Abstract
Waste newspaper fiber (WNF) was separated and deinked for use as an absorbent for removal of Malachite Green (MG) from aqueous solutions. The chemical composition of the deinked waste newspaper fiber (DWNF) was analyzed, and its morphology was observed by Scanning Electron Microscopy (SEM). A batch adsorption study was conducted under various adsorbent dosage, solution pH, and contact time. Kinetics and isotherms models were fitted; the thermodynamic parameters were also calculated. The results indicated that the main component in DWNF is cellulose. The SEM photographs showed that the surface became smoother and cleaner after deinking treatment. The equilibrium adsorption capacity was reached within 60 min, and the maximum adsorption capacity was around 27 mg/g. Alkaline pH (around 8) favored the adsorption process. The adsorption of MG was a spontaneous and exothermic process. It was found that the pseudo-second order kinetic equation and Langmuir adsorption isotherm model described the data of dye adsorption onto DWNF very well. The results show that DWNF is an effective absorbent for dye wastewater treatment.
Download PDF
Full Article
REMOVAL OF MALACHITE GREEN FROM AQUEOUS SOLUTION USING WASTE NEWSPAPER FIBER
Jia Tan, Xiaoyu Zhang, Xinhao Wei, and Lijuan Wang*
Waste newspaper fiber (WNF) was separated and deinked for use as an absorbent for removal of Malachite Green (MG) from aqueous solutions. The chemical composition of the deinked waste newspaper fiber (DWNF) was analyzed, and its morphology was observed by Scanning Electron Microscopy (SEM). A batch adsorption study was conducted under various adsorbent dosage, solution pH, and contact time. Kinetics and isotherms models were fitted; the thermodynamic parameters were also calculated. The results indicated that the main component in DWNF is cellulose. The SEM photographs showed that the surface became smoother and cleaner after deinking treatment. The equilibrium adsorption capacity was reached within 60 min, and the maximum adsorption capacity was around 27 mg/g. Alkaline pH (around 8) favored the adsorption process. The adsorption of MG was a spontaneous and exothermic process. It was found that the pseudo-second order kinetic equation and Langmuir adsorption isotherm model described the data of dye adsorption onto DWNF very well. The results show that DWNF is an effective absorbent for dye wastewater treatment.
Keywords: Adsorption; Malachite green; Waste newspaper fiber; Kinetics; Isotherms; Thermodynamic analysis
Contact information: Key Laboratory of Bio-based Material Science and Technology of Ministry of Education, Northeast Forestry University, 26 Hexing Road, Harbin 150040, P. R. China;
* Corresponding author: donglinwlj@163.com
INTRODUCTION
Pollution is a worldwide problem that has led to a corresponding interest in pollution control. On the positive side, dyes can give beautiful color to various products, and therefore they are widely used in many fields such as textiles, paper, plastic, food, painting, and medicine (Altınışık et al. 2010; Safa and Bhatti 2011). However, the wastewater from industries using dye may still contain up to 10% of the dye (Moussavi and Khosravi 2011). Most synthetic dyes are toxic and can bring about serious water pollution, destroy community structure of aquatic organisms, and further become a hazard to all mankind. It is reported that around 25% of diseases facing humans today occur because of long-term exposure to environmental pollution (Tang et al. 2012). Malachite green (MG) is an N-methyl diaminotriphenylmethane dye, which is also called Basic Green 4. MG has been widely used in many industries: it can prevent fungal attacks, protozoan infections, and other diseases caused by parasites of fish and other aquatic organisms (Hameed and El-Khaiary 2008).
Adsorption has been found to be a more feasible process for most pollutant removal from industrial effluents in comparison to methods such as membrane filtration, electro-coagulation, electrochemical destruction, ion-exchange, irradiation, advanced oxidation, and precipitation (Jain and Sikarwar 2008). The reason is that most of those processes involve high costs and low efficiency. Activated carbon (AC) is the most popular absorbent due to its excellent performances in adsorption (Baccar et al. 2009). However, its high cost and difficulty in regeneration limits its applicability (Mahmoodi et al. 2011). In recent years, low-cost absorbents for wastewater treatment have attracted a lot of attention. Agricultural wastes (Bhattacharyya and Sharma 2005; Vadivelan and Kumar 2005) and by-products (Batzias and Sidiras 2007; Hamdaoui 2006; Jain and Jayaram 2010; Kumar and Sivanesan 2007;) have been studied in detail for the removal of dyes in aqueous media. For the removal of MG from waste water, some low-cost absorbents have been investigated, such as degreased coffee bean (Baek et al. 2010), waste material of Daucus carota (Kushwaha et al. 2011), treated ginger waste (Ahmad et al. 2010), beech sawdust (Witek-Krowiak 2011), potato plant waste (Gupta et al. 2011), and modified rice husk (Chowdhury et al. 2011).
Waste newspaper is a kind of domestic waste. Recycling waste newspaper can save resources and avoid pollution. Waste newspaper fiber (WNF) is a low-cost cellulosic material. Researchers have investigated the adsorption and porous properties of activated carbon from waste newspaper prepared by chemical and physical activation (Okada et al. 2003). WNF has been directly used as an adsorbent to absorb Cu(II) ions (Chakravarty et al. 2008) and Cr(VI) ions (Wang and Li 2009). Adsorption of zinc was studied using chemically modified WNF as an adsorbent in an aqueous medium (Chakravarty et al. 2007). However, the use of WNF for dye removal from aqueous effluents has not been previously investigated.
In the present work, deinked waste newspaper fiber was used as an adsorbent to remove MG from aqueous solution at various conditions. Kinetic and isotherm parameters also were evaluated.
EXPERIMENTAL
Preparation of Waste Newspaper Cellulose
The waste newspaper was torn up into small pieces and immersed in water for 2 days. Then the water-saturated paper piece was disintegrated in the beating machine (model ZQS 2-23, made in machinery plant of Shanxi University of Science and Technology, China), and the waster newspaper fibers were obtained. The WNF was treated with an aqueous solution containing 1.5% NaOH, 3.0% H2O2, 0.3% Na2SiO3, and 1.5% surfactant for removing black ink at 65°C for 1h. After the processing, WNF was washed several times with distilled water until the supernatant pH was 6.5 to 7.0. The pretreated WNF was dried to a constant weight in an oven at 103°C. The dried wastepaper cellulose was crushed and screened.
The fraction passing an 80-mesh screen and retained on a 120-mesh screen was used as the adsorbent. The chemical composition of waste newspaper was analyzed, and the results are listed in Table 1. In DWNF, the largest component is cellulose, and there is around 12.35% of lignin.
Table 1. The Content of Main Chemical Composition in DWNF (%)
DWNF Characterization
The point of zero charge (pHPZC) was determined by the solid addition method (Vieira et al. 2009). To a series of 100-mL conical flasks, 10.0-mL aliquots of NaCl solution were transferred with pH values ranging from 2 to 11. Then, DWNF (0.05 g) was added to each flask, which was securely capped immediately and shaken for 10 h. The differences between the final and initial pH values (ΔpH) were plotted against the initial pH, and the point of intersection on the X-axis corresponds to the point of zero charge (pHPZC). The surface morphology of DWNF was observed using a Quanta 200 SEM. Specimens were observed after spraying with gold.
Preparation of Dye Solution
To evaluate the applicability of DWNF as adsorbent for dye effluent treatment, MG was used as the adsorbate. MG (λmax 618 nm) used in this study was kindly supplied by Kermel (Tianjing) Trading Co., Ltd. Stock solution was prepared with a concentration of 100 mg/L. The different concentrations were obtained by further dilutions. Standard curves were developed via the absorbance measurement of the dye solution by UV-Visible spectrophotometer (TU-1900).
Adsorption Experiment
The adsorption of MG was conducted in batch model under various initial pH values, adsorbent doses, initial dye concentrations, and contact times. In the experiments, the DWNF was mixed with 100 mL dye solution in a 250 mL conical flask. The mixture was shaken at 190 rpm in a water bath oscillator at different temperatures. The effects of initial pH value were measured at pH value from 3 to 9. The initial pH of the solution was adjusted with 0.1M HCl or 0.1M NaOH solutions.
After the adsorption, the adsorbents were removed by filtration by using nylon screen with a pore size of 400-mesh. The residual MG concentration of the solution was analyzed.
The dye removal efficiency (R), amount of absorbed dye per unit mass of adsorbent at time t (qt, mg g-1), and at equilibrium (qe, mg g-1) were calculated by using the following equations,
R(%) = [(C0 – Ct) / C0]×100% (1)
qt = (C0 – Ct) V/W (2)
qe = (C0 – Ce) V/W (3)
where Ct (mg L-1) is the dye concentration at time t. C0 and Ce (mg L-1) are the initial and equilibrium concentration of dye solution, respectively. V is the volume of dye solution (mL), and W is the amount of the absorbent (g).
Kinetics and Isotherm Studies
The kinetics of the adsorption were determined by analyzing the quantity of dye adsorbed from aqueous solution at different time intervals. Isotherms of the adsorption at various dosages were analyzed to determine the equilibrium adsorption capacity. Thermodynamic parameters were calculated from the equilibrium adsorption data at different temperatures.
RESULTS AND DISCUSSION
Characterization of DWNF
The surface morphologies of WNF before and after deinking are shown in Fig.1. It can be seen that the surface of WNF was rough and stained with impurity substances. After deinking, the surface became smoother and cleaner. The pit structure indicates that newspaper used as raw material in this study was made from woody fibers. No other pores can be observed on the fiber except some pits.
Fig.1. SEM photographs of (a) WNF and (b) DWNF
Effect of Adsorbent Dose
The effect of adsorbent dosage on adsorption of MG is shown in Fig. 2. The adsorption capacity of dye decreased with increasing dosage of the DWNF. The adsorbents cannot play their role to their full potential due to there being more sorption sites available with the increase in the amount of solid. In fact, the removal of MG increased with the increase in the amount of fiber, and the solution became colorless when the amount was more than 0.06 g. The removal reached 90% at lower temperature.
Fig. 2. Effect of adsorbent dose on (a) the adsorption capacity and (b) removal of MG (pH = 5.7, t = 7h, C = 10mg·L-1, V = 100mL)
Effect of Initial pH
Initial pH is one of the most important factors affecting the adsorption ability of an absorbent. The effect of initial pH on the adsorption of MG onto DWNF is shown in Fig. 3. The adsorption capacity increased from 12.8 mg/g to 27.6 mg/g with an increase in pH from 2 to 7. Then, the adsorption changed slightly at pH beyond 7. It has been reported that the changes in adsorption depend on the surface properties of the adsorbent and the dye structure (Khattria and Singh 2009). As shown in Fig. 4, the point of zero charge of DWNF is around 8.2. In general, if pH < pHZPC, the surface of the absorbent would be positively charged, and the repulsive force would result in a decrease of adsorption. If pH > pHZPC, the surface would be negatively charged, and the attractive force would favor adsorption. However, the practical adsorption did not follow the theoretical prediction. It is attributed that MG structure changes in alkaline condition (Tang et al. 2012).
Fig. 3. Effect of pH on the adsorption of MG (T = 20℃, t = 3h, C = 10mg·L-1, V = 100mL)
Fig. 4. The plots of pH difference vs. initial pH
Effect of Contact Time
As shown in Fig. 5, the adsorption was completed about 88% in 10 min from the beginning. The equilibrium adsorption capacity can be reached within 60 min. The adsorption capacity changed little with increasing time. Therefore, the adsorption of MG onto DWNF is very rapid.
Fig. 5. Effect of contact time on the adsorption of MG (initial pH, C = 10mg·L-1, V = 100mL)
Sorption Kinetics
The kinetics of the adsorption process was analyzed by using pseudo-first-order and pseudo-second-order equations to fit the experimental data of MG onto DWNF. The pseudo-first-order kinetic model (Ahmad et al. 2007) is expressed as follows,
ln(qe-qt) = lnqe –k1t (4)
where qe and qt are the adsorption capacity (mg/g) at equilibrium and at time t, respectively. k1 is the rate constant (L/min) of the pseudo-first-order kinetic model.
The plot of log(qe-qt) as a function of t provides the k1 and qe values. As shown in Table 2 and Fig. 6 (a), the coefficients of determination at the three experimental temperatures were 0.938, 0.687, and 0.772, respectively. However, the linearity was not good, and the calculated value of qe was far lower than the experimental qe. This discrepancy shows that the adsorption of MG on NWF does not fit the pseudo-first-order kinetic model.
The pseudo-second-order kinetic model (Ho 2006; El-Sayed 2011) can be expressed as follows,
t/qt =1/k2qe2 + t/qe (5)
h= k2qe2 (6)
where k2 (g/mg min) is the rate constant for the pseudo-second-order kinetic model and h (mg/g min)is the initial adsorption rate at time approaching 0. The qe and k2 values were estimated from the slope (1/qe) and intercept (1/k2qe2) of the linear plot of t/qt versus t at different dye concentrations.
From the data in Table 2 and Fig. 6 (b), the plots according to the pseudo-second-order model provided high coefficients of determination of more than 0.999 and excellent linearity at the three temperatures. Moreover, the calculated value of qe is extremely close to the experimental qe, with deviations of less than 1.5%. Therefore, pseudo-second-order kinetic model described the adsorption process very well.
Fig. 6. Pseudo-first-order (a) and pseudo-second-order (b) plots of absorption of MG on DWNF at different temperatures
Table.2. Kinetic Parameters for sorption of MG on DWNF
Adsorption Isotherm
The Langmuir and Freundlich isotherm equilibrium models were used for the analysis of the adsorption of MG onto DWNF. The Langmuir isotherm is based on the hypothesis that uptake occurs on a homogeneous surface by monolayer adsorption without interaction between the absorbed materials (Li et al. 2009). The application of the Langmuir model to the experimental equilibrium isotherm data for MG adsorption on DWNF produces two parameters: the maximum adsorption capacity qm (mg/g) and the constant b (L/mg). The linear equation can be expressed as:
Ce/qe=1/(bqm) + Ce/qm (7)
The essential characteristic of the Langmuir isotherm can be represented by the equilibrium parameter, RL, calculated by,
RL=1/(1+bC0) (8)
where b is the Langmuir constant and C0 is the initial dye concentration (mg/L). RL is a dimensionless separation factor used to determine whether the adsorption process is favorable or unfavorable. The shapes of the isotherms for 0< RL <1, RL >1, RL=1, and RL=0 are favorable, unfavorable, linear, and irreversible (El Ashtoukhy 2009), respectively.
The Freundlich isotherm is commonly used for investigating the non-linear adsorption of a variety of dyes on various materials. The Freundlich isotherm (Freundlich 1906) is given by the following equation,
qe=kfCe1/n (9)
where kf (mg/g) is the coefficient for the adsorbed amount and n is the Freundlich constant.
The adsorption data were also found to fit the linear form of the Freundlich equation:
log qe =log kf + (1/n)logCe (10)
Figure 7 and Table 3 show the calculated parameters for the Langmuir and Freundlich isotherm models for MG adsorption on DWNF. R2 values of the Langmuir isotherms were uniform and higher than 0.99; however, those of the Freundlich isotherms were lower, indicating that the adsorption of MG on DWNF fits the Langmuir model well compared to the Freundlich model. Moreover, all RL values were in the range 0 to 1, which indicates that the adsorption of MG on DWNF is favorable. The results also suggest that the uptake of MG occurs on the homogeneous surface of DWNF by monolayer adsorption without interaction between the absorbed dye molecules.
Fig. 7. The Langmuir (a) and Freundlich (b) isotherms for adsorption of MG onto DWNF
Table 3. Adsoption Isotherm Parameters for Sorption of KMG on DWNF
Thermodynamic Analysis
In practice, thermodynamic parameters are very important and can be taken into consideration to evaluate the spontaneity of the adsorption process. The thermodynamic parameters such as standard enthalpy change (ΔH°), standard Gibbs free energy change (ΔG°), and standard entropy change (ΔS°) were calculated from the sorption of MG on DWNF temperature data (Huang et al. 2011).
According to the Van’t Hoff equation,
ΔG°=ΔH°-TΔS° = –RTlnkc (11)
kc=Cs/Ce (12)
lnkc=-(ΔH°/RT)+ ΔS°/R (13)
where kc is the equilibrium constant, Cs (mg/L) is the equilibrium concentration on the adsorbent, and Ce (mg/L) is the equilibrium concentration of dye in the solution. R is the universal gas constant (8.314 J mol-1 K-1), and T is the absolute temperature. The values of ΔG° and ΔH° were obtained from the slope and intercept of the Van’t Hoff curve (Fig. 8), and results are listed in Table 4. The negative value of ΔG° indicates the spontaneity and feasibility of the sorption of MG on the adsorbent. The negative value of ΔH° indicates that the sorption is exothermic in nature. The positive value of ΔS° shows that the increase in randomness at the interface during the sorption process.
Fig. 8. Van’t Hoff plot for MG adsorption on DWNF
Table 4. The Thermodynamic Parameters for Sorption of MG on DWNF
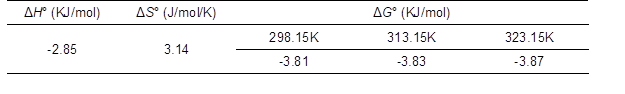
Waste newspaper fiber was used as an absorbent to remove malachite green from aqueous solution. The adsorption capacity reached 27 mg/g in alkaline condition and the adsorption was very fast. The adsorption followed the pseudo-second-order kinetic model well. The Langmuir isotherm provided the highest correlation of the experimental data for MG onto DWNF. Thermodynamic studies showed that the sorption was a spontaneous and exothermic process. DWNF can be a promising alternative for wastewater treatment.CONCLUSIONS
ACKNOWLEDGMENTS
The authors gratefully acknowledge Fundamental Research Funds for the Central Universities (DL12DB04) and National Innovation experiment program for university students in NEFU (111022536).
REFERENCES CITED
Ahmad, A. A., Hameed, B. H., and Aziz, N. (2007). “Adsorption of direct dye on palm ash: Kinetic and equilibrium modeling,” Journal of Hazardous Materials 141, 70-76.
Ahmad, R., and Kumar, R. (2010). “Adsorption studies of hazardous malachite green onto treated ginger waste,” Journal of Environmental Management 91, 1032-1038.
Altınışık, A., Gür, E., and Seki, Y. (2010). “A natural sorbent, Luffa cylindrica for the removal of a model basic dye,” Journal of Hazardous Materials 179, 658-664.
Baccar, R., Bouzid, J., Feki, M., and Montiel, A. (2009). “Preparation of activated carbon from Tunisian olive-waste cakes and its application for adsorption of heavy metal ions,” Journal of Hazardous Materials 162, 1522-1529.
Baek, M. H., Ijagbemi, C. O., Se-Jin, O, and Kim, D. S. (2010). “Removal of malachite green from aqueous solution using degreased coffee bean,” Journal of Hazardous Materials 176, 820-828.
Batzias, F. A., and Sidiras, D. K. (2007). “Simulation of dye adsorption by beech sawdust as affected by pH,” Journal of Hazardous Materials 141, 668-679.
Bhattacharyya, G. K., and Sharma, A. (2005). “Kinetics and thermodynamics of methylene blue adsorption on neem (Azadirachta indica) leaf powder,” Dyes and Pigments 65, 51-59.
Chakravarty, S., Bhattacharjee, S., Gupta, K. K., Singh, M., Chaturvedi, H. T., Maity, S. (2007). “Adsorption of zinc from aqueous solution using chemically treated newspaper pulp,” Bioresource Technology 98, 3136-3141.
Chakravarty, S., Pimple, S., Chaturvedi, H. T., Singh, S., and Gupta, K. K. (2008). “Removal of copper from aqueous solution using newspaper pulp as an adsorbent,” Journal of Hazardous Materials 159, 396-403.
Chowdhury, S., Mishra, R., Saha, P., and Kushwaha, P. (2011). “Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk,” Desalination 265,159-168.
El Ashtoukhy, E. S. Z. (2009). “Loofa egyptiaca as a novel adsorbent for removal of direct blue dye from aqueous solution,” Journal of Environmental Management 90, 2755-2761.
El-Sayed, G. O. (2011). “Removal of methylene blue and crystal violet from aqueous solution by palm kernel fiber,” Desalination 272, 225-232.
Freundlich, H. M. F. (1906). “Over the adsorption in solution,” Journal of Physical and Chemistry 57, 385-470.
Gupta, N., Kushwaha, A. K., and Chattopadhyaya, M. C. (2011). “Application of potato (Solanum tuberosum) plant wastes for the removal of methylene blue and malachite green dye from aqueous solution,” Arabian Journal of Chemistry, doi:10.1016/j.arabjc.2011.07.021.
Hamdaoui, O. (2006). “Batch study of liquid-phase adsorption of methylene blue using cedar sawdust and crushed brick,” Journal of Hazardous Materials B 135, 264-273.
Hameed, B. H., and El-Khaiary, M. I. (2008). “Kinetics and equilibrium studies of malachite green adsorption on rice straw-derived char,” Journal of Hazardous Materials 153, 701-708.
Ho, Y. S. (2006). “Review of second-order models for adsorption systems,” Journal of Hazardous Materials B136, 681-689.
Huang, X. Y., Bu, H. T., Jiang, G. B., and Zeng, M. H. (2011). “Cross-linked succinyl chitosan as an adsorbent for the removal of methylene blue from aqueous solution,” International Journal of Biological Macromolecules 49, 643-651.
Jain, S., and Jayaram, R. V. (2010). “Removal of basic dyes from aqueous solution by low-cost adsorbent: Wood apple shell (Feronia acidissima),” Desalination 250, 921-927.
Jain, R., and Sikarwar S. (2008). “Removal of hazardous dye congored from waste material,” Journal of Hazardous Materials 152, 942-948.
Khattria, S. D., and Singh, M. K. (2009). “Removal of malachite green from dye wastewater using neem sawdust by adsorption,” Journal of Hazardous Materials 167, 1089-1094.
Kumar, K. V., and Sivanesan S. (2007). “Isotherms for Malachite Green onto rubber wood (Hevea brasiliensis) sawdust: Comparison of linear and non-linear methods,” Dyes and Pigments 72, 124-129.
Kushwaha, A. K., Gupta N., and Chattopadhyaya, M. C. (2011). “Removal of cationic methylene blue and malachite green dyes from aqueous solution by waste materials of Daucus carota,” Journal of Saudi Chemical Society, doi:10.1016/j.jscs.2011.06.011.
Li, Y. J., Gao, B. Y., Wu, T., Wang, B., and Li, X. (2009). “Adsorption properties of aluminum magnesium mixed hydroxide for the model anionic dye reactive Brilliant Red K-2BP,” Journal of Hazardous Materials 164, 1098-1104.
Mahmoodi, N. M., Salehi, R., Arami, M., and Bahrami, H. (2011a). “Dye removal from colored textile wastewater using chitosan in binary systems,” Desalination 267, 64-72.
Moussavi, G., and Khosravi, R. (2011). “The removal of cationic dyes from aqueous solutions by adsorption onto pistachio hull waste,” Chemical Engineering Research and Design89(10), 2182-2189.
Okada, K., Yamamoto, N., Kameshima, Y., and Yasumori, A. (2003). “Adsorption properties of activated carbon from waste newspaper prepared by chemical and physical activation,” Journal of Colloid and Interface Science 262, 194-199.
Safa, Y., and Bhatti, H. N. (2011). “Adsorptive removal of direct textile dyes by low cost agricultural waste: Application of factorial design analysis,” Chemical Engineering Journal 167, 35-41.
Tang, H., Zhou, W. J., and Zhang, L. N. (2012). “Adsorption isotherms and kinetics studies of malachite green on chitin hydrogels,” Journal of Hazardous Materials 209-210, 218-225.
Vadivelan, V., and Kumar, K. V. (2005). “Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk,” Journal of Colloid Interface Science286, 90-100.
Vieira, A. P., Santana, S. A. A., Bezerra, C. W. B., Silva, H. A. S., Chaves, J. A. P., Melo, J. C. P., Silva Filho, E. C., and Airold, C. (2009). “Kinetics and thermodynamics of textile dye adsorption from aqueous solutions using babassu coconut mesocarp,” Journal of Hazardous Materials 166, 1272-1278.
Wang, X., and Li, Z. (2009). “Removal of Cr (VI) from aqueous solution by newspapers,” Desalination 249, 175-181.
Witek-Krowiak, A. (2011). “Analysis of influence of process conditions on kinetics of malachite green biosorption onto beech sawdust,” Chemical Engineering Journal 171, 976-985.
Article submitted: May 14, 2012; Peer review completed: July 3, 2012; Revised version received and accepted: July 22, 2012; Published: July 25, 2012.
