Abstract
Furfural residues produced from the furfural industry were investigated as a substrate for lactic acid production by simultaneous saccharification and fermentation (SSF). Alkaline peroxide was used for delignification of furfural residues to improve the final lactic acid concentration. The residue was treated with 1.3% to 1.7% hydrogen peroxide at 80 °C for 1 h with a substrate concentration of 3.33%. SSF of furfural residues with different delignification degrees were carried out to evaluate the effect of delignification degree on lactic acid production. Using corn hydrolysates/ furfural residues as substrates, SSF with different media were carried out to investigate the effect of lignin on the interaction between enzymes and lactic acid bacteria. Lactic acid bacteria had a negative effect on cellulase, thus resulting in the reduction of enzyme activity. Lignin and nutrients slowed down the decreasing trend of enzyme activity. A higher delignification resulted in a slower fermentation rate and lower yield due to degradation products of lignin and the effect of lignin on the interaction between enzymes and lactic acid bacteria. For the purpose of lactic acid production, a moderate delignification (furfural residues with the lignin content of 14.8%) was optimum.
Download PDF
Full Article
The effect of delignification process with alkaline peroxide on lactic acid production from furfural residues
Yong Tang, Lingxi Bu, Lihong Deng, Liwei Zhu, and Jianxin Jiang*
Furfural residues produced from the furfural industry were investigated as a substrate for lactic acid production by simultaneous saccharification and fermentation (SSF). Alkaline peroxide was used for delignification of furfural residues to improve the final lactic acid concentration. The residue was treated with 1.3% to 1.7% hydrogen peroxide at 80 °C for 1 h with a substrate concentration of 3.33%. SSF of furfural residues with different delignification degrees were carried out to evaluate the effect of delignification degree on lactic acid production. Using corn hydrolysates/ furfural residues as substrates, SSF with different media were carried out to investigate the effect of lignin on the interaction between enzymes and lactic acid bacteria. Lactic acid bacteria had a negative effect on cellulase, thus resulting in the reduction of enzyme activity. Lignin and nutrients slowed down the decreasing trend of enzyme activity. A higher delignification resulted in a slower fermentation rate and lower yield due to degradation products of lignin and the effect of lignin on the interaction between enzymes and lactic acid bacteria. For the purpose of lactic acid production, a moderate delignification (furfural residues with the lignin content of 14.8%) was optimum.
Keywords: Lactic acid; Delignification; Furfural residue; Enzyme activity; Interaction
Contact information: Department of Chemical Engineering, Beijing Forestry University, Beijing 100083, China. *Corresponding author: jiangjx@bjfu.edu.cn
INTRODUCTION
As one of the most versatile organic acids, lactic acid (LA) has applications in food, leather, and chemical industries (John et al. 2007). Lactic acid can be converted into various chemicals via chemical and biotechnological routes that modify its carboxylic and hydroxyl groups (Gao et al. 2011). The most important application of lactic acid is perhaps its use as a precursor for the production of poly-lactic acid, a biodegradable and biocompatible polymer (Wee et al. 2006). Lactic acid can be produced either by chemical synthesis or microbial fermentation and about 90% of total worldwide LA is fermented from starch materials.
Presently, cellulosic materials have received increasing attention as potential feedstocks to replace edible starch materials (Okano et al. 2010). Cheap cellulosic materials such as agricultural waste, industrial solid waste, and forestry waste are regarded as economically attractive feedstocks for large-scale fermentation. Investigators have discussed lactic acid fermentation allowing the utilisation of waste from agriculture or industry as source of carbohydrate (John et al. 2007; Tang et al. 2011a). The bioconversion of lignocellulosic biomass to lactic acid occurs in two steps. The cellulose is first depolymerised by cellulase to produce glucose, which is subsequently fermented to produce lactic acid (Marques et al. 2008).
As a result of furfural production by hydrolyzing hemicelluloses of corncob, huge amounts of furfural residues (FR) are generated annually in China (Sun et al. 2011; Tang et al. 2011b). A potential use could be as raw materials for LA production because FR are rich in cellulose that can be easily hydrolyzed by cellulase (Tang et al. 2011a). Lignin is the second most abundant component of lignocellulosic materials, especially in FR. The accumulation of lignin in a biorefinery process makes it difficult to operate a fermentation process with a high substrate concentration, which will limit the final LA concentration. Technologies have been developed to increase the final LA concentration to decrease the process cost of downstream process.
This paper deals with the fermentative production of L(+)-lactic acid from delignified FR as main carbon source. The alkaline hydrogen peroxide method was employed in this study to remove the lignin in FR, and the effect of delignification on lactic acid production was evaluated. Using corn hydrolysates/furfural residues as substrates, SSF with different media were carried out to investigate the effect of lignin on the interaction between enzymes and lactic acid bacteria.
To study the effect of lignin on interaction between enzymes and lactic acid bacteria, materials without lignin should be used as substrates in SSF to investigate the interaction of enzymes and lactic acid bacteria. It is hard to obtain lignin-free FR by alkaline peroxide. Corn and glucose are the potential materials because they are free of lignin. Corn saccharification liquid was therefore used as material for comparison.
EXPERIMENTAL
Materials
Furfural residue (FR) was kindly provided by Chunlei Company (Xingtai, China), and had been water-rinsed before being used. The average contents of glucan, lignin, and ash were 48.17%, 43.29%, and 6.42%, respectively. Corn kernels with a starch content of 75.2% and a nonstarch polysaccharides content of 11.9% were purchased from Zhongliang Company (Beijing, China).
Methods
Lignin removal
The alkaline hydrogen peroxide method was employed to remove the lignin in FR, as had been described in a previous study (Su et al. 2012). The hydrogen peroxide had been added to the pressure bottles with FR slurry in water (3.33%, w/v), followed by pH adjustment to 11.5 with 6 M sodium hydroxide. The pretreatments were performed in a water bath at 80 °C for 1 h. The solid residues were subsequently water-rinsed until neutral (water amount of 20 ml/ g raw FR) and stored in sealed bags at 4 °C before being used.
A series of samples with progressive lower lignin contents were obtained as the amount of H2O2 increased. Samples treated by 1.3, 1.5, and 1.7% H2O2 (w/v) were labeled as R1, R2, and R3, respectively. The raw material FR, without delignification, was labeled as R0.
Preparation for corn saccharification liquid
The saccharification of corn starch was realized by the double enzymes method. Corn kernels were liquefied at 85 C for 2 h using the commercial thermostable -amylase with an enzyme activity of 4KU/g, followed by 1 hour of saccharification (pH 4.0, 60 C) with amyloglucosidase. Before being used, the pH of corn saccharification liquid (CR) was adjusted to 6.35. The reducing sugars, cellulose, and protein (weight ratio about 70:11:4) are the main components in the corn hydrolysate. The glucose monomer fraction after pre-saccharification was close to 60%. The level of release of glucose and nutrients (including protein) depended on saccharification time. Complete starch hydrolysis required more than 7 h of pre-saccharification time.
Microorganisms and enzyme preparation
The microorganism used for lactic acid fermentation was freeze-dried lactic acid bacteria (Meihua Company, Haerbin, China), mainly comprising Streptococcus thermophilus and Lactobacillus bulgariaicus. Cellulase (Celluclast 1.5 L, Novozymes) and β-glucosidase (Novozyme 188, Novozymes) were used. Celluclast 1.5 L had an activity of 75 filter paper units (FPU)/mL, measured using the IUPAC protocol (Ghose 1986), and 35 IU/mL β-glucosidase activity according to the method of Berghem and Petterson (Berghem and Petterson 1974). Novozym 188 had a β-glucosidase activity of 174 IU/mL. -amylase (150 U/g corn) and glucoamylase (20 U/g corn) (Aoboxing Universeen Bio-Tech Company Ltd, Beijing, China) were used for corn liquefaction and saccharification, respectively.
Simultaneous saccharification and fermentation
As nutrients, full medium (beef extract, 5g/L; KH2PO4, 0.5 g/L; MgSO4∙7H2O, 0.5 g/L; NaCl, 0.1g/L) and mineral salt medium (KH2PO4, 0.5 g/L; MgSO4∙7H2O, 0.5 g/L; NaCl, 0.1g/L) were used. Calcium carbonate was added as 110% (w/w) of glucan (starch) to the medium. FR, calcium carbonate, and fermentation medium were separately sterilized (121 °C, 20 min) before loading into the conical flask. The amounts of Celluclast 1.5 L and Novozyme 188 used in simultaneous saccharification and fermentation were 15 FPU/g and 17 IU/g glucan of FR, respectively. Fermentations were carried out at 40 °C (0 to 30 h) and 42 °C (30 to 144 h) with a shaking speed of 150 rpm in 100 mL Erlenmeyer flasks containing 60 mL medium. The inoculation amount of all the fermentations was 0.21% (W/V).
Analytical methods
The lignin and glucan contents of FR as well as delignified samples were analyzed according to the National Renewable Energy Laboratory (NREL) methods (Sluiter et al. 2004a). The total starch was determined when the saccharification of corn starch was finished (McCleary et al. 1994). The samples after LA fermentation were filtered (0.22-μm pores) to detect UV absorbance at 250 nm, lactic acid, filter paper activity, and enzymatic protein concentration. Samples were diluted by 32 times with water points to determine their absorbance at 250 nm by a spectrophotomer (UNICO Instruments Co., Ltd, Shanghai, China). A250 was detected to reflect the relative content of phenolic compounds (Bustos et al. 2004). The liquid fraction of the supernatant after fermentation were analysed according to an NREL procedure (Sluiter et al. 2004b). Lactic acid was analyzed by HPLC (Waters 2695e, USA) using an Aminex HPX-87H (300×7.8mm: Bio-Rad, USA) at 65°C and refractive index detection detector at 30°C. The injection volume of the sample was 10 µL, and 5 mM sulfuric acid was used as the eluent, at a flow rate of 0.6 mL/min. Filter paper activity was determined by the method described by Mandels et al. (1976). Enzymatic protein concentration was determined by the Bradford method using bovine serum album as a standard (Bradford 1976). Assuming that 1 g cellulose or starch present in the liquid theoretically gave 1.11 g of lactic acid, lactic acid yield was expressed as percentage of the theoretical yield based on FR cellulose or corn starch. Assays were performed in three repeated experiments, and the mean values are presented.
RESULTS AND DISCUSSION
Effect of Lignin on Lactic Acid Production
The chemical composition of FR and delignified FR are summarized in Table 1. SSFs of delignified FR were carried out with full medium. Figure 1 shows the lactic acid profiles in SSF of delignified FR.
Table 1. Major Components of FR and Delignified FR
Fig. 1. The lactic acid profiles in SSF of delignified FR
*R0 represents the raw material of neutralized furfural residue, while R1, R2, and R3 represent furfural residue treated by 1.3, 1.5, and 1.7% H2O2 (w/v), respectively.
Lignin removal improved lactic acid production. However, great differences were observed among these cases. An increase in delignification decreased the fermentation rate. The fermentation rate in SSF of R1 was obviously faster than the other ones. Though R3 had the lowest lignin and the highest cellulose, the fermentation rate of SSF of R3 was almost the same as that of R0. According to our previous study, two kinds of delignifi-cation methods, alkaline peroxide and sodium chlorite methods, had been investigated to improve enzymatic hydrolysis of different lignocellulosic materials (Su et al. 2011, 2012). Alkaline peroxide provides effective delignification of fibrous cellulose. The hydroperox-ide anion (HOO-) and the hydroxyl radical (HO•) are generally considered as the lignin-oxidizing inducer in the alkaline peroxide process (Su et al. 2012). Compared with alkaline peroxide, the sodium chlorite method obviously decreased the glucose yield in enzymatic hydrolysis as the delignification degree increased. Su et al. stated that compared with delignified sample with a lignin content of 10.2%, delignified sample with a lignin content of 5.4% had a slightly higher conversion rate during the first 24 h, and almost the same conversion rate after 24 h.
Figure 2 shows the UV absorbance as functions of fermentation time and delignification degree. The UV absorbance of fermentation broth using delignified FR was lower than that using raw FR. For SSF of delignified FR, the initial UV absorbance at 30 h was inversely proportional to the lignin content (y = -0.0089x+0.8679, R2 = 0.996, y: UV absorbance; x: lignin content). At 48 h, a decrease of UV absorbance was observed in all cases except SSF of R0, probably due to the adaptation growth of lactic acid bacteria. UV absorbance slightly increased with fermentation time due to the release of phenolic compounds as fermentation time was beyond 48 h. Meanwhile, an increase in delignification degree also increased UV absorbance. The results indicated that an increase in delignification leads to more degradation products of lignin. Less degradation products of lignin in SSF of R0 probably resulted in an increase of A250 from raw FR during the whole SSF process. Some form of pretreatment is always necessary to ensure the efficient bioconversion of lignocellulosic materials to lactic acid. However, pretreatment itself produces a broad range of compounds that have inhibitory effects on fermentation microorganisms. These inhibitors can be classified according to their chemical structure into organic acids, furans, and phenolic compounds. The composition of these inhibitors depends on the type of lignocellulosic materials used, as well as the chemistry and the nature of the pretreatment process. For the delignification process in this study, phenolic compounds are supposed as the main inhibitors produced during the delignification process. Using the same fermentation strains, the effect of inhibitors on lactic acid yield in SSF has been studied by adding inhibitors, including formic acid, acetic acid, furfural, and vanillin into fermentation media (Feng et al. 2012). Their results suggested that the most pronounced effect of furfural and phenolic compounds (ranged from 0.1 g/L to 0.5 g/L) were a prolonged lag phase, which could partly explain why an increase in delignification degree decreases the fermentation rate.
Figure 3 shows the effect of delignification degree on initial UV absorbance, the final LA concentration, and the final yield. The final concentration of lactic acid initially increased with the decrease of lignin content, and then leveled off, or more precisely, exhibited a reduction after the highest point. The yield decreased with the reduction of lignin content. The degradation product of lignin produced during lignin removal was supposed to be one of the reasons for the negative effect of delignification on LA production. Rinsing with water has been proven to be an effective detoxifying method, which could reduce water-soluble inhibitors. However, the lignin degradation products linked to or wrapped by the FR structure backbone would be released by the rupture of the structure as the enzymatic hydrolysis proceeded. According to our previous study, the most pronounced effect of furfural and phenolic compounds was a prolonged lag phase, and there were no obvious differences in the maximum yield of lactic acid when phenolic compounds were added (Feng et al. 2012), which means that phenolic compounds are not the only reason for the low LA yield in SSF of R2 and R3 in this work. However, a moderate delignification was optimum for the purpose of lactic acid production.
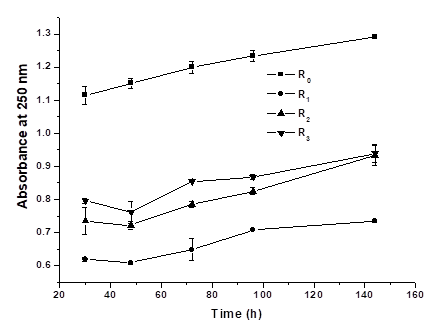
Fig. 2. The UV absorbance profiles in SSF of delignified FR
*R0 represents the raw material of neutralized furfural residue, while R1, R2, and R3 represent furfural residue treated by 1.3, 1.5, and 1.7% H2O2 (w/v), respectively.
Fig. 3. Effect of delignification degree on initial UV absorbance, final LA concentration, and yield
Effect of Lignin on Interaction Between Enzymes and Lactic Acid Bacteria
Using corn hydrolysates/furfural residue as substrates, SSF with different media were carried out to investigate the interaction between enzymes and lactic acid bacteria. SSF of 2.5% corn hydrolysates with mineral salt medium obtained 19.0% yield at 68 h, while that with full medium obtained 87.5% yield (Fig. 4). Cellulase (the amount is same as that used in SSF of 5% FR) was added to SSF of 2.5% corn hydrolysates to investigate the effect of enzymes on lactic acid bacteria. Cellulase increased the yield of SSF to 68.7%. Cellulase enabled the conversion of non-starch polysaccharides in corn hydroly-sates into glucose. However, the amount of non-starch glucan in corn kernels was found to be only 11.9%. 68.7% of the yield indicated that cellulase has the same positive effects as nitrogen sources. The small amount of cellulase added in SSF could not support LA fermentation. The level of release of nutrients in corn is dependent on saccharification time (Akerberg and Zacchi 2000). It is supposed that cellulase also has a positive effect on the release of nutrients in CR.
Fig. 4. The yield profiles in SSF of corn hydrolyzates with different media
To evaluate the influence of lactic acid bacteria on enzymes, the amount of remaining cellulase activity (filter paper activity unit, FPU) (Fig. 5) and the enzyme-based protein concentration (Fig. 6) in the liquid fraction were determined for selected samples during SSFs. FPU was measured at 42 h, 92 h, and 144 h in chosen SSFs (SSF of 5% FR with mineral salt medium, SSF of 5% FR with full medium, and SSF of 2.5% CR with cellulase). The enzyme-based protein concentration was measured at 92 and 144 h. The FPU decreased after 92 h in all SSFs (Fig. 5). FPU in SSF of 5% FR with mineral salt medium was lower than that with full medium. In all measured samples, FPU in the SSF with 2.5% CR with cellulase was the lowest. The enzyme-based protein concentration in the SSF with 2.5% CR was the lowest.
Fig. 5. The enzyme activity in the liquid fraction during chosen SSFs
Fig. 6. The enzymatic protein concentration in the liquid fraction during chosen SSFs
Cellulase would become unproductively bound to lignin (Alkasrawi et al. 2003). This explains why FPU and the enzyme-based protein concentration in SSF of FR decreased. However, the same trend was observed in SSF of 2.5% CR, and the enzyme-based protein concentration reached the lowest level. The results indicated that lactic acid bacteria would decrease the enzyme-based protein concentration, which probably results in decreasing the remaining enzyme activity. FPU and the enzyme-based protein concentration in the SSF with 5% FR with mineral salt medium were higher than that of the SSF with 2.5% CR. One could infer that, to a certain extent, lignin decreased the access of enzymes to lactic acid bacteria. SSF with full medium resulted in higher FPU than that with mineral salt medium, which suggested that nutrients could also slow down the decreasing trend of enzyme activity by lactic acid bacteria. Because lactic acid bacteria have a negative effect on enzyme, the positive effect of lignin on slowing down the decrease trend of enzyme activity can lead to a high LA yield of R1.
Delignification for Bioconversion
Enzymes constitute a significant contribution to the overall cost in bioconversion processes based on enzymatic hydrolysis of cellulose (Wingren et al. 2003). Delignifi-cation of lignocellulosic materials could provide a potential step for decreasing the enzyme loading and overcoming the disadvantage of low final product concentration in SSF. The efficiency of enzymatic hydrolysis may be decreased when the sodium chlorite method is used for delignification and the removal of lignin exceeds 50%, which also results in a reduction of yield in SSF for LA production. Lactic acid bacteria impacted the enzyme activity by decreasing protein concentration, and nutrient limitation led to more reduction, according to this study. Lignin would slow down the reduction of enzyme activity. Though the efficiency of enzymatic hydrolysis is not decreased, as high delig-nification is obtained by the alkaline hydrogen peroxide method, a higher delignification leads to a slower fermentation rate and a lower final LA concentration. The results indicated that inhibitors and the effect of lignin on the interaction between enzymes and fermentation strains should be considered when delignification is used to improve the final product concentration in SSF. However, more work is still needed for further study of the interaction between enzymes and fermentation strains by excluding the inhibiting factors. High delignification also means undesirable degradation of cellulose and high process cost. Therefore, moderate delignification would also be cost-effective for using FR to produce lactic acid.
CONCLUSIONS
- Cellulases have the same positive effects on the release of nutrients in corn and as nitrogen sources in lactic acid fermentation. Nutrients and lignin slowed down the decreasing trend of enzyme activity. The effect of lignin on the interaction between enzymes and lactic acid bacteria should be considered when delignification is used to improve the final product concentration in SSF.
- As the delignification degree increases, fermentation efficiency decreases, with a result of the increase of degradation products of lignin and the effect of lignin on interaction between enzymes and lactic acid bacteria. High delignification means more chemical consumption and higher process cost. For the purpose of lactic acid production, a moderate delignification is optimum.
ACKNOWLEDGMENTS
The authors are grateful for the financial support of this research from the Fundamental Research Funds for the Central Universities (BLYJ201212), National Science Foundation of China (31070510), and Major State Basic Research Projects of China (973-2010CB732204).
REFERENCES CITED
Akerberg, C., and Zacchi, G. (2000). “An economic evaluation of the fermentative
production of lactic acid from wheat flour,” Bioresour. Technol. 75, 119-126.
Alkasrawi, M., Eriksson, T., Borjesson, J., Wingren, A., Galbe, M., Tjerneld, F., and Zacchi, G. (2003). “The effect of Tween-20 on simultaneous saccharification and fermentation of softwood to ethanol,” Enzyme Microb. Technol. 33, 71-78.
Bradford, M. M. (1976). “A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding,” Anal. Biochem 72, 248-254.
Berghem, L. E. R., and Petterson, L. G. (1974). “Mechanism of enzymatic cellulose degradation-isolation and some properties of a beta-glucosidase from Trichoderma viride,” Eur. J. Biochem. 2, 295-305.
Bustos, G., Moldes, A. B., Cruz, J. M., and Dominguez, J. M. (2004). “Evaluation of vinification lees as a general medium for Lactobacillus strains,” J. Agric. Food Chem. 52, 5233-5239.
Feng, Y., Qi, X., Jian, H. L., Sun, R. C., and Jiang, J. X. (2012). “Effect of inhibitors on enzymatic hydrolysis and simultaneous saccharification fermentation for lactic acid production from steam explosion pretreated lespedeza stalks,” BioResources 7(3), 3755-3766.
Gao, C. Ma, C. Q., and Xu, P. (2011). “Biotechnological routes based on lactic acid production from biomass,” Biotechnology Advances 29, 930-939.
Ghose, T. K. (1986). “Measurement of cellulase activities,” Pure Appl. Chem. 59, 257-268.
John, R. P., Nampoothiri, K. M., and Pandey, A. (2007). “Fermentative production of lactic acid from biomass: An overview on process developments and future perspectives,” Appl. Microbiol. Biotechnol. 74, 524-534.
Marques, S., Santos, J. A. L., Girio, F. M., and Roseiro, J. C. (2008). “Lactic acid production from recycled paper sludge by simultaneous saccharification and fermentation,” Biochem. Eng. J. 41, 210-216.
McCleary, B. V., Solah, V., and Gibson, T. S. (1994). “Quantitative measurement of total starch in cereal flours and products,” J. Cereal Sci. 20, 51-58.
Mandels, M. Andreotti, R., and Roche, C. (1976). “Measurement of saccharifying cellulose,” Biotech. Bioeng. Symp. 6, 21-33.
Okano, K., Tanaka, T., Ogino, C., Fukuda, H., and Kondo, A. (2010). “Biotechnological production of enantiomeric pure lactic acid from renewable resources: Recent achievements, perspectives, and limits,” Appl. Microbiol. Biotechnol. 85, 413-423.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., and Templeton, D. (2004a). “Determination of structural carbohydrates and lignin in biomass,” National Renewable Energy Laboratory, Golden, CO, USA.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J. and Templeton, D. (2004). “Determination of sugars, byproducts, and degradation products in liquid fraction process samples,” National Renewable Energy Laboratory, Golden, CO, USA.
Sun, R., Song, X. L., Sun, R. C., and Jiang, J. X. (2011). “Effect of lignin content on enzymatic hydrolysis of furfural residues,” BioResources 6, 317-328.
Su, Z. Q., Bu, L. X., Zhao, D. Q., Sun, R. C., and Jiang, J. X. (2012). “Processing Lespedeza stalks by pretreatment with low severity steam and post-treatment with alkaline peroxide,” Industrial Crops and Products 36, 1-8.
Tang, Y., Zhao, D. Q., Zhu, L. W., and Jiang, J. X. (2011a). “Simultaneous saccharification and fermentation of furfural residues by mixed cultures of lactic acid bacteria and yeast to produce lactic acid and ethanol,” Eur. Food Res. Technol. 233, 489-495.
Tang, Y., Zhao, D. Q., Cristhian, C., and Jiang, J. X. (2011b). “Simultaneous saccharification and cofermentation of lignocellulosic residues from commercial furfural production and corn kernels using different nutrient media,” Biotechnology for Biofuels 4, Article No. 22.
Wingren, A., Galbe, M., and Zacchi, G. (2003). “Techno-economic evaluation of producing ethanol from softwood: comparison of SSF and SHF and identification of bottlenecks,” Biotechnol. Prog. 19, 1109-1117.
Wee, Y. J., Kim, J. N., and Ryu, H. W. (2006). “Biotechnological production of lactic acid and its recent applications,” Food technol. Biotechnol. 44, 163-172.
Article submitted: July 13, 2012; Peer review completed: August 17, 2012; Revised version received: August 25, 2012; Second revision received and accepted: September 6, 2012; Published: September 10, 2012.
