Abstract
Due to its different organizational structures, dense hard husk, and loose soft core, corn stover (CS) is more resistant to transformation into monosaccharides for biofuel production in comparison to rice straw or wheat straw. In this paper, before pretreatment with sulfur trioxide micro-thermal explosion and dilute alkali (STEX-DA), CS was cut into 2 cm to 3 cm lengths in the transverse direction and cross opening in the vertical direction. During the process of STEX-DA, the structures of cores of corn stover (CS-C) and husks of corn stover (CS-H) were separately studied via scanning electron microscopy (SEM), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FT-IR) analysis. Furthermore, the components content and reducing sugars yield were calculated. The results showed that the CS-C achieved 66.7% of lignin removal rate, and the reducing sugars yield (calculated by original dry weight of straw) was 70.8%, while the CS-H displayed values of 64.7% and 55.3%, respectively. This result indicated that STEX-DA pretreatment could facilitate corn stover usage as a renewable energy source.
Download PDF
Full Article
Structure and Saccharification of Corn Stover Pretreated with Sulfur Trioxide Micro-Thermal Explosion and Dilute Alkali (STEX-DA)
Zhijiang Dong,a,§ Fenghe Li,b,c,§ Huai Wang,a Shengsong Deng,a and Risheng Yao a,*
Due to its different organizational structures, dense hard husk, and loose soft core, corn stover (CS) is more resistant to transformation into monosaccharides for biofuel production in comparison to rice straw or wheat straw. In this paper, before pretreatment with sulfur trioxide micro-thermal explosion and dilute alkali (STEX-DA), CS was cut into 2 cm to 3 cm lengths in the transverse direction and cross opening in the vertical direction. During the process of STEX-DA, the structures of cores of corn stover (CS-C) and husks of corn stover (CS-H) were separately studied via scanning electron microscopy (SEM), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FT-IR) analysis. Furthermore, the components content and reducing sugars yield were calculated. The results showed that the CS-C achieved 66.7% of lignin removal rate, and the reducing sugars yield (calculated by original dry weight of straw) was 70.8%, while the CS-H displayed values of 64.7% and 55.3%, respectively. This result indicated that STEX-DA pretreatment could facilitate corn stover usage as a renewable energy source.
Keywords: Corn stover (CS); Pretreatment; Sulfur trioxide micro-thermal explosion (STEX); Reducing sugars yield; Lignin removal
Contact information: a: School of Biological and Medical Engineering, Hefei University of Technology, Hefei 230009, China; b: School of Chemistry, University of Science and Technology of China, Hefei 230026, China; c: Anhui Anson Biochemical Science and Technology Co., Ltd., Hefei 230601, China;
* Corresponding author: yaors@163.com
§ The authors contributed equally to this article
INTRODUCTION
Throughout the world, corn stover (CS) is an abundant renewable resource, and its annual output is about 250 million tons (Slade et al. 2014). CS can be used as raw material for biofuel production; however, it is necessary to pretreat CS to improve its utilization efficiency. The current CS pretreatments include steam blasting, hydrothermal treatment, and the thermocompression collaborative alkali method (Dogaris et al. 2009).
The steam explosion method normally pretreats the CS in harsh conditions, such as 205 °C/1.6 MPa, and CS has a conversion rate of 89% via 70 h of hydrolysis with 60 FPU/g of glucan cellulose supplementing with 15 CBU of β-glucosidase (Chen et al. 2014). The hydrothermal treatment of CS is conducted at 195 °C for 6 min after being pre-soaking in water at 80 °C for 6 min, and the CS saccharification rate achieved is 85.2% at 25 FPU/g enzyme loading for 72 h (Kaparaju and Felby 2010). Although there is a highly apparent enzyme saccharification rate, it is obtained only by high enzyme loading and long-term enzymatic hydrolysis (Sun and Cheng 2002; Taherzadeh and Karimi 2008).
To improve the efficiency of enzymatic saccharification and lignin stripping, pretreatment is combined with dilute alkali, such as in the thermocompression collaborative dilute alkali method. In this condition, the lignin is stripped to 41% by 10 wt.% alkali at 140 °C for 30 min, and the final saccharification rate reaches 85% with cellulase (20 FPU/g dry biomass) and β-glucosidase (10 IU/g dry biomass) loading for 48 h (Li et al. 2012a).
However, all of these pretreatments involve high temperature and/or high pressure, with low production efficiency and high energy consumption. Moreover, the high cellulose and hemicellulose loss rate (nearly 40%) (Li et al. 2012a) causes the treatment liquid to have high viscosity. As a result, it is difficult to use membrane separation, and this enhances the risk of waste and pollution.
The sulfur trioxide micro-thermal explosion with dilute alkali (STEX-DA) method is an environmentally friendly pretreatment method that is carried out at atmospheric pressure and room temperature conditions (Yao et al. 2011). STEX-DA can be used to pretreat the rough surface of rice straw and the waxy layer surface of wheat straw (Li et al. 2012b; Yao and Li 2013; Wang et al. 2016).
The treatment causes rapid thermal expansion with great heat release due to the reaction of SO3 gas (5 g/kg to 10 g/kg of dry straw) with water molecules, thus destroying the dense structure of straw. After the dilute alkali (2 wt% sodium hydroxide) pretreatment at 60 °C for 3 h, the lignin stripping reaches about 80%, and the total loss of cellulose and hemicellulose is less than 20%. At the same time, the enzymatic hydrolysis rate of rice or wheat straw increases more than 90% as a consequence of the direct STEX-DA pretreatment condition, without crushing-disposal. The low-viscosity black liquor obtained after the pretreatment can be easily separated by membrane systems. This approach avoids subsequent environmental pollution problems.
The pretreatment for CS is very difficult due to its dense hard shell and loose soft core. This paper explored the utilization of STEX-DA for CS pretreatment as a way to enhance the bioavailability (enzyme saccharification rate). The structure and appearance of CS-C and CS-H were investigated via elemental analysis, scanning electron microscopy (SEM), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FT-IR) to elucidate its principle and practical application value.
EXPERIMENTAL
Materials
Corn stover (CS), provided by local farmers in Fuyang, China, was chopped and stored in a dry environment for 1 to 2 months. The chemical composition of the raw CS was 32.2% cellulose, 22.8% hemicellulose, 19.0% lignin, 1.03% ash, and 17.2% water-soluble substances. Cellulose, hemicellulose, and lignin were purchased from Shanghai Macklin Biochemical Co. Ltd. (Shanghai, China). Cellulase enzyme (Aladdin®, Lot K1301016, Shanghai, China) had an activity of 88.16 filter paper unit (FPU)/g, as determined by a standard method (Sluiter et al. 2008).
Fig. 1. (a) The CS was diced in the transversal or longitudinal direction. (b) The CS sectional view of real products and mode pattern was separated into cores and husks.
Fig. 2. Scheme for pretreating of CS, CS-C, and CS-H
STEX with Dilute Alkali Pretreatment
The stored CS was cut into long fragments between 2 cm to 3 cm long (Fig. 1a, sample 1), and separated into CS-C and CS-H, as shown in Fig. 1b. They were stored in a transparent polyethylene self-contained bags for one week to ensure that the moisture content was as consistent as possible.
In a 500-mL beaker with ground-in glass stopper, approximately 0.15 g of sulfur trioxide gas was produced by chemical reaction of 0.9 g of P2O5 and 18 mL of 98% H2SO4 at 50 °C, and then 15 g of CS was quickly added and held over the upper portion of the beaker. In the beaker, CS and SO3 were allowed to react for 3 h at 50 °C (Yao et al. 2011). The lignin in CS was stripped at 60 °C for 3 h by 2% (w/v) NaOH (solid-liquid ratio of 1:15), and the mechanical stirring was selected at 150 rpm. Untreated waters (solid-liquid ratio of 1:15, only treated by water), and dilute alkali (solid-liquid ratio of 1:15, only treated by 2% NaOH) samples were used for comparison. The specific process is shown in Fig. 2.
Reducing Sugars Yield of CS
Enzymatic hydrolysis of different substrates was carried out at a substrate consistency of 2%. Cellulase (15 FPU/g or 30 FPU/g dry biomass) was added with 50 mL of 0.05 M sodium citrate buffer (pH 4.8) and 0.02% sodium azide, and hydrolysis took place at 50 °C in a conical flask (100 mL) placed in an incubator shaker at 150 rpm. Samples were taken by pipette from the flask, inactivated under 100 °C for 10 min immediately, and centrifuged at 8000 rpm for 10 min before the supernatant was collected. The reaction was monitored by periodically sampling and measuring the release of soluble reducing sugars by the 3,5-dinitrosalicylic acid (DNS) assay using D-glucose as a standard. The reducing sugars yield (calculated by the original dry weight of CS) was calculated as follows,
(1)
where RE is the reducing sugars after enzyme hydrolysis, and PS is the dry-weight CS before pretreatment.
Analytical Methods
Composition analysis of CS
This procedure was performed by two steps of acid hydrolysis procedure as described by Sluiter et al. (2008). Briefly, the sample was mixed with 72% sulfuric acid for 1 h at 30 °C. The acid concentration was diluted to 4% with deionized water, and the sample was heated at 121 °C for 1 h in an autoclave. After the two-step hydrolysis, the sample was filtered by vacuum using filter crucibles. Residues were analyzed for acid-insoluble lignin (AISL) and ash contents by drying at 105 °C for 24 h followed by drying at 575 °C for 4 h. The filtrate was analyzed for acid-soluble lignin (ASL) by recording absorbance at 310 nm and for sugar concentrations by HPLC (Aminex HPX-87P, Bio-Rad, Hercules, CA) (Sluiter et al. 2008; Zhang et al. 2011; Kim et al. 2016).
For surface morphology observation by SEM, a Hitachi S-4800 SEM instrument (Tokyo, Japan) operating at 20 kV accelerated voltage was used to examine morphology and size of different CS-C and CS-H samples for comparison of the effect of pretreatment. The samples were coated with a thin layer of gold before the test.
For FT-IR experiments, the dried plant biomass samples were mixed with KBr of spectroscopic grade and formed into pellets at a pressure of 1 MPa. The pellets were about 10 mm in diameter and 1 mm in thickness. FT-IR spectra were recorded from 3800 cm-1 to 500 cm-1 with a resolution of 4 cm-1 on a Thermo Scientific Nicolet 6700 Fourier transform infrared spectrometer (Nicolet 67, Madison, WI, USA).
The elemental analysis of C, H, N, S was accomplished by combustion analysis (Vario EL Cube, Hanau, Germany). During this process, each sample (0.02 to 50 mg) was treated with high temperature combustion or pyrolysis, the elements were converted into gas phase, and then the gases were separated and detected by Thermal Conductivity Detector (TCD) (Sahu et al. 2011).
For crystallinity analysis by x-ray diffraction (XRD), measurements were performed on a Bruker D8 Advance Diffractometer (X’Pert PRO MPO, Almelo, Netherlands) using Cu Ka radiation (k = 0.1541 nm) at 40 kV and 40 mA. Samples were scanned in the 2θ range from 5° to 50°. The crystallinity index (CrI) of samples was calculated by Eq. 2,
(2)
where CrI is crystallinity index, I200 represents both crystalline and amorphous material, and Iam represents amorphous material only (Segal et al. 1959).
All experiments were carried out three times, and the given values are the mean values ± standard deviation (SD).
RESULTS AND DISCUSSION
Yield and Compositions after Pretreatment of STEX-DA
CS was only cut as shown in sample 1 to process the subsequent STEX-DA pretreatment. The results of lignin content and lignin removal rate were 12.8% and 44.0%, respectively, which was lower than that of the recently reported lignin-stripping rate (40.9% to 71.6%) (Saha et al. 2016; Tang et al. 2017). CS had a large cross-sectional diameter and dense hard shell, which hindered the penetration of gas and dilute alkali solution. By increasing the longitudinal cross cutting or reducing the size, it was found that adding the longitudinal slicing made CS have a high lignin removal rate of 57.0%, as shown for sample 2 in Table 1a. The pretreatment effect was judged to be ideal under these conditions.
A possible reason for this could be that, without the slicing, the outside wax layer of CS blocked the direct SO3 diffusion from husk surface to the inner core of CS. Therefore, the only way of SO3 diffusion occurred was via the two cut ends of CS. After the crosscut operation for the CS, the increased surface area provided more pathways for SO3 diffusion, which improved the depth and efficiency of micro-thermal explosion.
At the same time, by comparing samples 3 and 4, there was not a significant change in the aspect of the lignin removal rate after pretreatment, with the cores and husks separating. There was also no meaningful difference in the amount of the composition as shown in Table 1a (samples 2 and 3) with further cutting pretreatment. On the whole, CS only needed to be exposed to get a better lignin stripping effect as much as possible, in which CS did not need to separate CS-C and CS-H, as well as further cutting or crushing from the original CS.
Table 1a. Compositions (%w/w) of Insoluble Solids from Different Cutting Stalk Samples after Pretreatment
Table 1b. Compositions (%w/w) of Insoluble Solids from Different Pretreatment of CS-C (about 2 cm to 3 cm) Pretreated by Different Pretreatment Ways (A: origin CS-C; B: CS-C after water pretreatment; C: CS-C after dilute alkali pretreatment; and D: CS-C after STEX-DA pretreatment)
Table 1c. Compositions (%w/w) of Insoluble Solids from Pretreatment of CS-H (about 2 cm to 3 cm) Pretreated by Different Ways: E: origin CS-H; F: CS-H after waters pretreatment; G: CS-H after dilute alkali pretreatment; and H: CS-H after STEX-DA pretreatment
To further investigate the effect of the STEX-DA pretreatment method for different composition lignocellulosic biomasses, the CS was separated into CS-C and CS-H and pretreated according to Fig. 2. As shown in Tables 1b and 1c, original CS-C and CS-H were different not only in morphology, but also in the main components. CS-H (20.41%) had a higher lignin content compared to CS-C (15.66%) and low holo-cellulose after pretreatment. But they had almost the same relative lignin removal ratio of the CS-H and CS-C whether the DA pretreatment (about 56%) or STEX-DA pretreatment (about 65%) was used. However, it was clear that the STEX-DA pretreatment was about 9% higher than DA in lignin-stripping rate. From Table 4, it can be seen that the lignin peeling effect was greater than that of the alkali pretreatment method that has been reported. The higher rate of individual stripping is mainly due to lower yield caused by high temperatures.
Surface Morphology Observation by Scanning Electron Microscopy (SEM)
Figure 3 shows the SEM micrographs of the original CS, the pretreated CS after STEX, and the pretreated CS after STEX-DA, respectively. The original CS-H had the original compact structure such as epidermis and vascular bundles, as well as parenchyma sticking to the bundle surface (Fig. 3a-1 and Fig. 3b-1) . The microfibril surface of the origin CS-H was relatively smooth, which was not suitable for subsequent enzyme treatment. After STEX pretreatment, different degrees of holes and cracks appeared inside or outside the surface of CS-H (Fig. 3a-2 and Fig. 3b-2), which promoted the subsequent infiltration of lye and ultimately increased the lignin-stripping rate. STEX-DA pretreatment increased concavity on the CS-H (Fig. 3a-3 and Fig. 3b-3). When the lignocellulosic material was soaked in the alkaline solution, the pores exhibited swelling. The bonds between lignin and the carbohydrate polymers were broken to solubilize partial free lignin via the pretreatment.
CS-C maintained integral plant cell wall structure (Fig. 3c-1 and Fig. 3d-1), and cell adhesion together formed a honeycomb structure. As shown in Fig. 3c-2 and Fig. 3d-2, the STEX pretreatment led to some cell wall slitting and collapse. These structural changes implied that the binding between the cell walls was damaged, which increased the exposure of lignin. It can be clearly seen that there was erosion (Fig. 3c-3 and Fig. 3d-3) on the CS-C, which was different with CS-H. The cell wall of CS-C contained less lignin to form hard net structure, which make easier to destroy the steady structure by STEX-DA pretreatment.
The main result was that new channels and holes appeared after STEX pretreatment, which could increase the contact rate between lignin and alkali to enhance the lignin removal rate. These holes and cracks can be mainly attributed to rapid thermal expansion with the vast heat releasing from the reaction of SO3 gas (5 g/kg to 10 g/kg of CS) with water molecules. Thus, these structural changes facilitated the destruction of the CS dense structure, which also presented direct evidence of a chemical reaction.
Fig. 3. SEM micrographs of CS-C and CS-H surface with different pretreatment methods: (a) Outer surface of the CS-H; (b) Inner surface of the CS-H; (c) Longitudinal section of CS-C; (d) transversal of CS-C; (1) Origin; (2) STEX pretreatment; (3) STEX-DA pretreatment
FT-IR Spectra Analysis
The 3800 cm-1 to 2000 cm-1 region (Fig. 4a) contained two main bands in the range around 2908 cm-1 and 3363 cm-1. The assignments of these bands are presented in Table 2, including seven peaks at 3567 cm-1, 3423 cm-1, 3342 cm-1, 3278 cm-1, 3106 cm-1, 2921 cm-1, and 2851 cm-1, which are consistent with the coverage of Popescu et al. (2007). The CS-C and CS-H existed in corresponding functional groups.
The 2000 cm-1 to 500 cm-1 IR spectra of different pretreatment of CS-C and CS-H are shown in Fig. 4b and Fig. 4c. The typical functional groups and the IR signal with the possible compounds are listed in Table 1 as a reference (Popescu et al. 2007). It could be observed that the different pretreatments for CS most likely consisted of organosilicon compounds, aromatics, ketones, alcohol, etc. Wang et al. reported that C-SO (772 cm-1) and C-O-S (800 cm-1 to 820 cm-1) formed at the wheat straw treated by sulfur trioxide at 60 °C for 1 h, and they inferred a reasonable pretreatment mechanism where the chemical bonds will be washed by alkali liquor (2016). The pretreatment samples mentioned above were finally pretreated by dilute alkali or water washing, so the final FT-IR spectra were nearly identical. The main difference is that the CS-C has a peak at 1736 cm-1, which may be attributed to unconjugated ketone carbonyls groups or conjugated acids/esters (Wayman and Chua 1979).
Table 2. The Main Functional Groups of the CS-C and CS-H Samples
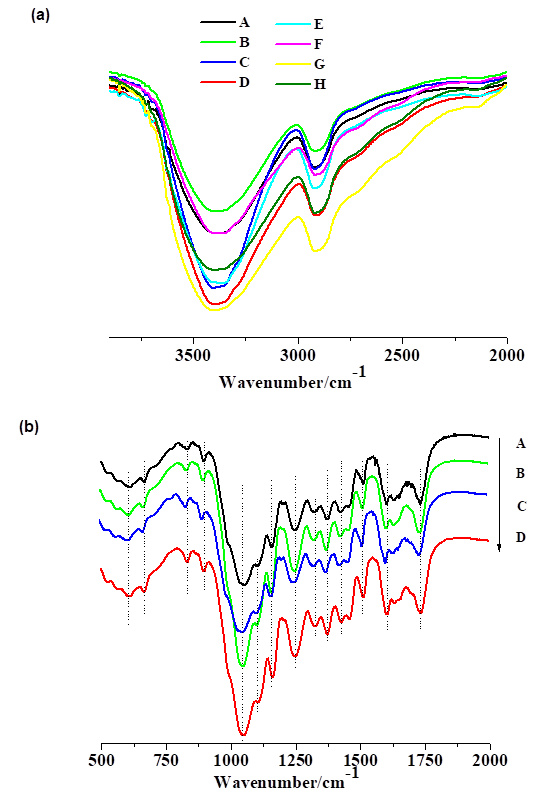
Fig. 4. FT-IR of (a) different CS materials in the wavenumber range from 2000 cm-1 to 3800 cm-1, (b) CS-C materials in the wavenumber range from 500 cm-1 to 2000 cm-1, and (c) CS-H materials in the wavenumber range from 500 cm-1 to 2000 cm-1. The samples within the panels are labeled as follows: A, original CS-C; B, CS-C after waters pretreatment; C, CS-C after dilute alkali pretreatment; D, CS-C after STEX-DA pretreatment; E, original CS-H; F, CS-H after waters pretreatment; G, CS-H after dilute alkali pretreatment; H, CS-H after STEX-DA pretreatment.
Table 3. The Carbon, Hydrogen, and Sulfur Contents of Different CS-C and CS-H Samples
Elemental Analysis
Although similar in composition and functional groups, the elemental analysis results in Table 3 show some differences of the carbon and hydrogen content between the cellulose and lignin. The ratio of C%/H% was calculated to show that lignocellulose contained different structures of cellulose and hemicellulose, which could be calculated as cellulose (6.97), hemicellulose (6.59), and lignin (9.62), as shown in Table 3. The C/H ratio values of the cellulose and hemicellulose compounds were less than 7, while the ratio of lignin was greater than 9. By the means of element composition analysis to reflect the changes of lignin content. At the same time, it was a better method to demonstrate the necessity of pretreatment, due to the lower C/H ratio and the higher calorific value for the energy fuel. Additionally, there was no sulfur in the holocellulose from the STEX-DA pretreatment. This result indicated that there was no inhibiting matter (such as sulfide or sulfuric acid) following fermentation via STEX-DA pretreatment.
Crystallinity of Pretreated CS
Among the various structural features affecting biomass enzymatic digestibility, cellulose crystallinity influences scarification kinetics and yields. To gain insight into the possible crystallinity affecting the CS biomass hydrolysis, the structure of native and pretreated CS was examined by XRD, as shown in Fig. 5a and Fig. 5b, respectively. The peaks at 2θ = 15° and 16.5° merged into a broad band, which was consistent with the literature data. The most prominent peak was 2θ = 22.16°, and it was used in the evaluations. This peak was assigned to the (200) plane, while 2θ = 18° was assigned to the (Am) plane.
Fig. 5a. XRD of (a) CS-C, (b) CS-H. The samples within the panels are labeled as follows: A, original CS-C; B, CS-C after waters pretreatment; C, CS-C after dilute alkali pretreatment; D, CS-C after STEX-DA pretreatment; E, original CS-H; F, CS-H after waters pretreatment; G, CS-H after dilute alkali pretreatment; H, CS-H after STEX-DA pretreatment.
Fig. 5b. XRD of (a) CS-C, (b) CS-H. The samples within the panels are labeled as follows: A, original CS-C; B, CS-C after waters pretreatment; C, CS-C after dilute alkali pretreatment; D, CS-C after STEX-DA pretreatment; E, original CS-H; F, CS-H after waters pretreatment; G, CS-H after dilute alkali pretreatment; H, CS-H after STEX-DA pretreatment.
The crystallinity values calculated by the empirical formula are listed in Table 3. CS-C and CS-H had the same changes after the pretreatment, in which dilute alkali treatment (62.6%/70.2%) was greater than STEX-DA (59.6%/68.9%), and original corn stalks was the lowest (46.8%/55.6%). With continued pretreatment, the degree of crystallinity greatly increased after dilute alkali treatment or STEX-DA pretreatment. The main reason is that the percentage of cellulose in the product increases after the removal of lignin and hemicellulose by the alkaline treatment with lye (Kim and Lee 2005; Bak et al. 2009a; Bak et al. 2009b). However, CS had a certain reduction after STEX-DA pretreatment compared to dilute alkali treatment. One reason was that that SO3 could react with the crystal water of CS to form micro-thermal explosions and transform into sulfuric acid. The other reason was that the micro-thermal explosions could destroy the crystallinity of cellulose from CS. While the degree of these impacts was less than the alkaline washing operation, they eventually led to a slight decline in crystallinity.
Reducing Sugars Yield of Pretreated CS
Reducing sugars yields (calculated by equation YRS) of pretreated CS are shown in Fig. 6a and Fig. 6b, which were used to represent the utilization of CS. The saccharification curve of the original CS was not listed, mainly due to the presence of a large amount of hydrolyzed reducing sugar, which prevented the comparison of different pretreatment effects. Water pretreatment of CS-C and CS-H was used as a blank control to reflect the effect of DA pretreatment and STEX-DA pretreatment for reducing sugars yield.
Fig. 6. Reducing sugars yield profiles for different pretreatment CS-C and CS-H at an enzyme loading of 15FPU (a) and 30 FPU(b) per gram substrate The samples within the panels are labeled as follows: B, CS-C after waters pretreatment; C, CS-C after dilute alkali pretreatment; D, CS-C after STEX-DA pretreatment; F, CS-H after waters pretreatment; G, CS-H after dilute alkali pretreatment; H, CS-H after STEX-DA pretreatment.
Reducing sugars yield were greatly increased after pretreatment, especially STEX-DA pretreatment, which had the highest curve. The reasons were that the CS treated by STEX could react with the lignin to increase the removal rate by following alkali treatment, and the micro-thermal explosion could generate some holes or concavities to facilitate the penetration of cellulase and enhance the enzymatic hydrolysis efficiency. At the same time, whichever cellulase loading, CS-C has a higher reducing sugars yield than CS-H, since its low lignin content, low crystallinity, and loose structure are conducive to enzymatic hydrolysis (Li et al. 2014; Meng and Ragauskas 2014). From Table 4, it can be seen that corncobs and cornhusks had higher levels of total sugar conversion than the lye pretreatment method that has been reported, and have an absolute advantage in conversion rate, which can reach a more stable value in 24 h.
To evaluate cellulose loading on the enzymatic scarification, a doubled amount of cellulase loading (30 FPU/g) was tested for comparison. As shown in Fig. 6a and Fig. 6b, the initial rate of hydrolysis (after 24 h) was about 1.7-fold faster than those groups with 15 FPU/g cellulase; higher lignin and large crystallinity inhibited the efficiency of enzymatic hydrolysis. By increasing the amount of enzyme, the rate of change was obviously increased in 24 h. In addition, after loading double the amount of cellulase, the reducing sugars yield for CS-H was 60.3%, which was 1.5 times greater than the result from using 15 FPU/g cellulase, while CS-C was only 1.2 times greater. This result indicated that CS-H had a stronger dependency on the enzyme loading compared to CS-C.
Table 4. Operating Conditions of Alkali Pretreatment and its Effectiveness
CONCLUSIONS
- Pretreatment of corn stover with sulfur trioxide micro-thermal explosion and dilute alkali (STEX-DA) was achieved at atmospheric pressure and room temperature conditions.
- STEX-DA pretreatment removed 57.0% of lignin in corn stover, and the length of the corn stover was about 2 cm to 3 cm without crushing.
- The structures of husks and cores of CS were different after pretreatment, as determined by SEM, FT-IR, and XRD.
- Corn stover was separated into CS-H and CS-C, which had the same lignin removal rate (about 65%), but the reducing sugars yield of CS-C was much higher than that of CS-H.
- CS-H had a stronger dependency on the enzyme loading than CS-C.
ACKNOWLEDGMENTS
This work was supported by the Program for The National 863 Program of China (Grant No. SS2014AA021902) and Anhui Province science and technology research major projects (Grant No. 1301032144). The authors are grateful to the Analysis and Test Center of Hefei University of Technology.
REFERENCES CITED
Bak, J. S., Ko, J. K., Choi, I.-G., Park, Y.-C., Seo, J.-H., and Kim, K. H. (2009a). “Fungal pretreatment of lignocellulose by Phanerochaete chrysosporium to produce ethanol from rice straw,” Biotechnol. Bioeng. 104(3), 471-482. DOI: 10.1002/bit.22423
Bak, J. S., Ko, J. K., Han, Y. H., Lee, B. C., Choi, I.-G., and Kim, K. H. (2009b). “Improved enzymatic hydrolysis yield of rice straw using electron beam irradiation pretreatment,” Bioresource Technol. 100(3), 1285-1290. DOI: 10.1016/j.biortech.2008.09.010
Chen, J., Zhang, W., Zhang, H., Zhang, Q., and Huang, H. (2014). “Screw extrude steam explosion: A promising pretreatment of corn stover to enhance enzymatic hydrolysis,” Bioresource Technol. 161, 230-235. DOI: 10.1016/j.biortech.2014.02.043
Chen, M., Zhao, J., and Xia, L.-M. (2009). “Comparison of four different chemical pretreatments of corn stover for enhancing enzymatic digestibility,” Biomass & Bioenergy. 33(10), 1381-1385. DOI: 10.1016/j.biombioe.2009.05.025
Dogaris, I., Karapati, S., Mamma, D., Kalogeris, E., and Kekos, D. (2009). “Hydrothermal processing and enzymatic hydrolysis of sorghum bagasse for fermentable carbohydrates production,” Bioresource Technol. 100(24), 6543-6549. DOI: 10.1016/j.biortech.2009.07.046
Kaparaju, P., and Felby, C. (2010). “Characterization of lignin during oxidative and hydrothermal pre-treatment processes of wheat straw and corn stover,” Bioresource Technol. 101(9), 3175-3181. DOI: 10.1016/j.biortech.2009.12.008
Kim, S. M., Dien, B. S., Tumbleson, M. E., Rausch, K. D., and Singh, V. (2016). “Improvement of sugar yields from corn stover using sequential hot water pretreatment and disk milling,” Bioresource Technol. 216, 706-713. DOI: 10.1016/j.biortech.2016.06.003
Kim, T. H., and Lee, Y. (2005). “Pretreatment of corn stover by aqueous ammonia,” Appl. Biochem. Biotech. 124(1-3), 1119-1131. DOI: 10.1385/abab:124:1-3:1119
Li, Q., Gao, Y., Wang, H., Li, B., Liu, C., Yu, G., and Mu, X. (2012a). “Comparison of different alkali-based pretreatments of corn stover for improving enzymatic saccharification,” Bioresource Technol. 125, 193-199. DOI: 10.1016/j.biortech.2012.08.095
Li, F., Yao, R., Wang, H., Hu, H., and Zhang, R. (2012b). “Process optimization for sugars production from rice straw via pretreatment with sulfur trioxide micro-thermal explosion,” BioResources 7(3), 3355-3366. DOI: 10.15376/biores.7.3.3355-3366
Li, H., Pu, Y., Kumar, R., Ragauskas, A. J., and Wyman, C. E. (2014). “Investigation of lignin deposition on cellulose during hydrothermal pretreatment, its effect on cellulose hydrolysis, and underlying mechanisms,” Biotechnol. Bioeng. 111(3), 485-492. DOI: 10.1002/bit.25108
Meng, X., and Ragauskas, A. J. (2014). “Recent advances in understanding the role of cellulose accessibility in enzymatic hydrolysis of lignocellulosic substrates,” Curr. Opin. Biotech. 27, 150-158. DOI: 10.1016/j.copbio.2014.01.014
Ouyang, J., Li, X., Dong Z.-W., and Lian, Z.-N. (2010). “Study on preparation of sugar by alkali pretreatment and enzymatic hydrolysis of corn stalk,” Journal of Nanjing Forestry University 34(3), 1-5. DOI: 10.3969/j.issn.1000-2006.2010.03.001
Popescu, C.-M., Popescu, M.-C., Singurel, G., Vasile, C., Argyropoulos, D. S., and Willfor, S. (2007). “Spectral characterization of eucalyptus wood,” Appl. Spectrosc. 61(11), 1168-1177. DOI: 10.1366/000370207782597076
Saha, B. C., Qureshi, N., Kennedy, G. J., and Cotta, M. A. (2016). “Biological pretreatment of corn stover with white-rot fungus for improved enzymatic hydrolysis,” Int. Biodeter. Biodegr. 109, 29-35. DOI: 10.1016/j.ibiod.2015.12.020
Sahu, R. C., Patel, R., and Ray, B. C. (2011). “Removal of hydrogen sulfide using red mud at ambient conditions,” Fuel Process. Technol. 92(8), 1587-1592. DOI: 10.1016/j.fuproc.2011.04.002
Segal, L., Creely, J., Martin Jr, A., and Conrad, C. (1959). “An empirical method for estimating the degree of crystallinity of native cellulose using the x-ray diffractometer,” Text. Res. J. 29(10), 786-794. DOI: 10.1177/004051755902901003
Slade, R., Bauen, A., and Gross, R. (2014). “Global bioenergy resources,” Nat. Clim. Change 4(2), 99-105. DOI: 10.1038/nclimate2097
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., and Crocker, D. (2008). Determination of Structural Carbohydrates and Lignin in Biomass (NREL/TP-510-42618), National Renewable Energy Laboratory, Golden, CO.
Sun, Y., and Cheng, J. (2002). “Hydrolysis of lignocellulosic materials for ethanol production: A review,” Bioresource Technol. 83(1), 1-11. DOI: 10.1016/s0960-8524(01)00212-7
Taherzadeh, M. J., and Karimi, K. (2008). “Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review,” Int. J. Mol. Sci. 9(9), 1621-1651. DOI: 10.3390/ijms9091621
Tang, C., Shan, J., Chen, Y., Zhong, L., Shen, T., Zhu, C., and Ying, H. (2017). “Organic amine catalytic organosolv pretreatment of corn stover for enzymatic saccharification and high-quality lignin,” Bioresource Technol. 232, 222-228. DOI: 10.1016/j.biortech.2017.02.041
Wang, H., Pan, C.-Y., Xu, F., Liu, L.-J., and Yao, R.-S. (2016). “Enhanced saccharification for wheat straw with micro-thermal explosion technology of in situ SO3 reaction,” Chem. Eng. J. 286, 394-399. DOI: 10.1016/j.cej.2015.11.006
Wayman, M., and Chua, M. G. S. (1979). “Characterization of autohydrolysis aspen (P. tremuloides) lignins. Part 4. Residual autohydrolysis lignin,” Can. J. Chem. 57(19), 2612-2616. DOI: 10.1139/v79-422
Yao, R.-S., Hu, H.-J., Deng, S.-S., Wang, H., and Zhu, H.-X. (2011). “Structure and saccharification of rice straw pretreated with sulfur trioxide micro-thermal explosion collaborative dilutes alkali,” Bioresource Technol. 102(10), 6340-6343. DOI: 10.1016/j.biortech.2011.02.073
Yao R.-S., and Li, F.-H. (2013). “Sulfur trioxide micro-thermal explosion for rice straw pretreatment,” in: Cellulose – Biomass Conversion, T. van de Ven and J. Kadla (eds.), IN TECH Press, Rijeka, Croatia. DOI: 10.5772/50428
Zhang, J., Wang, X., Chu, D., He, Y., and Bao, J. (2011). “Dry pretreatment of lignocellulose with extremely low steam and water usage for bioethanol production,” Bioresource Technol. 102(6), 4480-4488. DOI: 10.1016/j.biortech.2011.01.005
Article submitted: March 30, 2018; Peer review completed: May 5, 2018; Revised version received: May 16, 2018; Accepted: May 17, 2018; Published: May 22, 2018.
DOI: 10.15376/biores.13.3.5271-5288
