Abstract
Sulfomethylation of radiata pine kraft lignin was performed using formaldehyde and sodium sulfite under alkaline conditions to determine its potential as a mineral depressant for the separation of molybdenite and chalcopyrite by froth flotation. Optimal conditions for the sulfomethylation reaction were 20% (w/w) Na2SO3 at 130 °C for 4.2 h, which resulted in lignin with a sulfonate content of 2.2 mmol/g. Microflotation assays showed that the optimized sulfomethylated kraft lignin (OSKL) depressed the molybdenite in flotation by 93% using 200 mg/L OSKL, while only 5% chalcopyrite depression was achieved. The performance of OSKL was compared with those of commercial lignosulfonates, and the OSKL displayed a better ability to separate molybdenite and chalcopyrite, even at lower concentration doses. FTIR and 1H-NMR analyses showed that sulfonic groups were incorporated into the C5 position of the aromatic ring and into the aliphatic chain of the OSKL. The hydroxymethyl content of the OSKL was increased, and most β-O-4′, β-1′ and β-5′ bonds were broken, with the exception of the β-β’ bond. Sulfomethylation gives kraft lignin the chemical characteristics of a wood-based molybdenite depressant, making it an alternative to current reagents used in the chalcopyrite-molybdenite flotation process.
Download PDF
Full Article
Sulfomethylation of Radiata Pine Kraft Lignin and Its Use as a Molybdenite Depressant in Selective Chalcopyrite-Molybdenite Separation by Flotation
Claudia Vidal,a,b Isabel Carrillo-Varela,a Pablo Reyes-Contreras,c Leopoldo Gutierrez,d,e and Regis Teixeira Mendonça a,b,*
Sulfomethylation of radiata pine kraft lignin was performed using formaldehyde and sodium sulfite under alkaline conditions to determine its potential as a mineral depressant for the separation of molybdenite and chalcopyrite by froth flotation. Optimal conditions for the sulfomethylation reaction were 20% (w/w) Na2SO3 at 130 °C for 4.2 h, which resulted in lignin with a sulfonate content of 2.2 mmol/g. Microflotation assays showed that the optimized sulfomethylated kraft lignin (OSKL) depressed the molybdenite in flotation by 93% using 200 mg/L OSKL, while only 5% chalcopyrite depression was achieved. The performance of OSKL was compared with those of commercial lignosulfonates, and the OSKL displayed a better ability to separate molybdenite and chalcopyrite, even at lower concentration doses. FTIR and 1H-NMR analyses showed that sulfonic groups were incorporated into the C5 position of the aromatic ring and into the aliphatic chain of the OSKL. The hydroxymethyl content of the OSKL was increased, and most β-O-4′, β-1′ and β-5′ bonds were broken, with the exception of the β-β’ bond. Sulfomethylation gives kraft lignin the chemical characteristics of a wood-based molybdenite depressant, making it an alternative to current reagents used in the chalcopyrite-molybdenite flotation process.
Keywords: Microflotation; Lignosulfonates; Sulfonation degree; Kraft lignin; Optimization; 1H NMR; FTIR
Contact information: a: Centro de Biotecnología, Universidad de Concepción, Concepción, Chile; b: Facultad de Ciencias Forestales, Universidad de Concepción, Concepción, Chile; c: Centro de Excelencia en Nanotecnología Leitat Chile (CEN), LEITAT Chile, Santiago; d: Departamento de Ingeniería Metalúrgica, Facultad de Ingeniería, Universidad de Concepción, Concepción, Chile; e: Water Research Center for Agriculture and Mining (CRHIAM), Universidad de Concepción, Chile; * Corresponding author: rteixeira@udec.cl
GRAPHICAL ABSTRACT
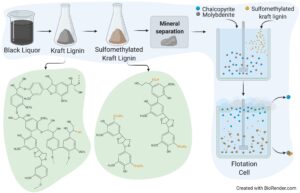
INTRODUCTION
Lignin is an amorphous, complex, and highly branched polyphenolic macro-molecule found in wood, annual plants, and agricultural residues, and it accounts for approximately 20 to 30% of the total biomass (Lange et al. 2013; Abdelaziz et al. 2016). Its cross-linked macromolecular network is polymerized by three precursors called monolignols: p-coumarilic alcohol (H unit), coniferyl alcohol (G unit), and sinapyl alcohol (S unit). These units differ in the number of methoxyl groups attached to the aromatic ring. The S unit has two methoxyl groups, the G unit has one methoxyl group, and the H unit has none. In particular, conifer species, such as pine, contain only G-type lignin (Li et al. 2015). The pulp and paper industries are the main source of lignin, with a current annual production of 70 million tons worldwide (Ahvazi et al. 2016; Bajwa et al. 2019). Lignins from kraft and sulfite pulp processes are the most abundant. However, lignin obtained from these processes undergoes significant structural changes and is not the same as the native molecules in wood (He and Fatehi 2015; Ahvazi et al. 2016; Bajwa et al. 2019).
Kraft lignin constitutes 85% of the total lignin produced worldwide and can be recovered from black liquor by acidification and precipitation, resulting in a low-molar mass and water-insoluble lignin with an increased amount of phenolic hydroxyl groups due to the cleavage of the β-aryl ether bonds (Sun et al. 2013; He and Fatehi 2015; Peretti et al. 2015). Kraft lignin is almost exclusively used as energy for pulp mills, but it can also be used as a dispersant and coagulant after chemical modifications (Pang et al. 2013; Ahvazi et al. 2016). However, the lignin in spent liquor from sulfite pulping, known as lignosulfonate (LS), is water soluble, has a high molar mass, and presents different amounts of sulfonic groups attached to the aliphatic chain, which facilitates its use in several applications without prior modification (He and Fatehi 2015; Peretti et al. 2015). LS can be used as binders, emulsion stabilizers, dispersants in oil drilling and coal-water slurry, paper coating, and cement admixtures (Ouyang et al. 2009; Yu et al. 2013; Ge et al. 2014; He and Fatehi 2015; Huang et al. 2018). Furthermore, because LS are strongly anionic polyelectrolytes that tend to interact with mineral surfaces, they have been investigated for mineral separation (Pradip and Fuerstenau 1991; Kelebek et al. 2001; Mu et al. 2016; Liu et al. 2018; Gutierrez et al. 2020). Sadowski (1995) used sodium LS for the selective separation of salt-like minerals (barite, calcite, dolomite, magnesite) in agglomeration with carbonate minerals, achieving the separation of barite by the addition of LS. The results suggested that the addition of sodium LS changes the hydrophobicity at the surface of the minerals. Ansari et al. (2007a, 2007b) demonstrated that it is possible to separate chalcopyrite from molybdenite using LS by depressing the molybdenite, indicating that electrostatic forces and chemical interactions between the anionic polyelectrolytes and metal-hydroxy sites on the mineral surfaces largely controlled the adsorption process.
The most important mineral byproduct from copper mining is molybdenum. To obtain copper and/or molybdenum, the recovery and separation of copper sulfides, such as chalcopyrite (CuFeS2), chalcocite (Cu2S), bornite (Cu5FeS4), and covellite (CuS), from molybdenite (MoS2) is needed; this recovery is performed through a two-stage flotation process (Ansari et al. 2007a,b; Gutierrez et al. 2020). In this process, sodium hydrosulfide (NaHS) is widely used for the selective separation of minerals, as its consumption could reach 20% of the total reagent consumption. As NaHS is a highly toxic reagent, there are environmental and economic incentives to use fewer chemicals in a more efficient process (Ansari et al. 2007a,b; Gutierrez et al. 2020). In this sense, the use of LS might represent an environmentally friendly alternative for mining activity; however, the limited worldwide production of LS represents a significant drawback (He and Fatehi 2015; Konduri and Fatehi 2015). Recently, Gutierrez et al. (2020) reported the separation of chalcopyrite and molybdenite by flotation using lignin derivatives, observing similar adsorption behavior among the different lignin types.
The sulfomethylation reaction of lignin involves the addition of a methylene sulfonate group (–CH2SO3) into the aromatic ring, i.e., the C5 position of G-type lignin. Briefly, the precursors formaldehyde (CH2O) and sodium sulfite (Na2SO3) under determined reaction parameters combine to form sodium hydroxymethyl sulfonate, the reactive species responsible for introducing the sulfonic group into lignin (Meister 2002; Konduri and Fatehi 2015; Huang et al. 2018; Kazzaz et al. 2019). However, due to the complex and highly branched polyphenolic structure of lignin along with the sulfomethylation reaction conditions, different degrees of sulfonation and structural changes of lignin can be promoted, which could affect the depression performance of the lignin-derived product. Accordingly, the main objective of this work was to investigate the sulfomethylation of radiata pine kraft lignin under different reaction conditions and determine the best conditions to depress molybdenite in microflotation assays. Hence, this work was divided into three sections: functionalization of kraft lignin under different conditions; microflotation assays of Mo with lignin derivatives; and chemical and structural characterization of lignin derivatives. The results addressed the ability of sulfomethylated kraft lignin to separate chalcopyrite and molybdenite by microflotation, which was compared to the separation ability of commercially available lignosulfonates.
EXPERIMENTAL
Kraft Lignin
Kraft black liquor from radiata pine (60% solid) was obtained from a cellulose pulp mill located in the Biobío Region (southern Chile). The liquor was diluted to 30% solid and acidified with H2SO4 at 60 °C until a pH of 3 was reached. The obtained suspension was allowed to decant for 24 h, and the precipitated lignin was recovered by vacuum filtration, washed thoroughly with tap water, and dried in a vacuum oven at 40 C for 48 h. The recovered lignin was stored in dry conditions and used for further chemical analysis and sulfomethylation procedures. Two commercial lignosulfonates were also used for comparison, sodium lignosulfonate (Na-LS, CAS number: 8061-51-6) and calcium lignosulfonate (Ca-LS, CAS number: 8061-52-7), both from Sigma-Aldrich (St. Louis, MO, USA).
Sulfomethylation of Kraft Lignin
Sulfomethylation of the radiata pine kraft lignin was conducted in a 1-L stainless steel reactor (Parr Instrument Co, Moline, IL, USA) in alkaline media. Solutions of sodium sulfite (Na2SO3 97%, Sigma-Aldrich) and formaldehyde (CH2O 37%, Sigma-Aldrich) were added. The Na2SO3/CH2O molar ratio was 0.7, and the solid:liquid ratio was 5:1. Different reaction conditions were evaluated according to a central composite factorial design. The parameters evaluated were reaction temperature (130 C to 160 C), reaction time (3 h to 6 h), and Na2SO3 dose (20% to 50% w/w of the dry weight of lignin), which were based on the conditions reported by Konduri and Fatehi (2015). After each reaction, the sulfomethylated lignin solution was recovered, cooled, and frozen. Once frozen, it was placed in a lyophilizer (ALPHA 2-4 LD plus, CHRIST, Osterode am Harz, Germany) to remove the aqueous phase. Finally, the modified lignin was recovered in powder form with a moisture content lower than 5%.
The experimental design was optimized to a defined response variable that was the ability to depress molybdenite in microflotation assays. The model was defined as a composite central factorial design. The variables were coded and normalized with unit values of -1 (defined as the lowest value) and +1 (defined as the highest value), and the focal point was defined as 0. The conditions for each experiment are described in Table 1. The influence of each variable was determined by response surface methodology using MODDE 7.0 software (Umetrics AB, Umeå, Sweden). Model equations included the first-order term to describe the main effects and the second-order term to describe the interactions. Variance analysis was used for the experimental results, and nonsignificant effects were excluded from the model regression. Contour diagrams from the model were used to define the optimal reaction conditions for molybdenite depression following the analytical procedures described by Norambuena et al. (2016). Pine kraft lignin was coded as KL, sulfomethylated kraft lignins were coded as SKL and sulfomethylated kraft lignin under optimized conditions was coded as OSKL.
Table 1. Experimental Design Used for the Sulfomethylation of Radiata Pine Kraft Lignin
Microflotation of Molybdenite and Chalcopyrite
Mineral sample preparation for microflotation procedures was detailed by Gutierrez et al. (2020). The concentration of chalcopyrite in the mineralized rock specimens was 98.9% with minor amounts of quartz (0.8%) and pyrite (0.3%). Molybdenite was purified from an industrial molybdenum concentrate to 99.1% with minor amounts of quartz (0.7%) and pyrite (0.2%). For the microflotation tests, chalcopyrite was ground to a particle size range of 75 to 50 μm, while the particle size of molybdenite ranged from 38 to 75 μm. The flotation of molybdenite and chalcopyrite by using lignin derivatives was evaluated through microflotation experiments, which were carried out in a 150 mL Partridge and Smith glass cell using 20 mL/min nitrogen (N2). Potassium amyl xanthate (PAX; Solvay, Santiago, Chile) was used as the collector, while methyl isobutyl carbinol (MIBC; Merck, Darmstadt, Germany) was used as the frother. All experiments were carried out at pH 9; when different pH conditions were previously evaluated (Gutierrez et al. 2020), it was observed that selective Cu-Mo separation using lignosulfonates and sulfomethylated kraft lignin as molybdenite depressant increased as the pH was increased.
Distilled water with an electrical conductivity 20 mS/cm was used in all the experiments, and the pH was adjusted to 9 using CaO and NaOH. All the experiments were performed in a 0.01 M NaCl solution using 25 ppm collector and 25 ppm MIBC. In the experiments, 1 g of the mineral (molybdenite or chalcopyrite) was first mixed in a beaker with 100 mL of solution for 5 min at pH 9. The lignin samples were added to the suspension and conditioned for an additional 10 min. Finally, the suspension was transferred to the microflotation glass cell, the nitrogen valve was opened, the flotation process was carried out for 2 min, and the froth was scraped off every 10 s. A constant pulp level in the microflotation cell was maintained by adding a background solution prepared at the same composition and pH of the original solution. Once the process was completed, the concentrate and tailings were filtered and dried in an oven at 100 °C for 1 h. The recovery was calculated as the mass of the concentrate divided by the mass of concentrate plus the mass of tailings. All tests were conducted in triplicate.
Elemental Analysis
The lignin samples were weighed in ceramic crucibles and then placed in a muffle furnace (NABERTHERM, N100/H), where they were converted to white or grayish ashes. The procedure was performed in two heating steps, the first at 380 °C for 120 min and the second at 525 °C for 180 min. After the reaction was completed, the sample was allowed to cool in a desiccator until a constant weight was reached. The ash content was determined by weight difference.
Degree of Sulfonation
The content of sulfonic groups (SO3-) was determined by conductometric titration (Stranel and Sebok 1999). To convert all the SO3– to HSO3– groups, 1 g of each sample was acidified by adding 0.1 M HCl until a pH of 5 was reached, and then 30 mL of the acidic lignosulfomethylated solution was titrated with 0.01 M NaOH. NaOH solution was added every 2 min, and the conductivity was recorded at each point by a conductivity meter (HI 2315, HANNA Co., Seoul, Korea). The obtained titration curves showed two points of change, where A indicated the neutralization of the free acid by NaOH, and B represented the titration of all the HSO3– groups. The content of sulfonic groups in each lignosulfomethylated group was calculated by Eq. 1,
where SO3– is the sulfonation degree (mmol/g), V (NaOH) is the volume (mL) of NaOH solution used for titration between points A and B, C (NaOH) is the concentration of NaOH solution (mmol/mL), and g is the dry mass of the sample used.
Molecular Weight Determination
Acetylation of the sulfomethylated lignin samples (30 mg) was conducted prior to the molecular weight determination by adding 4 mL of pyridine/anhydride acetic solution (1:1). Sulfomethylated lignin was also subjected to the same procedure, as it is soluble in THF (Konduri and Fatehi 2015). The reaction was left in the dark at room temperature, and after 10 h, 40 mL of distilled water was added. The mixture was centrifuged at 4000 rpm for 5 min, pyridine/anhydride acetic acid (supernatant) was removed, and the precipitate was vacuum-dried. The acetylated sample was dissolved in 2 mL of tetrahydrofuran (THF) and then filtered using 0.22 μm PTFE membranes for molecular weight determination using gel permeate chromatography (GPC, Shimadzu Co. Kyoto, Japan). The separation was carried out within Phenogel GPC columns (Phenomenex, Torrance, CA) filled with cross-linked polystyrene-divinylbenzene (PSDVB). Two 300 x 7.8 mm Phenogel columns (Phenogel 5u 10E3A and Phenogel 5u 10E6A) and a Phenogel 5u guard column were used. THF was used as the mobile phase at a flow rate of 1 mL/min. The fractionated sample in the column eluent was detected using a UV-VIS detector at 280 nm. The weight average molecular weight (Mw), number average (Mn), and polydispersity (Mw/Mn) were obtained after calibration with 10 polystyrene standards (GPC/SEC calibration kits).
Fourier Transform Infrared (FTIR) Spectrometry
The spectra were obtained on a PerkinElmer FTIR spectrometer (PerkinElmer, Inc., Waltham, MA, USA). The samples were dispersed in a matrix of KBr and pressed to form pellets. The sample collection was obtained in the range of 4000 to 400 cm-1 at a resolution of 4 cm-1 using ACD/Spectrus Processor software (ACD/Labs, Toronto, ON, Canada). The spectra were baseline-corrected and normalized at 1510 cm-1, the wavenumber shift that is associated with C=C stretching of the aromatic ring (G) in lignin (Faix 1991).
1H Nuclear Magnetic Resonance (NMR) Spectroscopy
Each sample was dissolved in deuterated dimethyl sulfoxide (DMSO-d6), as described by Qin et al. (2015). 1H NMR spectra were recorded on a Bruker 400 MHz NMR spectrometer (Karlsruhe, Germany) in the range of 0 to 10 ppm. The central solvent (DMSO-d6) peak was used as an internal chemical shift reference point. The obtained spectra were analyzed using ACD/Spectrus Processor software (ACD/LABS, Canada).
RESULTS AND DISCUSSION
Kraft Lignin
The elemental composition of the kraft lignin obtained from the black liquor of radiata pine pulping showed a CHO content commonly observed in kraft lignins (Table 2). The content of carbon predominated over that of the other elements (52.5% C), while the N, S, and ash contents remained relatively low (Cardoso et al. 2009; Hu et al. 2016; Zhang et al. 2018; Haz et al. 2019). Because Na2S was added during the kraft pulping procedure, a determined amount of sulfur was expected in the elemental composition of kraft lignins (Cardoso et al. 2009). The precipitated kraft lignin had a weight average molecular weight, Mw, of ~1670 g/mol, a number average molecular weight, Mn, of ~870 g/mol, and polydispersity (Mw/Mn) of 1.9 (Table 2).
Table 2. Elemental Analysis, Molecular Weight and Sulfonation Degree of Kraft Lignin, Commercial Lignosulfonates, and Sulfomethylated Kraft Lignin under Optimal Reaction Conditions
During kraft pulping, hydroxide and hydrosulfide ions act together to accelerate lignin degradation and dissociation, reducing its molecular mass (Cardoso et al. 2009). Thus, the molecular weight of kraft lignins can vary widely (García et al. 2009; Rashid et al. 2016; Jiang et al. 2017; Haz et al. 2019). The low polydispersity confirms the uniform character of the kraft lignin obtained through precipitation with H2SO4 (Zhou and Lu 2014).
Sulfomethylation of Radiata Pine Kraft Lignin
The sulfomethylated kraft lignins (SKLs) obtained under the different sulfomethylation conditions (Table 1) were evaluated according to their elemental composition, molecular weight, molybdenite depression ability and sulfonation degree, whose data are provided in Table 3. The C, H, O, N, and S contents ranged from 43.5 to 51.9%, 4.7 to 7.3%, 25.9 to 49.3%, 2.5 to 9.8%, and 2.5 to 11.5%, respectively. The Mw spanned from ~501 to ~1098 g/mol, while the molybdenite depression varied from 6.9% to 62.9%. The samples that showed the highest and lowest ability to depress molybdenite were selected for further determination of the sulfonation degree, which spanned from 0.552 to 1.846 mmol/g.
Table 3. Elemental Analysis, Molecular Weight, Molybdenite Depression, and Sulfonation Degree of Sulfomethylated Kraft Lignin (SKL)
As a first approach to the obtained data, correlations among the different variables were established. A positive and significant correlation was observed between the molybdenite depressing ability and the molecular weight of the SKL samples (Fig. 1a). This behavior has been reported earlier for commercial LS, where high-molecular weight fractions showed a stronger depressing effect during molybdenite flotation assays (Ansari et al. 2007a,b). Similar observations were made recently by Gutierrez et al. (2020) in commercially available LS. However, the depressive effect of those LSs was similar to that of low-molecular-weight sulfomethylated kraft lignin. The authors suggested that the sulfonation degree could explain the similar depression ability of LS and SKL compounds with different molecular weights (Gutierrez et al. 2020). In this regard, a greater number of sulfonic groups on the lignin molecules results in a higher anionic charge density (He and Fatehi 2015; Konduri and Fatehi 2015; Huang et al. 2018). Hence, as expected, a significant and positive correlation was observed between the molybdenite depression and the sulfonation degree (Fig. 1b). However, Deng et al. (2011) reported that the loading density of sulfonic groups decreased at elevated molecular weights of sodium-LS recovered from spent sulfite pulping liquor. In this study, the sulfonic group content increased as the molecular weight increased in sulfomethylated kraft lignin (SO3– content vs. Mw, r = 0.91, p<0.0001). However, the molecular weight magnitude of LS was much higher (54000 to 18000 g/mol) than that of the kraft and sulfomethylated lignins (501 to 1098 g/mol), as shown in Tables 2 and 3, respectively.
Fig. 1. Regression lines and correlation index of sulfomethylated kraft lignin (SKL) features. (a) Molybdenite depression vs molecular weight and (b) molybdenite depression vs. sulfonation degree.
Experimental Design to Optimize Sulfomethylation of Kraft Lignin
The main bottleneck of sulfomethylation reactions is the low reactivity of lignins. Herein, the reactivity is determined by the ability of the carbon-5 sites in the phenolic units of the lignin to react with the chemical precursors. Thus, the content of unsubstituted phenolic units reflects the reactivity of lignin in sulfomethylation (Wu et al. 2012). For this reason, G-type radiate pine lignin was used in this study. However, the formation of side reaction products has also been reported to affect the yield of sulfomethylation. The formation of these undesirable products can be influenced by the reaction conditions (Konduri and Fatehi 2015; Huang et al. 2018), which are assumed to affect the separation performance of the final product due to the relation of the charge density of lignin and sulfomethylate group content (Konduri and Fatehi 2015). Hence, the experimental design evaluated the influence of time, temperature and Na2SO3 concentration on the sulfomethylation reaction and allowed us to determine the optimal conditions for increasing molybdenite depression by the modified kraft lignin. The model equation was obtained using multiple linear regressions, where Y corresponded to the independent variable (response), defined as the ability to depress molybdenite. The linear, quadratic and interaction coefficients of the dependent variables X1 (time), X2 (temperature), and X3 (Na2SO3 concentration) were described according to Eq. 2:
The model equation was evaluated through ANOVA with a 95% confidence level. The statistical values for the model were F = 11.049, p-value=0.001, and R2=0.917, where interactions among the variables were observed, but not all the variables showed a significant influence in the model. The temperature variable (X2, p-value=0.38) did not have a significant influence on the model, while the time (X1, p-value=0.0042) and Na2SO3 concentration (X3, p-value = 0.0005) showed a significant influence on the molybdenite depression. The contour diagrams describing the estimated response surface for the ability to depress molybdenite are shown in Fig. 2. The interaction of time (3 to 6 h) and temperature (130 to 160 °C) was analyzed at 20% Na2SO3 (Fig. 2a), 35% Na2SO3 (Fig. 2b) and 50% Na2SO3 (Fig. 2c). The highest capacity to depress molybdenite (64.6%) could be obtained at 20% Na2SO3 (Fig. 2a). In contrast, high Na2SO3 concentrations in the sulfomethylation reaction might be detrimental to the depression ability of the modified kraft lignin (Fig. 2b and 2c). However, condition optimization showed that similar reaction temperatures (~130 °C) and times (~4.25 h) are needed to obtain maximum molybdenite depression at each Na2SO3 concentration evaluated.
Increased temperature and prolonged reaction time have a negative effect on the sulfomethylation yield (Huang et al. 2018) and the charge density of lignin (Konduri and Fatehi 2015) because the efficiency of the side reactions increases. The main undesirable product that affects the sulfomethylation yield is sodium thiosulfate, which is formed from a side reaction between CH2O and Na2SO3 (Pang et al. 2008; Konduri and Fatehi 2015; Huang et al. 2018). Similarly, a high Na2SO3 dose reduces the sulfomethylation yield, probably due to the enhancement of the mentioned side reactions (Wu et al. 2012; Konduri and Fatehi 2015). Furthermore, the increase in Na2SO3 concentration implies an increase in the CH2O dose. The addition of CH2O can introduce hydroxymethyl groups to the aromatic ring of lignin, allowing the incorporation of sulfonic groups into the lignin structure. However, an excessive dose of CH2O can produce a more condensed hydroxy-methylated lignin due to aldol condensation and disproportionation reactions (Yu et al. 2013), which could affect the sulfomethylation yield and depression ability of lignin.
The maximum molybdenite depression ability using 100 mg/L sulfomethylated kraft lignin was estimated to be 75%, obtained under sulfomethylation conditions of 130 °C, 4.2 h, and 20% (w/w) Na2SO3. Experimentally, 100 mg/L sulfomethylated kraft lignin obtained under the latter conditions depressed 80 ± 5% of molybdenite particles through microflotation assays.
Fig. 2. Estimated response surface for the ability to depress molybdenite (%) obtained as a result of the optimization of the experimental design using (a) 20% Na2SO3, (b) 35% Na2SO3 and (c) 50% Na2SO3. Molybdenite depression was determined with 100 mL/g SKL. SKL: sulfomethylated kraft lignin.
Microflotation of Molybdenite and Chalcopyrite
Sulfomethylated kraft lignin under optimized conditions (OSKL; sulfomethylation of kraft lignin at 130 °C, 4.2 h, with 20% Na2SO3) was prepared, and its potential as a mineral depressant was evaluated. To use sulfomethylated kraft lignin in the selective separation of molybdenite and chalcopyrite in flotation procedures, it must depress only the molybdenite particles and not the chalcopyrite. Microflotation experiments of molybdenite and chalcopyrite were carried out using different OSKL concentrations to depress the molybdenite and chalcopyrite minerals at a pH of 9, which was adjusted with lime (Fig. 3). The molybdenite depression increased as the OSKL concentration increased, reaching the maximum molybdenite depression (93%) at 200 mg/L OSKL (Fig. 3a). In contrast, the chalcopyrite depression showed slight increases with the different OSKL concentrations, reaching a maximum depression of 5% at 100 mg/L OSKL (Fig. 3b). At higher concentrations (200 mg/L), the chalcopyrite depression did not increase markedly.
The depression ability of the OSKL was compared with that of Na-LS and Ca-LS under the same microflotation conditions (Fig. 3). The molybdenite depression of Na-LS ranged from 4% to 56%, increasing the depression of molybdenite as the LS dose increased. Similar behavior was observed for Ca-LS (4 to 59%). Chalcopyrite depression ranged from 2 to 4% in Na-LS and 2 to 5% in Ca-LS. These results showed that sulfomethylated kraft lignin has a better ability to separate molybdenite and chalcopyrite minerals, even at lower concentration doses. Based on the structural features of these lignin samples (Table 2), the higher performance of the OSKL might be mainly due to its sulfonic degree, which is higher than that of the LS samples (Ansari et al. 2007a; He and Fatehi 2015; Huang et al. 2018; Gutierrez et al. 2020).
Fig. 3. Depression of molybdenite (a) and chalcopyrite (b) minerals by sulfomethylated kraft lignin and commercial lignosulfonates at different concentrations. OSKL: optimized sulfomethylated kraft lignin. Na-LS: Sodium lignosulfonate. Ca-LS: Calcium lignosulfonate. All microflotation experiments performed at a pH of 9.
In high pH ranges, LS molecules are negatively charged due to the dissociation of carboxylic, sulfonic, and phenolic groups (Nanthakumar et al. 2010). However, chalcopyrite and molybdenite surfaces are also negatively charged (Chander and Fuerstenau 1972; Castro et al. 2016). Less lignosulfonate adsorption is expected on these mineral particles as a result of electrostatic repulsion, which might explain the weak depression of chalcopyrite flotation at alkaline pH. However, it does not explain the strong depression of molybdenite. It has been proposed that at alkaline pH adjusted with lime, successive adsorption of Ca(OH)+ leads to the precipitation of calcium hydroxide on the molybdenite edges, which generate metallic sites that enhance lignosulfonate adsorption and promote the depression of molybdenite flotation (Gutierrez et al. 2020).
Ansari and Pawlik (2007b) tested several lignosulfonate types in flotation assays using ethyl xanthic acid as the chalcopyrite collector, dodecane as the molybdenite collector, and MIBC as the frother. They observed that chalcopyrite was slightly depressed (~10 to 25%) using a 50 mg/L concentration of several Na-LS types at a pH of 9 adjusted with lime, while 50 mg/L Ca-LS significantly depressed the chalcopyrite particles (~90%). Instead, 50 mg/L Na-LS depressed approximately 90 to 95% of molybdenite at a pH of 8, while very low concentrations of Ca-LS (5 to 10 mg/L) induced very strong depression of molybdenite (Ansari and Pawlik 2007b). These results agree with the flotation values reported in this work (Fig. 3); however, in addition to the particle surface behavior and pH conditions, the depression performance also depends on the type of collector used. In a previous work (Gutierrez et al. 2020), commercial LS and SKL samples were tested as molybdenite depressants in selective Cu-Mo separation by flotation using different collectors. The results indicated that LS can depress chalcopyrite flotation when sodium isopropyl xanthate is used as a collector, but this effect can be mitigated by using PAX as a collector, thus making the potential separation of both minerals possible. This result might be explained by the long chain of PAX, which prevents lignosulfonates from being adsorbed onto chalcopyrite but not the adsorption of these reagents on molybdenite, maintaining the hydrophobicity of the chalcopyrite and allowing its recovery (Leja 1982; Gutierrez et al. 2020).
Characterization of Sulfomethylated Kraft Lignin under Optimized Conditions
Chemical and structural characterization of the sulfomethylated kraft lignin under optimized reaction conditions (OSKL) was performed and compared to the characterization of the initial kraft lignin (KL). Table 2 shows the elemental composition results of the KL and OSKL. Noteworthy differences in the elemental composition between the samples were observed. The KL sample had a considerable amount of carbon (52.5%), and it was decreased remarkably after the sulfomethylation reaction (33.8%). This result is in accordance with previous studies that reported a low carbon content for lignosulfonates (Baker and Rials 2013; Hemmila et al. 2019; Gutierrez et al. 2020). A minor amount of nitrogen was observed (~2.8%), which might be due to some impurities of the samples. A meaningful increase (from 3.8 to 15.8%) in the sulfur amount was observed in the sulfomethylated kraft lignin; this increase was expected after the sulfomethylation reaction. Because lignosulfonates with high sulfur content are better dispersants for cement and coal-water slurry (Zhou et al. 2006; Ouyang et al. 2010), several studies have focused on increasing the sulfur content of lignin products (Wu et al. 2012; Yu et al. 2013; He and Fatehi 2015; Konduri and Fatehi 2015; Huang et al. 2018). Matsushita and Yasuda (2005) evaluated different chemical methods for the conversion of pine lignin into sulfonated products, achieving an increase in the sulfur content from 4% to 11% through sulfomethylation, and the magnitude of the increase was quite similar to our results. However, the oxygen and ash contents also increased, indicating that inorganic residues were incorporated during sulfomethylation. The Mw, Mn, polydispersity and the sulfonic degree of KL and OSKL are shown in Table 2. After the sulfomethylation reaction, the Mw and Mn decreased from 1650 to 940 g/mol and from 870 to 680 g/mol, respectively. The polydispersity decreased slightly, indicating that the uniform character of the lignin improved after the sulfomethylation reaction, as was reported in other investigations (He and Fatehi 2015; Ouyang et al. 2010). Regarding the sulfonic groups, sulfomethylated lignin with a sulfonation degree of 2.2 mmol/g was obtained under the optimized sulfomethylation conditions. Similar values were reported for sulfomethylation reactions under comparable conditions (Ouyang et al. 2010; He and Fatehi 2015; Qin et al. 2015).
FTIR Spectroscopy
FTIR spectra were obtained for LK and OSKL samples (Fig. 4) to distinguish the differences between the functional groups. The FTIR spectra can be separated into two regions: the 4000 to 2700 cm-1 “informative” region, which corresponds to OH and CH stretching vibrations, and the 1800 to 800 cm-1 “fingerprint” region, which is assigned to stretching vibrations of different groups. In the informative region, the strong broad band at 3400 cm-1 is assigned to different OH stretching modes, while the band at 2900 cm-1 is related to asymmetric and symmetric CH stretching (Collier et al. 1997; Schwanninger et al. 2004). In the OH stretching region (3400 cm-1), the broad band observed for both samples resulted from the stretching vibration of aromatic and aliphatic OH in lignin (Liu et al. 2014). After sulfomethylation, the relative absorbance intensity of the band at 3400 cm-1 was increased, revealing an increase in free and associated OH groups (Lu et al. 2017). It has been reported that under sulfomethylation conditions, the content of hydroxymethyl groups attached to the lignin molecule increases, and sulfonic groups can be introduced in the aromatic ring bonded to these groups (Matsushita and Yasuda 2005; Yu et al. 2013; Yang et al. 2014). This seems to be the reason for the observed increase in the absorbance intensity of the OH region (Table 4). The variation in wavelength, shape, and overall width of the OH region after the sulfomethylation reaction suggests a more uniform conformation of hydrogen bonding between the OH groups (Oh et al. 2005). The CH stretching region exhibited two well-resolved peaks at 2933 and 2845 cm-1, which are assigned to the stretching vibration of the CH band in the CH2, CH3, and CH3O groups of lignin (Collier et al. 1997). Both remained invariant in shape, wavelength, and intensity after sulfomethylation (Table 4).
In the fingerprint region, the bands that contribute to the peak at 1700 cm-1 were assigned to C=O stretching vibrations of the carboxyl, carbonyl, and acetyl groups (Faix 1991; Schwanninger et al. 2004; Ahvazi et al. 2016), whose absorbance intensity was drastically reduced after sulfomethylation (Table 4). The carbonyl is a reaction site during sulfonation (Ahvazi et al. 2016), and its decrease might indicate that the sulfomethyl groups may be introduced not only into the aromatic structure but also into the aliphatic chain of the lignin (Madzhidova et al. 1998). The bands at 1600 and at 1510 cm-1 are assigned to C=C stretching of the aromatic ring of lignin (Harrington et al. 1964; Faix 1991), and both remained relatively invariant after sulfomethylation, which confirms that the sample retains aromatic rings in its skeleton structure. The bands detected at 1460 and 1421 cm-1 are caused by stretching in the asymmetric bending in CH3 and C–H deformation and phenol-ether bonds of lignin, respectively (Liu et al. 2014), while the band at approximately 1369 cm-1 is assigned to the in-plane deformation vibration of phenolic hydroxyl and CH3 (non-etherified phenolic OH groups in lignin resulting from the cleavage of β-O-4 and α-O-4 linkages during kraft pulping) (Tejado et al. 2007; Sun et al. 2013). The band at 1273 cm-1 is assigned to the C–O stretching and C–O linkage in guaiacyl aromatic methoxyl groups (Pandey and Pitman 2003; Liu et al. 2014).
Fig. 4. FTIR spectra for radiata pine kraft lignin (KL) and optimized sulfomethylated kraft lignin (OSKL)
After sulfomethylation, the intensity of the latter mentioned bands remained invariant. The band at 1211 cm-1 was assigned to the C–O stretching vibration of phenolic hydroxyl and phenolic ether (Faix 1991; Sun et al. 2013), while the band at 1134 cm-1 was assigned to CH in-plane deformation (Faix 1991; Boeriu et al. 2004). After sulfomethylation, both bands at 1211 and 1134 cm-1 were masked by prominent bands contributing to a peak at 1145 cm-1, which can be ascribed to the contribution of S=O stretching (Qin et al. 2015; Li et al. 2018), where bands at 1169 and 1190 cm-1 have been reported for the asymmetric stretching vibration of SO3-2. The band at 1082 cm-1, assigned to C–O deformation in secondary alcohols and aliphatic ethers (Faix 1991), was absent after sulfomethylation. The absence of any absorption bands for secondary hydroxyl groups (~1085 cm-1) suggests the substitution of sulfonate groups at Cɑ during the modification of lignin with sulfonated sodium salt (Ahvazi et al. 2016). However, the band at 1032 cm-1, assigned to aromatic C–H in-plane deformation, C–O deformation in primary alcohols, C–H stretching, and S=O stretching (Yang et al. 2014; Li et al. 2018), persisted after chemical modification. The presence of the 1032 cm-1 band in the initial kraft lignin indicates the presence of sulfonate groups, which were probably incorporated into the lignin structure during the kraft pulping procedure (Hu et al. 2016). However, after sulfomethylation, the absorbance intensity of the band at 1032 cm-1 was notably increased (Table 4), indicating that additional sulfonic groups were attached to the lignin structure. Bands at approximately 858 and 810 cm-1 were assigned to the C–H out-of-plane deformation vibration of guaiacyl (Faix 1991; Sun et al. 2013), which shifted to higher wavenumbers and was slightly reduced in absorbance intensity (Table 4). The fingerprint region below 1400 cm−1 is difficult to analyze because several bands contribute to it. However, this region contains specific vibrations that allow the structural evaluation of lignins (Boeriu et al. 2004). In this case, no peaks related to S-type lignin were observed (bands at 1326 cm-1, 1115 cm-1, 825 cm-1, and 843 cm-1 are specific vibrations of S-units); this result confirms the G-type nature of the kraft lignin obtained in this work. Finally, the sulfomethylated sample showed a band at 677 cm-1, which is assigned to the S–O group of the sulfonic acid group that is absent in the initial kraft lignin. The band at 620 cm-1 is characteristic of the C=S linkage and has also been linked to the S–O group, which was increased after sulfomethylation (Table 4) (El-Araby et al. 2014; Giang et al. 2016; Hemmila et al. 2019). Additionally, the appearance of the band at 530 cm-1 after sulfomethylation is related to sulfonic acid groups grafted to lignin (Ahvazi et al. 2016).
1H Nuclear Magnetic Resonance (NMR) Spectroscopy
The 1H NMR spectra of KL and OSKL are shown in Fig. 5. 1H NMR is regularly used in the structural investigation of lignin and allows the chemical environment of protons to be detected. Signals at δ 9.4 to 8.0 are assigned to the more predominant phenolic hydroxyls (Li and Lundquist 1994), such as phenolic groups not conjugated with carbonyl groups. In detail, Li and Lundquist (1994) reported that signals at δ 9.4 to 8.5 are assigned to unsubstituted phenolic units, while signals at δ 8.5 to 8.0 are assigned to condensed phenolic structures (C5 substituted), such as biphenyl structures. In the range δ 9.4 to 8.0, signals were detected in KL, whose intensity was drastically decreased after the sulfomethylation reaction. The signals at δ 8.0 to 6.2 are attributed to the protons of the aromatic ring (Yang et al. 2014). The signals at δ 8.0 to 7.2 are attributable to the aromatic protons of the H units or vinylic groups (Yu et al. 2013). Low-intensity signals were detected in the KL sample at this region, probably due to the vinylic groups, but after sulfomethylation, the signals were almost unnoticeable. Signals at δ 7.2 to 6.8 are attributed to aromatic protons of G units, which were more intense in the KL sample than in the OSKL sample. At δ 6.8 to 6.2, signals associated with the protons in S units were expected, but they were not detected. The reduction in the signal intensity of the region assigned to the aromatic protons after sulfomethylation is evidence of the introduction of sulfonic groups into the aromatic ring of the lignin. The chemical shifts of Hɑ, Hβ and Hγ in β-bonded structures are in the region of δ 6.2 to 4.0, where the signals of aliphatic protons in the linkages of β-O-4′, β-1′, β-5′ and β-β’ are approximately δ 6.2 to 5.7, δ 5.7 to 5.2, δ 4.9 to 4.4, and δ 4.3 to 4.0, respectively (Yang et al. 2014; Lu et al. 2017). The protons associated with the latter mentioned structures were detected in the KL sample, but after sulfomethylation, the intensity in the region was dramatically decreased. The signals in the range of δ 4.0 to 3.2 are attributed to protons of the methoxyl and hydroxymethyl groups (Yang et al. 2014; Jiao et al. 2018), and their intensity increased after sulfomethylation. Signals at δ 3.9 to 3.6 are assigned to -OCH3 hydrogens and ~ δ 3.3 to -CH2 hydrogens from hydroxymethyl groups (Jiao et al. 2018). During sulfomethylation, formaldehyde introduces a hydroxymethyl group into the lignin at the C5 position of the aromatic ring, which is the main reaction location in the sulfonation reaction with sodium sulfite (Yu et al. 2013; Kazzaz et al. 2019). Thus, the increase in the signal intensity in this region is due to the introduction of hydroxymethyl groups in the OSKL. Furthermore, the weakness of the signal intensity from δ 3.9 to 3.6 might indicate the extent of demethoxylation during the sulfomethylation reaction. The signals from δ 2.2 to 0.6 are assigned to protons of the aliphatic chain of lignin (Hong et al. 2015), whose intensity was decreased after sulfomethylation. This observation agrees with the FTIR results that evidenced the aliphatic chain as a reaction site during sulfonation (Madzhidova et al. 1998; Ahvazi et al. 2016).
Fig. 5. 1H-NMR spectra of radiata pine kraft lignin (KL) and optimized sulfomethylated kraft lignin (OSKL)
CONCLUSIONS
- The experimental design, which evaluated the effect of temperature, time, and Na2SO3 concentration on the depressing ability of lignin, demonstrated that the optimal conditions of sulfomethylation for mineral flotation purposes are 20% Na2SO3 at 130 °C for 4.2 h.
- Microflotation assays demonstrated that the sulfomethylated kraft lignin obtained under the optimized conditions has a better performance for the selective separation of molybdenite and chalcopyrite than the commercial lignosulfonates studied.
- The results showed correlations between the molybdenite depression vs. molecular weight and sulfonation degree of the sulfomethylated kraft lignins.
- The Fourier transform infrared (FTIR) and proton nuclear magnetic resonance (1H NMR) results showed that after sulfomethylation under optimized conditions, the aromatic G-units of lignin prevailed, hydroxymethyl group content increased, and sulfonic groups were incorporated into the C5 position of the aromatic ring but also into the aliphatic chain of kraft lignin.
- Overall, sulfomethylation enables radiata pine kraft lignin to act as a molybdenite depression agent. Radiata pine kraft lignin has promising potential as an alternative reagent for the copper and/or molybdenum flotation process.
ACKNOWLEDGMENTS
The authors are grateful for the financial support from FONDEF IDeA grant (No. ID14I-10250). C. Vidal thanks FONDEF-VIU grant (No. 16E-0065). I. Carrillo-Varela thanks VRID-UdeC/Postdoctoral grant (No. 219.141.023-P) and ANID/Fondecyt-Postdoctoral grant (3200114). L. Gutierrez also wants to thank to the Water Research Centre for Agriculture and Mining (CRHIAM) of the Universidad de Concepción sponsored by the ANID/FONDAP/15130015 project.
REFERENCES CITED
Abdelaziz, O. Y., Brink, D. P., Prothmann, J., Ravi, K., Sun, M., García-Hidalgo, J., Sandahl, M., Hulteberg, C. P., Turner, C., Lidén, and G., Gorwa-Grauslund, M. F. (2016). “Biological valorization of low molecular weight lignin,” Biotechnol. Adv. 34 (8), 1318-1346. DOI: 10.1016/j.biotechadv.2016.10.001
Ahvazi, B., Cloutier, E., Wojciechowicz, O., and Ngo, T. D. (2016). “Lignin profiling: A guide for selecting appropriate lignin as precursors in biomaterials development”. ACS Sustainable Chem. Eng. 4(10), 5090-5105. DOI: 10.1021/acssuschemeng.6b00873
Ansari, A., and Pawlik, M. (2007a). “Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Part I. Adsorption studies,” Miner. Eng. 20(6), 600-608. DOI: 10.1016/j.mineng.2006.12.007
Ansari, A., and Pawlik, M. (2007b). “Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Part II. Hallimond tube flotation,” Miner. Eng. 20(6), 609-616. DOI: 10.1016/j.mineng.2006.12.008
Bajwa, D. S., Pourhashem, G., Ullah, A. H., and Bajwa, S. G. (2019). “A concise review of current lignin production, applications, products and their environmental impact,” Ind. Crops. Prod. 139(13), 111526. DOI: 10.1016/j.indcrop.2019.111526
Baker, D. A., and Rials, T. G. (2013). “Recent advances in low-cost carbon fiber manufacture from lignin,” J. Appl. Polym. 130(2), 713-728. DOI: 10.1002/app.39273
Boeriu, C. G., Bravo, D., Gosselink, R. J. A., and Van Dam, J. E. G. (2004). “Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy,” Ind. Crops. Prod. 20(2), 205-218. DOI: 10.1016/j.indcrop.2004.04.022
Cardoso, M., Oliveira, E. D., and Passos, M. L. (2009). “Chemical composition and physical properties of black liquors and their effects on liquor recovery operation in Brazilian pulp mills,” Fuel. 88(4), 756-763. DOI: 10.1016/j.fuel.2008.10.016
Castro, S., Lopez-Valdivieso, A., and Laskowski, J. S. (2016). “Review of the flotation of molybdenite. Part I: surface properties and floatability,” Int. J. Miner. Process. 148, 48e58. DOI: 10.1016/j.minpro.2016.01.003
Chander, S., and Fuerstenau, D. W. (1972). “On the natural floatability of molybdenite,” Trans. AIME. 252, 62e69.
Collier, W. E., Kalasinsky, V. F., and Schultz, T. P. (1997). “Infrared study of lignin: Assignment of methoxyl C-H bending and stretching bands,” Holzforschung 51(2), 167-168. DOI: 10.1515/hfsg.1997.51.2.167
Deng, Y., Zhang, W., Wu, Y., Yu, H., and Qiu, X. (2011). “Effect of molecular weight on the adsorption characteristics of lignosulfonates,” J. Phys. Chem. B. 115(49), 14866-14873. DOI: 10.1021/jp208312a
El-Araby, R., Attia, N.K., Eldiwani, G., Khafagi, M. G., Sobhi, S., and Mosta, T. (2014). “Characterization and sulfonation degree of sulfonated poly ether ether ketone using Fourier transform infrared spectroscopy,” World Appl. Sci. J. 32(11), 2239-2244. DOI: 10.5829/idosi.wasj.2014.32.11.14561
Faix, O. (1991). “Classification of lignins from different botanical origins by FT-IR spectroscopy,” Holzforschung. 45(s1), 21-27. DOI: 10.1515/hfsg.1991.45.s1.21
García, A., Toledano, A., Serrano, L., Egues, I., Gonzáles, M., Marín, F., and Labidi, J. (2009). “Characterization of lignins obtained by selective precipitation,” Sep. Purify. Technol. 68(2), 193-198. DOI: 10.1016/j.seppur.2009.05.001
Ge, Y., Li, D., and Li, Z. (2014). “Effects of lignosulfonate structure on the surface activity and wettability to a hydrophobic powder,” BioResources 9(4), 7119-7127. DOI: 10.15376/biores.9.4.7119-7127
Giang, N. T., Kien, T. T., Hoa, N. T., and Thiem, P. V. (2016). “A new synthesis process of lignosulfonate using lignin recovered from black liquor of pulp and paper mills,” Vietnam J. Sci. Technol. 54(4B), 1-10. DOI: 10.15625/2525-2518/54/4B/12017
Gutierrez, L., Uribe, L., Hernandez, V., Vidal, C., and Mendonça, R. T. (2020). “Assessment of the use of lignosulfonates to separate chalcopyrite and molybdenite by flotation,” Powder Technol. 359(1), 216-225. DOI: 10.1016/j.powtec.2019.10.015
Harrington, K. J., Higgins, H. G., and Michell, A. J. (1964). “Infrared spectra of Eucalyptus regnans F. Muell and Pinus radiata D. Don,” Holzforschung 18(4), 108-113. DOI: 10.1515/hfsg.1964.18.4.108
Haz, A., Jablonsky, M., Surina, I., Kacik, F., Bubenikova, T., and Durkovic, J. (2019). “Chemical composition and thermal behavior of kraft lignins,” Forests 10(6), 483. DOI: 10.3390/f10060483
He, W., and Fatehi, P. (2015). “Preparation of sulfomethylated softwood kraft lignin as a dispersant for cement admixture,” RSC Adv. 5, 47031-47039. DOI: 10.1039/C5RA04526F
Hemmila, V., Hosseinpourpia, R., Adamopoulos, S., and Eceiza, A. (2019). “Characterization of wood‐based industrial biorefinery lignosulfonates and supercritical water hydrolysis lignin,” Waste Biomass Valor. 11(6185), 5835-5845. DOI: 10.1007/s12649-019-00878-5
Hong, N., Yu, W., Xue, Y., Zeng, W., Huang, J., Xie, W., Qiu, X., and Li, Y. (2015). “A novel and highly efficient polymerization of sulfomethylated alkaline lignins via alkyl chain cross-linking method,” Holzforschung 70(4), 297-304. DOI: 10.1515/hf-2015-0043
Hu, Z., Du, X., Liu, J., Chang, H. M., and Jameel, H. (2016). “Structural characterization of pine kraft lignin: BioChoice lignin vs Indulin AT,” J. Wood. Chem. Technol. 36(6), 432-446. DOI: 10.1080/02773813.2016.1214732
Huang, C., Ma, J., Zhang, W., Huang, G., and Yong, Q. (2018). “Preparation of lignosulfonates from biorefinery lignins by sulfomethylation and their application as a water reducer for concrete,” Polymers. 10(8), 841. DOI: 10.3390/polym10080841
Jiang, X., Savithri, D., Du, X., Pawar, S., Jameel, H., Chang, H. M., and Zhou, X. (2017). “Fractionation and characterization of kraft lignin by sequential precipitation with various organic solvents,” ACS Sustainable Chem. Eng. 5(1), 835-842. DOI: 10.1021/acssuschemeng.6b02174
Jiao, G.J, Xu, Q., Cao, S.L., Peng, P., and She, D. (2018). “Controlled-release fertilizer with lignin used to trap urea/hydroxymethylurea/ urea-formaldehyde polymers,” BioResources 13(1), 1711-1728. DOI: 10.15376/biores.13.1.1711-1728
Kazzaz, A. E., Feizi, Z. H., and Fatehi, P. (2019). “Grafting strategies for hydroxy groups of lignin for producing materials,” Green Chem. 21, 5714. DOI: 10.1039/C9GC02598G
Kelebek, S., Yoruk, S., and Smith, G. W. (2001). “Wetting behavior of molybdenite and talc in lignosulfonate/MIBC solutions and their separation by flotation,” Separ. Sci. Technol. 36(2), 145-157. DOI: 10.1081/SS-100001072
Konduri, M., and Fatehi, P. (2015). “Production of water-soluble hardwood Kraft lignin via sulfomethylation using formaldehyde and sodium sulfite,” ACS Sustain. Chem. Eng. 3(6), 1172-1182. DOI: 10.1021/acssuschemeng.5b00098
Lange, H., Decina, S., and Crestini, C. (2013). “Oxidative upgrade of lignin. Recent routes reviewed,” Eur. Polym. J. 49(6), 1151-1173. DOI: 10.1016/j.eurpolymj.2013.03.002
Leja, J. (1982). Surface Chemistry of Froth Flotation, Plenum Press, New York. DOI: 10.1007/978-1-4757-4302-9
Li, C., Zhao, X., Wang, A., Huber, G. W., and Zhang, T. (2015). “Catalytic transformation of lignin for the production of chemicals and fuels,” Chem. Rev. 115(21), 11559-11624. DOI: 10.1021/acs.chemrev.5b00155
Li, S., and Lundquist, K. (1994). “A new method for the analysis of phenolic groups in lignins by 1H NMR spectrometry,” Nord. Pulp. Pap. Res. J. 9(3), 191-195. DOI: 10.3183/npprj-1994-09-03-p191-195
Li, X., Shu, F., He, C., Liu, S., Leksawasdi, N., Wang, Q., Qi, W., Alam, M. A., Yuan, Z., and Gao, Y. (2018). “Preparation and investigation of highly selective solid acid catalysts with sodium lignosulfonate for hydrolysis of hemicellulose in corncob,” RSC Adv. 8, 10922-10929. DOI: 10.1039/c7ra13362f
Liu, S., Chen, X., Lauten, R., Peng, Y., and Liu, Q. (2018). “Mitigating the negative effects of clay minerals on gold flotation by a lignosulfonate-based biopolymer,” Miner. Eng. 126(12), 9-15. DOI: 10.1016/j.mineng.2018.06.021
Liu, Y., Hu, T., Wu, Z., Zeng, G., Huang, D., Shen, Y., He, X., Lai, M., and He, Y. (2014). “Study on biodegradation process of lignin by FTIR and DSC,” Environ. Sci. Pollut. Res. Int. 21(24), 14004-14013. DOI: 10.1007/s11356-014-3342-5
Lu, Y., Lu, Y.C., Hu, H., Q., Xie, F. J., Wei, X. Y., and Fan, F. (2017). “Structural characterization of lignin and its degradation products with spectroscopic methods,” J. Spectrosc. Article ID 8951658. DOI: 10.1155/2017/8951658
Madzhidova, V., Dalimova, G., and Abduazimov, K. (1998). “Sulfomethylation of lignins,” Chem. Nat. Compd. 34, 179-181. DOI: 10.1007/BF02249140
Matsushita, Y., and Yasuda, S. (2005). “Preparation and evaluation of lignosulfonates as a dispersant for gypsum paste from acid hydrolysis lignin,” Bioresource Technol. 96(4), 465-470. DOI: 10.1016/j.biortech.2004.05.023
Meister, J. (2002). “Modification of lignin,” J. Macromol. Sci. Polymer. Rev. 42(2), 235-289. DOI: 10.1081/MC-120004764
Mu, Y., Peng, Y., and Lauten, R. (2016). “The depression of pyrite in selective flotation by different reagent systems – A literature review,” Miner. Eng. 96–97, 143-156. DOI: 10.1016/j.mineng.2016.06.018
Nanthakumar, B., Arinatwe, E., and Pawlik, M. (2010). “Adsorption of sodium lignosulfonates on hematite,” Adsorption 16, 447e455. DOI: 10.1007/s10450-010-9237-y
Norambuena, M., Vidal, C., Carrasco, L., Reyes, P., Parra, C., Contreras, D., and Mendonça, R. T. (2016). “Optimization of experimental variables to modify lignin from Eucalyptus globulus under alkaline catalysis,” BioResources 11(1), 1828-1842. DOI: 10.15376/biores.11.1.1828-1842
Oh, S. Y., Yoo, D. I., Shin, Y., Kim, H. C., Kim, H. Y., Chung, Y. S., Park, W. H., and Youk, J. H. (2005). “Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy,” Carbohydr. Res. 340(15), 2376-2391. DOI: 10.1016/j.carres.2005.08.007
Ouyang, X., Ke, L., Qiu, X., Guo, Y., and Pang, Y. (2009). “Sulfonation of alkali lignin and its potential use as dispersant for cement,” J. Disper. Sci. Technol. 30(1), 1-6. DOI: 10.1080/01932690802473560
Pandey, K. K., and Pitman, A. J. (2003). “FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi,” Int. Biodeterior. Biodegradation. 52(3), 151-160. DOI: 10.1016/S0964-8305(03)00052-0
Pang, Y. X., Gao, F., Lou, F. M., and Deng, Y. H. (2013). “Application performance of lignin-modified amino-sulfonic acid-based dispersant on the ceramic slurry,” AMR. 781–784, 952-956. DOI: 10.4028/www.scientific.net/amr.781-784.952
Pang, Y. X., Qiu, X. Q., Yang, D. J., and Lou, H. M. (2008). “Influence of oxidation, hydroxymethylation and sulfomethylation on the physicochemical properties of calcium lignosulfonate,” Colloids Surf. A Physicochem. Eng. Asp. 312(2-3), 154-159. DOI: 10.1016/j.colsurfa.2007.06.044
Pradip, and Fuerstenau, D. W. (1991). “The role of inorganic and organic reagents in the flotation separation of rare-earth ores,” Int. J. Miner. Process. 32(1-2), 1-22. DOI: 10.1016/0301-7516(91)90016-C
Peretti, S. W., Barton, R., and Mendonça, R. T. (2015). “Lignin as feedstock for fibers and chemicals,” in: Commercializing Biobased Products: Opportunities, Challenges, Benefits, and Risks, S. W. Snyder (ed.), RSC Green Chemistry, Cambridge. DOI: 10.1039/9781782622444-00132
Qin, Y., Yang, D., Guo, W., and Qiu, X. (2015). “Investigation of grafted sulfonated alkali lignin polymer as dispersant in coal-water slurry,” J. Ind. Eng. Chem. 27, 192-200. DOI: 10.1016/j.jiec.2014.12.034
Rashid, T., Kait, C. F., and Murugesan, T. (2016). “Effect of temperature on molecular weight distribution of pyridinium acetate treated kraft lignin,” Procedia Eng. 148, 1363-1368. DOI: 10.1016/j.proeng.2016.06.599
Sadowski, Z. (1995). “Selective spherical agglomeration of fine salt-type mineral particles in aqueous solution,” Colloids Surf. A Physicochem. Eng. Asp. 96(3), 277-285. DOI: 10.1016/0927-7757(94)03042-X
Schwanninger, M., Rodrigues, J. C., Pereira, H., and Hinterstoisser, B. (2004). “Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose,” Vib. Spectrosc. 36(1), 23-40. DOI: 10.1016/j.vibspec.2004.02.003
Stranel, O., and Sebok, T. (1999). “Relationships between the properties of ligninsulphonates and parameters of modified samples with cement binders Part III. Determination of sulphonated compounds content, characteristic of sulphonation, sorption studies,” Cem. Concr. Res. 29(11), 1769-1772. DOI: 10.1016/S0008-8846(99)00165-9
Sun, Y., Qiu, X., and Liu, Y. (2013). “Chemical reactivity of alkali lignin modified with laccase,” Biomass Bioenerg. 55, 198-204. DOI: 10.1016/j.biombioe.2013.02.006
Tejado, A., Peña, C., Labidi, J., Echeverria, J.M., and Mondragon, I. (2007). “Physico-chemical characterization of lignins from different sources for use in phenol-formaldehyde resin synthesis,” Bioresource Technol. 98(8), 1655-1663. DOI: 10.1016/j.biortech.2006.05.042
Wu, H., Chen, F., Feng, Q., and Yue, X. (2012). “Oxidation and sulfomethylation of alkali-extracted lignin from corn stalk,” BioResources 7(3), 2742-2751. DOI: 10.15376/ biores.7.3.2742-2751
Yang, D., Chang, Y., Wu, X., Qiu, X., and Lou, H. (2014). “Modification of sulfomethylated alkali lignin catalyzed by horseradish peroxidase,” RSC Adv. 4, 53855. DOI: 10.1039/C4RA07244H
Yu, G., Li, B., Wang, H., Liu, C., and Mu, X. (2013). “Preparation of concrete superplasticizer by oxidation-sulfomethylation of sodium lignosulfonate,” BioResources 8(1), 1055-1063. DOI: 10.15376/biores.8.1.1055-1063
Zhang, Y. S., Wang, C. G., Fang, X. Q., and Li, Y. Y. (2018). “Characterization of lignins from the black liquor of Australian Eucalyptus kraft pulping,” Wood Res. 64(4), 667-676.
Zhou, M., Qiu, X., Yang, D., and Lou, H. (2006). “Properties of different molecular weight sodium lignosulfonate fractions as dispersant of coal-water slurry,” J. Disper. Sci. Technol. 27(6), 851-856. DOI: 10.1080/01932690600719164
Zhou, X. F., Lu, and X.J. (2014). “Structural characterization of kraft lignin for its green utilization,” Wood Res. 59, 583-591.
Article submitted: April 9, 2021; Peer review completed: June 7, 2021; Revised version received and accepted: June 22, 2021; Published: June 24, 2021.
DOI: 10.15376/biores.16.3.5646-5666
