Abstract
Ground calcium carbonate (GCC) was modified by starch with the aid of sodium stearate and sodium hexametaphosphate. The GCC was encapsulated within the complex. The effect of the dosages of sodium stearate and sodium hexametaphosphate on the coating weight of modified GCC and the complex utilization rate were studied. The SEM images of modified GCC were compared with that of unmodified GCC. The results showed that the dosage of these two modifiers affected the coating weight of modified GCC and the complex utilization rate.
Download PDF
Full Article
Surface Modification of Ground Calcium Carbonate with Starch, Sodium Stearate, and Hexametaphosphate
Huiming Fan, Xueqin Wang, Jianan Liu,* and Binfeng Xu
Ground calcium carbonate (GCC) was modified by starch with the aid of sodium stearate and sodium hexametaphosphate. The GCC was encapsulated within the complex. The effect of the dosages of sodium stearate and sodium hexametaphosphate on the coating weight of modified GCC and the complex utilization rate were studied. The SEM images of modified GCC were compared with that of unmodified GCC. The results showed that the dosage of these two modifiers affected the coating weight of modified GCC and the complex utilization rate.
Keywords: Modified GCC; Starch; Sodium stearate; Sodium hexametaphosphate; Coating weight; Complex utilization rate
Contact information: State Key Laboratory of Pulp and Paper Engineering, South China University of Technology, 510640, Guangzhou, China; *Corresponding author: 191545996@qq.com
INTRODUCTION
In recent years, the use of high filler content has become an important trend in the papermaking industry. Paper production costs are reduced through high filler loading, and the market competitiveness of paper mills has also been strengthened (Feng et al. 2010; Wang et al. 2010; Wu and Zhang 2010). However, paper strength properties are inevitably reduced by mineral fillers, such as calcium carbonate, kaolin, and titanium dioxide (Zhu et al. 2007; Zhang et al. 2010; Bai and Fan 2011). This occurs because the elements and properties are different between the mineral fillers and the fibers. Fillers are difficult to combine with fibers; fiber-fiber bonding is obstructed with the addition of filler (Zhao et al. 2005; Lu et al. 2010; Fan et al. 2012; Huang et al. 2013; Zhang 2013). To overcome or alleviate the effects of fillers on paper strength, many methods have been examined, such as pre-flocculation and modification of the filler.
Filler modification technology is an effective method and an emerging topic. Starch is well known for its superior bonding ability because of the identical structural units between starch and fiber. In addition, the strong coating performance of starch in the gelatinized state is conducive to the formation of a starch-coating layer on the filler’s surface (Yan et al. 2005; Yong and Deng 2006; Shen et al. 2009; Sang et al. 2012). In comparison with other organic modifiers, starch has many advantages, such as being environmentally friendly and low-cost. Many studies have investigated the use of starch-modified fillers, mostly discussing the use-based effects of modified filler. Wand et al. (2007) studied the process of starch-modified GCC; the starch-modified GCC was used as papermaking filler to prepare handsheets and physical properties of those sheets were tested. The results showed that the paper strength properties were greatly improved. Luo (2010) studied the optical and physical properties of starch-sodium stearate modified GCC in handsheets. The results showed that compared with unmodified GCC filled papers, the physical properties of modified GCC were greatly improved, and the optical properties of modified GCC was slightly lower. Li et al. (2012) invented a method of cationic starch modified calcium carbonate; the physical properties and printability were improved, and the production cost was reduced. However, there are few studies concerning the modified filler itself.
In this study GCC was modified with starch, sodium stearate, and sodium hexametaphosphate. Using sodium stearate and sodium hexametaphosphate as modifiers, GCC could get more lipophilic properties, with activity improved, reagglomeration reduced, particle specific surface area increased, dispersibility improved in the water, and so on. In addition, in the modification, the starch can make strong hydrogen bonding with fibers. Sodium stearate was a hydrophobic agent, which can decrease the solubility of starch in water. Sodium hexametaphosphate was used as a cross-linking agent, which can increase the combination between modifiers and GCC. The effect of sodium stearate and sodium hexametaphosphate on the coating weight of the modified GCC and the complex utilization rate were studied in this work. In addition, the use-based effects of modified and unmodified GCC were investigated.
EXPERIMENTAL
Materials
Industrial grade ground calcium carbonate (GCC) was provided by Henan Tianbang Co., Ltd., China. Corn starch was supplied by the Shenzhen Taigang Food Co., Ltd., China. Sodium stearate (analytical reagent) was obtained from the Tianjin Kermel Chemical Reagent Co., Ltd., China. Sodium hexametaphosphate (analytical reagent) was provided by the Tianjin Yongda Chemical Reagent Development Center. Bleached eucalyptus pulp, with a drainage degree of 32° SR, was supplied by the Henan Tianbang Co., Ltd., China.
Methods
Preparation of modified GCC
A 20% corn starch and GCC suspension (starch: GCC weight ratio=1:5) was dispersed in cool water for 10 min under stirring at 200 rpm, then heated to 95 °C for 50 min. Afterwards, the sodium stearate solution was added to a starch and GCC suspension for 30 min. The dosage of sodium stearate was varied from 0% to 8.0% (based on the dry weight of GCC). Then, the temperature was decreased to 60 °C and 90 °C, respectively. A sodium hexametaphosphate solution was poured into the mixture solution for 30 min while stirring at 200 rpm. The dosage of sodium hexametaphosphate was varied from 0% to 3.0% (based on the dry weight of GCC).
The calculation of coating weight of modified GCC and the complex utilization rate
The coating weight of modified GCC and the complex utilization rate were calculated using the following equations,
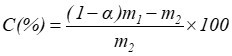 (1)
(1)
where C is the coating weight, m1(g) is the constant-weight of the modified GCC, m2(g) is the constant-weight of ash, and α is the loss on ignition of GCC at 575 °C for 4 h or more; and
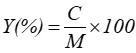 (2)
(2)
where Y is the complex utilization rate, M(g) is the total amount of added coated materials during the modification of GCC, and C is the coating weight (Fan et al. 2014).
RESULTS AND DISCUSSION
Coating Weight of Modified GCC and the Utilization Rate
Effects of the dosage of sodium stearate on the coating weight and complex utilization rate of modified GCC
As shown in Fig. 1, the coating weight and the complex utilization rate of modified GCC to which sodium stearate was not added were 16.68% and 77.58%, respectively. The coating weight of modified GCC and the complex utilization rate were 19.21% and 85.38%, respectively, when the dosage of sodium stearate was 1.0%, which was increased in comparison to modified GCC with no sodium stearate added. This may have occurred because a large amount of dissolved starch was not coated on the surface of the GCC when sodium stearate was not present. Therefore, the coating weight of modified GCC and the complex utilization rate were remarkably improved when sodium stearate was added. The coating weight of modified GCC increased greatly with increasing dosage of sodium stearate, and then increased more slowly beyond a certain threshold dose.
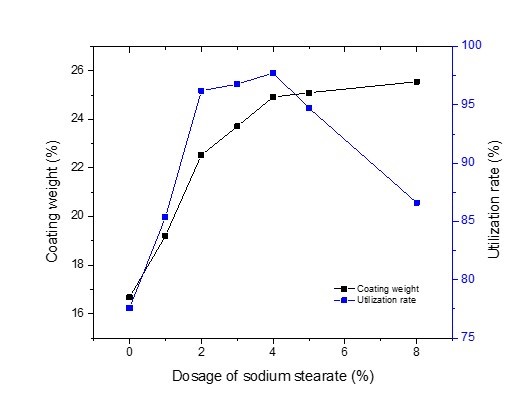
Fig. 1. Coating weight of the modified GCC and the complex utilization rate as a function of different dosages of sodium stearate. The dosage of sodium hexametaphosphate was 1.5%. (The average standard deviation of coating weight and utilization rate was 0.0025 and 0.0013, respectively.)
When the dosage of starch was small (≤ 4.0%), the composite reaction between the sodium stearate and starch was greatly improved with increasing dosage of sodium stearate. Therefore, the hydrophobicity was enhanced and the utilization rate increased. However, the composite reaction between sodium stearate and starch reached saturation when the dosage of starch was excessive (≥ 4.0%), and the resulting increment in the coating weight of modified GCC gradually slowed. However, the complex utilization rate increased greatly at first, as the dosage of sodium stearate increased, and then increased more slowly, and finally reversed. This occurred because the hydrophobicity of starch was enhanced at the beginning when the starch-based complexes were effectively coated on the surface of the GCC. Afterwards, the effect of sodium stearate decreased because of the saturation of the composite reaction between sodium stearate and starch. Finally, the complex utilization rate decreased because of the depletion of sodium stearate for the reaction.
Effects of the dosage of sodium hexametaphosphate on the coating weight and complex utilization rate of modified GCC
The coating weight and utilization rate of modified GCC increased when the dosage of sodium hexametaphosphate increased from 0% to 1.5% (Fig. 2). The coating weight and utilization rate of modified GCC were 24.45% and 95.88%, respectively, when the dosage of sodium hexametaphosphate was 1.5%, which increased by approximately 8.06% to 27.59% in comparison with the samples in which sodium hexametaphosphate was not added. This showed that the complex utilization rate or the starch utilization was greatly improved with the addition of sodium hexametaphosphate. The coating weight of the modified GCC increased when sodium hexametaphosphate was increased from 1.5% to 3.0%; however, the utilization rate decreased. This was because the increment of the coating weight of modified GCC was smaller than the increment of the dosage of sodium hexametaphosphate. For this situation, some of the sodium hexametaphosphate was not coated to the surface of GCC and may have dissolved in the water because of its high solubility with a large amount of sodium hexametaphosphate (> 1.5%).
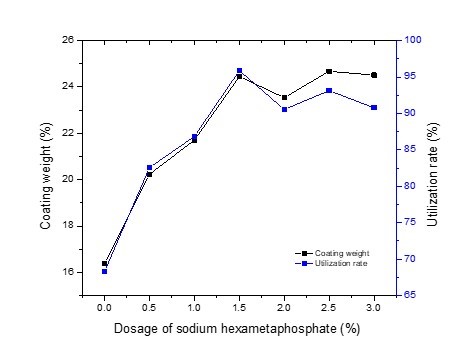
Fig. 2. Coating weight of the modified GCC and the complex utilization rate as a function of different dosages of sodium hexametaphosphate. The dosages of starch and sodium stearate were 20% and 1.5%, respectively. (The average standard deviation of coating weight and utilization rate was 0.0018 and 0.0011, respectively.)
Analysis of Zeta Potential and Water Contact Angle of modified GCC
The sodium hexametaphosphate was very easily solubilized in water, so the zeta potential and water contact angle of it could not be detected. As shown in Table 1, the zeta potential of modified GCC with 4.0% sodium stearate and 1.5% sodium hexametaphosphate was -2.7 mV, which was far lower than the zeta potential of unmodified GCC. This indicated that the zeta potential of modified GCC were determined by those modifiers. In addition, the zeta potential of modified GCC was affected by unmodified GCC to a certain extent. GCC with positively charged can be coated with positively charged starch, which indicated that gelatinized starch had strong coating performance, and then overcame the electrostatic repulsion between them. The water contact angle of starch was less than 90°, which indicated it was hydrophilic and easily solubilized in water. The water contact angle of GCC, sodium stearate, and modified GCC were more than 90°, which indicated that they were hydrophobic. As hydrophobic agent, the sodium stearate improved the hydrophobic properties of modified GCC, and decreased the solubility of starch.
Table 1. Zeta Potential and Water Contact Angle of Modified GCC
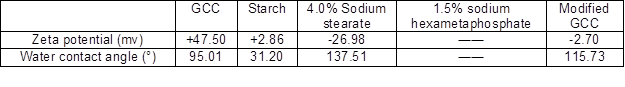
Analysis of the Modified GCC Sample and SEM Observations
As shown in Fig. 3, compared with the modification conditions of 60 °C and 0.0% sodium hexametaphosphate and 90 °C and 1.5% sodium hexametaphosphate, the suspension based on the 60 °C and 1.5% sodium hexametaphosphate was clearer and the sediment layer was thicker. This was attributed to the effects of the precipitation temperature and the dosage of sodium hexametaphosphate on the coating performance of modified GCC.

Fig. 3. Schematic diagram of Modified GCC. (Note: The precipitation temperatures of the three samples were 60 °C, 60 °C, and 90 °C, respectively, and the dosage of sodium hexametaphosphate of the three samples were 1.5%, 0.0%, and 1.5%, respectively.)
The lower precipitation temperature and use of sodium hexametaphosphate were conducive to the decrease in starch solubility; therefore, the utilization of starch increased. Consequently, the suspension was clearer, the particle size was larger when the amount of starch increased, and the sediment layer was thicker.
As shown in Fig. 4, the surface morphology of modified GCC was obviously different from that of the unmodified GCC. Modified GCC was coated by a layer of complex, which reduced the angular sharpness. Furthermore, the bonding between the modified GCC and the fibers was closer than that of the unmodified GCC. This occurred because the starch on the surface of the modified GCC had many hydroxyl groups, which could participate in hydrogen bonding with fibers.
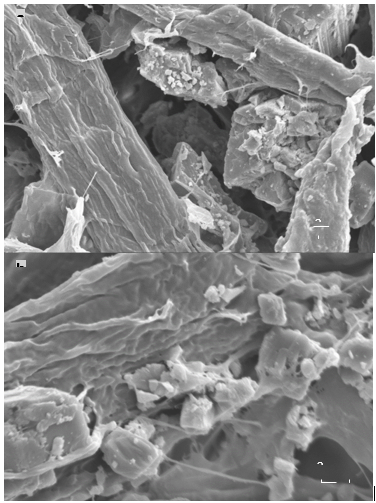
Fig. 4. SEM images of (a) unmodified GCC and (b) modified GCC
CONCLUSIONS
- To some extent, the coating weight of modified GCC was enhanced as the dosages of sodium stearate and sodium hexametaphosphate were increased.
- The complex utilization rate was reduced when excessive amounts of sodium stearate and sodium hexametaphosphate were added. This was attributed to the effective utilization rate of sodium stearate and sodium hexametaphosphate.
- The optimal dosages of sodium stearate and sodium hexametaphosphate were 4.0% and 1.5% (based on the dry weight of GCC), respectively.
ACKNOWLEDGMENTS
This work was supported by the Science and Technology Plan Projects of Guangdong Province (No.2013B010402010).
REFERENCES CITED
Bai, W. R., and Fan, H. M. (2011). “Preparation of starch-stearic complex modified PCC filler and its application as paper filler,” China Pulp and Paper Industry 30(1), 5-9.
Fan, H. M., Wang, D. X., Bai, W. R., and Liu, J. A. (2012). “Starch-sodium stearate complex modified PCC filler and its application in papermaking,” BioResources 7(3), 3317-3326. DOI: 10.15376/biores.7.3.3317-3326
Fan, H. M., Xu, B. F., Liu, J. A., and Zhang, C. (2014). “Effect of starch dosage on the properties of modified ground calcium carbonate,” BioResources 9(3), 4679-4689. DOI: 10.15376/biores.9.3.4679-4689
Feng, C., Chen, G., Chai, X. S., and Fang, Z. Q. (2010). “Effect of calcium carbonate- fiber fines composite fillers on the paper properties,” China Pulp and Paper Industry 29(2), 14-17.
Huang, X. J., Shen, J., and Qian, X. R. (2013). “Filler modification for papermaking with starch/oleic acid complexes with the aid of calcium ions,” Carbohydrate Polymers 9(1), 931-935. DOI: 10.1016/j.carbpol.2013.07.024
Li, X. L., Zhao, H., Wei, Q. M., Sun, Y. J., and Mao, X. D. (2012). “A method for cationic starch modified calcium carbonate filler,” China Patent No. 103073926.
Lu, Z. H., Li, M., and Liu, H. J. (2010). “Simple introduction of paper filler,” Journal of Hubei Papermaking 4(2), 40-44.
Luo, S. Q. (2010). “Surface modification of precipitated calcium carbonate and its application in papermaking process,” Master thesis, South China University of Technology, Guangzhou.
Sang, Y., McQuaid, M., and Englezos, P. (2012). “Pre-flocculation of precipitate calcium carbonate filler by cationic starch for highly filled mechanical grade paper,” BioResources 7(1), 354-373. DOI: 10.15376/biores.7.1.0354-0373
Shen, J., Song, Z., and Qian, X. (2009). “Investigations on the preparation of starch/sodium oleate/alum modified precipitated calcium carbonate filler and its use in papermaking,” Appita Journal 62(5), 360-364, 382.
Wang, H. P., Zhang, G. H., Lai, Z. C., and Shen, Y. (2010). “Preparation of cationic starch modified GCC filler and its application in high-filler paper,” China Pulp and Paper Industry 29(1), 6-8.
Wang, Y. Z., Pei, J. C., and Shi, S. L. (2007). “Surface modification of GCC and its application in papermaking,” Paper and Paper Making 26(6), 54-58.
Wu, H. Y., and Zhang, H. W. (2010). “Preparation of coated GCC and its effects on the property of paper,” China Pulp and Paper Industry 29(6), 24-27.
Yan, Z., Liu, Q., Deng, Y., and Ragauskas, A. (2005). “Improvement of paper strength with starch modified clay,” Journal of Applied Polymer Science 97(1), 44-50. DOI: 10.1002/app.21727
Yong, S. Y., and Deng, Y. (2006). “Starch-fatty complex modified filler for papermaking,” TAPPI Journal 5(9), 3-9.
Zhang, C. (2013). “The Fundamental Characteristics of Starch-Sodium Stearate Modified GCC and Its Influence on Paper Performance,” M.S. thesis, South China University of Technology, Guangzhou, China.
Zhang, G. H., Wang, H. P., Wang, X. X., Lai, Z. C., and Li, W. (2010). “Preparation and application of composite filler based on AKD-starch- calcium carbonate,” China Adhesives 19(1), 35-39.
Zhao, Y., Hu, Z., Ragauskas, A. J., and Deng, Y. (2005). “Improvement of paper properties using starch-modified precipitated calcium carbonate filler,” TAPPI Journal 4(2), 3-7.
Zhu, H. L., Chen, K. F., and Chen, G. (2007). “Modification and application technology of filler used in paper industry,” Papermaking Science and Technology 26(5), 46-48.
Article submitted: September 11, 2015; Peer review completed: November 8, 2015; Revised version received: November 13, 2015; Further revised version received and accepted: November 21, 2015; Published: December 3, 2015.
DOI: 10.15376/biores.11.1.957-964
