Abstract
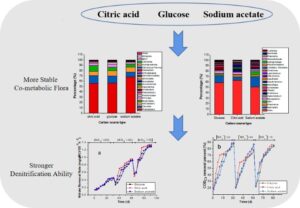
The effect of different carbon sources was studied relative to the treatment effect of aerobic denitrifying bacteria during the treatment of nitrate-containing wastewater as well as the influence of co-metabolism flora. Three carbon sources, i.e., glucose, citric acid, and sodium acetate, were selected to study the changes in the pH, nitrate nitrogen, chemical oxygen demand removal, nitrite nitrogen concentration, physical and chemical properties of sludge and volatile fatty acids, and high-throughput sequencing to study any changes in flora. The results showed that the denitrification ability of the system using citric acid as the carbon source was stronger than the denitrification ability of the system using glucose or sodium acetate as the carbon source. The removal of nitrate nitrogen in the system was the result of the co-metabolism of acid producing bacteria and aerobic denitrifying bacteria. Due to the greater number of types and greater amount of dominant bacteria in the sludge domesticated and cultured with citric acid as a carbon source, the co-metabolism flora formed was more stable, so it could better remove nitrate nitrogen.
Download PDF
Full Article
Treatment of Nitrate-Nitrogen-Containing Wastewater via Aerobic Denitrifying Bacteria Using Different Carbon Sources
Shanhong Lan,a,* Yingshan Lai,a Longyu Wang,b Wenbo Liu,b Jinhuan Liu,a Dejin Liu,b Ke Li,b Qiliang Cao,b Kun Wei,a and Huixia Lan b
The effect of different carbon sources was studied relative to the treatment effect of aerobic denitrifying bacteria during the treatment of nitrate-containing wastewater as well as the influence of co-metabolism flora. Three carbon sources, i.e., glucose, citric acid, and sodium acetate, were selected to study the changes in the pH, nitrate nitrogen, chemical oxygen demand removal, nitrite nitrogen concentration, physical and chemical properties of sludge and volatile fatty acids, and high-throughput sequencing to study any changes in flora. The results showed that the denitrification ability of the system using citric acid as the carbon source was stronger than the denitrification ability of the system using glucose or sodium acetate as the carbon source. The removal of nitrate nitrogen in the system was the result of the co-metabolism of acid producing bacteria and aerobic denitrifying bacteria. Due to the greater number of types and greater amount of dominant bacteria in the sludge domesticated and cultured with citric acid as a carbon source, the co-metabolism flora formed was more stable, so it could better remove nitrate nitrogen.
DOI: 10.15376/biores.17.2.1972-1987
Keywords: Carbon source; Aerobic denitrification; Nitrate nitrogen; Wastewater treatment
Contact information: a: School of Environment and Civil Engineering, Dongguan University of Technology, Dongguan 523808 China; b: College of Environment and Safe Engineering, Qingdao University of Science & Technology, Qingdao 266042 China;
* Corresponding author: lllssshhhh@126.com
GRAPHICAL ABSTRACT

INTRODUCTION
Nitrate nitrogen (NO3-), as one of the most common water pollutants, has caused serious water pollution problems in many parts of the world (Tang et al. 2018; Shi et al. 2019). In 2016 the consumption of chemical fertilizers was 197.5 million t in the world, including 110 million t of nitrogen fertilizers (Cui et al. 2021). It was reported to contaminate surface and ground water sources with around 293,000 tonnes/yr of nitrogen in Canada (Ritter et al. 2002). The treatment of nitrogenous wastewater has long been a research focus for domestic and foreign scholars. Compared to the physical and chemical methods, the biological denitrification method is not only economical and effective, with good treatment results, but it is also green and does not cause secondary pollution to the water body (Okada et al. 2005; Takebe et al. 2012). Biological nitrogen removal has been widely used in the field of sewage treatment. Common biological denitrification treatment methods include anoxic/aerobic (A/O), anaerobic-anoxic-aerobic (A2O), and intermittent activated sludge (SBR). The traditional view is that nitrous acid and nitrate bacteria will oxidize ammonia nitrogen into nitrate nitrogen and nitrite nitrogen under aerobic conditions (Coats et al. 2011; Zhang et al. 2011; Ge et al. 2012; Santos et al. 2016). Under anoxic conditions, in the presence of an external organic carbon source, denitrifying bacteria use nitrate as an electron acceptor to be reduced to nitrogen. Biological denitrification requires two processes, i.e., aerobic nitrification and anoxic denitrification. Microbial denitrification can only occur under conditions with limited oxygen (anoxic environment), and the addition of organic carbon sources at this stage increases the investment cost (Huang et al. 2013). The growth of microorganisms is slow under anoxic conditions, and the sensitivity to environmental conditions increases the complexity of the operation, which has become a restrictive factor for the widespread promotion of traditional biological denitrification methods.
In recent years, with the discovery of aerobic denitrifying bacteria, aerobic denitrification technology has increasingly become a hot spot in biological denitrification research (Obertson and Kuenen 1983; Zhang et al. 2011; Yang et al. 2020). Aerobic denitrification can realize nitrification and denitrification in the same aeration tank. Many studies have focused on the isolation of aerobic denitrifying strains with high denitrification efficiency, as well as studied the internal denitrification mechanism of its denitrification effect. Presently, a variety of aerobic denitrifying strains have been isolated, e.g., Azoarcus, Pseudomonas, Thauera, etc. (Li et al. 2021). Aerobic denitrifying bacteria are chemical-energy heterotrophic bacteria with a fast growth and reproduction rate. They use NO3- and O2 as electron acceptors to convert nitrate nitrogen into gaseous N2O or N2. Obertson and Kuenen (1983) discovered the first aerobic denitrifying bacteria that could use O2 and nitrate as electron acceptors at the same time. Due to the participation of oxygen, aerobic denitrification is different from the traditional anoxic denitrification process (Obertson and Kuenen 1983). Since then, more and more aerobic denitrifying bacteria have been isolated. The Acinetobacter YS2 isolated by Lang et al. (2020) from a petrochemical wastewater treatment process had excellent denitrification performance. Under the nitrification and denitrification processes, the removal percentages of NH4+-N and NO3-N after 24 h were 87.8% and 88.2%, respectively. Liu et al. (2020a) isolated a strain of Vibrio, and the removal of nitrate nitrogen reached up to 97.4%. Fu et al. (2019) isolated a new type of aerobic denitrifying bacteria Zobellella denitrificans A63 and found the addition of A63 could increase the removal percentage of nitrate nitrogen in saline wastewater (Hong et al. 1993).
Aerobic conditions enable aerobic denitrifying bacteria to maintain high activity, rapid growth and reproduction, and hold a high level of nitrogen removal efficiency (Chen and Ni 2011; Zheng et al. 2011; Ji et al. 2015). Therefore, aerobic denitrification treatment of nitrate nitrogen wastewater is increasingly favored by researchers. However, it is found that the denitrification rate is slow in actual application, primarily because the carbon source spectrum used by aerobic denitrifying bacteria is limited, and only short-chain fatty acids, e.g., acetic acid, can be utilized, while complex organic matter cannot (Yang et al. 2010; Yu et al. 2019). The organic matter contained in domestic sewage is often complex, which cannot provide the effective carbon source required by aerobic denitrifying bacteria. The presence of acid-producing bacteria in the sludge system can decompose complex organic matter to produce short-chain fatty acids and form a co-metabolism relationship with denitrifying bacteria. The lack of research data on co-metabolism flora under different carbon sources has become a bottleneck in the practical application of aerobic denitrification technology in engineering. In addition to glucose, which is often used as a carbon source, citric acid and sodium acetate are often used in studies of aerobic denitrification. Wen et al. (2010) studied nitrogen removal efficiency of Pseudomonas stutzeri strains isolated from an anaerobic/anoxic/oxic wastewater treatment process using citric acid as carbon source with a carbon-nitrogen ratio of 5.0. In addition, it was found that sodium acetate was the most favorable carbon source for ammonium oxidation by strain Y16 (Huang et al. 2013).
In this study, glucose, citric acid, and sodium acetate were selected as carbon sources to acclimatize sludge, enrich acid-producing bacteria and aerobic denitrifying bacteria, and study the effect of carbon sources on the performance of aerobic denitrification and denitrification. The co-metabolism relationship between the acid-producing bacteria and aerobic denitrifying bacteria were studied via high-throughput sequencing, which could provide a new method and theory for the treatment of nitrate-nitrogen-containing wastewater.
Experimental Water
In this experiment, glucose, citric acid, and sodium acetate were used as carbon sources to simulate wastewater, which had a chemical oxygen demand (CODCr) of approximately 300 mg/L. Three reactors were set up, with either glucose, citric acid, or sodium acetate as the carbon source, ammonium sulfate as the nitrogen source, and potassium dihydrogen phosphate as the phosphorus source. The BOD to N (ammonia nitrogen) to P ratio was 100 to 5 to 1, to maintain the growth and reproduction of microorganisms in each reactor. Carbon to nitrogen ratios of 15.0, 7.5 and 4.2 (nitrate concentrations of 20, 40, 70 mg/L) were used to ensure denitrification in the reactor. The sludge was taken from the return sludge from the secondary settling tank of a sewage treatment plant in Qingdao.
Analysis Methods
The pH value of the solution was measured with a PHS-3C pH meter. The CODCr was measured with a COD analyzer (DR1010, Shanghai Shilu Instrument Co., Ltd., Shanghai, China) via the rapid digestion method. The NO3–-N (nitrate nitrogen) content was determined via hydrochloric acid ultraviolet spectrophotometry (HJ/T 346-2007), while the NO2–-N (nitrosate nitrogen) content and MLSS concentration were determined according to the national standard method (National EPA 2002). The volatile fatty acid (VFAs) content was determined via gas chromatography. In addition, the bacterial population and abundance in the activated sludge were determined via high-throughput sequencing.
Sludge Acclimation
The activated sludge was divided into three 1000 mL glass beakers in equal amounts; 300 mL of activated sludge mixture and 300 mL of simulated wastewater from different carbon sources were added. Then, the pH was adjusted to approximately 7.0, and the solution was allowed to sit for 24 h. The acclimation process was divided into three stages, gradually increasing the concentration of sodium nitrate in the wastewater. The nitrate concentration was 20 mg/L in the first acclimation stage, 40 mg/L in the second acclimation stage, and 70 mg/L in the third acclimation stage. The removal percentage of nitrate nitrogen was measured during each acclimation stage. The next acclimation stage was entered when the removal reached a higher level. The pH, CODCr, and NO3–-N and NO2–-N concentrations of the inlet and outlet water were measured in each cycle. At the end of each acclimation stage, the physical and chemical indicators of the sludge, i.e., the MLSS were determined.
RESULTS AND DISCUSSION
Effluent pH and Chemical Oxygen Demand (CODCr)
During the acclimation process, the pH and CODCr removal percentages of the effluent treated by the aerobic activated sludge system cultured with three carbon sources were analyzed (as shown in Fig. 1).
Fig. 1. The pH (a); and CODCr removal percent (b) of the effluent during the acclimation process
The pH of the effluent water of the aerobic activated sludge system with glucose, citric acid, and sodium acetate as carbon sources showed an overall upward trend. There are two primary reasons for this: (1) during the process of organic matter treatment via the aerobic activated sludge system, the organic matter will eventually be decomposed to produce CO2 , as shown by Eq. 1 ,
(1)
and during aerobic treatment, aeration can blow off the CO2 produced, which increases the alkalinity of the effluent and the pH value; (2) during the process of denitrification, aerobic denitrifying bacteria use organic matter as electron donors to reduce nitrate nitrogen to nitrogen and produce OH–, as shown by Eqs. 2 and 3,
(2)
(3)
which will also increase the pH of the effluent.
During the acclimation process, citric acid and glucose were used as carbon sources, and the effluent pH fluctuated around 8.0 and 7.5. With sodium acetate as the carbon source, the effluent pH was relatively stable, fluctuating at approximately a pH of 7.3. Since the three carboxyl groups in citric acid were consumed during the metabolic process, the acidity was reduced, and the denitrification efficiency in the system was higher, which resulted in a considerably higher effluent pH. During the process of glucose metabolism, organic acids were produced, and the organic acids were used by aerobic denitrifying bacteria. Therefore, the pH of the effluent was lower than the pH of the citric acid system. However, short-chain organic acids were produced in both systems, and the accumulation of the latter was related to the denitrification effect. Therefore, in the initial stage of increasing the concentration of nitrate, when the denitrifying bacteria were inhibited, acid was accumulated, and the pH of the effluent considerably decreased. Sodium acetate as the carbon source system did not produce organic acids, so the pH of the effluent was relatively stable throughout the acclimation stage.
Figure 1b shows that in the first stage of acclimation, the removal percentages of the CODCr acclimated with the three different carbon sources gradually increased as the acclimation time increased. This was primarily because at this stage, the nitrate concentration was not high enough to inhibit microorganisms. Therefore, with the continuous acclimation of the sludge system, the microorganisms in the sludge continued to adapt to the environment. The maximum removal percentages of the three carbon sources in the three acclimation stages reached greater than 85.0%, which indicated that the aerobic denitrifying bacteria and other heterotrophic bacteria efficiently utilized the organic matter in the wastewater. When entering a new acclimation stage, the concentration of NO3– suddenly increased, and some bacteria in the system died due to not adapting to the higher NO3– concentration environment. This resulted in a decrease in metabolic capacity and a considerable decrease in removal percentages. After several days of continuous cultivation, the microorganisms gradually adapted to the high NO3– concentration conditions, and the microorganisms were suitable for survival under these conditions. The microorganisms then grew and multiplied in large numbers, causing the CODCr removal to rise. However, the microorganisms that were not adapted to the high NO3– concentration environment were gradually suppressed or even died. During the different stages of acclimation, the ability of the microorganisms to adapt to the environment gradually improved. As shown in Fig. 2, the maximum removal percentages of CODCr were as follows: citric acid was greater than sodium acetate, which was greater than glucose. Studies have shown that for aerobic activated sludge systems, different carbon sources had a greater impact on the effluent treatment effect (Wu and He 2012; Zuo et al. 2020). During the nitrate reduction process, the organic matter provided by the carbon source played an important role in the growth, respiration, and denitrification of the aerobic denitrifying bacteria. Compared with the glucose carbon source, the sodium acetate carbon source had a simpler chemical structure and smaller molecular weight. Therefore, sodium acetate as a carbon source had a higher removal of nitrate nitrogen and a higher denitrification efficiency. The ability of the microorganisms cultured with citric acid as a carbon source to utilize organic matter was greater and more stable than the microorganisms cultured with glucose or sodium acetate as carbon sources. This may be because citric acid was used as the carbon source when the sludge was activated. After a long-term acclimation process, more acid-producing bacteria that efficiently utilized citric acid were obtained in the sludge system, so that citric acid was effectively used.
Compared with the citric acid carbon source, the removal percentage of CODCr was slightly lower, which was primarily because too much sodium salt was added to the sodium acetate carbon source system. Excessive sodium salt destroyed the stability of the flora in the system and disturbed the degradation of the macromolecular organics by acid-producing bacteria.
Nitrate Removal Rate and Nitrite Nitrogen Concentration in the Effluent
During the acclimation process, the removal rate of nitrate nitrogen and the concentration of nitrite nitrogen in the effluent after treatment by the aerobic activated sludge system cultured with three carbon sources were analyzed (as shown in Fig. 2).
Fig. 2. NO3– removal rate (c) and changes in NO2– concentration (d)
At different stages of acclimation, as the acclimation progressed, the concentration of nitrite nitrogen gradually decreased, and the removal rates of nitrate nitrogen gradually increased. The three kinds of carbon source acclimation obtained a high denitrification effect, among which the system with citric acid as the carbon source was the best, with sodium acetate the second best, and glucose the worst.
During the first two stages of the acclimation process, the microorganisms quickly adapted to the environment and carried out denitrification reactions. All three reactors showed high denitrification efficiency. In the reactor with glucose, citric acid, and sodium acetate as carbon sources, the NO3– removal rate was 0.1463, 0.1518, and 0.1491 mg/gMLVSS-1·h-1 in the first stage, respectively, and 0.3034, 0.3175, and 0.3098 mg/gMLVSS-1·h-1 in the second stage, respectively. The aerobic denitrifying bacteria in the system used nitrate nitrogen to grow and multiply under aerobic conditions and continuously accumulated, which can effectively remove nitrate nitrogen in simulated wastewater. On the whole, the NO2– concentration in the effluent water of the three carbon sources showed a downward trend. During the process of aerobic denitrification, the NO3– was first converted into NO2–, and the NO2– was further converted into gaseous products such as N2O and N2. As shown in Fig. 3, Huang and Tseng (2001) proposed a hypothetical route of aerobic respiration and an electron transport model. When some microorganisms performed aerobic denitrification, they used nitrate nitrogen as the reaction substrate to convert nitrate nitrogen into nitrite nitrogen, and then into gaseous products under the action of a series of enzymes. Among them, nitrate reductase (NAR), nitrite reductase (NIR), nitric oxide reductase (NOR), and nitrous oxide reductase (NOS) were all important enzyme systems involved in the denitrification process (Huang and Tseng 2001). Therefore, with the progress of acclimation, the decrease of NO2– concentration in the water body may also be related to the higher denitrification level. After NO2– was produced, it could be further decomposed and transformed immediately by denitrifying bacteria.
Fig. 3. Hypothetical pathway of aerobic respiration and electron transport model (Hu et al. 2019) (Note: ① nitrate reductase (NAR); ② nitrite reductase (NIR); ③ nitric oxide reductase (NOR); and ④ nitrous oxide reductase (NOS))
After the system was stabilized, during the third stage of acclimation, the nitrate concentration reached a higher level. At the same time, the removal rate of nitrate nitrogen was considerably reduced, and the nitrite nitrogen concentration in the effluent was higher. The reason for this was that the high concentration had a greater toxic effect, which not only inhibited aerobic denitrifying bacteria, but also inhabited the decomposition of organic matter by the acid-producing bacteria. As a result, it was unable to provide an effective carbon source for aerobic denitrifying bacteria, resulting in a higher concentration of nitrate nitrogen and nitrite nitrogen in the effluent.
An effective carbon source is an indispensable energy material for the growth and reproduction of aerobic denitrifying bacteria. Aerobic denitrifying bacteria use carbon sources as electron donors and nitrate nitrogen as electron acceptors to reduce nitrate to nitrogen. The rate of nitrogen removal via aerobic denitrification strongly depends on the type of organic carbon source. Studies have shown that with different carbon sources, the denitrification rate of microorganisms is quite different, and the denitrification ability of the same strain varied from different carbon source conditions (Elefsiniotis et al. 2004; Hu et al. 2019; Wen et al. 2019).
Analysis of the Volatile Fatty Acid Content in the Effluent
The acetic acid content in the effluent water from the three carbon sources, i.e., glucose, citric acid, and sodium acetate, were 16.2, 35.8, and 25.4 mg/L, respectively. In addition, there was a certain amount of propionic acid, butyric acid, and valeric acid (Fig. 4).
Fig. 4. The VFAs content of the effluent from three different carbon source systems
Studies have shown that acetic acid in VFAs is a high-quality carbon source for accelerating the rate of denitrification, with the optimal carbon source selection order of denitrifying bacteria as follows: acetic acid is greater than butyric acid, which is greater than propionic acid (Elefsiniotis and Wareham 2007; Liu et al. 2020b). Moreover, the nitrate degradation efficiency of acetic acid is more than twice that of propionic acid. The citric acid carbon source had the highest acetic acid content in the three systems, which may be one of the reasons for the high denitrification of this system. Glucose is a simple sugar, which is easily absorbed and utilized by cells. The glucose carbon source contained the most short-chain fatty acids in the water. Among all the degradation intermediate products, the propionic acid content was the highest, while the acetic acid and butyric acid contents were relatively small. This may be due to the fact that most organic matter was first converted into propionic acid during the degradation process, and then it was converted to acetic acid after propionic acid accumulated to a certain amount. Therefore, the denitrification of the system using glucose as the carbon source was lower than the denitrification of the system using citric acid and sodium acetate as the carbon source. The overall short-chain fatty acids content in the sodium acetate carbon source system was less than the overall short-chain fatty acids content of the other two carbon source systems. The reason for this might be that sodium acetate itself is a short-chain fatty acid, which can be directly used by aerobic denitrifying bacteria as a carbon source, resulting in less short-chain fatty acids, e.g., acetic acid. Under the action of microbial synthase, the synthesis of propionic acid and valeric acid may be promoted, and the excessive sodium ion dosage in the sodium acetate system will affect the activity of microorganisms (Liu et al. 2020c). Meanwhile, under salt stress, the permeability of the cell membrane was affected, which in turn affected the intake of nutrients by the microorganisms, resulting in a very small amount of propionic acid and valeric acid in the system. It can be seen that the sodium acetate carbon source system may inhibit the metabolic activities of acid producing bacteria and aerobic denitrifying bacteria due to excessive sodium ions in the reactor. In summary, the citric acid carbon source was beneficial to aerobic denitrification.
Study on the Co-metabolism Flora
Using high-throughput sequencing technology to analyze the abundance of dominant bacteria in the microbial flora in the sludge, the coverage values of the sequencing results were all greater than 0.99, and the accuracy of the sequencing results can be guaranteed. The sequencing results showed that with glucose, citric acid, and sodium acetate as the carbon sources, the Chao1 indices were 2004.7, 2263.5, and 2366.7, respectively, and the Shannon indices were 8.1, 8.4, and 8.0, respectively. It can be seen that the abundance of activated sludge flora after acclimation of the three carbon sources was relatively high, which indicated that as the concentration of nitrate nitrogen continued to increase, the dominant bacteria were continuously enriched, and the unsuitable flora were gradually eliminated and died. The activated sludge acclimation with sodium acetate as a carbon source had a higher abundance of bacteria. This may be due to the simple and small molecular structure of sodium acetate, which made it easier to be absorbed and utilized by bacteria. Therefore, there were a variety of bacteria that effectively used the sodium acetate carbon source to promote the growth and reproduction of bacteria. However, the stability of the bacterial flora obtained by acclimation with citric acid as a carbon source was also relatively good. This may be because after a long period of acclimation, in the aerobic activated sludge system cultured with citric acid as a carbon source, the acid-producing bacteria reconciled well. The co-metabolism flora composed of oxygen denitrifying bacteria was basically stable, so the flora had good stability. The distribution law of the flora at the phylum level is shown in Fig. 5, and the distribution law at the genus level is shown in Fig. 6.
Fig. 5. The relative percentage of community composition of bacterial classification (phyla)
Fig. 6. The relative percentage of community composition of bacterial classification (genus)
The microbial flora in the three reactors was a co-metabolism flora composed of acid producing bacteria and aerobic denitrifying bacteria. It can be seen from Fig. 5 that at the phylum level, there were 7 phyla with a relative abundance of 1% or greater, arranged in order of abundance: Proteobacteria, Chloroflexi, Actinomycetes Actinobacteria, Bacteroidetes, Firmicutes, Planctomycetes, and Patescibacteria. Among the three systems, Proteobacteria, Chlorocurve, Actinomycetes, and Bacteroides were the common dominant phyla. Figure 6 showed that at the genus level, Skermanella, Roseomonas, Microbacterium, Hyphomicrobium, Amaricoccus, and Thauera (D) were the primary dominant genus.
Among the three carbon source systems, i.e., glucose, citric acid, and sodium acetate, the Proteobacteria had an absolute advantage, accounting for 56.6%, 56.0%, and 67.8%, respectively. Proteobacteria was the most common phylum in the nitrification and denitrification system, which belonged to the denitrification function bacteria, and its large amount allowed the nitrate nitrogen in the reactor to be effectively removed (Bruckner et al. 2012). Other studies have shown that during the process of nitrification and denitrification, Chloroflexus can promote the decomposition of COD and decompose macromolecular organic matter into small molecular organic acids. At the same time, it also plays an important role in the storage of endogenous substances. In addition, its filamentous structure was conducive to the formation of biofilms (Bachand and Horne 1999; Mulholland et al. 2008). The Bacteroides phylum also had a major advantage over the other microorganisms in terms of wastewater treatment. It can degrade complex organic matter and promote the hydrolysis and acidification of macromolecular organic matter into small molecular substances, e.g., acetic acid and propionic acid (Addison et al. 2011). Actinomycota played an important role in the degradation of macromolecular complex organics, e.g., aromatic compounds, cellulose, lignins, etc. (Manikkam et al. 2020). Among the three carbon source systems, i.e., glucose, citric acid, and sodium acetate, the phylum Chloroflexus and Bacteroides accounted for the largest proportion of the flora with citric acid as the carbon source. The system had certain advantages in degrading macromolecular organic matter; at the same time, aerobic denitrifying bacteria accounted for a relatively large proportion of the system. Therefore, the co-metabolism flora composed of acid-producing bacteria and aerobic denitrification can coordinate and cooperate to achieve effective degradation of nitrate nitrogen. Combined with the analysis of the nitrate nitrogen removal, this was also one of the reasons for the high nitrate nitrogen removal of the citric acid carbon source reactor. Since sodium acetate itself is a short-chain fatty acid that can be directly utilized by aerobic denitrifying bacteria, the abundance of acid-producing bacteria in the system was relatively small. However, there were a large number of aerobic denitrifying bacteria in the sodium acetate system, but the stability of the flora was poor. This may be an important reason why the removal of nitrate nitrogen in the sodium acetate carbon source reactor was lower than the removal of nitrate nitrogen in the citric acid carbon source reactor. The less abundant bacteria phyla, e.g., Acidobacteria and Gemmatimonadetes, played an important role in the co-metabolism system. The acid bacteria phylum can decompose and utilize the produced small molecular organic acids, thereby keeping the pH of the system relatively stable. At the same time, the acidity produced by the hydrolysis and acidification of macromolecular organic substances by other bacteria provides the necessary environmental conditions for the growth of the acid bacillus (Lee et al. 2013). Other studies have shown that Bacillus phylum not only had a strong denitrification performance, but it also can produce proteases, amylases, etc., and promote the hydrolysis and acidification of proteins, starches, and other macromolecular organic matter; at the same time, it also has the effect of purifying water quality (Hu et al. 2020; Han et al. 2021). The co-metabolic system composed of various bacterial phyla together maintained the relative stability of the system.
At the genus level, Skermanella can play a role in nitrogen fixation, while the remaining 5 species are all aerobic denitrifying bacteria. The aerobic denitrifying bacteria accounted for a large amount of the system, which indicated that nitrate nitrogen could be degraded and utilized, and a stable aerobic denitrifying bacterial flora was obtained through the acclimation process. In the three carbon source systems, i.e., glucose, citric acid, and sodium acetate, the relative abundance of Roseomonas was 12.9%, 6.3%, and 19.1%, respectively, which belonged to the phylum Proteobacteria. The Proteobacteria played an important role in the denitrification process and had the ability to remove nitrogen. Microbacteria can adapt to low temperature environments; under low temperature conditions, the metabolic process can be controlled by adjusting the fluidity of the membrane, so that nitrate nitrogen can also be removed under low temperature conditions (Zhang et al. 2013). Dauerella can use nitrate nitrogen as an electron acceptor and glucose as a carbon source to denitrify (Yang et al. 2019). The Dauerella genus accounted for the largest proportion (5.0%) in the system. Combined with the nitrite nitrogen concentration data of the effluent, the glucose carbon source effluent nitrite nitrogen concentration was the largest. It may be that part of the nitrate nitrogen is first transformed into nitrite nitrogen, and then the nitrite nitrogen is further transformed into gaseous products. However, Dauerella accounts for a relatively large proportion when the carbon source of glucose, so there is more nitrate nitrogen that is degraded by this metabolic method, resulting in more nitrite nitrogen accumulation. In addition, mycelial microbacterium and amaranth also have a denitrification effect, using nitrate as the primary electron acceptor (Meiberg et al. 1980; Chan et al. 2012). Studies have found that Pseudomonas can also perform aerobic denitrification. Feng et al. (2021) found that under the biological stimulation of Fe(III), it can promote the growth and aerobic denitrification of Pseudomonas stutzeri T13. After screening and isolation, Xie et al. (2021) isolated a strain X49 that was identified as Pseudomonas mendocina, which can rapidly degrade high concentrations of inorganic nitrogen and increase the rate of nitrogen biodegradation (Xie et al. 2021). Pseudomonas is a kind of aerobic denitrifying bacteria, and a type of heterotrophic denitrifying bacteria, that uses organic carbon as energy. Under the premise of an organic carbon source, nitrate nitrogen in water can be denitrified to achieve biological denitrification, which can realize simultaneous nitrification and denitrification under aerobic conditions and effectively remove nitrate nitrogen.
Among the genera with relatively small abundances, the primary genera were Altererythrobacter, Hydrogenophaga, and Sporobacter. The genus Alterobacter belonged to the phylum Proteobacteria and played an important role in degrading pollutants. Studies have found that Altererythrobacter are effective nitrate degrading bacteria (Zuo et al. 2020). Studies have found that Hydrogenphages are a new type of denitrifying bacteria and Park et al. (2005) used DGGE technology to isolate the dominant genus in biofilm reactors, i.e., Hydrophages, which can use hydrogen as an electron donor for denitrification. Sporobacterium can degrade macromolecular substances, e.g., aromatic compounds, and decompose macromolecular substances into small molecular organic acids (Liao et al. 2013). For the above four bacterial genera, relatively speaking, the citric acid carbon source had a greater abundance, which indicated that the co-metabolism flora composed of acid-producing bacteria and aerobic denitrifying bacteria when citric acid is the carbon source may have higher stability and be more resistant to the toxic effects of nitrate.
Based on the above analysis, with citric acid as the carbon source, there were a variety of denitrifying bacteria in the flora, with a relatively uniform abundance ratio, so the co-metabolism flora had stronger stability. This may also be an important reason for the high removal of CODCr and nitrate nitrogen with a citric acid carbon source. During the acclimation process, the dominant bacteria that efficiently degraded organic matter and nitrate nitrogen were continuously enriched and became the dominant bacteria group in the system, thereby reducing the effluent CODCr and nitrate concentration. Acid-producing bacteria decomposed macromolecular organic pollutants to produce a large number of small molecular organic acids. These small molecular organic acids further decomposed and mineralized, providing nutrition and energy for cell metabolism. Although the nitrate in the water body has an inhibitory effect on acid producing bacteria, aerobic denitrifying bacteria can convert the nitrate in the water body into N2O or N2. This removes the inhibitory effect on the acid-producing bacteria, which is conducive to the degradation of macromolecular organic pollutants by acid-producing bacteria. At the same time, the nutrients and energy produced by the acid-producing bacteria can promote the growth and reproduction of aerobic denitrifying bacteria. The removal of nitrate nitrogen can reduce the toxic effect on acid producing bacteria and promote the decomposition and utilization of macromolecular organic matter, as well as provide nutrition for aerobic denitrifying bacteria and promote the growth and reproduction of aerobic denitrifying bacteria.
Future Application
Aerobic denitrification can replace the traditional A/O process, improve denitrify-cation efficiency, and realize nitrification and denitrification in aerobic pools at the same time, especially for nitrate-containing organic wastewater, which has greater advantages.
CONCLUSIONS
- There were three acclimation stages when using glucose, citric acid, or sodium acetate as the carbon sources for sludge acclimation. The concentration of nitrate added for each stage was 20, 40, and 70 mg/L, respectively. After acclimatization, citric acid was the carbon source system with the highest removal of nitrate nitrogen and chemical oxygen demand (CODCr), and the lowest concentration of nitrite nitrogen in the effluent.
- Using gas chromatography (GC) to analyze the volatile fatty acids content in each reactor, the results showed that in the system using citric acid as the carbon source, the acetic acid content, a high-quality carbon source, available to denitrification bacteria was the highest. In the system using glucose as the carbon source, the propionic acid content, a carbon source that is difficult for denitrifying bacteria to use, was considerably higher than its content in the other two systems.
- High-throughput sequencing technology was used to analyze the abundance of the co-metabolizing flora. The co-metabolizing flora were primarily composed of acid-producing bacteria and aerobic denitrifying flora, and the co-metabolizing flora using citric acid as a carbon source had stronger stability.
CONFLICTS OF INTEREST
There are no conflicts to declare.
ACKNOWLEDGMENTS
Thanks to Guangdong Basic and Applied Fundamental Research Fund (Approval Number: 2020A1515110222), National College Student Innovation and Entrepreneurship Training Program (Approval Number: 202110426100) and Shandong Province College Student Innovation and Entrepreneurship Training Program (Approval Number: S202110426099)
REFERENCES CITED
Addison, S., Slade, A., and Dennis, M. (2011). “Effects of substrate composition on the structure of microbial communities in wastewater using fluorescence in situ hybridization,” Systematic and Applied Microbiology 34(5), 337-343. DOI: 10.1016/j.syapm.2010.10.006
Bachand, P. A. M., and Horne, A. J. (1999). “Denitrification in constructed free-water surface wetlands: I. Very high nitrate removal rates in a macrocosm study,” Ecological Engineering 14(2), 9-15. DOI: 10.1016/S0925-8574(99)00016-6
Bruckner, C. G., Mammitzsch, K., Gunter, J., Labrenz, M., and Jürgens, K. (2012). “Chemolithoautotrophic denitrification of epsilonproteobacteria in marine pelagic redox gradients,” Environmental Microbiology 15(5), 1505-1513. DOI: 10.1111/j.1462-2920.2012.02880.x
Chan, G. F., Rashid, N. A. A., Chua, L. S., Ab.llah, N., Nasiri, R., and Ikubar, M. R. M. (2012). “Communal microaerophilic–aerobic biodegradation of Amaranth by novel NAR-2 bacterial consortium,” Bioresource Technology 105(1), 48-59. DOI: 10.1016/j.biortech.2011.11.094
Chen, Q., and Ni, J. (2011). “Heterotrophic nitrification-aerobic denitrification by novel isolated bacteria,” Journal of Industrial Microbiology and Biotechnology 38(9), 1305-1310. DOI: 10.1007/s10295-010-0911-6
Coats, E. R., Mockos, A., and Loge, F. J. (2011). “Post-anoxic denitrification driven by PHA and glycogen within enhanced biological phosphorus removal,” Bioresource Technology 102(2), 1019-1027. DOI: 10.1016/j.biortech.2010.09.104
Cui, X., Guo, L., Li, C., Liu, M., Wu, G., and Jiang, G. (2021). “The total biomass nitrogen reservoir and its potential of replacing chemical fertilizers in China,” Renewable and Sustainable Energy Reviews 135. DOI: 10.1016/j.rser.2020.110215
Elefsiniotis, P., and Wareham, D. G. (2007). “Utilization patterns of volatile fatty acids in the denitrification reaction,” Enzyme and Microbial Technology 41(1-2), 92-97. DOI: 10.1016/j.enzmictec.2006.12.006
Elefsiniotis, P., Wareham, D. G., and Smith, M. O. (2004). “Use of volatile fatty acids from an acid-phase digester for denitrification,” Journal of Biotechnology 114(3), 289-297. DOI: 10.1016/j.jbiotec.2004.02.016
Feng, L., Yang, J., Ma, F., Xing, L., Pi, S., Cui, D., and Li, A. (2021). “Biological stimulation with Fe(III) promotes the growth and aerobic denitrification of Pseudomonas stutzeri T13[J],” Science of The Total Environment 17(2), 1-8. DOI: 10.1016/j.scitotenv.2021.145939
Fu, G., Zhao, L., Huangshen, L., and Wu, J. (2019). “Isolation and identification of a salt-tolerant aerobic denitrifying bacterial strain and its application to saline wastewater treatment in constructed wetlands,” Bioresource Technology 290(10), 1-9. DOI: 10.1016/j.biortech.2019.121725
GB 7493-87 (1987). “Water quality. Determination of nitrite nitrogen. Spectrophoto-metric method,” Standardization Administration of China, Beijing, China.
Ge, S., Peng, Y., Wang, S., Lu, C., Cao, X., and Zhu, Y. (2012). “Nitrite accumulation under constant temperature in anoxic denitrification process: The effects of carbon sources and COD/NO3-N,” Bioresource Technology 114(1), 137-143. DOI: 10.1016/j.biortech.2012.03.016
Han, B., Mo, L.-Y., Fang, Y.-T., Di, H. J., Wang, J.-T., Shen, J.-P., and Zhang, L.-M. (2021). “Rates and microbial communities of denitrification and anammox across coastal tidal flat lands and inland paddy soils in East China,” Applied Soil Ecology 157, 1-12. DOI: 10.1016/j.apsoil.2020.103768
Hong, Z., Hanaki, K., and Matsuo, T. (1993). “Greenhous gas-N2O production during denitrification in wastewater treatment,” Water Science & Technology 28(7), 203-207. DOI: 10.2166/wst.1993.0163
Hu, B., Wang, T., Ye, J., Zhao, J., Yang, L., Wu, P., Duan, J., and Ye, G. (2019). “Effects of carbon sources and operation modes on the performances of aerobic denitrification process and its microbial community shifts,” Journal of Environmental Management 239(6), 299-305. DOI: 10.1016/j.jenvman.2019.03.063
Hu, H., Deng, C., Wang, X., Chen, Z., Zhong, Z., Wang, R. (2020). “Performance and mechanism of urea hydrolysis in partial nitritation system based on SBR,” Chemosphere 258, 1-8. DOI: 10.1016/j.chemosphere.2020.127228
Huang, H. K., and Tseng, S. K. (2001). “Nitrate reduction by Citrobacter diversus under aerobic environment,” Applied Microbiology Biotechnology 55(1), 90-94. DOI: 10.1007/s002530000363
Huang, X., Li, W., Zhang, D., and Qin, W. (2013). “Ammonium removal by a novel oligotrophic Acinetobacter sp. Y16 capable of heterotrophic nitrification-aerobic denitrification at low temperature,” Bioresource Technology 146, 44-50. DOI: 10.1016/j.biortech.2013.07.046
Ji, B., Yang, K., Zhu, L., Jiang, Y., Wang, H., Zhou, J., and Zhang, H. (2015). “Aerobic denitrification: A review of important advances of the last 30 years,” Biotechnology and Bioprocess Engineering 20(4), 643-651. DOI: 10.1007/s12257-015-0009-0
Lang, X., Li, Q., Ji, M., Yan, G., and Guo, S. (2020). “Isolation and niche characteristics in simultaneous nitrification and denitrification application of an aerobic denitrifier, Acinetobacter sp. YS2,” Bioresource Technology 302(4), 1-7. DOI: 10.1016/j.biortech.2020.122799
Lee, S.-H., Kondaveeti, S., Min, B., and Park. H.-D. (2013). “Enrichment of Clostridia during the operation of an external powered bio-electrochemical denitrification system,” Process Biochemistry 48(2), 306-311. DOI: 10.1016/j.procbio.2012.11.020
Li, B., Jing, F., Wu, D., Xiao, B., and Hu, Z. (2021). “Simultaneous removal of nitrogen and phosphorus by a novel aerobic denitrifying phosphorus-accumulating bacterium, Pseudomonas stutzeri ADP-19,” Bioresource Technology 321(2), 1-11. DOI: 10.1016/j.biortech.2020.124445
Liao, R., Shen, K., Li, A.-M., Shi, P., Li, Y., Shi, Q., and Wang, Z. (2013). “High-nitrate wastewater treatment in an expanded granular sludge bed reactor and microbial diversity using 454 pyrosequencing analysis,” Bioresource Technology 134, 190-197. DOI: 10.1016/j.biortech.2012.12.057
Liu, C., Zhu, L., and Chen, L. (2020a). “Effect of salt and metal accumulation on performance of membrane distillation system and microbial community succession in membrane biofilms,” Water Research 177(5), 1-19. DOI: 10.1016/j.watres.2020.115805
Liu, W., Yang, H., Ye, J., Luo, J., Li, Y.-Y., and Liu, J. (2020b). “Short-chain fatty acids recovery from sewage sludge via acidogenic fermentation as a carbon source for denitrification: A review,” Bioresource Technology 311(9), 1-10. DOI: 10.1016/j.biortech.2020.123446
Liu, X., Wang, Q., Li, L., Sun, X., Lv, A., and Chen, C. (2020c). “Characterization of aerobic denitrification genome sequencing of Vibrio parahaemolyticus strain HA2 from recirculating mariculture system in China,” Aquaculture 526, 1-12. DOI: 10.1016/j.aquaculture.2020.735295
Manikkam, R., Imchen, M., Kaari, M., Angamuthu, V., Venugopal, G., Thangavel, S., Joseph, J., Ramasamy, B., and Kumavath, R. (2020). “Metagenomic insights unveil the dominance of undescribed Actinobacteria in pond ecosystem of an Indian shrine,” Meta Gene 23, 1-5. DOI: 10.1016/j.mgene.2019.100639
Meiberg, J. B. M., Bruinenberg, P. M., and Harder, W. (1980). “Effect of dissolved oxygen tension on the metabolism of methylated amines in Hyphomicrobium X in the absence and presence of nitrate: Evidence for aerobic denitrification,” Microbiology 120(2), 453-463. DOI: 10.1099/00221287-120-2-453
Mulholland, P. J., Helton, A. M., Poole, G. C., Hall, R. O., Hamilton, S. K., Peterson, B. J., Tank, J. L., Ashkenas, L. R., Cooper, L. W., Dahm, C. N., et al. (2008). “Stream denitrification across biomes and its response to anthropogenic nitrate loading,” Nature 452(7184), 202-205. DOI: 10.1038/nature06686
National EPA (2002). Monitoring Methods of Water and Wastewater, Environmental Scientifc Publishing Company, Peking.
Obertson, L. A., and Kuenen, J. G. (1983). “Thiosphaera pantotropha gen. nov. sp. nov., a facultatively anaerobic, facultatively autotrophic sulphur bacterium,” Microbiology 129(9), 2847-2855. DOI: 10.1099/00221287-129-9-2847
Okada, N., Nomura, N., Nakajima-Kambe, T., and Uchiyama, H. (2005). “Characterization of the aerobic denitrification in Mesorhizobium sp. strain NH-14 in comparison with that in related rhizobia,” Microbes and Environments 20(4), 208-215. DOI: 10.1264/jsme2.20.208
Park, H. I., Choi, Y.-J., and Pak, D. (2005). “Autohydrogenotrophic denitrifying microbial community in a glass beads biofilm reactor,” Biotechnology Letters 27(13), 949-953. DOI: 10.1007/s10529-005-7654-x
Ritter, L., Solomon, K., Sibley, P., Hall, K., Keen, P., Mattu, G., and Linton, B. (2002). “Sources, pathways, and relative risks of contaminants in surface water and groundwater: A perspective prepared for the Walkerton inquiry,” Journal of Toxicology and Environmental Health. Part A 65(1), 1-142. DOI: 10.1080/152873902753338572
Santos, C. E. D., Moura, R. B., Damianovic, M. H. R. Z., and Foresti, E. (2016). “Influence of COD/N ratio and carbon source on nitrogen removal in a structured-bed reactor subjected to recirculation and intermittent aeration (SBRRIA),” Journal of Environmental Management 166(1), 519-524. DOI: 10.1016/j.jenvman.2015.10.054
Shi, P., Zhang, Y., Song, J., Li, P., Wang, Y., Zhang, X., Li, Z., Bi, Z., Zhang, X., Qin, Y., et al. (2019). “Response of nitrogen pollution in surface water to land use and social-economic factors in the Weihe River watershed, northwest China,” Sustainable Cities and Society 50, 1-9. DOI: 10.1016/j.scs.2019.101658
Takebe, F., Hirota, K., Nodasaka, Y., and Yumoto, I. (2012). “Brevibacillus nitrificans sp. nov., a nitrifying bacterium isolated from a microbiological agent for enhancing microbial digestion in sewage treatment tanks,” International Journal of Systematic and Evolutionary Microbiology 62(9), 2121-2126. DOI: 10.1099/ijs.0.032342-0
Tang, Y., Li, M., Xu, D., Huang, J., and Sun, J. (2018). “Application potential of aerobic denitrifiers coupled with a biostimulant for nitrogen removal from urban river sediment,” Environmental Science and Pollution Research 25(6), 5980-5993. DOI: 10.1007/s11356-017-0903-4
Wang, X., Chen, Z., Shen, J., Kang, J., Zhang, X., Li, J., Zhao, X. (2020). “Effect of carbon source on pollutant removal and microbial community dynamics in treatment of swine wastewater containing antibiotics by aerobic granular sludge,” Chemosphere 260(12), 1-11. DOI: 10.1016/j.chemosphere.2020.127544
Wen, G., Wang. T., Li, K., Wang, H., Wang, J., and Huang, T. (2019). “Aerobic denitrification performance of strain Acinetobacter johnsonii WGX-9 using different natural organic matter as carbon source: Effect of molecular weight,” Water Research 164(11), 1-9. DOI: 10.1016/j.watres.2019.114956
Wen, Y., Ren, Y., Wei, C. H., Li, K. Y., Lin, F. M., and Chen, X. Y. (2010). “A study on nitrogen removal efficiency of Pseudomonas stutzeri strains isolated from an anaerobic/anoxic/oxic wastewater treatment process,” African Journal of Biotechnology 9(6), 869-873.
Wu, J., and He, C. (2012). “The effect of settlement on wastewater carbon source availability based on respirometric and granulometric analysis,” Chemical Engineering Journal 189(1), 250-255. DOI: 10.1016/j.cej.2012.02.066
Xie, F., Thiri, M., and Wang, H. (2021). “Simultaneous heterotrophic nitrification and aerobic denitrification by a novel isolated Pseudomonas mendocina X49,” Bioresource Technology 319(2), 1-7. DOI: 10.1016/j.biortech.2020.124198
Yang, J., Feng, L., Pi, S., Cui, D., Ma, F., Zhao, H., and Li, A. (2020). “A critical review of aerobic denitrification: Insights into the intracellular electron transfer,” Science of The Total Environment 731(44), 1-15. DOI: 10.1016/j.scitotenv.2020.139080
Yang, N., Zhan, G., Li, D., Wang, X., He, X., and Liu, H. (2019). “Complete nitrogen removal and electricity production in Thauera-dominated air-cathode single chambered microbial fuel cell,” Chemical Engineering Journal 356, 506-515. DOI: 10.1016/j.cej.2018.08.161
Yang, X.-P., Zhong, L., and Zhou, L.-X. (2010). “Effect of carbon source and dissolved oxygen on denitrification by aerobic denitrifier Pseudomonas mendocina AD6,” Huan Jing Ke Xue 31(6), 1633-1639.
Yu, G., Peng, H., Fu, Y., Yan, X., Du, C., and Chen, H. (2019). “Enhanced nitrogen removal of low C/N wastewater in constructed wetlands with co-immobilizing solid carbon source and denitrifying bacteria,” Bioresource Technology 208(5), 337-344. DOI: 10.1016/j.biortech.2019.02.043
Zhang, D., Li, W., Huang, X., Qin, W., and Liu, M. (2013). “Removal of ammonium surface water at low temperature by a newly isolated Microbacterium sp. strain SFA13,” Bioresource Technology 137, 147-152. DOI: 10.1016/j.biortech.2013.03.094
Zhang, J., Wu, P., Hao, B., and Yu, Z. (2011). “Heterotrophic nitrification and aerobic denitrification by the bacterium Pseudomonas stutzeri YZN-001,” Bioresource Technology 102(21), 9866-9869. DOI: 10.1016/j.biortech.2011.07.118
Zheng, H., Liu, Y., Sun, G., Gao, X., Zhang, Q., and Liu, Z. (2011). “Denitrification characteristics of a marine origin psychrophilic aerobic denitrifying bacterium,” Journal of Environmental Sciences 23(11), 1888-1893. DOI: 10.1016/S1001-0742(10)60615-8
Zuo, X., Zhang, H., and Yu, J. (2020). “Microbial diversity for the improvement of nitrogen removal in stormwater bioretention cells with three aquatic plants,” Chemosphere 244(4), 1-8. DOI: 10.1016/j.chemosphere.2019.125626
Article submitted: September 9, 2021; Peer review completed: November 30, 2021; Revised version received and accepted: January 16, 2022; Published: February 2, 2022.
DOI: 10.15376/biores.17.2.1972-1987
