Abstract
The thermogravimetric properties and chemical characterization of rice straw (RS) pretreated by mixed culture of white-rot fungi Phanerochaete chrysosporium (P. chrysosporium) and brown-rot fungi Gloeophyllum trabeum (G. trabeum) were investigated. The mixed fungal pretreatment showed a synergistic effect, which resulted in an energy-efficient pyrolysis of pretreated rice straw. The differences in thermochemical conversion of rice straw before and after fungal pretreatment were investigated using thermogravimetric analysis and the Flynn–Wall–Ozawa (FWO) method. Furthermore, the pretreated samples were also analyzed by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and scanning electron microscopy (SEM) to illuminate the changes in chemical composition and pyrolysis behavior. Compared to single fungal pretreatment, the mixed fungal pretreatment worked better and exhibited great potential in biomass pyrolysis.
Download PDF
Full Article
Synergistic Effect of Mixed Fungal Pretreatment on Thermogravimetric Characteristics of Rice Straw
Meng Li,a Zhinan Wang,a Jin Sun,a Wanjuan Chen,b Xianfeng Hou,a,* and Zhenzhong Gao a,*
The thermogravimetric properties and chemical characterization of rice straw (RS) pretreated by mixed culture of white-rot fungi Phanerochaete chrysosporium (P. chrysosporium) and brown-rot fungi Gloeophyllum trabeum (G. trabeum) were investigated. The mixed fungal pretreatment showed a synergistic effect, which resulted in an energy-efficient pyrolysis of pretreated rice straw. The differences in thermochemical conversion of rice straw before and after fungal pretreatment were investigated using thermogravimetric analysis and the Flynn–Wall–Ozawa (FWO) method. Furthermore, the pretreated samples were also analyzed by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and scanning electron microscopy (SEM) to illuminate the changes in chemical composition and pyrolysis behavior. Compared to single fungal pretreatment, the mixed fungal pretreatment worked better and exhibited great potential in biomass pyrolysis.
Keywords: Pyrolysis; White-rot fungi; Brown-rot fungi; Rice straw; Mechanism
Contact information: a: Key Laboratory for Biobased Materials and Energy of Ministry of Education, College of Materials & Energy, South China Agricultural University, Guangzhou 510642, China; b: College of Materials Science & Energy Engineering, Foshan University, Foshan 528000, China; *Corresponding author: xfhou@scau.edu.cn; zzgaoscau@163.com
GRAPHICAL ABSTRACT
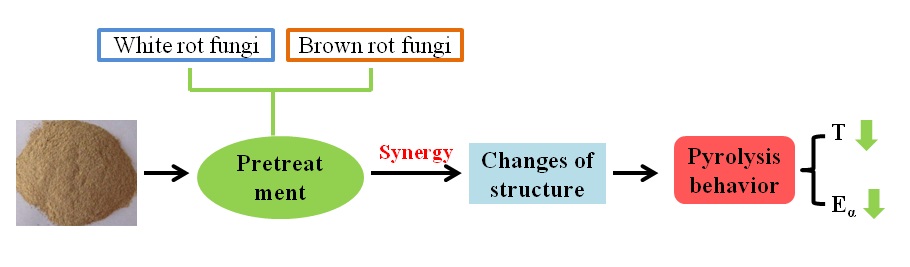
INTRODUCTION
The depletion of oil resources and continued increase in global energy demand are important factors driving researchers to develop renewable and sustainable biofuel resources (Sharma et al. 2020). Lignocellulosic biomass, which mainly includes agricultural residues, forestry residues, and energy crops, is widely used in the biofuel conversion field and is considered a potential replacement for traditional fossil fuels (Kumar and Verma 2020). Additionally, agricultural crop residues are a subset of the total biomass energy resource base to meet a large part of current energy demand (Yang et al. 2010). Rice straw (RS) is one of the most abundant agricultural castoffs. About 200 million tons are produced in China, causing serious waste and environmental problems (Ranjan and Moholkar 2013). Therefore, utilization of RS in an economically feasible way would not only prevent environmental pollution but also provide biofuels.
Pyrolysis is a promising thermochemical conversion route, converting biomass to energy-dense biofuels as well as chemical feedstocks (Moriana et al. 2014). In this way, reactor designs and thermal decomposition mechanisms on the pyrolysis of wood biomass have been extensively studied (Van de Velden et al. 2010; Zeng et al. 2011). In contrast, a limited amount of research has focused on green pretreatment before biomass pyrolysis to promote thermochemical reactions (Krutof and Hawboldt 2018). Pretreatment can break the high recalcitrant limits of lignocellulose material limits by modifying chemical structure or selectively removing lignocellulosic components, thereby rendering biomass more accessible for pyrolysis (Chen et al. 2019a). Meanwhile, several pretreatment methods used for biomass modification before pyrolysis have recently been investigated, mainly including physical (Wu et al. 2009; Wang et al. 2019), chemical (acid and alkali) (Liang et al. 2019; Wu et al. 2019), and a combined pretreatment. However, these methods require high temperature and operating pressure, corrosion resistant instruments, and the use of acid and alkali, which might cause serious environmental pollution problems. Thus, an effective, low-cost, and green pretreatment, with mild treatment conditions and low energy consumption, exhibits evident superiority (Lee et al. 2008).
Fungi has been proved to have excellent potential in breaking down lignocellulosic biomass (Chen et al. 2019b). Basidiomycete strains are the most efficient lignocellulose degraders (Sanchez. 2009). Therefore, fungal pretreatment has been widely explored in the production of bioethanol and wood pulp processing (Moriana et al. 2014; You et al. 2019). White-rot fungi have the capacity to selectively decompose intact lignin via integrate ligninolytic enzymes, which subsequently can be utilized to produce biofuels (Yang et al. 2011). Brown-rot fungi are often preferred for their use in depolymerizing polysaccharides (mainly cellulose and hemicelluloses), with minimal assimilation of degradation products (Shiny et al. 2018). Therefore, a mixed culture of white-rot fungi and brown-rot fungi was expected to achieve better degradation and destruction of biomass structure, which could effectively shorten pretreatment time or increase its degradation efficiency (Hermosilla et al. 2018). To date, studies of mixed culture of wood-rotting fungi treatment have focused on the secretion of ligninolytic enzymes or increasing sugar yields (Chen et al. 2018). A co-culture of white-rot and brown-rot fungi has been proved to be an efficient pretreatment for enzymatic hydrolysis (Rasmussen et al. 2010). However, the effect of mixed pretreatment of white-rot and brown-rot fungi on thermogravimetric and chemical characterization of RS has been poorly reported.
In the authors’ previous studies, the brown-rot fungi G. trabeum was shown to decrease the activation energy of Ep and Mp pyrolysis, and reduce initiation temperatures, as well as increase the proportion of aromatic hydrocarbons, which makes the pyrolysis more energy efficient (Gao et al. 2016). P. chrysosporium is one of the most commonly studied white-rot fungi, which showed potential to promote thermal degradation processes by breaking down the complex structure, and improving the low-temperature pyrolysis of RS. The aim of this study was to explore the effect of co-inoculation of white-rot fungus (P. chrysosporium) and brown-rot fungus (G. trabeum) pretreatment on thermal behavior and the chemical structure of RS.
EXPERIMENTAL
Materials and Fungal Strain
Rice straw, purchased from the suburb of Luoding (Guangdong province, China), was ground through a 60-mesh sieve, and then dried at an equilibrium moisture content of approximately 8% to 10% (oven-dry basis) for subsequent experiments. Moreover, all the chemicals were reagent grade and used as received without further purification.
White-rot fungi P. chrysosporium and brown-rot fungi G. trabeum were purchased from Guangdong Culture Collection Center (Guangzhou, China). The fungal strains were maintained on potato dextrose agar (PDA) plates (pH 6.8) at 28 °C for 5 to 7 days on a reciprocal shaker until the mycelia covered the entire agar plates. The fungal mycelium was then grown in 2-L Erlenmeyer flasks with a 200 mL liquid medium (sterilized at 121 °C, 20 min) containing 40 g potato (filter with gauze) and 5 g glucose. Twenty discs of pre-cultured strain (8 mm in diameter) cut from actively growing cultures in a solid-state PDA medium were inoculated in each flask and incubated for two weeks at 28 °C. The grown mycelium was washed and filtered with approximately 300 mL sterilized water, and then blended with 50 mL sterilized water in two cycles lasting approximately 10 s for each washed mycelium obtained from several strain pre-cultures. The mycelium suspension was used to inoculate the sterilized samples in bioreactors.
Bio-pretreatments of RS
P. chrysosporium and G. trabeum were used for biodegradation. Each bioreactor was loaded with 30 g of RS samples and inoculated with a suspension volume corresponding to 50 mg (25 mg P. chrysosporium and 25 mg G. trabeum) of fungal mycelium per 100 g of dry samples. The inoculated RS were incubated in an acclimatized room at 28 °C and 55% relative humidity for eight weeks. After bio-degradation, the superficial mycelia on the surface of RS were brushed away and decayed samples were dried at 40 °C to constant weight. Control samples of RS were prepared under the same conditions but without incubation of fungi. The mass loss of biomass was calculated according to Eq. 1,
Mass loss = (1)
where W0 is the dry quantity (g) of the sample before bio-degradation, and W1 is the dry quantity of the sample after bio-degradation.
Methods
Fourier transform infrared spectroscopy (FTIR) analysis
The FTIR spectra of the RS samples were recorded on a NEXUS 670 spectrometer (Thermo Nicolet Corporation, Madison, WI, USA). Samples for the FTIR analysis were ground and sieved in a Wiley mill with a mesh size of 0.2 mm (IKA MF10; IKA-Werke, Staufen, Germany). The KBr pellets used for FTIR spectroscopy were prepared by tableting a mixture of powder samples and KBr (2 mg of sample in 200 mg of KBr) (Pandey and Pitman 2003). The spectra scope was between 4000 and 400 cm-1 with a spectral resolution of 4 cm-1.
X-ray diffractometry (XRD) analysis
X-ray diffraction data of the RS samples were recorded on a Rigaku-Ultima IV diffractometer (Rigaku Corp., Tokyo, Japan) using Cu-Kα radiation (λ = 0.154 nm) at 40 KV and 40 mA in a 2θ range of 5° to 40° with a step size of 2°/min. The crystallinity index (CrI) was calculated by following equation,
Crystallinity index (CrI, %) = (I002 – Iam) / I002 × 100 (2)
where I002 is the intensity of the peak (002) at approximately 22.5°, and Iam is the intensity of the background at approximately 15.7° (Segal et al. 1959).
Scanning electron microscope (SEM) analysis
After drying in the oven at 105 ℃ for 2 h, an S-570 scanning electron microscope (Carl Zeiss Ag, Jena, Germany) was used for recording surface morphologies of samples. To prevent charging on the surface, a thick layer of gold was first sprayed on samples.
Chemical composition analysis
The component analysis was performed according to the Van Soest method (Van Soest 1963). Neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) were obtained using sequential abstersion neutral detergent reagent, acid detergent reagent, and 72% H2SO4. The content of hemicellulose, cellulose, and Klason lignin were calculated as the differences between NDF and ADF, ADF and ADL, and ADL and ash content, respectively (Wang et al. 2018a). The experiment was repeated three times for the mean value of each group of samples.
Thermogravimetric analysis
The thermogravimetric analysis was performed in a sensitive thermobalance (PerkinElmer, Diamond, Shanghai, China). Powdered RS samples mainly from the surface were sieved, and the fraction with an average diameter less than 0.2 mm was retained for analysis. Each sample (5.0 mg) was loaded in an aluminum crucible and heated from 40 °C to 800 °C with a steady insert nitrogen flow of 30 mL/min. Dynamic heating rates of 5, 10, 15, and 20 °C/min were used to research pyrolysis behavior. The deconvolution of derivative thermogravimetric (DTG) curves was plotted according to the Grams/32 program with Log-Normal functions (Galactic Industry Corporation, V4.14, Shenzhen, China). The thermogravimetric experiments were performed three times, and the average results were used for plotting TG and DTG curves.
Kinetic Parameters Modeling
Based on relevant study, pyrolysis of biomass can be described using Eq. 3 (Gokul et al. 2019). Therefore, a wide range of kinetic methods were used to analyze pyrolysis kinetics of lignocellulose. In this study, it was assumed that conversion of raw materials into product is the only single process (Idris et al. 2012). According to the Arrhenius equation, a kinetic model of pyrolysis can be established, and kinetic parameters can be determined. Equation 3 is as follows:
(3)
RESULTS AND DISCUSSION
Thermal Decomposition Characteristic
Thermal decomposition behaviors of bio-pretreated and un-pretreated RS samples were investigated by thermogravimetry (TG). Each sample exhibited three distinct stages: dehydration, active pyrolysis, and passive pyrolysis, which ranged from 40 °C to approximately 190 °C, 200 °C to 410 °C, and approximately 400 °C to 800 °C (He et al. 2018). As shown in Fig 1b, the DTG peaks of all samples maintained essentially the same shape on the temperature axis as was observed by comparing the RS samples before and after pretreatment. In detail, a shoulder (the fastest conversion of hemicellulose), a peak (decomposition of cellulose), and a long tailing (lignin pyrolysis) can be observed for each DTG curve. From Fig. 1a, weight loss (at the same temperature) became lower after pretreatment. Concurrently, after single and mixed fungal pretreatment, a lower thermal degradation rate was shown before the initial decomposition. However, in the initial decomposition stage (degradation of hemicellulose), reaction rate at the same temperature was followed by mixed fungi pretreated RS > brown-rot fungi pretreated RS (in close proximity to mixed fungi pretreated RS) > untreated RS > white-rot fungi pretreated RS. This sequence indicated that mixed fungal pretreatment tended to make it easier for RS to be pyrolyzed. The untreated RS showed a higher maximum degradation rate of cellulose than the pretreated samples, which was followed by untreated RS > white-rot fungi pretreated RS > brown-rot fungi pretreated RS > mixed fungi pretreated RS; the temperatures required to reach the maximum degradation rate of hemicellulose were in the opposite order. These changes in thermal decomposition behaviors were related to degradation of carbohydrates caused by brown-rot fungi. Furthermore, the tendency of the weight loss curve became mild in the stage of passive pyrolysis, due to the slow decomposition of solids and some lignin residue, which is associated with endothermic reaction (Mishra and Mohanty 2018).
Fig. 1. TG (a) and DTG (b) curves of untreated (black), white-rot fungi (red) and brown-rot fungi (green) and mixed fungi (blue)-pretreated RS samples; the heating rate was 10 °C/min
Analyzed by the -d2X/dt2 curves, the thermal decomposition characteristics of lignocellulose can be quantified through several parameters (listed as below): Tonset(hc) is the extrapolated temperature for the beginning of hemicellulose decomposition; Tshoulder is the temperature corresponding to the hemicellulose shoulder, marking the peak top of hemicellulose decomposition; Tpeak is the temperature of the maximum devolatilization rate; and Toffset(c) is the extrapolated temperature for the termination of cellulose decomposition (and the beginning of the lignin tail). As seen in Table 1, pretreated samples possessed a lower Tonset(hc) than the raw sample. Specifically, the Tonset(hc) of the samples were followed by mixed fungi-pretreated RS < brown-rot fungi-pretreated RS < white-rot fungi-pretreated RS < unpretreated RS, which meant that bio-pretreatment, especially the mixed fungal pretreatment, can decrease the beginning temperature of hemicellulose degradation. Similarly, mixed fungi-pretreated RS showed the lowest Tshoulder and Tpeak, which indicated that the mixed fungal pretreatment made pyrolysis of RS energy efficient. These results can be attributed to the synergistic effect of white-rot fungi and brown-rot fungi. In terms of Toffset(c), the Toffset(c) of the bio-pretreated samples were higher than that of the unpretreated samples. This can be attributed to degradation of carbohydrates by white-rot and brown-rot fungi. In addition, charcoal yields of samples were calculated. Results showed that biopretreated samples possessed higher charcoal yield (17.5%, 9.8%, 12.8% for white-rot fungi-pretreated RS, brown-rot fungi-pretreated RS and mixed fungi-pretreated RS, respectively) in comparison with unpretreated RS (4.8%). This can be attributed to removal of the vulnerable part caused by fungi.
Fig. 2. Mass fraction: the first and the second time derivatives of the mass fraction as functions of temperature for the pretreated RS sample
Table 1. Thermal Degradation Characteristic of Rice Straw Samples at the Heating Rate of 10 °C/min
Kinetic Parameters
Activation energy Eα, one of the most important dynamic factors to describe reaction equations, is the minimum energy when a chemical reaction occurs. The first-order decomposition reaction was supposed to occur in the co-pyrolysis process, and the isoconversional method was used to analyze the pyrolysis dynamics that could obtain the certain Eα uninfluenced by mechanism function (Jeguirim and Trouve 2009). In this study, the Eα of RS before and after pretreatment with two kinds of fungi were investigated by the method of Flynn–Wall–Ozawa (FWO) at four different heating rates (5, 10, 15, and 20 °C /min). In the FWO method, there is a linear relationship between ln(β) and 1/T, and the kinetic parameters can be calculated at different conversion rates (0.1 ≤ α ≤ 0.7).
The detailed data are listed in Table 2. The Eα values of untreated RS were around 78.3 to 223.0 kJ/mol at conversion rates from 0.1 to 0.7, respectively, which were markedly higher than that of the pretreated samples. Higher values of Eα corresponded to more difficult thermal decomposition of the samples, which may illustrate that bio-treatment has a positive influence on the decrease of activation energy, which is consistent with the thermal decomposition characteristic. Moreover, the mixed fungal pretreatment possesses a lower Eα value compared to white-rot fungi (P. chrysosporium) pretreatment or brown-rot fungi (G. trabeum) pretreatment. Because activation energy is defined as the minimum energy required to start a chemical reaction and driving force (Kannaiyan et al. 2015), this suggested that it is easier to initiate a pyrolysis reaction for RS treated with mixed fungus than with single fungal pretreatment. Compared to single fungal pretreatment, the mixture of white-rot fungi and brown-rot fungi seemed to have a positive synergetic interaction during the degradation. Therefore, based on decomposition mechanisms of white-rot fungi and brown-rot fungi, it was assumed that simultaneous decomposition of cellulose, hemicellulose, and lignin made pyrolysis of mixed fungi-pretreated RS easier.
Table 2. Activation Energies of Pretreated RS Sample at Different Conversation Rates Based on the FWO Method
The changes in the chemical components of bio-pretreated RS were different. As shown in Table 3, the single fungal pretreatment had a great selective removal ability on RS. A significant decrease in the lignin content after white-rot (P. chrysosporium) pretreatment can be observed, which could be attributed to the delignification of white-rot. For the single brown-rot (G. trabeum) pretreated sample, cellulose and hemicellulose contents decreased 13.49% and 18.42%, respectively, in comparison with untreated RS. It is widely believed that brown-rot fungi can mainly decompose polysaccharides (cellulose and hemicellulose). At the same time, pretreatment with P. chrysosporium led to an apparent increase in the percentage of cellulose and hemicellulose in RS, which was attributed to the removal of the lignin component during degradation. For the mixed fungal pretreatment, the pretreated sample showed a decline in the component of carbohydrates (61.2% to 50.43%) and an increase in the lignin component (21.51% to 22.45%) compared to untreated RS. In term of weight loss, the mixed fungal pretreatment resulted in a weight loss at 45.87%, which was higher than that of the single fungi pretreated sample. This could suggest that the composition of the mixed fungi pretreated sample was the result of the combined action of white-rot and brown-rot fungi. In other words, the mixed fungal pretreatment can decompose polysaccharides and lignin simultaneously. The reduced carbohydrates content revealed the strong delignification of mixed fungal pretreatment. These changes in the composition accounted for the thermal decomposition characteristic of the mixed fungi pretreated sample to some extent.
Table 3. Compositional Analysis wt% (on a Dry Basis) of Untreated and Bio-pretreated RS Samples
Effect of Pretreatment on Chemical Composition of RS
FTIR is a useful approach for describing changes in composition (Zeng et al. 2011). It is well known that 1375 cm-1 (unconjugated C=O in xylans), 1158 cm-1 (C–O–C vibration in cellulose and hemicellulose), and 898 cm-1 (C-H deformation in cellulose) belong to carbohydrates in biomass (Gao et al. 2016). As shown in Fig. 3, for brown-rot fungi-pretreated samples, decreases in carbohydrate bands can be attributed to the degradation of polysaccharides caused by brown-rot fungi (Wang et al. 2018b).
Fig. 3. FTIR spectra of the RS with different fungal pretreatments
In terms of lignin bands, such as 1268 cm-1 (guaiacyl ring breathing, C–O stretch in lignin, and for C–O linkage in guaiacyl aromatic methoxyl groups) and 1112 cm-1 (aromatic skeletal and C–O stretch), the white-rot fungi-pretreated RS sample showed a decline, and brown-rot fungi-pretreated RS exhibited an increase. For pretreatment with a mixture of P. chrysosporium and G. trabeum, a similar tendency to the brown-rot fungi-pretreated sample emerged. In combination with the composition analysis, the authors speculated that brown-rot fungi play a dominant role in the mixed fungal pretreatment.
Effect of Pretreatment on Surface Morphology and Crystallinity of RS
To further investigate the influence of fungal pretreatment on RS, SEM was executed to record microscopic photos of the samples. An obvious difference in morphology between the samples can be observed. Figure 3a illustrates a smooth and plain surface structure of the untreated RS. White-rot fungi-pretreated RS shows a breakage and a collapsed surface (Fig. 4b). This change is attributed to delignification caused by white-rot fungi (Zeng et al. 2011). Meanwhile, a loose and fragmented surface can be observed from brown-rot fungi-pretreated RS (Fig. 4c), which is attributed to degradation of polysaccharides caused by brown-rot fungi. Under the combined effect of white-rot fungi and brown-rot fungi, micro-morphology of the mixture pretreated sample was heavily destroyed, and a collapsed, cracked, and alveolate surface morphology emerged (Fig. 4d). Lignin plays an important role in the integral cell wall structure, while hemicelluloses connect celluloses and lignin by various ways (Kim et al. 2018). Therefore, the disrupted structure may be attributed to the removal of lignin and hemicellulose, which has been confirmed by chemical composition analysis and FTIR analysis.
Fig. 4. Scanning electron microscopic images of raw (a), white-rot (b), brown-rot (c), and mixed fungi (d)-treated RS samples (500×)
To further explore the effect of pretreatment on the crystal structure of cellulose, XRD was employed to analyze the RS samples. As shown in Table 5, the CrI of the brown-rot-pretreated sample exhibited a decline, which may be due to conversion of some crystalline cellulose to glucose by brown-rot fungi after micro-fibrils are exposed over a long pretreatment time (Yiin et al. 2018). Furthermore, the CrI of the RS sample pretreated by mixed fungi increased, which can be attributed to the cooperative effect of white-rot and brown-rot fungi. This phenomenon can be ascribed to the removal of amorphous fraction (Wu et al. 2016). With alteration of the crystal structure, the mixed fungi-pretreated sample has a high possibility to produce more uniform and desirable intermediates as fast pyrolysis products (Mukarakate et al. 2016).
Table 4. Crystallinity Index of RS Samples
CONCLUSIONS
- The results showed that the mixed fungal pretreatment can effectively reduce recalcitrance of biomass by degrading main components, removing amorphous regions of cellulose, and destroying micromorphology. It can decrease temperatures of key pyrolysis steps (beginning of initial decomposition, maximum rate of hemicellulose degradation, and maximum devolatilization rate) and activation energy at a different conversion rates.
- The mixed fungal pretreatment, equipped with the synergistic effect, worked better than the single fungal pretreatment on the basis of reducing the beginning temperature of hemicellulose degradation, as well as start a pyrolysis reaction for RS with less energy.
- This work demonstrated a green and energy-efficient method in the pyrolysis of biomass and provided valuable reference information for pretreatment techniques.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the financial support by the National Key R&D Program of China (2018YFD0600305), the Science and Technology Project of Guangzhou, China (201803030031), and the Open Fund for Key Lab of Guangdong High Property and Functional Macromolecular Materials, China (20190001).
REFERENCES CITED
Chen, Y., Chen, Y., Li, Y., Wu, Y., Zhu, F., Zeng, G., Zhang, J., and Li, H. (2018). “Application of Fenton pretreatment on the degradation of rice straw by mixed culture of Phanerochaete chrysosporium and Aspergillus niger,” Ind. Crop. Prod. 112, 290-295. DOI: 10.1016/j.indcrop.2017.12.005
Chen, X., Che Q., Li S., Liu Z., Yang H., Chen Y., Wang X., Shao J., and Chen H. (2019a). “Recent developments in lignocellulosic biomass catalytic fast pyrolysis: Strategies for the optimization of bio-oil quality and yield,” Fuel Processing Technology 196, article no.106180. DOI: 10.1016/j.fuproc.2019.106180
Chen, Y., Wang, Y., Xu Z, Liu, Y., and Duan H. (2019b). “Enhanced humification of maize straw and canola residue during composting by inoculating Phanerochaete chrysosporium in the cooling period,” Bioresource Technol. 293, article no. 122075. DOI: 10.1016/j.biortech.2019.122075
Gao, Z., Fan, Q., He, Z., Wang, Z., Wang, X., and Sun, J. (2016). “Effect of biodegradation on thermogravimetric and chemical characteristics of hardwood and softwood by brown-rot fungus,” Bioresource Technol. 211, 443-450. DOI: 10.1016/j.biortech.2016.03.128
Gokul, P. V., Singh, P., Singh, V. P., and Sawarkar, A. N. (2019). “Thermal behavior and kinetics of pyrolysis of areca nut husk,” Energy Sources Part A-Recovery Utilization and Environmental Effects. 41(23), 2906-2916. DOI: 10.1080/15567036.2019.1582733
He, Y., Chang, C., Li, P., Han, X., Li, H., Fang, S., Chen, J., and Ma, X. (2018). “Thermal decomposition and kinetics of coal and fermented cornstalk using thermogravimetric analysis,” Bioresource Technol. 259, 294-303. DOI: 10.1016/j.biortech.2018.03.043
Hermosilla, E., Rubilar, O., Schalchli, H., Da Silva, A. S., Ferreira-Leitao, V., and Diez, M. C. (2018). “Sequential white-rot and brown-rot fungal pretreatment of wheat straw as a promising alternative for complementary mild treatments,” Waste Manage. 79, 240-250. DOI: 10.1016/j.wasman.2018.07.044
Idris, S. S., Rahman, N. A., and Ismail, K. (2012). “Combustion characteristics of Malaysian oil palm biomass, sub-bituminous coal and their respective blends via thermogravimetric analysis (TGA),” Bioresource Technol. 123, 581-591. DOI: 10.1016/j.biortech.2012.07.065
Jeguirim, M., and Trouve, G. (2009). “Pyrolysis characteristics and kinetics of Arundo donax using thermogravimetric analysis,” Bioresource Technol. 100(17), 4026-4031. DOI: 10.1016/j.biortech.2009.03.033
Kannaiyan, R., Mahinpey, N., Kostenko, V., and Martinuzzi, R. J. (2015). “Nutrient media optimization for simultaneous enhancement of the laccase and peroxidases production by coculture of Dichomitus squalens and Ceriporiopsis subvermispora,” Biotechnol. Appl. Biochem. 62(2), 173-185. DOI: 10.1002/bab.1263
Kim, M., Kim, B., Nam, K., and Choi, Y. (2018). “Effect of pretreatment solutions and conditions on decomposition and anaerobic digestion of lignocellulosic biomass in rice straw,” Biochem. Eng. J. 140, 108-114. DOI:10.1016/j.bej.2018.09.012
Krutof, A., and Hawboldt, K. A. (2018). “Upgrading of biomass sourced pyrolysis oil review: Focus on co-pyrolysis and vapour upgrading during pyrolysis,” Biomass Conversion and Biorefinery 8(3), 775-787. DOI: 10.1007/s13399-018-0326-6.
Kumar, B., and Verma, P. (2020). “Enzyme mediated multi-product process: A concept of bio-based refinery,” 154, 112607. DOI: 10.1016/j.indcrop.2020.112607
Lee, J. W., Kim, H. Y., Koo, B. W., Choi, D. H., Kwon, M., and Choi, I. G. (2008). “Enzymatic saccharification of biologically pretreated Pinus densiflora using enzymes from brown rot fungi,” J. Biosci. Bioeng. 106(2), 162-167. DOI: 10.1263/jbb.106.162
Liang, J., Xu, X., Yu, Z., Chen, L., Liao, Y., and Ma, X. (2019). “Effects of microwave pretreatment on catalytic fast pyrolysis of pine sawdust,” Bioresource Technol. 293, article no. 122080 DOI: 10.1016/j.biortech.2019.122080
Mishra, R. K., and Mohanty, K. (2018). “Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis,” Bioresource Technol. 251, 63-74. DOI: 10.1016/j.biortech.2017.12.029
Moriana, R., Zhang, Y., Mischnick, P., Li, J., and Ek, M. (2014). “Thermal degradation behavior and kinetic analysis of spruce glucomannan and its methylated derivatives,” Carbohyd. Polym. 106, 60-70. DOI: 10.1016/j.carbpol.2014.01.086
Mukarakate, C., Mittal, A., Ciesielski, P. N., Budhi, S., Thompson, L., Iisa, K., Nimlos, M. R., and Donohoe, D. S. (2016). “Influence of crystal allomorph and crystallinity on the products and behavior of cellulose during fast pyrolysis,” ACS Sustain. Chem. Eng. 4(9), 4662-4674. DOI: 0.1021/acssuschemeng.6b00812
Pandey, K. K., and Pitman, A. J. (2003). “FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi,” Int. Biodeter. Biodegr. 52(3), 151-160. DOI: 10.1016/S0964-8305(03)00052-0
Ranjan, A., and Moholkar, V. S. (2013). “Comparative study of various pretreatment techniques for rice straw saccharification for the production of alcoholic biofuels,” Fuel 112(2), 567-571. DOI: 10.1016/j.fuel.2011.03.030
Rasmussen, M. L., Shrestha, P., Khanal, S. K., Pometto, A. L., and Hans van Leeuwen, J. (2010). “Sequential saccharification of corn fiber and ethanol production by the brown rot fungus Gloeophyllum trabeum,” Bioresource Technol. 101(10), 3526–3533. DOI: 10.1016/j.biortech.2009.12.115
Sanchez, C. (2009). “Lignocellulosic residues: Biodegradation and bioconversion by fungi,” Biotechnol. Adv. 27(2), 185-194. DOI: 10.1016/j.biotechadv.2008.11.001
Segal, L., Creely, J. J., Martin, A. E., and Conrad, C. M. (1959). “An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer,” Text. Res. J. 29(10), 786-794. DOI: 10.1177/004051755902901003
Sharma, A., Singh, Y., Ahmad Ansari, N., Pal, A., and Lalhriatpuia, S. (2020). “Experimental investigation of the behaviour of a DI diesel engine fuelled with biodiesel/diesel blends having effect of raw biogas at different operating responses,” Fuel (Guildford). 279, 118460. DOI: 10.1016/j.fuel.2020.118460
Shiny, K. S., Sundararaj, R., and Vijayalakshmi, G. (2018). “Potential use of coconut shell pyrolytic oil distillate (CSPOD) as wood protectant against decay fungi,” European Journal of Wood and Wood Products 76 (2), 767-773. DOI: 10.1007/s00107-017-1193-8
Van de Velden, M., Baeyens, J., Brems, A., Janssens, B., and Dewil, R. (2010). “Fundamentals, kinetics and endothermicity of the biomass pyrolysis reaction,” Renew. Energ. 35(1), 232-242. DOI: 10.1016/j.renene.2009.04.019
Van Soest, P. J. (1963). “Use of detergents in the analysis of fibrous feeds. II. A rapid method for the determination of fiber and lignin,” J. Assoc. Off. Agric. Chem. 46(5), 829-835. DOI: 10.1093/jaoac/46.5.829
Wang, T. P., Ai, Y. N., Peng, L., Zhang, R. H., Lu, Q., and Dong, C. Q. (2018a). “Pyrolysis characteristics of poplar sawdust by pretreatment of anaerobic fermentation,” Ind. Crop. Prod. 125, 596-601. DOI: 10.1016/j.indcrop.2018.09.033
Wang, Z., Hou, X., Sun, J., Li, M., Chen, Z., and Gao, Z. (2018b). “Comparison of ultrasound-assisted ionic liquid and alkaline pretreatment of Eucalyptus for enhancing enzymatic saccharification,” Bioresource Technol. 254, 145-150. DOI: 10.1016/j.biortech.2018.01.021
Wang, Z., Qu, L., Qian, J., He, Z., and Yi, S. (2019). “Effects of the ultrasound-assisted pretreatments using borax and sodium hydroxide on the physicochemical properties of Chinese fir,” Ultrason. Sonochem. 50, 200-207. DOI: 10.1016/j.ultsonch.2018.09.017
Wu, M., Varhegyi, G., and Zha, Q. (2009). “Kinetics of cellulose pyrolysis after a pressurized heat treatment,” Thermochim. Acta 496(1-2), 59-65. DOI: 10.1016/j.tca.2009.06.024
Wu, R., Zhao, X., and Liu, D. (2016). “Structural features of formiline pretreated sugar cane bagasse and their impact on the enzymatic hydrolysis of cellulose,” ACS Sustain. Chem. Eng. 4(3), 1255-1261. DOI: 10.1021/acssuschemeng.5b01298
Wu, Y., Jiang, L., Lin, Y., Qian, L., Xu, F., Lang, X., Fan, S., Zhao, Z., and Li. H. (2019). “Novel crude glycerol pretreatment for selective saccharification of sugarcane bagasse via fast pyrolysis.” Bioresource Technol. 294, article no. 122094 DOI:10.1016/j.biortech.2019.122094
Yang, X., Zeng, Y., Ma, F., Zhang, X., and Yu, H. (2010). “Effect of biopretreatment on thermogravimetric and chemical characteristics of corn stover by different white-rot fungi,” Bioresource Technol. 101(14), 5475-5479. DOI: 10.1016/j.biortech.2010.01.129
Yang, X., Ma, F., Yu, H., Zhang, X., and Chen, S. (2011). “Effects of biopretreatment of corn stover with white-rot fungus on low-temperature pyrolysis products,” Bioresource Technol. 102(3), 3498-3503. DOI: 10.1016/j.biortech.2010.11.021
Yiin, C. L., Yusup, S., Quitain, A. T., Uemura, Y., Sasaki, M., and Kida, T. (2018). “Thermogravimetric analysis and kinetic modeling of low-transition-temperature mixtures pretreated oil palm empty fruit bunch for possible maximum yield of pyrolysis oil,” Bioresource Technol. 255, 189-197. DOI: 10.1016/j.biortech.2018.01.132
You, T. T., Li, X., Wang, R. Z., Zhang, X. M., and Xu, F. (2019). “Effects of synergistic fungal pretreatment on structure and thermal properties of lignin from corncob,” Bioresource Technol. 272, 123-129. DOI: 10.1016/j.biortech.2018.09.145
Zeng, Y., Yang, X., Yu, H., Zhang, X., and Ma, F. (2011). “Comparative studies on thermochemical characterization of corn stover pretreated by white-rot and brown-rot fungi,” J. Agr. Food Chem. 59(18), 9965-9971. DOI: 10.1021/jf202451q
Article submitted: April 23, 2020; Peer review completed: July 25, 2020; Revised version received and accepted: March 18, 2021; Published: April 20, 2021.
DOI: 10.15376/biores.16.2.3978-3990
