Abstract
The co-pyrolysis of Salix psammophila and coal was conducted in a pyrolysis reactor. The interaction between coal and S. psammophila at different ratios was investigated. It was found that the promotion and inhibition effects were more obvious at 20 wt.% and 40 wt.% Salix psammophila, respectively, indicating a certain synergistic effect. The results of the N2 adsorption-desorption experiment showed that the addition of S. psammophila increased the surface area and decreased the pore size of the co-pyrolysis char. The Fourier transform infrared (FT-IR) spectra indicated that the pyrolysis tar contained mainly aromatic rings and fatty compounds. The addition of S. psammophila was beneficial to the decomposition of phenolic hydroxyls, ether bonds, and oxygen-containing heterocyclic rings in the coal tar. However, the addition of 50 wt.% S. psammophila blocked the pores of the coal and thus obstructed its pyrolysis, while the porosity of the co-pyrolysis char became more abundant. Richer porosity of the char implies great potential to be widely used in the sewage treatment industry.
Download PDF
Full Article
Synergetic Effect and Product Characteristics of Coal and Salix psammophila Co-pyrolysis
Juhui Gong, Tingting Shao, and Kebing Wang *
The co-pyrolysis of Salix psammophila and coal was conducted in a pyrolysis reactor. The interaction between coal and S. psammophila at different ratios was investigated. It was found that the promotion and inhibition effects of the co-pyrolysis experiment were more obvious at 20 wt.% and 40 wt.% Salix psammophila, respectively, indicating a certain synergistic effect. The results of the N2 adsorption-desorption experiment showed that the addition of S. psammophila increased the surface area and decreased the pore size of the co-pyrolysis char. The Fourier transform infrared (FT-IR) spectra indicated that the pyrolysis tar contained mainly aromatic rings and fatty compounds. The addition of S. psammophila was beneficial to the decomposition of phenolic hydroxyls, ether bonds, and oxygen-containing heterocyclic rings in the coal tar. However, the addition of 50 wt.% S. psammophila blocked the pores of the coal and thus obstructed its pyrolysis, while the porosity of the co-pyrolysis char became more abundant. Richer porosity of the char implies great potential to be widely used in the sewage treatment industry.
Keywords: Biomass; Co-pyrolysis; Oil; Char; Synergetic effect
Contact information: College of Science, Inner Mongolia Agricultural University, Huhhot 010018, China; * Corresponding author: wkb0803@163.com
INTRODUCTION
Environmental pollution and a shortage of energy are increasingly prominent and serious issues motivating the need to find new energyresources to replace diminishing fossil fuels. China is rich in coal, but poor in oil and gas, making coal the primary energy source, thus requiring its clean utilization (Li and Suzuki 2010; Tian and Sang2013; Zhang et al. 2015). However, coal has a low hydrogen content and pyrolysis conversion rate. Biomass, the fourth most prominent energy source after coal, oil, and natural gas, is currently the only renewable energy that can be stored and transported. Biomass also contains low amounts of sulfur and nitrogen, has almost zero CO2emission, is highly volatile, and is rich in alkaline oxides and hydrogen. When co-pyrolysis of inexpensive and hydrogen-rich Salix psammophila and coal is conducted, hydrogen in the S. psammophilastructure may transfer to the coal structure and connect to carbon atoms in the coal. Thus, the conversion rate of pyrolysis is improved and biomass resources are saved (Guo and Bi 2015). The purpose of this experiment was to investigate the role of S. psammophila in coal pyrolysis, utilize the coal efficiently, and reduce the resource waste of S. psammophila.
Coal pyrolysis technology, as an independent thermochemical conversion process, especially pyrolysis of low-grade coal, yields high yields of tar and gas that can play a role in efficient coal utilization and pollutant control. However, due to the characteristics of hydrogen depletion and structural features of coal itself, hydrogen in coal pyrolysis mainly escapes with water of combination and stable light aliphatic hydrocarbon. This situation leads to aromatic clusters at higher temperature that are hard to be cracked in the absence of hydrogen, and mainly a mixture of heavy coal tar, char, and coke are produced. Therefore, there is a need to introduce external hydrogen to increase the volatile oil production. Conventional hydrogenation pyrolysis requires hydrogen production and gas circulation device. This costs more money. So the search for cheap hydrogen source is one of the directions of coal hydrogenation pyrolysis. Biomass is a natural renewable fuel that contains abundant hydrogen. It has the characteristics of volatility, charcoal activity, and high alkaline oxide content. If hydrogen in biomass can be effectively transferred to coal, or if it can impart a catalytic effect, then it can achieve the purpose of co-pyrolysis to improve coal’s conversion rate.
S. psammophila has the characteristics of drought resistance, resistance to wind and sand, easy reproduction, strong sand retention, and strong soil formation. And the more it is cut, the more it grows, or it dies. Therefore, it is necessary to take advantage of the agricultural and forestry waste. Biomass is the only clean and renewable energy that can be stored and transported. The content of nitrogen and sulfur is low, the growth needs of CO2 and combustion emissions of CO2 content is roughly equal, so the net for atmospheric CO2 emissions to zero. Pyrolysis technology of biomass samples refers to the process of biomass thermal degradation of three products of solid, liquid, and gas under the condition of complete hypoxia of biomass. Therefore, it is a mature technique to use biomass in pyrolysis process.
Several studies have investigated the co-pyrolysis of different mixtures of coal and biomass. Some researchers have observed synergistic effects. Krerkkaiwan et al. (2013) studied the interaction between biomass and coal in a fixed bed reactor under a N2atmosphere. The results showed a synergetic effect in lower tar and char yields and higher gas yield, especially in the blend ratio of biomass and coal=1:1. Haykiri-Acma and Yaman (2010) studied the co-pyrolysis of lignite and hazelnut shell via thermogravimetric analysis. The experimental results showed an obvious synergistic effect between lignite and hazelnut shell in the temperature range of 200 °C to 300 °C, but no such effects were reported above 500 °C. The addition of biomass led to some increases in the volatilization rates of coals, especially at temperatures below 500 °C. Wu et al. (2017) reported synergistic effects during the co-pyrolysis of low-rank coal and microalgae biomass. Glycine showed positive synergistic effects. With lower-rank coal under 25% glycine mass ratio, the yield of volatile was higher than the calculated value. The value of WCalculated was higher than that of WExperimental.
Some previous studies indicate an inhibitive effect between coal and biomass during the co-pyrolysis process. Li et al. (2015) studied the co-pyrolysis characteristics of biomass and bituminous coal by changing the heating rate and biomass blending ratio at 900 °C. The results showed that the co-pyrolysis of biomass and coal had an interaction that was influenced by the biomass heat transfer. The residue weight fractions were higher than the calculated values. Some studies think that synergistic and inhibition co-exist in co-pyrolysis. Yi et al. (2016) studied the co-pyrolysis of lignite and bagasse at different mixing ratios and reported an interaction between lignite and bagasse; at a blending ratio of 20%, co-pyrolysis played the most important role in promoting tar. Cheng et al. (2016) studied the co-pyrolysis of lignite and ulva at low temperatures and found that the amount of pyrolysis oil decreases after a prior increase with the increase of biomass blending ratio. The interaction effect of ligniteand ulva was first promoted and then inhibited. The synergistic effect was highest with a 30% blending ratio of ulva.
In this study, the authors performed a co-pyrolysis experiment at 600 °C at different blend ratios of coal to S. psammophila (2:8, 4:6, 5:5, 6:4, and 8:2). The authors studied the effect of increasing amounts of S. psammophila on the pyrolysis of coal, thus providing a theoretical basis for improving the clean utilization of coal and S. psammophila, mitigating energy shortages, and preventing environmental degradation.
EXPERIMENTAL
Materials
The Shengli coal and Salix psammophila were bought from the Inner Mongolia Autonomous Region (Ordos, China) and NingXia province (Ningxia Hedong Sandy, Ningxia Hui Autonomous Region, China), respectively. The materials were pulverized, sieved, and dried for 6 h at 105 °C before use. The particle sizes of the materials were in the range of 2.50 mm to 5.00 mm. The proximate and ultimate analyses of the coal and S. psammophila are summarized in Table 1.
Table 1. Proximate and Ultimate Analyses of Coal and Salix psammophila
Experimental apparatus
Pyrolysis experiments were conducted in a pyrolysis reactor system, depicted in Fig. 1. All of the products could be collected. The solid product was collected in a self-sealing bag after cooling in the tube. The liquid product was received by the receiving flasks after passing through the condensing tube. The gas product could enter the gas chromatograph through a six-way valve on the instrument or pass through a wet gas flow meter, collected in the air bag. The experimental reactor has a three-stage heated tube furnace made of 2520 stainless steel material with an inner diameter of 25 mm and length of 700 mm. The temperature of the middle stage was controlled by the reactor’s temperature program and the upper and lower stages used direct temperature control. The pyrolysis temperature was measured using a thermocouple. A Büchner funnel was connected to the outlet of the reactor under the heating furnace to collect the cooled liquid of the high temperature pyrolyzed products from the condenser. The amounts of gas products from the outlet of the Büchner funnel were measured by a wet gas flowmeter, and the composition of the gas was analyzed by a gas chromatograph (GC; Shanghai KeChuang Gas Chromatography Instrument Co., Ltd., ShangHai, China). The atmosphere of the experiment was high purity nitrogen (99.999%). The infrared spectrum was measured using a Spectrum 65 Fourier transform infrared spectrometer (FT-IR; PerkinElmer, Waltham, MA, USA). The specific surface area and pore structure were determined using a JW-BK static nitrogen adsorber (Beijing Jingwei Gaobo Science and Technology Co., Ltd., Beijing, China). The morphology of the co-pyrolysis char was obtained from scanning electron microscope (SEM; Hitachi, Beijing, China) images. The elemental analysis was determined using an Italy EA3000 Elemental Analyzer (Leeman China, Beijing, China). The mass ratio of co-pyrolysis in Figs. 3, 4, and 5 are 50% coal +50% S. psammophlia.
Fig. 1. Schematic of pyrolysis reactor system: 1. Temperature controller; 2. Carrier gas; 3. Gas rotor meter; 4. Reaction tube; 5. Process furnace; 6. Cold finger; 7. Receiving Flasks; 8. Rotameter; 9. Six-way valve; 10. Gas chromatograph; 11. Chromatogram; and 12. Argon
Methods
A certain amount of the sample was loaded into the reactor to ensure raw materials were in the middle part of the reaction tube, which can be subjected to temperature programming. Approximately 35 g coal was used for the pyrolysis of coal. An amount of 8 g was used for the pyrolysis of the Salix psammophila. The blend ratios of co-pyrolysis of coal and S. psammophila are listed in Table 2. High purity N2(99.999%) was introduced to the reaction system for 30 min to eliminate residue air in the system. The sample was heated from room temperature to 600 °C at a heating rate of 50 °C/min and held for 120 min. The flow rate of N2 was 300 mL/min. The pyrolysis products at different coal to S. psammophila blend ratios were analyzed and studied. The mass ratios of S. psammophila in the mixtures were 0.20, 0.40, 0.50, 0.60, and 0.80. The reason for choosing 600 °C is because pyrolysis of biomass is not complete at lower temperatures. Thus, macromolecules of woody raw materials do not break sufficiently and will only break at sites where the bond energy is small, so the product has higher residual solids and less liquid yield. As the temperature increases, the pyrolysis reaction becomes more and more complete. The volatile components precipitated in the solid are continuously precipitated. At the same time, there may be a reaction between the carbon, the volatile components and the gas. Therefore, the remaining amount of solids decreases and the liquid yield increases. When the temperature is higher than 600 °C, the site of molecular rupture further increases. The second pyrolysis reaction of the initial product (steam) formed by pyrolysis aggravates and forms the small molecule gas of the secondary oil, resulting in the decrease of liquid yield. The second stage of coal pyrolysis (350 to 600 °C) is the thermal decomposition stage to depolymerization and decomposition reactions, generating large amounts of gas and tar. In summary, the maximum liquid yield was 600 °C, so the temperature 600 °C was judged to be most favorable.
Table 2. Blend Ratio of Co-pyrolysis of Coal and S. psammophila
Definition of theoretical yield/measured yield
Equations 1 through 3 were used to find the measured yield, theoretical yield, and difference or error between the two quantities. The αmeasured is the yield (wt.%) of coal, S. psammophila, and blended coal-Salix psammophila particles. The mt is the residue mass (g) of coal, S. psammophila, and blended coal-Salix psammophilaafter pyrolysis. The m0 is the mass (g) of coal, S. psammophila, and blended coal-Salix psammophila. The αtheoretical is the theoretical yield (wt.%) of the linear weighted average calculation of coal and Salix psammophila. The αbiomass, αcoal, and αcoal-biomass are the measured yields (wt.%) of the pyrolysis of S. psammophila, coal, and the mixture of coal and S. psammophila, respectively. The measured yields were obtained using Eq. 1,
αmeasured (%) = mt / m0 × 100 (1)
αtheoretical = αbiomass × ω + αcoal × (1 – ω) (2)
Δα = αmeasured – αtheoretical (3)
where, αmeasured is the yield (wt.%) of coal, Salix psammophila, and blended coal-Salix psammophila particles; mt is the residue mass (g) of coal, S. psammophila, and blended coal-S. psammophila after pyrolysis; m0 is the mass (g) of coal, S. psammophila, and blended coal-S. psammophila; αtheoretical is the theoretical yield (wt.%) of the linear weighted average calculation of coal and S. psammophila; αbiomass and αcoal are the measured yields (wt.%) of the pyrolysis of S. psammophila and coal, respectively; ω is the mass ratio of S. psammophila in the mixtures; and Δα is the difference between the measured and theoretical yields (wt.%). A Δα > 0 indicated that S. psammophila and coal had a mutual promoting effect, Δα = 0 indicated that S. psammophila had no interaction with coal, and Δα < 0 indicated that S. psammophila had a mutual inhibitory effect with coal.
RESULTS AND DISCUSSION
Product Yield and Analysis
From Table 1, it can be seen that Salix psammophila contained minimal sulfur and nitrogen. This meant that almost no harmful, environmentally unfriendly gas was released when S. psammophilawas used. Typical unfriendly gases are NOX, SOX, and CO2. The changes in the carbon and hydrogen contents after pyrolysis are summarized in Table 3, which shows the value of chars.
Calculation of the values in the third column of Table 3 was done as follows: The value 40.44 means the carbon content before and after S. psammophila pyrolysis, whereas 16.47 means the value of carbon content before and after coal pyrolysis. The value 3.65 means the co- pyrolysis carbon content than the coal pyrolysis carbon content increases. Finally, 28.59 is the theoretical value of the difference between coal and Salix before and after pyrolysis, i.e.(40.44+16.74)*0.5.
The second column difference calculation method empathy, but the C content change into H content. After the pyrolysis, the carbon content of the samples increased while the hydrogen content decreased. The mass ratios of hydrogen to carbon for S. psammophila and coal were 0.122 and 0.072, respectively. The hydrogen to carbon ratio of S. psammophila was 1.696 times that of coal. Moreover, the mass of carbon increased and that of hydrogen decreased after pyrolysis of S. psammophila. Thus, S. psammophila has great potential to serve as a hydrogen provider for coal pyrolysis. In the pyrolysis process for coal, most hydrogen existed in the form of combined water, stable methane, and stable ethane.
Table 3. Changes in Carbon and Hydrogen Contents After Pyrolysis
The measured values of the carbon and hydrogen content for the co-pyrolysis sample were lower than corresponding theoretical values. And the carbon content was reduced while the hydrogen content increased.
The hydrogen in S. psammophila will likely transfer to coal to bind with carbon atoms, which is beneficial to the release of hydrogen in coal during pyrolysis, satisfies the demand of hydrogen required for coal pyrolysis, and inhibits the free radical re-polymerization and reaction with pyrolysis char. Thus, it can improve the conversion rate of pyrolysis process.
Fig. 2. The theoretical and measured values of the (a) solid yield; (b) tar yield; and (c) gas yield under the different S. psammophila blend ratios
The initial pyrolysis temperature of S. psammophila was lower than that of coal. The initial pyrolysis S. psammophila produces hydrogen-rich gases such as CH4 and H2 that react with carbon in the coal to form hydrocarbons. The organic liquid can be used as a solvent for coal pyrolysis (Yi et al. 2016).
Figure 2 illustrates the difference between the theoretical and measured values of the co-pyrolysis product yield at 600 °C. As shown, there was a different numeric between the theoretical and measured values of the tar yield. It was apparent that S. psammophilahad a certain promotion and inhibition effect on coal because Δα was almost not equal to 0. This analysis of the char yield and the gas yield in this study reveals that for the co-pyrolysis char yield, Δα < 0 and for the co-pyrolysis gas yield, Δα > 0. Accordingly, hydrogen in S. psammophila was transferred and connected to carbon atoms in coal. Thus, the hydrogen in coal was better pyrolyzed, improving the pyrolysis conversion rate.
Tar is greatly important for the graded conversion and the cascade utilization of coal oil and energy. Therefore, the interaction between coal and S. psammophila is mainly based on the difference between the measured value and the theoretical value of the tar yield. With a S. psammophila ratio of 40%, the theoretical and measured values for tar reached their maximum negative deviation, Δα < 0, and the inhibitory effect was the most obvious. With a S. psammophila ratio of 20%, the theoretical and measured values for tar reached the maximum positive deviation, Δα > 0, and the promoting effect was the most obvious. When the Salix psammophila ratio was small, the promotion effect was due to the catalysis of CaO and H, a result of the pyrolysis of S. psammophila. The amount of S. psammophila was not great enough to soften, adhere, and cover the surface of the coal to inhibit its pyrolysis. Therefore, the co-pyrolysis of the sample with a S. psammophila content of 20% had a mutual promoting effect. With increasing S. psammophila ratios, S. psammophila block the coal pores before it is volatilized. The addition of Salix psammophilainhibited the pyrolysis of coal, so the co-pyrolysis showed mutual inhibition. When the blend ratio of Salix psammophila was greater than 50%, S. psammophila were not conducive to the release of the coal volatiles. It gradually reduced the promoting effect on the volatilization of the coal, which was the mutual promoting action between the coal and the S. psammophila. As the content of S. psammophila increased, the promoting effect was gradually reduced. Yan and Chen (2007) also reached the same conclusions.
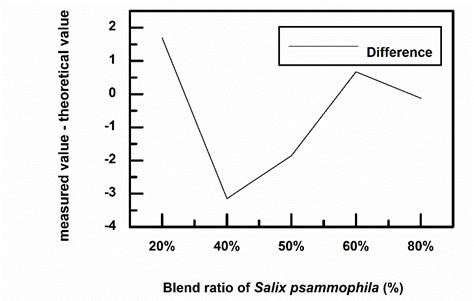
Fig. 3. The magnitude of Δα with respect to blending ratio
Figure 3 illustrates the magnitude of Δα with respect to blending ratio. As can be seen from the figure, with a S. psammophila ratio of 20% and 60%, the value of Δα was greater than 0. With a S. psammophila ratio of 40% and 50%, Δα < 0. With a S. psammophilaratio of 80%, Δα ≈ 0.
The roles of CaO and H
(1) Promoting effect of CaO in biomass
There is a large amount of CaO in the biomass, which has great influence on the release of sulfur in the gas phase of coal. The following reactions occur:
H2S+CaO=CaS+H2O
COS+CaO=CaS+CO2
CaO can react with H2S and COS in the pyrolysis of coal, so that the pyrolysis reaction can be carried out in the direction of volatile analysis, which is beneficial to the co-pyrolysis of biomass and coal.
(2) Promoting effect of H in biomass
According to coal chemical theory, if hydrogen is properly assigned to carbon atoms during pyrolysis of coal, the amount of hydrogen in the coal is almost enough to volatilize it. However, due to the structural characteristics of coal, hydrogen is mainly released in the form of water (derived from hydroxyl groups) and highly stable light aliphatic hydrocarbons (such as methane and ethane), but the remaining carbon atoms, which are in great need of hydrogen, are unable to react with such hydrogen. In coal chemical processing, hydrogenation pyrolysis can improve the conversion rate of coal pyrolysis, increase tar yield and improve tar quality. The H / C ratio of biomass is high, the internal hydrogen is enough to make it completely volatilize, and the hydrogen atmosphere has a great influence on the pyrolysis of coal, which can be a good hydrogen donor for coal.
Fig. 4. XRD patterns of S. psammophila and pyrolyzed S. psammophila
In the process of biomass with coal pyrolysis, biomass pyrolysis produce hydrogen in advance. Coal is poor in hydrogen, such that hydrogen in the biomass may be transferred to coal in the process of coal pyrolysis. It is advantageous to the coal pyrolysis. Figure 4 shows the XRD patterns of S. psammophila before and after pyrolysis, indicating CaO in the samples.
N2 Adsorption-desorption Analysis of Pyrolysis Char
Table 4 summarizes the pore volume, pore size, and specific surface area of coal char, Salix char, and co-pyrolysis char (pyrolyzed at 600 °C). The co-pyrolysis char was obtained from the sample with a 50:50 ratio of coal to S. psammophila. The average pore sizes of coal char, Salix char, and co-pyrolysis char were 10.87 nm, 9.10 nm, and 9.28 nm, respectively, which can be ascribed to mesopores.
The average pore size of coal char was the largest, and its pore volume and specific area were the smallest. The specific area of the co-pyrolysis char was 35.7 m2/g, which was lower than that of the S. psammophila char. The theoretical value of the specific area of the co-pyrolysis char was only 24.868 m2/g, and its measured value was slightly higher. The average pore size of the co-pyrolysis char was 9.28 nm, which was smaller than that of coal char and theoretical value of co-pyrolysis char. This indicated that the interaction between S. psammophila and coal improved both the surface area of the co-pyrolysis char and the pore size distribution.
Table 4. Pore Structure Parameters of Char
FT-IR Spectra Analysis
Figure 5 shows the FT-IR spectra of the samples. The mass ratios of S. psammophila in the mixtures was 0.50. The band at 3300 cm-1 to 3500 cm-1 can be attributed to the telescopic vibration absorption of -OH functional groups of phenols and alcohols. The band around 2840 cm-1 to 3000 cm-1 can be attributed to the stretching vibration of the C-H bond of the aliphatic, and that near 1600 cm-1 can be attributed to the absorption of hydrogen bonds associated with the -C=C skeleton. The band around 1500 cm-1 is attributed to the absorption of the aromatic ring skeleton. The absorption band near 1450 cm-1 is due to the bending vibration of methyl and methylene groups (Edreis et al. 2014; Zhang et al. 2015). The absorbance bands within 1500 cm-1 to 1750 cm-1 are from C=C, C=O, and COOH and are mainly ketones, aldehydes, acids, and esters (Song et al. 2014).
The absorption band from 1000 cm-1 to 1350 cm-1 can be attributed to the telescopic vibration of C-O, C-C carbon skeleton, and C-N (aliphatic ammonia) stretching vibrations. The band at 600 cm-1 to 820 cm-1 is due to the bending vibration of the C-H benzene ring (Yi et al. 2016).
Fig. 5. FT-IR spectra of (a) coal tar and co-pyrolysis tar and (b) Salix psammophila tar and co-pyrolysis tar
The FT-IR results indicated that the pyrolysis tar contained primarily aromatic rings and fatty acid compounds. The total amount of aliphatic compounds in co-pyrolysis tar was less than that of coal tar, evidenced by the decrease in the absorption band characteristic of the fatty acid compounds in co-pyrolysis tar. The reason was that the addition of S. psammophila was beneficial to the decomposition of phenolic hydroxyl, ether bonds, and oxygen-containing heterocyclic rings in coal tar. Characteristic absorption bands of aromatic compounds in the co-pyrolysis tar were enhanced because H and OH in S. psammophila occupied the free radical active sites of the aromatic structure of coal and inhibited crosslinking and polymerization in the co-pyrolysis process, thus resulting in an increase in aromatic ring compounds in the co-pyrolysis tar. Because the biomass pyrolysis produces hydrogen, the long-chain alkanes are further hydrocracked into alkanes or other substances with small molecular weight (Wang et al. 2012), so it can be concluded that the addition of S. psammophila improved the quality of tar, which was beneficial to the light tar products.
Figure 5 shows the FT-IR spectra of coal tar, S. psammophila tar, and total co-pyrolysis tar. The -OH telescopic vibration at 3394 cm-1 in Salix tar was significantly higher than that of coal. This was because S. psammophila is composed mainly of cellulose, hemicellulose, and lignin, with many more -OH and other oxygen-containing functional groups than coal. The absorption band for the co-pyrolysis tar was between those of S. psammophila tar and coal tar, which showed that the two had certain additivity. The main functional groups for FT-IR spectra was added in table 5.
Table 5. Absorption Peak Attributable of FT-IR Spectra of Oil
SEM Data Analysis of Material and Char
The SEM images of the materials and their pyrolysis char can be seen in Figs. 6 (a through f). Coal char had a collapsed structure and more crack pores than raw coal. The surface of coal char was relatively loose and rough, and there were more foreign substances than raw coal. The crack and pore structures are caused by the release of volatile matter during the pyrolysis of coal (He et al. 2016). The S. psammophila char had a clearer tubular structure and pore structure than S. psammophila that were conducive to the release of pyrolysis gases. Figure 6(e) shows that co-pyrolysis char had abundant pores and a disordered structure, which can be widely used as an active carbon adsorbent in the sewage treatment industry. This is because the addition of S. psammophila forms the pore structure due to the volatilization of the volatiles, or in the pyrolysis process of coal itself leads to uneven contraction between coal particles (Cheng et al. 2016). It can be seen from Fig. 6(e) that S. psammophila blocked the pores of coal and inhibited the volatilization of coal. The same conclusion as before can also be drawn: when the S. psammophilacontent was 50%, the pyrolysis of coal was inhibited.
Fig. 6. SEM analyses of the char samples: (a) coal, (b) S. psammophila, (c) coal char, (d) S. psammophila char, (e) co-pyrolysis char
CONCLUSIONS
- In the co-pyrolysis process with Salix psammophila mixing ratios of 20% and 40%, promotion and inhibition are exhibited, respectively. When the Salix psammophila mixing ratio is small, CaO and H play a dominant role in biomass pyrolysis. When the S. psammophila mixing ratio increased, the coal pores were blocked before volatilization occurred.
- It can be seen from the pore structure that the measured value of the specific surface area of the blend coal-S. psammophila was higher than the theoretical value, the specific surface area of the co-pyrolysis char was lower than that of the S. psammophila char, and the average pore size of co-pyrolysis char was smaller than that of both coal char and S. psammophila char. This shows that the interaction between S. psammophila and coal improved the surface area of the co-pyrolysis char and the pore diameter distribution, creating a tendency to decrease the pore diameter of the co-pyrolysis char.
- It can be seen from the SEM images that when the content of S. psammophila was 50%, the pore structure of the co-pyrolysis char was abundant, allowing it to be used as an industrial activated carbon adsorbent. Increased S. psammophila content blocked the pores of coal and inhibited its pyrolysis.
ACKNOWLEDGMENTS
Project No. 21366018 is supported by the National Natural Science Foundation of China.
REFERENCES CITED
Cheng, X. H., He, X. M., Chai, J., and Rong, F. (2016). “Characteristics of low-temperature co-pyrolysis products of ulva and lignite,” Chemical Industry and Engineering Progress 35(1), 105-109. DOI: 10.16085/j.issn.1000-6613.2016.01.014
Edreis, E. M. A., Luo, G. Q., and Yao, H. (2014). “Investigations of the structure and thermal kinetic analysis of sugarcane bagasse char during non-isothermal CO2 gasification,” Journal of Analytical and Applied Pyrolysis 107(12), 107-115. DOI: 10.1016/j.jaap.2014.02.010
Haykiri-Acma, H., and Yaman, S. (2010). “Interaction between biomass and different rank coals during co-pyrolysis,” Renewable Energy 35(1), 288-292. DOI: 10.1016/j.renene.2009.08.001
He, X. M., Yi, S., Fu, P. R., Zeng, X. C., Zhang, D., and Cheng, X. H. (2016). “Combustion reactivity of biochar and char generated from co-pyrolysis of coal and four additives: Application in blast furnace,” Journal of Energy Engineering 143(1), Article ID 04016023. DOI: 10.1061/(ASCE)EY.1943-7897.0000369.
Krerkkaiwan, S., Fushimi, C., Tsutsumi, A., and Kuchonthara, P. (2013). “Synergetic effect during co-pyrolysis/gasification of biomass and sub-bituminous coal,” Fuel Processing Technology 115(11), 11-18. DOI:10.1016/j.fuproc.2013.03.044
Li, S., Chen, X., Liu, A., Li, W., and Yu, G. (2015). “Co-pyrolysis characteristic of biomass and bituminous coal,” Bioresource Technology 179, 414-420. DOI: 10.1016/j.biortech.2014.12.025
Song, Y. Y., Tahmasebi, A., and Yu, J. L. (2014). “Co-pyrolysis of pine sawdust and lignite in a thermogravimetric analyzer and a fixed-bed reactor,” Bioresource Technology 174, 204-11. DOI: 10.1016/j.biortech.2014.10.027
Wu, Z., Yang, W., Tian, X., and Yang, B. (2017). “Synergistic effects from co-pyrolysis of low-rank coal and model components of microalgae biomass,” Energy Conversion and Management 135, 212-225. DOI: 10.1016/j.enconman.2016.12.060
Yan, W. P., and Chen, Y. Y. (2007). “Interaction performance of co-pyrolysis of biomass mixture and coal of different rank,” Proceedings of the CSEE 27(2), 80-86.
Yi, S., He, X. M., Zheng, H., Lin, H. T., Li, C. H., and Li, C. (2016). “Characteristics of -pyrolysis char of sugarcane bagasse and lignite,” Chemical Industry and Engineering Progress 35(10), 3149-3154. DOI: 10.16085/j.issn.1000-6613.2016.10.019
Zhang, J., Quan, C., Qiu, Y., and Xu, S. (2015). “Effect of char on co-pyrolysis of biomass and coal in a free fall reactor,” Fuel Processing Technology 135(9), 73-79. DOI: 10.1016/j.fuproc.2014.10.022
Article submitted: October 17, 2017; Peer review completed: December 29, 2018; Revised version received: February 17, 2018; Accepted: February 19, 2018; Published: February 26, 2018.
DOI: 10.15376/biores.13.2.2846-2860
