield of biochar was obtained at a heating rate of 25 °C/min, pyrolysis temperature of 350 C, and slow nitrogen flow rate. In order to expect optimum pyrolysis condition for biochar yield, recent studies adapted the Taguchi method (Vieira et al. 2020). The study evaluated four parameters influencing biochar yield produced from rice husk biomass. The parameters were the heating temperature (5, 10, 20 °C/min), operating temperature (300, 400, 500 °C), residence time (3600, 5400, 7200 s), and input mass (125, 250, and 500 g). The maximum biochar production was 37% under following conditions: 20 °C/min, 300 °C, 5400 s, and 500 g of biomass.
The investigation of potential feedstock for biochar production has been carried out in various fields. For example, Kim et al. (2018) developed the biochar pyrolyzed at 600 C that originated from phosphorus-rich Escherichia coli-based fermentation waste used for the monosodium glutamate production. The yield of biochar pyrolyzed by 2 h, which ranged from 12 to 18%, was the highest among three different residence time (2 h, 3 h, and 4 h) conditions. The amount of produced biochar from dried-state feedstock was ca. 8% higher than that from wet-state. Although the water content of wet-state biomass was removed at heating about 120 C during the pyrolysis process, biochar yield was influenced by the moisture content of feedstock.
Biochar production is significantly affected by the composition of the feedstock causing various phenomena of thermal decomposition. In particular, the yield of biochar from second, third, and fourth-generation biomass was critical at an operating temperature of 500 C. Recently, several studies have focused on an effective design to produce biochar, such as the Taguchi method. In order to produce the constant quality and yield of biochar, various pyrolysis parameters should be investigated.
Biochar Production from Mixed Feedstock
In biochar production, the feedstock is one of the important economic factors, and the process cost is significantly affected by seasonal security and transportation costs by region. In order to solve this problem, a strategy has been suggested to perform thermal decomposition by mixing feedstock that is easy to supply and inexpensive. However, since the heterogeneous raw materials have significant effect on the quality control of the product, it is very important to understand the relationship between the operating conditions of co-pyrolysis and the results (biochar yield and quality, etc.). For example, Masek et al. (2013) carried out co-pyrolysis of mixed larch and spruce chips for the feedstock to make biochar at 350 and 500 C with the use of N2 gas for a residence time of 60 min. The yield of biochar was decreased by 42 to 29% with increasing operating temperatures. Each biomass component has a different thermal decomposition temperature, which is known to be 300 to 400 C for cellulose and more than 400 C for lignin (Chun et al. 2019). When the total ratio of cellulose and lignin in the mixed feedstock and the ratio of a single feedstock are similar under the same pyrolysis conditions, the biochar yield was found to follow a similar trend. In addition, Yang et al. (2019) prepared the feedstock mixed with vegetable waste and pine cone (1:1, w/w) for biochar production via co-pyrolysis at the temperatures of 200 and 500 C for 2 h. The reduction of the biochar yields derived from the mixed feedstock was 79% (at 200 C) to 33% (at 500 C). Sewu et al. (2017) co-pyrolyzed the mixed feedstock that is consisting of Saccharina japonica (10 wt%) and a spent mushroom substrate (90 wt%) at a temperature of 500 C with a heating rate of 10 C/min under a nitrogen atmosphere, producing a 28% yield of biochar. Reduction of biochar production with increasing operating temperature was also observed in the co-pyrolysis of mixed-feedstock owing to the composition of biomass. In the relationship between feedstock and biochar production, as mentioned in Table 3, the ash content of feedstock is a factor that greatly influences increasing the yield of the solid product. Ash content not only increases biochar yield but also has a positive effect on applications (Tumuluru et al. 2011). These substances in biochar may provide biological or physical advantages when using soil remediation and become effective components of electrode materials for electron transfer (Lee et al. 2018). For example, sewage sludge with high ash content (22-35% of feedstock) that is continuously increasing in quantity may be a promising feedstock for high biochar production (Leng et al. 2018; Prajitno et al. 2018; Mujahid et al. 2020). In addition, it has been reported that the ash content of the feedstock serves as a catalyst in the formation of char during pyrolysis (Nowakowski et al. 2007). Therefore, many studies have attempted to produce biochar by inducing different thermochemical reactions (dehydration, decarboxylation, cracking, polymerization) through co-pyrolysis mixed with other biomass compared to single pyrolysis. For example, Huang et al. (2017) produced co-pyrolyzed biochar prepared by mixing rice straw or sawdust with sewage sludge and compared it with single sewage sludge derived biochar production. Biochar production was carried out by pyrolyzing a mixed feedstock consisting of sewage sludge and sawdust in a 1:1 mass ratio. Yields decreased with increasing temperature, as follows: 93% (300 C), 69% (400 C), 54% (500 C), 49% (600 C), and 46% (700 C). In addition, the production of feedstock mixed with sewage sludge and rice straw decreased from 88% to 45% in the temperature range of 300 to 700 C. It was observed that the yield of biochar from co-pyrolysis was a little more reduced compared to that of single sewage biochar (88%, 72%, 66%, 61%, and 55%) due to the low ash content of the added feedstock (rice straw and sawdust; second-generation). Jin et al. (2017) compared the yield of biochar derived from single sewage sludge and sewage sludge added with bamboo sawdust (1:1, w/w) produced through pyrolysis from 400 to 600 C for 1 h under a nitrogen atmosphere. The yields of sewage sludge biochar was reduced slightly from 60%, 57%, and 53% with increasing pyrolysis temperature because of its high ash contents in the feedstock. However, the addition of bamboo sawdust, mainly consisting of carbohydrates decomposed during the operating temperatures of 400 to 600 C, resulted in the reduction of biochar yields compared to that of single sewage sludge. Sewage sludge-derived biochar production through co-pyrolysis with feedstock having low ash content influenced the yield. Furthermore, there has been biochar research based on different feedstock mixed at various weight ratios with sewage sludge. Wang et al. (2019) prepared biochar by co-pyrolysis of sewage sludge and cotton stalks with different mixing ratios (cotton stalks/sewage sludge, w/w; from 1:9 to 9:1) under nitrogen atmosphere at an operating temperature of 650 C for 2 h. The biochar yields decreased from 57% (1:9, w/w) to 32% (9:1, w/w), which shows that biochar production is highly dependent on mixed feedstock with sewage sludge.
During pyrolysis, carrier gas in the reactor has been found to affect the elimination of volatile substances, influencing the yield of biochar production. Konczak et al. (2019) also produced biochar pyrolyzed at 500, 600, and 700 °C from sewage sludge and willow (w/w; 8:2 and 6:4). As the amount of willow in the feedstock increased, and the pyrolysis reaction temperature increased, the yield of co-pyrolysis biochar was decreased. In addition, the effect of carrier gas type (N2 or CO2) on the production of biochar was investigated. During pyrolysis in the presence of CO2, various volatile compounds are produced and affect the reduction of biochar yield. This has been confirmed in other mixed feedstock-based biochar production. For example, Song et al. (2020) carried out N2, N2/CO2, and CO2-assisted co-pyrolysis of Pteris vittata and textile dyeing sludge for 30 min at 550, 750, and 950 °C at the three heating conditions. Biochar yield from mixed feedstock (7:3, w/w) decreased 36% (at N2), 20% (at N2/CO2), and 19% (at CO2) at 950 C heating as the CO2 volume of carrier gas increased.
Table 4. Summary of Mixed Feedstock and Co-pyrolysis Conditions for Biochar Production
The yield of biochar production under nitrogen gas was 17% less than that of carbon dioxide. CO2 atmosphere obviously affected biochar yield, which can be attributed to easy release decomposed molecules such as CO, CO2, and CH4 from feedstock in response to atmospheric gas type.
In addition to sewage sludge, there has been research on biochar production through co-pyrolysis using pig manure as feedstock with high ash content. Meng et al. (2018) produced biochar co-pyrolyzed from mixed feedstock that follows mass ratios 1:0, 0:1, 1:3, and 3:1 of rice straw and pig manure. The co-pyrolysis yields decreased markedly with increasing pyrolysis temperature due to the decomposition of cellulose, lignin, and hemicellulose in feedstock. Under the same co-pyrolysis condition, biochar derived from manure (1:0) alone had the highest yield of 35 to 61% at 300 to 700 C. This was attributed to higher ash contents in the feedstock, resulting in higher biochar yields at the same pyrolysis temperature. Biochar yields decreased with the increasing mass ratio of rice straw in mixed feedstock due to the lower ash content of rice straw (ash: 11%) than pig manure (ash: 36%). Biochar yield is related to a mass ratio of rice straw in the mixed feedstock.
High ash content in feedstock increased the yield of biochar production due to its high thermal decomposition property. High ash content in sewage sludge and manure classified in fourth-generation biomass has been found to be critical for producing a high yield of biochar. This is because the ash content has higher thermal stability than the carbohydrates. Although significant merit of co-pyrolysis with mixed feedstock was not observed, various biomass for biochar production was expanded widely.
BIOCHAR CHARACTERIZATION AND APPLICATION
Analytical Methods for Biochar Characterization
Biochar has unique physical and chemical properties such as specific surface area, surface functional groups, and degree of graphitization, and its characteristics are highly affected by the type of feedstock (first to fourth generation of biomass) and the pyrolysis conditions (pyrolysis type, operating temperature, retention time, etc.). Therefore, understanding of charring properties of the produced biochar is essential to its use in various fields. Physical characterization (moisture content, density, particle size and morphology) and chemical characterization (elemental analysis, surface functional groups, and surface charge) are determined by scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy dispersive X-ray spectroscopy (EDX), Fourier transform infrared spectrometry (FT-IR), X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), elemental analysis (EA), Brunauer, Emmett, and Teller (BET), and Barrett-Joyner-Halenda (BJH) (Fig 2).
Fig. 2. Analytical methods for biochar characterization
BET analysis is used to determine the specific surface area in the units of m2/g by measuring the quantity of gas adsorbed as a function of pressure at a low temperature. Pore volume and pores size distribution are determined by BJH calculation of the adsorption and desorption isotherms. SEM is used to observe the surface morphology and the pore of the produced biochar. TEM is used to determine the lattice constant of biochar produced by high-temperature pyrolysis. EDX, which is equipped with the electron microscope, is used to identify the various elemental compositions of biochar. In addition, a quantitative analysis of main components of C, H, N, O, S is obtained by EA. The surface chemical property of biochar can be determined by FT-IR and XPS. FT-IR is used to investigate the functional groups present on the surface of biochar. Each functional group is determined as a vibration at a specific position in infrared spectroscopy. In general, the functional groups present on the biochar surface have a peak indicating the stretching of hydroxyl at 3400 cm-1, stretching of carbonyl at 1600 cm-1, and vibration of the carboxylate at 1260 cm-1. XPS is utilized to determine the chemical composition and binding interactions by analyzing the energy distribution of photoelectrons of biochar surfaces. XRD is used to analyze the carbon crystallinity and identify the crystalline minerals. Typically, a narrow and sharp reflection pattern represents the graphitic carbon of biochar, while a broad reflection pattern indicates the non-graphitized carbon.
Biochar Application in Various Fields
The myriad uses of fossil fuels and the resulting environmental pollution have created severe problems for humans and ecosystems. Challenges for an eco-friendly and sustainable environment have been performed, such as the development of adsorbents, energy storage devices, and biofuels, and one of the promising materials for these applications is biochar. Application of biochar in various fields based on feedstock, pyrolysis condition, and characterization was summarized in Table 5.
Biochar, which is derived from low-cost biomass via thermochemical conversion, is widely investigated to use for adsorbent from the aqueous and atmosphere systems. Biochar as an adsorbent should be modified according to the state of the adsorbate for exhibiting the superb adsorption capacity. In addition, the maximum adsorption capacity of biochar is one of the important factors in adsorbent development. These properties are highly dependent on feedstock and pyrolysis conditions; therefore, the optimum parameters for manufacturing high performance adsorbent should be investigated thoroughly. Recently, biochar production according to various feedstock’s (including mixed feedstock) and pyrolysis conditions and the application of improved adsorbents (aqueous and atmosphere) have been reported. Wang et al. (2020) prepared cork-based biochar for Cu ion adsorbent prepared by slow pyrolysis at 750 °C for 0.5 h under N2 atmosphere with a gas flow of 0.3 L/min. Especially, the physicochemical properties of the biochar from different pyrolysis temperatures (450, 550, 650, and 750 °C) and pyrolysis times (0.5, 1.0, 1.5, and 2.0 h) were demonstrated by EA, FT-IR, BET, and SEM. Surface chemical properties determined by EA and FT-IR appeared to have high aromaticity and lower polarity as increasing pyrolysis temperature. The maximum specific surface area of 392.5 m2/g and pore volume of 0.24 m3/g were obtained by the heating temperature at 750 °C, which were 50-fold and 25-fold higher than feedstock, respectively. In addition, the thin cell walls and many pores produced by high pyrolysis temperature and long pyrolysis time affected the increase of specific surface area. The increased surface area analyzed by BET measurement and SEM was a significant factor for showing 18.5 mg/g of Cu ion adsorption. Gao et al. (2020) produced jujube pit-based biochar for Pb ion adsorbent using in aqueous solution. A feedstock is a byproduct of agricultural waste that is considered as fourth-generation biomass. In this work, biochar was produced by a pyrolysis at 800 °C for 2 h under oxygen-limited condition and characterized by SEM, BET, BJH, and FT-IR. The surface morphology observed by SEM is rough, and the layered structure enlarges the surface area, providing more adsorption sites.
Table 5. Summary of Biochar Applications Based on Feedstock, Pyrolysis Conditions, and Characterization
Enough active site of biochar for adsorption was measured as the specific surface area of 247 m2/g and average pore volume of 0.054 m3/g calculated by BET and BJH methods. FT-IR confirmed the hydroxyl groups on the surface derived from carboxylic acids. The maximum adsorption of Pb ion was 137 mg/g due to the large surface area created by pyrolysis. Chen et al. (2018) prepared macroalgae-derived biochar for making dye adsorbent under different pyrolysis temperatures (400, 600, and 800 °C) with a heating rate of 15 °C/min at a nitrogen atmosphere. A feedstock is a third-generation biomass. The highest yield of biochar was 46.2% at pyrolysis of 400 °C among other conditions (35.2% at 600 °C and 22.6% at 800 °C). The physicochemical properties were demonstrated by EA, FT-IR, SEM, and BET to determine pyrolysis conditions for a promising dye adsorbent. According to EA and FT-IR, the surface properties of biochar were confirmed to have high aromaticity interacting with dye molecules as the pyrolysis temperature increased. Also, surface morphology observed by SEM showed the effect of pyrolysis temperatures. The smooth surface of the biochar from 400 °C was changed to a rough and porous structure with increasing temperature (800 °C). This was related to the increasing specific surface area from 33.2 m2/g (at 400 °C) to 133.2 m2/g (at 800 °C) measured by BET. Physicochemical properties of the biochar at 800 °C positively affect the outstanding malachite green adsorption of 5306.2 mg/g. Lgalavithana et al. (2020) made biochar derived from the combination of food waste and wood waste by co-pyrolysis of gasification. The produced biochar using food waste (fourth-generation biomass) was developed for CO2 adsorbent. Most of all, the enlarged specific surface area and pore size distribution characterized by BET and BJH methods were significant factors for CO2 adsorbent. High ash contents in biochar derived from food waste reduced specific surface area, however, the textural property was improved about 8-fold by KOH activation. Due to chemical treatment, biochar derived from the mixed feedstock via gasification is a promising CO2 adsorbent for a sustainable environment.
In the development of energy storage devices, carbon is a typical substance for electrode materials due to its high level of electrical conductivity. Therefore, biochar, mainly composed of high carbon content, is one of the promising materials as electrode for batteries (Li-ion, Na-ion, Li-S, and Metal-air) and supercapacitors. Biochar derived from biomass is expected to massively supply a core material for energy storage devices and contribute to a sustainable environment. Murali et al. (2019) reported that the peanut shells were utilized for carbonization by a scalable and low-power microwave method. Before carbonization, sample was washed with hot deionized water (DI) to remove dust, dirt, and other impurities and then dried and powdered in a household mixer grinder. Then, it was transformed into a porous material by sulfuric acid and potassium hydroxide activation. This was confirmed by SEM. The low-temperature method produced a surface area of 525 m2/g, and it was confirmed by BET, XRD, Raman, XPS, and TEM. The peanut shell-derived carbon delivered a reversible specific capacity of 680 mAh/g at a 0.05 C rate after 100 cycles. Similarly, Wang et al. (2018) utilized the egg yolk-derived carbon to fabricate nitrogen-doped carbon dots (N-CDs) through pre-carbonization and hydrothermal treatment. The feedstock was mixed with borax to prepare for N-doping. Then the mixture was calcined at 700 °C for 2 h under N2 atmosphere, and through XPS, it was confirmed. Likewise, various analysis methods (SEM, XRD, Raman, TEM) confirmed that the surface of calcined egg yolk was porous, and the crystallinity was analyzed. Also, the egg yolk derived carbon dots exhibited reversible capacities of 600 and 712 mAh/g at the current densities of 500 and 100 mA/g, respectively. Supercapacitors (SC) are attracting considerable attention in energy storage applications, including hybrid electric vehicles, memory backup devices, etc., due to their high-power capability, fast charge-discharge rates, and long cycle life. SC can be classified as electric double-layer capacitors (EDLCs), pseudocapacitors, and hybrid capacitors based on their energy storage mechanism. In EDLC, porous carbon materials such as activated carbon, mesoporous carbon, and their derivatives have been requested to facilitate the storage of static electricity. A recent study proposes biomass-derived carbon as an interesting candidate for developing a wide range of SC electrodes or substrates with effective pseudocapacitive behavior (Senthil and Lee 2021). There have been several reports on pore restructuring and surface modification to develop high-performance SCs.
According to Lee et al. (2018), a study was conducted on the carbonization of microalga sludge (MS) resulting from microalga extraction and evaluating its potential as an electrode feedstock. Biochar was obtained by operating temperature of 800 °C under N2 (0.3 L/min) for 3 h. Surface morphology and carbon crystalline were investigated by SEM and TEM. Also, the surface chemical property was analyzed with XPS. The composition of the mediator was analyzed using FT-IR. As a result, the performance of the enzymatic fuel cell (EFC) system was estimated to be a power density of 3.1 mW/cm2 and a current density of 9.7 mA/cm2.
Likewise, there have been reports using abundant agricultural waste biomass (second-generation) as a feedstock for SC. Kim et al. (2021) reported on carbonization of a rice straw as a feedstock for SC. To utilize this, raw rice straw was pretreated with HNO3 before pyrolysis to enhance the structure of graphite carbon. As a result, the specific surface area and graphitic carbon structure of biochar were increased, and it was confirmed by SEM. After that, pyrolysis was performed at 900 °C for 2 h at a heating rate of 10 °C/min in a nitrogen atmosphere. The surface modification and evaluation of biochar components were confirmed by FT-IR, XRD, XPS, and Raman methods.
Biodiesel (fatty acid methyl ester; FAME), which is converted from triacylglycerol, is attracting attention as an alternative to petroleum diesel. Typically, FAME is converted through the transesterification of oils and alcohols (mostly methanol) using a homogeneous basic or acid as a catalyst, such as KOH, NaOH, and H2SO4. Conventional catalysts used for biodiesel conversion have high conversion rates, but they cannot be recycled, and thus a separation process is essential. Recently, acid/alkali functionalized biochar as a solid supporter has been reported as a reusable catalyst for biodiesel conversion. Bhatia et al. (2020) prepared acid-functionalized biochar via slow pyrolysis for conversion of waste cooking oil into biodiesel. Biochar obtained at operating temperature of 600 °C was determined among pyrolysis conditions (400, 600, and 800 °C) based on SEM, BET, and BJH analyses. These analytical techniques presented the porous structure and large specific surface area which are beneficial to the catalytic activity. Biochar catalyst activated by H2SO4 was analyzed by FT-IR and XPS for investigating the surface chemical property. As a result, acid-functionalized biochar performed 98% of FAME conversion and showed an 86% conversion after 5 cycles. Gonzalez et al. (2017) developed a microwave reactor for biodiesel production using acid-functionalized biochar derived from lignocellulosic biomass. The specific surface area of biochar produced at 600 °C was 48.3 m2/g and it decreased to 5.43 m2/g after acid activation. However, FAME conversion increased about 70% due to the acidic activation, which was demonstrated by the surface chemical property, as confirmed by FT-IR and XPS. It is noteworthy that biochar as an acidic solid catalyst successfully performed biodiesel production under the harsh condition of microwave irradiation. Zhao et al. (2018) synthesized alkali-functionalized biochar derived from pomelo peel through pyrolysis at 600 °C for 2 h. The pomelo peel biochar increased the specific surface area identified by BET by KOH activation by 41.5 times compared to the initial state. The presence of K2CO3, an alkaline modification to provide catalytic activity, was confirmed by XRD. BET and SEM were used to demonstrate that K2CO3 had an effect on reduction of the specific surface area by blocking the pores of biochar. Although specific surface area associated with catalytic activity became lower, the highest FAME conversion was 98% due to K2CO3 on biochar surface, and reusability was 82% conversion after 8 cycles. Zhong et al. (2019) used coconut shell powder for making a biochar catalyst produced by slow pyrolysis at 550 °C for 1 h. Designing of acid-functionalized hydrophobic surface was suggested to prevent the side reaction of hydrolysis while esterification and transesterification proceeded for biodiesel production. Acidic activation on the surface performed by hydrothermal method was analyzed by FT-IR showing an O-S bond in the sulfonic group and by EA exhibiting S content. Hydrophobicity was demonstrated by measuring the water contact angle (WCA), which determines the surface whether hydrophilic or hydrophobic. A novel surface modification by amino-arenesulfonic acids showed high conversion (96.7% for esterification and 86.3% for transesterification) compared with commercial sulfonated material (amberlyst-15; 86.7% and 39.9%). Therefore, biochar can be a promising supporter of catalyst for biodiesel conversion through chemical and physical modifications.
CONCLUDING STATEMENTS
Biomass is an attractive feedstock in the bioindustry to replace the petroleum-based materials, as it is carbon neutral and abundant in nature. Biomass has been used to produce bioenergy and biomaterials, and in recent years, the scope of application is expanding to biochar production. Most of the biochar studies reported to date have been carried out on a laboratory-scale and focused on the analysis of the products without standardized process parameters of pyrolysis. In order to produce biochar with consistent yield and quality on an industrial scale, an understanding of the whole process is essential. In this review, the following types of published studies were considered to satisfy the required characteristics of biochar, which has a high potential for application in various fields in near future, and to suggest a strategy for producing it more effectively.
- In order to establish a systematic strategy for biochar production, the feedstock biomass was classified into first, second, third, and fourth generations, respectively, and its composition was investigated focusing on non-edible biomass.
- Various pyrolysis methods for biochar production were introduced, and production yields according to feedstock and process parameters were investigated.
- Biochar production technology using mixed feedstock, which has recently been highlighted, were discussed, and reports on production yield according to mixed feedstock and co-pyrolysis conditions were summarized.
- The analytical methods for the unique physicochemical properties of biochar were investigated and the applications based on feedstock, pyrolysis conditions, and characterization were summarized.
Securing the diversity of feedstock and systematic determination of pyrolysis conditions will play a key role in the economic production of biochar with required characteristics on an industrial scale.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT (MSIT) (NRF-2019R1A2C1006793 and NRF-2020R1C1C1005060).
REFERENCES CITED
Abo-State, A., Ragab, A. M. E., EL-Gendy, N. S., Farahat, L. A., and Madian, H. R. (2014). “Bioethanol production from rice straw enzymatically saccharified by fungal isolates, Trichoderma viride F94 and Aspergillus terreus F98,” Soft 3(2), 19-29. DOI: 10.4236/soft.2014.32003
Ahmadzadenia, Y., Nazera, K., Hezave, S. G., Hejazi, M. A., Ghavidel, S. Z., Hassanpour, S., and Chaichisemsari, M. (2011). “Effect of replacing fishmeal with Spirulina on carcass composition of rainbow trout,” ARPN J Agric Biol Sci. 6(6), 1-6.
Allen, J. A., and Downie, A. E. (2020). “Predicting slow pyrolysis process outcomes with simplified empirical correlations for a consistent higher heating temperature: Biochar yield and ash content,” Energy Fuels 34, 14223-14231. DOI: 10.1021/acs.energyfuels.0c02597
Amiri, H., Karimi, K., and Zilouei, H. (2014). “Organosolv pretreatment of rice straw for efficient acetone, butanol, and ethanol production,” Bioresour. Technol. 152, 450-456. DOI: 10.1016/j.biortech.2013.11.038
An, H. E., Lee, K. H., Jang, Y. W., Kim, C. B., and Yoo, H. Y. (2021). “Improved glucose recovery from Sicyos angulatus by NaOH pretreatment and application to bioethanol production,” Processes 9, 245. DOI: 10.3390/pr9020245
Antar, M., Lyu, D., Nazari, M., Shah, A., Zhou, X., and Smith, D. L. (2021). “Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization,” Renew. Sust. Energ. Rev. 139, 110691. DOI: 10.1016/j.rser.2020.110691
Arapoglou, D., Varzakas, Th., Vlyssides, A., and Israilides, C. (2010). “Ethanol production from potato peel waste (PPW),” Waste Manage. 30(10), 1898-1902. DOI: 10.1016/j.wasman.2010.04.017
Aurore, G., Parfait, B., and Fahrasmane, L. (2009). “Bananas, raw materials for making processed food products,” Trends Food Sci. Technol. 20(2), 78-91. DOI: 10.1016/j.tifs.2008.10.003
Bardhan, S. K., Gupta, S., Gorman, M. E., and Haider, M. A. (2015). “Biorenewable chemicals: Feedstocks, technologies and the conflict with food production,” Renew. Sust. Energ. Rev. 51, 506-520. DOI: 10.1016/j.rser.2015.06.013
Bhatia, S. K., Gurav, R., Choi, T., Kim, H. J., Yang, S., Song, H., Park, J. Y., Park, Y., Han, Y., Choi, Y., Kim, S., Yoon, J., and Yang, Y. (2020). “Conversion of waste cooking oil into biodiesel using heterogenous catalyst derived from cork biochar,” Bioresor. Tech. 302, 122872. DOI: 10.1016/j.biortech.2020.122872
Bhattacharjee, N., and Biswas, A. B. (2019). “Pyrolysis of orange bagasse: comparative study and parametric influence on the product yield and their characterization,” J. Environ. Eng. 7, 102903. DOI: 10.1016/j.jece.2019.102903
Binda, G., Spanu, D., Bettinetti, R., Magagnin, L., Pozzi, A., and Dossi, C. (2020). “Comprehensive comparison of microalgae-derived biochar from different feedstocks: A prospective study for future environmental applications,” Algal Res. 52, 102103. DOI: 10.1016/j.algal.2020.102103
Biswas, B., Singh, R., Krishna, B. B., Kumar, J., and Bhaskar, T. (2017). “Pyrolysis of azolla, sargassum tenerrimum and water hyacinth for production of bio-oil,” Bioresour. Technol. 242, 139-145. DOI: 10.1016/j.biortech.2017.03.044
Cassales, A., Souza-Cruz, P. B. d., Rech, R., and Ayub, M. A. Z. (2011). “Optimization of soybean hull acid hydrolysis and its characterization as a potential substrate for bioprocessing,” Biomass Bioenerg. 35(11), 4675-4683. DOI: 10.1016/j.biombioe.2011.09.021
Chen, Y. Lin, Y., Ho, S., Zhou, Y., and Ren, N. (2018). “Highly efficient adsorption of dyes by biochar derived from pigments-extracted macroalgae pyrolyzed at different temperature,” Bioresour. Tech. 259, 104-110. DOI: 10.1016/j.biortech.2018.02.094
Choi, J.-S., Bae, H.-J., Kim, Y.-C., Kim, T.-B., Choi, Y.-J., Choi, E.-Y., Park, S.-M., and Choi, I.-S. (2008). “Nutritional composition and biological activities of the methanol extracts of sea mustard (Undaria pinnatifida) in market,” Kor. J. Life Sci. 18(3) 387-394. DOI: 10.5352/JLS.2008.18.3.387
Chu, K. H., and Feng, X. (2013). “Enzymatic conversion of newspaper and office paper to fermentable sugars,” Process Saf. Environ. Protect. 91(1-2), 123-130. DOI: 10.1016/j.psep.2011.12.003
Chun, Y., Ko, Y. G., Do, T., Jung, Y., Kim, S. W., Chun, Y, J., and Choi, U. S. (2018). “Electrorheological properties of algae dispersed suspension: New application of harmful algae,” Colloid. Surf. A – Physicochem. Eng. Asp. 539, 354-363. DOI: 10.1016/j.colsurfa.2017.12.022
Chun, Y., Ko, Y. G., Do, T., Jung, Y., Kim, S. W., and Choi, U. S. (2019). “Spent coffee grounds: Massively supplied carbohydrate polymer applicable to electrorheology,” Colloid. Surf. A – Physicochem. Eng. Asp. 562, 392-401. DOI: 10.1016/j.colsurfa.2018.11.005
Ciolkosz, D., and Wallace, R. (2011). “A review of torrefaction for bioenergy feedstock production,” Biofuels Bioprod. Biorefining 5(3), 317-329. DOI: 10.1002/bbb.275
Dalena, F., Senatore, A., Iulianelli, A., Di Paola, L., Basile, M., and Basile, A. (2019). “Ethanol from biomass: Future and perspectives,” in: Ethanol, pp. 25-59. Elsevier. DOI: 10.1016/B978-0-12-811458-2.00002-X
Demirbas, A. (2010). “Fuels from biomass,” in: Biorefineries. Green Energy and Technology, Springer, London. DOI: 10.1007/978-1-84882-721-9_2
Deng, S., Tan, H., Wang, X., Yang, F., Cao, R., Wang, Z., and Ruan, R. (2017). “Investigation on the fast co-pyrolysis of sewage sludge with biomass and the combustion reactivity of residual char,” Bioresour. Technol. 239, 302-310. DOI: 10.1016/j.biortech.2017.04.067
Devi, N. M., Prasad, R. V., and Palmei, G. (2018). “Physico-chemical characterisation of pumpkin seeds,” Int. J. Chem. Stud. 6(5), 828-831.
Dhillon, G. S., Brar, S. K., Verma, M., and Tyagi, R. D. (2011). “Utilization of different agro-industrial wastes for sustainable bioproduction of citric acid by Aspergillus niger,” Biochem. Eng. J. 54(2) 93-92. DOI: 10.1016/j.bej.2011.02.002
Do, J.-R., Nam, Y.-J., Park, J.-H., and Jo, J.-H. (1997). “Studies on chemical composition of red algae,” J. Korean Fish. Soc. 30(3), 428-431.
Eylen, D. V., Dongen, Fv., Kabel, M., and Bont, Jd. (2011). “Corn fiber, cobs and stover: Enzyme-aided saccharification and co-fermentation after dilute acid pretreatment,” Bioresour. Technol. 102(10), 5995-6004. DOI: 10.1016/j.biortech.2011.02.049 10.1016/j.biortech.2011.02.049
Gan, Y. Y., Ong, H. C., Show, P. L., Ling, T. C., Chen, W., Yu, K. L., and Abdullah, R. (2018). “Torrefaction of microalgal biochar as potential coal fuel and application as bio-adsorbent,” Energy Convers. Manag. 165, 152-162. DOI: 10.1016/j.enconman.2018.03.046
Gao, J., Liu, Y., Yang, M., Wang, J., and Chen, Y. (2020). “A promising and cost-effective biochar adsorbent derived from jujube pit for the removal of Pb(2) from aqueous solution,” Sci. Rep. 10, 7473. DOI: 10.1038/s41598-020-64191-1
Garcia-Nunez, J. A., Pelaez-Samaniego, M. R., Garcia-Perez, M. E., Fonts, I., Abrego, J., Westerhof, R. J. M., and Garcia-Perez, M. (2017). “Historical developments of pyrolysis reactors: A review,” Energy Fuels 31(6), 5751-5775. DOI: 10.1021/acs.energyfuels.7b00641
Gonzalez, M. E., Cea, M., Reyes, D., Romero-Hermoso, L., Hidalgo, P., Meier, S., Benito, N., and Navia, R. (2017). “Functionalization of biochar derived from lignocellulosic biomass using microwave technology for catalytic application in biodiesel production,” Energy Convers. Manag. 137, 168-173. DOI: 10.1016/j.enconman.2017.01.063
Gundekari, S., Mitra, J., and Varkolu, M. (2020). “Classification, characterization, and properties of edible and non-edible biomass feedstocks,” in: Advanced Functional Solid Catalysts for Biomass Valorization, Elsevier, Netherlands, Chapter 4. DOI: 10.1016/B978-0-12-820236-4.00004-0
Hassan, M. A. M., and Hussein, S. M. (2017). “Chemical and technological studies on pink grapefruit (Citrus paradise L.) peels. 1-effect of storage conditions on gross chemical composition, phytochemical components and oil stability of pink grapefruit peels,” World J. Dairy & Food Sci. 12(2) 115-123. DOI: 10.5829/idosi.wjdfs.2017.115.123
He, X., Liu, Z., Niu, W., Yang, L., Zhou, T., Qin, D., Niu, Z., and Yuan, Q. (2018). “Effects of pyrolysis temperature on the physicochemical properties of gas and biochar obtained from pyrolysis of crop resides,” Energy 143, 746-756. DOI: 10.1016/j.energy.2017.11.062
Hersh, B., Mirkouei, A., Sessions, J., Rezaie, B., and You, Y. (2019). “A review and future directions on enhancing sustainability benefits across food-energy-water systems: the potential role of biochar-derived products,” AIMS Environ. Sci. 6(5), 379-416. DOI: 10.3934/environsci.2019.5.379
Huang, H., Yang, T., Lai, F., and Wu, G. (2017). “Co-pyrolysis of sewage sludge and sawdust/rice straw for the production of biochar,” J. Anal. Appl. Pyrolysis 125, 61-68. DOI: 10.1016/j.jaap.2017.04.018
Hwang, J.-H., Kim, N.-G., Woo, H.-C., Rha, S.-J., Kim, S.-J., and Shin, T.-S. (2014). “Variation in the chemical composition of Saccharina Japonica with harvest area and culture period,” J. Aquac. Res. Development 5(7), 2155-9546. DOI: 10.4172/2155-9546.1000286
Ibrahim, M. F., Kim, S. W., and Abd-Aziz, S. (2018). “Advanced bioprocessing strategies for biobutanol production from biomass,” Renew. Sustain. Energy Rev. 91, 1192-1204. DOI: 10.1016/j.rser.2018.04.060
Jacobsson, S., and Johnson, A. (2000). “The diffusion of renewable energy technology: an analytical framework and key issues for research,” Energy Policy 28(9), 625-640. DOI: 10.1016/S0301-4215(00)00041-0
Jang, Y. W., Lee, K. H., and Yoo, H. Y. (2021). “Improved sugar recovery from orange peel by statistical optimization of thermo-alkaline pretreatment,” Processes 9(3), 409. DOI: 10.3390/pr9030409
Jiang, B., Zhang, Y., Guo, T., Zhao, H., and Jin, Y. (2018). “Structural characterization of lignin and lignin-carbohydrate complex (LCC) from ginkgo shells (Ginkgo biloba L.) by comprehensive NMR spectroscopy,” Polymers 10(7), 736. DOI: 10.3390/polym10070736
Jin, J., Wang, M., Cao, Y., Wu, S., Liang, P., Li, Y., Zhang, J., Zhang, J., Wong, M. H., Shan, S., and Christie, P. (2017). “Cumulative effects of bamboo sawdust addition on pyrolysis of sewage sludge: Biochar properties and environmental risk from metals,” Bioresour. Technol. 228, 218-226. DOI: 10.1016/j.biortech.2016.12.103
Jung, Y. H., Kim, I. J., Kim, J. J., Oh, K. K., Han, J.-I., Choi, I.-G., and Kim, K. H. (2011). “Ethanol production from oil palm trunks treated with aqueous ammonia and cellulase,” Bioresour. Technol. 102(15), 7307-7312. DOI: 10.1016/j.biortech.2011.04.082
Jung, K., Jeong, T., Kang. H., and Ahn, K. (2016). “Characteristics of biochar derived from marine macroalgae and fabrication of granular biochar by entrapment in calcium-alginate beads for phosphate removal for aqueous solution,” Bioresour. Technol. 211, 108-116. DOI: 10.1016/j.biortech.2016.03.066
Kim, K. H., Kim, J., Cho, T., and Choi, J. W. (2012). “Influence of pyrolysis temperature on physicochemical properties of biochar obtained from the fast pyrolysis of pitch pine (Pinus rigida),” Bioresour. Technol. 118, 158-162. DOI: 10.1016/j.biortech.2012.04.094
Kim, J. H., Affan, A., Jang, J., Kang, M.-H., Ko, A.-R., Jeon, S.-M., Oh, C., Heo, S.-J., Lee, Y.-H., Ju, S.-J., and Kang, D.-H. (2015). “Morphological, molecular, and biochemical characterization of astaxanthin-producing green microalga Haematococcus sp. KORDI03 (Haematococcaceae, Chlorophyta) isolated from Korea,” J. Microbiol. Biotechnol. 25(2) 238-246. DOI: 10.4014/jmb.1410.10032.
Kim, C. K., Choi, H. S., Lee, S. J., Lee, J. H., Lee, J. H., Yoo, H. Y., Han, S. O., and Kim, S. W. (2018a). “Production of xylanase from a novel engineered Pichia pastoris and application to enzymatic hydrolysis process for biorefinery,” Process Biochem. 65, 130-135. DOI: 10.1016/j.procbio.2017.11.001
Kim, J. A., Vijayaraghavan, K., Reddy, D. H. K., and Yun, Y. (2018b). “A phosphorus-enriched biochar fertilizer from bio-fermentation waste: A potential alternative source for phosphorus fertilizers,” J. Clean. Prod. 196, 163-171. DOI: 10.1016/j.jclepro.2018.06.004
Kim. H. R., Lee, J. H., Lee, S. K., Chun, Y., Park, C., Jin, J., Lee, H. U., and Kim, S. W. (2021). “Fabricating a modified biochar-based all-solid-state flexible microsupercapacitor using pen lithography,” J. Clean. Prod. 284(15), 125449. DOI: https: 10.1016/j.jclepro.2020.125449
Konczak, M., Oleszczuk, P., and Rozylo, K. (2019). “Application of different carrying gases and ratio between sewage sludge and willow for engineered (smart) biochar production,” J. CO2 Util. 29, 20-28. DOI: 10.1016/j.jcou.2018.10.019
Laird, D. A., Brown, R. C., Amonette, J. E., and Lehmann, J. (2009). “Review of the pyrolysis platform for coproducing bio-oil and biochar,” Biofuels Bioprod. Biorefining 3, 547-562. DOI: 10.1002/bbb.169
Lee, J. H., Kim, D. S., Yang, J. H., Chun, Y. S., Yoo, H. Y., Han, S. O., Lee, J. Y., Park, C., and Kim, S. W. (2018). “Enhanced electron transfer mediator based on biochar from microalgal sludge for application to bioelectrochemical systems,” Bioresour. Technol. 264, 387-390. DOI: 10.1016/j.biortech.2018.06.097
Lee, J. H., Lee, H. U., Lee, J. H., Lee, S. K., Yoo, H. Y., Park, C., and Kim, S. W. (2019). “Continuous production of bioethanol using microalgal sugars extracted from Nannochloropsis gaditana,” Korean J. Chem. Eng. 36(1), 71-76. DOI: 10.1007/s11814-018-0173-y
Lee, K. H., Chun, Y. Jang, Y. W., Lee, S. K., Kim, H. R., Lee, J. H., Kim, S. W., Park, C., and Yoo, H. Y. (2020). “Fabrication of functional bioelastomer for food packaging from Aronia (Aronia melanocarpa) juice processing by-products,” Foods 9(11), 1565. DOI: 10.3390/foods9111565
Lee, K. H., Jang, Y. W., Kim, H., Ki, J. S., and Yoo, H. Y. (2021a). “Optimization of lutein recovery from Tetraselmis suecica by response surface methodology,” Biomolecules 11, 182. DOI: 10.3390/biom11020182
Lee, K. H., Jang, Y. W., Lee, J., Kim, S., Park, C., and Yoo, H. Y. (2021b). “Statistical optimization of alkali pretreatment to Improve sugars recovery from spent coffee grounds and utilization in lactic acid fermentation,” Processes 9(3), 494. DOI: 10.3390/pr9030494
Lee, K. H., Lee, S. K., Lee, J., Kim, S., Park, C., and Kim, S. W. (2021c). “Improvement of enzymatic glucose conversion from chestnut shells through optimization of KOH pretreatment,” Int. J. Environ. Res. Public Health 18(7), 3772. DOI: 10.3390/ijerph18073772
Leng, L., Li, J., Yuan, X., Han, P., Hong, Y., Wei, F., and Zhou, W. (2018). “Beneficial synergistic effect on bio-oil production from co-liquefaction of sewage sludge and lignocellulosic biomass,” Bioresour. Technol. 251, 49-56. DOI: 10.1016/j.biortech.2017.12.018.
Lgalavithana, A. D., Choi, S. W., Dissanayke, P. D., Shang, J., Wang, C., Yang, X., Kim. S., Tsang, D. C. W., Lee, K. B., and Ok, Y. S. (2020). “Gasification biochar from biowaste (food waste and wood waste) for effective CO2 adsorption,” J. Hazard. Mater. 391, 121147. DOI: 10.1016/j.jhazmat.2019.121147
Li, S., Zhu, D., Li, K., Yang, Y., Lei, Z., and Zhang, Z. (2013). “Soybean curd residue: Composition, utilization, and related limiting factors,” Int. Sch. Res. Notices 2013, 8. DOI: 10.1155/2013/423590
Li, S., Harris, S., Anandhi, A., and Chen, G. (2019). “Predicting biochar properties and functions based on feedstock and pyrolysis temperature. A review and data syntheses,” J. Clean Prod. 215, 890-902. DOI: 10.1016/j.jclepro.2019.01.106
Li, Y., Xing, B., Ding, Y., Han, X., and Wang, S. (2020). “A critical review of the production and advanced utilization of biochar via selective pyrolysis of lignocellulosic biomass,” Bioresour. Technol. 312, 123614. DOI: 10.1016/j.biortech.2020.123614
Masek, O., Brownsort, P., Cross, A., and Sohi, S. (2013). “Influence of production conditions on the yield and environmental stability of biochar,” Fuel 103, 151-155. DOI: 10.1016/j.fuel.2011.08.044
Meng, J., Liang, S., Tao, M., Liu, X., Brookers, P. C., and Xu, J. (2018). “Chemical speciation and risk assessment of Cu and Zn in biochars derived from co-pyrolysis of pig manure with rice straw,” Chemosphere 200, 344-350. DOI: 10.1016/j.chemosphere.2018.02.138
Meyer, S., Glaser, B., and Quicker, P. (2011). “Technical, economical, and climate-related aspects of biochar production technologies: A literature review,” Envrion. Sci. Technol. 45(22), 9473-9483. DOI: 10.1021/es201792c
Meyer, S., Genesio, L., Vogel, I., Schmidt, H.-P., Soja, G., Someus, E., Shackley, S., Verheijen, F. G. A., and Glaser, B. (2017). “Biochar standardization and legislation harmonization,” J. Environ. Eng. Landsc. Manag. 25(2), 175-191. DOI: 10.3846/16486897.2016.1254640
Mhgub, I. M., Hefnawy, H. T., Gomaa, A. M., and Badr, H. A. (2018). “Chemical composition, antioxidant activity and structure of pectin and extracts from lemon and orange peels,” Zagazig J. Agric. Res. 45(4), 1395-1404. DOI: 10.21608/zjar.2018.48589
Mohsenzadeh, A., Jeihanipour, A., Karimi, K., and Taherzadeh, M. J. (2012). “Alkali pretreatment of softwood spruce and hardwood birch by NaOH/thiourea, NaOH/urea, NaOH/urea/thiourea, and NaOH/PEG to improve ethanol and biogas production,” J. Chem. Technol. Biotechnol. 87(8), 1209. DOI: 10.1002/jctb.3695
Murali, G., Harish, S., Ponnusamy, S., Ragupathi, J., Therese, H. A., Navaneethan, M., and Muthamizhchelvan, C. (2019). “Hierarchically porous structured carbon derived from peanut shell as an enhanced high rate anode for lithium ion batteries,” Appl. Surf. Sci. 492(30), 464-472. DOI: 10.1016/j.apsusc.2019.06.142.
Murray, D., Parsons, S. A., Jarvis P., and Jefferson, B. (2010). “The impact of barley straw conditioning on the inhibition of Scenedesmus using chemostats,” Water Res. 44(5), 1373-1380. DOI: 10.1016/j.watres.2009.11.014
Mujahid, R., Riaz, A., Insyani, R., and Kim, J. H. (2020). “A centrifugation-first approach for recovering high-yield bio-oil with high calorific values in biomass liquefaction: A case study of sewage sludge,” Fuel 262(15), 116628. DOI: 10.1016/j.fuel.2019.116628
Naik, S. N., Goud, V. V., Rout, P. K., and Dalai, A. K. (2010). “Production of first and second generation biofuels: a comprehensive review,” Renew. Sustain. Energy Rev. 14(2), 578-597. DOI: 10.1016/j.rser.2009
.10.003
Naik, D. K., Monika, K., Prabhakar, S., Parthasarathy, R., and Satyavathi, B. (2017). “Pyrolysis of sorghum bagasse biomass into bio-char and bio-oil products.” J. Therm. Anal. Calorim. 127, 1277-1289. DOI: 10.1007/s10973-016-6061-y
Nowakowski, D. J., Jones, J. M., Brydson, R. M. D., and Ross, A. B. (2007). “Potassium catalysis in the pyrolysis behavior of short rotation willow coppice,” Fuel 86, 2389-2403. DOI: 10.1016/j.fuel.2007.01.026
Park, C., Lee, A. H., Yang, X., Yoo, H. Y., Lee, J. H., Lee, S. K., and Kim, S. W. (2016). “Enhancement of hydrolysis of Chlorella vulgaris by hydrochloric acid,” Bioprocess Biosyst Eng. 39, 1015-1021. DOI: 10.1007/s00449-016-1570-4
Pandey, D., Daverey, A., and Arunachalam, K. (2020). “Biochar: Production, properties and emerging role as a support for enzyme immobilization,” J. Clean. Prod. 255, 120267. DOI: 10.1016/j.jclepro.2020.120267
Prasad, S., Singh, A., and Joshi, H. C. (2007). “Ethanol as an alternative fuel from agricultural, industrial and urban residues,” Resour. Conserv. Recycl. 50(1), 1-39. DOI: 10.1016/j.resconrec.2006.05.007
Prasetyo, J., and Park, E. Y. (2013). “Waste paper sludge as a potential biomass for bio-ethanol production,” Korean J. Chem. Eng. 30, 253-261. DOI: 10.1007/s11814-013-0003-1
Prajitno, H., Park, J., Ryu, C., Park, H. Y., Lim, H. S., and Kim, J. (2018). “Effects of solvent participation and controlled product separation on biomass liquefaction: A case study of sewage sludge,” Appl. Energy 218, 402-416. DOI: 10.1016/j.apenergy.2018.03.008
Qin, L., Wu, Y., Hou, Z., and Jiang, E. (2020). “Influence of biomass components, temperature and pressure on the pyrolysis behavior and biochar properties of pine nut shells,” Bioresour. Technol. 313, 123682. DOI: 10.1016/j.biortech.2020.123682
Queirós, C. S. G. P., Cardoso, S., Lourenço, A., Ferreira, J., Miranda, I., Lourenço, M. J. V., and Pereira, H. (2019). “Characterization of walnut, almond, and pine nut shells regarding chemical composition and extract composition,” Biomass Convers. Biorefinery 10, 175-188. DOI: 10.1007/s13399-019-00424-2
Ruiz-Gomez, N., Quispe, V., Abrego, J., Atienza-Martinez, M., and Murillo, M. B. (2017). “Co-pyrolysis of sewage sludge and manure,” Waste Manage. 59, 211-221. DOI: 10.1016/j.wasman.2016.11.013
Sareena, C., Sreejith, M. P., Ramesan, M. T., and Purushothaman, E. (2013). “Biodegradation behaviour of natural rubber composites reinforced with natural resource fillers – Monitoring by soil burial test,” J. Reinf. Plast. Compos. 33(5), 412-429. DOI: 10.1177/0731684413515954
Selvarajoo, A., and Oochit, D. (2020). “Effect of pyrolysis temperature on product yields of palm fibre and its biochar characteristics,” Mater. Sci. Energy Technol. 3, 575-583. DOI: 10.1016/j.mset.2020.06.003
Senthil, C. and Lee, C. W. (2021). “Biomass-derived biochar materials as sustainable energy sources for electrochemical energy storage devices,” Renew. Sustain. Energy Rev. 137, 110464. DOI: 10.1016/j.rser.2020.110464
Sewu, D. D., Boakye, P., Jung, H., and Woo, S. H. (2017). “Synergistic dye adsorption by biochar from co-pyrolysis of spent mushroom substrate and Saccharina japonica,” Bioresour. Technol. 244, 1142-1149. DOI: 10.1016/j.biortech.2017.08.103
Silva, M. P., Nieva Lobos, M. L., Piloni, R. V., Dusso, D., Quijon, M. E. G., Scopel, A. L., and Moyano, E. L. (2020). “Pyrolytic biochars from sunflower seed shells, peanut shells and Spirulina algae: Their potential as oil amendment and natural growth regulators,” SN Appl. Sci. 2, 1926. DOI: 10.1007/s42452-020-03730-x
Siripong, P., Doungporn, P, Yoo, H. Y., and Kim, S. W. (2018). “Improvement of sugar recovery from Sida acuta (Thailand Weed) by NaOH pretreatment and application to bioethanol production,” Korean J. Chem. Eng. 35, 2413-2420. DOI: 10.1007/s11814-018-0170-1
Song, Y., Hu, J., Liu, J., Evrendile, K. F., and Buyukada, M. (2020). “CO2-assisted co-pyrolysis of textile dyeing sludge and hyperaccumulator biomass: Dynamic and comparative analyses of evolved gases, bio-oils, biochars, and reaction mechanisms,” J. Hazard. Mater. 400, 123190. DOI: 10.1016/j.jhazmat.2020.123190
Suman, S., and Gautam, S. (2017). “Pyrolysis of coconut husk biomass: Analysis of its biochar properties,” Energy Sources Part A-Recovery Util. Environ. Eff. 8, 761-767. DOI: 10.1080/15567036.2016.1263252
Sundar, S., Bergey, N. S., Salamanca-Cardona, L., Stipanovic, A., and Driscoll, M. (2014). “Electron beam pretreatment of switchgrass to enhance enzymatic hydrolysis to produce sugars for biofuels,” Carbohydr. Polym. 100, 195-201. DOI: 10.1016/j.carbpol.2013.04.103
Taha, F. S., Wagdy, S. M., Hassanein, M. M. M., and Hamed, S. F. (2012). “Evaluation of the biological activity of sunflower hull extracts,” Grasas y Aceites 63(2), 184-192. DOI: 10.3989/gya.072111
Tumuluru, J. S., Sokhansanj, S., Wright, C. T., Boardman, R. D., and Yancey, N. A. (2011). “A review on biomass classification and composition, co-firing issues and pretreatment methods,” Am. Soc. Agric. Biol. Eng. 2011. DOI: 10.13031/2013.37191
Tursi, A. (2019). “A review on biomass: Importance, chemistry, classification, and conversion,” Biofuel Res. J. 22, 962-979. DOI: 10.18331/BRJ2019.6.2.3
Vieira, F. R., Luna, C. M. R., Arce, G. L. A. F., and Avila, I. (2020). “Optimization of slow pyrolysis process parameters using a fixed bed reactor for biochar yield from rice husk,” Biomass Bioenerg. 132, 105412. DOI: 10.1016/j.biombioe.2019.10541
Waghmare, A. G., Salve, M. K., LeBlanc, J. G., and Arya, S. S. (2016). “Concentration and characterization of microalgae proteins from Chlorella pyrenoidosa,” Bioresour. Bioprocess. 3, 16. DOI: 10.1186/s40643-016-0094-8
Wang, Z., Xu, J., Pandey, P., Cheng, A. J., Li, R., and Qu, R. (2012). “Improvement of sugar production from transgenic switchgrass with low-temperature alkali pretreatment,” Energy Fuels 26(5), 3054-3061. DOI: 10.1021/ef3004575
Wang, T., Zhai, Y., Zhu, Y., Li, C., and Zheng, G. (2018a). “A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties,” Renew. Sust. Energ. Rev. 90, 223-247. DOI: 10.1016/j.rser.2018.03.071
Wang, S., Wang, H., Zhang, R., Zhao, L., Wu, X., Xie, H., Zhang, J., and Sun, H. (2018b). “Egg yolk-derived carbon: Achieving excellent fluorescent carbon dots,” J. Alloys Compd. 746(25), 567-575. DOI: 10.1016/j.jallcom.2018.02.293
Wang, Z., Xie, L., Liu, K., Wang, J., Zhu, H., Song, Q., and Shu, X. (2019). “Co-pyrolysis of sewage sludge and cotton stalks,” Waste Manage. 89, 430-438. DOI: 10.1016/j.wasman.2019.04.033
Wang, Z., Lai, Z., Mun, J., Chu, D., and Zhang, X. (2020). “Conversion industrial waste cork to biochar as Cu (Ⅱ) adsorbent via slow pyrolysis,” Waste Manag. 105, 102-109. DOI: 10.1016/j.wasman.2020.01.041
Wang, F., Ouyang, D., Zhou, Z., Page, S. J., Liu, D., and Zhao, X. (2021). “Lignocellulosic biomass as sustainable feedstock and materials for power generation and energy storage,” J. Energy Chem. 57, 247-280. DOI: 10.1016/j.jechem.2020.08.060
Weldekidan, H., Strezov, V., He, J., Kumar, R., Asumadu-Sarkodie, S., Doyi, I. N. Y., Jahan, S., Kan, T., and Town, G. (2019). “Energy conversion efficiency of pyrolysis of chicken litter and rice husk biomass,” Energy fuels 33(7), 6509-6514. DOI: 10.1021/acs.energyfuels.9b01264
Wi, S. G., Chung, B. Y., Lee, Y. G., Yang, D. J., and Bae, H.-J. (2011). “Enhanced enzymatic hydrolysis of rapeseed straw by popping pretreatment for bioethanol production,” Bioresour. Technol. 102(10), 5788-5793. DOI: 10.1016/j.biortech.2011.02.031
Yan, W., Hastings, J. T., Acharjee, T. C., Coronella, C. J., and Vasquez, V. R. (2010). “Mass and energy balances of wet torrefaction of lignocellulosic biomass,” Energy Fuels 24(9) 4738-4742. DOI: 10.1021/ef901273n
Yang, H.-C., Jung, K.-M., Gang, K.-S., Song, B.-J., Lim, H.-C., Na, H.-S., Mun, H., and Heo, N.-C. (2005). “Physicochemical composition of seaweed fulvescens (Capsosiphon fulvescens),” Korean J. Food Sci. Technol. 37(6), 912-917.
Yang, X., Lee, J. H., Yoo, H. Y., Shin, H. Y., Thapa, L. P., Park, C., and Kim, S. W. (2014). “Production of bioethanol and biodiesel using instant noodle waste,” Bioprocess. Biosyst. Eng. 37, 1627-1635. DOI: 10.1007/s00449-014-1135-3.
Yang, S. J., Yoo, H. Y., Choi, H. S., Lee, J. H., Park, C., and Kim, S. W. (2015a). “Enhancement of enzymatic digestibility of Miscanthus by electron beam irradiation and chemical combined treatments for bioethanol production,” Chem. Eng. J. 275, 227-234. DOI: 10.1016/j.cej.2015.04.056
Yang, X., Choi, H. S., Park, C. H., and Kim, S. W. (2015). “Current states and prospects of organic waste utilization for biorefineries,” Renew. Sustain. Energy Rev. 49, 335-349. DOI: 10.1016/j.rser.2015.04.114
Yang, X., Wang, H., Strong, P. J., Xu, S., Liu, S., Lu, K., Sheng, K., Guo, J., Che, L., He, L., Ok, Y. S., Yuang, G., Shen, Y., and Chen, X. (2017). “Thermal properties of biochars derived from waste biomass generated by agricultural and forestry sectors,” Energies 10, 469-480. DOI: 10.3390/en10040469
Yang, X., Ng, W., Wong, B. S. E., Baeg, G. H., Wang, C., and Ok, Y. S. (2019). “Characterization and ecotoxicological investigation of biochar produced via slow pyrolysis: effect of feedstock composition and pyrolysis conditions,” J. Hazard. Mater. 365, 178-185. DOI: 10.1016/j.jhazmat.2018.10.047
Yang, X., Kang, K., Qiu, L., Zhao, L., and Sun, R. (2020). “Effects of carbonization conditions on the yield and fixed carbon content of biochar from pruned apple tree branches,” Renew. Energy 146, 1691-1699. DOI: 10.1016/j.renene.2019.07.148
Yoo, H. Y., Pradeep, G. C., Lee, S. K., Park, D. H., Cho, S. S., Choi, Y. H., Yoo, J. C., and Kim, S. W. (2015). “Understanding β-mannanase from Streptomyces sp. CS147 and its potential application in lignocellulose based biorefining,” Biotechnol. J. 10, 1894-1902. DOI: 10.1002/biot.201500150
Yoo, H. Y., Lee, J. H., Kim, D. S., Lee, J. H., Lee, S. K., Lee, S. J., Park, C., and Kim, S. K. (2017). “Enhancement of glucose yield from canola agricultural residue by alkali pretreatment based on multi-regression models,” J. Ind. Eng. Chem. 51, 303-311. DOI: 10.1016/j.jiec.2017.03.018
Yoo, H. Y., and Kim, S. W. (2021). “The next-generation biomass for biorefining,” BioResources 16(2), 2188-2191.
Yuan, P., Wang, J., Pan, Y., Shen, B., and Wu, C. (2019). “Review of biochar for the management of contaminated soil: preparation, application and prospect,” Sci. Total Environ. 659, 473-490. DOI: 10.1016/j.scitotenv.2018.12.400
Zhao, C., Lv, P., Yang, L., Xing, S., Luo, W., and Wang, Z. (2018). “Biodiesel synthesis over biochar-based catalyst from biomass waste pomelo peel,” Energy Convers. Manag. 160, 477-485. DOI: 10.1016/j.enconman.2018.01.059
Zhang, Y., Yang, L., Wang, D., and Li, D. (2017). “Structure elucidation and properties of different lignins isolated from acorn shell of Quercus variabilis Bl,” Int. J. Biol. Macromol. 107, 1193-1202. DOI: 10.1016/j.ijbiomac.2017.09.099
Zhang, Z., Zhu, Z., Shen, B., and Liu, L. (2019). “Insights into biochar and hydrochar production and applications: A review,” Energy 171, 581-598. DOI: 10.1016/j.energy.2019.01.035
Zhang, R., Zhang, X., Tang, Y., and Mao, J. (2020). “Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: A review,” Carbohydr. Polym. 228(15), article no. 115381. DOI: 10.1016/j.carbpol.2019.115381
Zhong, Y., Deng, Q., Zhang, P., Wang, J., Wang, R., Zeng, Z., and Deng, S. (2019). “Sulfonic and functionalized hydrophobic mesoporous biochar. Design, preparation and acid-catalytic properties,” Fuel 240, 270-277. DOI: 10.1016/j.fuel.2018.11.152
Article submitted: March 2, 2021; Peer review completed: March 28, 2021; Revised version received and accepted: July 16, 2021; Published: July 21, 2021.
DOI: 10.15376/biores.16.3.Chun
