Abstract
Different thermo-mechanical extrusion pretreatments were evaluated as alternatives to traditional biomass pretreatments for lignocellulosic ethanol production. Wheat straw, a commonly available agricultural co-product, was chosen as the substrate model for the study. Five thermo-mechanical pretreatments were evaluated: one purely thermo-mechanical (TM) using just water, and the rest thermo-mechano-chemical (TMC), thus using acid, alkaline, oxidant in alkaline medium, and organic solvent. The parietal constituents, hemicelluloses, cellulose, and lignin were quantified to enable the amounts extracted by the pretreatment to be estimated. The digestibility of cellulose was evaluated by quantifying the hydrolysability with an enzyme cocktail. Water thermomechanical treatment gave strong straw defibration; however the digestibility only attained 35%, whereas ground wheat straw was already 22%. This improvement is insufficient to prepare material for direct enzymatic hydrolysis; thus a combination of the thermo-mechanical and chemical treatment is required. All chemical treatments produced greater improvements in cellulose digestibility. For the acidic treatments, hydrolysability was between 42 and 50%, and reached 89% with alkaline pretreatment.
Download PDF
Full Article
Thermomechanical and Thermo-mechano-chemical Pretreatment of Wheat Straw using a Twin-screw Extruder
Virginie Vandenbossche,a,b,* Catherine Doumeng,a,b and Luc Rigal a,b
Different thermo-mechanical extrusion pretreatments were evaluated as alternatives to traditional biomass pretreatments for lignocellulosic ethanol production. Wheat straw, a commonly available agricultural co-product, was chosen as the substrate model for the study. Five thermo-mechanical pretreatments were evaluated: one purely thermo-mechanical (TM) using just water, and the rest thermo-mechano-chemical (TMC), thus using acid, alkaline, oxidant in alkaline medium, and organic solvent. The parietal constituents, hemicelluloses, cellulose, and lignin were quantified to enable the amounts extracted by the pretreatment to be estimated. The digestibility of cellulose was evaluated by quantifying the hydrolysability with an enzyme cocktail. Water thermomechanical treatment gave strong straw defibration; however the digestibility only attained 35%, whereas ground wheat straw was already 22%. This improvement is insufficient to prepare material for direct enzymatic hydrolysis; thus a combination of the thermo-mechanical and chemical treatment is required. All chemical treatments produced greater improvements in cellulose digestibility. For the acidic treatments, hydrolysability was between 42 and 50%, and reached 89% with alkaline pretreatment.
Keywords: Wheat straw; Twin-screw extruder; Pretreatment; Enzymatic hydrolysis; Bio-ethanol; Alkaline; Acid; Glycerol; Hydrogen peroxide
Contact information: a: Université de Toulouse, INP, Laboratoire de Chimie Agro-industrielle, ENSIACET, 4 Allée Emile Monso, BP 44362, 31030 Toulouse Cedex 4, France; b: INRA, UMR 1010, Laboratoire de Chimie Agro-industrielle, 31030 Toulouse Cedex 4, France;
* Corresponding author: Virginie.Vandenbossche@ensiacet.fr
INTRODUCTION
Concern about increasing energy demands, CO2 emissions, and depletion of fossil fuel energy highlight the need to find new renewable and carbon-neutral energy sources. Lignocellulosic materials from agriculture and forest by-products represent an important clean energy source. They are cheap, available in large quantities, and are relatively independent of geographical location. They are also carbon neutral, renewable, and can be used to produce bio-ethanol.
The use of by-products for ethanol production makes the process more competitive than the first generation schemes. However, such a substrate is recalcitrant to enzyme accessibility (Himmel 2007) and requires the use of a pretreatment; thus, researchers have been encouraged to develop cellulosic biomass adapted technologies for the production of bio-ethanol.
Pretreatment has a very important role. Simple and inexpensive, it must reduce particle size and improve enzyme accessibility by limiting the onset of inhibitory fermentation. Merely reducing size mechanically is an energy-intensive process and insufficient to obtain acceptable cellulose digestibility (Himmel 2007). Pore size is a major limiting factor for the enzymatic hydrolysis of cellulose (Chandra et al. 2007), and an increase of porosity can be obtained by removing the hemicelluloses. This increases substrate’s accessibility to cellulases and in turn increases the likelihood of hydrolysing the cellulose. Other factors are also important to obtain good digestibility; for example cellulose crystallinity (Chang and Holtzapple 2000) and lignin content (Mansfield et al. 1999).
Ogier et al. (1999) reviewed lignocellulosic biomass pretreatment methods for ethanol production, and many of these have been already studied such as: Liquid hot water (Mosier et al. 2005; Laser et al.2002; Pérez et al. 2008), steam explosion (Oliva et al. 2003; Cara et al. 2006; Varga et al. 2004; Ballesteros et al. 2006), alkaline pretreatment (Carvalheiro et al. 2008; Kumar et al. 2009; McIntosh and Vancov 2011; Taherzadeh and Karimi 2008), alkaline pretreatment with an oxidant (Carvalheiro et al. 2008), pretreatment in organic solvents such as glycerol or alkaline glycerol (Adeeb 2004), dilute acid pretreatment (Saha et al. 2005), ammonia fibre explosion (Galbe and Zacchi 2007), oxidation pretreatment, microwave pretreatment (Keshwani 2009), ultrasound pretreatment (Yachmenev et al.2009), and supercritical CO2 pretreatment (Kim and Hong 2001).
Among the processes used to carry out pretreatment with a minimum number of steps, extrusion has many advantages. It produces a high shear, rapid heat transfer, and effective and rapid mixing, in a continuous operation, with good modulation of treatment steps. Co-penetrating and co-rotating twin-screw extruders are the most commonly used equipment for this (Dziezak 1989).
The performance of extrusion and the influence of the operating parameters have been studied on biomass such as poplar (N’Diaye and Rigal 2000), Miscanthus sp. (De Vrije et al. 2002), sugar beet pulp (Rouilly et al. 2006), sunflower (Evon et al. 2007), soybean hulls (Yoo et al. 2011), rice straw (Chen et al. 2011), wheat straw, and bran (Marechal et al. 2004; Zeitoun et al. 2010; Jacquemin et al.2012).
Extrusion can in particular be used to pretreat different biomass for the production of sugar, and several authors have reported this type of application over the last few years. Lamsal et al. (2010) showed that extrusion led to higher reducing sugar yields compared to grinding, and they studied the process in two steps: impregnation with water followed by mechanical treatment (grinding or extrusion) on wheat bran. Lee et al. (2010) used extrusion after hot-compressed water treatment, and obtained a greatly improved monosaccharide production yield with Douglas fir and eucalyptus. They also used ethylene glycol, glycerol, and DMSO to open up the cell wall structure and improve enzymatic accessibility (Lee et al. 2009).
The combination of extrusion with chemical treatment can also improve results. A pretreatment in a dilute acid medium at low temperature can reach the wall structure by hydrolysis of certain components, especially hemicelluloses. This combination of extrusion and dilute acid pretreatment has been tested on rice straw (Chen et al. 2011). Aqueous alkaline treatment can reach the fibre structure by solvation of certain components, accompanied by the solubilisation and the extraction of hemicelluloses and lignins, to a greater or lesser degree depending on the operating conditions (alkali concentration, temperature, and contact time) (Mosier et al. 2005; Balat and Balat 2008; Zhao et al.2008). Alkaline pretreatment, which also decreases cellulose crystallinity and swelling, is one of the most widely used methods for enhancing enzymatic digestibility of the lignocellulosic biomass. The combination of both extrusion and alkaline pretreatment has been explored more recently by Lamsal et al. (2010) and Karunanithy and Muthukumarappan (2011), and in both studies, alkali soaking preceded extrusion.
The objective of this study was to assess the impact of thermo-mechanical and thermo-mechano-chemical pretreatments in a twin-screw extruder, on the digestibility of wheat straw. This has been selected as a model substrate to study enzymatic hydrolysis of polysaccharides destined for ethanolic fermentation of sugars. The pretreatments selected for the study are thermo-mechanical and thermo-mechano-chemical with acid, alkali, oxidant, and organic solvent reagents.
Thermo-mechanical treatment was used to cause intense defibration of wheat straw, in order to increase the surface directly accessible for liquid / solid exchange during the enzymatic attack of cell wall polysaccharides. The coupling of the thermo-mechanical treatment with a chemical treatment was intended to enhance the defibration of the fibres and the destructuration of the straw by directly attacking the cell wall biopolymers.
EXPERIMENTAL
Material
Pre-shredded Apache variety wheat straw was provided by ARD (France). Pre-milled wheat straw was crushed using a hammer mill (Electra VS 1, France) fitted with a 6 mm screen. Raw wheat straw contains 35% of hemicelluloses, 11% of lignin and 42% of cellulose.
Twin-screw Extruder Material
Thermo-mechanical and thermo-mechanico-chemical pretreatment was conducted with two co-penetrating and co-rotating twin-screw extruders: Clextral BC 45 and Clextral BC 21 (France). The BC 45 was used for neutral and alkaline pretreatments, and BC 21, equipped with acid resistant metal, was used for acid pretreatments. The extruders had seven modular barrels, each 200 mm in length for BC 45 and 100mm in length for BC21, plus different twin-screws with segmental screw elements each 50 and 100 mm in length for BC 45, and 12.5, 25, and 50 mm in length for BC21 (Fig. 1).
Fig. 1. Schematic modular barrel of the Clextral BC 45 and BC 21 twin-screw extruders used for pretreatment of wheat straw
Modules were thermo-regulated by thermal induction for BC45 and by heater band for BC21, and cooled by water circulation. To enable the filtrate to be collected, a filter section could be used on module 6, and this consisted of six hemispherical dishes with conical holes (1 mm entry, 2 mm exit) on both extruders. Experiments were carried out using six screw profiles adapted for each pretreat-ment (Fig. 2).
Thermo-mechanical Pretreatment of Wheat Straw in the Twin-screw Extruder
The thermo-mechanical pretreatment used only water, and wheat straw was fed into the BC 45 extruder inlet port via a volumetric screw feeder (Clextral 40, France) in the first module. The water was injected at two points: at the end of module 2 using a piston pump (Clextral DKM K202 PP16, France) and at the beginning of module 5 using a second piston pump (Clextral DKM K202 P32) (Fig. 1). Screw profile 1 was used in this study (Fig. 2), and operating conditions are given in Table 1. A first zone of mechanical pressure applied in module 3 ensured grinding and good mixing of the material with the liquid. It consisted of a succession of 10 bilobe paddles, 10 cm apart, and angled at 90° to each other. The defibreing zone, using a reverse pitch screw, was situated in the module 7.
Table 1. Operating Conditions for TM Pretreatment
Twin-screw extrusion was performed for 30 min before any sampling to ensure stabilisation of operating conditions. The filtrate and the cake were then immediately collected for a period of 30 min to avoid any variation in the outlet flow rates. Sample collection was carried out once and timed with a stopwatch. The filtrate and the cake were then weighed.
The energy consumed by the motor was determined using to the following formula,
P = U x I x cos φ (Ss / Smax) (1)
where P is the electric power supplied by the motor (W), U is the motor’s operating voltage (U = 460V), I is the current feeding the motor (A), cos φ is the theoretical yield of the extruder motor (cos φ = 0.95), and SS and Smax are the test speed and maximum speed (600 rpm) of the rotating screws (rpm), respectively. The specific mechanical energy (SME) is given by,
SME = P / Qs (2)
where Qs is the wheat straw feed rate (kg/h) and SME is the specific mechanical energy consumed by the motor per unit weight of wheat straw (W h/kg).
Acid Thermo-mechanico-chemical Pretreatment of Wheat Straw in the Twin-screw Extruder
Sulphuric acid (H2SO4) was used, and experiments were carried out in BC21 in two different modes: mode 1 was impregnation only, using profile 2 (Fig. 2) and mode 2 impregnation and filtration using profile 3 (Fig. 2). Operating conditions are shown in Table 2. Wheat straw was fed into the extruder using a volumetric screw feeder (K-tron Soder KCL-KT-20, Switzerland), and water was injected using a piston pump (Clextral DKM K202-AMP16, France) at the beginning of module 3 (Fig. 2). A first zone of mechanical pressure applied in module 3 ensured grinding and good mixing of the material with the acid. It consisted of a succession of 10 bilobe paddles 5 cm apart, angled at 90° to each other in profile 2 and at 45° in profile 3. In impregnation mode, the defibreing zone was situated in module 7, and used a succession of reverse pitch screws and bilobe paddles. In impregnation and filtration mode, the filtration zone was situated in module 6, and used two reverse pitch screws in module 7, which carried the contents in the opposite direction, and guaranteed intense shearing of the matter and the formation of a so-called “dynamic plug”. It is the formation of this plug that generates the flow of the filtrate in module 6. The rest of the operating conditions are similar to those used with BC45.
Alkaline Thermo-mechanico-chemical Pretreatment of Wheat Straw in the Twin-screw Extruder
Sodium hydroxide (NaOH) was used as the alkali for this trial, and experiments were carried out in BC45 in impregnation and filtration mode with profile 4 (Fig. 2). Operating conditions are given in Table 3. Wheat straw was fed into the extruder using a volumetric screw feeder (Clextral 40, France) in the first module.
Fig. 2. Screw profiles. Profile 1: TM pretreatment, Profile 2: acid TMC pretreatment in impregnation mode, Profile 3: acid TMC pretreatment in impregnation-extraction mode, profile 4: alkaline TMCpretreatment, profile 5: oxidant TMC pretreatment in alkaline medium, profile 6: TMC pretreatment in organic solvent medium. (Screw configuration for pretreatment of wheat straw. T2F = trapezoidal double-thread screw; C2F = conveying double-thread screw; C1FT = conveying trapezoidal simple-thread screw; BB = Bilobe paddle screw; CF2C = reverse pitch double-thread screw; CF1T = reverse pitch simple-thread screw. The numbers following the type of screw indicate the pitch of T2F, C2F, C1FT, CF2C and CF1T screws and the length of the BB screws. Heated modules are shown in grey).
Table 2. Operating Conditions for Acid TMC Pretreatment
The alkaline solution was injected using a piston pump (Clextral DKM K202 PP16, France) at the end of module 2 (Fig. 1), and a first zone of mechanical pressure applied in module 3 ensured grinding and good mixing of the material with this solution. It consisted of the succession of 10 bilobe paddles, 10 cm apart, and angled at 90° to each other. Water was injected in module 5 using a piston pump (Clextral DKM K202 P32, France) to flush out compounds solubilised by the alkaline treatment. The filtration zone situated in module 6, depended on a reverse pitch screw in module 7. Finally, the extrudates obtained were immersed in a large volume of water and 1M H2SO4 was added until neutralisation.
Oxidant Thermo-mechanico-chemical Pretreatment of Wheat Straw in the Twin-screw Extruder
This pretreatment used hydrogen peroxide (H2O2) in the presence of NaOH. Experiments were carried out in BC21 with profile 5 (Fig. 2), and operating conditions are given in Table 3. Wheat straw was fed into the extruder using a volumetric screw feeder (K-tron Soder KCL-KT-20, Switzerland), and NaOH solution was injected using a piston pump (Clextral DKM K202-AMP16, France) at the beginning of module 3 (Fig. 2). A first zone of mechanical pressure applied in module 3 ensured grinding and good mixing of the material with the NaOH. It consisted of a succession of 10 bilobe paddles, 5 cm apart and angled at 45° to each other. H2O2 was injected at the beginning of module 5 using a second piston pump (Clextral DKM K202 P32, France). Two reverse pitch screws were used in this profile, the first at the end of module 4 to form a light plug and limit liquid return along the barrel, and the second at the beginning of module 7, to form the dynamic plug essential for filtrate flow in module 6.
Table 3. Operating Conditions for Alkaline and Oxidant TMC Pretreatment
Thermo-mechanico-chemical Pretreatment of Wheat Straw in Organic Solvent Medium in the Twin-screw Extruder
Glycerol was used as an organic solvent for this trial, in association with NaOH. Experiments were carried out in BC45 with profile 6 (Fig. 2), and operating conditions are given in Table 1. Wheat straw was fed into the extruder using a volumetric screw feeder (Clextral 40, France) in the first module, and the glycerol solution was injected using a piston pump (Clextral DKM K202 PP16, France) at the end of module 2 (Fig. 1). A first zone of mechanical pressure applied in module 3 ensured grinding and good mixing of the material with the glycerol solution. It consisted of a succession of 10 bilobe paddles, 10 cm apart and angled at 45° to each other. Water was injected in module 5 using a piston pump (Clextral DKM K202 P32, France) to flush out compounds solubilised by alkaline treatment. The filtration zone was situated in module 6, and used a reverse pitch screw in module 7.
Analytical Methods
Dry matter and parietal compounds
Moisture contents were determined according to French standard NF V 03-903, and mineral contents according to French standard NF V 03-322. An estimation of the three parietal constituents (cellulose, hemicelluloses, and lignins) contained in the solids was made using the ADF-NDF method of Van Soest and Wine (1967, 1968). Solubility in the ADF, NDF, and ADF-KMNO4 solutions was also extrapolated. All determinations have been carried out in triplicate. The standard deviation was less than 1.5% for all measurements.
An estimation of the water-soluble components contained in the cake, was made by measuring the mass loss of the test sample after 1 h in boiling water. This method has been adapted according to standard TAPPI 204 cm-97 on the apparatus Fibertec Tecator M1017. All determinations were carried out in duplicate.
Granulometric analysis
Particle size distribution of the material was obtained after drying and passing through 0.25 to 2.36 mm mesh sieves.
Analysis of fermentation inhibitors
Two grams of matter was added to 100 mL of water and refluxed at 100 °C for 1 h. HPLC–UV-MS was used to analyse the liquid recovered by filtration. The HPLC (high performance liquid chromatography) system was a Dionex, including a P680 pump with an ASI-100 autosampler coupled to a UVD 170/340U photodiode array detector, and a Surveyor MSQ (Thermo Electron Corporation) mass spectrometer. Separation was performed on an Omnispher (Varian) C18 stainless steel column (100 x 3.0 mm i.d. and particle size of 3 µm) at 30 °C and a flow rate of 0.6 mL/min. The gradient elution used two solvents, Sa (0.5% acetic acid in water) and Sb (0.5% acetic acid in 60/40 (v/v, %) methanol and acetonitrile), and was as follows: 10 min of equilibration at 100% Sa, then from 0 to 5 min, 100 to 90% Sa, from 5 to 8 min, 90 to 73% Sa, maintained at 73% for 6min, from 14 to 25 min, 73 to 40% Sa, and from 25 to 30 min, 0% Sa. The injection volume was 10 µL, and detection was by UV at 273 nm and MS. The scan measurements, using positive APCI mode, were performed with the following settings: heater temperature of nitrogen gas, 550 °C; corona needle current, 10 µA; mass range, 1 to 400 m/z. Nitrogen was used for nebulisation of the HPLC mobile phase at a pressure of 0.31MPa.
Enzymatic hydrolysis
The substrate was introduced as 25 g/L dry matter (DM) in 50 mM citrate–phosphate buffer (pH 4.6) with 0.1% sodium azide as preservative. After 2 h of impreg-nation, Celluclast 1.5 L (16%/DM substrate) and cellobiase N188 (4%/DM substrate) were added. The experiments were carried out in 100 mL Erlenmeyer flasks at 50 °C with magnetic stirring. A flask was withdrawn at different times (every hour up to 6 h, and every 24 h up to 92 h) to monitor the hydrolysis, and the liquid phase (hydrolysate) was immediately heated for 5 min in a boiling water bath to inactivate cellulase and prevent further hydrolysis. The mixture was centrifuged at 14,000 × g for 2 min to remove solids. Glucose assay was by HPLC with an HPX 87 PB column.
The yield of cellulose digestibility has been determined as the percentage of free glucose released by enzymatic hydrolysis relative to total glucose content in matter.
RESULTS AND DISCUSSION
Thermo-mechanical Pretreatment
The thermomechanical pretreatment is a hydrothermal treatment that does not employ any catalyst or chemicals. The objective of this thermomechanical pre-treatment is to induce a physical destructuration of the material without chemical additives to avoid the formation of inhibitors. When applied on wheat straw, it produced a significant change in the size of the straw particles (Fig. 3), leading to defibration of the fibres and eventually to destructuration of the straw. This is manifested by an increase in the amount of hot water soluble components: up to 37% more (Table 4).
Table 4. Solubility in Hot Water and Van Soest Reagents
The increase in temperature had little effect on the solubility in hot water. However, it produced an increase in solubility in the “ADF” and “ADF-KMnO4” reagents, reflecting a change in the cell wall structure, which increased with increasing temperature. Assay of ethanolic fermentation inhibitor compounds indicates that those identified are present in quantities that are too small to interfere with the alcoholic fermentation (Table 5) (Palmqvist and Hahn-Hagerdal 2000; Delgenes et al. 1996; Ando et al. 1986).
Fig. 3. Comparison of the appearance of the extrudates from the various pretreatments with that of raw wheat straw
The hydrolysability of the extrudates increased by 60% for the highest temper-ature conditions (Fig. 4). However, it still only attained 35%, which is too low to consider for use as a substrate for ethanolic fermentation.
Table 5. Inhibitor Content
Fig. 4. Cellulose digestibility of dried extrudates obtained after thermo-mechanical pretreatment of wheat straw
Acid Thermo-mechanico-chemical Pretreatment
A thermomechanical treatment alone does not provide sufficient destructuration of the material. It is necessary to consider the coupling of pretreatment with a chemical action. The use of acid conditions is one of the possibilities. It allows the hydrolysis of hemicelluloses, leading to an improvement in the accessibility of the cellulose. This type of treatment has been extensively studied in the past and needs to work at low concentration to avoid inhibitor formation (Saha et al. 2005) and material corrosion. The use of this treatment in twin screw extruder reduces the contact time and thus the formation of degradation products. Two treatments were examined: an impregnation treatment and a combined impregnation extraction treatment. For the two modes, optical microscopic observation of the finest particles present in the extrudate revealed significant lysis of the cells (Fig. 5), up to the point where individual cells and fibres could be seen.
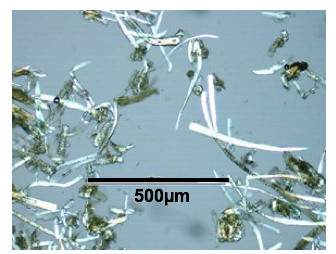
Fig. 5. Optical microscopic view of the finest particles present in the extrudate from the two modes of acid TMC pretreatment
Impregnation mode
The acid pretreatment in impregnation mode produced strong destructuration of the material (Fig. 3). Solubility in water and in NDF increased with increasing acid concentration and temperature (Table 4), thus the cell wall components were strongly impacted.
HPLC analysis of the hot water-soluble fraction showed the presence of furfural and hydroxymethylfurfural (Table 5), indicating a breakdown of sugars released by hydrolysis of hemicellulosic polysaccharides.
The material balance on 1 kg of dry straw confirmed that this treatment resulted in the hydrolysis of hemicelluloses (Table 6), and this impact was greater with higher acid concentration or temperature. Loss of hemicelluloses attained 37%, and lignin was also affected by this type of treatment, with a loss of 27%, depending on operating conditions.
Table 6. Quantity of Hemicelluloses, Lignin, and Cellulose Obtained in the Extrudate from 1 kg of Dry Raw Wheat Straw
Impregnation- extraction mode
Compared to the impregnation mode, the impregnation-extraction mode led to slightly finer particles (Fig. 3). The elimination of the liquid fraction via the filter and the addition of the reverse pitch screws allows a stronger plug to be formed. This extruder profile leads to stronger mechanical action and pressure, which is reinforced by the increase in the acid / straw ratio. More hemicelluloses were extracted (Table 6) and the cell walls were better broken down.
In the impregnation-extraction mode, a part of the soluble fraction is driven into the filtrate. Therefore the water-soluble and NDF-soluble percentages measured in the extrudate were logically lower than in the impregnation mode (Table 4).
In impregnation-extraction mode, the extraction of the hemicelluloses increased slightly with increasing acid / straw ratio and temperature. The amount extracted was between 60 and 74% (Table 6).
The acid thermo-mechanico-chemical pretreatment of wheat straws led to improved digestibility of the cellulose in both modes (Fig. 6). Hydrolysability reached 50% for temperatures of 150 °C or for a high acid / straw ratio (50%). An assay of alcoholic fermentation inhibitors in the extrudates was made for both acid pretreatment modes (Table 5), but their concentrations were too low to reveal any negative effect (Palmqvist and Hahn-Hagerdal 2000; Delgenes et al. 1996; Ando et al. 1986).
Fig. 6. Cellulose digestibility of dried extrudates obtained after acid TMC pretreatment of wheat straw
Alkaline Thermo-mechanico-chemical Pretreatment
Another possibility of coupling thermomechanical pretreatment with a chemical action is to use alkaline conditions. This way has been initially developed for the delignification of lignocellulosic material for the manufacture of pulp and purified cellulose (Gierer 1986). Simultaneously with the extraction of lignin, alkaline treatment causes the extraction of the hemicelluloses (Sun and Sun 2002) and allows a better swelling of cellulose by solvation of hydrogen bonds and thus helps to improve the accessibility of cellulose. Compared to acid pretreatment, mechanical defibration was low for alkaline pretreatment (Fig. 3), due to the high viscosity of the medium. The fibres present in the alkaline extrudate had a very wrinkled appearance (Fig. 7). This indicates an attack not only on the tissues that hold the fibres together, but also of the fibres’ components. The hemicelluloses and a part of the lignin were significantly solubilised (Table 6). Almost 50% of the hemicelluloses were extractable at a low NaOH /straw ratio (7.3%), and they were almost all extracted with a longer extruder residence time and a NaOH/ straw ratio of 35%. At the same time, 36% of the lignin was solubilised.
No molecules identified as having an inhibitor effect on fermentation with S. cerevisiae were generated by this pretreatment (Table 5).
This alkaline pretreatment led to a significant increase in the digestibility of the wheat straw (Fig. 8). Thus, the hydrolysability of the cellulose reached 73% in the case of treatment with a moderate concentration of caustic soda (NaOH / straw ratio of 7.3%) and reached 89% with an excess of caustic soda (NaOH / straw ratio of 35%).
Fig. 7. Electron micrograph of fibres present in the extrudate after alkaline TMC pretreatment
Fig. 8. Cellulose digestibility of dried extrudates of wheat straw obtained after alkaline TMCpretreatment, oxidant TMC pretreatment and TMC pretreatment in organic solvent
Oxidant thermo-mechanico-chemical pretreatment in alkaline medium
Gould and Freer (1984) demonstrated that it was possible to improve the hydrolyzability of the cellulose by delignification of the material in the presence of hydrogen peroxide in an alkaline medium. This treatment is normally used for bleaching paper pulp. The alkaline activation of hydrogen peroxide is used to form the perhydroxyl HOO- anion, and this acts on the lignin side chains leading to the elimination of their chromophore groups. This oxidant pretreatment produced a discoloration of the straw (Fig. 3).
Adapting this approach to the twin screw extruder, tests were carried out with an H2O2/NaOH ratio of 2, to avoid excess alkalinity detrimental to the stability of the perhydroxyl anion. This led to the formation of radicals capable of inducing breakdown of cellulose by radical oxidation.
Twenty-five percent of the hemicelluloses were extracted under these conditions (Table 6). A fraction of the lignin was solubilised (15 to 20%), and the hemicellulose extraction yields were lower with hydrogen peroxide than in its absence. Thus the introduction of H2O2 reduced the effectiveness of the caustic soda but allowed the extraction of more lignin.
This oxidant pretreatment did not generate molecules identified as having an inhibitor effect on fermentation with S. cerevisiae (Table 5).
Finally, the hydrolysability of the cellulose reached 67% with this pretreatment (Fig. 8).
Thermo-mechanico-chemical pretreatment in organic solvent medium.
Another way to delignify the lignocellulosic material, developed from so-called organosolv processes in the 70’s, consists of solubilizing the lignin by the action of the organic solvent in alkaline medium at high temperature (Adeeb 2004). This type of treatment is applied in twin screw extruder at lower temperature with a green solvent, the glycerol, which has a very low toxicity. The mechanical defibration was low as in case of the other alkaline medium pretreatment (Fig. 3). The solubility of the cell wall constituents of the extrudate was improved by alkaline pretreatment in a glycerol medium (Table 6). Seventy percent of the hemicelluloses were extracted at a glycerol / straw ratio of 1.5 and caustic soda/ straw ratio of 3.4%. The hemicelluloses content in the washed extrudate was less than 15% and the wheat straw was also partially de-lignified. The hydrolysability of the cellulose reached 81% (Fig. 8).
Alkaline medium: Correlation between digestibility of cellulose and hemicelluloses content
Talking all alkaline pretreatments into account, in the case of alkaline pretreatment, results reveal a simple correlation, almost linear, between the digestibility of cellulose and the percentage of hemicelluloses in the extrudates (Fig. 9). This highlights the existence of a common mechanism for these three pretreatments, which is based on partial extraction of hemicelluloses and lignin, and is probably associated with the phenomenon of conversion of cellulose I into cellulose II in alkaline medium, called mercerisation. These modifications of the cell wall are known to improve the accessibility of the cellulose resulting in an increase of the enzymatic digestibility (Mansfield et al. 1999 and Chandra et al. 2007).
Fig. 9. Correlation between the digestibility of cellulose and the percentage hemicelluloses in the extrudates (trials: 8, 9, 10, and 11)
CONCLUSIONS
- The use of different pretreatments by twin-screw extrusion leads to a variable breakdown of the wheat straw lignocellulosic assembly and allows modulation of the cellulose digestibility percentage of the treated materials.
- Thermomechanical treatment with water produces a strong physical defibration of the straw. It reaches to the level of the straw fibre bundles’ assembly and greatly increases the surface available for the liquid / solid exchange. However, it does not significantly alter the solubility of cell wall components or the structure of cellulose fibrils. The cellulose accessibility to enzymatic hydrolysis is only slightly improved, and the digestibility reaches about 35%, whereas it is 22% for the ground straw. Nonetheless, this pretreatment could be a useful method for preparing material. It would allow the liquid / solid ratio and contact time required for a soft acid or enzymatic hydrolysis of hemicelluloses to be reduced.
- The acid thermo-mechanico-chemical pretreatment enables the actual structure of the wall polymers to be degraded, causing the hydrolysis of hemicelluloses. Extruder residence time was too short to directly obtain significant extraction of pentoses, and similarly, mild acid hydrolysis of the hemicelluloses using the twin screw extruder required an increase in contact time before acid extraction of the sugars. Nonetheless, this acid pretreatment is interesting for enzymatic hydrolysis of cellulose because it enables the latter’s enzymatic digestibility to be greatly improved without causing the formation of inhibitor in annoying amount.
- The alkaline thermo-mechanico-chemical pretreatment allows the structure of the wall polymers to be degraded. The results are different depending on the operating conditions (concentration of the sodium hydroxide solution, NaOH/straw ratio, liquid/solid ratio, aqueous or glycerol medium, presence of an oxidizing agent (H2O2), temperature and residence time, etc.). The results reveal a simple correlation, almost linear, between the digestibility of cellulose and the percentage of hemicelluloses in the extrudates. Under some conditions the hydrolysability of the cellulose reached 89%. Therefore, these alkaline pretreatments are highly interesting and could be studied and optimised as pretreatment steps for an ethanol production process.
ACKNOWLEDGMENTS
This work is in memory of Catherine Doumeng, who left us in 2011. The authors thank Frédéric Martel and the ARD society for contributions to this work.
REFERENCES CITED
Adeeb, Z. (2004). “Glycerol delignification of poplar wood chips in aqueous medium,” Energy Educ. Sci. Technol. 13(2), 81-88.
Ando, S., Arai, I., Kiyoto, K., and Hanai, S. (1986). “Identification of aromatic monomers in steam-exploded poplar and their influences on ethanol fermentation by Saccharomyces cerevisiae,” J. Ferment. Technol. 64(6), 567-570.
Balat, M., and Balat, H. (2008). “Progress in bioethanol processing. Progress in energy and combustion,” Science 34(5), 551-573.
Ballesteros, I., Negro, M., Oliva, J. M., Cabañas, A., Manzanares, P., and Ballesteros, M. (2006). “Ethanol production from steam-explosion pretreated wheat straw,” Appl. Biochem. Biotechnol. 130(1), 496-508.
Cara, C., Ruiz, E., Ballesteros, I., Negro, M. J., and Castro, E. (2006). “Enhanced enzymatic hydrolysis of olive tree wood by steam explosion and alkaline peroxide delignification,” Process Biochem. 41(2), 423-429.
Carvalheiro, F., Duarte, L. C., and Gírio, F. M., (2008). “Hemicellulose biorefineries: A review on biomass pretreatments,” J. Sci. Ind. Res. 67(11), 849-864.
Chandra, R. P., Bura, R., Mabee, W. E., Berlin, A., Pan, X., and Saddler, J. N. (2007). “Substrate pretreatment: The key to effective enzymatic hydrolysis of lignocellulosics?” Adv. Biochem. Eng. Biotech. 108, 67-93.
Chang, V. S., and Holtzapple, M. T. (2000). “Fundamental factors affecting enzymatic reactivity,” Appl. Biochem. Biotechnol. 84-86(1-9), 5-37.
Chen, W. H., Xu, Y. Y., Hwang, W. S, and Wang, J. B. (2011). “Pretreatment of rice straw using an 10 extrusion/extraction process at bench-scale for producing cellulosic ethanol,” Bioresource Technol. 102(22), 10451-10458.
Delgenes, J. P., Moletta, R., and Navarro, J. M. (1996). “Effects of lignocellulose degradation products on ethanol fermentations of glucose and xylose by Saccharomyces cerevisiae, Zymomonas mobilis, Pichia stipitis, and Candida shehatae,” Enzyme Microb.Tech. 19(3), 220-225.
Dziezak, J. D. (1989). “Single and twin-screw extruders in food processing,” Food Technol. 43(4), 164-174.
Evon, P., Vandenbossche, V., Pontalier, P. Y., and Rigal, L. (2007). “Direct extraction of oil from sunflower seeds by twin-screw extruder according to an aqueous extraction process: Feasibility study and influence of operating conditions,” Ind. Crops Prod. 26(3), 351-359.
Gogoi, B. K., Choudhury, G. S, and Oswalt, A. J. (1996). “Effects of location and spacing of reversed screw and kneading element combination during twin-screw extrusion of starchy and proteinaceous blends,” Food Res. Int. 29(5-6), 505-512.
Himmel, M. E., Ding, S.-Y., Johnson, D. K., Adney, W. S., Nimlos, M. R., Brady, J. W., and Foust, T. D. (2007). “Biomass recalcitrance. Engineering plants and enzymes for biofuels production,” Science315(5813), 804-807.
Jacquemin, L., Zeitoun, R., Sablayrolles, C., Pontalier, P. Y., and Rigal, L. (2012). “Evaluation of the technical and environmental performances of extraction and purification processes of arabinoxylans from wheat straw and bran,” Process Biochem. 47(3), 373-380.
Keshwani, D. R. (2009). Microwave Pretreatment of Switchgrass for Bioethanol Oroduction, M.S. thesis, North Carolina State University, Raleigh, NC.
Galbe, M., and Zacchi, G. (2007). “Pretreatment of lignocellulosic materials for efficient bioethanol production,” Adv. Biochem. Eng. Biotech. 108, 41-65.
Gierer, J. (1986). “Chemistry of delignification,” Wood Sci. and Technol. 20, 1-33.
Gould, J. M., and Freer S. N. (1984). “High-efficiency ethanol production from lignocellulosic residues pretreated with alkaline H2O2,” Biotechnol. and Bioeng. 26(6), 628-631.
Karunanithy, C. and Muthukumarappan, K. (2011). “Optimization of switchgrass and extruder parameters for enzymatic hydrolysis using response surface methodology,” Ind. Crops Prod. 33(1), 188-199.
Kim, K., and Hong, J. (2001). “Supercritical CO2 pretreatment of lignocellulose enhances enzymatic cellulose hydrolysis,” Bioresource Technol. 77(2), 139-144.
Kumar, R., Mago, G., Balan, V., and Wyman, C. E. (2009). “Physical and chemical characterizations of corn stover and poplar solids resulting from leading pretreatment technologies,” Bioresource Technol. 100(17), 3948-3962.
Lamsal, B., Yoo, J., Brijwani, K., and Alavi, S. (2010). “Extrusion as a thermo-mechanical pre-treatment for lignocellulosic ethanol,” Biomass Bioenerg. 34(12), 1703-1710.
Laser, M., Schulman, D., Allen, S. G., Lichwa, J., Antal, M. J., and Lynd, L. R. (2002). “A comparison of liquid hot water and steam pretreatments of sugar cane bagasse for conversion to ethanol,” Bioresource Technol. 81(1), 33-44.
Lee, S. H., Inoue, S., Teramoto, Y., and Endo, T. (2009). “Enzymatic saccharification of woody biomass micro/nanofibrillated by continuous extrusion process I: Effect ofadditives with cellulose affinity,” Bioresource Technol. 100(1), 275-279.
Lee, S. H., Inoue, S., Teramoto, Y., and Endo, T. (2010). “Enzymatic saccharification of woody biomass micro/nanofibrillated by continuous extrusion process II: Effect of hot-compressed water treatment,” Bioresource Technol. 101(24), 9645-9649.
Mansfield, S. D., Mooney, C., and Saddler, J. N. (1999). “Substrate and enzyme characteristics that limit cellulose hydrolysis,” Biotechnol. Prog. 15(5), 804-816.
Marechal, P., Jorda, J., Pontalier, P. Y., and Rigal, L. (2004). “Twin-screw extrusion and filtration for xylan production from wheat straw and bran,” in Hemicelluloses: Sciences and Technology – ACS Symposium Series 864, P. Gatenholm and M. Tenkanen, (eds.), Washington, DC.
McIntosh, S., and Vancov, T. (2011). “Optimisation of dilute alkaline pretreatment for enzymatic saccharification of wheat straw,” Biomass Bioenerg. 35(7), 3094-3103.
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y. Y., Holtzapple, M., and Ladisch, M. (2005). “Features of promising technologies for pre-treatment of lignocellulosic biomass,” Bioresource Technol. 96(6), 673-686.
N’Diaye, S., and Rigal, L. (2000). “Factors influencing the alkaline extraction of poplar hemicelluloses in a twin-screw reactor: correlation with specific mechanical energy and residence time distribution of the liquid phase,” Bioresource Technol. 75(1), 13-18.
Ogier, J.-C., Ballerini, D., Leygue, J.-P., Rigal, L., and Pourquié, J. (1999). “Production d’éthanol à partir de biomasse lignocellulosique,” Oil Gas Sci. Technol. 54(1), 67-94.
Oliva, J. M., Sáez, F., Ballesteros, I., Gónzalez, A., Negro, M. J., Manzanares, P., and Ballesteros, M. (2003). “Effect of lignocellulosic degradation compounds from steam explosion pretreatment on ethanol fermentation by thermotolerant yeast Kluyveromyces marxianus,” Appl. Microbiol. Biotechnol. 105(1), 141-154.
Palmqvist, E., and Hahn-Hägerdal, B. (2000). “Fermentation of lignocellulosic hydrolysates II: Inhibitors and mechanism of inhibition,” Bioresource Technol. 74(1), 25-33.
Pérez, J. A., Ballesteros, I., Ballesteros, M., Sáez, F., Negro, M. J., and Manzanares, P. (2008). “Optimizing liquid hot water pretreatment conditions to enhance sugar recovery from wheat straw for fuel-ethanol production,” Fuel 87(17-18), 3640-3647.
Rouilly, A., Jorda, J., and Rigal, L. (2006). “Thermo-mechanical processing of sugar beet pulp. I. Twin-screw extrusion process,” Carb. Polym. 66(1), 81-87.
Saha, B. C., Iten, L. B., Cotta, M. A., and Wu, Y. V. (2005). “Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol,” Process Biochem. 40(12), 3693-3700.
Sun, R. C., and Sun, X. F. (2002). “Fractional and structural characterization of hemicelluloses isolated by alkali and alkaline peroxide from barley straw,” Carbohyd. Polym. 49(4), 415-423.
Taherzadeh, M. J., and Karimi, K. (2008). “Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review,” Int. J. Mol. Sci. 9(9), 1621-1651.
Varga, E., Reczey, K., and Zacchi, G. (2004). “Optimization of steam pretreatment of corn stover to enhance enzymatic digestibility,” Appl. Biochem. Biotechnol. 113-116, 509-523.
Van Soest, P. J., and Wine, R. H. (1967). “Use of detergents in the analysis of fibrous feeds. IV. Determination of plant cell wall constituents,” J. AOAC Int. 50(1), 50-55.
Van Soest, P. J., and Wine, R. H. (1968). “Determination of lignin and cellulose in acid detergent fiber with permanganate,” J. AOAC Int. 51(4), 780-784.
Yachmenev, V., Condon, B., Klasson, T., and Lambert, A. (2009). “Acceleration of the enzymatic hydrolysis of corn stover and sugar cane bagasse celluloses by low intensity uniform ultrasound,” J. Biobased Mater. Bioenergy 3(1), 25-31.
Yoo, J., Alavi, S., Vadlani, P. and Amanor-Boadu, V. (2011). “Thermo-mechanical extrusion pretreatment for conversion of soybean hulls to fermentable sugars,” Bioresource Technol. 102(16), 7583-7590.
Zhao, X., Zhang, L., and Liu, D. (2008). “Comparative study on chemical pretreatment methods for improving enzymatic digestibility of crofton weed stem,” Bioresource Technol. 99(9), 3279-3736.
Zeitoun, R., Pontalier, P.-Y., Marechal, P., and Rigal, L. (2010). “Twin-screw extrusion for hemicellulose recovery: Influence on extract purity and purification performance,” Bioresource Technol. 101(23), 9348-9354.
Article submitted: November 29, 2013; Peer review completed: January 6, 2014; Revised version received and accepted: January 24, 2014; Published: January 30, 2014.
