Abstract
Technical lignins are becoming an attractive natural, renewable, and non-toxic ingredient in sunscreens, having the capability for replacing synthetic compounds. Researchers have reported that lignin can increase the solar protection factor (SPF) of sunscreens and provide sun protection to body creams. However, to achieve the valorization of lignin in the fabrication of personal care products, it is necessary to overcome several challenges related to their molecular complexity and unattractive color. Fractionation, chemical modification, whitening, particle size reduction, and the synthesis of nanocomposites and copolymers are strategies reported to overcome the lignin challenges in the development of lignin-based sunscreens. This paper summarizes and analyzes previous research studies and outstanding findings (from 2016 to 2022) directed at the reduction of the problems that limit the extensive applications of lignin in skincare products such as sunscreens.
Download PDF
Full Article
Recent Progress in the Production of Lignin-based Sunscreens: A Review
José Luis Espinoza-Acosta,* Evelyn Guadalupe Figueroa-Espinoza, and María de los Ángeles De la Rosa-Alcaraz *
Technical lignins are becoming an attractive natural, renewable, and non-toxic ingredient in sunscreens, having the capability for replacing synthetic compounds. Researchers have reported that lignin can increase the solar protection factor (SPF) of sunscreens and provide sun protection to body creams. However, to achieve the valorization of lignin in the fabrication of personal care products, it is necessary to overcome several challenges related to their molecular complexity and unattractive color. Fractionation, chemical modification, whitening, particle size reduction, and the synthesis of nanocomposites and copolymers are strategies reported to overcome the lignin challenges in the development of lignin-based sunscreens. This paper summarizes and analyzes previous research studies and outstanding findings (from 2016 to 2022) directed at the reduction of the problems that limit the extensive applications of lignin in skincare products such as sunscreens.
DOI: 10.15376/biores.17.2.Espinoza
Keywords: Lignin; Skincare products; Natural sunscreens; Sustainable resources; Sun protection factor (SPF); UV shielding activity; Skin protection; Cosmetic applications
Contact information: Department of Food Technology Engineering, State University of Sonora, P. O. Box 85875, Navojoa, Sonora, México; *Corresponding authors: jose.espinoza@ues.mx; angeles.rosa@ues.mx
GRAPHICAL ABSTRACT

INTRODUCTION
Personal care products have become an essential part of our lives for maintaining good personal hygiene and beautification. In recent years, products for hair, nails, and skincare have gained importance globally (Savary et al. 2016). Among these, hair and skincare products are the most used and encompass more than half of the worldwide market (Owh et al. 2016). Skincare products help keep skin healthy, enhance its appearance, and help to prevent skin conditions. For that, the cosmetic industry offers many skincare products such as exfoliators, facial treatments, serums, face oils, anti-aging beauty masks, moisturizers, eye creams, chemical peels, sunscreens, and so on.
Regarding skincare, protection against UV radiation has become a great interest topic for many persons. Prolonged periods of UV radiation can trigger skin problems that can appear quickly as sunburns, melanomas, premature aging, spots, freckles, or after a long time, like skin cancer. Using physical barriers such as umbrellas, adequate clothing, or hats helps reduce UV radiation to the skin. Each year, the UV radiation that reaches the earth obtains higher values in some areas of the planet. For this reason, the use of sunscreen is necessary together with some physical barriers to achieve complete protection of the skin. By definition, sunscreens are personal care products (cream, lotions, gels, sticks, and/or sprays) whose objective is to protect the skin against UV radiation from the sun rays. Sunscreens began to be developed and studied only since the second half of the last century, and since that time the use of sunscreens on a population-wide scale started to spread rapidly (Trivedi and Murase 2017). According to their active ingredients, commercial sunscreens can be separated into two categories; (1) minerals sunscreens and (2) organic chemical sunscreens (Qian et al. 2015). In mineral sunscreens, the active ingredients are mainly inorganic particles, such as zinc oxide (ZnO) and titanium dioxide (TiO2), which provide protection from the sunlight by sitting on top of the skin and blocking or reflecting UV radiation (Lewicka et al. 2011). In addition, rutile and anatase, two important types of TiO2 have the ability to absorb UV radiation. Although mineral sunscreen has demonstrated good photostability and low photoallergic reaction, the integration of ZnO and TiO2 on a nanometric scale in sunscreens has raised interesting questions regarding dermal penetration, systemic absorption, toxicity, as well as contamination of water by inorganic compounds from sunscreens (Johnson et al. 2011; Gondikas et al. 2014; Sharma et al. 2019). By contrast, organic chemical sunscreens contain synthetic molecules (e.g., aminobenzoic acid derivatives, benzophenones, cinnamates, salicylates, avobenzone, ecamsule, ensulizole, bemotrizole, and bisoctrizole) with conjugated carbonyl groups that absorb the long wave UV (UVA) and/or medium wave UV (UVB) radiation (Chrétien et al. 2006). For instance, cinnamates show good protection action against UVB and UVA rays; however, they are photo-unstable and have poor resistance to water. Aminobenzoates and salicylates have a low molar absorbance coefficient in the UVA region, while benzophenones have a low molar absorbance coefficient in the UVB region (Dondi et al. 2006; Brugè et al. 2014). Furthermore, unpleasant feelings on the skin are caused by mineral-based sunscreens, and although chemical sunscreens are comfortable on the skin, the chemical compounds may cause unexpected problems on the skin, especially for people who have skin conditions.
With the growing concern about personal safety, health risk, and the environmental impact of synthetic ingredients, the search for natural compounds for applications in personal care products, especially in sunscreens has increased. In this context, biopolymers from animals and plants (Sionkowska et al. 2020) such as chitosan (Casadidio et al. 2019), cellulose (Bianchet et al. 2020), and alginates (Savary et al. 2016) have gained attention for replacing thickeners, active agent carriers, emulsifiers, rheology modifiers, stabilizers gelling agents, and antibacterial active agents in skincare products and cosmetics.
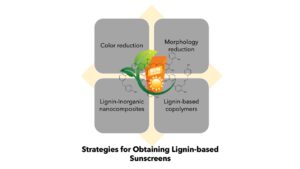
In contrast, in less than a decade, lignin, a natural complex phenolic polymer obtained in large quantities as a waste of paper and bioethanol production has gained a lot of attention as an interesting biopolymer for applications in sunscreens. Qian et al. (2015) demonstrated the efficacy of lignin for increasing the solar protection factor (SPF) of commercial sunscreens. Subsequent research found that lignin has a synergistic effect with active ingredients in these commercial sunscreens (Qian et al. 2016). A remarkable aspect of lignin is its ability to reduce the formation of free radicals. This antioxidant activity is relevant for sunscreen and cosmetics applications. Following up in the research in Qian’s group, several researchers have been studying the use of different lignins in the formulation of innovative lignin-based sunscreens. In addition to the excellent antioxidant capacity of lignin, biological activities, such as antimicrobial activity, antiviral activity, antimutagenic activity, and UV absorption properties (Espinoza-Acosta et al. 2016), could make it suitable for developing broad-spectrum sunscreens (Gordobil et al. 2018, 2020). However, problems associated with the nature of lignin, such as the molecular complexity, the great variety of linkages and functional groups, the dark color, as well as the lack of evidence regarding lignin toxicity, have limited the exhaustive use as a bio-based ingredient in sunscreen formulations (Sadeghifar and Ragauskas 2020). Physical or chemical whitening, chemical modification, size reduction, fractionation/modification, and the synthesis of lignin-based nanocomposites are strategies that are helping to introduce the technical lignins in skincare applications. In this work, the current information of the strategies aimed at boosting the use of lignin in sunscreen is summarized. Additionally, the safety and cytotoxicity of lignin as a greener alternative to petroleum-based ingredients in the fabrication of these skincare products were reviewed.
Background of Lignin
Lignin is a complex, amorphous, three-dimensional, and highly branched network of macromolecules (Figueiredo et al. 2018). Woody and non-woody plants are mainly made up of carbohydrate-type polymers (cellulose and hemicellulose 70 to 80 wt%), followed by lignin (around 20 to 30 wt%), and other minor compounds including lipids, proteins, and minerals (5 to 10 wt%). In plants, lignin serves as a natural glue to hold the adjacent cells together, and it is responsible for providing strength and rigid structure to plant cell walls due to multiple chemical bondings of hemicellulose and cellulose microfibrils (Haq et al. 2020). In the cell wall of plants, the presence of lignin offers resistance to decay, resistance against chemical and biological attacks, as well as water impermeability. Unlike other polymers, lignin exhibits a lack of uniformity and repeatability of its building blocks. The building blocks of lignin polymer (so-called monolignols) are mainly three types of phenylpropanoid structures: para-coumaryl, coniferyl, and sinapyl alcohols. These three aromatic alcohols are transported to the plant cell wall, where they are called guaiacyl (G), p-hydroxyphenyl (H), and syringyl (S). The radical random coupling of these monolignols gives as a result the lignin polymer. The proportions of H, G, and S units are highly variable according to the plant type (Billa et al. 1998). For instance, the hardwoods are abundant in GS units, gymnosperms are rich in G units, and HGS-units are present only in grasses (Buranov and Mazza 2008). The dominant linkages in the lignin polymer are β–O–4´, α–O–4´, β–β´, β–5´, 5–O–4´, and 5–5´ (Fig. 1). In addition, the molecule of lignin contains various functional groups such as O–CH3, phenolic and aliphatic –OH, C=O groups, and phenyl groups (Tang et al. 2020).
In the cell wall of plants, lignin is found covalently bound to cellulose and hemicellulose, and these multiple interactions make separation of lignin especially difficult. In the papermaking industries, and cellulosic ethanol production, different separation methodologies or treatments are applied to separate the lignin from the rest of the lignocellulosic polymers (Espinoza-Acosta et al. 2018). For many years, the lignins obtained from papermaking have been under the sights of research to provide added value. In pulp and paper industries, traditionally, lignin is burned to produce steam, heat, and energy. However, in the last few decades, the valorization of lignin has received more attention due to the introduction of modern biorefineries that produce cellulosic fuels and generate large amounts of lignin with high purity. High-purity lignins could be the key to the development of advanced, high-quality products for the cosmetic, pharmaceutical, and food industries, as well as the skincare industry.
Fig. 1. (a) Monomeric lignin precursors (monolignols), (b) the corresponding general structural units in lignin, and (c) the most common covalent linkages depicted in lignin. Main reference: Österberg et al. (2020)
Technical Lignins
In the different lignin extraction processes, the lignocellulosic biomass is submitted to treatment with solvents, heat, and pressure in the presence or absence of catalyst for hours. Under such conditions, lignin undergoes partial depolymerization, solubilization, and in this way, it can be recovered as an insoluble fraction rich in aromatic compounds.
The lignin obtained as an insoluble fraction after the treatment of lignocellulosic biomass (herbaceous and woody biomasses) with organic acids, alkaline solutions, or alcohols is known as industrial lignin or technical lignins. The most common technical lignins are lignosulphonates, kraft lignin, soda lignin, and organosolv lignin, and all of them are highly available in large amounts (Vishtal and Kraslawski 2011). There are other types of lignins obtained using alternative treatments, such as enzymes, or ionic liquids, that can also be considered technical lignins; however, they are produced in small amounts and do not constitute part of the potentially valuable lignins. The name assigned to each technical lignin denotes the process used for its extraction. Table 1 shows the critical characteristics of the technical lignins.
Because the focus of this work is not the detailed review of the extraction processes for obtaining technical lignins, this issue is only briefly discussed. Plenty of information about the extraction process for obtaining technical lignins can be found already available in original research or reviews (Vishtal and Kraslawski 2011; Berlin and Balakshin 2014; Demuner et al. 2019; Kazzaz and Fatehi 2020).
Table 1. Main Characteristics and Physical Properties of Technical Lignins. Adapted from Berlin and Balakshin (2014)
Among other topics, these studies documented the importance and the impact of the extraction processes on the properties and characteristics of the technical lignin, presenting new information about the composition and structure of technical lignins from new lignocellulosic biomasses as well as the challenges that lignins must overcome to expand their uses in industrial applications. Over several years technical lignins have shown applications in various fields. These applications take advantage of one or more characteristics of each technical lignin. For example, lignosulfonates are preferred for dispersing and binding applications because of their specific solubility in water (Hu et al. 2011; Yu et al. 2013). Kraft lignins are used in the formulation of phenolic resins (Demuner et al. 2019). Organosolv lignins have found applications as starting material for obtaining fine chemicals due to their high purity (Rodrigues Pinto et al. 2012). Reported potential applications of technical lignins are shown in Table 2.
Table 2. Technical Lignin and Their Potential Applications
Until now, the application market for technical lignins has been extremely limited (around 1% and 2%). Many of the applications are for developing low-value products. However, the search for new applications is being pursued in pharmacological and biomedical industries (Domínguez-Robles et al. 2020), foods (Gil-Chávez et al. 2021), cosmetics (Gordobil et al. 2020), and human health areas (Vinardell and Mitjans 2017). Many such studies are focusing their interest on the exploration of the beneficial properties of technical lignins in skin care applications.
The enormous availability, the phenolic nature, and the biological activity of the different technical lignins has attracted considerable attention due to their application as a multifunctional ingredient in the development of sunscreens. A lot of the information available in the literature reports the incorporation of lignin as a bio-based ingredient in sunscreens. The incorporation of low amounts of lignin in sunscreens can act as a broad-spectrum UV blocker (protecting against UVA and UVB rays), has a synergistic effect when it is combined with synthetic and mineral UV absorbers, and is capable of providing sun protection in commercial body creams (Qian et al. 2015). Additionally, due to its antimicrobial and antioxidant activity, the incorporation of lignin can act as a preservative, inhibiting the growth of harmful microorganisms and replacing the use of antioxidants of chemical origin, which have been considered as environmental contaminants to water (Shu et al. 2021).
However, the use of lignin in the skincare area is a big challenge due to the great structural complexity, polydispersity, and the presence of impurities that characterize the different types of technical lignins. In addition, the intense dark brown pigmentation of lignin is transferred to the products where it is applied, such as body creams or sun blockers. The interesting dark brown color of lignin-based skincare products is in stark contrast to the white color of commercial skincare products. To face the complications that the use of lignin represents for the development of skincare products, lignin has been subjected to chemical modifications, the alteration of its morphology, the reduction of the particle size (micro- and nanometric size), reducing its color, and it has also been combined with synthetic and mineral active ingredients used in commercial sunscreens.
Technical Lignins as Ultraviolet Blocking
Technical lignins are gaining attention in sunscreen formulations due to their high abundance, non-toxicity, biodegradability, antioxidant and antimicrobial properties, UV-absorbing ability, and excellent oxidation resistance. The UV-absorbing ability of technical lignins has been attributed to their numerous functional groups, such as ketones, quinoid structure, phenolics, and other chromophores, which are able to absorb UV light in the range of 250 to 400 nm (Paulsson and Parkås 2012). In addition, the chromophores and auxochromic groups make the lignin color reddish-brown to black. Auxochromic groups are defined as a group of atoms bearing non-bonding electrons able to increase the chromophore effect. The aromatic rings have been described as the main chromophoric moiety of the structural units of lignin (Widsten 2020). A spectrum of lignin light absorption in the UV and visible light range and different chromophore groups with their approximate wavelength absorptions are indicated in the Fig. 2.
Fig. 2. Chromophores in lignin structure and their UV absorption spectra. Main references: Polcin and Rapson (1971); Paulsson et al. (2012); Sadeghifar and Ragauskas (2020).
As is well known, UV radiation can be placed into three categories as UVC, UVB, and UVA rays. The UVC rays (200 to 280 nm) have the shortest wavelength and 99% of their energy is blocked by the ozone layer. Meanwhile, UVB (280 to 315 nm) and UVA (315 to 400 nm) rays penetrate Earth’s atmosphere. Early studies have shown that the lignin polymer has the capacity of absorb UVB and small amounts of UVA rays. In the protection against UV, phenolic hydroxyl groups play a crucial role. Free and etherified phenolic hydroxyl groups in the lignin structures absorb near the UVB region, where the double bonds and carbonyl groups conjugated with phenyl groups contribute to the absorption of UVA radiation (Jablonsky et al. 2015; Ratanasumarn and Chitprasert 2020). Recent reports have mentioned that hydroxycinnamic acids, caffeic acid, coumaric acid, and ferulic acid are of great interest for the formulation of cosmetics and sunscreens (Taofiq et al. 2017). The hydroxycinnamic acids contain a specific structure (a phenolic nucleus and three-carbon side chain) which give, as a result, a resonance-stabilized phenoxy radical that is highly capable of stopping oxidation reactions, thus helping to protect against skin damage caused by UV rays exposure (Tosato et al. 2016).
Regarding the monolignols, of the HGS units that are present in each technical lignins, the S units have higher UV resistance compared to G and H units because they contain more functional methoxy groups (Zhang and Naebe 2021). Technical lignins contain more chromophoric and auxochromic structures than other lignins, such as milled wood lignin, making them more absorbent in the target UVB to UVA wavelength area (Capanema et al. 2005; Lee et al. 2019; Widsten et al. 2020a). The capacity of lignin for blocking UV light has been previously tested in varnish, oil-coated materials, microorganisms, packaging, and transparent films (Sadeghifar and Ragauskas 2020). Although the use of commercial lignins as a natural ingredient for lignin-based sunscreens is a relatively new topic, the results obtained thus far have encouraged the cosmetics industry (Taofiq et al. 2017; Tran et al. 2021). However, the correct application of lignin in industries directly related to human health and wellness requires overcoming some challenges that are present in each type of technical lignin. The main problems are associated with the heterogeneous and complex structure, poor solubility, unpleasant color and smell, as well as the lack of information about lignin cytotoxicity in applications that directly involve contact with human skin (Gao and Fatehi 2019; Piccinino et al. 2021; Schneider et al. 2021; Zhang and Naebe 2021). In the following sections, the challenges and solutions to increase the functionality of technical lignins as functional bio-based ingredients for sunscreen will be presented and discussed.
Color Reduction
Lignin is almost colorless when it is present in plants, but technical lignins, such as alkali lignin, lignosulfonates, and organosolv lignins, have a brown color. As mentioned previously, various chromophores are mainly responsible for the characteristic color of the lignin, and these chromophores are generated and introduced during the lignin extraction process (Wang et al. 2016). Chromophores in industrial lignins mostly include quinones, stilbenes, aromatic ketones, catechol, metal complexes, and conjugated carbonyl with phenolics (Azadfallah et al. 2008). The extreme complexity of lignin structure and structural variability hinders a complete elucidation and quantification of the chromophores with the current technology (Guerra et al. 2006).
To decrease the dark color of technical lignins and achieve their applications in sunscreens, the use of chemical (Zhang et al. 2017b) and physical treatments has been reported (Wang et al. 2016; Zhang et al. 2017a, 2020). An overview of the procedures to reduce the color of lignin is shown in Fig. 1. A fast procedure for the color reduction of kraft lignin (KL) was developed by Zhang et al. (2017b). In their study, KL was sulfonated using 1,4-butane under mild conditions with the addition of sodium borohydride (SB) as a catalyst and whitening agent. As a date, the free phenolic hydroxyls of lignin molecules can be transformed into quinoid structures, which will result in darker colors of lignin. During KL sulfonation, free phenolic hydroxyls are replaced by sulfonated groups (–SO3) while SB is endowed with brightness to the modified lignin.
The re-organization of the chromophores present in lignin is another pathway to regulate their color. This re-organization of the chromophores can be accomplished by turning lignin into colloidal nanospheres. Qian et al. (2014) reported changing the color of AL from dark brown to light yellow through the re-organization of their chromophores.
The fractionation of lignin in solvents such as methanol and water mixtures is a facile and economical way that allows an elimination selective of chromophores and in this way to obtain light-colored lignin (Zhang et al. 2017a, 2019b). Although the mentioned methodologies are relatively effective, the restriction or elimination of chromophore from lignin needs tedious steps and consume too much time. This has led to the question of whether there any other ways to modify the color of lignin not related to deleting its chromophores.
The application of physical methods such as drying, grinding, and sieving has shown promising results in lignin discoloration without blocking the chromophores. For example, Zhang et al. (2018) applied several drying methods to eucalyptus kraft lignin. It was found that spray and freeze-drying generate lignin as finer powders in comparison to other drying methods. Based on visual appearance, spray-drying and freeze-drying lignin showed differences in their color but not in their chemical structure. Therefore, the color variations were related to changes in the micromorphology of lignin. The formation of small particles creates a high specific surface area and large interval spaces, leading to a low bulk density. This decreased the concentration of chromophores on the microscopic scale hence the color of the lignin lightens macroscopically. Ultraviolet light (UV) irradiation is another physical treatment for the color reduction in lignin. A long-time exposure of lignin under UV gave, as a result, a highly lightened lignin. Ultraviolet irradiation produced the transformation of phenolic hydroxyls in phenoxyl radicals that form quinone structures, and these quinone structures were converted into colorless aliphatic structures by photo-oxidation (Wang et al. 2020). An overview of the main procedures to reduce the color of lignin are shown in Fig. 3.
The color of lignin can be affected by the extraction, purification, and drying process. Under this premise, Zhang et al. (2020) mentioned that if the color of lignin is taken into account from the first separation processes, subsequent color reduction treatments would be less necessary. They mentioned that condensed structures with high molecular weight and low solubility are formed during the pulping process. These condensed structures enlarge the conjugation and unsaturation degree of lignin. To separate the condensed structures from lignin, their fractionation required pH values higher than 6 and gave rise to lignin fractions with the darker color.
Fig. 3. Overview of the main procedures to reduce the color of lignin
The information reported by different researchers about lignin color reduction has shown promising results. Different methodologies for reducing the characteristic color of technical lignins have been explored. In some cases, more than one procedure to reduce the color can be applied to guarantee the best result. However, the procedure for changing the color of lignin should not cause the degradation or destruction of their aromatic structures in such a way that it does not allow a subsequent application in skin care products.
The modification of the color of technical lignins may lead the way to the cosmetics and skincare industry. However, the change in the color of the lignin should not be strictly necessary. Therefore, in attempting to incorporate colorless lignin into skincare applications, its effectiveness may be reduced or lost. Hence an attractive strategy could be the incorporation of lignin into products like sunscreens and creams for consumers with dark skin tones. It is important to remember that not everyone needs skincare products or cosmetics with light colors.
Lignin-based Copolymers
Modification of lignin can take place without depolymerizing its typical structure (Sugiarto et al. 2022). The synthesis of lignin-based copolymers through polymerization have been applied as another pathway for reducing lignin limitations in cosmetic applications. Lignin-based copolymers can be synthesized via controlled radical polymerization (Fig. 4). The main controlled radical polymerization (CRP) for obtaining lignin copolymers are atom transfer radical polymerization (ATRP), reversible addition-fragmentation chain-transfer (RAFT), ring-opening polymerization (ROP), and free radical polymerization (FRA) (Zhai et al. 2019). Among the various CRP methods, ATRP is the most common due to the tolerance of a wide range of functional monomers, narrow molecular weight distribution of grafted polymer, chemically active end groups of polymers, relatively ease control of grafted polymer lengths, and convenience in synthesis.
In ATRP, an important step to obtain lignin-based copolymers is the synthesis of an initiator. This initiator is attached to the lignin polymer backbone, followed by their polymerization. The numerous hydroxyl groups on the lignin molecule are sites for placing the initiator. In other words, lignin acts as a core unit, and new polymer chains grow on the initiating sites. CRP can be applied to different lignins to improve their miscibility, reduce their dark color, increase the content of hydroxyl groups, increase the antioxidant activity as well as improve their UV absorption.
Fig. 4. Controlled radical polymerization methods to generate novel lignin-based copolymers. Main reference: Ganewatta et al. (2019)
Lignin-poly(ethylene glycol) methacrylate (PEGMA) copolymers were synthesized via ATRP and blended in different concentrations into a commercial sunscreen (Kai et al. 2016a). The addition of lignin-PEGMA copolymer (10 wt%) doubled the SPF of commercial sunscreen creams. Furthermore, lignin-PEGMA copolymer showed better dispersion efficiency than unmodified KL, maintaining excellent radical scavenging capability after 48 h. Similarly, Zhou et al. (2020) synthesized copolymers-based lignin and polydopamine via FRA under alkaline conditions. The copolymer polydopamine-grafted alkali lignin (AL-PDA) was first applied to emulsify organic UV filters (avobenzone and ethyl methoxycinnamate), and then the organic UV filters were encapsulated in AL-PDA to prevent their penetration and damage to the human skin. The incorporation of 10 wt% of AL-PDA in the formulation of sunscreens demonstrated an unexpected SPF195. Even the SPF value was maintained after 8 h under UV irradiation. The encapsulation of organic UV filters in AL-PDA gave them good photostability and excellent resistance to washing with water. The polymerization of PDA and AL produced biocompatible and antioxidant nano-capsules with innovative active sun protection (Zhou et al. 2020). The most relevant researchers related to the synthesis of lignin copolymers and the applications as ingredient in sunscreen are shown in Table 3.
A simple, green, and effective method to improve the reactivity of enzymatic lignin (EL) was reported by Shi et al. (2020). In this study, EL was oxidized with ozone under mild conditions for generating new carbonyl groups in the aromatic ring at the surface of the lignin molecule. The carbonyl groups in ozonated lignin (OzEL) were used as esterification points to graft polyethylene glycol (PEGs). OzEL grafted to PEGs (OzEL-PEGs) afforded satisfactory water solubility, good surface activity, and good emulsifying properties. The remaining phenolic hydroxyl groups in OzEL-PEGs also maintained excellent antioxidant and UV absorbing properties.
Table 3. Lignin Copolymers Applied as Ingredient for Sunscreens
Up to the present, many efforts have been made to reduce the limitations of the use of lignin in skin care products as sunscreens. In this section, grafting modifications for endowing lignin with different properties are presented. This is a relatively new and effective pathway that takes advantage of the lignin chemical structure (especially of their aliphatic and aromatic functional groups) for creating active sites where new compounds (polymers, UV filters, and others) can be attached. The presence of such compounds in the lignin molecule improves its reactivity, reduces its limited solubility, and increases the UV absorbing properties while preserving the antioxidant activity present in the native lignin. Lignin-based copolymers are another alternative for developing a new generation of sunscreen creams.
Morphology Reduction
Disorganized and complicated lignin morphology is another obstacle encountered during the development of lignin-based cosmetics and skincare products. The synthesis of lignin particles with ordered and uniform structures has generated interest in the development of new sunscreens (Schneider et al. 2021). Emulsions and colloids based-lignin on spherical nanoparticles have been evaluated in sunscreens formulations (Nypelö et al. 2015; Österberg et al. 2020).
Lignin nanoparticles can be fabricated from different lignin types by physical, biological, and chemical methods, which produce diverse characteristics for their size, morphology, shape, stability, and yield (Tang et al. 2020). In addition, it has been mentioned that lignin micro and nanoparticles are biodegradable, non-toxic, and biocompatible compared with typical chemical ingredients used in the fabrication of commercial sunscreens (Beisl et al. 2017). Reducing the size of lignin at the micro or nano level improves its compatibility and dispersion as well as improves the ability to protect against UV radiation. Piccinino et al. (2021) mentioned that the high surface area on the lignin nanoparticles helps to increase the UV blocking activity. Furthermore, the presence of π–π stacking interaction between aromatic moieties decreases the energy gap of the electron transition and it improves the UV absorbability of the lignin nanoparticles.
In the literature, the use of micro and nanoparticles as a green antioxidant, photo-protection agent, and UV shielding for natural broad-spectrum sunscreen has been reported. Li et al. (2019) reported the lignin extraction from bamboo using different organic acid processes; then these lignins were converted into sub-micrometer particles. The preparation of the lignin particles was carried out in tetrahydrofuran (THF), recovered by dialysis in water, and their potential as a natural broad-spectrum and photo-protection agent in pure lotions was evaluated. These submicron lignin particles exhibited higher SPF than lignin-poly(ethylene glycol) methacrylate (PEGMA) copolymers, even at low concentrations. Trevisan and Rezende (2020) obtained spherical lignin nanoparticles from elephant grass (Pennisetum purpureum) with high antioxidant activity incorporated in neutral creams. The evaluation of the lignin-nanoparticles into the cream indicated great UV-vis (ultraviolet-visible) absorption, biocompatibility, and biodegradability, which is highly useful for applications in tinted sunscreens.
It has been mentioned that lignin can boost the SPF when added to conventional sunscreens (Qian et al. 2015). To know whether lignin nanoparticles have the same capability, Lee et al. (2020) studied the effect of cellulolytic enzyme lignin (CEL) and cellulolytic enzyme lignin nanoparticles (CEL-NP) in the SPF of creams and sunscreens. The authors found a remarkable synergistic effect between CEL-NP and the active ingredients of the sunscreen Biotherm SPF15. This effect was explained by the π–π interaction present in lignin nanoparticles previously reported for lignin powders (Deng et al. 2011; Ma et al. 2018). These interactions formed J-aggregates that required low energy for π–π* transition and as a consequence increased the UV absorption (Qian et al. 2016). Conversely, no synergistic effect between CEL-NP and the component of mineral sunscreen (Attitude SPF15) was found, confirming that aromatic active components in sunscreen are responsible for the synergy.
The method for preparation of lignin nanoparticles is an important factor that must be taken into account to ensure the valorization of lignin in sunscreen applications. There are many preparation methods for obtaining lignin nanoparticles with specific shapes and sizes, but most of these methodologies use toxic compounds and need extensive time for preparation (up to 24 h). Furthermore, in some cases the attention is on specific characteristics such as their size or functionality, leaving aside the yield. To solve this problem, an environmentally friendly and economic method to prepare uniform lignin nanoparticles with high yield and broad-spectrum protection for application in sunscreen was reported. A fast and easy production of lignin nanoparticles by acetylation using only acetic anhydride as both reaction agent and dispersion solvent was reported by Wang et al. (2019). Furthermore, the nanoparticles were prepared using solvent shifting accompanied by different ultrasonic intensities. As a result, uniform and stable lignin nanoparticles with a yield of up to 80% were obtained when the lignin content was 4.0 mg/mL and under intensive sonication treatment (200 W). The incorporation of these LNP in pure cream increased their SPF, but these values were still lower than those reported in other investigations. The authors attributed the low SPF to the acetylation of the lignin, which decreases the phenolic hydroxyl groups and plays an important role during the sun blocking process. In other research, a green and scalable procedure for obtaining colloidal lignin nanoparticles (cLNP) was reported by Piccinino et al. (2021). In this case, cLNP were produced using dimethyl isosorbide (DMI) and isopropylidene glycerol (IPG) as alternative solvents for those toxic and flammable organic solvents, such as THF or dimethyl sulfoxide (DMSO), commonly applied for nanoparticles synthesis. Regarding the total yield of nanoparticles, this procedure achieved recovery yields close to 90%. These values were higher than those previously reported by Wang et al. (2019).
Lignin-Inorganic Nanocomposites
Another promising route for the development broad-spectrum sunscreens with lignin involves the use of nanocomposites where synergistic interactions are highly beneficial (Lizundia et al. 2021). Titanium dioxide (TiO2) is an inorganic agent utilized in sunscreens formulation, because it possesses the ability to reflect, scatter, and absorb a wide range of UV radiation in sunlight (Sambandan and Ratner 2011). Nevertheless, the generation of reactive species such as superoxides, hydroxyl radicals, and reactive oxygen species (ROS) when TiO2 (anatase and rutile) is exposed to UV light has been associated with damage to the human skin and DNA (Jacobs et al. 2010). The photocatalytic activity of TiO2 particles has generated controversy regarding their safety for its applications in skincare products. Due to this problem, several strategies have been applied to reduce the photocatalytic activity of TiO2. Coating and encapsulation of TiO2 have demonstrated benefits for this purpose. The surface coating of TiO2 quenched its photocatalytic activity by improving the recombination of electron holes (Picatonotto et al. 2007), while the encapsulation of TiO2 particles increases their bandgap, changing the wavelength where the excitation takes place (Shen et al. 2006). Similarly, the use of antioxidants to minimize the photocatalytic activity of TiO2 has been reported (Chen et al. 2010; Zrinski et al. 2021). Antioxidants prevent and reduce the release of ROS in surrounding media maintaining the protection of TiO2 against UV irradiation.
Studies have reported the synthesis of nanocomposites based on a lignin-TiO2 combination, taking advantage of the excellent antioxidant activity of lignin (Morsella et al. 2015; Morsella et al. 2016; Nair et al. 2016; Ibrahim et al. 2019; Fournier et al. 2021). Lignin-TiO2 nanocomposites have been prepared using various methods and different types of lignins. In all the cases, the combination of lignin and TiO2 offers several advantages, such as the inhibition of the production of ROS under UV radiation, and it diminishes the photocatalytic activity of TiO2 while maintaining their photoprotection activity. These new hybrid nanomaterials take advantage of the free radical scavenging and antioxidant properties of lignin, which is effectively used as a sacrificial scavenger for ROS anticipated from TiO2 (Morsella et al. 2016). In all the above-mentioned studies, lignin always was effective for reducing the photoactivity of TiO2. However, some of these lignin-TiO2 nanocomposites were obtained using large volumes of toxic compounds, such as tetrahydrofuran (THF) and glutaraldehyde, organic solvents, and rarely, water (Nair et al. 2016). If the objective of the new materials is to be incorporated into the formulation of skincare products its production may preferably be carried out using a minimum of toxic solvents. In an attempt for developing lignin-TiO2 reducing the use of toxic solvents, Guo et al. (2020) utilized nanofibrillated cellulose (NFC) to inhibit the growth and aggregation of TiO2 crystals. The spatial structure and phenolic hydroxyls groups of lignin promote and induce the crystallization of TiO2. Lignin-TiO2@NFC were incorporated in unmodified hand creams in various percentages. The addition of 10 wt% of lignin-TiO2@NFC absorbed UV light more than 85%.
Zinc oxide (ZnO) is another ingredient used in the formulation of sunscreens due to its biological and chemical properties as well as its excellent UV protection. Researchers reported a green and one-step procedure for the synthesis of lignin-ZnO nanoparticles (Kaur et al. 2020). In this study, two types of lignin demonstrated the ability to be used as a capping and stabilizing agent for the formation and functionalization of ZnO nanocomposites. Lignin-ZnO nanoparticles incorporated as an additive in neutral creams showed UV blocking efficiency and SPF values 5 times more than native lignins and 2.5 times more than ZnO nanoparticles. The antimicrobial and UV-blocking properties in this new material could have potential applications as additives in sunscreens. Lignin-ZnO nanoparticles with coverage against the full UV spectrum was reported by Gutiérrez-Hernández et al. (2016). Soda and organosolv lignin from Agave tequilana bagasse were combined with ZnO and formaldehyde in an alkaline medium. Soda lignin and ZnO materials demonstrated the best results. The nanoparticles showed more photoprotection retaining the SPF values even after 3 h. The results of the performance of each type of lignin in combination with ZnO were related to their extraction process. An important issue is that the use of formaldehyde was not necessary to achieve high SPF values.
The combination of lignin-TiO2 can be a breakthrough for solving the photocatalytic reaction problems of the main inorganic agent utilized in sunscreen formulation. In the above-mentioned studies, the lignin-TiO2 nanocomposites showed good performance to increase the SPF of commercial sunscreens incorporated even below 10%. It is expected that in the future the cosmetics industry will adopt the use of lignin to increase the performance of its products without compromising the peace of mind of its consumers. The challenges of scale-up processes to generate, for example, hybrid materials-based lignin-TiO2 will need to be considered in advance. In this sense, Fournier et al. (2021) reported the scale-up of the photochemical synthesis of lignin@TiO2 being able to produce 50 g of lignin@TiO2 per day using a flow photoreactor with capacities of 10 to 25 L made up of multiple tubes in parallel. In addition, these authors mentioned that this photoreactor produced lignin@TiO2 with a particle size of 100 nm, reducing the liberation of free radicals and retaining the protection in light of TiO2.
Safety and Cytotoxicity of Lignin
In the last 30 years, the exploration of different types of lignin in applications that do not involve their consumption or contact directly with a human has been widely researched. These applications include the synthesis of biomaterials, pesticides, fine chemicals, and biofuels as a key step for lignin valorization (Ragauskas et al. 2014). Furthermore, the intrinsic biological activity (antioxidant, antibacterial, antitumoral, and antiviral activity) of lignins are helping the foray into applications related to pharmaceutical industries (Gil-Chávez et al. 2021), human health (Vinardell and Mitjans 2017), animal feeds (Baurhoo et al. 2008), drug delivery (Machado et al. 2020), and tissue engineering (Witzler et al. 2018). This have been a heavily pursued topic area for different research groups. However, the exploration of lignin in applications such as functional foods, food additives, and the cosmetics industry, is still limited. The absence of exploration in cosmetic has been related to the lack of regulations for the observance of its use in applications related to human health (Gil-Chávez et al. 2021). Another limiting factor is that each type of lignin shows specific characteristics related to its extraction process. This implies the undoubted need to analyze the possible consequences of using lignin in high added-value products for the cosmetics industry.
Safety and toxicity are vital aspects to evaluate when new compounds appear with the potential to provide beneficial effects on human health. For this, each of the new compounds must undergo a series of tests required by the health authorities to prove not only its effectiveness but also guarantee the safety of consumers. For example, cytotoxicity is one of the most frequent reasons for the withdrawal of approved drugs. Even natural compounds must be shown under assay to be harmless to humans during their usage. Cytotoxicity assays are the best indicators for this purpose. The evaluation of the cytotoxicity of lignins through in vitro tests has become a competitive alternative to in vivo experimentation as a consequence of ethical considerations.
Relevant results regarding the toxic effects of lignin in areas closely related to human health have been obtained (Quraishi et al. 2015; Figueiredo et al. 2017; Pishnamazi et al. 2019; Czaikoski et al. 2020). Frangville et al. (2012) reported the preparation of environmentally biodegradable lignin nanoparticles non-toxic for microalgae and yeast. More recently, Stine et al. (2021) found that plain lignin nanoparticles are safe compounds after in vivo toxicity assessment in embryonic zebrafish (Danio rerio).
The influence of variables, such as the lignin extraction process (Gil-Chávez et al. 2019), its size and morphology (Kai et al. 2016b), and the lignin concentration (Ugartondo et al. 2008), on the cytotoxicity of living cells have been evaluated. These studies are supported by the use of in vitro cytotoxicity testing that determines the viability of lignin against animal and human cells.
One of the first studies about the biological toxicity of lignin was carried out by Vinardell et al. (2008). They investigated the potential of bagasse lignin, lignosulfonates, steam explosion lignin, and curan100 lignin to irritate eyes and skin. The study demonstrated that neither of the lignins was harmful to the skin and eyes. Lignosulfonate was the least toxic of all the lignins analyzed (Vinardell et al. 2008). At the same time, all the lignins demonstrated high UV stability even at 60 days before UV irradiation, ensuring a good conservation in cosmetic formulations (Ugartondo et al. 2008). The results obtained by Ugartondo et al. (2008) opened the way for the exploration of lignin as ingredient in topical and cosmetic formulations.
Kraft or organosolv lignin with high antioxidant capacity and an effective sun protection factor was proposed as a natural additive for sun lotions (Gordobil et al. 2018). Furthermore, the capacity of kraft lignin for inducing apoptosis and necrosis on tumor and normal cells was reported (Gordobil et al. 2019). These results were obtained evaluating the kraft lignin over the in vitro cytotoxic of healthy animal cell lines (mouse hepatoma MH-22A and Chinese hamster ovary), and tumors cell lines (melanoma B16). However, an exact biological mechanism on the studied cell was not reported. Regarding the lignin extraction process effect on the cytotoxicity, sulfur-free aquasolv lignin (lignin extracted using hot water and high pressure) revealed the lowest cytotoxicity compared to alkali and organosolv lignin evaluated in Caco-2 cells (Gil-Chávez et al. 2019). Barapatre et al. (2016) evaluated the cytotoxicity of lignin fractions extracted from Acacia nilotica against breast cancer cells (MCF-7) and human hepatic stellate cells (HHSteCs). The results exhibited a cytotoxic effect against human hepatic stellate cells at concentrations up to 100 μg/mL. The lignin exploration as a new ingredient of personal care products is a recent issue. Even many people have no idea that lignin can be used in personal care products. This was corroborated in the study in an exploratory study of consumers’ knowledge and attitudes about lignin-based sunscreens (Sajinčič et al. 2021).
Up to the present, cytotoxic effects of sunscreen-based-lignin on health consumers are unknown. Nevertheless, several studies have analyzed the cytotoxicity of lignin nanodrug carriers in recent years. In this case, lignin helps in carrying pharmaceutically active ingredients to the site of action and maintains its stability during the whole process. These lignin nanoparticles exhibit good biocompatibility with normal and lung cancer cells even at very high concentrations. Studies have shown that under specific conditions lignins are not toxic. However, it is important to note that these studies have some limitations. More research is required to know the suitability of lignins, such as organosolv lignin, which are being heavily investigated to develop personal care products like broad-spectrum sunscreens, topical formulations with SPF, and lignin-based sun blockers.
The information reviewed in this paper indicates that various types of lignin could be a safe natural additive for application in cosmetic industries. The study of the effect of lignin micro- and nanoparticles incorporated in the formulation of broad-spectrum sunscreens is a topic that deserves urgent attention. Although the clinical importance of skin absorption of these lignins is not yet known, further research is suggested to determine whether there are any potential health sequelae from the absorption of lignin-based sunscreen. In this context, lignin molecules could offer a great contribution to the sunscreen field, not only in substituting synthetic products, but also because of its high availability, low price, and because its chemical properties contribute to the development of new and valuable uses.
CONCLUSIONS
- From this review, several recent advances involving the use of lignin as a potential ingredient in skin care products were analyzed. The authors focused on reviewing the strategies applied to reduce the problems related to the use of lignin in the cosmetic industry.
- One of the main problems is the characteristic brown-dark color of lignin. Light-colored lignin has been obtained by fractionation in different solvents, synthesis of lignin copolymers via polymerization reactions (ATRP and FRA), filtration and pH changes, and UV irradiation. However, it must be noted that some of these procedures can cause the degradation or destruction of the macromolecules and aromatic structures present on lignin in such a way that does not allow a subsequent utilization in skincare products.
- Another strategy is the reduction of the size of lignin at the micro or nanoscale. Lignin nanoparticles have been reported as a green antioxidant, photo-protection agent, and UV shielding agent for a broad-spectrum sunscreen. The reduction of the size of lignin at the micro or nanoscale also improves the compatibility and dispersion of lignin in lotions, creams, and commercial sunscreen as well as improves the ability to protect against UV radiation.
- Nanocomposites based on lignin-TiO2 with excellent antioxidant activity offer several advantages, such as the inhibition in the production of reactive oxygen species (ROS) under UV radiation and the diminishing of the photocatalytic activity of TiO2 particles while maintaining their photoprotection activity. These new hybrid nanomaterials take advantage of the free radical scavenging and antioxidant properties of lignin, which is effectively used as a sacrificial scavenger for ROS anticipated from TiO2.
- Synthetic additives utilized as antioxidants, preservatives, and sunlight-protectives can be reduced because of the multi-functional nature of lignin. Thus, the substitution of chemical and synthetic ingredients by lignin in sunscreen formulations will allow mitigating health and environmental impacts.
- Finally, many studies have demonstrated that under certain conditions the technical lignin showed low cytotoxicity in mammalian cells although the clinical importance of the skin absorption of these lignins is not yet known. Further research is suggested to determine whether there are any potential health sequelae from the absorption of lignin-based sunscreen.
REFERENCES CITED
Azadfallah, M., Mirshokraei, S. A., and Latibari, A. J. (2008). “Photodegradation of acidolysis lignin from BCMP,” Molecules 13(12), 3129-3139. DOI: 10.3390/molecules13123129
Barapatre, A., Meena, A., Mekala, S., Das, A., and Jha, H. (2016). “In vitro evaluation of antioxidant and cytotoxic activities of lignin fractions extracted from Acacia nilotica,” International Journal of Biological Macromolecules 86, 443-453. DOI: 10.1016/j.ijbiomac.2016.01.109
Baurhoo, B., Ruiz-Feria, C. A., and Zhao, X. (2008). “Purified lignin: Nutritional and health impacts on farm animals—A review,” Animal Feed Science and Technology 144(3-4), 175-184. DOI: 10.1016/j.anifeedsci.2007.10.016
Beisl, S., Friedl, A., and Miltner, A. (2017). “Lignin from micro- to nanosize: Applications,” International Journal of Molecular Sciences 18(11), article no. 2367. DOI: 10.3390/ijms18112367
Berlin, A., and Balakshin, M. (2014). “Chapter 18 – Industrial lignins: Analysis, properties, and applications,” in: Bioenergy Research: Advances and Applications, V. K. Gupta, M. G. Tuohy, C. P. Kubicek, J. Saddler, and F. Xu (eds.), Elsevier, Amsterdam, Netherlands, pp. 315-336. DOI: 10.1016/B978-0-444-59561-4.00018-8
Bianchet, R. T., Vieira Cubas, A. L., Machado, M. M., and Siegel Moecke, E. H. (2020). “Applicability of bacterial cellulose in cosmetics – Bibliometric review,” Biotechnology Reports 27, Article ID e00502. DOI: 10.1016/j.btre.2020.e00502
Billa, E., Koukios, E. G., and Monties, B. (1998). “Investigation of lignins structure in cereal crops by chemical degradation methods,” Polymer Degradation and Stability 59(1-3), 71-75. DOI: 10.1016/S0141-3910(97)00152-3
Brugè, F., Tiano, L., Astolfi, P., Emanuelli, M., and Damiani, E. (2014). “Prevention of UVA-induced oxidative damage in human dermal fibroblasts by new UV filters, assessed using a novel in vitro experimental system,” PLOS One 9(1), Article ID e83401. DOI: 10.1371/journal.pone.0083401
Buranov, A. U., and Mazza, G. (2008). “Lignin in straw of herbaceous crops,” Industrial Crops and Products 28(3), 237-259. DOI: 10.1016/j.indcrop.2008.03.008
Camiré, A., Espinasse, J., Chabot, B., and Lajeunesse, A. (2020). “Development of electrospun lignin nanofibers for the adsorption of pharmaceutical contaminants in wastewater,” Environmental Science and Pollution Research 27(4), 3560-3573. DOI: 10.1007/s11356-018-3333-z
Capanema, E. A., Balakshin, M. Y., and Kadla, J. F. (2005). “Quantitative characterization of a hardwood milled wood lignin by nuclear magnetic resonance spectroscopy,” Journal of Agricultural and Food Chemistry 53(25), 9639-9649. DOI: 10.1021/jf0515330
Casadidio, C., Peregrina, D. V., Gigliobianco, M. R., Deng, S., Censi, R., and Di Martino, P. (2019). “Chitin and chitosans: Characteristics, eco-friendly processes, and applications in cosmetic science,” Marine Drugs 17(6), article no. 369. DOI: 10.3390/md17060369
Çetin, N. S., and Özmen, N. (2002). “Use of organosolv lignin in phenol-formaldehyde resins for particleboard production: II. Particleboard production and properties,” International Journal of Adhesion and Adhesives 22(6), 481-486. DOI: 10.1016/S0143-7496(02)00059-3
Chantapet, P., Kunanopparat, T., Menut, P., and Siriwattanayotin, S. (2013). “Extrusion processing of wheat gluten bioplastic: Effect of the addition of kraft lignin,” Journal of Polymers and the Environment 21(3), 864-873. DOI: 10.1007/s10924-012-0557-8
Chen, X., Liu, Y., Shi, H., Wang, X., Qi, K., Zhou, X., and Xin, J. H. (2010). “Carboxymethyl chitosan coating to block photocatalytic activity of TiO2 nanoparticles,” Textile Research Journal 80(20), 2214-2222. DOI: 10.1177/0040517510376265
Chrétien, M. N., Migahed, L., and Scaiano, J. C. (2006). “Protecting the protectors: Reducing the biological toxicity of UV sunscreens by zeolite encapsulation,” Photochemistry and Photobiology 82(6), 1606-1611. DOI: 10.1562/2006-07-11-RA-967
Ciobanu, C., Ungureanu, M., Ignat, L., Ungureanu, D., and Popa, V. I. (2004). “Properties of lignin-polyurethane films prepared by casting method,” Industrial Crops and Products 20(2), 231-241. DOI: 10.1016/j.indcrop.2004.04.024
Czaikoski, A., Gomes, A., Kaufmann, K. C., Liszbinski, R. B., de Jesus, M. B., and da Cunha, R. L. (2020). “Lignin derivatives stabilizing oil-in-water emulsions: Technological aspects, interfacial rheology and cytotoxicity,” Industrial Crops and Products 154, article ID 112762. DOI: 10.1016/j.indcrop.2020.112762
de la Torre, M. J., Moral, A., Hernández, M. D., Cabeza, E., and Tijero, A. (2013). “Organosolv lignin for biofuel,” Industrial Crops and Products 45, 58-63. DOI: 10.1016/j.indcrop.2012.12.002
Demuner, I. F., Colodette, J. L., Demuner, A. J., and Jardim, C. M. (2019). “Biorefinery review: Wide-reaching products through kraft lignin,” BioResources 14(3), 7543-7581. DOI: 10.15376/biores.14.3.Demuner
Deng, Y., Feng, X., Zhou, M., Qian, Y., Yu, H., and Qiu, X. (2011). “Investigation of aggregation and assembly of alkali lignin using iodine as a probe,” Biomacromolecules 12(4), 1116-1125. DOI: 10.1021/bm101449b
Domínguez-Robles, J., Cárcamo-Martínez, Á., Stewart, S. A., Donnelly, R. F., Larrañeta, E., and Borrega, M. (2020). “Lignin for pharmaceutical and biomedical applications – Could this become a reality?,” Sustainable Chemistry and Pharmacy 18, article ID 100320. DOI: 10.1016/j.scp.2020.100320
Dondi, D., Albini, A., and Serpone, N. (2006). “Interactions between different solar UVB/UVA filters contained in commercial suncreams and consequent loss of UV protection,” Photochemical & Photobiological Sciences 5(9), 835-843. DOI: 10.1039/B606768A
Espinoza-Acosta, J. L., Torres-Chávez, P. I., Olmedo-Martínez, J. L., Vega-Rios, A., Flores-Gallardo, S., and Zaragoza-Contreras, E. A. (2018). “Lignin in storage and renewable energy applications: A review,” Journal of Energy Chemistry 27(5), 1422-1438. DOI: 10.1016/j.jechem.2018.02.015
Espinoza-Acosta, J. L., Torres-Chávez, P. I., Ramírez-Wong, B., López-Saiz, C. M., and Montaño-Leyva, B. (2016). “Antioxidant, antimicrobial, and antimutagenic properties of technical lignins and their applications,” BioResources 11(2), 5452-5481. DOI: 10.15376/biores.11.2.Espinoza_Acosta
Fache, M., Boutevin, B., and Caillol, S. (2016). “Vanillin production from lignin and its use as a renewable chemical,” ACS Sustainable Chemistry and Engineering 4(1), 35-46. DOI: 10.1021/acssuschemeng.5b01344
Figueiredo, P., Lintinen, K., Kiriazis, A., Hynninen, V., Liu, Z., Bauleth-Ramos, T., Rahikkala, A., Correia, A., Kohout, T., Sarmento, B., et al. (2017). “In vitro evaluation of biodegradable lignin-based nanoparticles for drug delivery and enhanced antiproliferation effect in cancer cells,” Biomaterials 121, 97-108. DOI: 10.1016/j.biomaterials.2016.12.034
Figueiredo, P., Lintinen, K., Hirvonen, J. T., Kostiainen, M. A., and Santos, H. A. (2018). “Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications,” Progress in Materials Science 93, 233-269. DOI: 10.1016/j.pmatsci.2017.12.001
Frangville, C., Rutkevičius, M., Richter, A. P., Velev, O. D., Stoyanov, S. D. and Paunov, V. N. (2012). “Fabrication of environmentally biodegradable lignin nanoparticles,” Chem. Phys. Chem. 13, 4235-4243. DOI: 10.1002/cphc.201200537
Fournier, K., Marina, N., Joshi, N., Berthiaume, V. R., Currie, S., Lanterna, A. E., and Scaiano, J. C. (2021). “Scale-up of a photochemical flow reactor for the production of lignin-coated titanium dioxide as a sunscreen ingredient,” Journal of Photochemistry and Photobiology 7, article ID 100040. DOI: 10.1016/j.jpap.2021.100040
Ganewatta, M. S., Lokupitiya, H. N., and Tang, C. (2019). “Lignin biopolymers in the age of controlled polymerization,” Polymers 11(7), article no. 1176. DOI: 10.3390/polym11071176.
Gao, W., and Fatehi, P. (2019). “Lignin for polymer and nanoparticle production: Current status and challenges,” The Canadian Journal of Chemical Engineering 97(11), 2827-2842. DOI: 10.1002/cjce.23620
Gil-Chávez, G. J., Padhi, S. S. P., Pereira, C. V., Guerreiro, J. N., Matias, A. A., and Smirnova, I. (2019). “Cytotoxicity and biological capacity of sulfur-free lignins obtained in novel biorefining process,” International Journal of Biological Macromolecules 136, 697-703. DOI: 10.1016/j.ijbiomac.2019.06.021
Gil-Chávez, J., Gurikov, P., Hu, X., Meyer, R., Reynolds, W., and Smirnova, I. (2021). “Application of novel and technical lignins in food and pharmaceutical industries: Structure-function relationship and current challenges,” Biomass Conversion and Biorefinery 11(6), 2387-2403. DOI: 10.1007/s13399-019-00458-6
Gondikas, A. P., von der Kammer, F., Reed, R. B., Wagner, S., Ranville, J. F., and Hofmann, T. (2014). “Release of TiO2 nanoparticles from sunscreens into surface waters: A one-year survey at the Old Danube recreational lake,” Environmental Science & Technology 48(10), 5415-5422. DOI: 10.1021/es405596y
Gordobil, O., Herrera, R., Yahyaoui, M., Ilk, S., Kaya, M., Labidi, J., İlk, S., Kaya, M., and Labidi, J. (2018). “Potential use of kraft and organosolv lignins as a natural additive for healthcare products,” RSC Advances 8(43), 24525-24533. DOI: 10.1039/c8ra02255k
Gordobil, O., Oberemko, A., Saulis, G., Baublys, V., and Labidi, J. (2019). “In vitro cytotoxicity studies of industrial Eucalyptus kraft lignins on mouse hepatoma, melanoma and Chinese hamster ovary cells,” International Journal of Biological Macromolecules 135, 353-361. DOI: 10.1016/j.ijbiomac.2019.05.111
Gordobil, O., Olaizola, P., Banales, J. M., and Labidi, J. (2020). “Lignins from agroindustrial by-products as natural ingredients for cosmetics: Chemical structure and in vitro sunscreen and cytotoxic activities,” Molecules 25(5), article no. 1131. DOI: 10.3390/molecules25051131
Guerra, A., Filpponen, I., Lucia, L. A., and Argyropoulos, D. S. (2006). “Comparative evaluation of three lignin isolation protocols for various wood species,” Journal of Agricultural and Food Chemistry 54(26), 9696-9705. DOI: 10.1021/jf062433c
Guo, D., Zhang, J., Sha, L., Liu, B., Zhang, X., Zhang, X., and Xue, G. (2020). “Preparation and characterization of lignin-TiO2 UV-shielding composite material by induced synthesis with nanofibrillated cellulose,” BioResources 15(4), 7374-7389. DOI: 10.15376/biores.15.4.7374-7389
Gutiérrez-Hernández, J. M., Escalante, A., Murillo-Vázquez, R. N., Delgado, E., González, F. J., and Toríz, G. (2016). “Use of Agave tequilana-lignin and zinc oxide nanoparticles for skin photoprotection,” Journal of Photochemistry and Photobiology B: Biology 163, 156-161. DOI: 10.1016/j.jphotobiol.2016.08.027
Haq, I., Mazumder, P., and Kalamdhad, A. S. (2020). “Recent advances in removal of lignin from paper industry wastewater and its industrial applications – A review,” Bioresource Technology 312, article ID 123636. DOI: 10.1016/j.biortech.2020.123636
Hayashi, J., Kazehaya, A., Muroyama, K., and Watkinson, A. P. (2000). “Preparation of activated carbon from lignin by chemical activation,” Carbon 38(13), 1873-1878. DOI: 10.1016/S0008-6223(00)00027-0
Hu, L., Zhou, Y., Zhang, M., and Liu, R. (2011). “Characterization and properties of a lignosulfonate-based phenolic foam,” BioResources 7(1), 554-564. DOI: 10.15376/biores.7.1.0554-0564
Ibrahim, M. N. M., Iqbal, A., Shen, C. C., Bhawani, S. A., and Adam, F. (2019). “Synthesis of lignin-based composites of TiO2 for potential application as radical scavengers in sunscreen formulation,” BMC Chemistry 13, Article Number 17. DOI: 10.1186/s13065-019-0537-3
Jablonsky, M., Kočiš, J., Haz, A., and Šima, J. (2015). “Characterization and comparison by UV spectroscopy of precipitated lignins and commercial lignosulfonates,” Cellulose Chemistry and Technology 49(3-4), 267-274.
Jacobs, J., van de Poel, I., and Osseweijer, P. (2010). “Sunscreens with titanium dioxide (TiO2) nano-particles: A societal experiment,” Nanoethics 4(2), 103-113. DOI: 10.1007/s11569-010-0090-y
Johnson, A. C., Bowes, M. J., Crossley, A., Jarvie, H. P., Jurkschat, K., Jürgens, M. D., Lawlor, A. J., Park, B., Rowland, P., Spurgeon, D., et al. (2011). “An assessment of the fate, behaviour and environmental risk associated with sunscreen TiO2 nanoparticles in UK field scenarios,” Science of The Total Environment 409(13), 2503-2510. DOI: 10.1016/j.scitotenv.2011.03.040
Kadla, J. F., Kubo, S., Venditti, R. A., Gilbert, R. D., Compere, A. L., and Griffith, W. (2002). “Lignin-based carbon fibers for composite fiber applications,” Carbon 40(15), 2913-2920. DOI: 10.1016/S0008-6223(02)00248-8
Kai, D., Chua, Y. K., Jiang, L., Owh, C., Chan, S. Y., and Loh, X. J. (2016a). “Dual functional anti-oxidant and SPF enhancing lignin-based copolymers as additives for personal and healthcare products,” RSC Advances 6(89), 86420-86427. DOI: 10.1039/C6RA21433A
Kai, D., Tan, M. J., Chee, P. L., Chua, Y. K., Yap, Y. L., and Loh, X. J. (2016b). “Towards lignin-based functional materials in a sustainable world,” Green Chemistry 18(5), 1175-1200. DOI: 10.1039/C5GC02616D
Kaur, R., Thakur, N. S., Chandna, S., and Bhaumik, J. (2020). “Development of agri-biomass based lignin derived zinc oxide nanocomposites as promising UV protectant-cum-antimicrobial agents,” Journal of Materials Chemistry B 8(2), 260-269. DOI: 10.1039/c9tb01569h
Kazzaz, A. E., and Fatehi, P. (2020). “Technical lignin and its potential modification routes: A mini-review,” Industrial Crops and Products 154, article ID 112732. DOI: 10.1016/j.indcrop.2020.112732
Lee, S. C., Tran, T. M. T., Choi, J. W., and Won, K. (2019). “Lignin for white natural sunscreens,” International Journal of Biological Macromolecules 122, 549-554. DOI: 10.1016/j.ijbiomac.2018.10.184
Lee, S. C., Yoo, E., Lee, S. H., and Won, K. (2020). “Preparation and application of light-colored lignin nanoparticles for broad-spectrum sunscreens,” Polymers 12(3), article no. 699. DOI: 10.3390/polym12030699
Lewicka, Z. A., Benedetto, A. F., Benoit, D. N., Yu, W. W., Fortner, J. D., and Colvin, V. L. (2011). “The structure, composition, and dimensions of TiO2 and ZnO nanomaterials in commercial sunscreens,” Journal of Nanoparticle Research 13(9), 3607-3617. DOI: 10.1007/s11051-011-0438-4
Li, J., Wang, M., She, D., and Zhao, Y. (2017). “Structural functionalization of industrial softwood kraft lignin for simple dip-coating of urea as highly efficient nitrogen fertilizer,” Industrial Crops and Products 109, 255-265. DOI: 10.1016/j.indcrop.2017.08.011
Li, R. J., Gutierrez, J., Chung, Y., Frank, C. W., Billington, S. L., and Sattely, E. S. (2018). “A lignin-epoxy resin derived from biomass as an alternative to formaldehyde-based wood adhesives,” Green Chemistry 20(7), 1459-1466. DOI: 10.1039/c7gc03026f
Li, S., Li, M., Bian, J., Wu, X., Peng, F., and Ma, M. (2019). “Preparation of organic acid lignin submicrometer particle as a natural broad-spectrum photo-protection agent,” International Journal of Biological Macromolecules 132, 836-843. DOI: 10.1016/j.ijbiomac.2019.03.177
Lima, R. B., Raza, R., Qin, H., Li, J., Lindström, M. E., and Zhu, B. (2013). “Direct lignin fuel cell for power generation,” RSC Advances 3(15), 5083-5089. DOI: 10.1039/c3ra23418e.]
Lizundia, E., Sipponen, M. H., Greca, L. G., Balakshin, M., Tardy, B. L., Rojas, O. J., and Puglia, D. (2021). “Multifunctional lignin-based nanocomposites and nanohybrids,” Green Chemistry 23(18), 6698-6760. DOI: 10.1039/D1GC01684A.
Ma, Z., Liu, C., Niu, N., Chen, Z., Li, S., Liu, S., and Li, J. (2018). “Seeking brightness from nature: J-aggregation-induced emission in cellulolytic enzyme lignin nanoparticles,” ACS Sustainable Chemistry & Engineering 6(3), 3169-3175. DOI: 10.1021/acssuschemeng.7b03265
Machado, T. O., Beckers, S. J., Fischer, J., Müller, B., Sayer, C., de Araújo, P. H. H., Landfester, K., and Wurm, F. R. (2020). “Bio-based lignin nanocarriers loaded with fungicides as a versatile platform for drug delivery in plants,” Biomacromolecules 21(7), 2755-2763. DOI: 10.1021/acs.biomac.0c00487
Morsella, M., D’Alessandro, N., Lanterna, A. E., and Scaiano, J. C. (2016). “Improving the sunscreen properties of TiO2 through an understanding of its catalytic properties,” ACS Omega 1(3), 464-469. DOI: 10.1021/acsomega.6b00177
Morsella, M., Giammatteo, M., Arrizza, L., Tonucci, L., Bressan, M., and D’Alessandro, N. (2015). “Lignin coating to quench photocatalytic activity of titanium dioxide nanoparticles for potential skin care applications,” RSC Advances 5(71), 57453-57461. DOI: 10.1039/c5ra05232g
Mukhopadhyay, A., Hamel, J., Katahira, R., and Zhu, H. (2018). “Metal-free aqueous flow battery with novel ultrafiltered lignin as electrolyte,” ACS Sustainable Chemistry and Engineering 6(4), 5394-5400. DOI: 10.1021/acssuschemeng.8b00221
Nair, V., Dhar, P., and Vinu, R. (2016). “Production of phenolics via photocatalysis of ball milled lignin–TiO2 mixtures in aqueous suspension,” RSC Advances 6(22), 18204-18216. DOI: 10.1039/C5RA25954A
Nypelö, T. E., Carrillo, C. A., and Rojas, O. J. (2015). “Lignin supracolloids synthesized from (W/O) microemulsions: Use in the interfacial stabilization of Pickering systems and organic carriers for silver metal,” Soft Matter 11(10), 2046-2054. DOI: 10.1039/C4SM02851A.
Österberg, M., Sipponen, M. H., Mattos, B. D., and Rojas, O. J. (2020). “Spherical lignin particles: A review on their sustainability and applications,” Green Chemistry 22(9), 2712-2733. DOI: 10.1039/d0gc00096e.
Ouyang, X., Ke, L., Qiu, X., Guo, Y., and Pang, Y. (2009). “Sulfonation of alkali lignin and its potential use in dispersant for cement,” Journal of Dispersion Science and Technology 30(1), 1-6. DOI: 10.1080/01932690802473560
Owh, C., Chee, P. L., and Loh, X. J. (2016). “A global analysis of the personal care market,” in: Polymers for Personal Care Products and Cosmetics, X. J. Loh (ed.), Royal Society of Chemistry, London, UK, pp. 1-17. DOI: 10.1039/9781782623984-00001
Paulsson, M., and Parkås, J. (2012). “Review: Light-induced yellowing of lignocellulosic pulps – mechanisms and preventive methods,” BioResources 7(4), 5995-6040. DOI: 10.15376/biores.7.4.5995-6040
Picatonotto, T., Vione, D., and Carlotti, M. (2007). “Effect of some additives used in the cosmetic field on the photocatalytic activity of rutile,” Journal of Dispersion Science and Technology 23(6), 845-852. DOI: 10.1081/DIS-120015981
Piccinino, D., Capecchi, E., Tomaino, E., Gabellone, S., Gigli, V., Avitabile, D., and Saladino, R. (2021). “Nano‐structured lignin as green antioxidant and UV shielding ingredient for sunscreen applications,” Antioxidants 10(2), article no. 274. DOI: 10.3390/antiox10020274
Pishnamazi, M., Hafizi, H., Shirazian, S., Culebras, M., Walker, G. M., and Collins, M. N. (2019). “Design of controlled release system for paracetamol based on modified lignin,” Polymers 11(6), article no. 1059. DOI: 10.3390/polym11061059
Polcin, J., and Rapson, W. H. (1971). “Effects of bleaching agents on the absorption spectra of lignin in groundwood pulps part II. Oxidative-reductive bleaching,” Pulp & Paper Magazine Canada 72, 80-91.
Qian, Y., Deng, Y., Li, H., and Qiu, X. (2014). “Reaction-free lignin whitening via a self-assembly of acetylated lignin,” Industrial & Engineering Chemistry Research 53(24), 10024-10028. DOI: 10.1021/ie5010338
Qian, Y., Qiu, X., and Zhu, S. (2015). “Lignin: A nature-inspired sun blocker for broadspectrum sunscreens,” Green Chemistry 17(1), 320-324. DOI: 10.1039/c4gc01333f
Qian, Y., Qiu, X., and Zhu, S. (2016). “Sunscreen performance of lignin from different technical resources and their general synergistic effect with synthetic sunscreens,” ACS Sustainable Chemistry and Engineering 4(7), 4029-4035. DOI: 10.1021/acssuschemeng.6b00934
Quraishi, S., Martins, M., Barros, A. A., Gurikov, P., Raman, S. P., Smirnova, I., Duarte, A. R. C., and Reis, R. L. (2015). “Novel non-cytotoxic alginate–lignin hybrid aerogels as scaffolds for tissue engineering,” The Journal of Supercritical Fluids 105, 1-8. DOI: 10.1016/j.supflu.2014.12.026
Ragauskas, A. J., Beckham, G. T., Biddy, M. J., Chandra, R., Chen, F., Davis, M. F., Davison, B. H., Dixon, R. A., Gilna, P., Keller, M., et al. (2014). “Lignin valorization: Improving lignin processing in the biorefinery,” Science 344(6185), article ID 1246843. DOI: 10.1126/science.1246843
Ratanasumarn, N., and Chitprasert, P. (2020). “Cosmetic potential of lignin extracts from alkaline-treated sugarcane bagasse: Optimization of extraction conditions using response surface methodology,” International Journal of Biological Macromolecules 153, 138-145. DOI: 10.1016/j.ijbiomac.2020.02.328
Rodrigues Pinto, P. C., Borges da Silva, E. A., and Rodrigues, A. E. (2012). “Lignin as source of fine chemicals: Vanillin and syringaldehyde,” in: Biomass Conversion: The Interface of Biotechnology, Chemistry and Materials Science, C. Baskar, S. Baskar, and R. S. Dhillon (eds.), Springer, Berlin, Germany, pp. 381-420. DOI: 10.1007/978-3-642-28418-2_12.
Sadeghifar, H., and Ragauskas, A. (2020). “Lignin as a UV light blocker—A review,” Polymers 12(5), article no. 1134. DOI: 10.3390/polym12051134
Sajinčič, N., Gordobil, O., Simmons, A., and Sandak, A. (2021). “An exploratory study of consumers’ knowledge and attitudes about lignin-based sunscreens and bio-based skincare products,” Cosmetics 8(3), 78. DOI: 10.3390/cosmetics8030078.
Sambandan, D. R., and Ratner, D. (2011). “Sunscreens: An overview and update,” Journal of the American Academy of Dermatology 64(4), 748-758. DOI: 10.1016/j.jaad.2010.01.005
Sathawong, S., Sridach, W., and Techato, K. (2018). “Lignin: Isolation and preparing the lignin-based hydrogel,” Journal of Environmental Chemical Engineering 6(5), 5879-5888. DOI: 10.1016/j.jece.2018.05.008
Savary, G., Grisel, M., and Picard, C. (2016). “Cosmetics and personal care products,” in: Natural Polymers, O. Olatunji (ed.), Springer, Cham, Switzerland, pp. 219-261. DOI: 10.1007/978-3-319-26414-1_8
Schneider, W. D. H., Dillon, A. J. P., and Camassola, M. (2021). “Lignin nanoparticles enter the scene: A promising versatile green tool for multiple applications,” Biotechnology Advances 47, article ID 107685. DOI: 10.1016/j.biotechadv.2020.107685
Sharma, S., Sharma, R. K., Gaur, K., Cátala Torres, J. F., Loza-Rosas, S. A., Torres, A., Saxena, M., Julin, M., and Tinoco, A. D. (2019). “Fueling a hot debate on the application of TiO2 nanoparticles in sunscreen,” Material 12(14), article no. 2317. DOI: 10.3390/ma12142317
Shen, B., Scaiano, J. C., and English, A. M. (2006). “Zeolite encapsulation decreases TiO2-photosensitized ROS generation in cultured human skin fibroblasts,” Photochemistry and Photobiology 82(1), 5-12. DOI: 10.1562/2005-05-29-RA-551
Shi, C., Zhang, S., Wang, W., Linhardt, R. J., and Ragauskas, A. J. (2020). “Preparation of highly reactive lignin by ozone oxidation: Application as surfactants with antioxidant and anti-UV properties,” ACS Sustainable Chemistry and Engineering 8(1), 22-28. DOI: 10.1021/acssuschemeng.9b05498
Shu, F., Jiang, B., Yuan, Y., Li, M., Wu, W., Jin, Y., and Xiao, H. (2021). “Biological activities and emerging roles of lignin and lignin-based products─A review,” Biomacromolecules 22(12), 4905-4918. DOI: 10.1021/acs.biomac.1c00805
Sionkowska, A., Adamiak, K., Musial, K., and Gadomska, M. (2020). “Collagen based materials in cosmetic applications: A review,” Materials 13(19), Article Number 4217. DOI: 10.3390/MA13194217
Stine, J. S., Harper, B. J., Conner, C. G., Velev, O. D., and Harper, S. L. (2021). “In vivo toxicity assessment of chitosan-coated lignin nanoparticles in embryonic zebrafish (Danio rerio),” Nanomaterials 11(1), article no. 111. DOI: 10.3390/nano11010111
Sugiarto, S., Leow, Y., Tan, C. L., Wang, G., and Kai, D. (2022). “How far is lignin from being a biomedical material?,” Bioactive Materials 8, 71-94. DOI: 10.1016/j.bioactmat.2021.06.023
Tang, Q., Qian, Y., Yang, D., Qiu, X., Qin, Y., and Zhou, M. (2020). “Lignin-based nanoparticles: A review on their preparations and applications,” Polymers 12(11), article no. 2471. DOI: 10.3390/polym12112471
Taofiq, O., González-Paramás, A. M., Barreiro, M. F., and Ferreira, I. C. F. R. (2017). “Hydroxycinnamic acids and their derivatives: Cosmeceutical significance, challenges and future perspectives, a review,” Molecules 22(2), article no. 281. DOI: 10.3390/molecules22020281
Teng, N., Dallmeyer, I., and Kadla, J. F. (2013). “Effect of softwood kraft lignin fractionation on the dispersion of multiwalled carbon nanotubes,” Industrial and Engineering Chemistry Research 52(19), 6311-6317. DOI: 10.1021/ie303261z
Tosato, M. G., Orallo, D. E., Fangio, M. F., Diz, V., Dicelio, L. E., and Churio, M. S. (2016). “Nanomaterials and natural products for UV-photoprotection,” Surface Chemistry of Nanobiomaterials 3, 359-383. DOI: 10.1016/B978-0-323-42861-3.00012-1
Tran, M. H., Phan, D., and Lee, E. Y. (2021). “Review on lignin modifications toward natural UV protection ingredient for lignin-based sunscreens,” Green Chemistry 23(13), 4633-4646. DOI: 10.1039/D1GC01139A
Trevisan, H., and Rezende, C. A. (2020). “Pure, stable and highly antioxidant lignin nanoparticles from elephant grass,” Industrial Crops and Products 145, article ID 112105. DOI: 10.1016/j.indcrop.2020.112105
Trivedi, M., and Murase, J. (2017). “Titanium dioxide in sunscreen,” in: Application of Titanium Dioxide, M. Janus (ed.), IntechOpen, Rijeka, Croatia, pp. 61-71. DOI: 10.5772/intechopen.68886
Ugartondo, V., Mitjans, M., and Vinardell, M. P. (2008). “Comparative antioxidant and cytotoxic effects of lignins from different sources,” Bioresource Technology 99(14), 6683-6687. DOI: 10.1016/j.biortech.2007.11.038
Vinardell, M. P., and Mitjans, M. (2017). “Lignins and their derivatives with beneficial effects on human health,” International Journal of Molecular Sciences 18(6), article no. 1219. DOI: 10.3390/ijms18061219
Vinardell, M. P., Ugartondo, V., and Mitjans, M. (2008). “Potential applications of antioxidant lignins from different sources,” Industrial Crops and Products 27(2) 220-223. DOI: 10.1016/j.indcrop.2007.07.011
Vishtal, A. G., and Kraslawski, A. (2011). “Challenges in industrial applications of technical lignins,” BioResources 6(3), 3547-3568. DOI: 10.15376/biores.6.3.3547-3568
Wang, B., Sun, D., Wang, H., Yuan, T., and Sun, R. (2019). “Green and facile preparation of regular lignin nanoparticles with high yield and their natural broad-spectrum sunscreens,” ACS Sustainable Chemistry and Engineering 7(2), 2658-2666. DOI: 10.1021/acssuschemeng.8b05735
Wang, H., Wang, Y., Fu, F., Qian, Y., Xiao, Y., Yang, D., and Qiu, X. (2020). “Controlled preparation of lignin/titanium dioxide hybrid composite particles with excellent UV aging resistance and its high value application,” International Journal of Biological Macromolecules 150, 371-379. DOI: 10.1016/j.ijbiomac.2019.12.185
Wang, J., Deng, Y., Qian, Y., Qiu, X., Ren, Y., and Yang, D. (2016). “Reduction of lignin color via one-step UV irradiation,” Green Chemistry 18(3), 695-699. DOI: 10.1039/c5gc02180d
Widsten, P. (2020). “Lignin-based sunscreens—State-of-the-art, prospects and challenges,” Cosmetics 7(4), Article Number 85. DOI: 10.3390/cosmetics7040085
Widsten, P., Liitiä, T., Immonen, K., Borrega, M., Jääskeläinen, A., Wikberg, H., Ohra-aho, T., and Tamminen, T. (2020a). “Potential of lignin as antioxidant for thermoplastics and other materials,” Lignin 1, 11-19.
Widsten, P., Tamminen, T., and Liitiä, T. (2020b). “Natural sunscreens based on nanoparticles of modified kraft lignin (CatLignin),” ACS Omega 5(22), 13438-13446. DOI: 10.1021/acsomega.0c01742
Witzler, M., Alzagameem, A., Bergs, M., El Khaldi-Hansen, B., Klein, S. E., Hielscher, D., Kamm, B., Kreyenschmidt, J., Tobiasch, E., and Schulze, M. (2018). “Lignin-derived biomaterials for drug release and tissue engineering,” Molecules 23(8), Article Number 1885. DOI: 10.3390/molecules23081885
Wu, Y., Qian, Y., Lou, H., Yang, D., and Qiu, X. (2019). “Enhancing the broad-spectrum adsorption of lignin through methoxyl activation, grafting modification, and reverse self-assembly,” ACS Sustainable Chemistry and Engineering 7(19), 15966-15973. DOI: 10.1021/acssuschemeng.9b02317
Wu, Y., Qian, Y., Zhang, A., Lou, H., Yang, D., and Qiu, X. (2020). “Light color dihydroxybenzophenone grafted lignin with high UVA/UVB absorbance ratio for efficient and safe natural sunscreen,” Industrial and Engineering Chemistry Research 59(39), 17057-17068. DOI: 10.1021/acs.iecr.9b06970
Yao, C., Yongming, F., Jianmin, G., and Houkun, L. (2012). “Coloring characteristics of in situ lignin during heat treatment,” Wood Science and Technology 46(1), 33-40. DOI: 10.1007/s00226-010-0388-5
Ye, J., Cheng, Y., Sun, L., Ding, M., Wu, C., Yuan, D., Zhao, X., Xiang, C., and Jia, C. (2019). “A green SPEEK/lignin composite membrane with high ion selectivity for vanadium redox flow battery,” Journal of Membrane Science 572, 110-118. DOI: 10.1016/j.memsci.2018.11.009
Yu, G., Li, B., Wang, H., Liu, C., and Mu, X. (2013). “Preparation of concrete superplasticizer by oxidation-sulfomethylation of sodium lignosulfonate,” BioResources 8(1), 1055-1063. DOI: 10.15376/BIORES.8.1.1055-1063
Zhai, J., Hu, X., Liu, C., Zhu, N., and Guo, K. (2019). “Grafting modification of lignin via atom transfer radical polymerization,” Progress in Chemistry 31(9), 1293-1302. DOI: 10.7536/PC190106
Zhang, H., Bai, Y., Yu, B., Liu, X., and Chen, F. (2017a). “A practicable process for lignin color reduction: Fractionation of lignin using methanol/water as a solvent,” Green Chemistry 19(21), 5152-5162. DOI: 10.1039/c7gc01974b
Zhang, H., Bai, Y., Zhou, W., and Chen, F. (2017b). “Color reduction of sulfonated eucalyptus kraft lignin,” International Journal of Biological Macromolecules 97, 201-208. DOI: 10.1016/j.ijbiomac.2017.01.031
Zhang, H., Chen, F., Liu, X., and Fu, S. (2018). “Micromorphology influence on the color performance of lignin and its application in guiding the preparation of light-colored lignin sunscreen,” ACS Sustainable Chemistry & Engineering 6(9), 12532-12540. DOI: 10.1021/acssuschemeng.8b03464
Zhang, H., Fu, S., and Chen, Y. (2020). “Basic understanding of the color distinction of lignin and the proper selection of lignin in color-depended utilizations,” International Journal of Biological Macromolecules 147, 607-615. DOI: 10.1016/j.ijbiomac.2020.01.105
Zhang, H., Liu, X., Fu, S., and Chen, Y. (2019a). “High-value utilization of kraft lignin: Color reduction and evaluation as sunscreen ingredient,” International Journal of Biological Macromolecules 133, 86-92. DOI: 10.1016/j.ijbiomac.2019.04.092
Zhang, H., Liu, X., Fu, S., and Chen, Y. (2019b). “Fabrication of light-colored lignin microspheres for developing natural sunscreens with favorable UV absorbability and staining resistance,” Industrial & Engineering Chemistry Research 58(31), 13858-13867. DOI: 10.1021/acs.iecr.9b02086
Zhang, Y., and Naebe, M. (2021). “Lignin: A review on structure, properties, and applications as a light-colored UV absorber,” ACS Sustainable Chemistry & Engineering 9(4), 1427-1442. DOI: 10.1021/acssuschemeng.0c06998
Zhou, Y., Qian, Y., Wang, J., Qiu, X., and Zeng, H. (2020). “Bioinspired lignin-polydopamine nanocapsules with strong bioadhesion for long-acting and high-performance natural sunscreens,” Biomacromolecules 21(8), 3231-3241. DOI: 10.1021/acs.biomac.0c00696
Zrinski, I., Martinez, S., and Gospić, E. (2021). “Catalytic and photocatalytic effects of TiO2 nanoparticles on electrooxidation of common antioxidants on carbon paste,” Journal of Solid-State Electrochemistry 25(5), 1591-1600. DOI: 10.1007/s10008-021-04937-7
Article submitted: January 13, 2022; Peer review completed: February 20, 2022; Revised version received and accepted: March 29, 2022; Published: March 31, 2022.
DOI: 10.15376/biores.17.2.Espinoza
