Abstract
The bleaching plant of a kraft pulp mill is the sector that consumes water and generates effluent with the highest volume. Water recycling is an attractive option to reduce water consumption and effluent generation. This study evaluated the technical feasibility of using treated effluent as washing water in the bleaching stages. The bleaching sequence was simulated in the laboratory using four types of washing water: deionized water, whitewater, low organic load effluent, and high organic load effluent. To achieve 90% ISO pulp brightness, the ClO2 consumption increased from 8.1 kg ClO2 odt-1 when using water to 13.8 and 16.3 kgClO2 odt-1 for the low and high organic effluents. Physical and optical tests of the hand-sheet papers did not show any statistical difference between various washing waters. The filtrates showed values that did not burden the efficiency of the effluent treatment plant. It was possible to use effluent in the bleaching stages, considering that the filtrates and the produced paper complied with the quality standards.
Download PDF
Full Article
Recirculation of Treated Effluent in the Bleaching of Kraft Pulp
Erika Nascimben Santos,a,*,1 Claudio Mudadu Silva,a Jorge Luiz Colodette,a Samilly B. Zanith de Almeida,a Antonio José Vinha Zanuncio,b Thiago Oliveira de Souza,a Karyna da Silva Menezes,a Bruna Luíza Pesso da Silveira,a and Yesenia Belén Llumiquinga Paucar a
The bleaching plant of a kraft pulp mill is the sector that consumes water and generates effluent with the highest volume. Water recycling is an attractive option to reduce water consumption and effluent generation. This study evaluated the technical feasibility of using treated effluent as washing water in the bleaching stages. The bleaching sequence was simulated in the laboratory using four types of washing water: deionized water, whitewater, low organic load effluent, and high organic load effluent. To achieve 90% ISO pulp brightness, the ClO2 consumption increased from 8.1 kg ClO2 odt-1 when using water to 13.8 and 16.3 kgClO2 odt-1 for the low and high organic effluents. Physical and optical tests of the hand-sheet papers did not show any statistical difference between various washing waters. The filtrates showed values that did not burden the efficiency of the effluent treatment plant. It was possible to use effluent in the bleaching stages, considering that the filtrates and the produced paper complied with the quality standards.
Keywords: Bleaching plant; ECF bleaching; Effluent recirculation; Water recycling; Washing water
Contact information: a: Pulp and Paper Laboratory, Department of Forest Engineering, Universidade Federal de Viçosa, 36570-900, Viçosa, Minas Gerais, Brazil; b: Instituto de Ciências Agrárias, Universidade Federal de Uberlândia, Monte Carmelo, 38500-000, Brazil; 1Present address: Institute of Process Engineering, University of Szeged, 6725, Szeged, Hungary;
* Corresponding author: erikansantos@outlook.com
GRAPHICAL ABSTRACT
INTRODUCTION
The demand for water in a kraft pulp mill with an annual production of one million tons is comparable to a city with over 500 thousand inhabitants. Besides its huge pollutant potential, the pulp sector invests in new technologies to reduce its environmental impacts. It is worth noticing that the specific water consumption of kraft pulp mills decreased from 50 m³ odt-1 in the 1980s to currently 25 m3 odt-1 (European Commission 2014).
Bleaching is the most water-consuming stage in a pulp mill, often consuming up to 50% of the water of the whole mill and generating up to 80% of the total effluent of a pulp mill effluents (Reeve and Silva 2000; NCASI 2009). The main cause of the significant water consumption in the bleaching sequence is the necessity of washing the pulp after each stage, to assure that all the oxidated material (lignin, extractives, etc.) are removed efficiently prior to the following bleaching stage. The bleaching filtrates are a complex mixture composed of several organic and inorganic compounds that are extracted from the pulp during the process. The suspended solids in this effluent are mainly composed of fibers and additives, while the dissolved solids are mostly organic, derived from the wood and inorganics from the chemical reagents (Mounteer et al. 2002).
The changes in raw material, and types of process and reagents used can generate effluents with significantly diverse quality. Also, diverse wastewater streams have different characteristics depending on its origin such as: debarking, pulping, bleaching or drying, and therefore, they can be treated with different treatment techniques (Hubbe et al. 2016). The challenges for the chosen treatment depend on the nature of the wastewater and the required quality of the treated effluent. The state-of-the-art treatment of effluent from pulp mills consists basically of two steps: primary treatment (clarification) followed by biological treatment (Cabrera 2017). Some pre- or post-treatment can be necessary, depending on the required quality of the treated effluent, such as advanced oxidation processes or membrane filtration (Morais et al. 2008; Kamali and Khodaparast 2015). Moreover, separated treatment can be done in different in-plant sectorial mill streams that do not require biological treatment due to their low biodegradable organic content, e.g. the chemical plant effluent, which requires only the clarification treatment.
Several studies have focused on reducing water consumption and the negative impacts of the pulp industries (Frigieri et al. 2016; Johnson 2019). There are different ways to minimize or even eliminate the effluent, based on the minimal impact industries approach, bleaching filtrate recovery, bleaching water circuit closure, and partial water circuit closure (Axegård et al. 1997). Water reuse may allow a reduction of more than 50% of the mill consumption (Huber et al. 2014). For instance, the counter-current jump-stage method of washing the pulp contributes significantly to the reduction of fresh water consumption. Closing the water circuit reduces water consumption and effluent generation, but the improper use of recycling may cause negative effects to the process and to the product quality (Nuortila-Jokinen et al. 2003), accumulating contaminants in the effluent, increasing odors, and damaging the quality of the final product (Hubbe et al. 2016). Therefore, it is essential to evaluate the quality of the water (solids, COD, pH, etc.) and the accumulation of non-process elements (NPE), such as K, Cl, and Cr that are the main sources responsible for corrosion, as well as for Ca, Al, Si, Mg and Mn that can cause scaling on the equipment (Furley and De Oliveira Filho 2000). Often purges are necessary to avoid problems with NPE accumulation while reusing the water (Gleadow et al. 1997).
In addition to reducing effluent generation, pulp mills seek to generate effluents with lower organic and inorganic loads. The elemental chlorine free (ECF) bleaching sequence is an important technology to generate lower contaminating load effluents and facilitate possible water reuse (Axegård et al. 1997; Gleadow et al. 1997). Nevertheless, the bleaching filtrates are still directed to the wastewater treatment plant (WTP) and cannot be reused in other stages due to the amount of NPE that can jeopardize the quality of the pulp and the corrosive compounds that are harmful for equipment (Parsad et al. 1996; Reeve and Silva 2000). Without the utilization of ClO2, the filtrates could be reused in other stages, for instance the pos-delignification washer (O2) (Costa et al. 2006).
Currently, many mills do not use fresh water in the pulp bleaching washers, but white water from the pulp dryer and black liquor evaporation condensates (Sillanpää 2005). The use of treated effluents as wash water in the bleaching stages also has been considered recently as an alternative for reducing water consumption (Vehmaa et al. 2016), but research is still needed in order to make feasible the recirculation of water and guarantee the quality of the pulp, equipment, and effluent.
Based on these considerations, the objective of the present research was to evaluate the technical feasibility of using two different treated kraft pulp effluents streams to wash pulp between the bleaching stages in order to reduce water consumption of the process, while keeping acceptable the pulp quality and the effluent.
EXPERIMENTAL
Materials
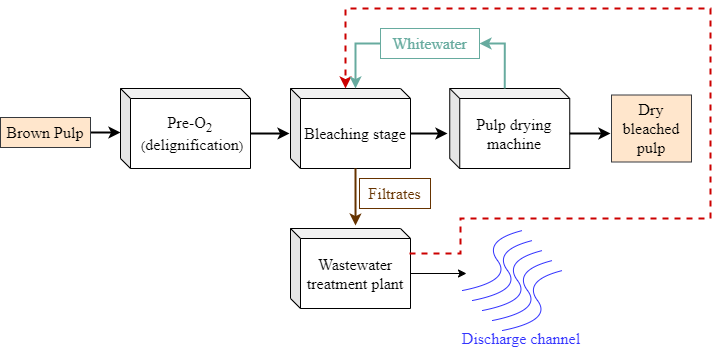
The material was obtained from a bleached kraft pulp mill that produces 1.2 million tons of pulp annually from eucalyptus wood. The mill specific water consumption is approximately 40 m³ adt-1 supplied by a river. The bleaching plant consumes 16 m³ adt-1 of water, of which 6 m³ adt-1 is for washing between the bleaching stages. The used bleaching sequence after pre-oxygen delignification (Pre-O2) is Dht(EP)DP – hot chlorine dioxide (Dht), alkaline extraction with hydrogen peroxide (EP), chlorine dioxide (D), and finally hydrogen peroxide (P). The washing is performed counter-current in a jump-stage type, and the generated filtrates are directed to the wastewater treatment plant (WTP). After the bleaching sequence, the pulp has 10% consistency, and it goes to a consistency control tank where white water from the pulp drying machine is added until reaching 1% consistency prior to the pulp-forming machine, where the pulp is dried to a consistency of 90% for packaging and distribution (Fig. 1).
The effluents from the mill are divided in two streams that are treated separately and mixed prior to being discharged into the river: i) high organic load effluent (HE), from bleaching and the chemical recovery of black liquor, treated by primary sedimentation followed by an activated sludge process; and ii) low organic load effluent (LE), originated from the wood yard and the chemical plant, treated solely by sedimentation on a separate clarifier.
Fig. 1. Flowchart of the bleaching and drying process in the mill. Dht: ClO2 stage in high temperature, EP: alkaline extraction and peroxidation (NaOH and H2O2), D: ClO2 stage, P: peroxidation (H2O2), W: pulp washing system, C: consistency control system
The high and low organic load (HE and LE) treated effluents were sampled after the ending of their respective treatments, and the white water (WW) was sampled at the pulp drying machine. Pre-O2 delignified pulp was also collected in order to carry out the bleaching lab simulation.
The effluents and the pulp were characterized. Bleaching simulations were performed using the different types of washing water (HE, LE, WW and deionized water). After bleaching, the resulting pulps and filtrates were characterized and compared. Hand-sheets of the bleached pulp were fabricated, characterized and compared statically.
Test Standards
The sampled pulps were characterized according to brightness, kappa number, viscosity, and hexenuronic acid content using methodologies TAPPI T525 om-17 (2017), TAPPI T236 om-13 (2013), TAPPI T230 om-19 (2019), and TAPPI T282 om-19 (2019), respectively.
The sampled effluents and generated filtrates after bleaching simulation were characterized according to chemical oxygen demand (COD), pH, total dissolved and total suspended solids (TDS and TSS), color, turbidity, electrical conductivity, metal and chloride content. These analyzes were carried out according to standard methods (APHA 2012).
The bleached pulp was characterized by the same mentioned parameters; in addition, brightness reversion, and metal content were evaluated according to TAPPI UM200 (2012) (4 h, 105 °C, 0% relative moisture) and TAPPI T266 om-18 (2018). The bleached pulp was refined in a PFI mill (model MARK VI, Hamjern Maskjn, Hamar, Norway, City, Country) following the TAPPI T248 sp-15 (2015) standard. The refining levels were measured from the Schopper-Riegler freeness (°SR) by the ISO 5267-1 standard (1999).
Handsheet papers of each refining level were generated using the TAPPI T205 sp-18 (2018) methodology with a weight of 60 g m-2, and their physical and optical characteristics were analyzed according to their thickness (TAPPI T551 om-18 (2018)), grammage (TAPPI T410 om-19 (2019)), resistance to passage of air (TAPPI T536 om-18 (2018)), bursting strength (TAPPI T403 om-15 (2015)), internal tearing resistance (TAPPI T414 om-12 (2012)), tensile properties (TAPPI T494 om-13 (2013)), and diffuse brightness (TAPPI T525 om-17 (2017)). The analysis of the tensile index, tensile energy absorption (TEA) and modulus of elasticity (MOE) tests of the fabricated hand-sheets were performed on a universal testing machine Instron model 4204 (Norwood, MA, USA).
Methods
A Dht(EP)DP bleaching sequence alternating stages with chlorine dioxide (ClO2) and hydrogen peroxide (H2O2) to achieve 90% ISO brightness in the final bleached pulp was carried out. Four polyethylene bags with 200 g of pre-O2 delignified oven dried pulp in each were used. The bleaching conditions were established according to the current conditions of the mill (Table 1), and water was used for pulp consistency adjustment.
The amount of chlorine dioxide used in D stage was determined from smaller tests with 5 g of pulp to reach, at the end of the completed sequence, 90% ISO brightness. The H2SO4 and NaOH applied were determined in initial tests with 5 g of pulp until, at the end of each bleaching stage, the required final pH was reached.
The pulp was removed from the bag at the end of the defined period. The residual pH and chlorine dioxide or hydrogen peroxide were measured by the extracted liquor. Then the pulp was washed manually with the proportion of 9 m³ odt-1 with the different washing waters (DW, WW, LE, HE). The initial four pulp samples from stages Dht and EP were washed with deionized water. The initial four pulp samples from stages D and P were washed with DW, WW, LE, HE. The experiment was repeated using the acid filtrates of stage D to wash the pulps of stages Dht, and alkaline filtrates of stage P to wash the pulps of stages EP, defined as countercurrent jump-stage (Fig. 1).
Table 1. Conditions in the Laboratory Bleaching Simulation Using Different Washing Waters
The filtrates from the stages Dht and EP were collected to COD measurement in each bleaching experiment. The sequence was repeated until the COD levels of the filtrates from Dht and EP stages remained constant (steady-state). A final complete bleaching sequence was performed to obtain the final bleached pulp for each used washing water and the final Dht and EP filtrates for characterization.
Statistical tests with the 4 bleached pulps were performed in R software using the Tukey test at 5% significance to set the significant difference between their viscosities. Then, the statistically different pulps were refined and a refining curve was generated for each pulp. The physical-mechanical properties of the hand-sheets were compared using the Identity Model (Regazzi 1996, 2003) and evaluated with the F test at 5% significance level. The tested hypotheses were: i. acceptance of H0: no differences among the averages, generating a common curve for the both samples; and ii. rejection of H0: at least one different average from the others.
The final acidic and alkaline filtrates, collected after the last bleaching repetition, were mixed in the proportion of 1:1 and characterized for further comparison of its quality and the impact on the industrial effluent treatment plant (ETP).
RESULTS AND DISCUSSION
Initial Characterization
The sampled pulp after pre-O2 delignification presented 56% ISO brightness and a kappa number of 8.3, which represents the residual lignin and chromophore groups, responsible to increase the reactivity and the light absorption characteristics (Theliander 2009). This value is typical for eucalyptus pulps ranging from 8 to 12 for eucalyptus pulps (Hart and Rudie 2012). The hexenuronic acids (hexA) level of 58 mmol kg-1 is typical for eucalyptus pulps; however, it may vary with the raw material, cooking and delignification applied conditions, reducing the brightness stability (Yang et al. 2003; Theliander 2009). The viscosity of the pulp (808 dm³ kg-1) was lower than usual (1000 and 1200 dm³ kg-1) (Hart and Rudie 2012), due to the fact that the mill produces pulp for tissue, which involves a more severe cooking condition and reduced level of polymerization of carbohydrates compared to normal conditions. For tissue paper production, the viscosity must be reduced to maintain high softness due to the reduction of interactions and resistance to movement (McKay et al. 2004).
The characteristics of the sampled white water, high (HE) and low organic (LE) load effluents are shown in Table 2.
Table 2. Characterization of the White Water (WW), Low Organic Load Effluent (LE), and High Organic Load Effluent (HE)
The TSS concentration of 20 mg L-1 in white water (WW) is due to the presence of suspended fibers that are not retained in the drying machine (Thompson et al. 2001). Between 48 and 58% of the total COD (295 mg L-1) in this effluent is due to the fiber particles and the soluble organic matter is due to unidentified compounds, mostly lignin and wood extractives (Rintala and Lepistö 1992), which are mainly responsible for its color and TDS (100 CU and 680 mg L-1).
The low organic load effluent (LE) has a concentration of TSS of 70 mg L-1, which was not removed by sedimentation. The component responsible of the COD and TDS values (190 and 800 mg L-1) is the organic matter of the wood that is leached from the woodyard, which is not removed by the primary treatment.
The TSS of the high organic load effluent (HE) was 10 mg L-1, showing an adequate efficiency of the secondary clarifiers. Considering that the biological effluent treatment decomposes biodegradable organic matter and toxic components (Hubbe et al. 2016) and does not remove recalcitrant organic material, there was a residual COD, color and TDS of 360 mg L-1, 575 CU and 1900 mg L-1, respectively, in the HE originated mainly from wood organic matter (Bajpai and Bajpai 1994). The high color originates from the lignin of wood degraded during the pulping and bleaching processes, with high aromatic content and unsaturated structures (Hubbe et al. 2016), in addition to hemicelluloses, resins, fatty acids, tannins, phenols, among others (Pokhrel and Viraraghavan 2004). These recalcitrant materials are found in high-load effluents due mainly to compounds with high molecular weight (Mounteer et al. 2007). There are other ways to remove the recalcitrant material and achieve a better quality of the treated effluent. Sonkar et al. (2019) enhanced a biological treatment with novel bacterium strains and reached COD removal efficiency of 89%; Mainardis et al. (2020) used ozonation before and after the biological treatment and removed COD can be removed up to 46% and 81% respectively; while Mainardis and Goi (2019) treated separately the black liquor evaporation condensate with UASB reactor and achieved 54% COD removal.
Although there were high values of COD for both treated effluents (LE and HE), they were still below several international standards and limitations for final discharge, including the European Commission which limits COD between 7 and 20 kg adt-1 (European Commission 2014) and the national limits from Brazil (COD<20 kg adt-1) (Minas Gerais 2008). Besides the legal limitations, the treated effluents are also in agreement with other pulp treated effluents characteristics, which are in the range of 4.5 and 10 kg COD adt-1 (mainly eucalyptus wood) in Brazil (Rodrigues et al. 2010; Cabrera 2017).
The metals are mostly originated from the wood and discharged to the ETP throughout the production process (Table 3). The degree of debarking of the wood is one of the steps that most influences the metal content that goes to the effluent or into the pulp (Vehmaa et al. 2016), because of the metals content in the bark.
Table 3. Metals Content in White Water (WW), Low Organic Load Effluent (LE), and High Organic Load Effluent (HE)
The metal content in white water is due to the metals presented in the wood that are not retained in the pulp drying machine, and the values depend on the raw material and on the reagents used in the process (Lacorte et al. 2003).
The metal content in the HE demonstrates the efficiency removal by the biological treatment, allowing the effluent to be discharged into the river. However, in order to be reused, it is necessary to assess the accumulation of these metals in the process. The kraft process uses cooking liquor to digest the pulp (NaOH and Na2S), generates green liquor in the boiler (Na2CO3 and Na2S), and recovers cooking liquor through causticizing and calcination (Ca(OH)2 and CaCO3). Therefore, the highest concentrations of Na and Ca in the HE were expected (Achoka 2002). In addition, the higher chloride content of this effluent was also expected since the bleaching plant uses ClO2 as the main bleaching reagent. In the effluent, all the chorine-based chemicals react very quickly with the organic matter, generating organochlorine compounds, which are assessed as adsorbable organic halides (AOX). Likewise. the amount of formed chlorinated compounds can be controlled by manipulation in the pH of the water; therefore, there is no active chlorine on it (Kumar et al. 2017; Solomon 1996).
Potassium and chloride are the main elements responsible for corrosion in equipment and pipes and calcium, manganese, and magnesium can cause scaling in the bleaching washers (Furley and De Oliveira Filho 2000). The high metal levels in the HE could limit a closed water circuit in bleaching. Nevertheless, with the used recirculation, there is still a continuous purge of these elements in the filtrates and no accumulation during the reuse is expected. The metals content in the water circuit tends to remain constant after steady-state condition.
The content of metals and chloride in the low-load effluent (LE) is justified by its origin—the wood yard and the chemical plant—but it does not serve as a limitation for its recirculation.
Bleaching Experiment
To achieve the final required pH at D-stage, sulfuric acid was used in the pulp washed with WW and DW, while sodium hydroxide was used for the pulp washed with LE and HE. In addition, the amount of chlorine dioxide required increased when using effluents LE and HE to achieve the brightness 90% ISO (Table 4).
The consumption of ClO2 for the complete bleaching sequence, considering the use of defined 6.3 kgClO2 odt-1 in the first Dht stage, was 8.1, 8.0, 16.3, and 13.8 kgClO2 odt-1 when using deionized water (DW), white water (WW), low (LE) and high (HE), organic load effluents respectively.
The complete bleaching sequence was repeated eleven times until the filtrate COD had become stabilized (steady-state). The COD of the Dht and EP filtrates started at 520 and 470 mg L-1 and remained, on average, in the range 600 to 850 and in the range 450 to 600 mg L-1 (Fig. 2), respectively. After the second repetition, there was a higher increment in the COD value because the first repetition was washed with water and from the second one, such that all the washing was carried with effluents. The COD was considered stable after the 11th repetition, and the last bleaching sequence was performed to obtain and characterize the bleached pulp.
Table 4. Quantity of Used Reagents in Stage D for Bleaching Pulp Washed with Deionized Water (DW), White Water (WW), Low (LE) and High (HE) Organic Load Effluents
Fig. 2. COD of filtrates of stage Dht (a), COD of filtrates of stage EP (b) along the repetitions. LE: low-load effluent; HE: high load effluent; DW: deionized water, WW: white water
Chlorine dioxide has a high interaction with organic materials (Narkis et al. 1995) and, due to its high organic matter content, the high organic load effluent (HE) reacts more easily with ClO2, justifying the increase in the consumption from 8.1 to 13.8 kgClO2 odt-1 when using this effluent in bleaching instead of deionized water. The presence of organic matter in the effluent competes with the lignin in the reaction with ClO2, requiring more reagent to be able to remove properly the lignin, which reduces the oxidation efficiency (Simões et al. 2010). These results contrast with the results of Vehmaa et al. (2016), who used effluent as dilution water in stages Dht and D and achieved 90% ISO brightness without increasing chlorine dioxide consumption. However, in the study of these authors, the complete bleaching sequence consumed 17 kgClO2 odt-1 in the reference experiment (with deionized water), value much higher than all of those found in the present study.
The copper, manganese, and iron content increases the brightness reversion processes (Buchert et al. 1997) and reduces the efficiency of the hydrogen peroxide stage (P). These components cause a catalytic decomposition of hydrogen peroxide by the simultaneous generation of hydroxyl and hydroperoxyl free radicals (Gellerstedt and Pettersson 1982). Therefore, the use of effluents with a high content of Cu, Mn, and Fe in the washing of stages D and P increases the demand for ClO2 in stage D to reach, at the end of stage P, 90% ISO brightness. Therefore, it is interesting to rearrange the amount of chlorine dioxide used in the sequence (Dht and D), to increase efficiency in the early stages and not affect negatively the final P-stage.
HE has a higher content of Mn and Fe (0.3 and 1.3 mg L-1) than WW (0.1 and 0.4 mg L-1), also corroborating to the increase of ClO2 consumption in the following process. LE has less organic matter than HE, but the consumption of chlorine dioxide in D-stage was higher (16.3 kgClO2 odt-1) to reach the 90% ISO brightness at the end of P-stage, justified by the high content of Mn and Fe (0.2 and 4.6 mg L-1, respectively).
Metals must be removed prior to using effluents (HE and LE) or chelators should be added to the pulp to stabilize the performance of P-stage. The negative aspect of chelation is the requirement for strict pH control in a slightly acidic to neutral range and the limited biodegradability of these chelators (Fowles et al. 2007). The production cost of bleached kraft pulp increases with the increase of the bleaching reagents.
The consumption of ClO2 increased by 100% when using LE (from 8.1 to 16.3 kgClO2 odt-1) and by 70% when using HE (from 8.1 to 13.8 kgClO2 odt-1), compared to the use of deionized water. However, the total amount consumed is still acceptable, as many industries use values up to 20 kgClO2 odt-1 according to the literature (Colodette et al. 2005) and practical experiences. The rearrangement of ClO2 loads between Dht and D is a possibility to reduce the total consumption of reagent, by increasing the efficiency of Dht-stage and, therefore, reducing the demand of ClO2 in the following D-stage.
Final Characterization
Bleached pulp
The brightness of the bleached pulp was fixed at 90% ISO and the brightness-reversion increased when using LE and HE. The viscosity, kappa number and hexenuronic acids content in the pulps bleached with effluents decreased compared to the bleached with deionized water and white water (DW and WW) (Table 5).
Table 5. Characterization of Bleached Pulps Washed with Deionized Water (DW), White Water (WW), Low and High Organic Load Effluent (LE and HE)
Bleached pulps with LE and HE had higher brightness reversion due to the presence of metals, mainly copper, manganese and iron, which reduce the efficiency of the stage with P-stage, decreasing the brightness stability (Gellerstedt and Pettersson 1982). The association of lignin with the pulp polysaccharides leads to an exposure of the carbohydrates during delignification and bleaching. Chlorine dioxide is selective, although its selectivity is limited; therefore, carbohydrates are also partially degraded in addition to lignin (Gierer 1986). The reaction of chlorine with lignin forms carbonyl groups in celluloses and hemicelluloses, creating structures that fragment, resulting in peeling of carbohydrates (Gargulak et al. 2015). Thus, the increase in chlorine dioxide to bright the pulp increases the degradation of lignin and, consequently, more carbohydrates are also degraded (Lehtimaa et al. 2010). This justifies the reduction in the kappa number and viscosity while using effluent instead of water in the pulp bleaching. Chlorine dioxide in an acidic stage during bleaching reacts with lignin and carbohydrates and hydrolyzes the hexenuronic acids presented in the pulp, justifying the decrease when bleaching has higher ClO2 dosage (Yang et al. 2003). The K, Na, Cu, and Mn contents in the bleached pulps were higher when bleaching was carried out with LE and HE than with white water (WW) and deionized water (Table 6).
Table 6. Metal Levels in Bleached Pulps Washed with Deionized Water (DW), White Water (WW), Low and High Organic Load Effluent (LE and HE)
The Fe content was quite high for pulp bleached with LE. The levels of Fe, K, Na, Cu, and Mn in bleached pulps with HE and LE were higher due to their higher concentration in the effluents, which were retained in the pulp during bleaching due to the complexation with carboxyl groups – chelating agents (Fowles et al. 2007). The amount of copper, manganese and mainly iron must be restricted, as they interfere in the brightness reversion (Buchert et al. 1997).
The bleaching with WW had higher Ca content than those with deionized water and effluents, demonstrating that this parameter does not limit the use of the effluents, since white water is already often used in pulp bleaching with no harm on the process. The Mg content can limit the recycling by delaying the oxidation of the pulp (Zeronian and Inglesby 1995). However, because this value was higher when using white water (currently used in the mill), this parameter would not be a limitation for the reuse of HE and LE.
Refining process and paper manufacturing
After the Tukey tests at 5% significance comparing the viscosities of the 4 different final pulps (DW, WW, LE, and HE), there was no significant difference between the pairs of deionized water and white water (meaning that Ag = AB), as well as pairs of low and high organic load effluent (meaning that LE = HE). However, all the other pairs differed from each other (DW and WW ≠ LE and HE) (Fig. 3).
Fig. 3. Tukey test results at 5% significance level made in the R software comparing bleaching with white water (WW), deionized water (DW), low (LE) and high (HE) organic load effluent
Deionized water and high organic load effluent (DW and HE) were chosen to move forward with the experiments, as their pulp viscosity values, signifying molecular weight, were significantly different. With the characterized physical-mechanical properties of the fabricated papers (Table S1) and the application of Identity Model proposed by Regazzi (1996 2003) (Table S2 and Fig. 4), it was possible to account for the differences between the papers.
The Schopper Riegler freeness increases with the increasing of refining due to the formation of a fiber mat better arranged (González et al. 2012). In addition, energy consumption for the HE paper was very close to the reference pulp (DW), showing that the production cost will not be changed when using this effluent.
The tensile index, bursting strength, and tensile energy absorption (TEA) increased as a second-degree polynomial function when increasing the refining. These measured indexes are mainly affected by the bonding capacity between the fibers, which can account for the similarity in the observed trend for all parameters (Potulski et al. 2014).
All indexes decreased when adding effluent to the pulp, especially when the degree of refining was increased. This is explained by the decrease in viscosity of the pulp washed with effluent, which reduces the bonding between fibers and increases the empty spaces between them during the formation of the paper (Garg and Singh 2006). In addition, the curves are distanced along the increase in the refining degree due to the lower viscosity of the pulp in the HE handsheets, making the fiber mesh formed with the refining not very compacted.
The resistance to passage of air consists of the time a determined volume of air passes through the paper under certain conditions. This property is influenced exponentially by the degree of fiber compaction (Silva et al. 2013), which accounts for the behavior of both samples.
The more severe the bleaching conditions, the lower was the viscosity of the pulp and the greater the difficulty of compacting the fibers in the course of refining; therefore, the greater was the porosity of the network and lower was the resistance to the passage of air (González et al. 2012), which was demonstrated by the HE pulp. In addition, it is noted that at a higher the degree of refining, there was more separation of the curves, which means that the DW paper, which had higher viscosity, produced a more compacted fiber mesh than the HE papers.
Both fabricated papers described similar behaviors with similar rupture points in the modulus of elasticity (MOE) and internal tearing resistance (ITR) tests, even though the HE paper had, along the refining degree, smaller MOE than the DW paper. As the paper specimens were being subjected to continuously increasing tension, they exhibited the expected MOE behavior: initially, a linear behavior (elastic deformation region) and, after overcoming this region, plastic behavior (plastic deformation region) until reaching the maximum load that they were capable of absorbing (maximum rupture load), reaching rupture (Garg and Singh 2006). The ITR increased with refining up to maximum values, thereafter tending to stabilize and decrease in value. The maximum value, in all treatments, was close to the 17 Wh refining energy consumption and, after this refining level, there was a loss of the intrinsic strength of the fibers, causing a reduction of tear strength of the paper.
The brightness of the papers maintained the same trend: decrease in brightness with the increase in the degree of refining, up to a constant level. However, the brightness of the sample prepared with effluent remained greater than the sample with the addition of fresh water along almost the entire curve.
According to the Identity Model (Regazzi 1996, 2003), the papers were similar with respect to all the mentioned parameters, since it was possible to obtain an average common curve between both samples within the 5% significance level. Although the bleached pulp washed with different types of washing water (DW, WW, LE, HE) had different levels of metals, viscosity, and kappa number, all the physical and optical characteristics of the bleached hand-sheet had not been changed. This demonstrates that the quality of the manufactured papers was not altered when using effluent as washing water.
Fig. 4. Identity model curves of papers made from pulp washed with deionized water (DW) and high organic load effluent (HE). °SR: Schopper Riegler freeness; MOE: modulus of elasticity; TEA: tensile energy absorption
Resulted filtrates
The Dht and EP filtrates of the 12th bleaching repetition had average values of electric conductivity, color, turbidity, COD, and TDS that were higher when bleaching was carried out with HE and LE compared to the references (DW and WW) (Table 7).
Table 7. Dht and EP Filtrates Characterization of Bleaching using Deionized Water (DW), White Water (WW), Low (LE) and High Organic Load Effluent (HE)
The generated filtrates are complex mixtures that present uncountable chemical compounds, derived from all the extract materials from the pulp during bleaching (Pokhrel and Viraraghavan 2004). The higher values of COD, color, and TDS when HE and LE were used are due to the more severe bleaching conditions. The increase of chlorine dioxide application favored a greater degradation of organic matter in the pulp, such as carbohydrates, lignin, which ends up in the filtrates.
The COD increased from 7.9 kg odt-1 (deionized water) to 9.1 kg odt-1 (HE), which does not significantly burden the industrial Effluent Treatment Plant (ETP). The activated sludge treatment can reduce COD between 40 and 85% (Cabrera 2017; Mounteer et al. 2007) and, therefore, the increase of 1.2 kg odt-1 in the HE bleaching filtrate is diluted with the rest of the high organic load effluent, and will be responsible for a rise of less than 0.2 kg odt-1 after biological treatment. This low increase in COD level is further diluted with the rest of the effluent from the entire mill. These values will not legally limit the discharging (maximum 20 kg COD adt-1). Furthermore, all the values were below the already mentioned limits from Europe Commission and Brazilian Government (Minas Gerais 2008; European Commission 2014).
The electrical conductivity was higher for bleaching with LE and HE due to the carry-over of the inorganic salts and ionic compounds, demonstrated by the high content of metals in these effluents (Achoka 2002).
The levels of Ca, Fe, K, and Na were higher for bleaching with LE and HE compared to the references (deionized water and white water). The chloride content, Cu, Mn, and Mg did not change (Table 8).
Table 8. Dht and EP Filtrates Metal Levels of Bleaching Using Deionized Water (DW), White Water (WW), Low (LE) and High Organic Load Effluent (HE)
Because the kraft pulping process uses NaOH, Na2S, and Ca(OH)2, higher concentrations of Na and Ca in the bleaching filtrates were expected. As LE and HE bleaching uses more NaOH to reach the appropriate final pH, more Na is released into the filtrates as well. The increase in the content of other metals in the effluent bleaching filtrates is justified by the severe bleaching conditions that dissolve the existing compounds in wood in higher proportion.
The filtrate contains chloride, which is generally proportional to the consumption of chlorine in the bleaching (Savant et al. 2006). The more ClO2 used in bleaching, the higher the formation of chlorate and chloride, but the stoichiometry of the reaction remains stable (Lehtimaa et al. 2010). The chloride content is higher for bleaching with LE and HE due to the higher consumption of ClO2 in stage D.
CONCLUSIONS
This research evaluated the feasibility of using treated kraft pulp effluents (low and high organic load effluent) in the washing stage of the Dht(EP)DP bleaching sequence of a pulp mill. The following conclusions follow from the work:
- Low and high organic load effluent was able to be used to wash the pulp between the bleaching stages.
- The quality of the generated filtrates, the bleached pulp, and the manufactured papers was adequate for all evaluated parameters.
- The consumption of reagents increased when using low organic load effluent (LE) and biologically treated effluent (HE), from 8.1 kg ClO2 odt-1 (reference value) to 16.3 kg ClO2 odt-1 and 13.8 kg ClO2 odt-1, respectively, which might lead to a considerable rise in the cost of bleached pulp production.
- The results of the present research show a potential of recycling treated effluents in the bleaching of kraft pulp.
ACKNOWLEDGEMENTS
The authors ensure there are no conflicts of interests for this research and they are grateful for the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), CENIBRA, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES- Finance Code 001) to support this work.
REFERENCES CITED
Achoka, J. D. (2002). “The efficiency of oxidation ponds at the Kraft pulp and paper mill at Webuye in Kenya.” Water Res. 36(5), 1203-1212. DOI: 10.1016/S0043-1354(01)00325-6
American Public Health Association (APHA). (2012). Standard Methods for the Examination of Water and Wastewater, Washington, D.C.
Axegård, P., Folke, J., Carey, J., Gleadow, P., and George, P. (1997). “Minimum-impact mills: Issues and challenges the environmental significance of eliminating bleaching effluent,” in: TAPPI Proceedings – Environmental Conference & Exhibition, Minneapolis, MN, USA.
Bajpai, P., and Bajpai, P. K. (1994). “Biological colour removal of pulp and paper mill wastewaters,” J. Biotechnol. 33(3), 211-220. DOI:10.1016/0168-1656(94)90069-8
Buchert, J., Bergnor, E., Lindblad, G., and Viikari, L. (1997). “Significance of xylan and glucomannan in the brightness reversion of kraft pulps.” Tappi J. 80, 15-171.
Cabrera, M. N. (2017). “Pulp mill wastewater: Characteristics and treatment,” in: Biological Wastewater Treatment and Resource Recovery, IntechOpen, pp. 119-139. DOI: 10.5772/67537
Colodette, J. L., Gomes, C. M., Rabelo, M., and Eiras, K. M. M. (2005). “Progress in eucalyptus kraft pulp bleaching,” 2nd Int. Colloq. Eucalyptus Pulp, pp. 1-18. http://www.eucalyptus.com.br/icep02/jorge_colodette.pdf
Costa, M. M., Colodette, J. L., Landim, A., Silva, C. M., and Macêdo, A. M. L. C. (2006). “A novel bleaching technology adapted to partial bleach plant closure,” Rev. Árvore 30(1), 129-139. DOI: 10.1590/S0100-67622006000100016
European Commission, E. (2014). “2014/687/EU: Commission Implementing Decision of 26 September 2014 establishing the best available techniques (BAT) conclusions, under Directive 2010/75/EU of the European Parliament and of the Council, for the production of pulp, paper and board (notifie.” Off. J. Eur. Union, 284, 76.
Fowles, E. H., Gilbert, B. C., Giles, M. R., and Whitwood, A. C. (2007). “The effects of chelating agents on radical generation in alkaline peroxide systems, and the relevance to substrate damage,” Free Radic. Res. 41(5), 515-522. DOI: 10.1080/10715760601148475
Frigieri, T. C., Ventorim, G., and Favaro, J. S. C. (2016). “The effect of water reduction in kraft pulp washing in ECF bleaching,” Rev. Árvore 40(6), 1091-1098. DOI: 10.1590/0100-67622016000600015
Furley, T. H., and De Oliveira Filho, A. C. (2000). “Biomonitoring of heavy metals and organo-chlorinated compounds in a pulp mill effluent using introduced mussels,” Aquat. Ecosyst. Heal. Manag. 3(4), 499-507. DOI: 10.1080/14634980008650686
Garg, M., and Singh, S. P. (2006). “Reasons of strength loss in recycled pulps,” Appita Technol. Innov. Manuf. Environ. 59(4), 274-279.
Gargulak, J. D., Lebo, S. E., and McNally, T. J. (2015). “Lignin,” in: Kirk-Othmer Encycl. Chem. Technol., Wiley, Hoboken, NJ, USA, 5, pp. 1-26. DOI: 10.1002/0471238961.12090714120914.a01.pub3
Gellerstedt, G., and Pettersson, I. (1982). “Chemical aspects of hydrogen peroxide bleaching. Part II the bleaching of kraft pulps,” J. Wood Chem. Technol. 2(3), 231-250. DOI: 10.1080/02773818208085133
Gierer, J. (1986). “Chemistry of delignification – Part 2: Reactions of lignins during bleaching,” Wood Sci. Technol. 20(1), 1-33. DOI: 10.1007/BF00350692
Gleadow, P., Hastings, C., Barynin, J., Schroderus, S., and Warnqvist, B. (1997). “Towards closed-cycle kraft: ECF versus TCF case studies,” Pulp Pap. Canada 98(4), 27-37.
González, I., Boufi, S., Pèlach, M. A., Alcalà, M., Vilaseca, F., and Mutjé, P. (2012). “Nanofibrillated cellulose as paper additive in eucalyptus pulps.” BioResources 7(4), 5167-5180. DOI: 10.15376/biores.7.4.5167-5180
Hart, B. P. W., and Rudie, A. W. (2012). The Bleaching of Pulp (5th Ed.), TAPPI Press, Atlanta, GA, USA.
Hubbe, M. A., Metts, J. R., Hermosilla, D., Blanco, M. A., Yerushalmi, L., Haghighat, F., Lindholm-Lehto, P., Khodaparast, Z., Kamali, M., and Elliott, A. (2016). “Wastewater treatment and reclamation: A review of pulp and paper industry practices and opportunities,” BioResources 11(3), 7953-8091. DOI: 10.1016/j.seppur.2011.07.002
Huber, P., Burnet, A., and Petit-Conil, M. (2014). “Scale deposits in kraft pulp bleach plants with reduced water consumption: A review.” J. Environ. Manage. 141, 36-50. DOI: 10.1016/j.jenvman.2014.01.053
ISO 5267-1 (1999). “Pulps – Determination of drainability,” International Organization for Standardization, Geneva, Switzerland.
Johnson, T. (2019). “Is water use reduction still relevant in P and P mills?: An update on process closure with reference to the circular economy,” Appita 3, 52-55.
Kamali, M., and Khodaparast, Z. (2015). “Ecotoxicology and environmental safety review on recent developments on pulp and paper mill wastewater treatment,” Ecotoxicol. Environ. Saf. 114, 326-342. DOI: 10.1016/j.ecoenv.2014.05.005
Kumar, S., Bhardwaj, N. K., and Kumar, P. (2017). “Pollution reduction from pulp bleaching effluents by process change,” J. Indian Pulp Pap. Tech. Assoc. 28(2), 68-73.
Lacorte, S., Latorre, A., Barceló, D., Rigol, A., Malmqvist, A., and Welander, T. (2003). “Organic compounds in paper-mill process waters and effluents,” TrAC – Trends Anal. Chem. 22(10), 725-737. DOI: 10.1016/S0165-9936(03)01009-4
Lehtimaa, T., Tarvo, V., Kuitunen, S., Jääskeläinen, A. S., and Vuorinen, T. (2010). “The effect of process variables in chlorine dioxide prebleaching of birch kraft pulp. Part 1. Inorganic chlorine compounds, kappa number, lignin, and hexenuronic acid content,” J. Wood Chem. Technol. 30(1), 1-18. DOI: 10.1080/02773810903276676
Mainardis, M., Buttazzoni, M., Bortoli, N. De, Mion, M., and Goi, D. (2020). “Evaluation of ozonation applicability to pulp and paper streams for a sustainable wastewater treatment,” J. Clean. Prod. 258, e120781. DOI: 10.1016/j.jclepro.2020.120781
Mainardis, M., and Goi, D. (2019). “Pilot-UASB reactor tests for anaerobic valorisation of high-loaded liquid substrates in friulian mountain area,” J. Environ. Chem. Eng. 7, 2-7. DOI: 10.1016/j.jece.2019.103348
McKay, D. D., Rice, J. E., Vinson, K. D., McFarland, J. R., Hamilton, A. J., Wahl, E. H., and Frankenbach, G. M. (2004). “Low viscosity bilayer disrupted softening composition for tissue paper,” U.S. Patent 6797117/2004.
Minas Gerais. (2008). Deliberação Normativa Conjunta COPAM/CERH-MG N.o 1, de 05 de Maio de 2008. http://www.mma.gov.br/port/conama/processos/EFABF603/DeliberaNormativaConjuntaCOPAM-CERHno01-2008.pdf
Morais, A. de A., Mounteer, A., and Silveira, D. S. A. (2008). “Improvement of eucalyptus bleached kraft pulp effluent treatment through combined ozone-biological,” Tappi J. 7(2), 26-31.
Mounteer, A. H., Pereira, R. O., Morais, A. A., Ruas, D. B., Silveira, D. S. A., Viana, D. B., and Medeiros, R. C. (2007). “Advanced oxidation of bleached eucalypt kraft pulp mill effluent,” Water Sci. Technol. 55(6), 109-116. DOI: 10.2166/wst.2007.218
Mounteer, A. H., Colodette, J. L., and Silva, D. O. (2002). “Treatment efficiency of eucalypt kraft pulp bleaching effluents : influence of dissolved organic matter.” Tappi J. 1(2), 26-32.
Narkis, N., Katz, A., Orshansky, F., Kott, Y., and Friedland, Y. (1995). “Disinfection of effluents by combinations of chlorine dioxide and chlorine,” Water Sci. Technol. 31(5–6), 105-114. DOI: 10.1016/0273-1223(95)00249-M
National Council for Air and Stream Improvement (NCASI) (2009).” Environmental footprint comparison tool. A tool for understanding environmental decisions related to the pulp and paper industry,” (http://www.paperenvironment.org/ PDF/water/Water_General_Overview.pdf), Washington, D. C.
Nuortila-Jokinen, J., Huuhilo, T., and Nystrom, M. (2003). “Closing pulp and paper mill water circuits with membrane filtration,” Ann. N Y Acad. Sci. 984, 39-52. DOI: 10.1111/j.1749-6632.2003.tb05991.x
Parsad, B., Kirkman, A., Jameel, H., Gratzl, J., and Magnotta, V. (1996). “Mill closure with high-kappa pulping and extended oxygen delignification,” Tappi J. 79(9), 144-152.
Pokhrel, D., and Viraraghavan, T. (2004). “Treatment of pulp and paper mill wastewater – A review,” Sci. Total Environ. 333, 37-58. DOI: 10.1016/j.scitotenv.2004.05.017
Potulski, D. C., Muniz, G. I. B. de, Klock, U., and Andrade, A. S. de. (2014). “The influence of incorporation of microfibrillated cellulose on mechanical strength properties of paper,” Sci. For. 42(103), 345-351.
Reeve, D., and Silva, C. M. (2000). “Closed cycle systems for manufacture of bleached chemical wood pulp,” in: Chemical Pulping, Book 6B. J. Gullichsen & C.J. Fogelholm. Fapet Oy, Jyväskylä, pp. B440-B473.
Regazzi, A. J. (1996). “Teste para verificar a identidade de modelos de regressão,” in: Pesqui. Agropecu. Bras. 31(1), 1-17.
Regazzi, A. J. (2003). “Teste para verificar a igualdade de parâmetros e a identidade de modelos de regressão não-linear,” Ceres 50(287), 9-26.
Rintala, J. A., and Lepistö, S. S. (1992). “Anaerobic treatment of thermomechanical pulping whitewater at 35-70°C,” Water Res. 26(10), 1297-1305. DOI: 10.1016/0043-1354(92)90124-M
Rodrigues, C. L. S., Mounteer, A. H., Stoppa, T. V, and Dalvi, L. C. (2010). “Chemical components of bleached eucalypt kraft pulp effluent COD and treatment removal efficiency during normal mill operation and maintenance shutdowns,” Water Sci. Technol. 62(7), 1567-1573. DOI: 10.2166/wst.2010.941
Savant, D. V., Abdul-Rahman, R., and Ranade, D. R. (2006). “Anaerobic degradation of adsorbable organic halides (AOX) from pulp and paper industry wastewater,” Bioresour. Technol. 97(9), 1092-1104. DOI: 10.1016/j.biortech.2004.12.013
Sillanpää, M. (2005). “Studies on washing in kraft pulp bleaching,” University of Oulu, Finland.
Silva, J. C. da, Oliveira, R. C. de, Batalha, L. R., and Manfredi, M. (2013). “Combination of enzymatic, mechanical and ultrasonic treatments for improvement of the properties of secondary pulps,” Cerne 19(4), 653-669.
Simões, R. M. dos S., Barroca, M. J. M. C., and Castro, J. A. A. M. (2010). “Effect of carry-over on the kinetics of chlorine dioxide delignification of an unbleached hardwood kraft pulp,” Appita 55, 60-64.
Solomon, K. R. (1996). “Chlorine in the bleaching of pulp and paper,” Pure Appl. Chem. 68(9), 1721-1730. DOI: 10.1351/pac199668091721
Sonkar, M., Kumar, M., Dutt, D., and Kumar, V. (2019). “Biocatalysis and agricultural biotechnology treatment of pulp and paper mill effluent by a novel bacterium Bacillus sp . IITRDVM-5 through a sequential batch process,” Biocatal. Agric. Biotechnol. 20, e101232. DOI: 10.1016/j.bcab.2019.101232
TAPPI T525 om-17 (2017). “Diffuse brightness of paper, paperboard and pulp,” TAPPI Press, Atlanta, GA.
TAPPI T236 om-13 (2013). “Kappa number of pulp,” TAPPI Press, Atlanta, GA.
TAPPI 230 om-19 (2019). “Viscosity of pulp (capillary viscometer method),” TAPPI Press, Atlanta, GA.
TAPPI T282 om-19 (2019). “Hexeneuronic acid content of chemical pulp,” TAPPI Press, Atlanta, GA.
TAPPI T266 om-18 (2018). “Determination of sodium, calcium, copper, iron, and manganese in pulp and paper by atomic absoption spectroscopy,” TAPPI Press, Atlanta, GA.
TAPPI T248 sp-15 (2015). “Laboratory beating of pulp (PFI mill method),” TAPPI Press, Atlanta, GA.
TAPPI T205 sp-18 (2018). “Forming handsheets for physical tests of pulp,” TAPPI Press, Atlanta, GA.
TAPPI T551 om-18 (2018). “Thickness of paper and paperboard (soft platen method),” TAPPI Press, Atlanta, GA.
TAPPI T410 om-19 (2019). “Grammage of paper and paperboard (weight per unit area),” TAPPI Press, Atlanta, GA.
TAPPI T536 om-18 (2018). “Resistance of paper to passage of air (high-pressure Gurley method),” TAPPI Press, Atlanta, GA.
TAPPI T403 om-15 (2015). “Bursting strength of paper,” TAPPI Press, Atlanta, GA.
TAPPI T414 om-18 (2018). “Internal tearing resistance of paper (Elmendorf-type method),” TAPPI Press, Atlanta, GA.
TAPPI T494 om-13 (2013). ” Tensile breaking properties of paper and paperboard (using constant rate of elongation apparatus),” TAPPI Press, Atlanta, GA.
TAPPI T525 om-17 (2017). “Diffuse brightness of paper, paperboard and pulp (d/0),” TAPPI Press, Atlanta, GA.
TAPPI UM200 (2012). “Brightness loss of bleached pulp,” TAPPI Press, Atlanta, GA.
Theliander, H. (2009). “Recovery of cooking chemicals: The treatment and burning of black liquor,” in: Pulp Chem and Technol., M. Ek, G. Gellerstedt, and G. Henriksson (eds.), De Gruyter, Berlin, pp. 297-334. DOI: 10.1515/9783110213423.297
Thompson, G., Swain, J., Kay, M., and Forster, C. F. (2001). “The treatment of pulp and paper mill effluent: A review,” Bioresour. Technol. 77(3), 275-286. DOI: 10.1016/S0960-8524(00)00060-2
Vehmaa, J., Råmark, H., and Tervola, P. (2016). “Utilizing purified mill effluent in a low-effluent concept after secondary treatment.” International Forest, Pulp and Paper Conference, pp. 1-9.
Yang, R., Lucia, L., Ragauskas, A. J., and Jameel, H. (2003). “Oxygen delignification chemistry and its impact on pulp fibers.” J. Wood Chem. Technol. 23(1), 13-29. DOI: 10.1081/WCT-120018613
Zeronian, S. H., and Inglesby, M. K. (1995). “Bleaching of cellulose by hydrogen peroxide,” Cellulose, 2(4), 265-272. DOI: 10.1007/BF00811817
Article submitted: July 13, 2020; Peer review completed: September 5, 2020; Revised version received and accepted: September 24, 2020; Published: October 12, 2020.
DOI: 10.15376/biores.15.4.8944-8964
SUPPLEMENTARY
Table S1. Characterization of the Fabricated Papers with Deionized Water (DW) and High Organic Load Effluent (HE)
Table S2. Statistical Analysis of the Physical and Optical Properties of the Fabricated Papers
Notes: YDW: Equation for the DW hand-sheets, YHE: Equation for the HE hand-sheets, YC: Common equation for both hand-sheets. Null hypothesis (H0): YDW = YHE = YC
