Abstract
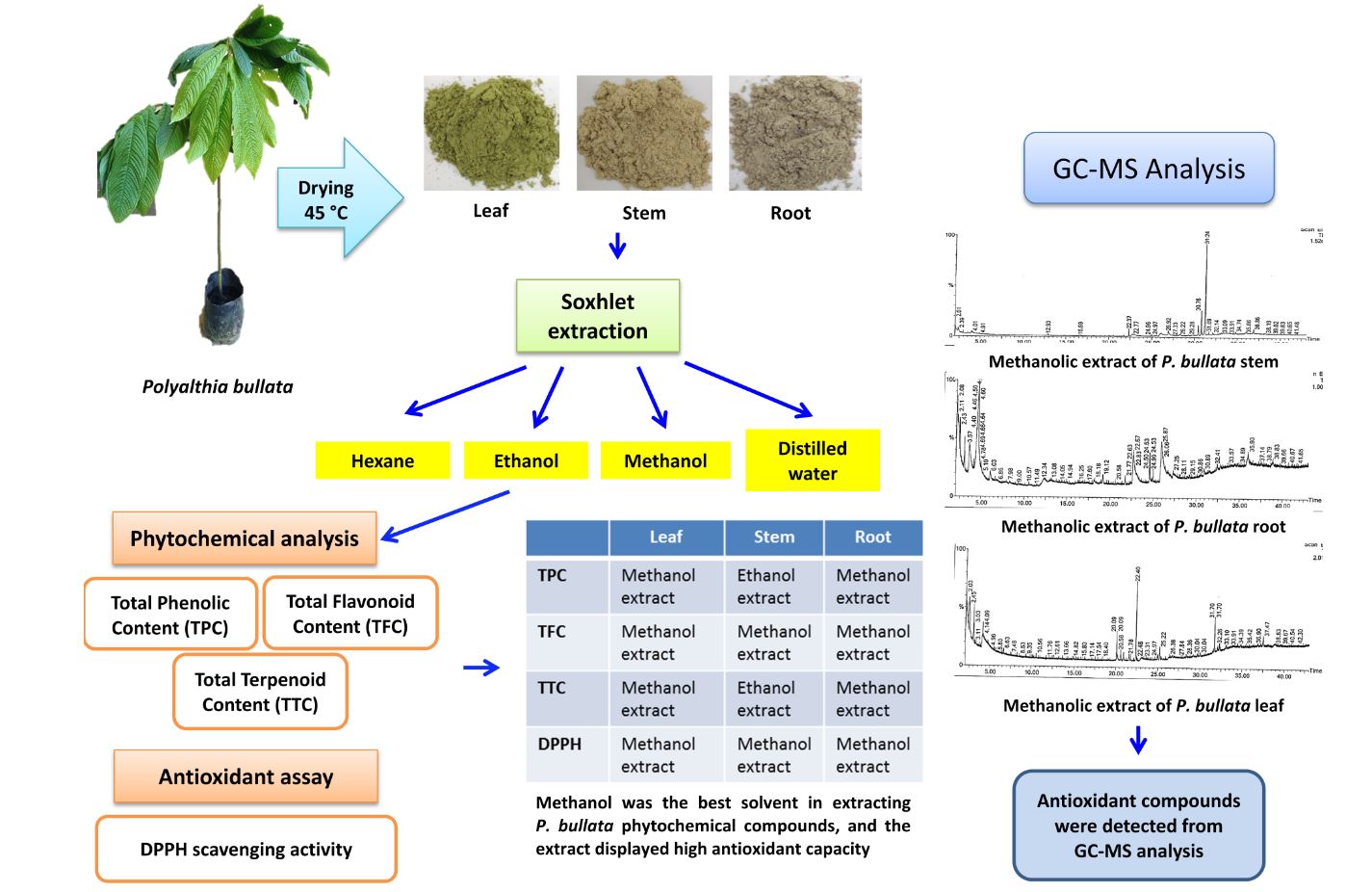
Polyalthia bullata is a woody medicinal plant that contains antioxidant compounds. Finding a suitable solvent is important to obtain a high yield of antioxidants in the phenolic, flavonoid, and terpenoid families. In this study, from different solvent extracts, the leaf methanolic extract exhibited the highest total phenolic content (TPC), total flavonoid content (TFC), total terpenoid content (TTC), and total antioxidant activity. For woody parts of stem and roots, methanol was the best solvent for all phytochemicals except for phenolics, which accumulated in the roots and were extracted more efficiently using ethanol. However, the methanolic extracts from both tissues displayed the best antioxidant capacity. Gas chromatography-mass spectrometry (GC-MS) profiling data showed the presence of antioxidant compounds such as thymol, phytol, and neophytadiene in the leaf; trans-farnesol, n-hexadecanoic acid, and 9-Octadecenamide in the stem; and fatty acid (cis-vaccenic) and its methyl ester (11-Octadecanoic acid, methyl ester and [1,1’-bicyclopropyl]-2-octanoic acid, 2’-hexyl-methyl ester) in the roots. These findings reveal important compounds that are present in different plant parts of P. bullata.
Download PDF
Full Article
Solvent Extraction and Its Effect on Phytochemical Yield and Antioxidant Capacity of Woody Medicinal Plant, Polyalthia bullata
Munirah Adibah Kamarul Zaman,a Azzreena Mohamad Azzeme,a,* Siti Nurhafizah Ramli,a Noor Azmi Shaharuddin,a,c Syahida Ahmad, and Siti Nor Akmar Abdullah b,c
Polyalthia bullata is a woody medicinal plant that contains antioxidant compounds. Finding a suitable solvent is important to obtain a high yield of antioxidants in the phenolic, flavonoid, and terpenoid families. In this study, from different solvent extracts, the leaf methanolic extract exhibited the highest total phenolic content (TPC), total flavonoid content (TFC), total terpenoid content (TTC), and total antioxidant activity. For woody parts of stem and roots, methanol was the best solvent for all phytochemicals except for phenolics, which accumulated in the roots and were extracted more efficiently using ethanol. However, the methanolic extracts from both tissues displayed the best antioxidant capacity. Gas chromatography-mass spectrometry (GC-MS) profiling data showed the presence of antioxidant compounds such as thymol, phytol, and neophytadiene in the leaf; trans-farnesol, n-hexadecanoic acid, and 9-Octadecenamide in the stem; and fatty acid (cis-vaccenic) and its methyl ester (11-Octadecanoic acid, methyl ester and [1,1’-bicyclopropyl]-2-octanoic acid, 2’-hexyl-methyl ester) in the roots. These findings reveal important compounds that are present in different plant parts of P. bullata.
Keywords: Polyalthia bullata; Woody medicinal plant; Extraction solvent polarities; Phytochemical compounds; Antioxidant capacity
Contact information: a: Department of Biochemistry, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor; b: Department of Agriculture Technology, Faculty of Agriculture, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor; c: Institute of Plantation Studies, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor;
* Corresponding author: azzreena@upm.edu.my; azzreena@yahoo.com.my
GRAPHICAL ABSTRACT
INTRODUCTION
Polyalthia is a distinguished genus of the Annonaceae family. In its Greek origin, “poly” means much or many, and althea is derived from the word altheo, meaning “to cure” (Katkar et al. 2010). The genus comprises 120 species of shrubs and trees. Most of them are grown in tropical and subtropical regions in South and South-Eastern Asia, Australia, Africa, and New Zealand (Katkar et al. 2010; Mohamad et al. 2017). The genus Polyalthia contains numerous phytochemicals including alkaloids, flavonoids, triterpenoids, and lipids (Paarakh and Khosa, 2009; Jothy et al. 2013). The presence of clerodane diterpenoids and alkaloids are significantly associated with the medicinal importance of Polyalthia (Katkar et al. 2010). Various parts of the Polyalthia genus are used in traditional medication to treat ailments such as helminthiasis, stomach ache, pharynx neurosis, dysmenorrhoea, diabetes, skin disease, rheumatic fever, hypertension, gastrointestinal ulcer, and generalized body pain (Wu et al. 2016; Yao et al. 2019).
Polyalthia bullata is a woody plant species. The plant grows to two- to three-meters in height, mainly in lowland of primary and secondary forests located in Peninsular Malaysia and Sabah. In Southeast Asia, particularly in Malaysia and Indonesia, the P. bullata flower, root, and leaf are used to treat diabetes, high blood pressure, and liver diseases. Also, the root is well known to have an aphrodisiac property, which can boost male sexual desires (Virmala 2013). Findings by Connolly et al. (1996) and Paarakh and Khosa (2009) revealed three alkaloids in P. bullata stem: urabaine, 7,7′-bisdehydro-O-methylisopiline, and 7-dehydronornuciferine-7′-dehydro-O-methylisopiline. Nantapap et al. (2017) reported three types of flavones extracted from the aerial part of P. bullata: 5-hydroxy-3,7,4’-trimethoxyflavone, 5,3’-dihydroxy-3,7,4’-trimethoxyflavone, and 5,3’,4’-trihydroxy-3,7-dimethoxyflavone, which exhibit anticancer activities.
Some phytochemicals are antioxidants, and some of them are unique to plant species. Humans consume antioxidants for reducing chances of getting oxidative damages due to elevated production of reactive oxygen species (ROS). The imbalance of ROS and antioxidants in cells causes damage to primary biomolecules such as proteins, nucleic acids, and lipids (Srinivasan 2014; Phaniendra et al. 2015; Ramlan et al. 2017). This condition can certainly cause abnormal cell function, tissue damage, and induction of diseases like cancer, diabetes, Alzheimer’s, inflammation, and obesity (Liguori et al. 2018). As prevention, consumers are searching for medicinal plant-based supplements to replenish antioxidant compounds in the body (Saeed et al. 2012). However, the extraction yields are always affected by the solvent used.
Selecting the best solvent for phytochemical extraction is crucial due to the presence of phytochemicals with different chemical structures and polarities, which may influence their solubility in the selected solvent. Choosing the best solvent extraction can maximize the yield of phytochemicals and antioxidants (Fatiha et al. 2012; Pham et al. 2015). Water, methanol, ethanol, acetone, and a mixture of these organic solvents with water are commonly used for phytochemical extraction (Boeing et al. 2014). The increase of solvent polarity from hexane to distilled water (hexane < ethanol < methanol < distilled water) further suggests the influence of solvent polarity towards solubility of phytochemical compounds. In this study, phytochemicals in different P. bullata tissues were quantified by extracting them using different extraction solvent polarities. The extracts were examined their antioxidant capacity, and the best solvent extract was used for phytochemical profiling.
EXPERIMENTAL
Plant Materials and Crude Extract Preparation
The P. bullata plant was obtained from Herbal Nursery located at Pahang, Malaysia. The plants were acclimatized in a greenhouse located at Universiti Putra Malaysia under ambient temperature for a month prior to analysis. The leaf, root, and stem were randomly sampled and oven dried at 45 °C until a constant weight was obtained. The dried leaf, root, and stem were ground using a blender, and 10 g of dried P. bullata powder was weighed and placed in a dark container containing 250 mL of solvents with different polarities (100% v/v methanol, 100% v/v ethanol, and 100% v/v hexane) and distilled water. Each mixture was heated using a Soxhlet apparatus for 8 h to extract phytochemical compounds. The extract was evaporated using a rotary evaporator at 45 °C. The concentrated extract was stored at 4 °C in a dark container.
Determination of Total Phenolics Content (TPC)
The TPC of dried leaf, root, and stem extracts of P. bullata was determined using Folin-Ciocalteu method as described by Dian-Nashiela et al. (2015). The absorbance was read at 765 nm using a UV-Visible spectrophotometer. The TPC was expressed as mg of gallic acid equivalents (GAE) per gram dry weight (mg GAE/g DW).
Determination of Total Flavonoid Content (TFC)
The TFC was determined using aluminum chloride colorimetric assay as described by Kaur and Mondal (2014). The absorbance was read at 510 nm using a UV-Visible spectrophotometer. The TFC was expressed as mg of quercetin equivalents (QE) per gram of dry weight (mg QE/g DW).
Determination of Total Terpenoid Content (TTC)
The TTC was determined using a sulfuric acid calorimetric assay (Geetha et al. 2015). The absorbance was read at 538 nm using UV-Visible spectrophotometer. The total terpenoid content was expressed as linalool equivalent per gram (mg LE/g DW) of dry weight.
Antioxidant Assay
The antioxidant activity was determined using a 2,2-diphenyl-1-picryl-hydrazyl (DPPH) free radical scavenging activity assay adapted from Sumazian et al. (2010). The initial absorbance of DPPH solution was measured without sample at 517 nm. Approximately 0.2 mL of each sample extract was mixed with 3 mL of 0.1 mM DPPH solution. The mixture was incubated at room temperature in the dark for 30 min. The change in absorbance was measured after 30 minutes of incubation at 517 nm using a UV-Visible spectrophotometer. The results obtained were calculated and expressed in percent of DPPH free radical scavenging activity using the following formula,
(1)
where Acontrol is the absorbance of DPPH solution without sample, and Asample is the absorbance of sample with DPPH solution.
Phytochemical Profiling of Dried Leaf, Stem, and Root of P. Bullata Using Gas Chromatography-Mass Spectrometry (GC-MS)
The phytochemical constituents in concentrated methanolic extract of dried leaf, stem, and root of P. bullata was analyzed using GC-MS (Geetha et al. 2013). The relative percentage of the extract constituents was expressed as percentage with peak area normalization. The identity of the components in the extract was assigned by the comparison of their retention indices and mass spectra fragmentation pattern with those data stored on the computer library and also with published literatures.
Statistical Analysis
The data of different parameters were subjected to one-way analysis of variance (ANOVA), while the significance of difference between means was determined by Tukey’s multiple range tests using SAS 9.4 software (Cary, NC, USA). Values expressed were means of triplicate determination standard error (SE). Different letters indicate the values of significant difference at p ≤ 0.05.
RESULTS AND DISCUSSION
Plants produce diverse phytochemicals at different concentrations in leaf, stem, flowers, fruits and roots (Altemimi et al. 2017; Almeida et al. 2019). The yield of extracted phytochemicals also depends on solubility of the compounds in extraction solvents. In this study, different amounts of phenolics, flavonoids, and terpenoids from different plant parts and extraction solvents were observed (Tables 1, 2, and 3). The results suggest the presence of different compound polarities in each of extraction solvent. Among the solvents, methanol displayed the highest capacity in extracting phenolics from P. bullata leaf and stem, while ethanol was the best solvent in extracting phenolics from roots. A similar trend was observed for flavonoids in P. bullata leaf and stem except for the root; the flavonoids content was highest in the ethanolic extract. These findings suggest a high amount of polar phenolics and flavonoids in P. bullata, which might be due to the hydrogen bond formation between hydroxyl groups with electronegative oxygen of methanol and ethanol. The formation of hydrogen bond might also form between hydroxyl group of the methanol and ethanol with oxygen atom located at phenolic and flavonoid structures (Galanakis et al. 2013; Thavamoney et al. 2018).
Table 1. Total Phenolic Content of P. bullata Leaf, Stem, and Root in Different Extraction Solvents
Note: Different letters indicate significant difference at P ≤ 0.05 Tukey’s range test
Table 2. Total Flavonoid Content of P. bullata Leaf, Stem, and Root in Different Extraction Solvents
Note: Different letters indicate significant difference at P ≤ 0.05 Tukey’s range test
Table 3. Total Terpenoid Content of P. bullata Leaf, Stem, and Root in Different Extraction Solvents
Note: Different letters indicate significant difference at P ≤ 0.05 Tukey’s range test
The methanol and ethanol were the best in extracting phenolics including flavonoids from other plants. The highest amount of phenolic was observed in leaf methanolic extracts of Zaravschanica membranacea (268.12 ± 1.04 mg GAE/g DW) and Ferulago angulata (72.33 ± 1.14 mg GAE/g DW), followed by leaf ethanolic extracts of Z. membranacea (243.38 ± 1.01 mg GAE/g DW) and F. angulate (63.72 ± 2.03 mg DAE/g DW) (Rezaei and Ghasemi Pirbalouti (2019). However, the phenolics contents in these plants are lower than in P. bullata. In Mentha spicata, the flavonoids content was detected higher than P. bullata at the amount of 267.33 ± 3.12 mg/g DW in methanolic extract (Bimakr et al. 2011) and 218 ± 4.24 mg/g DW in ethanolic extract. The high amount of flavonoids was also observed in methanolic and ethanolic extracts of Pluchea indica leaves at concentration of 911.9 ± 65.4 mg CE/g DW and 93.1 ± 2.1 mg CE/g DW, respectively (Widyawati et al. 2014). Hence, the present findings display the high amount of total phenolics compared with that of total flavonoids in P. bullata, and low amount of flavonoids in the plant.
The solubility of phenolics and flavonoids in extraction solvents depends on functional groups attached to the main structure of these phytochemicals, the molecular size, and the length of hydrocarbon (Iloki-Assanga et al. 2015; Enneb et al. 2020). For example, the ideal solubility of kaempferol, hesperidin, ferreirin, and 4-hydroxycoumarin extracted from M. spicata differed in different extraction solvents, which therefore contributes to the different yields of phenolics and flavonoids in the solvents used (Li and Tian 2018). Moreover, the solvation capacity influences the solubility of phytochemicals. For instance, methanol has better solvation of phenolics and flavonoids than ethanol due to the presence of shorter methyl radical in methanol compared to long ethyl radical in ethanol (Boeing et al. 2014).
For terpenoid, even though terpenes usually exhibit high solubility in nonpolar solvents, the presence of the hydroxyl group in terpenes structure does influence the polarity of the compounds, and make terpenes solubilize in polar solvents (Jiang et al. 2016). In P. bullata, terpenes were quantified in methanolic, ethanolic, hexanic, and distilled water extracts from all plant parts. The presence of terpenes in methanolic extract was also observed in S. buxifolia branches (1.25% w/w), followed by ethanolic extract (0.97% w/w) and distilled water (0.43% w/w) (Truong et al. 2019).
Phenolics, flavonoids, and terpenes possess antioxidant properties (Takaidza et al. 2018). The presence of these compounds in P. bullata makes the plant a natural source of antioxidant. Different antioxidant capacity was observed from different solvent extracts, as presented in Table 4. This might be due to the presence of different polar and nonpolar phenolics, flavonoids, and terpenoids, and they might exist at different amounts in different plant parts.
Table 4. DPPH Scavenging Activity of P. bullata Leaf, Stem, and Root in Different Extraction Solvents
Note: Different letters indicate significant difference at P ≤ 0.05 Tukey’s range test
The existence of different side chains and substituents in the phytochemical structure may lead to the different hydrogen-donating capacity. In S. buxifolia, the presence of phenolics, flavonoids, and terpenes was responsible for high antioxidant activity (Truong et al. 2019), and a study conducted by Mamphiswana et al. (2010) showed the relationship between phenolic content and antioxidant activity in M. burkeana.
The highest antioxidant activity in methanolic extract of P. bullata could be attributed to the presence of polar antioxidant compounds. A GC-MS analysis was used to identify the responsible phytochemicals, and the results showed the phenolic terpenes such as thymol and other terpenoids (phytol and neophytadiene) were present in the leaf extract (Table 5). In stem, trans-farnesol terpenoid and fatty acids (n-hexadecanoic acid and 9-octadecenamide) were detected in the extract. While in root, fatty acid (cis-vaccenic) and its methyl ester (11-octadecanoic acid, methyl ester and [1,1’-bicyclopropyl]-2-octanoic acid, 2’-hexyl-methyl ester) were profiled from the extract. All of these compunds has been demonstrated to have antioxidant activity (Anyasor et al. 2014; de Moraes et al. 2014; Rahman et al. 2014; Vinholes et al. 2014; Memar et al. 2017; Tyagi and Agarwal 2017; Singh and Chaturvedi 2019; Kim et al. 2020).
Based on the literature, other compounds profiled from GC-MS analysis might show their association with other biological activities. Phthalic acid, di(2-propylpentyl) ester (Khatiwora et al. 2012; Ahsan et al. 2017; Shobi and Viswanathan 2018), which was detected the highest in the stem, is reported to have antimicrobial properties. Other antimicrobial compounds detected in P. bullata were thymol (Swamy et al. 2017), 9-octadecenamide, (Z)- (Meenakshi et al. 2012), 4H-pyran-4-one, 2,3-dihydro-6-methyl (Mujeeb et al. 2014), 5H-indeno[1,2-b]pyridin-5one, 3-methyl (Hussain et al. 2014), and 9(10H)-acridone, 4-methoxy- (Gensicka-Kowalewska et al. 2017). Two alkaloid compounds, 5H-indeno[1,2-b]pyridin-5one,3-methyl and 9(10H)-acridone,4-methoxy-, were detected at a lower percentage (<1%) in the P. bullata root. These compounds are reported to have antimicrobial and antimalarial activities (Addla et al. 2012; Anand et al. 2017). The 9(10H)-acridone derivatives possess anti-inflammatory, anticancer, antimicrobial, antiparasitic, antimalarial, antiviral, and fungicidal activities (Gensicka-Kowalewska et al. 2017; Kukowska 2017). The findings show the neutraceutical potential of P. bullata that can benefit human health.
Table 5. List of Bioactive Compounds Identified in Methanolic Extract of Leaf, Stem, and Root of P. bullata
From this study it is clear that methanol was efficient in extracting phytochemical compounds of P. bullata. However, one concern about the finding is the toxicity of methanol. Methanol is reported to be able to deteriorate human health if the ingestion exceeds 30 mg/day (Food and Drug Administration 2018). But many studies have demonstrated that there is no sign of toxicity in animal models after feeding the animals with plant methanolic extracts (Viswanad et al. 2012; Abbas et al. 2018; Ebohon et al. 2020; Nguenang et al. 2020). The methanol traces can also be eliminated by heating the extracts using a rotary evaporator or nitrogen stream.
CONCLUSIONS
- Methanol showed the highest capacity to extract phytochemicals from P. bullata leaf, stem, and root, and each of the methanolic extracts displayed the highest antioxidant activity.
- The GC-MS analysis profiled some antioxidant compounds in methanolic extracts, and others were potentially associated with other biological activities as described in previous reports.
ACKNOWLEDGMENTS
This study was supported by the Putra Graduate Initiative (IPS) grant (GP-IPS/2018/9630400) and Putra Young Initiative (IPM) grant (GP-IPM/2017/9533800). Also, the authors wish to thank Universiti Putra Malaysia for funding the Master of Science Degree of Munirah Adibah Kamarul Zaman under Graduate Research Fellowship (GRF) scheme.
REFERENCES CITED
Abbas, M. Y., Ejiofor, J. I., and Yakubu, M. I. (2018). “Acute and chronic toxicity profiles of the methanol leaf extract of Acacia ataxacantha DC (Leguminosae) in Wistar rats,” Bulletin of Faculty of Pharmacy, Cairo University 56(2), 185-189. DOI: 10.1016/j.bfopcu.2018.09.001
Addla, D., Sridhar, B., Devi, A., and Kantevari, S. (2012). “Design, synthesis and antimicrobial evaluation of novel 1-benzyl 2-butyl-4-chloroimidazole embodied 4-azafluorenones via molecular hybridization approach,” Bioorg. Med. Chem. Lett. 22(24), 7475-7480. DOI: 10.1016/j.bmcl.2012.10.042
Ahsan, T., Chen, J., Zhao, X., Irfan, M., and Wu, Y. (2017). “Extraction and identification of bioactive compounds (eicosane and dibutyl phthalate) produced by Streptomyces strain KX852460 for the biological control of Rhizoctonia solani AG-3 strain KX852461 to control target spot disease in tobacco leaf,” AMB Express. 7(1), 54-62. DOI: 10.1186/s13568-017-0351-z
Almeida, É. S., de Oliveira, D., and Hotza, D. (2019). “Properties and applications of Morinda citrifolia (Noni): A review,” Compr. Rev. Food Sci. Food Saf. 18(1), 883-909. DOI: 10.1111/1541-4337.12456
Altemimi, A., Lakhssassi, N., Baharlouei, A., Watson, D. and Lightfoot, D. (2017). “Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts,” Plants 6(4), 42-65. DOI: 10.3390/plants6040042
Anand, D. Yadav, P. K., Patel, O. P., Parmar, N., Maurya, R. K., Vishwakarma, P., Raju, K. S., Taneja, I., Wahajuddin, M., Kar, S. and Yadav, P. P. (2017). “Antileishmanial activity of pyrazolopyridine derivatives and their potential as an adjunct therapy with miltefosine,” J. Med. Chem. 60(3), 1041-1059. DOI: 10.1021/acs.jmedchem.6b01447
Anyasor, G. N., Funmilayo, O., Odutola, O., Olugbenga, A. and Oboutor, E. M. (2014). “Chemical constituents in n-butanol fractions of Castus afer ker Gawl leaf and stem,” J. Intercult. Ethnopharmacol. 3(2), 78-84. DOI: 10.5455/jice.20140112010648
Bimakr, M., Rahman, R. A., Taip, F. S., Ganjloo, A., Salleh, L. M., Selamat, J., Hamid, A., and Zaidul, I. S. M. (2011). “Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves,” Food Bioprod. Process. 89(1), 67-72. DOI: 10.1016/j.fbp.2010.03.002
Boeing, J. S., Barizao, E. O., e Silva, B. C., Montanher, P. F., de Cinque Almeida, V., and Visentainer, J. V. (2014). “Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: Application of principal component analysis,” Chem. Cent. J. 8(1), 48-56. DOI: 10.1186/s13065-014-0048-1
Connolly, J. D., Haque, M. E., and Kadir, A. A. (1996). “Two 7,7′-bisdehydroaporphine alkaloids from Polyalthia bullata,” Phytochemistry 43(1), 295-297. DOI: 10.1016/0031-9422(96)00219-1
de Moraes, J. de Oliveira, R. N., Costa, J. P., Junior, A. L., de Sousa, D. P., Freitas, R. M., Allegretti, S. M., and Pinto, P. L. (2014). “Phytol, a diterpene alcohol from chlorophyll, as a drug against neglected tropical disease Schistosomiasis mansoni,” PLoS Neglect. Trop. D. 8(1), e2617-e2628. DOI: 10.1371/journal.pntd.0002617
Dian-Nashiela, F., Abdullah, N., Hashim, N., and Abdul Hamid, A. (2015). “Antioxidant activity of herbal tea prepared from Cosmos caudatus leaves at different maturity stages,” Int. Food Res. J. 22(3), 1189-1194.
Ebohon, O., Irabor, F., and Omoregie, E. S. (2020). “Sub-acute toxicity study of methanol extract of Tetrorchidium didymostemon leaves using biochemical analyses and gene expression in Wistar rats,” Heliyon 6(6), e04313. DOI: 10.1016/j.heliyon.2020.e04313
Enneb, S. Drine, S., Bagues, M., Triki, T., Boussora, F., Guasmi, F., Nagaz, K., and Ferchichi, A. (2020). “Phytochemical profiles and nutritional composition of squash (Cucurbita moschata D.) from Tunisia,” S. Afr. J. Bot. 130, 165-171. DOI: 10.1016/j.sajb.2019.12.011
Fatiha, B., Khodir, M., Farid, D., Tiziri, R., Karima, B., Sonia, O., and Mohamed, C. (2012). “Optimisation of solvent extraction of antioxidants (phenolic compounds) from Algerian mint (Mentha spicata L.),” Phcog. Commn. 2(4), 72-86. DOI: 10.5530/pc.2012.4.10
Food and Drug Administration (2018). Q3C-Tables and List Guidance for Industry (Revision 4).
Galanakis, C. M., Goulas, V., Tsakona, S., Manganaris, G. A., and Gekas, V., (2013). “A knowledge base for the recovery of natural phenols with different solvents,” Int. J. Food Prop. 16(2), 382-396. DOI: 10.1080/10942912.2010.522750
Geetha, D. H., Rajeswari, M., and Jayashree, I. (2013). “Chemical profiling of Elaeocarpus serratus L. by GC-MS,” Asian Pac. J. Trop. Biomed. 3(12), 985-987. DOI: 10.1016/S2221-1691(13)60190-2
Geetha, N., Subha, D., and Chandralega, N. (2015). “Phytochemical screening of Tanacetum Parthenium L. (Feverfew) leaves: an important medicinal plant,” Int. J. Pharm. Pharm. Sci. 2(2), 98-126.
Gensicka-Kowalewska, M., Cholewiński, G., and Dzierzbicka, K., (2017). “Recent developments in the synthesis and biological activity of acridine/acridone analogues,” RSC Advances 7(26), 15776-15804. DOI: 10.1039/c7ra01026e
Hussain, A. M., Mansoor, S. S., Aswin, K., and Sudhan, S. P. N. (2014). “Pentafluorophenylammonium triflate: An effective and reusable organocatalyst for the one-pot preparation of 2,4-diaryl-5H-indeno [1,2-b] pyridin-5-one derivatives,” J. King Saud Univ. Sci. 26(3), 213-221. DOI: 10.1016/j.jksus.2013.08.007
Iloki-Assanga, S. B., Lewis-Luján, L. M., Lara-Espinoza, C. L., Gil-Salido, A. A., Fernandez-Angulo, D., Rubio-Pino, J. L., and Haines, D. D. (2015). “Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum,” BMC Res. Notes 8(1), 396-410. DOI: 10.1186/s13104-015-1388-1
Jiang, Z., Kempinski, C., and Chappell, J. (2016). “Extraction and analysis of terpenes/terpenoids,” Curr. Protoc. Plant Biol. 1(2), 345-358. DOI: 10.1002/cppb.20024
Jothy, S. L., Yeng, C., and Sasidharan, S. (2013). “Chromatographic and spectral fingerprinting of Polyalthia lobgifolia, a source of phytochemicals,” BioResources 8(4), 5102-5119. DOI: 10.15376/biores.8.4.5102-5119
Katkar, K. V., Suthar, A. C., and Chauhan, V. S. (2010). “The chemistry, pharmacologic, and therapeutic applications of Polyalthia longifolia,” Pharmacogn. Rev. 4(7), 62-68. DOI: 10.4103/0973-7847.65329
Kaur, S., and Mondal, P. (2014). “Study of total phenolic and flavonoid content, antioxidant activity and antimicrobial properties of medicinal plants,” J. Microbiol. Exp. 1(1), 23-28. DOI: 10.15406/jmen.2014.01.00005
Khatiwora, E., Adsul, V. B., Kulkarni, M., Deshpande, N. R., and Kashalkar, R. V. (2012). “Antibacterial activity of dibutyl phthalate: a secondary metabolite isolated from Ipomoea carnea stem,” J. Pharm. Res. 5(1), 150-152.
Kim, B. R., Kim, H. M., Jin, C. H., Kang, S. Y., Kim, J. B., Jeon, Y. G., Park, K. Y., Lee, I. S., and Han, A. R. (2020). “Composition and antioxidant activities of volatile organic compounds in radiation-bred Coreopsis cultivars,” Plants 9(6), 717-725. DOI: 10.3390/plants9060717
Kukowska, M. (2017). “Amino acid or peptide conjugates of acridine/acridone and quinoline/quinolone-containing drugs. A critical examination of their clinical effectiveness within a twenty-year timeframe in antitumor chemotherapy and treatment of infectious diseases,” Eur. J. Pharm. Sci. 109, 587-615. DOI: 10.1016/j.ejps.2017.08.027
Li, X., and Tian, T. (2018). “Phytochemical characterization of Mentha spicata L. under differential dried-conditions and associated nephrotoxicity screening of main compound with organ-on-a-chip,” Front. Pharmacol. 9, 1067-1076. DOI: 10.3389/fphar.2018.01067
Liguori, I., Russo, G., Curcio, F., Bulli, G., Aran, L., Della-Morte, D., Gargiulo, G., Testa, G., Cacciatore, F., Bonaduce, D., and Abete, P. (2018). “Oxidative stress, aging, and diseases,” Clin. Interv. Aging. 13, 757-772. DOI: 10.2147/CIA.S158513
Mamphiswana, N. D., Mashela, P. W., and Mdee, L. K. (2010). “Distribution of total phenolics and antioxidant activity in fruit, leaf, stem and root of Monsonia burkeana,” Afr. J. Agr. Res. 5(18), 2570-2575. DOI: 10.5897/AJAR.9000177
Memar, M. Y., Raei, P., Alizadeh, N., Aghdam, M. A., and Kafil, H. S. (2017). “Carvacrol and thymol: Strong antimicrobial agents against resistant isolates,” Rev. Med. Microbiol. 28(2), 63-68. DOI: 10.1097/MRM.0000000000000100
Mohamad, M., Mohsin, H. F., and Singh, G. K. S. (2017). “A study on the effect of Eurycoma longifolia and Polyalthia bullata on reproductive organs and androgens of male sprague-dawley rats,” J. Eng. Appl. Sci. 12(5), 6994-6999. DOI: 10.36478/jeasci.2017.6994.6999
Mujeeb, F., Bajpai, P., and Pathak, N. (2014). “Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos,” BioMed Res. Int. 2014, 1-11. DOI: 10.1155/2014/497606
Nantapap, S. S., Punyanitya, N., Nuntasaen, W., Pompimon, W., and Meepowpan, P. (2017). “Flavones from aerial parts of Polyalthia bullata and cytotoxicity against cancer cell lines,” Chem. Nat. Compd. 53(4), 762-763. DOI: 10.1007/s10600-017-2114-0
Nguenang, G. S., Ntyam, A. S., and Kuete, V. (2020). “Acute and subacute toxicity profiles of the methanol extract of Lycopersicon esculentum L. Leaves (Tomato), a botanical with promising in vitro anticancer potential,” Evid. Based Complement. Altern. Med.2020, 1-10. DOI: 10.1155/2020/8935897
Paarakh, P. M., and Khosa, R. L. (2009). “Phytoconstituents from the genus Polyalthia – A review,” J. Pharm. Res. 2(4), 594-605.
Pham, H. N. T., Nguyen, V. T., Vuong, Q. V., Bowyer, M. C., and Scarlett, C. J. (2015). “Effect of extraction solvents and drying methods on the physicochemical and antioxidant properties of Helicteres hirsuta Lour. leaves,” Technologies 3(4), 285-301. DOI: 10.3390/technologies3040285
Phaniendra, A., Jestadi, D. B., and Periyasamy, L. (2015). “Free radicals: Properties, sources, targets, and their implication in various diseases,” Ind. J. Clin. Biochem. 30(1), 11-26. DOI: 10.1007/s12291-014-0446-0
Rahman, M. M., Ahmad, S. H., Mohamed, M. T. M., and Ab Rahman, M. Z. (2014). “Antimicrobial compounds from leaf extracts of Jatropha curcas, Psidium guajava, and Andrographis paniculata,” The Scientific World Journal 2014, 1-8. DOI: 10.1155/2014/635240
Ramlan, N. N., Azzeme, A. M., Padzil, K. N. M., and Mahmood, M. (2017). “Influence of different extraction solvents on phytochemical content and antioxidant capacity extracted from pulp and flower of dessert and cooking bananas,” Malaysian J. Biochem. Mol. Biol. 20(2&3), 10-16.
Rezaei, M., and Ghasemi Pirbalouti, A., (2019). “Phytochemical, antioxidant and antibacterial properties of extracts from two spice herbs under different extraction solvents,” J. Food Meas. Charact. 13(3), 2470-2480. DOI: 10.1007/s11694-019-00167-8
Saeed, N., Khan, M. R., and Shabbir, M. (2012). “Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L,” BMC Complem. Altern. Med. 12(1), 221-233. DOI: 10.1186/1472-6882-12-221
Shobi, T. M., and Viswanathan, M. B. G. (2018). “Antibacterial activity of di-butyl phthalate isolated from Begonia malabarica,” J. Appl. Biotechnol. Bioeng. 5(2), 101-104. DOI: 10.15406/jabb.2018.05.00123
Singh, R., and Chaturvedi, P. (2019). “Phytochemical characterization of rhizome, fruit, leaf and callus of Rheum emodi Wall. using GC-MS,” Pharmacogn. J. 11(3), 617-623. DOI: 10.5530/pj.2019.11.99
Srinivasan, K. (2014). “Antioxidant potential of spices and their active constituents,” Crit. Rev. Food Sci. 54(3), 352-372. DOI: 10.1080/10408398.2011.585525
Sumazian, Y., Syahida, A., Hakiman, M., and Maziah, M. (2010). “Antioxidant activities, flavonoids, ascorbic acid and phenolic contents of Malaysian vegetables,” J. Med. Plants Res. 4(10), 881-890. DOI: 10.5897/JMPR10.011
Swamy, M. K., Arumugam, G., Kaur, R., Ghasemzadeh, A., Yusoff, M. M., and Sinniah, U. R. (2017). “GC-MS based metabolite profiling, antioxidant and antimicrobial properties of different solvent extracts of Malaysian Plectranthus amboinicus leaves,” Evid.-Based Complement. Altern. Med. 2017, 1-10. DOI: 10.1155/2017/1517683
Takaidza, S., Mtunzi, F., and Pillay, M. (2018). “Analysis of the phytochemical contents and antioxidant activities of crude extracts from Tulbaghia species,” J. Tradit. Chin. Med. 38(2), 272-279. DOI: 10.1016/j.jtcm.2018.04.005
Thavamoney, N., Sivanadian, L., Tee, L. H., Khoo, H. E., Prasad, K. N., and Kong, K. W. (2018). “Extraction and recovery of phytochemical components and antioxidative properties in fruit parts of Dacryodes rostrata influenced by different solvents,” J. Food Sci. Tech. 55(7), 2523-2532. DOI: 10.1007/s13197-018-3170-6
Truong, D. H., Nguyen, D. H., Ta, N. T. A., Bui, A. V., Do, T. H., and Nguyen, H. C. (2019). “Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia,” J. Food Quality 2019, 1-9. DOI: 10.1155/2019/8178294
Tyagi, T., and Agarwal, M. (2017). “Phytochemical screening and GC-MS analysis of bioactive constituents in the ethanolic extract of Pistia stratiotes L. and Eichhornia crassipes (Mart.) solms,” J. Pharmacogn. Phytochem. 6(1), 195-206.
Vinholes, J., Gonçalves, P., Martel, F., Coimbra, M. A., and Rocha, S. M. (2014). “Assessment of the antioxidant and antiprolifferative effects of sesquiterpenic compounds in in vitro Caco-2 cell models,” Food Chem. 156, 204-211. DOI: 10.1016/j.foodchem.2014.01.106
Virmala, S. (2013). “Malaysian herbal heritage,” Forest Research Institute Malaysia (FRIM), Kuala Lumpur, pp. 60-61.
Viswanad, V., Aleykutty, N. A., Jayakar, B., Zacharia, S. M., and Thomas, L. (2012). “Development and evaluation of antimicrobial herbal formulations containing the methanolic extract of Samadera indica for skin diseases,” J. Adv. Pharm. Tech. Res. 3(2), 106-111. DOI: 10.4103/2231-4040.97285
Widyawati, P. S., Budianta, T. D. W., Kusuma, F. A., and Wijaya, E. L. (2014). “Difference of solvent polarity to phytochemical content and antioxidant activity of Pluchea indicia less leaves extracts,” Int. J. Pharmacogn. Phytochem. Res. 6(4), 850-855.
Wu, L. J., Zheng, C. J., Wang, L. K., Han, C. R., Song, X. P., Chen, G. Y., Zhou, X. M., Wu, S. Y., Li, X. B., Bai, M., and Liu, C. X. (2016). “One new berberine from the branches and leaves of Polyalthia obliqua Hook. f. & Thomson,” Nat. Prod. Res. 30(20), 2285-2290. DOI: 10.1080/14786419.2016.1164699
Yao, L. J., Jalil, J., Attiq, A., Hui, C. C., and Zakaria, N. A. (2019). “The medicinal uses, toxicities and anti-inflammatory activity of Polyalthia species (Annonaceae),” J. Ethnopharmacol. 229, 303-325. DOI: 10.1016/j.jep.2018.10.001
Article submitted: July 21, 2020; Peer review completed: October 10, 2020; Revised version received and accepted: October 24, 2020; Published: October 29, 2020.
DOI: 10.15376/biores.15.4.9555-9568
