Abstract
Carbon microsphere was successfully synthesized from waste office paper via hydrothermal method and high temperature treatment in nitrogen atmosphere. The process of carbon microsphere synthesis from waste office paper fiber using concentrated sulfuric acid was studied, and the influence of H2SO4 concentration and reaction time on the hydrolysis reaction was considered. To investigate the mechanism of conversion of waste office paper to carbon microsphere, Fourier transform infrared spectrometry was used to analyze the chemical composition of the supernatant liquid product and the non-carbonized carbon microsphere. The weight loss of the non-carbonized carbon microsphere was assessed through thermogravimetry. Scanning electron microscopy was used to analyze the morphology. The hydrolysis reaction of waste office paper fiber and synthesis mechanism of carbon microsphere under different conditions were evaluated. The results showed that when the reaction time reached 24 h, the product had uniform particle size and was well dispersed, and the more sulfuric acid present led to the waste office paper fiber being more thoroughly hydrolyzed.
Download PDF
Full Article
Synthesis Mechanism of Carbon Microsphere from Waste Office Paper via Hydrothermal Method
Mannan Yang,a Changqing Fang,a,b,* Jian Su,b,* Youliang Cheng,b Qi Zhang,b and Ming Liu c
Carbon microsphere was successfully synthesized from waste office paper via hydrothermal method and high temperature treatment in nitrogen atmosphere. The process of carbon microsphere synthesis from waste office paper fiber using concentrated sulfuric acid was studied, and the influence of H2SO4 concentration and reaction time on the hydrolysis reaction was considered. To investigate the mechanism of conversion of waste office paper to carbon microsphere, Fourier transform infrared spectrometry was used to analyze the chemical composition of the supernatant liquid product and the non-carbonized carbon microsphere. The weight loss of the non-carbonized carbon microsphere was assessed through thermogravimetry. Scanning electron microscopy was used to analyze the morphology. The hydrolysis reaction of waste office paper fiber and synthesis mechanism of carbon microsphere under different conditions were evaluated. The results showed that when the reaction time reached 24 h, the product had uniform particle size and was well dispersed, and the more sulfuric acid present led to the waste office paper fiber being more thoroughly hydrolyzed.
DOI: 10.15376/biores.17.4.5568-5577
Keywords: Waste office paper; Carbon microsphere; Hydrolysis; Hydrothermal method
Contact information: a: School of Mechanical and Precision Instrument Engineering, Xi’an University of Technology, Xi’an 710048, China; b: Faculty of Printing, Packaging Engineering and Digital Media Technology, Xi’an University of Technology, Xi’an 710048, China; c: Jiangsu Weixing New Materials Co., Ltd., Gaoyou 225600, China; *Corresponding authors: fcqxaut@163.com; sujian@xaut.edu.cn
GRAPHICAL ABSTRACT

INTRODUCTION
An office can hardly avoid the use of paper, and office paper consumption is huge around the world (Hasanin et al. 2020). At present, the waste office paper is mainly used for secondary papermaking and other paper products such as paperboard, pulp molding, etc. (Savitha et al. 2007; Liang et al. 2012). As the secondary beating process further cuts and breaks down the fiber in the pulp, the performance of recycled paper products is significantly reduced (Nazhad and Paszner 1994). It is known that good quality pulp is usually used for office paper, where most of the lignin in the biomass materials has been removed during the papermaking process. The cellulose and hemicellulose are the main chemical components of the office paper. Therefore, as a cellulose raw material, waste paper can have a broader application prospect (Lark et al. 1997; Rajput et al. 2012; Nishimura et al. 2017; Mikhailidi et al. 2019).
Carbon microsphere has excellent physical and chemical properties and can be used in many fields in the future; hence it has attracted the attention of many experts around the word (Su et al. 2020). The development of new cheap carbon sources and simple preparation methods of carbon materials are research hotspots (Shang et al. 2018a,b; Zhang et al. 2022). Many studies have shown that saccharides, such as sucrose, fructose, xylose, arabinose, etc., can be used to prepare carbon microsphere (Cai et al. 2017; Cheng et al. 2017; Zhang et al. 2017; Bai et al. 2021). Most of these saccharides can be obtained from a wide range of inexpensive biomass sources (Samayam and Schall 2010; Jagannathan et al. 2017).
Research shows that cellulose and hemicellulose can be hydrolyzed into mono sugars such as glucose, pentose, etc. (Wang et al. 2013; Shaikh et al. 2011). The authors’ previous research showed that cellulose is the most abundant component in waste office paper. Therefore, using waste office paper as a carbon resource to prepare carbon microsphere was judged to be feasible and has practical significance in many aspects such as ecological protection and resource conserving.
In this study, the waste office paper was used as carbon resource to synthesize carbon microsphere via hydrothermal method using sulfuric acid and followed by a high temperature treatment process in nitrogen atmosphere. The influence of H2SO4 concentration and reaction time on the hydrothermal reaction and the synthesis mechanism from waste office paper to carbon microsphere were evaluated.
EXPERIMENTAL
All of the waste office paper fiber used in this study was extracted from the same batch of waste office paper (M&G Chenguang Stationery Co., Ltd., Shanghai, China). The chemical contents of the waste office paper were tested by the Van Soest method, and the results are shown in Table 1. Concentrated sulfuric acid (98 wt% H2SO4) and the deionized water were purchased from Shaanxi Dexiang Experimental Equipment Co., Ltd., China. Absolute ethyl alcohol (C2H6O, 99.7 wt% Tianli Chemical Tianjin, China) was used to wash the hydrothermal products. Potassium bromide (KBr, purity ≥ 99.9 wt%) used for Fourier transform infrared (FT-IR) analysis was purchased from Tianjin Kemiou Chemical Reagent Co., Ltd., China. The sodium hydrogen carbonate (NaHCO3, 99.5 wt%) used for neutralize the waste filtrate was purchased from Tianjin Fuchen Chemical Reagents Factory, China.
Table 1. Chemical Components Content of the Waste Office Paper Fiber (wt%)
The waste office paper was soaked in deionized water for 24 h, then filtered, beaten, and dried to remove the additives other than biomass. Thus, the waste office paper fiber was obtained. The paper fiber was added into deionized water with stirring to prepare paper pulp with the solid-to-liquid ratio (g/L) of 12.5:1. Sulfuric acid was added into the paper pulp and fully and evenly stirred. Then the above mixed solutions were transferred into 100 mL para-polyphenylene (PPL) liner of the high-temperature and high-pressure reaction kettle and heated at 210 ℃ for different holding times. The detailed preparation conditions are shown in Table 2. After the reaction kettles had cooled down naturally to room temperature, the reaction solution was filtrated and washed with deionized water, and ethanol until the filtrate was neutral. Finally, the above obtained products were carbonized in a tubular furnace under the protection of nitrogen atmosphere (100 mL/min). The temperature of tube furnace was programmed to increase the temperature from room temperature at a heating rate of 10 ℃/min to 350 ℃ and held for 1 h, then increased to 800 ℃ at a heating rate of 5 ℃/min and held for 2 h. The carbon microsphere was obtained after the tube furnace was cooled down at 5 ℃/min to room temperature.
Table 2. The Synthesis Conditions of Carbon Microsphere
A field emission scanning electron microscope (FE-SEM, JEOL JSM-6700F, Jeol Ltd., Tokyo, Japan) was used to analyze the morphology of carbon microsphere. Fourier transform infrared spectroscopy in the wavelength range of 4000 to 400 cm-1 with an average of 20 scans and a resolution of 2 cm-1 was used to characterize the functional groups of the non-carbonized hydrothermal products. The functional groups of non-carbonized hydrothermal products were characterized using a Shimadzu FTIR-8400S infrared spectrometer (Kyoto, Japan) with KBr pellets as the sample matrix. The liquid supernatant products were tested by a Shimadzu IRSpirit spectrometer (Kyoto, Japan) in attenuated total refraction mode (ATR). Thermogravimetric analysis (TG, NETZSCH TG209F3, NETZSCH-Gerätebau GmbH (NGB), Selb, Germany) was performed at standard atmospheric pressure with N2 as the protective atmosphere. The particle size distribution was measured by the Nano Measurer 1.2.5 software (Fudan University, Shanghai, China). The yields of the synthesized carbon microsphere were calculated by the following formula, where Y is the yield, m1 is the weight of carbon microspheres after drying, and m2 is the weight of raw materials.
(1)
RESULTS AND DISCUSSION
Table 3 lists the yields and mean size of the carbon microsphere that was obtained under different conditions corresponding to the sample codes.
Figure 1 shows the morphologies of the waste office paper fiber and the carbon microsphere synthesized from waste office paper. Figure 2 shows the particle size distribution of the obtained carbon microsphere. It can be seen that the morphology of the product (C0-24) without adding concentrated sulfuric acid was irregular, and no spherical product was observed. However, when sulfuric acid was added, spherical products formed at the same reaction time (24 h). When 5 mL (C5-24) and 10 mL (C10-24) solutions of sulfuric acid were used, the spherical products with a uniform particle size and less impurity were obtained, but C10-24 was more dispersed and the size was more uniform, which was demonstrated by the particle size distribution curve (Fig. 2(a)).
Table 3. Yields and Mean Size of Carbon Microsphere at Various Conditions
Fig. 1. SEM images of the waste office paper fiber and the synthesized carbon microsphere
As shown in Figs. 1 and 2(b), when 10 mL of sulfuric acid was used, the product was spherical at the reaction time of 4 h (C10-4), but still irregular and undispersed, and there were some irregular shaped impurities present. With the increase of reaction time, the spherical form of carbon microsphere increased gradually and the impurities decreased. The range of particle size distribution became narrower, which confirmed that the particle size became more uniform. The reaction time of 24 h was sufficient, and still a few impurities were found on the surface of the obtained carbon microsphere. As shown in Table 3, the yield of the obtained carbon microsphere was highest when the H2SO4 content was 10 mL and the reaction time was 24 h.
Figure 2(c) shows the electrochemical impedance spectroscopy diagram of C10-24, the intersection of the high-frequency semicircle and the real axis indicates that the resistance value was 2.16 Ω, which confirms that the synthesized carbon microsphere achieved good electrical conductivity.
Fig. 2. Particle size distribution of the synthesized carbon microsphere with different H2SO4 concentration (a) and different reaction time (b). The electrochemical impedance spectroscopy diagram of C10-24 (c)
Figure 3(a) shows the FT-IR spectra of the non-carbonized hydrothermal products. The peaks at 590 and 881 cm-1 were attributed to the α- cellulose and β-cellulose units that were not reacted, whereas the bands at 1053 cm-1 were assigned to the -C-O (epoxy) stretching vibration.
Fig. 3. (a) FT-IR spectra of the non-carbonized hydrothermal products after 24 h; (b) FT-IR spectra of the liquid products with 10 mL H2SO4 at various reaction times; (c) FT-IR spectra of the liquid products with different H2SO4 contents for 24 h
The peak at 1211 cm-1 was due to the C-O-C stretching. The peak at 1635 cm-1 was because of the -C=O and C=C stretching vibrations of furan rings. The peak at 3465 cm-1 was assigned to O-H stretching, the oxygen-containing functional groups of gels that have been removed after the carbonization at 800 ℃. However, when the reaction time was 4 h, several peaks at 590 cm-1, 881 cm-1, 1053 cm-1, and 1211 cm-1 did not appear in the spectrum of C10-4, which indicated that the components of waste office paper were not completely hydrolyzed, and the result was consistent with the morphological analysis.
The FT-IR spectra of the liquid products of different reaction times are shown in Fig. 3(b). The peaks at 1094, 1641, and 3255 to 3348 cm-1 were attributed to the C-O (epoxy) stretching, the -C=O stretching vibrations, and the vibration peaks of -O-H groups, respectively. It can be seen that the components produced by the hydrolysis of waste office paper fiber in the hydrothermal reaction in different holding times were approximately the same. Figure 3(c) shows the FT-IR spectra of the liquid products with various H2SO4 contents at 24 h holding. All the peaks (located at 1092, 1639, and 3277 to 3329 cm-1) appeared at the same positions in Figs. 3(b) and (c). However, these peaks were weak in the spectrum of C20-24 and C25-24, which indicated that more sulfuric acid could promote the hydrolysis of waste paper fiber.
Figure 4 shows the TG curves of the non-carbonized hydrothermal products obtained under different reaction conditions.
Fig. 4. TG curves of the waste office paper fiber and the synthesized carbon microsphere
The slight weight loss before 260 ℃ was attributed to the removal of the partial moisture in the product and other small molecules. The weight loss in the range of 260 to 680 ℃ was due to further polycondensation and the removal of oxygen-containing functional groups. When the temperature reached 650 ℃, the mass tended to be stable and a vast majority of C element was left. The weight loss trend of the hydrothermal reaction product (C0-24) without sulfuric acid was similar to that of the office paper raw material. However, the weight loss between 260 ℃ to 370 ℃ was less than that of raw material, which indicated that the chemical composition had changed remarkably after hydrothermal reaction. The rapid weight loss between 650 and 730 ℃ of C0-24 and raw material was attributed to the volatilizations of inorganic fillers that were added in the papermaking process. The weight loss between 260 and 800 ℃ of C10-4 was less than that of other products, which was attributed to the lower hydrolysis degree. The functional groups on the surface of non-carbonized carbon microspheres were less, compared to others because of the short reaction time, which was consistent with the results of the FT-IR analysis.
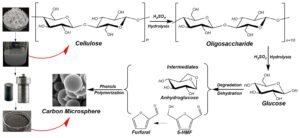
A schematic diagram of the synthesis mechanism of carbon microsphere prepared by hydrothermal method from waste office paper is shown in Fig. 5. Cellulose and hemicellulose in the waste office paper fiber were hydrolyzed in acidic solution to produce glucose, pentose, and some disaccharide components. These hydrolyzed products formed spherical gel through hydrothermal reaction. The gel was carbonized under the protection of nitrogen atmosphere and turned into carbon microsphere. Most of the lignin in raw material were removed during the pulp manufacturing process. Results showed that the contents of cellulose and hemicellulose in the fibers of waste office paper was higher than 72%. Studies have shown that cellulose can be hydrolyzed to glucose, and that cellulose can be converted to glucose in large quantities under acidic conditions. Sulfuric acid was the most commonly used acid catalyst for hydrolysis of cellulose. Limited swelling of cellulose can occur in aqueous solution of sulfuric acid with a certain concentration. The aqueous solution of sulfuric acid can penetrate into the crystallization zone of the paper fiber, which can result in swelling and dissolution of cellulose. With the increase of temperature, the oxygen bridge bond of cellulose began to break, and the long chain molecule became short chain molecules. Glucose, pentose, disaccharides, and intermediate products were generated with the participation of water. The generated glucose by hydrolysis was continued to be held in the heat and pressure of hydrothermal conditions and was subjected to hydrothermal reactions. In the course of the hydrothermal reactions, the glucose was converted to 5-hydroxymethylfurfural (HMF) via intramolecular dehydration (Sevilla and Fuertes 2009). The HMF can form a 3D network structure by polycondensation, which was the precursor compound of carbon microsphere.
Fig. 5. The schematic diagram of the synthesis mechanism for carbon microsphere
CONCLUSIONS
- In this study, carbon microsphere was successfully prepared from fibers of waste office paper, of which the main content was cellulose and hemicellulose.
- Cellulose and hemicellulose were first hydrolyzed into oligosaccharides and monosaccharides under the condition of sulfuric acid catalysis. The hydrolyzed products were further converted into 5-HMF by intermolecular dehydration in hydrothermal reaction to form spherical products, and carbon microspheres were obtained after carbonization of these at high temperature.
- The results showed that the content of sulfuric acid and reaction time affected the formation of carbon microsphere by affecting the hydrolysis process of the waste paper fiber. When 10 mL of sulfuric acid was used, the paper fiber can be hydrolyzed within 4 h at 210 ℃ and can form spherical hydrothermal products. More sulfuric acid can make the waste office paper fiber more thoroughly hydrolyzed. There were almost no cellulose and hemicellulose detected in the reaction liquid when above 20 mL of sulfuric acid was used.
- This work provides a new way for high value recycling of waste paper. The synthesized carbon microsphere had good electrical conductivity, and its application in conductive inks needs further study.
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program of China (No. 2021YFD1600402), Natural Science Basic Research Program of Shaanxi Province (Young Talents Program, 2021JQ-484), National Natural Science Foundation of China (No. 52000151) and Central Guidance on Local Science and Technology Development Fund of Shaanxi Province (2020-ZYYD-NCC-9).
REFERENCES CITED
Bai, L. H., Xing, H. L., Ji, X. L., and Yang, P. (2021). “D-xylose-derived carbon microspheres modified by CuFe2O4 nanoparticles with excellent microwave absorption properties,” J. Mater. Sci. Mater. Electron. 32(22), 26726-26739. DOI: 10.1007/s10854-021-07050-7
Cai, H. M., Lin, X. Y., Qin, Y., and Luo, X. G. (2017). “Hydrothermal synthesis of carbon microsphere from glucose at low temperature and its adsorption property of uranium (VI),” J. Radioanal. Nucl. Chem. 311, 695-706. DOI: 10.1007/s10967-016- 5106-9
Cheng, Y. L., Yang, M. N., Fang, C. Q., Chen, J., Bai, M., and Su, J. (2017). “Controllable morphologies of carbon microspheres via green hydrothermal method using fructose and xylose,” Chem. Lett. 46(9), 1400-1402. DOI: 10.1246/cl.170592
Hasanin, M. S., Hashem, A. H., El-Sayed, E., and El-Saied, H. (2020). “Green ecofriendly bio-deinking of mixed office waste paper using various enzymes from Rhizopus microsporus AH3: Efficiency and characteristics,” Cellulose 27(8), 4443- 4453. DOI: 10.1007/s10570-020-03071-3
Jagannathan, P., Muthukumaran, C., and Tamilarasan, K. (2017). “A sequential pretreatment of lignocelluloses in bamboo biomass to fermentable sugars by acid/enzymatic hydrolysis,” 3Biotech 7(4), article no. 260. DOI: 10.1007/s13205-017-0892-5
Lark, N., Xia, Y., Qin, C. G., Gong, C. S., and Tsao, G. T. (1997). “Production of ethanol from recycled paper sludge using cellulase and yeast, Kluveromyces marxianus,” Biomass Bioenerg. 12(2), 135-143. DOI: 10.1016/S0961-9534(96)00069-4
Liang, S., Zhang, T. Z., and Xu, Y. (2012). “Comparisons of four categories of waste recycling in China’s paper industry based on physical input-output life-cycle assessment model,” Waste Manage. 32(3), 603-612. DOI: 10.1016/j.wasman.2011.10.020
Mikhailidi, A. M., Saurov, S. K., Markin, V. I., and Kotelnikova, N. E. (2019). “Functional materials from paper wastes: I. From waste newsprint paper and cardboard to high-grade cellulose fibers,” Russ. J. Bioorg. Chem. 45(7), 888-894. DOI: 10.1134/S1068162019070069
Nazhad, M. M., and Paszner, L. (1994). “Fundamentals of strength loss in recycled paper,” TAPPI J. 77(9), 171-179. DOI: 10.1007/BF00813509
Nishimura, H., Tan, L., Kira, N., Tomiyama, S., Yamada, K., Sun, Z. Y., Tang Y. Q., Morimura S., and Kida, K. (2017). “Production of ethanol from a mixture of waste paper and kitchen waste via a process of successive liquefaction, presaccharification, and simultaneous saccharification and fermentation,” Waste Manage. 67, 86-94. DOI: 10.1016/j.wasman.2017.04.030
Rajput, D., Bhagade, S. S., Raut, S. P., Ralegaonkar, R. V., and Mandavgane, S. A. (2012). “Reuse of cotton and recycle paper mill waste as building material,” Constr. Build Mater. 34, 470-475. DOI: 10.1016/j.conbuildmat.2012.02.035
Samayam, I. P., and Schall, C. A. (2010). “Saccharification of ionic liquid pretreated biomass with commercial enzyme mixtures,” Bioresource Technol. 101(10), 3561- 3566. DOI: 10.1016/j.biortech.2009.12.066
Savitha, S., Sadhasivam, S., and Swaminathan, K. (2007). “Application of Aspergillus fumigatus xylanase for quality improvement of waste paper pulp,” Bull. Environ. Contam. Toxicol. 78(3-4), 217-221. DOI: 10.1007/s00128-007-9132-8
Sevilla, M. and Fuertes, A. B. (2009). “The production of carbon materials by hydrothermal carbonization of cellulose,” Carbon 47(9), 2281-2289. DOI: 10.1016/j.carbon.2009.04.026
Shaikh, H. M., Adsul, M. G., Gokhale, D. V., and Varma, A. J. (2011). “Enhanced enzymatic hydrolysis of cellulose by partial modification of its chemical structure,” Carbohydr. Polym. 86(2), 962-968. DOI: 10.1016/j.carbpol.2011.05.067
Shang, J. B., Lin, J., and Zhao, G. J. (2018a). “Effects of chemical curing on the properties of wood-based precursors and activated carbon microspheres,” BioResources 13(4), 7175-7187. DOI: 10.15376/biores.13.4.7175-7187
Shang, J. B., Lin, J., and Zhao G. J. (2018b) “Preparation and characterization of wood- based pre-oxidized precursors and activated carbon microspheres,” BioResources 13(4), 7983-7997. DOI: 10.15376/biores.13.4.7983-7997
Su, J., Fang, C. Q., Yang, M. N., Cheng, Y. L., Wang, Z., Huang, Z. G., and You, C. Y. (2020). “A controllable soft-templating approach to synthesize mesoporous carbon microspheres derived from d-xylose via hydrothermal method,” J. Mater. Sci. Technol. 38, 183-188. DOI: 10.1016/j.jmst.2019.03.050
Wang, Y., Yuan, B., Ji, Y. C., and Li, H. (2013). “Hydrolysis of hemicellulose to produce fermentable monosaccharides by plasma acid,” Carbohydr. Polym. 97(2), 518-522.
DOI: 10.1016/j.carbpol.2013.05.017
Zhang, C. Y., Lin, S., Peng, J. J., Hong, Y. Z., Wang, Z. Y., and Jin, X. B. (2017). “Preparation of highly porous carbon through activation of NH4Cl induced hydrothermal microsphere derivation of glucose,” RSC Adv. 7(11), 6486-6491. DOI: 10.1039/c6ra26141h
Zhang, Q. Y., Wu, R., Zhou, Y. H., Lin, Q. L., and Fang, C. Q. (2022). “A novel surface- oxidized rigid carbon foam with hierarchical macro-nanoporous structure for efficient removal of malachite green and lead ion,” J. Mater. Sci. Technol. 103, 15-28. DOI: 10.1016/j.jmst.2021.07.012
Article submitted: April 30, 2022; Peer review completed: July 17, 2022; Revised version received and accepted: July 29, 2022; Published: August 3, 2022.
DOI: 10.15376/biores.17.4.5568-5577
