Abstract
Hydroxyl radicals (HO•) and hydrogen radicals (H•) produced from sub/supercritical ethanol have an obvious contribution on cellulose liquefaction for bio-oil production. Salicylic acid was employed as the HO• trap and CCl4 was employed as the H• trap to investigate the role of HO• and H• on the formation pathways of dominant chemical components in bio-oil during cellulose liquefaction in sub/supercritical ethanol (mostly ketones and esters). The yield of bio-oil decreased from 24.7% to 20.7% with the addition of CCl4, while the bio-oil yield increased from 29.3% to 47.9% with the addition of salicylic acid. Gas chromatography/mass spectrometry results showed that the yields of ketones, esters, and phenols in the bio-oil were 22.3%, 8.8%, and 4.7%, respectively, without salicylic acid or CCl4. The highest yields of esters and phenols increased to 21.6% and 36.9%, respectively, in the presence of salicylic acid. The yield of ketones decreased to 14.1%. Experimental data indicated that the cleavage of C-O-C and C-C bonds in the cornstalk cellulose initially generated many active cellulose fragments. Then, platform chemicals were formed from these fragments through aromatization, isomerization, aldol condensation, Baeyer-Villiger oxidation, and trans-Diels-Alder ring-opening with the redox of HO• and H•.
Download PDF
Full Article
The Distribution of Bio-Oil Components with the Effects of Sub/Supercritical Ethanol and Free Radicals during Cellulose Liquefaction
Wei Li, Xin-an Xie,* Cheng-zheng Tang, Yan Li, Lu Li, Ya-li Wang, Di Fan, and Xing Wei
Hydroxyl radicals (HO•) and hydrogen radicals (H•) produced from sub/supercritical ethanol have an obvious contribution on cellulose liquefaction for bio-oil production. Salicylic acid was employed as the HO• trap and CCl4 was employed as the H• trap to investigate the role of HO• and H• on the formation pathways of dominant chemical components in bio-oil during cellulose liquefaction in sub/supercritical ethanol (mostly ketones and esters). The yield of bio-oil decreased from 24.7% to 20.7% with the addition of CCl4, while the bio-oil yield increased from 29.3% to 47.9% with the addition of salicylic acid. Gas chromatography/mass spectrometry results showed that the yields of ketones, esters, and phenols in the bio-oil were 22.3%, 8.8%, and 4.7%, respectively, without salicylic acid or CCl4. The highest yields of esters and phenols increased to 21.6% and 36.9%, respectively, in the presence of salicylic acid. The yield of ketones decreased to 14.1%. Experimental data indicated that the cleavage of C-O-C and C-C bonds in the cornstalk cellulose initially generated many active cellulose fragments. Then, platform chemicals were formed from these fragments through aromatization, isomerization, aldol condensation, Baeyer-Villiger oxidation, and trans-Diels-Alder ring-opening with the redox of HO• and H•.
Keywords: Cellulose liquefaction; Sub/Supercritical ethanol; Hydroxyl radical; Hydrogen radical
Contact information: College of Food Science, South China Agricultural University, Guangzhou 510640, China; *Corresponding author: xinanxie@scau.edu.cn
INTRODUCTION
Energy supply is one of the world’s most concerning problems with respect to the theme of sustainable development in the 21st century (Gallucci et al. 2015). With fossil fuel supplies dwindling, biomass energy reserves, as a kind of alternative energy, have received much attention because of their advantages, including being renewable nature and having enormous reserves, which are both necessary for sustainable development (Chinese Ministry of Agriculture & US Department Energy 1998; Song et al. 2004; Japan Institute of Energy 2007).
Cellulosic biomass is the most abundant type of biomass and serves as an important feedstock for research. Investigations of cellulose pyrolysis science could provide meaningful methods for biomass reserve utilization. Cellulose pyrolysis has been shown to be an applicable method for producing biomass energy (such as bio-oil and gas products) and platform chemicals, but the yield of the bio-oil has been relatively low and cellulose pyrolysis has a high char content. In recent years, supercritical liquefaction of cellulose has been considered as a promising method for producing bio-oil and platform chemicals (such as esters, ketones, etc.), and some investigations have confirmed that supercritical organic solvents have the advantages of remaining in the liquid phase (higher dissolving capacity) and gas phase (lower viscosity). These solvents have a great contribution to cellulose decomposition into bio-oil and also show a critical inhibition of the repolymerization of liquefaction products (Li et al. 2010; Zheng et al. 2012). Additionally, some experimental results have shown that the bio-oil produced from supercritical liquefaction has a higher yield and higher selectivity in comparison with those of a pyrolysis method (Yamazaki et al. 2006; Mazaheri et al. 2010; Zheng et al. 2012; Tao et al. 2014). Researchers have found that ketones and esters are dominant components in the liquid products of cellulose liquefaction in a sub/supercritical solvent, and experiments have indicated that the ketone contents derived from rice straw, spruce, bamboo, and cornstalk cellulose with supercritical liquefaction are as high as 27% to 50% (Xie et al. 2008; Cao 2008; Chang et al. 2011), 25% to 35% (Demirbas 2000, 2007), 20% to 25% (Shao et al. 2007; Peng et al. 2009), and 54% (Zheng et al. 2012), respectively, in heavy oil. Cornstalk cellulose liquefaction in sub/supercritical ethanol has shown to have an ester yield of 26.9% to 42.7% (Tang 2009; Zheng et al. 2013). Experimental results have also shown that levulinic acid esters account for 13.8% to 40.7% of produced bio-oil (Mao et al. 2010; Rataboul and Essayem 2011), and the yield of ethyl esters in a light oil and a heavy oil were 26.9% and 29.6%, respectively (Zheng et al. 2012). These important platform chemicals are used in many fields, such as pesticides and daily chemicals and materials.
The free radicals HO• and H• have strong redox abilities with active fragments during chemical reactions (Sun et al. 1999, 2002). Free radicals exist for a very short time, but these unstable and active transition-state fragments (free radicals) are important for clarifying the mechanism of chemical reactions; thus, spin traps have been employed to trap free radicals for further investigation of their effects on chemical reactions. One thing to consider is that HO• can react with aromatic compounds. By detecting the yield of hydroxylation products (dihydroxy-benzoic acid) with high-performance liquid chromatography (HPLC) or gas chromatography (GC), the yield of HO• can then be indirectly determined with salicylic acid as a HO• spin trap (Richmond and Halliwell 1982; Zhou and Dong 1995; Yao et al. 2006). One work (Nie et al. 2007) reported that CCl4 could trap H• inside a cavitation bubble for investigation of the effect of trace CCl4 on styrene emulsion polymerization with ultrasonic radiation. Studies have confirmed that ethanol can produce HO• and H• under a sub/supercritical state (Chen 2008), and these free radicals have obvious effects on promoting cellulose liquefaction. A study on rice straw liquefied in supercritical ethanol showed that cellulose can produce ethyl and hydroxyl radicals, which can then attack C-O bonds and C-H bonds in lignin, respectively, thus enhancing rice straw liquefaction (Chen 2008). The cleavage of C-C, C-O, and -OH bonds of cellulose is shown to be enhanced under the effects of ethanol free radicals (Tao et al. 2014).
Presently, the roles of HO• and H• generated from sub/supercritical ethanol in bio-oil and main chemicals formation routes have not been sufficiently clarified, and only little information is available about how HO• and H• contribute to target bond cleavage in cellulose liquefaction with a higher yield of bio-oil. In this work, salicylic acid was selected as the HO• trap and CCl4 was selected as the H• trap to investigate the influence of HO• and H• concentration and activity on cornstalk cellulose liquefaction in sub/supercritical ethanol. The aim of this work was to better understand the performances of HO• and H• in the formation pathways of bio-oil and platform chemicals (such as ketones, esters, etc.). The liquid products were characterized by gas chromatography/mass spectrometry (GC/MS) to further verify the selectivity of HO• and H• on the cellulose liquefaction product distribution. This work provides a theoretical basis for the optimization of biomass liquefaction in supercritical solvents. It also provides process design approaches to higher yields and selectivity for bio-oil and important platform chemical production.
EXPERIMENTAL
Materials and Reagents
Cornstalk was collected from a farm of South China Agricultural University, Guangzhou, China. The feedstock was milled to obtain a fine powder, and the powder that passed through a 40-mesh sieve was used in the experiments. The powder was dried at 105 °C for 12 h before use. Anhydrous ethanol (Tianjin Fuyu Fine Chemical Co, China), acetone (Tianjin Hongda Chemical Reagent Co, China), salicylic acid (Tianjin Fuchen Chemical Reagent Co, China), carbon tetrachloride (CCl4) (Tianjin Fuyu Fine Chemical Co, China), and sodium hydroxide (Guangdong Guanghua Science and Technology Co, China) were of analytical grade. Sodium chlorite (Aladdin Reagent Co, China) was of industrial grade.
Cellulose Preparation
Water-soluble products in the cornstalk cellulose were removed according to GB/T 2677.1 (1993) and GB/T 2677.10 (1995). The cornstalk was treated with a sodium chlorite solution to remove lignin and obtain holocellulose. An insoluble residue was then prepared by treating the holocellulose with sodium hydroxide. This residue was dried at 105 °C for 12 h and kept in a desiccator at room temperature. According to GB/T 744 (2004) and GB/T 2677.3 (1993), the cellulose content and ash content of the residue were 97.51±1.04% and 1.23±0.19%, respectively.
Experimental Procedures
The dosage of powdered cornstalk cellulose was 8 g, the volume of ethanol was 100 mL, the salicylic acid volume range was 0 to 4 mL, and the carbon tetrachloride volume range was 0 to 2 mL. The feedstock and liquid were loaded into an autoclave (PARR 4521M, USA). The liquefaction experiments were conducted in a 1.0-L intermittent autoclave at 280 or 320 °C for 60 min. The autoclave was rated up to a working pressure of 13 MPa and a working temperature of 350 °C.
The critical temperature and critical pressure of ethanol are 243 °C and 6.34 MPa, respectively. The experimental procedure flow chart is shown in Fig. 1 (Gas products, GAS; Bio-oil, BO; Cellulose residue, RE).
The volatile compounds (VO) were classified for mass balance with cornstalk cellulose feedstock. The results obtained in this study were reported using the following parameters:
where is the yield of bio-oil, is the yield of the solid residue, is the yield of the gas products, is the yield of volatile compounds, is the weight of the bio-oil, is the weight of the cellulose residue, is the weight of the gas products, and is the weight of the raw material.
Fig. 1. Flow diagram of the cellulose liquefaction procedure in sub/supercritical ethanol
Chemical Analysis
The BO was analyzed by a gas chromatograph equipped with a mass selective detector (GC-MS, Finnigan Co, USA). Both the injector and detector were kept at 250 °C, and the velocity of the carrier gas (He) was 1.0 mL·min–1. An HP-1 column (30 mm × 0.25 mm) was also used. The oven program was 10 min isothermal at 40 °C, followed by a heating rate of 10 °C·min–1 to 120 °C, where it was held for 1 min. The oven temperature then increased at a heating rate of 5 °C·min-1 to 250 °C and was held for 10 min. The injected volume was 0.5 μL. The mass range scanned was from 35 to 335 amu in electron-impact (70 eV) mode. Data were acquired and processed using Chemstation software (B.04.01, Finnigan Co, USA). The compounds in the samples were identified by comparing the mass spectra results with those in NIST library data.
RESULTS AND DISCUSSION
Effects of H• Trap (CCl4) Dosage on Cellulose Liquefaction
Cornstalk cellulose was liquefied in 100 mL of ethanol for 60 min at 280 °C. The concentration of H• was influenced by the dosage of CCl4. The maximum pressure in the autoclave was 11 MPa. The effects of H• concentration on the yields of GAS, BO, VO, and RE from cellulose liquefaction are shown in Fig. 2.
Figure 2 shows that the gas yield increased from 2.0% to 5.0% as the CCl4 dosage increased from 0 to 2 mL. Gases (such as H2O, CO2, CH4, CO, and C2H4) are formed in large amounts during the initial stage of cellulose liquefaction (Guo et al. 2011). Cellulose liquefaction was inhibited by H• being trapped by the CCl4, and the gas content increased because part of the fragments were transformed to gas under the effect of CCl4. The residue yield increased from 54.1% to 59.1%, bio-oil yield decreased from 24.7% to 20.7%, and volatile compounds yield decreased from 19.2% to 15.3%. This may have occurred because the CCl4 and fragments had a competitive reaction with H•. Fragment liquefaction through secondary reactions was inhibited as the dosage of CCl4 increased, resulting in a decreased yield of bio-oil and volatile compounds, while the residue yield increased. Part of the bio-oil and volatile compounds were transformed to residue through condensation as the concentration of H• decreased, and cellulose liquefaction was not efficient as the dosage of CCl4 increased.
Fig. 2. Effect of H• concentration (CCl4 dosage) on the yield of liquefaction products
GC/MS Analysis of Bio-Oil with Various Dosages of CCl4
The bio-oil obtained from cellulose liquefaction in 100 mL of ethanol at 280 °C for 60 min with various dosages of CCl4 (0 mL, 1 mL, and 2 mL) were characterized by GC/MS to investigate the performance of H• concentration and its activity on dominant chemical formation (ketones, esters, phenols, furans, and aldehydes). The yields of the dominant components identified in the bio-oil are presented in Table 1. The dominant components of the bio-oil were ketones, esters, furans, and aldehydes.
The yields and compositions of the bio-oil components were different at different dosages of CCl4. At 280 °C without CCl4, there were few types of dominant compounds in the bio-oil. In the presence of CCl4, there were various types and yields of ketones, esters, furans, and aldehydes.
This result is supported by the fact that the H• was trapped by CCl4; thus, the redox between the cellulose fragments and intermediates was inhibited. At a lower H• concentration the cellulose liquefaction was not efficient and more intermediates (ketones, esters, furans, and aldehydes) were produced. The dominant ketone components were 4-hydroxy-4-methyl-2-pentanone and cyclopentenones, and the main esters were ethyl esters and lactones.
Table 1. GC-MS Analysis Results for the BO Obtained from Cellulose Liquefaction in Sub/Supercritical Ethanol with Various Dosages of CCl4
– : Not detected or less than 0.1%
Effects of HO• Trap (Salicylic Acid) Dosage on Cellulose Liquefaction
Salicylic acid was added to trap the hydroxyl radicals (HO•) and H•/H+ donor under the sub/supercritical state. The concentration and activity of HO• and H•/H+ during cellulose liquefaction were influenced by the dosage range of salicylic acid and the reaction temperature. Supercritical liquefaction of cellulose was conducted in the autoclave at 320 °C for 60 min in 100 mL of ethanol. The dosage range of salicylic acid was 0 to 4 mL. The maximum pressure in the autoclave was 12 MPa. The effects of salicylic acid dosage on the cellulose liquefaction product distribution are shown in Fig. 3.
Experimental results showed that cellulose was more easily liquefied with a higher dosage of salicylic acid. The concentration of HO• decreased and the concentration of H•/H+ increased with an increase in salicylic acid dosage. The yield of bio-oil increased from 29.3% to 47.9% with a salicylic acid dosage increase from 0 mL to 3 mL. The yield of volatile compounds decreased from 39.6% to 23.2%, and the yield of residue decreased from 26.7% to 24.3%. These results occurred because of the enhancement in the redox ability of H•/H+ and HO• on cellulose fragments, as there was a higher concentration and higher activity of H•/H+ in the presence of salicylic acid. More fragments and free radicals were transformed to the bio-oil components under the strong redox of H•/H+ and HO•. The cellulose conversion rate also improved, i.e., the yield of residue decreased, with the addition of salicylic acid.
Fig. 3. Effects of salicylic acid dosage on the yields of cellulose liquefaction products
The yield of residue increased slightly as the salicylic acid dosage increased from 3 to 4 mL. This is because some of the bio-oil components became transformed to residue through condensation, cyclization, and redox when the H•/H+ concentration and activity further increased with a salicylic acid dosage up to 4 mL.
The overall yield of gas slightly decreased as the volume of salicylic acid increased from 0 to 4 mL. More cellulose fragments transformed into either bio-oil or residue at a higher concentration and activity of H•/H+, as gas production from low molecular weight cellulose fragments was inhibited.
GC/MS Analysis of the Bio-oil at Various Dosages of Salicylic Acid
The bio-oil obtained from cellulose liquefaction in 100 mL of ethanol at 320 °C for 60 min with different dosages of salicylic acid (0, 1, 2, and 4 mL) was characterized by GC/MS. GC/MS allowed for the investigation of the performances of HO• and H•/H+ concentration and activity on dominant chemical formation (ketones, esters, and phenols). The yields of the dominant components identified in the bio-oil are presented in Table 2.
Table 2. GC/MS Analysis Results for the BO Obtained from Cellulose Liquefaction in Sub/Supercritical Ethanol at Various Dosages of Salicylic Acid
– : Not detected or less than 0.1%
As shown in Table 2, the dominant components of the bio-oil were ketones, esters, and phenols. The yields and compositions were different at the different dosages of salicylic acid. Table 2 shows that the ketones were mostly aliphatic ketones (4-hydroxy-4-methyl-2-pentanone) and aromatic ketones (2-formyloxy-1-phenyl ethanone). The esters were primarily ethyl esters, and phenol was the dominant component of the phenols.
In the absence of salicylic acid, the bio-oil yield was 29.3%, and lower yields of the dominant components was also seen. The concentration and activity of H•/H+ increased rapidly with the increase in salicylic acid dosage. The yield of bio-oil increased to 47.9%, with the yields of ketones, esters, and phenols also increasing. However, the yield of furans decreased in the presence of salicylic acid. These results are attributed to depolymerization, deoxidization, decarboxylation, and oxidation of the various active cellulose fragments being enhanced under the strong redox of H•/H+ and HO• in the process of cellulose liquefaction. This enhancement thus resulted in higher yields of ketones, esters, and phenols.
Effects of Salicylic Acid Dosage on the Yields of the Dominant Components
The yields of ketones, esters, and phenols in the bio-oil are shown in Fig. 4.
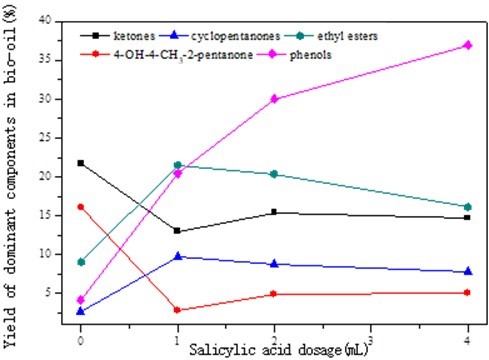
Fig. 4. Effects of salicylic acid dosage on the yields of the dominant components in the bio-oil
With a salicylic acid dosage increase from 0 to 4 mL, the overall yield of ketones decreased from 22.3% to 14.1%. However, the yield of the ketone 4-hydroxy-4-methyl-2-pentanone decreased from 16.1% to 2.6%, and the yield of cyclopentenones increased from 2.25% to 9.3%. This may have been caused by the HO• trap (salicylic acid), which increased the concentration of H•/H+and the reaction activity of cellulose fragments under the sub/supercritical state and produced ketones from active fragments. Acids, esters, and other small molecules were generated from aliphatic ketones through decomposition and strong redox under a higher concentration of H•/H+. Additionally, some of the -OH groups (hydroxyl compounds) transformed into cyclopentenoes via the isomerization of enolate structures, dehydration, and cyclization (Chang et al. 2011).
The yield of esters increased from 8.8% to 21.6%. This may be due to the fact that the esterification between hydroxyl compounds and acids was enhanced with the increasing concentration of H•/H+ in the presence of salicylic acid. The esters further decomposed into small molecular weight ketones and acids under the strong redox of H•/H+ with an excessive dosage of salicylic acid (4 mL). In the presence of salicylic acid, the yield of phenols increased largely from 4.7% to 36.9%. The yield of phenol in the phenols was 31.79%. The high phenol yield was because of the higher concentration of H•/H+ enhancing the formation of aromatic compounds, hydroxyl compounds, and phenols through oxidation and aromatization (Cartson et al. 2010).
Effects of Salicylic Acid and CCl4 on Cellulose Liquefaction
Supercritical liquefaction of cornstalk cellulose with salicylic acid or CCl4 was conducted in the autoclave at 280 °C for 60 min in 100 mL of ethanol. The dosage of salicylic acid or CCl4 were 1 mL. The maximum pressure in the autoclave was 10 MPa. The effects of salicylic acid or CCl4 on the cellulose liquefaction product distribution are shown in Fig. 5.
Fig. 5. The comparison of cellulose liquefaction products with salicylic acid and CCl4
Figure 5 shows that in the presence of CCl4, the yield of GAS and RE were higher than with salicylic acid. But the yield of VO and BO with CCl4 were lower than with salicylic acid. The decreasing of H• concentration had an inhibiting effect on cellulose liquefaction with H• trapped by CCl4, while the HO• and H•/H+ concentration were higher with salicylic acid which had an enhancement on cellulose liquefaction. Various of active fragments were produced with the C-O-C and -OH in cellulose cleaved under the attack of HO• and H•/H+, thus a higher content of VO and BO were formed from these fragments via redox, addition, cyclization, and hydrogenation.
Pathways of Ketones Formation
As shown in Tables 1 and 2 and Fig. 4, one of the dominant components in the bio-oil were ketones. The yield of ketones was higher than 14.1% in the presence of salicylic acid. The dominant ketones observed were 4-hydroxy-4-methyl-2-pentanone and cyclopentenones.
Liquefaction and pyrolysis were two dominant reactions that took place during the cellulose decomposition to bio-oil in sub/supercritical ethanol. In the initial stage, active cellulose was produced from raw cellulose through bond cleavage and dehydration (Huang et al. 2011). Trans-Diels-Alder ring-opening, dehydration, isomerization, and aldol condensation between the active cellulose and free radicals had great contributions to the formation of ketones, esters, and acids (such as 4-hydroxy-4-methyl-2-pentanone). The pathways of aliphatic ketone formation are shown in Fig. 6.
Fig. 6. Pathways of ketone formation with the effects of ethanol free radicals
Fig. 7. Pathways of ketone decomposition with the effect of ethanol free radicals
Alicyclic ketones (such as cyclopentenone) in ketones were formed from active cellulose via C-C bond cleavage and cyclization. Aromatic ketones (such as 2-formyl-1-phenyl ethyl ketone) in ketones were formed through ring-opening, condensation, and cyclization between the active cellulose, alicyclic ketones, free radicals, and various fragments (Tao et al. 2013). Under the sub/supercritical state of ethanol, some of the aromatic ketones decomposed to acids and alcohols, with further bonds experiencing cracking, decarbonylation, and isomerization. At a higher salicylic acid dosage, these reactions were enhanced by the stronger redox of a higher concentration and higher activity of HO• and H•/H+. The pathways of ketone decomposition are shown in Fig. 7.
Pathways of Esters Formation
It can be concluded from Tables 1 and 2 and Fig. 4 that esters were one of the dominant components in the bio-oil.
Salicylic acid enhanced the conversion rate of the cellulose. The yield of ethyl esters in esters increased to 21.6%. Many of the active cellulose fragments were transformed to acids through esterification under the redox of HO• and H•/H+. As the salicylic acid dosage increased, the esters further decomposed into smaller molecules via redox, cracking, and isomerization under the higher concentration and activity of H•/H+. In the sub/supercritical ethanol, the cellulose transformed to active cellulose by dehydration, and its degree of polymerization declined rapidly (Tan 2005). Esters, ketones, and acids were produced from the active cellulose through trans-Diels-Alder ring-opening, dehydration, redox, and isomerization mechanisms, and these formation routes were all enhanced by the strong redox of various free radicals (Bicker et al.2005; Tao et al. 2013). Ethyl esters in esters were formed largely through esterification among active cellulose fragments and ethanol radicals. At the same time, lactones and esters were formed from ketones via Baeyer-Villiger reaction, ring-opening, and redox mechanisms with anhydride as the medium (Berkessel and Andreae 2001; Yamada et al. 2007). The pathways of ester formation are shown in Fig. 8.
Fig. 8. Pathways of ester formation with the effects of ethanol free radicals
Pathways of Phenols Formation
It can be seen from Table 2 and Fig. 4 that the yield of phenols rapidly increased to 36.9%. The yield of phenol in phenols was as high as 31.79%. In the presence of salicylic acid, the concentration and activity of H•/H+ increased rapidly. Various fragments and free radicals were produced from cellulose liquefaction through redox, dehydration, and aromatization, which were enhanced under the strong redox of HO• and H•/H+ (Cartson et al. 2010). A higher yield of phenols was produced under the stronger redox at a higher concentration and higher activity of H•/H+ as the dosage of salicylic acid increased to 4 mL. And part of phenol were produced from salicylic acid through decomposition at 320 C. Possible pathways of phenol formation from cellulose are shown in Fig. 9.
Fig. 9. Pathways of phenol formation with the effects of ethanol free radicals
CONCLUSIONS
- Ethanol produced HO• and H• under a sub/supercritical state, and cellulose was more easily liquefied for bio-oil production in sub/supercritical ethanol with salicylic acid. Cellulose liquefaction was inhibited by the use of CCl4 for sub/supercritical ethanol. A higher concentration and higher activity of HO• and H•/H+ enhanced the formation of esters and phenols in the bio-oil, but the yields of various ketones decreased.
- Ketones in the bio-oil included aliphatic ketones, cyclopentenones, and aromatic ketones. Under the strong redox of HO• and H•/H+, the aliphatic ketones were generated from active cellulose through trans-Diels-Alder ring-opening, dehydration, isomerization, and aldol condensation. The cyclopentenones were formed from some of the aliphatic ketones via an enol structure mechanism. The aromatic ketones were produced through condensation and cyclization between cyclopentenones and active cellulose fragments.
- Ethyl esters and lactones were the dominant esters found in the bio-oil. Ethyl esters were generated through trans-Diels-Alder ring-opening, dehydration, isomerization, redox, condensation, and esterification of active cellulose and various cellulose fragments with HO• and H•/H+. Lactones and esters were formed by ketones that decomposed via a Baeyer-Villiger reaction under the strong redox of HO• and H•/H+.
- Phenols were generated from the active cellulose fragments and free radicals through redox, dehydration, and aromatization. These reactions were enhanced by a higher concentration and higher activity of H•/H+ and HO•.
ACKNOWLEDGMENTS
The authors sincerely acknowledge the financial support of the National Natural Science Foundation of China (21576107, 21176097) and the Guangdong Provincial Science and Technology Program Foundation of China (2014A010106024).
REFERENCES CITED
Berkessel, A., and Andreae, M. R. (2001). “Efficient catalytic methods for the Baeyer-Villiger oxidation and epoxidation with hydrogen peroxide,” Tetrahedron Letters 42(12), 2293-2295. DOI: 10.1016/S0040-4039(01)00141-1
Bicker, M., Endres, S., Ott, L., and Vogel, H. (2005). “Catalytical conversion of carbohydrates in subcritical water: A new chemical process for lactic acid production,” Journal of Molecular Catalysis A: Chemical 239(1-2), 151-157. DOI: 10.1016/j.molcata.2005.06.017
Cao, H. T. (2008). The Research on the Liquefaction of Biomass in Sub-and Supercritical Water, Master’s thesis, Hunan University, Changsha, China.
Cartson, T. R., Jae, J., Lin, Y. C., Tompsett, G. A., and Huber, G. W. (2010). “Catalytic fast pyrolysis of glucose with HZSM-5: The combined homogeneous and heterogeneous reactions,” Journal of Catalysis 270(1), 110-124. DOI: 10.1016/j.jcat.2009.12.013
Chang, S., Zhao, Z. L., Zhang, W., Zheng, A. Q., Wu, W. Q., and Li, H. B. (2011). “Comparison of chemical composition and structure of different kinds of bio-oils,” Journal of Fuel Chemistry and Technology 39(10), 746-753. DOI: 10.3969/j.issn.0253-2409.2011.10.005
Chen, X. F. (2008). Analysis of the Products from Alkanolysis of the Rice-Stalk Powder and Related Mechanism Study, Master’s thesis, Wuhan University of Science and Technology, Wuhan, China.
Chinese Ministry of Agriculture & US Department Energy. (1998). Chinese Biomass Resources Availability Evaluation, China Environmental Science Press, Beijing, China.
Demirbas, A. (2000). “Mechanism of liquefaction and pyrolysis reactions of biomass,” Energy Conversion and Management 41(6), 633-646. DOI: 10.1016/S0196-8904(99)00130-2
Demirbas, A. (2007). “The influence of temperature on the yields of compounds existing in bio-oils obtained from biomass samples via pyrolysis,” Fuel Processing Technology 88(6), 591-597. DOI: 10.1016/j.fuproc.2007.01.010
Gallucci, F., Hamers, H. P., and Zanten, M. (2015). “Experimental demonstration of chemical-looping combustion of syngas in packed bed reactors with ilmenite,” Chemical Engineering Journal 274, 156-168. DOI: 10.1016/j.cej.2015.03.081
GB/T 744 (2004). “Pulps-determination of alkali resistance,” General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China & China National Standardization Management Committee, Beijing, China.
GB/T 2677.1 (1993). “Fibrous raw material of sampling for analysis,” China State Bureau of Technical Supervision, Beijing, China.
GB/T 2677.3 (1993). “Fibrous raw material-determination of ash,” China State Bureau of Technical Supervision, Beijing, China.
GB/T 2677.10 (1995). “Fibrous raw material-determination of holocellulose,” China State Bureau of Technical Supervision, Beijing, China.
Guo, Z., Bai, Z., Bai, J., Wang, Z., and Li, W. (2011). “Co-liquefaction of lignite and sawdust under syngas,” Fuel Processing Technology 92(1), 119-125. DOI: 10.1016/j.fuproc.2010.09.014
Huang, J. B., Liu, C., Wei, S. A., Huang, X. L., and Li, H. J. (2011). “A theoretical study on the mechanism of levoglucosan formation in cellulose pyrolysis,” Journal of Fuel Chemistry and Technology 08, 590-594. DOI: 10.3969/j.issn.0253-2409.2011.08.006
Japan Institute of Energy. (2007). Manual of Biomass and Bioenergy, Chemical Industry Press, Beijing, China.
Li, H., Yuan, X., Zeng, G., Huang, D., Huang, H., Tong, J., You, Q., Zhang, J., and Zhou, M. (2010). “The formation of bio-oil from sludge by deoxy-liquefaction in supercritical ethanol,” Bioresource Technology 101(8), 2860-2866. DOI: 10.1016/j.biortech.2009.10.084
Mao, R., Zhao, Q., Dima, G., and Petraccone, D. (2010). “New process for the acid-catalyzed conversion of cellulosic biomass (AC3B) into alkyl levulinates and other esters using a unique one-pot system of reaction and product extraction,” Catalysis Letters 141(2), 271-276. DOI: 10.1007/s10562-010-0493-y
Mazaheri, H., Lee, K. T., Bhatia, S., Mohamed, A. R. (2010). “Sub/supercritical liquefaction of oil palm fruit press fiber for the production of bio-oil: Effect of solvents,” Bioresource Technology 101(19), 7641-7647. DOI: 10.1016/j.biortech.2010.04.072
Nie, M., Wang, Q., Qiu, and G. H. (2007). “Effect of Hydrogen radical scavenger on ultrasonically initiated emulsion polymerization of styrene,” Acta Polymerica Sinica 7, 633-637. DOI: 10.3321/j.issn:1000-3304.2007.07.008
Peng, J. X., Shao, Q. J., Chen, F. N., Chen F.X., and Yuan, B. Z. (2009). “Experimental study on co-liquefaction of bamboo and PE in supercritical ethanol,” Acta Energiae Solaris Sinica 30(8), 1139-1144. DOI: 10.3321/j.issn:0254-0096.2009.08.027
Rataboul, F., and Essayem, N. (2011). “Cellulose reactivity in supercritical methanol in the presence of solid acid catalysts. Direct synthesis of methyl-levulinate,” Industrial & Engineering Chemistry Research 50(2), 799-805. DOI: 10.1021/ie101616e
Richmond, R., and Halliwell, B. (1982). “Formation of hydroxyl radicals from the paraquat radical cation, demonstrated by a highly specific gas chromatographic technique. The role of superoxide radical anion, hydrogen peroxide, and glutathione reductase,” Journal of Inorganic Biochemistry 17(2), 95-107. DOI: 10.1016/S0162-0134(00)80078-1
Shao, Q. J., Peng, J. X., Xiu, S. D., and Wen, X. H. (2007). “Analysis of oil products by pyrolysis of bamboo in supercritical methanol,” Acta Energiae Solaris Sinica 28(9), 984-987. DOI: 10.3321/j.issn:0254-0096.2007.09.009
Song, C. C., Wang, G., and Hu, H. Q. (2004). “Progress in thermochemical liquefaction of biomass,” Energiae Solaris Sinica 25 (2), 242-247. DOI: 10.3321/j.issn:0254-0096.2004.02.022
Sun, P. C., Zhang, J. Z., and Duan, S. F. (1999). Introduction to Free Radical Biology, China University of Science and Technology Press, Hefei, China.
Sun, X. B., Zhao, Q. X., Cao, G. M., and Zhou, J. (2002). “The characteristics and applications of advanced oxidation process,” China Water & Wastewater 18(5), 33-35. DOI: 10.3321/j.issn:1000-4602.2002.05.010
Tan, H. (2005). Mechanism Study of Biomass Pyrolysis, PhD dissertation, Zhejiang University, Zhejiang, China.
Tang, S. R. (2009). “Analysis of depolymerization product of cornstalk in supercritical ethanol,” Journal of Anhui Agriculture Science 37(11), 4869-4870. DOI: 10.3969/j.issn.0517-6611.2009.11.014
Tao, H. X., Xie, X. A., Tang, C. Z., and Tian, W. G. (2013). “Mechanism of ketones formation from cellulose liquefaction in sub- and supercritical ethanol,” Journal of Fuel Chemistry and Technology 41(1), 60-66. DOI: 10.1016/S1872-5813(13)60010-9
Tao, H. X., Xie, X. A., Zheng, C. Y., and Zhan, X. Q. (2014). “Liquefaction of cornstalk cellulose in sub/super-critical ethanol,” Journal of Northwest University A.&F. (Natural Science Edition)01, 196-204.
Xie, W., Yuan, X. Z., Zeng, G. M., Tong, J. Y., and Li, H. (2008). “Effects of catalysts on biomass liquefaction in subcritical water,” Resources Science 30(1), 129-133. DOI: 10.3321/j.issn:1007-7588.2008.01.019
Yamada, T., Aratani, M., Kubo, S., and Hirokuni O. (2007). “Chemical analysis of the product in acid-catalyzed solvolysis of cellulose using polyethylene glycol and ethylene carbonate,” Journal of Wood Science 53(6), 487-493. DOI: 10.1007/s10086-007-0886-8
Yamazaki, J., Minami, E., and Saka, S. (2006). “Liquefaction of beech wood in various supercritical alcohols,” Wood Science 52(6), 527-532. DOI: 10.1007/s10086-005-0798-4
Yao, B., Zhu, T., and Lin, W. L. (2006). “Silanization of DHBAs and measurement of gas phase hydroxyl radicals using gas chromatography-mass spectrometry,” Environmental Chemistry25(6), 773-775. DOI: 10.3321/j.issn:0254-6108.2006.06.025
Zheng, C. Y., Xie, X. A., Tao, H. X., Zheng, L. S., and Li, Y. (2012). “Depolymerization of stalk cellulose during its liquefaction in sub-and supercritical ethanol,” Journal of Fuel Chemistry and Technology 40(5), 526-532. DOI: 10.3969/j.issn.0253-2409.2012.05.003
Zheng, C. Y., Tao, H. X., and Xie, X. A. (2013). “Distribution and characterizations of liquefaction of celluloses in sub- and super-critical ethanol,” BioResources 8(1), 648-662. DOI: 10.15376/biores.8.1.648-662
Zhou, J. Z., and Dong, H. J. (1995). “Determination of hydroxyl radical in Fenton reaction by using high performance liquid chromatograph (HPLC) connected with electrochemical detector,” Chinese Journal of Pharmacology and Toxicology 9(4), 299-302. DOI: 10.3321/j.issn:1000-3002.1995.04.018
Article submitted: July 22, 2016; Peer review completed: September 11, 2016; Revised version received: September 17, 2016; Accepted: September 19, 2016; Published: September 29, 2016.
DOI: 10.15376/biores.11.4.9771-9788
