Abstract
The surface chemistry of gravure printed décor paper and its effect on the adhesion of melamine formaldehyde (MF) coatings were studied. Two industrially printed decor papers with different designs were used for the study. A combination of the attenuated total reflectance Fourier transform Infrared (ATR FT-IR) and FT-IR spectroscopy techniques were employed to determine the effect of the gravure printing ink on the printed paper surface chemistry. Then, the influence of the surface chemistry on the adhesion of MF coatings was characterized according to the abrasion resistance test. The ATR IR results suggested that the printing ink components had a noticeable effect on the surface characteristics of the printed décor paper. In addition, it was indicated that the use of an organosilane adhesion promoter in the gravure ink formulation could significantly affect the adhesion strength of the MF coatings through the formation of ring siloxane structures. It seemed that siloxane bridges formed between the molecules of ink binder and UF resin could enhance the adhesion strength of subsequent MF coatings and could reveal better Taber abrasion resistance performance.
Download PDF
Full Article
Surface Chemistry of Gravure Printed Décor Paper and Adhesion of Melamine Formaldehyde Resin Coatings
Abouzar Hatam,* Mohammadreza Dehghani Firouzabadi,a and Hossein Resalati
The surface chemistry of gravure printed décor paper and its effect on the adhesion of melamine formaldehyde (MF) coatings were studied. Two industrially printed decor papers with different designs were used for the study. A combination of the attenuated total reflectance Fourier transform Infrared (ATR FT-IR) and FT-IR spectroscopy techniques were employed to determine the effect of the gravure printing ink on the printed paper surface chemistry. Then, the influence of the surface chemistry on the adhesion of MF coatings was characterized according to the abrasion resistance test. The ATR IR results suggested that the printing ink components had a noticeable effect on the surface characteristics of the printed décor paper. In addition, it was indicated that the use of an organosilane adhesion promoter in the gravure ink formulation could significantly affect the adhesion strength of the MF coatings through the formation of ring siloxane structures. It seemed that siloxane bridges formed between the molecules of ink binder and UF resin could enhance the adhesion strength of subsequent MF coatings and could reveal better Taber abrasion resistance performance.
Keywords: Surface chemistry; ATR IR spectroscopy; Décor paper; Gravure printing; MF coatings adhesion
Contact information: Department of Pulp and Paper, Gorgan University of Agricultural Sciences and Natural Resources, Gorgan, Iran; *Corresponding author: abhatam@yahoo.com
INTRODUCTION
Décor paper is made from alpha cellulose-rich pulp, and it can be characterized as technically advanced and highly engineered specialty paper. These alpha cellulose-based papers are known as the exclusive base print papers, to which various patterns are applied using gravure printing processes. Such décor paper products are widely used in laminated board production because of their resistance to photo-yellowing (Enzensberger 1961; Rydholm 1965; Tocchio et al. 1993; Roberts and Evans 2005; Bardak et al. 2011). The decorative paper is impregnated with thermosetting synthetic resins, which include urea formaldehyde (UF) and melamine formaldehyde (MF) resins, and converted into a technologically sensitive cellulosic composites through an impregnation process, i.e. melamine impregnated paper or decorative foils. Decorative foils are also used extensively as facing materials for the coating of wood-based panels in the furniture and decoration industries (Hiziroglu 1996; Istek et al. 2010).
The widely used aminoplastic resins for the manufacture of low-pressure melamine (LPM) are the saturating grade UF and MF thermosetting resins. Preliminarily, MF resin has been applied to paper treating and manufacture of decorative laminates in a one-stage impregnation process. Later, UF resin as the most economical amino resin was introduced into the process to reduce the production costs. Therefore a double-stage process was developed for this purpose. While the UF resin provides inferior durability to the MF resin for exterior applications, properties such as hardness, scratch resistance, good moisture resistance, and excellent performance of the cured MF resin render it suitable for most of such applications. For this reason, usage of UF resin for the impregnation of décor paper is limited only to the first bath, where the core of the paper is to be fully saturated with resin. Then the process is followed by coating of the partially dried decorative paper with a coat of 100% MF resin to completely seal the resin in the core of paper away from its surface (Composite Panel Association 2007; Bardak et al. 2011).
In an industrial two-stage impregnation line, as illustrated in Fig. 1, gravure printed décor paper is saturated with UF resin in the first stage and is dried to a volatiles content of approximately 10%. This is done with the so-called S-type multiple hot air ovens (first dryer), which typically operate within the temperature range of 110 °C to 140 °C, depending on the production rate. In the second stage, the UF-saturated décor paper is coated with MF resin on the top and bottom sides simultaneously by means of two gravure roll coaters, and is precisely dried to a controlled B-stage (partially cured) level using the same ovens (Bardak et al. 2011; Hatam et al. 2015).
The process of décor paper impregnation with UF/MF resins involves several physicochemical and thermodynamic phenomena. Most of the physicochemical phenomena, such as wetting, wicking, and adhesion, are mainly affected by the surface chemistry of the décor paper. One of the most fundamental surface phenomena is adhesion, which is often used as a criterion to determine the durability and performance of the coatings. Adhesion is obtained when interfacial molecules achieve adsorption and form chemical bonds during wetting. Because it is defined as the strength of the bonds and interactions between the coatings and substrate, practical adhesion is usually measured as the force or energy required to break various physical and chemical bonds, and to completely detach the contacting materials (Baghdachi 2014).
The effects of the décor paper properties on the adhesion of MF resin coatings have not yet been exhaustively investigated (Bardak et al. 2011). In particular, the influence of the surface chemistry of industrially gravure printed décor paper on the adhesion of MF resin has not been studied. The surface chemistry of décor paper can originate from both the papermaking and gravure printing processes. Papermaking conditions can contribute to the structural (physical) and chemical properties of the décor paper, such as porosity and pH. The cross-linking reactions of the thermosetting UF and MF resins in the impregnation process take place at an acidic condition (Blank 1979; Xing et al. 2007). Even a slight deviation of the décor paper pH from a neutral value (7.0 ± 0.1) might significantly impinge on the reactivity and curing degree of the impregnated paper by accelerating or decelerating the polymerization reactions of these resins. Therefore, the décor paper’s pH can have a crucial effect on the curing of the UF/MF-saturated décor paper and its subsequent affinity for developing interfacial molecular interactions with MF resin coatings,
A printing process, on the other hand, exhibits an explicit effect on the surface chemistry of printed paper through the chemical functional groups of printing ink constituents including color pigments, binders, solvents, and chemical additives, maybe in a more complicated manner than papermaking conditions. Therefore, with regards to the key contributions of printing ink components to the surface chemistry of printed paper, this study aims to examine how the chemical interactions between the printed décor paper surface and UF/MF thermosetting resins during the impregnation process can be manipulated by the gravure water-based ink constituents.
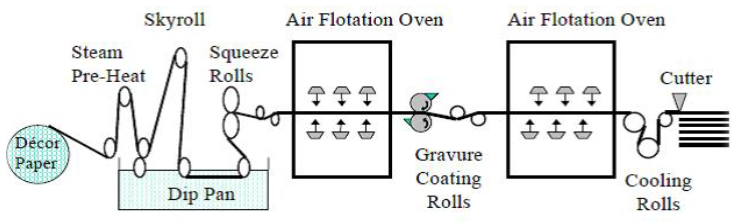
Fig. 1. Schematic representation of a two-stage impregnation line with gravure roll coaters (Adopted from Marshall and Parent (1998); reproduced with permission from TAPPI)
EXPERIMENTAL
Materials
This study was conducted on industrial décor papers. Two printed décor papers, with the commercial names of Latin Ceviz (Latin Walnut) and Ceviz (Walnut), were obtained from Aryak Decor local impregnation factory located in Mahmoodabad, Mazandaran Province, Iran, and are shown in Fig. 2. Various properties of these décor papers were measured and are summarized in Tables 1 and 2. From the tables, it was inferred that the illustrated papers were different décor papers.
In addition to the measured properties, the selected papers were different in the color of their printed designs, which is associated to the different inks used in the printing process. This difference between these two printed samples provided the main motivation for the present study. As shown in Fig. 2, the selected décor papers were clearly different in their colors before and after printing.
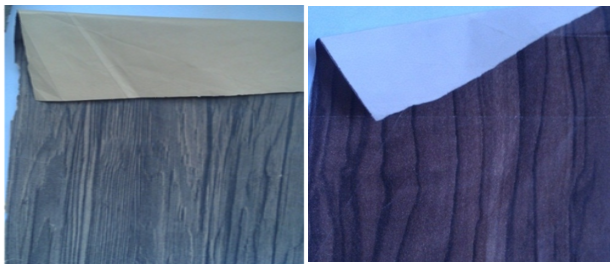
Fig. 2. Industrially gravure printed décor papers used for the study: (A) Walnut (Ceviz) and (B) Latin Walnut (Latin Ceviz)
Table 1. Physical and Chemical Properties of the Studied Décor Papers
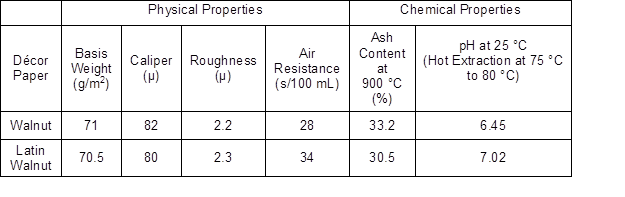
Table 2. Mechanical Properties of the Studied Décor Papers
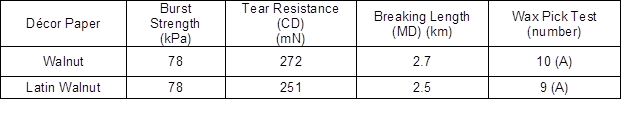
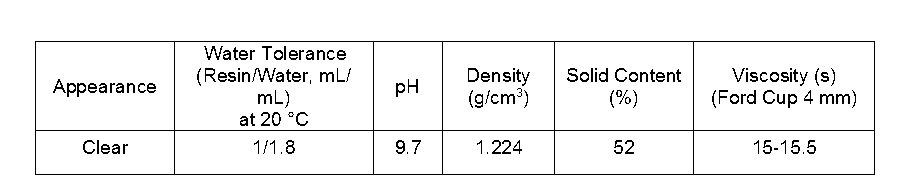
The UF and MF resins were impregnation resins purchased by the factory as raw materials for the production of melamine impregnated paper. The technical specifications of these resins are given in Tables 3 and 4. Additives were also used in this study, which included the wetting agent (Trade Name : MA11), anti-blocking agent (FA), and catalyst (KSN) for the UF resin, and the surface smoothing and releasing agent (GMEcp), plasticizer (THP), and catalyst (KS) for the MF resin. These additives were exactly the same as those that are normally added to the UF and MF resins in the industrial impregnation process. All of the additives were products of the Deurowood Company (Lustenau, Vorarlberg state, Austria). Unfortunately, because the detailed chemical and physical specifications of these additives are patents of the Deurowood Company, no further data were provided.
Table 3. Specifications of the UF Resin Used for the Saturation of the Décor Paper
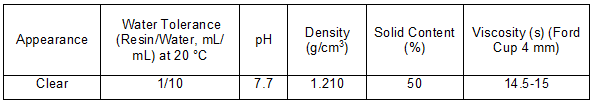
Table 4. Specifications of the MF Resin Used for the Coating of the Décor Paper
Methods
Determination of the paper properties
The physical, chemical, and mechanical properties of the décor papers were determined according to the ISO and TAPPI standard test methods. Tables 5 and 6 present the standard test methods used for measuring the different properties of the décor papers.
Table 5. Standard Test Methods Used for Measuring the Décor Paper Properties
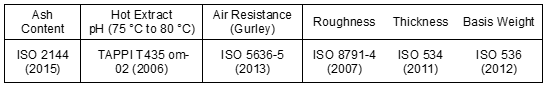
Table 6. Standard Test Methods Used for Measuring the Décor Paper Properties
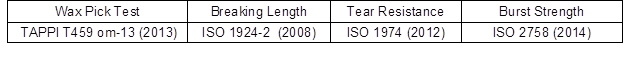
Characterization by FT-IR spectroscopy
The surface chemistry characteristics of the unprinted (back) and printed (front) sides of the décor paper were characterized by attenuated total reflectance Fourier transform infrared (ATR FT-IR) spectroscopy to identify the differences between their chemical functional groups. The ATR FT-IR spectroscopy was also performed for the UF-saturated paper samples on the printed side only to detect the chemical changes of the paper surface during the UF saturation stage. Further insights into the chemical characterization of the décor paper were provided by a FT-IR analysis of the paper and their ashes (obtained by ignition of the décor paper at 900 °C in air) using the KBr method. The FT-IR spectra were acquired in absorption mode, over the region of 4000 cm-1 to 400 cm-1 at a spectral resolution of 4 cm-1 using a Nicolet 670 FTIR spectrometer (Thermo Nicolet, Madison, USA). The ATR measurements were conducted using a 45° ZnSe crystal element.
Examination of the UF saturated paper surface morphology by optical microscope
To eliminate the effects of the factors relevant to the saturation stage on the final results, it was necessary to use the coating substrates that had been produced in the same saturation conditions in terms of the impregnation machine speed and UF resin formulation. Thus, it was required to wait until it would be possible to be provided by the impregnation factory with the suitable opportunity for taking the samples according to the production planning. It took a period of around two weeks to obtain the UF-saturated samples from the running impregnation line. After the samples were prepared, both the Walnut and Latin Walnut saturated papers had been stored in the same conditions in a refrigerator until they were used for coating. However, prior to coating it was observed that the two saturated papers were discernibly different in terms of their flexibility. The UF-saturated Walnut paper tended to be less flexible compared to the saturated Latin Walnut paper. Therefore, their surfaces were surveyed with an optical microscope to see whether any changes in the surface morphology of the saturated papers could be observed that might be connected to the effect of the décor paper pH.
Preparation of the melamine prepregs
The UF-saturated paper samples were obtained from the end of the first stage immediately behind the MF coating station in a running two-stage impregnation line. The samples, which had a basis weight of approximately 140 gram per square meter (GSM) and volatiles content of approximately 10%, were then cut into 15 cm × 15 cm sheets, put into plastic bags, and kept in a refrigerator to prevent any changes in their volatiles content until they were used for the coating purpose. The coatings composition was then formulated by adding different additives to the MF resin. As shown in Table 7, all of the additives were added by weight.
Table 7. Composition of the MF Resin Coatings

Due to the lack of a laboratory gravure roll coater, a flat painting brush with a very thin bristle head was employed for coating the UF-saturated papers. The coating was applied on the bottom surface first. After a partial drying for 1 h at room temperature, the top surface was then coated. The coated papers were kept at room temperature for 1 h, and then procured using two ovens. The samples were put in the first oven at 90 °C for 1 min, and in the second oven at 125 °C for 45 s to obtain the final volatiles of 5.5% to 6.0%. The prepreg samples were also put into the plastic bags and stored in a refrigerator to keep the volatiles content constant. It should be noted that application of the coating substance to the top and bottom surfaces was followed according to the ratio of 60% to 40% by weight, which is a typical procedure for the coating of UF-saturated paper in the impregnation industry. A final basis weight of 165 GSM to 170 GSM was achieved at the end of the process.
Evaluation of the coatings adhesion strength
The strength of the coatings adhesion was characterized by the Taber abrasion test. The melamine prepregs were laminated on 8-mm High-density Fiberboards (HDF) by a Burkle laboratory press machine (BURKLE Process Technologies, Freudenstadt, Germany). Table 8 presents the conditions of the laboratory press parameters for preparing the laminate samples. The laminates were cut into 10 cm × 10 cm samples to be used for the Taber test. The abrasion tests were conducted using a 5135 rotary Taber Abraser (TABER Industries, New York, USA). Typically, the abraser is equipped with a 250-g weight on each arm (set by default) to determine the abrasion resistance of the melamine surfaced engineered wood composites, such as medium-density fiberboard (MDF), particleboard, and laminate flooring fabricated by the Low Pressure Laminating (LPL) process. However, because it was intended to assess the strength of the MF coatings adhesion, exertion of a more severe force on the laminates was required. Therefore, 500 g of additional weight was loaded on each abraser arm to achieve the total exerted load of 750 g on each wheel. The Calibrase CS-17 abrasive wheels, which are commonly used for measuring the abrasion resistance of hard organic coatings, were employed as the abrasive media in this test.
Table 8. Laboratory Press Conditions for Preparing Different Laminate Samples
RESULTS AND DISCUSSION
FT-IR Spectroscopy
ATR FT-IR analysis of the unprinted (print base) décor papers
The ATR FT-IR spectra were obtained from the unprinted surfaces (back side) of the Walnut and Latin Walnut décor papers, and are compared in Fig. 3. As was already shown in Fig. 2, the two unprinted décor papers were different in their base colors due to the application of dissimilar color pigments in the papermaking process. However, no noticeable differences were observed in their ATR FT-IR spectra over the wavenumber range of 400 cm-1 to 4000 cm-1.
Absorption bands located in the “fingerprint region” of both spectra that ranged from 1200 cm-1 to 800 cm–1 corresponded well to the vibrations of the main functional groups or bonds in the chemical structure of cellulose. These functional groups were as follows: a strong absorption band at 1025 cm-1 due to C-O stretching of C-OH and C-O-C in pyranose rings, a small sharp peak at 894 cm-1 attributed to the glycosidic C-H deformation with ring vibration contribution, a sharp band at 1158 cm-1 assigned to the stretching vibration of β-glycosidic linkage between pyranose rings, and a rather sharp band at 1313 cm-1 attributed to CH2 wagging vibration (Brugnerotto et al. 2001; Manso and Carvalho 2009; Chen et al. 2011; Kotelnikova and Mikhailidi 2011; Jahan et. al. 2012; Caro et al. 2013; Gorassini et al. 2016). According to the ATR spectra shown in Fig. 3, only the functional groups of the cellulose component were revealed in the spectra of the unprinted décor papers, which accentuated the fact that the pulp (alpha pulp) used for making décor paper is essentially devoid of hemicelluloses and lignin, and was in good agreement with the reports from previous publications (Roberts and Evans 2005; Bardak et al. 2011).
It is worth mentioning that the pigments that are normally used for the coloration of the print base décor papers are inorganic pigments due to their superior lightfastness relative to organic pigments (Lanxess Corporation 2006). It was reported by Vahur et al. (2010) that most of these inorganic color pigments have characteristic absorption bands in the wavenumber region lower than 400 cm-1, which is inaccessible for routine ATR FT-IR spectroscopy.
This may be a reasonable explanation for why there were no specific peaks attributable to the inorganic color pigments in the ATR FT-IR spectra of the base décor papers in this study.
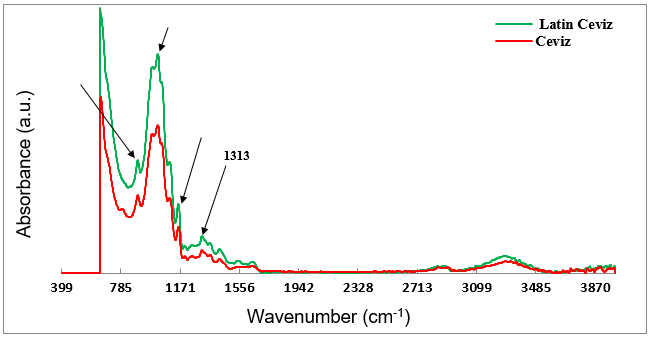
Fig. 3. ATR FT-IR spectra obtained from the unprinted surfaces of the Latin Walnut and Walnut décor papers
ATR FT-IR analysis of the printed décor papers
In contrast to the ATR spectra of the unprinted surfaces of the décor papers depicted in Fig. 3, noticeable differences were observed in the spectral responses of their printed surfaces. Figure 4 shows the ATR FT-IR spectra acquired for the printed surfaces of the Walnut and Latin Walnut décor papers. As shown, the positions of the cellulose characteristic peaks in both spectra were slightly shifted towards lower frequencies, i.e. 886 cm-1, 1021 cm-1, and 1152 cm-1, which indicated that a chemical interaction occurred between the printing ink components and cellulosic fibers in both décor papers. Additionally, some new peaks were also observed in both spectra, which could have been associated with the formation of new functional groups after printing. As was evident in Fig. 4, the cellulose characteristic peaks of both spectra had shifted in a similar manner. However, a clear distinction was observed when comparing the spectra of the two printed papers, which indicated that each printed sample had its own individual spectrum. This result suggested that there should have been remarkable differences in terms of the surface chemistry features between the two printed décor papers.
The FT-IR characteristic bands at 1257 cm-1, 1326 cm-1, and 1448 cm-1 were only observed in the ATR spectrum of the Latin Walnut printed paper, and were assigned to aryl-O bond stretching, aryl sulphone bond stretching, and C-H bending in the molecular structure of the organic printing ink components, namely the organic pigments and binders, respectively. In contrast, the absorption frequency at approximately 1500 cm-1, which appeared in the spectrum of both printed papers, could most likely have been attributed to the stretching vibration of benzene rings in the molecular structure of the organic ink pigments. However, its intensity was markedly higher in the spectrum of the Latin Walnut paper compared to that of the Walnut paper, which confirmed the higher concentration of benzene rings on the surface of the Latin Walnut printed paper. Also, as one of the most widely used binders in the formulation of water-based gravure ink for décor paper is acrylic resin (Podhajny 2004), the appearance of an IR band at 1448 cm-1 could probably be assigned to the C-H bending vibration of vinyl groups in the chemical structure of the acrylic resin binder.
It should be noted that these findings properly supported the results of previous studies, which reported that the ATR FT-IR absorption bands associated with the functional groups of printing ink organic constituents could be predominantly detected in the wavenumber region of 1200 cm-1 to 1600 cm-1 (Vikman and Sipi 2003; Bouchard et al. 2009; Stenger et al. 2010; Doncea and Ion 2014; Nastova et al. 2015; Del Hoyo-Meléndez et al. 2016). Therefore, it was concluded that these results could provide adequate supporting evidence of the contribution of printing ink components to the main differences in the surface chemistry characteristics of the two printed décor papers in this study.
Although an extremely thin layer of ink was printed on the paper during gravure printing, the ink-related absorption frequencies in the ATR spectrum of the Walnut printed paper were weaker and relatively fewer compared to that of the Latin Walnut paper. One possible reason for the poorly detected ink-related peaks in the ATR spectrum of the Walnut printed paper was that more ink might have transferred onto the surface of the Latin Walnut paper compared to Walnut paper (Vikman and Sipi 2003). This claim could be further supported by the fact that the gravure printing method for décor paper was a multistage process. Each stage was referred to as color station. Depending on the decorative design, the paper might pass through up to 4 stations of the printing machine. Therefore, the number of stations that the paper passed through or the number of colors which were used for printing gave rise to differences in the amount of ink that was transferred onto the paper. The amount of ink that was applied onto the decor paper could probably affect the ink coverage and the surface chemistry of the final printed paper.
Therefore, it seemed that appearance of the ATR IR spectrum with more ink-related bands and higher intensities in the Latin Walnut paper compared to Walnut paper could likely be attributed to the presence of further amounts of printing ink on the Latin Walnut paper surface and its higher ink coverage as a result of passing through more color stations during printing. Although surface porosity of the print base décor paper might have played a role in respect of ink holdout characteristic in the Latin Walnut paper surface, more information was required to be obtained to verify its effect.
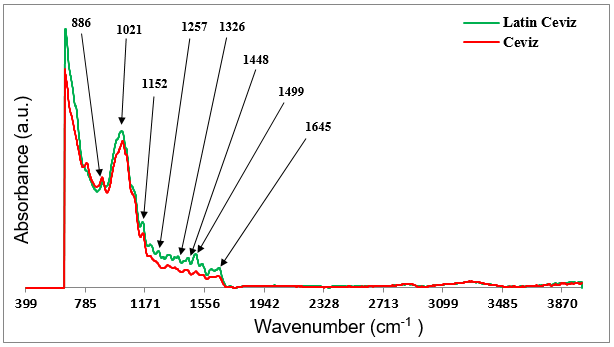
Fig. 4. ATR FT-IR spectra obtained from the printed surfaces of the Latin Walnut and Walnut décor papers
ATR FT-IR analysis of the UF-saturated décor papers
As mentioned earlier, the UF-saturated samples of the Latin Walnut and Walnut décor papers were taken from the end of the first stage in a running two-stage impregnation line. The surface chemistry of these saturated samples was characterized by ATR FT-IR spectroscopy. Figure 5 presents the ATR spectra obtained from the surface of both samples. It can be clearly seen that, regardless of the intensity, both spectra had peaks at almost identical positions, which demonstrated that these two saturated papers contained exactly the same functional groups on their surfaces. In the ATR FT-IR spectra, the strong sharp band at 991 cm-1 that was a cellulose characteristic peak shifted to a lower wavenumber, which indicated that chemical interactions occurred between the -OH group of the cellulosic fibers and UF resin during the saturation stage.
The new absorption bands at 1240 cm-1 due to C-N and N-H stretching, 1530 cm-1 corresponded to C=O stretching in the secondary amide, 1632 cm-1 belonged to N-H bending vibration in the primary amide, and 1379 cm-1 due to C-N stretching of -CH2-N (methylene bridge) were attributed to the chemical structure of the UF resin pre-polymers (Zorba et al. 2008; Šmidriaková and Laurová 2011; Singru et al. 2011; Chiang et al. 2016; Dontulwar et al. 2016). However, the appearance of a relatively small band at 1379 cm-1 suggested that a mild condensation reaction occurred among monomers of the UF resin when the saturated décor paper was subjected to drying in the first stage of the impregnation process. Therefore, it was inferred from the view of polymer chemistry that the cross-linking reactions between the molecules of the UF resin monomers advanced to the partially cured (B stage) level or pre-polymeric state in both of the saturated décor papers.
Additionally, it was found that the overall absorption bands revealed in the ATR spectrum of the UF-saturated Latin Walnut paper were remarkably stronger than those observed in the spectrum of the saturated Walnut paper. This result might also be assigned to the contribution of ink coverage to surface chemistry of the UF-saturated décor paper as well as the printed paper. In other words, it seemed that, due to the presence of more ink on the Latin Walnut paper surface, a more homogeneous layer of ink might have formed which had led to retaining a greater amount of UF resin on its surface during the saturation stage. Hence, stronger bands were detected in its spectrum compared to that of the Walnut paper.
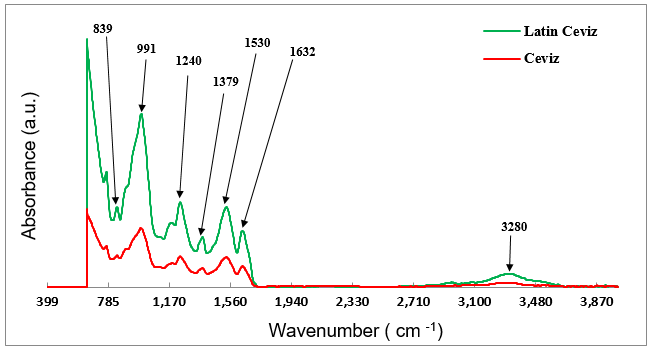
Fig. 5. ATR FT-IR spectra obtained from the UF-saturated Latin Walnut and Walnut décor papers
It should be mentioned that a weak broad peak at approximately 3280 cm-1 was also revealed in each spectrum, which might have been attributed to the formation of intermolecular hydrogen bonding between the functional groups of the pre-polymers, including CH2OH, NH, and NH2, and the by-products of the condensation reaction, such as water and free formaldehyde (Zorba et al. 2008; Singru and Gurnule 2010; Šmidriaková and Laurová 2011). This band was remarkably stronger in the spectrum of the Latin Walnut paper than that in the spectrum of the Walnut paper, which indicated that noticeably higher contents of water and reactive groups for hydrogen bonding existed in the Latin Walnut-saturated paper surface.
FT-IR analysis of the printed décor papers
A more detailed chemical characterization of the differences between the Latin Walnut and Walnut printed samples was performed using FT-IR spectroscopy. As Fig. 6 shows, there was a strong similarity between the FT-IR spectra obtained from the two printed papers in terms of peak positions and intensities. However, small differences were seen between the spectra of the two papers, which required special attention.
According to the literature, in the 1000 cm-1 to 1250 cm-1 range of the FT-IR spectra, the appearance of strong broad bands represented the presence of Si-O-Si (siloxane) structures in the silicon-containing compounds (Jing et al. 2002). As clearly shown in Fig. 6, the peak associated with the band at 1061 cm-1 only appeared in the Latin Walnut décor paper spectrum, and it could have been attributed to the Si-O-C bond stretching in the Si-O-Si ring link (network) structure (Jing et al. 2002). The band at 1105 cm-1 was observed in the spectra of both papers, which corresponded to the Si-O-C bond stretching in the Si-O-Si open link structure (Jing et al. 2002). These results suggested that both printed papers contained the Si-O-Si structure in their chemical compositions, though the Si-O-Si ring structure was only formed in the Latin Walnut printed décor paper. The results also suggested that the FT-IR bands at 1061 cm-1 and 1105 cm-1 were frequencies of key importance in identifying the siloxane structures formed in the gravure printed décor papers.
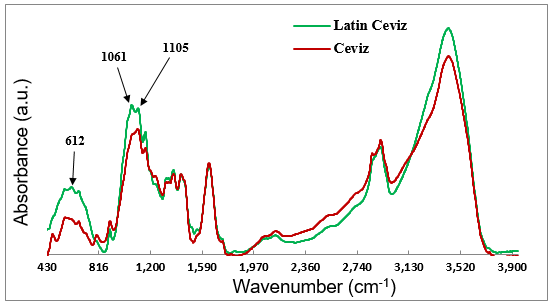
Fig. 6. FT-IR spectra obtained from the printed Latin Walnut and Walnut décor papers
It should be noted that the silicon-containing compounds in the composition of the gravure printed décor paper can originate from the organosilane compounds, which are widely used as an adhesion promoter additive in the gravure printing ink formulation (Witucki 1993; Sathyanarayana and Yaseen 1995; Guichenuy et al. 2004). As presented in Fig. 7, the molecules of organosilanes served as molecular bridges through establishing a cross Si-O link at the interface between the inorganic or organic substrates (such as minerals, fillers, metals, and cellulose) and organic or polymeric matrices (such as rubber, thermoplastics, thermosets, and printing ink binders), and can dramatically ameliorate adhesion between them (Materne et al. 2005). The mechanism of adhesion promotion in the presence of organosilanes was also illustrated in Fig. 8. As clearly shown, the molecules of alkoxysilane were subjected to the hydrolysis reaction with atmospheric moisture or water adsorbed on the surface to form silanols. These silanols could then condense with other silanols to form stable siloxane bonds (-Si-O-Si-). In the presence of hydroxyl groups at the surface of the substrate, silanols (Si-OH) would form stable Si-O- covalent cross bonds with the substrates, and water molecules would be liberated. This is the key chemistry that allows silanes to function as valuable adhesion promoting agents in the industry (Witucki 1993; Materne et al. 2005).
It is believed that at the interface between the décor paper and gravure printing ink, the molecules of organosilanes can promote adhesion strength through the formation of Si-O-C bridges between the cellulose and printing ink binder, which act as the substrate and polymeric matrix, respectively (Materne et al. 2005). However, depending on the gravure printing process parameters, such as water-based ink pH and drying temperature, the extent of silanol condensation and final cross-linking can change (Witucki 1993; Gelest Inc. 2006). As a result, two configurations of polysiloxane structures can be created, i.e. an open structure (less cross-linked) and a ring or network structure (highly cross-linked). The more cross-linking, then a higher adhesion can be attained (Oh and Choi 2010).
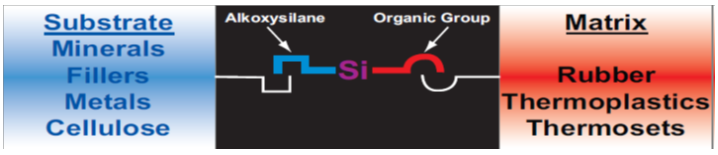
Fig. 7. Schematic representation of organosilane molecules (Materne et al. 2005)
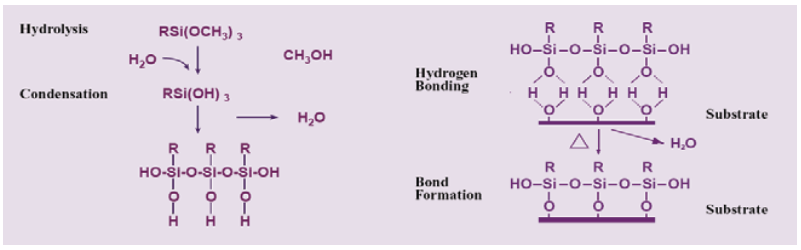
Fig. 8. The mechanism of hydrolysis, condensation, and bonding of organosilanes to a substrate (Materne et al. 2005)
As mentioned earlier, the FT-IR analysis indicated that, unlike in the Latin Walnut paper, the ring (network) polysiloxane structure was not formed in the Walnut décor paper, and an open structure occurred. As the curing reactions and formation of the final covalent bonds between the organosilanes and décor paper were mostly affected by the drying temperature of the printed papers, it was inferred that the Walnut printed paper was probably not subjected to the required temperature for the occurrence of a remarkable extent of cross-linking covalent bonds. Therefore, an absorption peak at 1061 cm-1 associated with the ring siloxane structure was not observed in the FT-IR spectrum of the Walnut paper.
FT-IR analysis of the ashes of the printed papers
A further examination of the chemical differences of the Walnut and Latin Walnut papers was performed by a FT-IR analysis of their ashes. Some interesting results were achieved by this analysis. As presented in Fig. 9, a strong peak at 1090 cm-1 with an asymmetric shape near 1200 cm–1 was observed in both FT-IR spectra, which could have been attributed to Si-O bond stretching of silicate ions present in the chemical composition of both ashes (Derrick et al. 2000). This result indicated that both décor papers contained silicon atoms in their ashes, which most likely originated from the organosilane compound used as an adhesion promoter in their printing ink formulations, as discussed earlier in the previous section.
It should be mentioned that the most widely used filler for décor paper production is titanium dioxide, which provides its opacity, whiteness, durability, and aging resistance (permanency). Therefore, it was expected that the décor paper ash would have titanium dioxide as the main component. However, no specific bands associated with titanium dioxide were observed in the FT-IR spectra of the ashes to provide evidence of its presence. This might be explained as due to the fact that titanium dioxide particles generally escape the FT-IR analysis (Gorassini et al. 2008).
Another interesting observation, with respect to the ashes, was their colors. The ashes were obtained by igniting the two printed décor papers at 900 °C, and the corresponding results are shown in Fig. 10. Although the titanium dioxide filler added to the décor paper was white in color, the ashes obtained from the printed papers were not white. As shown in Fig. 10, the ashes had specific colors, which was most likely due to the inorganic color pigments added to the corresponding pulp during the décor paper making process in the paper machine. Generally, these inorganic pigments were added in the production process of the print base décor papers to provide colors that matched the ink pigments used to print each design. In fact, the inorganic nature of these color pigments rendered them resistant against high temperatures, so that they were retained in the ash composition.
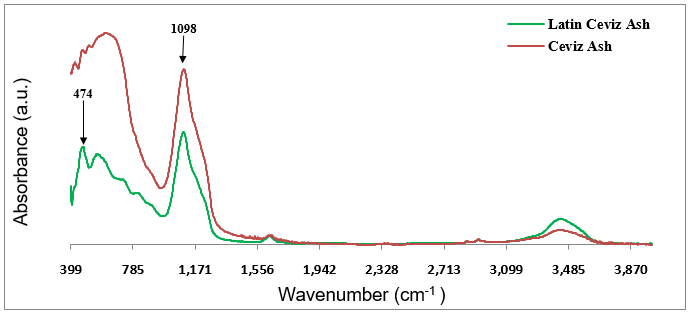
Fig. 9. FT-IR spectra obtained from the ashes of the printed Latin Walnut and Walnut décor papers
Fig. 10. Walnut (A) and Latin Walnut (B) paper ashes obtained at 900 °C
Surface morphology characterization of the UF-saturated paper by optical microscope
As presented in Table 1, the measured chemical characteristics of the two décor papers revealed considerable differences between them with respect to their pH. The two UF-saturated papers were found to have discernible differences in terms of their flexibility. Because the condensation reaction of the UF and MF resins that led to the brittleness and shelf life reduction of the saturated papers was strongly impinged by the pH value of the décor paper, and that of the resin itself, an examination of the surface of the UF-saturated paper samples was conducted. An optical microscope was used to elucidate whether, in addition to the flexibility, the surface morphology of the UF-saturated samples was also affected by the décor paper pH.
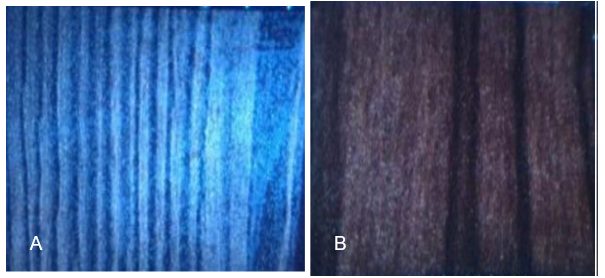
Fig. 11. Optical microscope photographs of the surfaces of the UF-saturated Walnut (A) and Latin Walnut (B) papers
The surface morphologies of the two UF-saturated papers are illustrated in Fig. 11. As clearly shown, their surface appearances were not the same. The UF resin molecules made a smooth homogeneous transparent film on the saturated Latin Walnut paper surface. In contrast, no uniform film of the UF resin was formed on the surface of the Walnut décor paper after the saturation stage. This result can be analyzed by further focusing on the influence of the décor paper hot extract pH on the condensation reaction of the UF resin and brittleness of the saturated paper.
According to the pH values of the two décor papers presented in Table 1, it seemed that an approximate pH difference of 0.5 units (7.0 versus 6.45) resulted in an accelerating effect on the condensation reaction of the UF resin in the Walnut décor paper and rendered the saturated Walnut paper more brittle than the saturated Latin Walnut paper. Similarly, the resin molecules on the surface of the Walnut paper also underwent the same accelerated curing reaction. Because the polymerization reaction of the thermosetting resins involved shrinkage of the condensed molecules, the shrunk molecules formed numerous minute particles of the cured resin on the Walnut paper surface and decreased the transparency of the resin film. Therefore, the surface morphology of the Walnut paper under the optical microscope had a lot of white spots, as shown in Fig. 11(A). It should be noted that the effect of the décor paper pH stability on the brittleness and shelf life of laminate papers was also reported on by other researchers (Goodrow and Nero 2007).
Evaluation of the MF coatings adhesion strength
The adhesion strength of the MF coatings on the UF-saturated décor paper was evaluated according to the abrasion resistance performance of the laminates in the Taber abrasion test. The laminates were prepared at the same conditions as described in Table 8 and were used for the Taber test to assess the coatings adhesion.
The results of the abrasion resistance performance test of the laminates at 1000 revolutions of the CS-17 abrasive wheels are illustrated in Fig. 12. It was observed that the surface appearances of the two abraded (damaged) samples represented the existence of a noticeable difference between the adhesion strength of the MF coatings on the UF-saturated samples. The comparison of the damaged areas of the two abraded laminates clearly indicated that the Walnut laminate was abraded on a larger area than the Latin Walnut specimen. This result suggested that the adhesion of the MF coatings to the Walnut saturated paper was not as robust as that of the Latin Walnut saturated paper.

Fig. 12. Surface appearance of the abraded laminates: Walnut (A) and Latin Walnut (B)
The likely reasons for the poor adhesion of the MF resin coatings to the Walnut saturated paper could be further discussed from two points of view. First, from the point of view of the décor paper chemical characteristics, specifically hot extract pH in this study, and second, from the viewpoint of the printed paper surface chemistry.
The influence of the lower pH value of the Walnut décor paper compared to the Latin Walnut paper resulted in a much less flexible UF-saturated Walnut foil, which could have been attributed to the catalytic effect of the low décor paper pH on the curing reactions of the UF resin. Hence, it seemed that such a brittle Walnut saturated foil, which had a surface morphology as displayed in Fig. 11(A), might not have been able to provide a suitable substrate to bind to the MF resin and to establish the tightly adhered coatings in the final laminate. However more studies should be done to gain further understanding on the effect of décor paper pH on the adhesion strength of the MF coatings in the impregnation process of décor paper.
According to the present study’s results, the surface chemistry of the gravure printed décor paper could probably be affected by the amount of printing ink, which were transferred onto the paper during printing. The appearance of the absorption bands with remarkably higher intensities in the ATR spectra demonstrated that in comparison with Walnut paper, the Latin Walnut paper had contained a greater amount of ink and a higher content of functional groups belonged to the ink constituents on its surface. Therefore, it could be inferred that the presence of more chemical functional groups might promote the adhesion, firstly between the UF resin layer and Latin Walnut paper surface, and secondly between the UF resin layer and MF coatings through the formation of more chemical bonds.
The adhesion strength between the MF coatings and UF-saturated paper could also be affected by the ink formulation and printing process conditions. In this respect, the role of the adhesion promoter additives in improving the adhesion of coatings in the ink composition should be taken into consideration. The results of this study indicated that the effectiveness of the adhesion promoters in enhancing the adhesion strength of the UF and subsequent MF coatings to the gravure printed décor paper most probably depended on the printing process conditions. As was discussed in the results of the FT-IR analysis of the printed décor papers, it seemed that the chemical bonds and siloxane structures that were formed between the printed paper and the UF resin layer determined the adhesion strength of the subsequent MF resin coatings. The ring siloxane structure, which was only identified in the FT-IR spectrum of the Latin Walnut décor paper, was found to have a noticeable contribution in enhancing the adhesion strength of MF coatings, and eventually a better abrasion resistance performance of the Latin Walnut laminate in the Taber abrasion test was achieved.
CONCLUSIONS
- The results of the ATR FT-IR analysis suggested that the surface chemistry of gravure printed décor paper could probably be affected by the ink coverage and the amount of ink transferred onto the paper surface during the printing.
- The hot extract pH as a chemical property of the print base décor paper seemed to play a significant role in the flexibility and surface quality of the UF-saturated décor paper. However, this study could not indicate the specific influence of décor paper pH on the adhesion strength of the MF coatings in the impregnation process.
- The FT-IR spectra obtained from the printed décor papers and their ashes confirmed the presence of an organosilane adhesion promoter in the printing ink formulation and formation of both the open and ring siloxane structures in the printed décor papers.
- The enhancement in the adhesion strength of the MF coatings to Latin Walnut printed paper was most likely due to the presence of more functional groups belonging to the printing ink components as well as the formation of ring siloxane structures that probably resulted from the successful curing of the molecules of organosilane adhesion promoter additive during the gravure printing.
- The findings of the present study accentuate the importance of using the organosilane adhesion promoter additive in the formulation of water-based gravure ink used for printing of décor paper.
ACKNOWLEDGEMENTS
The authors would like to thank the Aryak Décor and the Mazandaran Wood & Paper Industries (MWPI) Companies for providing the required materials and facilities and the Department of Textile Engineering at Amirkabir University of Technology for performing Spectroscopy tests.
REFERENCES CITED
Baghdachi, J. (2014). “Adhesion of coatings,” Coatings Research Institute, Eastern Michigan University, Ypsilanti, Michigan.
Bardak, S., Sarı, B., Nemli, G., Kırcı, H., and Baharoğlu, M. (2011). “The effect of decor paper properties and adhesive type on some properties of particleboard,” Int. J. Adhes. Adhes. 31(6), 412-415. DOI: 10.1016/j.ijadhadh.2011.02.008
Blank, W. J. (1979). “Reaction mechanism of amino resins,” J. Coatings Technol. 51(656), 61-70.
Bouchard, M., Rivenc, R., Menke, C., and Learner, T. (2009). “Micro-FTIR and micro-raman study of paints used by Sam Francis,” e-Preservation Sci. 6, 27-37.
Brugnerotto, J., Lizardi, J., Goycoolea, F. M., Argüelles-Monal, W., Desbrières, J., and Rinaudo, M. (2001). “An infrared investigation in relation with chitin and chitosan characterization,” Polymer 42(8), 3569-3580. DOI: 10.1016/S0032-3861(00)00713-8
Caro, C. A., Cabello, G., Landaeta, E., Pérez, J., Zagal, J. H., and Lillo, L. (2013). “Synthesis and spectroscopic and electrochemical studies of chitosan Schiff base derivatives,” Russ. J. Appl. Chem. 86(11), 1791-1797. DOI: 10.1134/S1070427213110268
Chen, X., Yu, J., Zhang, Z., and Lu, C. (2011). “Study on structure and thermal stability properties of cellulose fibers from rice straw,” Carbohyd. Polym. 85(1), 245-250. DOI: 10.1016/j.carbpol.2011.02.022
Chiang, T. C., Hamdan, S., and Osman, M. S. (2016). “Urea formaldehyde composites reinforced with Sago fibres analysis by FTIR, TGA, and DSC,” Adv. Mater. Sci. Engin. 2016, 1-10. DOI: 10.1155/2016/5954636
Composite Panel Association (2007). Laminating Composite Panels: Technical Bulletin, Composite Panel Association, Ontario, USA.
Del Hoyo-Meléndez, J. M., Gondko, K., Mendys, A., Król, M., Klisińska-Kopacz, A., Sobczyk, J., and Jaworucka-Drath, A. (2016). “A multi-technique approach for detecting and evaluating material inconsistencies in historical banknotes,” Forensic Sci. Int. 266, 329-337. DOI: 10.1016/j.forsciint.2016.06.018
Derrick, R. M., Stulik, D., and Landry, M. J. (2000). Infrared Spectroscopy in Conservation Science, Getty Publications, Los Angeles, CA, USA.
Doncea, S. M., and Ion, R. M. (2014). “FTIR (DRIFT) analysis of some printing inks from the 19th and 20th centuries,” Rev. Roum. Chim. 59(3-4), 173-183.
Dontulwar, J., Nagmote, M., and Singru, R. (2016). “Kinetics study of thermal degradation of resin derived from 1-naphthol-4-sulphonic acid, hexamethylene diamine and formaldehyde,” J. Chem. Pharm. Res. 8(5), 713-720.
Enzensberger, W. (1961). “On the surface finishing of particleboard with resin-impregnated paper layers,” Holz Roh. Werkst. 19(10), 394-398. DOI: 10.1007/BF02630378
Gelest Inc. (2006). Silane Coupling Agents: Connecting Across Boundaries, Gelest, Inc., Morrisville, PA.
Goodrow, J., and Nero, P. (2007). “Laminate paper having increased pH stability and method of making same,” U. S. Patent No. 20070012412 A1.
Gorassini, A., Adami, G., Calvini, P., and Giacomello, A. (2016). “ATR-FTIR characterization of old pressure sensitive adhesive tapes in historic papers,” J. Cult. Herit. 21, 775-785. DOI: 10.1016/j.culher.2016.03.005
Gorassini, A., Calvini, P., and Baldin, A. (2008). “Fourier transform infrared spectroscopy (FTIR) analysis of historic paper documents as a preliminary step for chemometrical analysis,” in: CMA4CH, Mediterraneum Meeting, Multivariate Analysis and Chemometrics Applied to Environment and Cultural Heritage, Ventotene Island, Italy, pp. 1-4.
Guichenuy, M., Abel, M. L., Audenaert, M., Vineer, A., and Watts, J. F. (2004). “Mechanism of delamination of a thick coating on untreated steel,” in: Proceedings of the 27th Annual Meeting of The Adhesion Society, Inc., Wilmington, NC, pp. 200-201.
Hatam, A., Dehghani Firouzabadi, M., and Resalati, H. (2015). “Preparation, characterization and abrasion resistance property of melamine formaldehyde/montmorillonite nanocomposite coatings,” Progress in Color, Colorants and Coatings 8(4), 267-281.
Hiziroglu, S. (1996). “Surface roughness analysis of wood composites: A stylus method,” Forest Prod. J. 46(7-8), 67-72.
Istek, A., Aydemir, D., and Aksu, S. (2010). “The effect of décor paper and resin type on the physical, mechanical, and surface quality properties of particleboards coated with impregnated décor papers,” BioResources 5(2), 1074-1083.
ISO 534 (2011). “Determination of thickness, density and specific volume for paper and board,” International Standardization Organization, Geneva, Switzerland.
ISO 536 (2012). “Determination of grammage for paper and board,” International Standardization Organization, Geneva, Switzerland.
ISO 1924-2 (2008). “Determination of tensile properties for paper and board using elongation method,” International Standardization Organization, Geneva, Switzerland.
ISO 1974 (2012). “Determination of tearing resistance for paper and board using the Elmendorf method,” International Standardization Organization, Geneva, Switzerland.
ISO 2144 (2015). “Determination of residue (ash) on ignition of pulps, papers and boards at 900 degrees C,” International Standardization Organization, Geneva, Switzerland.
ISO 2758 (2014). “Determination of bursting strength for paper and board,” International Standardization Organization, Geneva, Switzerland.
ISO 5636-5 (2013). “Determination of air permeance for paper and board using the Gurley method,” International Standardization Organization, Geneva, Switzerland.
ISO 8791-4 (2007). “Determination of roughness/smoothness for paper and board using the Print-surf method,” International Standardization Organization, Geneva, Switzerland.
Jahan, M. S., Saeed, A., and Ni, Y. (2012). “Extraction of cellulose and microfibrils from non-wood lignocellulosic material using biorefinery concept,” Lignocellulose 1(3), 185-195.
Jing, S. Y., Lee, H. J., and Choi, C. K. (2002). “Chemical bond structure on Si-O-C composite films with a low dielectric constant deposited by using inductively coupled plasma chemical vapor deposition,” J. Korean Phys. Soc. 41(5), 769-773.
Kotelnikova, N. E., and Mikhailidi, A. M. (2011). “Hydrate cellulose films and preparation of film composites with nickel nano- and microparticles. I. Properties of hydrate cellulose films,” Cell. Chem. Technol. 45(9), 585–592.
Lanxess Corporation (2006). Inorganic Pigments for the Paper Industry, Lanxess Corporation, Cologne, Germany.
Manso, M., and Carvalho, M. L. (2009). “Application of spectroscopic techniques for the study of paper documents: A survey,” Spectrochim. Acta B 64(6), 482-490. DOI: 10.1016/j.sab.2009.01.009
Marshall, P., and Parent, B. (1998). “What is a laminate? How decorative foils, low pressure laminates and high pressure laminate are produced,” in: Proceedings of TAPPI Plastic Laminates Symposium, Atlanta, GA.
Materne, T., de Buyl, F., and Witucki, G. L. (2005). Organosilane Technology in Coating Applications: Review and Perspectives, Dow Corning Corporation, Midland, MI.
Nastova, I., Grupče, O., Minčeva-Šukarova, B., Kostadinovska, M., and Ozcatal, M. (2015). “Spectroscopic analysis of pigments and inks in manuscripts. III. Old-Slavonic manuscripts with multicolored rubication,” Vib. Spectrosc. 78, 39-48. DOI: 10.1016/j.vibspec.2015.03.005
Oh, T., and Choi, C. K. (2010). “Comparison between SiOC thin films fabricated by using plasma enhanced chemical vapor deposition and SiO2 thin films by using Fourier transform infrared spectroscopy,” J. Korean Phys. Soc. 56(4), 1150-1155.
Podhajny, M. R. (2004). “Water-based printing inks & pH,” (http://www.pffc-online.com/magazine/2956-printing-inks-ph-0105), 31 December 2004.
Roberts, R. J., and Evans, P. D. (2005). “Effects of manufacturing variables on surface quality and distribution of melamine formaldehyde resin in paper laminates,” Compos. Part A-Appl. S. 36(1), 95-104. DOI: 10.1016/j.compositesa.2003.05.001
Rydholm, S. A. (1965). Pulping Processes, Interscience Publishers, New York, NY.
Sathyanarayana, M. N., and Yaseen, M. (1995). “Role of promoters in improving adhesion of organic coatings to a substrate,” Prog. Org. Coat. 26, 275-313.
Singru, R. N., and Gurnule, W. B. (2010). “Chelating ion-exchange properties of copolymer resins derived from p-cresol, oxamide and formaldehyde,” Iran. Polym. J. 19(3), 169-183.
Singru, R. N., Khati, V. A., Gurnule, W. B., Zade, A. B., and Dontulwar, J. R. (2011). “Studies on semiconducting, chelating and thermal properties of P-cresol–oxamide-formaldehyde terpolymer resin,” Analytical & Bioanalytical Electrochemistry 3(1), 67-86.
Šmidriaková, M., and Laurová, M. (2011). “ATR-FTIR spectral analysis of modified UF adhesive,” Forestry and Wood Technology 76, 49-53.
Stenger, J., Kwan, E. E., Eremin, K., Speakman, S., Kirby, D., Stewart, H., Huang, S. G., Kennedy, A. R., Newman, R., and Khandekar, N. (2010). “Lithol red salts: Characterization and deterioration,” e-Preservation Science 7, 147-157.
TAPPI T435 om-02 (2006). “Hydrogen ion concentration (pH) of paper extracts (hot extraction method),” TAPPI Press, Atlanta, GA.
TAPPI T459 om-13 (2013). “Surface strength of paper (wax pick test),” TAPPI Press, Atlanta, GA.
Tocchio, U., Negro, P., O’Neill, G. P., and Srl, T. (1993). “Melamine treaters/coaters,” in: European Plastic Laminates Forum, Koln, Germany, pp. 47-55.
Vahur, S., Teearu, A., and Leito, I. (2010). “ATR-FT-IR spectroscopy in the region of 550-230 cm−1 for identification of inorganic pigments,” Spectrochim. Acta A 75(3), 1061-1072. DOI: 10.1016/j.saa.2009.12.056
Vikman, K., and Sipi, K. (2003). “Applicability of FTIR and Raman spectroscopic methods to the study of paper–ink interactions in digital prints,” J. Imaging Sci. Techn. 47(2), 139-148.
Witucki, G. L. (1993). “A silane primer: Chemistry and applications of alkoxy silanes,” J. Coating. Technol. 65(822), 57-60.
Xing, C., Zhang, S. Y., Deng, J., and Wang, S. (2007). “Urea-formaldehyde-resin gel time as affected by the pH value, solid content, and catalyst,” J. Appl. Polym. Sci. 103(3), 1566-1569. DOI: 10.1002/app.25343
Zorba, T., Papadopoulou, E., Hatjiissaak, A., Paraskevopoulos, K. M., and Chrissafis, K. (2008). “Urea formaldehyde resins characterized by thermal analysis and FTIR method,” J. Therm. Anal. Calorim. 92(1), 29-33. DOI: 10.1007/s10973-007-8731-2
Article submitted: January 27, 2017; Peer review completed: March 29, 2017; Revised version received: April 12, 2017; Accepted: April 15, 2017; Published: April 26, 2017.
DOI: 10.15376/biores.12.2.4239-4258
