Abstract
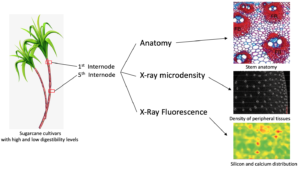
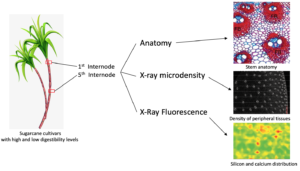
Sugarcane is widely used as feed for cattle, buffalo, goats, and sheep, primarily during drought periods. Some sugarcane cultivars contain low digestibility fibers, which compromises animal performance. Thus, the present study reports on anatomical, chemical, and elemental analysis along stem internodes of two sugarcane cultivars to better understand the structure-digestibility relationship of industrial cultivar cv. IACSP95-5000 compared to a forage cultivar (cv. IAC86-2480). X-ray microdensitometry assays revealed that the peripheral tissues of IACSP95-5000 were denser than IAC86-2480. In the first internode, cv. IACSP95-5000 has more vascular bundles and occupy a larger area. In addition, it had more fibers surrounding the vascular bundles compared to cv. IAC86-2480. However, fibers are prominent at the fifth internode in both cultivars but are more evident in cv. IACSP95-5000. The microprobe X-ray fluorescence spectroscopy analysis showed that silicon and calcium elemental distribution were similar for both cultivars. The structural features of the forage sugarcane presented herein are able to explain the digestibility differences between cultivars.
Download PDF
Full Article
Sugarcane as a Forage Plant: Structural and Chemical Traits that Affect Fiber Quality
João Paulo Rodrigues Marques,a,†,* Gabriela Aferri,b Gabriel Sgabieiro Montanha,a Fernanda Trilstz Perassolo Guedes,c Marli Misaki Soares,d Larissa Fernando Muniz,d Mario Tomazello Filho,c Mauro Alexandre Xavier,e and Hudson Wallace Pereira de Carvalho a
Sugarcane is widely used as feed for cattle, buffalo, goats, and sheep, primarily during drought periods. Some sugarcane cultivars contain low digestibility fibers, which compromises animal performance. Thus, the present study reports on anatomical, chemical, and elemental analysis along stem internodes of two sugarcane cultivars to better understand the structure-digestibility relationship of industrial cultivar cv. IACSP95-5000 compared to a forage cultivar (cv. IAC86-2480). X-ray microdensitometry assays revealed that the peripheral tissues of IACSP95-5000 were denser than IAC86-2480. In the first internode, cv. IACSP95-5000 has more vascular bundles and occupy a larger area. In addition, it had more fibers surrounding the vascular bundles compared to cv. IAC86-2480. However, fibers are prominent at the fifth internode in both cultivars but are more evident in cv. IACSP95-5000. The microprobe X-ray fluorescence spectroscopy analysis showed that silicon and calcium elemental distribution were similar for both cultivars. The structural features of the forage sugarcane presented herein are able to explain the digestibility differences between cultivars.
Keywords: Fibers; Plant anatomy; Microdensity; Microscopy; Saccharum spp.; Structural marker; X-ray; XRF
Contact information: a: University of São Paulo/CENA, DVTEC, Center for Nuclear Energy in Agriculture, Piracicaba, São Paulo 13400-970 Brazil; b: Animal Science Institute, SAA-SP, IZ, Nova Odessa, São Paulo 13380-011 Brazil; c: University of São Paulo, Luiz de Queiroz College of Agriculture, Department of Forest Sciences, Piracicaba, São Paulo 13418–260 Brazil; d: University of São Paulo, Luiz de Queiroz College of Agriculture, Department of Biological Sciences, Piracicaba, São Paulo 13418–260 Brazil; e: Agronomic Institute, SAA-SP, IAC, Ribeirão Preto, São Paulo 14001-970 Brazil;
†, Current Address: Section of Plant Biology, Institute of Biosciences, São Paulo State University (UNESP), Botucatu 18618-689, Brazil; *Corresponding author: joaoanatomia@gmail.com
GRAPHICAL ABSTRACT
INTRODUCTION
Sugarcane is an agricultural crop of great importance for the economy of Brazil, which stands out as the largest sugarcane producer in the world. For the 2020 to 2021 harvest, sugar and ethanol production is estimated at 642.1 million tons (CONAB 2020). Sugarcane benefits not only humans, but also animals, as it is used as feed for beef cattle, dairy cows, sheep, goats, buffalo, etc., primarily as source of bulk to feed during seasonal forage scarcity and normally corrected for protein content (Amorim et al. 2017). As such, approximately 4.2 million tons are currently used for animal feed in the state of São Paulo (IEA 2019). The sugar cane utilized for animal feeding represents approximately 10% of the total production of the sugarcane crops for industry. This meets the demand for bulk sources for animal feeding, which may favor sugarcane use (Marengo 2014), without causing problems to animal health, when used with other feeds in a balanced diet (Sousa et al. 2017). In general, whole cane has around 30% dry matter and approximately 20% of the juice are sugars.
One of the primary factors regulating the consumption in ruminants is the fiber contents and quality in the diet (Ellis 1978; Berchielli et al. 2006). When cv. IAC86-2480 was used to feed lambs, in a diet with 60% whole crushed sugarcane plus 40% concentrate, the results indicated that other genotypes should be evaluated, due to the difference that occurs in animal performance with different cultivars, which have similar neutral detergent fibers (NDF) and acid detergent fibers (FDA) (Aferri et al. 2015). This indicates that auxiliary methods for bromatological analysis should be developed to assist the identification of improved cultivars for animal feed.
The sugarcane plant has different cell types with structural and ultrastructural characteristics that may be associated with a lower digestibility rate. With the exception of sugarcane mesophyll, all the leaf and stem tissues have lignified cells. In addition, the epidermis of the leaves and stems may have lignified, siliceous, and suberous cells (Sakai and Sanford 1984). Silicon and suberin are important reducers in the rumen microbiota capacity to access cellulose and hemicellulose, therefore reducing their digestibility (Smith and Nelson 1975; Shewmaker et al. 1989; Figueiredo et al. 2016). Silicon accumulation has already been observed in the stoma guard cells, trichomes, siliceous cells, in the walls of long epidermal cells, and in the intercellular spaces between sub-epidermal cells (Sakai and Sanford1984; Evert 2006).
The primary inhibitory mechanism for cell wall degradation refers to the chemical and physical impediment of access to the reaction center of potentially digestible constituents, e.g., hemicellulose and cellulose, which reduces forage digestibility. The principal chemical component to the indigestible fraction of the cell wall is lignin (Berchielli et al. 2006). Forages contain a 3% to 20% lignin content, depending on their maturation stage (Paciullo et al. 2002). Inside the rumen, a rich diversity of microorganisms participates in the cell wall degradation process. Fungi, protozoa, and bacteria act in the degradation of cell walls, obeying a sequence of softer tissues, such as the fundamental parenchyma of the leaf and stem of grasses, and later more lignified tissues (Akin 1989).
Therefore, structural studies are necessary to better understand how stem tissue anatomy and composition influence digestibility. Hence, in this study the authors investigated the anatomical and physical features, as well as describe the distribution of chemical element in the stems, of two cultivars of Saccharum spp. and tried to correlate these features to cultivars with more digestible traits.
EXPERIMENTAL
Plant Material
The field phase was conducted at the Jaú Research and Development Unit of the Agronomic Institute (22º17’S latitude and 48º34’W longitude). The region is 580 m above sea level and the climate is Cfa subtropical humid, i.e., hot, rainy summers and dry winters (Köppen classification). Cultivars IAC86-2480 (feed use) and IACSP95-5000 (industry use) were cultivated using the pre-sprouted seedling system in two experimental plots composed of eight plants for each cultivar. Representative samples of the stems were collected from the tussocks. The soil preparation and correction procedures, as well as fertilization, were performed according to the proposal by Raij et al. (1997) and Maria et al. (2016). Planting occurred in May and irrigation was used to establish the crop. The basal, median, and apical portions (including leaves) of the shoots were collected 10 months after planting, representing the initial phase of the sugarcane use cycle for animal feeding.
The stems were separated from the plants and subjected to bromatological analysis, structural analysis via light microscopy, X-ray density measurements, and elemental distribution assessment via microprobe X-ray fluorescence spectroscopy (µ-XRF). The 1st and 5th internodes were collected, just below the old and basal chlorophylled leaf, and the median internode region was used to perform cross sections.
The experiment, included the harvesting and the experimental data, was repeated two times.
Biochemical Analysis
Whole stalks were harvested and chopped using a forage chipper. Representative samples were oven dried with forced ventilation at a temperature of 55 °C to determine the dry matter (DM) content. Subsequently, they were ground in a Willey mill, with 2 mm porosity sieves, for bromatological determinations of the fiber fraction. The cultivars were evaluated for their neutral detergent fiber, acid detergent fiber, hemicellulose, cellulose, and lignin contents, according to the method described in Soest et al. (1991).
Light Microscopy
The median internode region was sectioned longitudinally into four parts, which were immersed in an FAA fixative solution (Formaldehyde 37%–90:5:5, glacial acetic acid, and ethyl alcohol 70%) and immediately subjected to vacuum for better fixation (Johansen 1940). The 1st and 5th internodes below the last chlorophylled leaf were collected, divided into four longitudinal parts, and then fixed. After 1 week in the fixative solution, the samples were washed in 70% ethanol 3 times for 10 min each, and then sectioned into 20 µm thick pieces in the slide microtome (Leica MS 2000R). The cuts were placed in watch glasses with distilled water. Afterwards, the tissues were cleared by immersion in NaOH 5% in water for 1 h, washed (3 times for 1 min each) in distilled water and stained with 1% safranin in water for 5 min, and then washed in distilled water and stained with 0.5% Astra Blue in water for 3 min. The excess dye was removed by two washes in distilled water. The slides were mounted on glycerin gelatin (Kaiser 1890). The slides were analyzed using a Leica IM50 microscope (LEICA, Wetzlar, Germany).
Chemical Imaging via Microprobe X-ray Fluorescence Spectroscopy (µ-XRF)
The sugarcane stems were longitudinally cut using a razor blade and subsequently dried in a laboratory oven at a temperature of 60 °C for 24 h. The chemical images were acquired through microprobe X-ray fluorescence spectroscopy (Orbis PC, EDAX, Mahwah, NJ, USA). The µ-XRF samples were fixed using a Kapton® tape on a 6 µm thin polypropylene film previously laid in an acrylic sample holder and then loaded into the µ-XRF. The samples were investigated using X-rays generated by an Rh anode operating at 15 kV and 500 µA and focused by a polycapillary 30 µm beam under a vacuum (greater than 73.3 Pa) atmosphere. The chemical images were recorded using a 32 pixel x25 pixelmatrix. The dwell time was 1.5 s per pixel, the X-ray spectra were acquired via a silicon drift detector (SDD), and the dead time was smaller than 8%.
Density Measurement
To analyze the tissue density, the sugarcane internodes were fixed in the FAA and then 3 mm thick sections were created using a metallographic cutter adapted for histological cuts. The section was kept on a Petri dish with 70% ethanol. Then, the internodes were dehydrated with 100% ethanol and then dried to the CO2 critical point in an EM CPD 300 (LEICA, Wetzlar, Germany). The critical point drier was used to avoid the shrinking of the sugarcane sections during the X-ray analysis. Digital radiographic images of the sugarcane sections were obtained using LX-60 Faxitron digital X-ray equipment, followed by calibration and automatic reading within the range of 30 kV for a maximum time of 19 s. The sugarcane digital radiographs were then analyzed using the WindendroTM software (WinDENDRO version 2019a, Quebec, Canada) to obtain the microdensitometric profiles.
Statistical Analyses
Quantitative data regarding the anatomical traits were collected from ten replicates. The microdensity analyses had five replicates, while the chemical assays were obtained with three replicates. The data were drawn from a normally distributed population, i.e., the number and area of vascular bundles, and the fiber area surrounding them, under a Ryan-Joiner Test (p-value greater than 0.01) were compared through an analysis of variance (ANOVA) followed by Tukey’s posthoc test at a 0.05 significance level. Furthermore, Pearson’s product-moment correlation coefficient (R) was employed to investigate the correlation between the Si and Ca spatial distribution. All statistical tests were carried out on Minitab (version 19.2.0.0, LCC, State College, USA) and Origin (version 2021, OriginLab Corp., Northampton, USA) software.
RESULTS AND DISCUSSION
Biochemical Data of the Fibers
The fibrous fraction is composed of fibrous carbohydrates, i.e., cellulose, hemicellulose, and lignins, which are polymers that participate in the composition of the plant cell wall. The fibers from the sugarcane industry are interesting for energy cogeneration (the more the better), and the fibers from the foraged sugarcane are important for the digestibility of the diet (the fiber chemical composition is better). This difference is fundamental in evaluating cultivars for different uses, e.g., as animal feed (Aferri et al. 2015). The bromatological analysis of the sugarcane fiber fraction showed differences in the NDF, ADF, hemicellulose, and lignin contents.
Table 1. Characteristics of the Fiber Fraction of Two Sugarcane Cultivars as a Percentage of Dry Matter
In general, cv. IACSP95-5000 presented higher DM (4.45%), ADF (32%), NDF (27%), hemicellulose (20%), cellulose (23%), and lignin (139%) contents. This meant that cv. IACSP95-5000, i.e., industrial cultivar, contained more than twice the lignin content compared to cv. IAC86-2480, i.e., forage cultivar (Table 1). The way these compounds are arranged in plant tissues influences digestibility. The cross-arrangement of the bonds in polymers that form the vegetal fiber, as hemicellulose, for example, gives less resistance to the degradation of this carbohydrate in the rumen in comparison to cellulose. In sugarcane bagasse, the fibrous fraction is well organized with smooth, compact fibers and without rupture, due to the presence of the protective layer formed by lignins and hemicellulose (Assumpção et al. 2016). Therefore, the greater the presence of these components, the more difficult it is to digest the material because the presence of lignin in vegetal tissue impair fiber digestibility as a whole. Ash content was 1.74% and 1.46%, respectively for IAC84-2480 and IACSP95-5000. The cultivar IACSP95-5000 presented the highest ADF, NDF, hemicellulose, cellulose, and lignin contents. In general, the NDF from roughage has greater rumen repletion than that from non-roughage sources. The effect of the NDF content and the proportion of lignins on intake, digestibility of nutrients, and animal performance depends on the source used, which reflects the chemical and physical differences between these components (Oliveira et al. 2016).
Fig. 1. Density variation in the internodes between two different sugarcane cultivars; the density range in cv. IACSP95-5000 is higher than cv. IAC86-2480 (the red line indicates the density profile)
Structural Features of the Sugarcane
The degradation rate of the cell wall of forages by ruminants is largely determined by the capacity of the rumen biota to cross anatomical barriers. In this study, the authors found that cv. IACSP95-5000 has a wide range of chemical and structural factors that may be associated with low fiber digestibility (Akin1989; Queiroz et al. 2000; Brito et al. 2004; Valente et al. 2011).
As plant development is related to lignin deposition, the goal was to investigate whether there would be a difference between the oldest and youngest internodes in the same stem. Images of transversal sections of the stems (Fig. 1) showed that both cultivars were denser in the peripheral tissues in comparison to the central tissues. In general, the older internodes were denser than the young internodes. Cultivar IACSP95-5000 had a greater density index than cv. IAC86-2480. The maximum density reached 0.58 g·cm2 and 0.65 g·cm2 in the 1st and 5th internodes of cv. IACSP95-5000, respectively, and 0.44 g·cm2 and 0.52 g·cm2 in the 1st and 5th internodes of cv. IAC86-2480, respectively.
Fig. 2. The cross sections and quantitative analysis of the first internode of the two cultivars of Saccharum spp. with different bromatological fiber features (Note that cv. IACSP95-5000 has a larger number of vascular bundles as well as more fibers (walls stained red) and FB – Fiber; VB – Vascular Bundle)
The general anatomical analysis showed that the epidermis was unstratified and their cell walls were lignified. In addition, silicate crystals were observed. The 1st internode had vascular bundles evolved by fiber sheaths with sclerenchyma cells forming a ring around vascular bundles (Fig. 2a and 2b), constituting a fibrovascular region. The 1st internode (Fig. 2) showed that the vascular bundles from the stem periphery of cv. IACSP95-5000 were surrounded by a fiber sheath that enveloped the vascular bundle. The cv. IAC86-2480 showed the beginning of the thickening of the fibers cell walls surrounding the vascular bundle.
For both cultivars, the 5th internode (Fig. 3) fibers are distributed in an area surrounding the vascular tissues larger than in 1st internode. Figure 4 indicated the comparisons between the cultivars and showed that cv. IACSP95-5000 has more vascular bundles, which occupy a larger area, and has more fibers when compared to cv. IAC86-2480 for both the 1st and 5th internodes.
Fig. 3. The cross sections and quantitative analysis of the fifth internode of two Saccharum spp. with different bromatological fiber features (Note that cv. IACSP95-5000 has a larger number of vascular bundles as well as more fibers (walls stained red) and FB – Fiber; VB – Vascular Bundle)
The differences found in the fibrous fraction were also related to the genotypic characteristics of sugarcane. Bottcher (2013) observed that, in relation to the internode diameter, cv. IACSP04-065 (with a lower lignin content) demonstrated, on average, a larger diameter when compared to cv. IACSP04-627 (with a higher lignin content), which showed a greater internode length.
This structural feature of the stem reveals a link between the biometric and chemical features. The greater internode length is supported by the higher lignin concentration, as observed in the cultivar IACSP95-5000; while a decreasing lignin content is associated with a larger diameter, as observed in the cultivar IAC84-2480, more digestible due to the lower content of FDA, NDF and lignin. The physiological function is the same, conferring resistance to the stem; however, it can be achieved in different ways. This was observed in the analysis of internode density after the X-ray analysis, which demonstrated a greater density in the peripheral regions of the internodes studied. The authors observed that cv. IACSP95-5000 had higher densities compared to cv. IAC86-2480. This method showed an important way to determine the potential digestibility rate.
Cultivar IACSP95-5000 showed a greater number of bundles with more fibers when compared to cv. IAC86-2480. There was also lignin deposition in the parenchyma of cv. IACSP95-5000, which may be the determining factor of the higher lignin content, implying less digestibility. In addition, the anatomical feature of vascular bundles could be used as an indicator of genetic diversity for lignin deposition.
Fig. 4. Quantitative analysis of the number of bundles, vascular bundle area, and the fiber area surrounding the vascular bundles in both the first and fifth internodes of two cultivars (2480 and 5000) of Saccharum spp. (Note: statistical analysis was performed using one-way ANOVA with Tukey’s test (p-value is less than 0.05) and in each graph, the capital letters refer to the comparison between the cultivar within the same internode, whereas the lower case letters compare the effect of a single cultivar across different internodes)
Microprobe X-ray Fluorescence Spectroscopy Silicon and Calcium Assessment
Figure 4 shows the spatial distribution of Si and Ca at the sugarcane peripheral region of the 1st and 5th internodes. The count rate (cps) is directly proportional to the elemental concentration in the tissue. The cv. IAC86-2480 contained more Si in the younger internode (1323 cps to 3360 cps) than in the older internodes (948 cps to 2360 cps). The opposite was observed in cv. IACSP95-5000 (100 cps to 1900 cps in the young internodes and 1173 cps to 2960 cps in the old ones). The calcium distribution displayed the same pattern. Comparing the spatial distribution of Ca and Si, the authors observed that Si was distributed in spots of high cps that did not match with the Ca detection (as shown in Fig. 5). Figure 6 shows a heatmap of Pearson product-moment correlation coefficients between Si and Ca intensities. An absence of spatial association between these elements is shown. The XRF analysis showed the distribution of Si and Ca on the surface of the sugarcane stem, which are minerals also linked to the plant resistance factor (Meyer and Keeping 2000). In general, cv. IAC86-2480 showed a more homogeneous Si distribution in the tissues of the stem periphery, which could be a compensation mechanism in relation to the lignin content in order to build an external protection barrier for the stems. Therefore, the detection of Ca in the internode proved to be less effective in the discrimination of cultivars. However, the distribution of other elements in both cultivars is something to be further explored.
Fig. 5. Silicon and calcium elemental XRF volume maps made after analyzing the periphery of the Saccharum spp. cv. IAC86-2480 and cv. IACSP95-5000 (A, D, G, and J – Internode surface viewed under magnifying glass; B, E, H, and K – Ca Kα distribution map; and C, F, I, and L – Si Kα distribution map)
Fig. 6. Heatmaps of Person’s correlations coefficient for Ca and Si spatial distribution patterns obtained in the first and fifth internodes of cv. 2480 and cv. 5000of Saccharum spp through microprobe XRF on three independent biological replicates. The clustering bars indicate the correlations affinities amongst the internodes and the replicates.
CONCLUSIONS
- The fiber fraction in sugarcane cv. IACSP95-5000 had a high amount of lignins comparing to cv. IAC86-2480.
- The anatomical and the X-ray analysis confirmed structural changes in the density as well as the size and area of the sclerenchyma in the fibrous vascular bundles.
- No correlation between the Si and Ca in the sugarcane internode tissues was determined within this study.
- This integrative study demonstrated that the anatomy and the density of the sugarcane internodes can be considered as an important structural marker for qualifying the fiber fraction of sugarcane cultivars.
ACKNOWLEDGMENTS
This work was supported by the São Paulo Research Foundation (FAPESP) (Grant No. 2018/18851-0, 2015/05942-0, and 2015/19121-8) and FAPESP Young Investigators Award No. 2015/05942-0, and FAPESP Multiuser Equipment Program No. 2015/19121-8.The authors are grateful to the Laboratory of Electron Microscopy “Prof. Elliot Watanabe Kitajima” for the infrastructure for the light microscopy analysis. The authors also thank Prof. Dr. Beatriz Appezzato da Glória for allowing the usage of the sliding microtome.
REFERENCES CITED
Aferri, G., Campana, M. P., Anjos, I. A., Henrique, W., Xavier, M. A., Silva, D. N., Nascimento, J. P., and Landell, M. G. A. (2015). “Interferência de genótipos de cana-de-açúcar no desempenho de cordeiros em terminação [Interference of sugarcane genotypes on the performance of finishing lambs],” in: Proceedings of the XXV Brazilian Congress of Zootechny Zootec, 27-29 May, Fortaleza, Brazil.
Akin, D. E. (1989). “Histological and physical factors affecting digestibility of forages,” Agronomy Journal 81(1), 17-25. DOI:10.2134/agronj1989.00021962008100010004x
Amorim, D. S., Silva, A. L., Sousa, S. V. Sousa, P. H. A. A. d., Lima, B. S. L. d., and Reis Á. L. A. (2017). “Caracterização e restrições de forrageiras indicadas para as diferentes espécies de animais de produção [Characterization and restrictions of forage indicated for the different species of farm animals],” Revista Eletrônica Científica UERGS 3(1), 215-237. DOI: 10.21674/2448-0479.31.215-237
Assumpção, S. M. N. d., Pontes, L. A. M., Carvalho, L. S. d., Campos, L. M. A., Andrade, J. C. F. d., and Silva, E. G. d. (2016). “Pre-treatment combined H2SO4/H2O2/NaOH to obtain the lignocellulosic fractions of sugarcane bagasse,” Revista Virtual de Quimica 8(3), 8803-822. DOI: 10.5935/1984-6835.20160059
Berchielli, T. T., Pires, A. V., and Oliveira, S. G. (2006). Nutrição de Ruminantes [Ruminant Nutrition], Funep, Jaboticabal, Brazil.
Bottcher, A. (2013). Systematic Study of Lignin Deposition in Contrasting Sugarcane Genotypes, Ph.D. Dissertation, Universidade Estadual de Campinas, Campinas, Brazil.
Brito, C. J. F. A. d., Rodella, R. A., and Deschamps, F. C. (2004). “Quantitative anatomy of leaves and stems of Brachiaria brizantha (Hochst. ex A. Rich.) Stapf and B. humidicola (Rendle) Schweick,” Revista Brasileira de Zootecnia 33(3), 519-528. DOI: 10.1590/S1516-35982004000300001
CONAB (2020). “Acompanhamento da safra brasileira de cana-de-açúcar, v.2 – Safra 2020/21, n.2 – Segundo Levantamento [Monitoring of the Brazilian sugarcane harvest, v.2 – 2020/21 Harvest, n.2 – Second Survey],” (https://www.conab.gov.br/info-agro/safras/cana/boletim-da-safra-de-cana-de-acucar), Accessed 23 October 2020).
Ellis, W. C. (1978). “Determinants of grazed forage intake and digestibility,” Journal of Dairy Science 61, 327-336. DOI:10.3168/jds.S0022-0302(78)83809-0
Evert, R. F. (2006). Esau’s Plant Anatomy, Meristems, Cells, and Tissues of the Plant Body: their Structure, Function, and Development, John Wiley & Sons, Hoboken, NJ.
Figueiredo, R., Cesarino, I., and Mazzafera, P. (2016). “Suberin as an extra barrier to grass digestibility: A closer look to sugarcane forage,” Tropical Plant Biology 9, 96-108. DOI:10.1007/s12042-016-9166-3
IEA (2020). “Levantamento de área e produção dos principais produtos da agropecuária do estado de São Paulo [Survey of the area and production of the main agricultural products in the state of São Paulo],” (http://www.iea.agricultura.sp.gov.br/out/previsao.php), Accessed 7 November 2020.
Johansen, D. A. (1940). Plant Microtechnique, McGraw-Hill Publications, New York, NY.
Marengo, J. A. (2014). “O futuro clima do Brasil [Brazil’s future climate],” Revista USP 103, 25-32. DOI: 10.11606/issn.2316-9036.v0i103p25-32
Maria, I. C. D., Drugowich, M. I., Bortoletti, J. O., Vitti, A. C., Rossetto, R., Fontes, J. L., Tcatchenco, J., and Margatho, S. M. F. (2016). Recomendações Gerais Para a Conservação do Solo na Cultura da Cana-de-açúcar [General Recommendations for Soil Conservation in Sugarcane Culture] (Boletim Técnico IAC No. 216), Instituto Agronômico, Campinas, Brazil.
Meyer, J. H., and Keeping, M. G. (2000). “Review of research into the role of silicon for sugarcane production,” Proceeding of South African Sugarcane Technology Association 74, 29-40.
Oliveira, V. d. S., Neto, J. A. S., Valença, R. d. L., Silva, B. C. D. d., and Santos, A. C. P. d. (2016). “Carboidratos fibrosos e não fibrosos na dieta de ruminantes e seus efeitos sobre a microbiota ruminal [Fibrous and non-fibrous carbohydrates in the diet of ruminants and their effects on the ruminal microbiota],” Veterinária Notícias 22(2),1-18. DOI:10.14393/VTv22n2a2016.32660
Paciullo, D. S. C., Gomide, J. A., Silva, E. A. M. d., Queiroz, D. S., and Gomide, C. A. M. (2002). “Anatomical traits of leaf blade and stem of tropical forage grasses, according to level of insertion on the grass tiller, age and season of growth,” Revista Brasileira de Zootecnia 3(S2), 890-899. DOI: 10.1590/S1516-35982002000400012
Queiroz, D. S., Gomide, J. A., and Maria, J. (2000). “Evaluation of top and bottom leaf and stem fractions from tiller of three forages grasses: 1. In vitro disappearance and chemical composition,” Revista Brasileira de Zootecnia 29(1), 61-68. DOI: 10.1590/S1516-35982000000100008
Raij, B. v., Cantarella, H., Quaggio, J. A., and Furlani, A. M. C. (1997). Boletim Técnico 100 – Recomendações de Adubação e Calagem Para o Estado de São Paulo [Technical Bulletin 100 – Fertilization and Liming Recommendations for the State of São Paulo], Instituto Agronômico de Campinas (IAC), Campinas, Brazil.
Sakai, W. S., and Sanford, W. G. (1984). “A developmental study of silicification in the abaxial epidermal cells of sugarcane leaf blades using scanning electron microscopy and energy dispersive x-ray analysis,” American Journal of Botany 71(10), 1315-1322. DOI: 10.2307/2443698
Shewmaker, G. E., Mayland, H. F., Rosenau, R. C., and Asay, K. H. (1989). “Silicon in C-3 grasses: Effects on forage quality and sheep preference,” Journal of Range Management 42(2), 122-127. DOI: 10.2307/3899308
Smith, G. S., and Nelson A. B. (1975). “Effects of sodium silicate added to rumen cultures on forage digestion, with interactions of glucose, urea and minerals,” Journal of Animal Science 41(3), 891-899. DOI:10.2527/jas1975.413891x
Sousa, D. O., Mesquita, B. d. S., Pires, A. V., Santana, M. H. d. A, and Silva, L. F. P. (2017). “Effects of fiber digestibility and level of roughage on performance and rumen fermentation of finishing beef cattle,” Tropical Animal Health and Production 49, 1503-1510. DOI:10.1007/s11250-017-1353-1
Valente, T. N. P., Lima, E. d. S., Henriques, L.T. d., Machado-Neto, O. R., Gomes, D. I., Sampaio, C. B., and Costa, V. A. C. (2011). “Anatomy of forage plants and the availability of nutrients for animal ruminants,” Vetetinaria e Zootecia 18(3), 347-358.
Soest, P. J. V., Robertson, J. B., and Lewis, B. A. (1991). “Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition,” Journal of Dairy Science 74, 3583-3597. DOI: 3168/jds.S0022-0302(91)78551-2
Article submitted: May 19, 2021; Peer review completed: August 28, 2021; Revised version received: September 9, 2021; Accepted: September 20, 2021; Published: September 28, 2021.
DOI: 10.15376/biores.16.4.7623-7634
