Abstract
The purpose of this study was to use biomass silicon resources in rice straw to synthesize high value-added organosilicon products to solve the problem of low utilization of rice straw and high pollution and energy consumption in the silicon chemical industry. In this work, spirocyclic alkoxysilane was successfully synthesized by an environmentally friendly method from rice straw ash for the first time. The spirocyclic alkoxysilane yield per gram of rice straw ash can reach up to 1.9 g. Spirocyclic alkoxysilane was used to modify silicone resin coating. This coating can withstand 150 applications of friction and still maintain good hydrophobic effect. The maximum water contact angle after friction can reach 104°. This study broadens the application of rice straw. Furthermore, this research lays the foundation for the improvement of energy-saving and emission reduction process of silicon chemical industry.
Download PDF
Full Article
Spirocyclic Alkoxysilane Synthesized from Rice Straw Ash for Preparation of Eco-friendly Hydrophobic Silicone Coatings
Wenlong Liu,a,b Xingwen Zhang,b,* Hongyu Ren,c,* Xingcheng Hu,b Jie Li,b and Hui Liu a
The purpose of this study was to use biomass silicon resources in rice straw to synthesize high value-added organosilicon products to solve the problem of low utilization of rice straw and high pollution and energy consumption in the silicon chemical industry. In this work, spirocyclic alkoxysilane was successfully synthesized by an environmentally friendly method from rice straw ash for the first time. The spirocyclic alkoxysilane yield per gram of rice straw ash can reach up to 1.9 g. Spirocyclic alkoxysilane was used to modify silicone resin coating. This coating can withstand 150 applications of friction and still maintain good hydrophobic effect. The maximum water contact angle after friction can reach 104°. This study broadens the application of rice straw. Furthermore, this research lays the foundation for the improvement of energy-saving and emission reduction process of silicon chemical industry.
DOI: 10.15376/biores.17.3.5065-5078
Keywords: Plant biomass; Rice straw ash; Hydrophobic Silicone Coating; Biosilica
Contact information: a: School of Energy Science and Engineering, Harbin Institute of Technology, No. 92, West Dazhi Street, Harbin 150001, China; b: School of Chemistry and Chemical Engineering, Harbin Institute of Technology, No. 92, West Dazhi Street, Harbin 150001, China; c: College of Resources and Environment, Northeast Agriculture University, No. 600, Changjiang Street, Harbin 150001, China;
* Corresponding authors: zhangxingwen@hit.edu.cn; renhongyu@163.com
GRAPHICAL ABSTRACT

INTRODUCTION
Rice is one of the major crops in the world. The cultivating area of rice is 163 million hectares, and the yield is 752 million tons per year (Mirmohamadsadeghi and Karimi 2020). Rice straw is the main by-product of rice production (Liu et al. 2021). The annual yield of rice straw is about 740 million to 1.11 billion tons (Baramee et al. 2020). However, due to the lack of high value-added utilization routes, the most common way to dispose of rice straw resources is to burn them in open fields (Mirmohamadsadeghi and Karimi 2020). This disposal method cannot utilize the heat energy in the combustion process, which causes huge energy waste. Moreover, there is no method to deal with the open burning waste gas properly, which leads to serious environmental pollution problems (Satlewal et al. 2018) and presents a great threat to human health and sustainable development. It is therefore essential to find a way to utilize rice straw with high added value and promote the recycling of rice straw.
At the same time, silicon-based materials play an important role in the field of high technology. The silicon chemical industry is a high consumer of energy (Putro et al. 2020) and high generator of pollution (Furgal and Lenora 2019). Thus, it is important to find green and environmentally friendly routes for the synthesis of silicon-based materials. Laine et al. (2016) found that silica with high specific surface area has high reactivity, which could solve this problem. The main component of rice straw ash is silica with high specific surface area. However, there are relatively few studies on the synthesis of organosilicon from rice straw ash with green and low energy consumption method.
In this study, an environmentally friendly method was used to synthesize spirocyclic alkoxysilane (SP) from rice straw ash. The influence factors of the synthesis condition and the optimization of yield were investigated. The novelty of the present work is mainly in the use of rice straw ash, which is cheap and has an autocatalytic effect, and the procedures and findings closely follow known cited work (Laine et al. 2016). The spirocyclic alkoxysilane was then used to modify the silicone coating, and the hydrophobic performance and wear resistance of the coatings were investigated.
This research realized the low cost and green preparation of spirocyclic alkoxysilane from rice straw ash for the first time. Furthermore, the spirocyclic alkoxysilane modified silicone coatings were found to have good wear resistance and hydrophobic performance. This study laid the foundation for the exploration of high value-added utilization routes of rice straw. We can envision that the incineration will take place at a village or regional facility that will product electrical energy (or maybe steam too) in addition to producing the silica-rich ash.
EXPERIMENTAL
Materials
Triethoxyphenylsilane, lithium hydroxide, methanol, tetrahydrofuran, sodium hydroxide, triethoxymethylsilane, 2-methyl-2,4-pentanediol and tetrabutylammonium fluoride solution were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd (Shanghai, China). Rice straw ash was received from the Xing Fu Farm (Qiqihaer, China). Glass substrates (76.2 mm * 25.4 mm * 1.2 mm) were purchased from Yancheng Fei Zhou Glass & Plastic Co., Ltd.
Methods
Synthesis of spirocyclic alkoxysilane
Rice straw ash (500 g) was placed in a 2 L three-neck flask with a mechanical stirrer under N2. Then, 1.2 L of 2-methyl, 2,4-pentanediol was added into the three-neck flask. Sodium hydroxide (2.66 g) was dissolved in 20 mL of methanol. The sodium hydroxide methanol solution was added into the three-neck flask, and then the solution was stirred for 0.5 h to promote dissolution. After 0.5 h, the solution was heated to 180 °C for 48 h. The spirocyclic alkoxysilane was distilled out and collected by the Laine method (Laine et al. 2016). The rice straw ash (RS) was pyrolyzed in a muffle furnace which was preheated to a predetermined temperature (400, 500, 600, or 700 °C) for a certain duration (1, 3, 5, or 7 h). The resulting samples were referred to as RS-T-d (T: pyrolysis temperature (400, 500, 600, or 700 °C); d: duration of pyrolysis (1, 3, 5, or 7 h)), respectively.
Preparation procedure for the water-washed rise straw ash.
Rice straw ash was rinsed with deionized water three times. It was named as water-washed rice straw ash (WRS).
Preparation procedure for coatings
The silicone coatings were synthesized by fluoride rearrangement method (Krug and Laine 2017). The mass ratio of phenyltriethoxysilane and methyltriethoxysilane was 1:1. Tetrahydrofuran was used as the solvent. TBAF was used as the catalyst. The coating concentration is 15 wt%. The reaction was carried out under sealed and magnetically stirred conditions at room temperature for 7 days. The mass percentages of spirocyclic alkoxysilane are, respectively, 5%, 10%, 15%, 20%, and 25% in the coatings. And spin coating was carried out by using a KW-4A spin coater. The glass substrates were ultrasonically washed with deionized water for 10 min, ethanol for 10 min, and acetone for 10 min before spin coating. Then, all the glass substrates were dried in an oven and prepared for use. The speed of the KW-4A spin coater was 2000 rpm, and the process of spin coating lasted for 15 seconds. The coatings were cured at 80 °C for 8 h and 120 °C for 2 h.
Characteristics of spirocyclic alkoxysilane
Fourier transform infrared spectroscopy of the as-obtained spirocyclic alkoxysilane was analyzed with a NICOLET IS50 Fourier transform infrared spectrometer (Waltham, America) by the KBr squash method. Mass spectrum of the as-obtained spirocyclic alkoxysilane was analyzed with a waters ACQUITY UPLC H-Class/Xevo TQD (Milford, America).
Water contact angle measurement
The water contact angle of the coating was measured by a contact angle meter (FCA2000A) manufactured by the company of Shanghai Aifis Precision Instruments Co. (Shanghai, China). Three different areas in each coating were selected to repeat the water contact angle measurements. The average of the three tests was taken as the water contact angle for each coating, and the error bars represent the standard deviation of the three tests for each coating.
Wear resistance
An H-18 device purchased from Taber (North Tonawanda, America) was used to test the wear resistance of coatings. The coating was subjected to a pressure of 2 kPa, and the rubbing speed was 50 mm/s. The number of rubbing times was 50, 100, 150, and 200. Three different areas in each coating were selected to repeat the wear resistance test. The average of the three tests was taken as the water contact angle for each coating, and the error bars represent the standard deviation of the three tests for each coating. In a review of the durability of hydrophobic coatings, Milionis et al. (2016) suggest that this method may become a universal standard for evaluating the durability of hydrophobic coatings in the future.
RESULTS AND DISCUSSION
Characterization of Spirocyclic Alkoxysilane
Figure 1(a) is the infrared spectrum of the product, in which the peaks at 962, 1023, and 1377 cm-1 correspond to the absorption peaks of Si-O-C stretching vibration, Si-O stretching vibration, and -CH3 deformation vibration, respectively. Figure 1(b) is the mass spectrum of the product, in which the peak at 283.19 corresponds to the molecular weight of spirocyclic alkoxysilane (Na+ adduct). Combined with the IR spectrum analysis of the product in Fig. 1(a), it can be inferred that the structure of the product is spirocyclic alkoxysilane.
Fig. 1. Spirocyclic alkoxysilane: (a) Infrared Spectroscopy; (b) Mass Spectrum
Figure 2 (a) is the 1H NMR spectrum of spirocyclic alkoxysilane.
Fig. 2. NMRs of spirocyclic alkoxysilane (a)1H NMR (b) 29Si NMR (c)13C NMR
It can be seen that the peaks at 1.32 ppm and 1.20 ppm correspond to CH3; the peaks at 1.67 ppm and 1.49 ppm correspond to CH2; and the peak at 3.57 ppm corresponds to C(H,Me)O. Figure 2(b) is the 29Si NMR spectrum of spirocyclic alkoxysilane. It can be seen that there is a sharp peak at -81.35 ppm, which is at the same position of chemical shift as Q-type siloxane. The spirocyclic alkoxysilane is a Q-type siloxane. Figure 2(c) shows the 13C NMR spectrum of spirocyclic alkoxysilane. It can be seen that the peak at 24.06 ppm corresponds to CH3; the peaks at 32.54 ppm and 27.42 ppm correspond to (CH3)2; the peaks at 49.16 ppm and 48.10 ppm correspond to CH2; the peak at 67.37 ppm corresponds to C(H,Me)O; and the peak at 74.48 ppm corresponds to C(Me)2O. The above results combined with the infrared spectroscopy and mass spectrometry results indicate that the spirocyclic alkoxysilane structure was successfully synthesized. Part b of Fig. 1 includes a chemical structure of spirocyclic alkoxysilane.
Synthesis Condition Optimization
Figure 3(a) shows the yield data of spirocyclic alkoxysilane synthesized from rice straw ash at different heat treatment temperatures. The spirocyclic alkoxysilane yield per gram of rice straw ash increased with the increase of heat treatment temperature at first. After it reached the maximum yield of 1.57 g at 500 °C, the yield gradually decreased with the increase of heat treatment temperature. The reasons for this are as follows: firstly, rice straw ash contains a portion of carbon. Appropriately increasing the heat treatment temperature can further reduce the proportion of carbon content in rice straw ash. Moreover, the heat treatment at 500 °C for one hour cannot affect the activity of amorphous silica in the rice straw ash. With the further increase of the heat treatment temperature, the residual carbon in the rice straw ash could be further removed, and the silica content could be increased. However, the excessive high temperature led to the reduction of the specific surface area of silica in the rice straw ash, and the high temperature may lead to the phenomenon of crystallization (Chandrasekhar et al. 2006) and crystalline transformation (Dizaji et al. 2019). These changes result in the reduction of the reaction activity of silica in the rice straw ash. Thus, when the heat treatment temperature was higher than 500 °C, the yield gradually decreased with the increase of heat treatment temperature. Figure 3(b) shows the effect of heat treatment time on the yield of spirocyclic alkoxysilane. The effect of heat treatment time and heat treatment temperature on yield was shown to be similar. So, when the pretreatment condition of rice straw ash was 500 °C for 1 h, the yield was optimal.
Figure 3(c) shows the effect of NaOH concentrations on the yield of spirocyclic alkoxysilane synthesized from rice straw ash. It shows that the highest spirocyclic alkoxysilane yield per gram of rice straw ash could reach 1.9 g when the NaOH concentration was 0.0025 mol/L. After that, with the increase of NaOH concentration, the yield gradually decreased. The reaction of rice straw ash with 2-methyl-2,4-pentanediol to form spirocyclic alkoxysilane can occur without the additional alkali. This indicates that the alkali contained in rice straw ash could catalyze the reaction.
However, while the washed rice straw ash reacted with 2-methyl-2,4-pentanediol, the yield was substantially reduced to only 0.18 g. Therefore, there is an optimal concentration of alkali catalyst in the synthesis of spirocyclic alkoxysilane from rice straw ash. When the alkali concentration exceeds optimal concentration, high concentration alkali may destroy the structure of spirocyclic alkoxysilane. Furthermore, it can also lead to a side reaction. When the NaOH concentration exceeds 0.25 mol/L, 2-methyl-2,4-pentanediol itself will undergo an elimination reaction, and the corresponding ether with an irritating smell will be produced. A great many by-products will be evaporated from the reaction, which greatly interferes with the main reaction and reduces the yield. Moreover, it creates great difficulties for the subsequent separation and purification.
NaOH was used as the alkali catalyst in the above experiments. Additionally, the effect of other alkali catalysts on the yield was also studied. Generally considering the cost and the convenience of operation, NaOH was judged to be the most suitable as an alkali catalyst. It is noteworthy that the catalytic efficiency of LiOH is significantly lower than that of NaOH. Only 1.23 g of spirocyclic alkoxysilane can be synthesized per gram of rice straw ash, under the reaction conditions with 0.25 mol/L LiOH as the alkali catalyst.
Fig. 3. Synthesis condition optimization (a) temperature (b) time (c) NaOH concentration
Analyzing the reaction mechanism, the possible reason is that the rate-determining step in this reaction may depend on the properties of the alkali catalyst cation. Li+ can interact with 2-methyl-2,4 pentanediol to form an intermediate compound, which is very stable. The potential barrier of the reaction between the oxygen-negative part of activated SiO2 and 2-methyl-2,4 pentanediol is higher than that catalyzed by the Na+ alkali catalyst. (Butera et al. 2018) Thus, the catalytic efficiency of LiOH is significantly reduced.
Characterization of Coatings
Figure 4 shows the infrared spectrum of the silicone coatings. The absorbance peaks around 770 to 730 cm-1 and 710 to 690 cm-1 are the out-of-plane bending vibration absorbance peaks of C-H in the benzene ring. The absorbance peaks around 1100 to 1000 cm-1 are the stretching vibration absorption peaks of Si-O. The absorbance peaks around 1125 cm-1 are the vibration absorbance peaks of aromatic ring in Si-Ph and stretching vibration absorbance peaks of Si-C. The absorbance peaks around 1260 cm-1 are the symmetric deformation vibration absorbance peaks of CH3 in Si-Me. The absorbance peaks around 1428 cm-1 are the vibration absorbance peaks of aromatic ring in Si-Ph. The broad absorbance peaks around 3500 to 3300 cm-1 are the stretching vibration absorbance peaks of hydroxyl groups. The presence of absorbance peaks of Si-Ph, Si-Me, and Si-O can be seen in all groups of IR spectra, which indicates the successful synthesis of the silicone resin. However, a larger hydroxyl absorbance peak in the control group can be seen around 3500 to 3300 cm-1. And the hydroxyl absorption peak became smaller with the gradual increase of the addition ratio of spirocyclic alkoxysilane. When the addition ratio was 20%, the absorbance peak of hydroxyl group disappeared. The control group produced a large amount of active silicone hydroxyl groups after the hydrolysis of methyltriethoxysilane and phenyltriethoxysilane. So, the silicone hydroxyl groups failed to be completely condensed and cured. With the introduction of spirocyclic alkoxysilane, the proportion of silicone hydroxyl groups in the reaction was reduced. So, the curing effect gradually increased and the absorbance peaks of hydroxyl groups in the infrared spectrum gradually weakened. When the spirocyclic alkoxysilane addition ratio was 20%, the absorbance peak of the hydroxyl group disappeared, and the silicone resin film was completely cured.
Fig. 4. Infrared spectra of coatings
Figure 5 shows the SEM image of the silicone resin coatings modified with spirocyclic alkoxysilane. The control group coating was uneven. Moreover, the control group coating was not dense and homogeneous. The IR results indicates that the curing effect of control group was poor. With the increase of spirocyclic alkoxysilane addition ratio, the surface of the film gradually became homogeneous and dense. When the addition ratio reached 20%, the surface morphology of the film was the best. This indicates that the compatibility between spirocyclic alkoxysilane and silicone was the best at this time, and so was the curing effect of the film. When the addition ratio of spirocyclic alkoxysilane reached 25%, some fine particles appeared on the surface of the coating. Due to the high reactivity of phenyltriethoxysilane and methyltriethoxysilane, their hydrolysis generated a large number of active silicone hydroxyl groups. They could not be cured completely, which resulted in the uneven surface morphology of the film. Spirocyclic alkoxysilane is mainly introduced as a modifier by physical doping. So, the proportion of silicon hydroxyl groups in the film after hydrolysis was reduced with the increase of doping ratio. The film is cured more completely; thus, the coating became more homogeneous and denser. When the spirocyclic alkoxysilane addition ratio reached 25%, excess spirocyclic alkoxysilane agglomerated in the film and formed fine particles.
Fig. 5. SEM images of coatings. (a) control, (b) 5%, (c) 10%, (d) 15%, (e) 20% and (f) 25%. Magnification 100×, scale bar 1 mm
Hydrophobic Performance
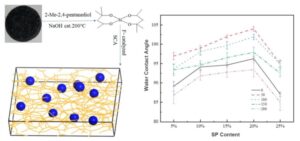
Figure 6 shows the water contact angle test data of the spirocyclic alkoxysilane modified silicone resin. It shows that with the increase of spirocyclic alkoxysilane doping ratio, the water contact angle of the modified silicone resin first increased and then decreased. When the doping ratio of spirocyclic alkoxysilane was 20%, the water contact angle of the modified silicone resin was the largest. After that, the water contact angle decreased with the increase of the spirocyclic alkoxysilane doping ratio. The main reasons are as follows: firstly, the one spirocyclic alkoxysilane molecule contains six methyl groups. So, the introduction of spirocyclic alkoxysilane into the silicone resin significantly increases the proportion of methyl groups. Secondly, the unique double ring and rigid skeleton structure of spirocyclic alkoxysilane contribute to the upgradation of hydrophobicity of the silicone resin. Because spirocyclic alkoxysilane does not contain reactive groups, it modifies the silicone resin mainly by physical mixture. When the spirocyclic alkoxysilane doping ratio exceeds 20%, particle collision and agglomeration can occur. This results in changes in the surface micro-nano structure of the modified silicone resin, and the hydrophobicity decreases.
Fig. 6. Water contact angle of coatings
Figure 7 shows the contact angle hysteresis data of the coatings. The addition ratio of spirocyclic alkoxysilane increased as the contact angle hysteresis of the film gradually decreased. When the addition ratio of spirocyclic alkoxysilane was 20%, the contact angle hysteresis of the coating reached its minimum, before subsequently increasing. The main reason for this is that the hydrolysis of methyltriethoxysilane and phenyltriethoxysilane produced a large number of silicone hydroxyl groups. Due to the relatively high content of silicone hydroxyl groups in the control group, the condensation and curing process could not occur completely. As a result, the cured film still contained some silicon hydroxyl groups, which can combine with water. This reaction induces molecular rearrangement on the coating surface. With the increase of the addition ratio of spirocyclic alkoxysilane, the proportion of silicone hydroxyl groups after hydrolysis in the film was relatively reduced and the curing degree gradually improved. As such, the polymer composition became more fixed and the water-induced molecular rearrangement was greatly suppressed. Thus, the contact angle hysteresis of the modified silicone resin film was gradually reduced. When the spirocyclic alkoxysilane addition ratio exceeded 20%, the surface morphology and roughness of the film were changed due to the agglomeration of spirocyclic alkoxysilane. As a result, the contact angle hysteresis of the film increased.
Fig. 7. Contact angle hysteresis of coatings
Wear Resistance
Figure 8 shows the wear resistance performance of the spirocyclic alkoxysilane modified silicone resin. The water contact angle of each group of silicone resin showed an increasing data trend with the increase of friction times, and the water contact angle of each group of silicone resin reached the corresponding maximum value when the friction application times was 150. After that, the water contact angle of each group of silicone resin gradually decreased with the increase of friction applications. The membrane surface of silicone resin showed a tendency to be destroyed at this time. When the friction applications reached 200, the water contact angle of each group of silicone resin was smaller than the corresponding group without friction. This indicates that the hydrophobic effect of the coating was destroyed at this time. These results show that the coating can withstand 150 applications of friction and still maintain good hydrophobic effect. The water contact angle of the 20% spirocyclic alkoxysilane modified silicone coating was able to reach 104° after 50 applications of friction. The main reason for the good wear resistance performance is that the unique double-ring and rigid skeleton structure of spirocyclic alkoxysilane plays a role as an “anchor point” (Grassie et al. 1980) in the silicone resin.
Fig. 8. Wear resistance performance of coatings
Figure 9 shows SEM images of the coatings after 200 applications of friction. The doping ratio of 5% and 10% spirocyclic-alkoxysilane-modified silicone resin films showed severe breakage and large-pieces flaking off after 200 applications of friction. In contrast, the doping ratio of 15% and 20% spirocyclic-alkoxysilane-modified silicone resin films showed less breakage and less large-pieces flaking than the other films after friction. This indicates that the adhesion of these films was better than the others. The doping ratio of 25% spirocyclic-alkoxysilane-modified silicone resin film showed more breakage and more large-pieces flaking off than the doping ratio of 20% spirocyclic-alkoxysilane-modified silicone resin film. Excess spirocyclic alkoxysilane agglomerated, which makes the doping ratio of 25% spirocyclic-alkoxysilane-modified silicone resin film weaken. The silicone resin film without spirocyclic alkoxysilane added was completely destroyed and flaked off after 50 times of friction. Combined with the IR and SEM results, the main reason for this is that the content of silicone hydroxyl groups in the film was too high after the hydrolysis of methyltriethoxysilane and phenyltriethoxysilane in the film. So, the film was not completely cured, which made the adhesion of the film weaker. The mechanism of adhesion of coatings is the condensation of free silicone hydroxyl groups in the silicone resin with the silicone hydroxyl groups of the substrate. Because there are more free silicone hydroxyl groups on the surface of wood, we believe that the coatings in this study can also be applied to the surface of wood (Wei et al. 2019).
Fig. 9. SEM images of coatings after 200 wear cycles. (a) control, (b) 5%, (c) 10%, (d) 15%, (e) 20% and (f) 25%. Magnification 100×, scale bar 1 mm
Table 1 shows the results of tape adhesion test for silicone resin film. The result of the control group was the worst, which was 0 B. This indicates that the adhesion of the control group was very poor. The same conclusion can be also drawn from the IR results and wear resistance test. The main reason is that the proportion of silicon hydroxyl in the silicone resin film without spirocyclic alkoxysilane added was relatively high. So, the film was not cured completely. With the increase of the spirocyclic alkoxysilane addition ratio, the adhesion of the film gradually improved. Moreover, when the addition ratio was 20%, the adhesion of the film reached its best, which was 3 B. The main reason is that the curing effect of the film was the best at this time and no agglomeration had occurred. When the spirocyclic alkoxysilane addition ratio reached higher than 20%, the adhesion of the silicone resin film was weakened due to the agglomeration of spirocyclic alkoxysilane in the film.
Table 1. Tape Adhesion Test of Coatings
CONCLUSIONS
- Spirocyclic alkoxysilane was prepared using rice straw ash as the reactant for the first time, and the spirocyclic alkoxysilane yield per gram of rice straw ash was able to reach 1.9 g. This study realized the efficient and clean utilization of rice straw ash.
- The modified silicone hydrophobic coatings were prepared by using spirocyclic alkoxysilane as a modifier. This modified coating was able to withstand 150 applications of friction and still maintain good hydrophobic effect. The maximum water contact angle after friction reached 104°.
- This is the first time low-cost and green synthesis of spirocyclic alkoxysilane using rice straw ash as a reactant. It can improve the efficiency of rice straw utilization. This work can reduce the cost of high value-added silicone chemicals and reduce the pollution caused by open burning of rice straw and the production of traditional silicone chemicals.
- Potassium carbonate in rice straw ash can be used as a catalyst to catalyze this reaction. The processes of water washing step and alkali addition can be removed in the industrial production.
ACKNOWLEDGMENTS
The authors are grateful for the support of Major Project of the Ministry of Science and Technology of China, Grant No. 2017YFB0307700.
REFERENCES CITED
Baramee, S., Siriatcharanon, A., Ketbot, P., Teeravivattanakit, T., Waeonukul, R., Pason, P., Tachaapaikoon, C., Ratanakhanokchai, K., and Phitsuwan, P. (2020). “Biological pretreatment of rice straw with cellulase-free xylanolytic enzyme-producing Bacillus firmus K-1: Structural modification and biomass digestibility,” Renewable Energy 160, 555-563.
Butera, V., Fukaya, N., Choi, J. C., Sato, K., and Choe, Y. K. (2018). “Alkoxysilane production from silica and dimethylcarbonate catalyzed by alkali bases: A quantum chemical investigation of the reaction mechanism,” Inorganica Chimica Acta 482, 70-76. DOI: 10.1016/j.ica.2018.05.036
Chandrasekhar, S., Pramada, P. N., and Majeed, J. (2006). “Effect of calcination temperature and heating rate on the optical properties and reactivity of rice husk ash,” Journal of Materials Science 41(23), 7926-7933. DOI: 10.1007/s10853-006-0859-0
Dizaji, H. B., Zeng, T., Hartmann, I., Enke, D., Schliermann, T., Lenz, V., and Bidabadi, M. (2019). “Generation of high quality biogenic silica by combustion of rice husk and rice straw combined with pre- and post-treatment strategies – A review,” Applied Sciences 9(6), article no. 1083. DOI: 10.3390/app9061083
Furgal, J. C., and Lenora, C. U. (2019). “Green routes to silicon-based materials and their environmental implications,” Physical Sciences Reviews 5(1). DOI: 10.1515/psr-2019-0024
Grassie, N., Francey, K. F., and Macfarlane, I. G. (1980). “The thermal degradation of polysiloxanes—Part 4: Poly(dimethyl/diphenyl siloxane),” Polymer Degradation and Stability 2(1), 67-83. DOI: 10.1016/0141-3910(80)90016-6
Krug, D. J., and Laine, R. M. (2017). “Durable and hydrophobic organic-inorganic hybrid coatings via fluoride rearrangement of phenyl T 12 silsesquioxane and siloxanes,” ACS Applied Materials & Interfaces 9(9), 8378-8383. DOI: 10.1021/acsami.6b16121
Laine, R. M., Furgal, J. C., Doan, P., Pan, D., Popova, V., and Zhang, X. (2016). “Avoiding carbothermal reduction: Distillation of alkoxysilanes from biogenic, green, and sustainable sources,” Angewandte Chemie International Edition 55(3), 1065-1069. DOI: 10.1002/anie.201506838
Liu, H., Zhang, F., Wu, Z., Cui, E., Yue, L., Hou, G., and Wang, L. (2021). “Nitrogen-doped porous carbon derived from cellulose microfibers of rice straw for high-performance electrodes of supercapacitors,” Energy & Fuels 35(12), 10190-10198. DOI: 10.1021/ACS.ENERGYFUELS.1C00323
Milionis, A., Loth, E., and Bayer, I. S. (2016). “Recent advances in the mechanical durability of superhydrophobic materials,” Advances in Colloid and Interface Science 229, 57-79. DOI: 10.1016/J.CIS.2015.12.007
Mirmohamadsadeghi, S., and Karimi, K. (2020). “Recovery of silica from rice straw and husk,” in: Current Developments in Biotechnology and Bioengineering: Resource Recovery from Wastes, Elsevier, Amsterdam, pp. 411-433. DOI: 10.1016/B978-0-444-64321-6.00021-5
Putro, W. S., Fukaya, K., Choi, J.-C., Choi, S. J., Horikoshi, T., Sato, K., and Fukaya, N. (2020). “Direct transformation of silica from natural resources to form tetramethoxysilane,” Bulletin of the Chemical Society of Japan 93(8), 958-962. DOI: 10.1246/bcsj.20200054
Satlewal, A., Agrawal, R., Bhagia, S., Das, P., and Ragauskas, A. J. (2018). “Rice straw as a feedstock for biofuels: Availability, recalcitrance, and chemical properties,” Biofuels, Bioproducts and Biorefining 12(1), 83-107. DOI: 10.1002/bbb.1818
Wei, S., Meng, L., Liu, W., Guo, S., and Zhang, X. (2019). “Polyhedral oligomeric silsesquioxane (POSS) as reinforcing agent for waterborne polyurethane coatings on wood,” Materials Research 22(2). DOI: 10.1590/1980-5373-MR-2018-0278
Article submitted: February 20, 2022; Peer review completed: May 21, 2022; Revised version received and accepted: July 12, 2022; Published: July 14, 2022.
DOI: 10.15376/biores.17.3.5065-5078
