Abstract
Extraction of aromatic substances obtained during oxidation of lignin with ionic liquids (ILs)-based molecular oxygen was investigated. It was found that virtually no aromatic substances could be directly extracted with organic solvent until water had been added to the system. The amount of extracted substances increased sharply with an increase in the molar ratio of water to ILs. The distribution constants of aromatic monomers between ILs/water and extracting solvent was obtained by Nernst partition function analysis. The constants fitted well to a second order exponential function model (ExpDec2), which indicated that the activity coefficients of reaction products between ILs and extracting solvent could be tuned by water addition. Among the seven organic solvents tested, ethyl acetate was the most effective, on account of its suitable polarity, while temperature more prominently affected extraction efficiency than time.
Download PDF
Full Article
Tuning Solute Partitioning Coefficients in a Biphasic Ionic Liquid/Water System to Facilitate Extraction of Lignin-Oxidized Aromatics
Jingyang Tian,a Shiyu Fu,a,* Chunhui Zhang,b,* and Lucian A. Lucia a,c
Extraction of aromatic substances obtained during oxidation of lignin with ionic liquids (ILs)-based molecular oxygen was investigated. It was found that virtually no aromatic substances could be directly extracted with organic solvent until water had been added to the system. The amount of extracted substances increased sharply with an increase in the molar ratio of water to ILs. The distribution constants of aromatic monomers between ILs/water and extracting solvent was obtained by Nernst partition function analysis. The constants fitted well to a second order exponential function model (ExpDec2), which indicated that the activity coefficients of reaction products between ILs and extracting solvent could be tuned by water addition. Among the seven organic solvents tested, ethyl acetate was the most effective, on account of its suitable polarity, while temperature more prominently affected extraction efficiency than time.
Keywords: Extraction of aromatics; Ionic liquid; Lignin oxidation; Partitioning coefficients
Contact information: a: State Key Laboratory of Pulp and Paper Engineering, South China University of Technology, Guangzhou, 510640, China; b: School of Light Industry and Food Science, South China University of Technology, Guangzhou 510640, China; c: The Laboratory of Soft Materials & Green Chemistry, Departments of Chemistry, Forest Biomaterials, North Carolina State University, Raleigh, NC, 27695, USA; *Corresponding authors: shyfu@scut.edu.cn; chunhui@scut.edu.cn
INTRODUCTION
After cellulosics and chitin, lignin is the most abundant renewable biomaterial, owing chiefly to its status as an essential structural biopolymer in all woody plants. Kraft lignin, a by-product in the kraft pulping process, is usually burned to provide energy, but its potential to serve as a source of high-value, low molecular weight chemicals (the lignin valorization model) is currently very attractive (Ragauskas et al. 2006). Lignin is an amorphous, polyphenolic polymer arising from the polymerization of three phenylpropanoid monomers: coniferyl, sinapyl, and p-coumaryl alcohol. Although it undergoes harsh reactions during the cooking process, kraft lignin retains its original monomers, and it is possible to produce aromatic aldehydes or acids by oxidation degradation (e.g., commercial vanillin) (Villar et al. 2001).
Lignin oxidation is a traditional method used to generate aromatic aldehydes from lignin (e.g., oxidation with alkaline-nitrobenzene) (Argyropoulos 2001). However, most lignin oxidation reactions require alkaline conditions and are not generally justifiable from a green chemistry perspective because of the low yield and need for by-product remediation.
The largest obstacle, pertaining to the low yield of products, is that lignin does not dissolve in most solvents. Recently, there have been a few methods used to dissolve biomass and isolate lignin (Kim and Lee 2005; Lloyd and Wyman 2005; Mosier et al. 2005). The use of ionic liquids (ILs) as a pretreatment has been promising for biomass transformations (Lee et al. 2009; Fu et al. 2010; Kim et al. 2011; Cox and Ekerdt 2012). Ionic liquids are often considered green because of the advantages they provide in the separation and for reusability. Because of their tunable and powerful solubilizing capacity, low vapor pressure, and tunable physicochemical properties, ILs have received considerable attention for biomass processing. Lignin and lignin models can be degraded in ILs (Binder et al. 2009; Cox and Ekerdt 2012).
When lignin is both dissolved and reacted with ILs, the next important process involves extracting the products from the reaction system. The efficiency of extracting aromatic products from ILs in a biphasic system is important with respect to the yield of aromatic products. The objective of this work was to create a process for extraction of aromatic substances from lignin oxidation products in an ILs-based system by tuning the solvent partitioning coefficients. Seven organic solvents and extraction conditions were used with the intent of being able to reuse the ILs.
EXPERIMENTAL
Materials
Syringic acid (98%), vanillic acid, vanillin, syringaldehyde, and acetosyringone (99%) were purchased from Aladdin (Shanghai, China). 1-butyl-3-methyl imidazolium chloride ([BMIM]Cl, 99%) was purchased from the Lanzhou Institute of Chemical Physics (Lanzhou, China). Sulfuric acid, dimethyl sulfoxide (98%), acetonitrile, acetic acid, ethyl acetate, dichloromethane, toluene, tetrachloromethane, carbon disulfide (99%), petroleum (60-90°C), sodium hydroxide, and n-hexane (97%) were purchased from Kermel. (Tianjin, China).
Methods
Lignin preparation
The kraft pulping process was performed in a 10L D2072/2005 cooker (M/K Systems Inc, America). For each pulping experiment, 1.2 kg of oven-dried Eucalyptus wood chips were treated as follows: 20% alkali charge (based on Na2O), 25% sulfidity, and 4:1 liquor-to-wood ratio. The pulping temperature was raised to 150 °C within 1 h and was maintained at this temperature for 2 h. After the pulping process was complete, the black liquor was collected. The lignin samples were precipitated using sulfuric acid from the excess, acidified black liquor. The supernatant was discarded, and the precipitate was separated using centrifugation (SIGMA 3K15, Laborzentrifugen GmbH, Germany). Then, the lignin was washed using acidified water and separated using centrifugation. Finally, the lignin was washed three times using deionized water and collected after centrifugation at 4000 rpm. After that, the product was dried at 50 °C in a vacuum oven and ground into powder.
Extraction of aromatic products
Seven organic solvents were used for the extraction process based on their degree of solubility and polarity. Water was added before the extraction with organic solvents. Mixtures of organic solvents and water/ILs (1:1, v/v) were shaken at a specified temperature (30 to 80 °C) for a certain time (1 to 8 min) and transferred into a separation funnel. After settling for 10 min, the aqueous phase (water and ILs) and solids were removed. Then, the organic solvent, including aromatic monomer products, was collected and dried by rotary evaporation. The products, such as vanillin, vanillic acid, syringaldehyde, syringic acid, and acetosyringone were obtained. The aqueous phase (water and ILs) was separated by centrifugation at 10,000 rpm. The extraction process was repeated three times for each of the organic solvent samples.
Process of lignin oxidation using ILs
A specific amount of ILs (30 g) and lignin (500 mg) were added into a 50 mL precision reaction kettle (SLM-50, Beijing, China) and heated to 150 °C under a nitrogen atmosphere followed by oxygen sparging. The oxygen partial pressure was 6 MPa, which is the most effective pressure for producing aromatic products. The total reaction time was 120 min, starting with the introduction of O2 through an air pump. The kettle was cooled to 30 °C after the reaction. Ionic liquids and water (aqueous phase) were separated from the reaction products following the procedures described in the section “Extraction of aromatic products”. Water was further removed by vacuum evaporation to recycle the ILs.
Detection of products using high-performance liquid chromatography (HPLC)
The quantitative analysis of aromatic monomers was performed using high-performance liquid chromatography (Lobbes et al. 1999). Six standard solutions (p-hydroxybenzaldehyde, vanillin, vanillic acid, syringaldehyde, syringic acid, and acetosyringone) were diluted to 20-300 mg/L with 5:95(v/v) acetonitrile-water containing 1.5% (v/v) acetic acid and used for the HPLC quantification analysis. The extracted products, such as vanillin, vanillic acid, syringaldehyde, syringic acid, and acetosyringone, were dissolved in a buffer of 5:95 (v/v) acetonitrile-water containing 1.5% (v/v) glacial acetic acid to 250 mL. The detection of oxidation products was carried out using an Agilent 1100 HPLC system with a diode array detector (Agilent 1100 DAD, USA) set at λ= 280 nm. The acetic acid aqueous solution (1.5%, v/v), with the addition of acetic acid (A) and acetonitrile (B), were used as the mobile phase at a rate of 0.8 mL/min. The concentration of aromatic monomer products were calculated based on standard curves.
RESULTS AND DISCUSSION
Effect of Water on Aromatic Product Extraction
A liquid-liquid extraction technique was applied to separate the aromatic substances from the reaction products using organic solvents. In the present work, lignin was dissolved in ILs and oxidized to aromatic monomers. It was conjectured that the aromatic products could be extracted using an extracting organic solvent such as ethyl acetate after oxidation. Organic solvents generally can dissolve aromatic products of similar polarity. However, a very small amount of aromatic substance could be extracted using ethyl acetate. When water was added to the reaction system, it was found that the aromatic products could be extracted from the IL system using organic solvents. The product compounds included vanillic acid, vanillin, acetosyringone, syringic acid, syringaldehyde, and acetosyringone.
With an increase in the molar ratio of water to ILs, the yield of aromatic product extracted substantially increased, as shown in Fig. 1. It was found that the concentration of guaiacyl products was lower than that of syringyl products at the same ratio of water to ILs. This was because eucalyptus lignin has more syringyl than guaiacyl moieties (Bland et al. 1950). The ratio of syringyl to guaiacyl was 6.7/1 when no water was added. The ratio of syringyl to guaiacyl decreased to 3/1 when the ratio of water to ILs was increased to 16/1. Overall, the total yield of monomeric guaiacyl and syringyl products increased by 480% and 215%, respectively, when the ratio of water to ILs increased from 0/1 to 16/1. The addition of water had a more pronounced effect on the extraction efficiency of acidic aromatic substances than for aldehydes and ketones (Fig. 1). The result may be explained according to the partition law (Xu 1984). In general, Nernst defined the partition law (distribution law) within solvents by summarizing numerous two-phase equilibria. The ratio of the concentration of a solute between two non-miscible solvents, i.e., the partition coefficient, is constant at a given temperature.
Fig. 1. Effects of molar ratio of water to ILs on the product concentrations; a) guaiacyl products and b) syringyl products
Investigations on the molecular dynamics of ionic liquids have indicated they contain micelle-like structures in the pure state that may be reactive with water molecules (Canongia Lopes and Pádua 2006; Jiang et al. 2007). The charged imidazolium rings and anions can form a polar network, while the charged-neutral nonpolar groups form a nonpolar domain. Water-induced changes to the IL can accelerate the ion diffusion (Schröder et al. 2000), resulting in the spontaneous self-organization of ionic liquids with high order (Firestone et al. 2002). Water can accelerate the formation of a water-anion-water network and the ordered nonpolar group aggregation. As the amount of water increases, the polar network is continuously broken up by intruding water molecules. Both the ordered micelle structure and water network are formed at the turnover point. Thereafter, the structural organization abates drastically and a loose micellar structure forms for the dominant water-water interactions. Therefore, the inter-structure between the ILs and other substances (products in ionic liquid) depends on the water content. This micelle-like structure of ILs is stable when the water content is low, but it tends to collapse as the water content rises (Hanke and Lynden-Bell 2003; Jiang et al. 2007). This is because of the hydrogen bonding between water and ions (Cammarata et al. 2001; Zhang et al. 2008). Most water molecules in 1-allyl-3-methyl imidazolium chloride ([Amim]Cl) ILs tend to associate to the anions, thereby probably forming symmetrically H-bonded complexes (Wu et al. 2009). Thus, the solubility of aromatic products remaining in ILs after the reaction is reduced by the addition of water, which acts to facilitate the extraction process.
According to Nernst (Xu 1984), the ratio of concentrations of a solute between two non-miscible solvents, i.e., the distribution constant (Λ), is constant at a given temperature, and was calculated using Eq. 1,
(1)
where C1 and C2 represent the solute concentrations in the two non-miscible solvents. Theoretically, the chemical potentials of the solute in the two solvents are equal when equilibrium between phases is achieved at a constant temperature and pressure, as expressed using Eq. 2:
(2)
The chemical potential relevant to the activity (a) is described by Eqs. 3 and 4,
where and
are the standard chemical potentials of solutes in the two solvents.
The thermodynamics distribution constant is defined in Eq. 5 as follows:
(5)
where and
are the activity coefficients of a solute in the two solvents, and
is the constant when the two solvents are immiscible.
Fig. 2. Effect of molar ratio of water to ILs on the vanillin distribution constants; 1) is the distribution constants in ILs, and 2)
is the distribution constants in ethyl acetate
Based on Fig. 1, it appeared that all of the aromatic substances were extracted when the ratio of water to ILs reached 32/1. The effect of the distribution constants on aromatic monomer products in ILs and ethyl acetate at different molar ratios of water to ILs is shown in Fig. 2. All the distribution constants were well fitted by second order exponential decay models (ExpDec2) given by Eq. 6, as follows,
(6)
where x is the molar ratio of water to ILs, A1 and A2 are the pre-exponential factors for the two solutes, y0 is the constant dependent on specific models, and t1 and t2 are exponential constants for the two solutes. The correlation coefficients (R2) and parameters obtained from these models are listed in Table 1. Using Eq. 5 and Eq. 6, the following equation was created (Eq. 7),
(7)
where the proportion of activity coefficients of aromatic products in the two solvents is related to the ratio of water to ILs (x), when holding constant the values of Λ0, A1, A2, t1, t2, and y0.
Actually, the addition of water prior to the extraction process changed the activity coefficients of the reaction products in ILs and organic solvents. As the ratio of water to ILs increased, the proportion of the activity coefficients subsequently decreased . This indicated that the addition of water facilitated the distribution of reaction products into the organic solvent phases (e.g., EtOAc). The extraction solvents chosen here are immiscible with the ILs and water, which is in agreement with the Nernst separation law.
Table 1. Coefficients of Determination (R2) and Parameters Obtained from the Organic Solvents
Based on these models, it may be deduced that there are two main steps involved in the extraction process. As mentioned above, molecular products may bind to the IL’s micelle-like structure after the reaction process. The addition of water destroys the binding behavior because ILs prefer to bind to water through hydrogen bonding. It is the debonding process (t1), followed by the transfer of the reaction products from ILs to ethyl acetate, which can be considered a diffusion process (t2). The exponential constant t1 was one order of magnitude higher than t2, as shown in Table 1, which indicated that the debonding process was the rate limiting step in the extraction process. The extraction process included a series of steps. First, the extraction phase (extracting solvent) was brought within close contact to the sample phase, which comprised the diffusion process. During this process, there is generally no problem for samples in liquid form. Then, the compounds of interest from the extraction products diffuse into the extracting solvent. However, if the compound being extracted to the extracting solvent is in solid form, the energy of interaction between the compounds of interest and the sample substrate (the solid bulk) must be overcome, which is the rate-limiting step in the extraction process (Raynie 2000). Here, similar to solid sample extraction, the products have a strong interaction potential with ILs, which may limit the extraction process.
Effect of Solvent Type on Aromatic Product Extraction
Seven commonly-used organic solvents (e.g., petroleum, n-hexane, carbon disulfide, tetrachloromethane, toluene, dichloromethane, and ethyl acetate) with different polarities were selected to extract aromatic substances from the degradation products of lignin. It was found that four of the organic solvents (i.e., ethyl acetate, dichloromethane, toluene, and tetrachloromethane) could extract aromatic monomers. The concentrations of aromatic monomers extracted from the reaction products by these four solvents at 30 ± 0.5 °C are shown in Table 2. Based on the total concentration of the monomers, the order of extraction ability for the four solvents was ethyl acetate > dichloromethane > toluene > tetrachloromethane. This order is consistent with the order of the solvents’ polarity (ethyl acetate) > dichloromethane > toluene > tetrachloromethane). However, the other three organic solvents with lower polarities did not show any observable extraction abilities in this system. A possible explanation would be that acids normally have a higher polarity than aldehydes and ketones, and a higher polarity may affect the analyte-matrix complex, which lowers the activation energy of the debonding process (Alexandrou et al. 1992). Thus, this increases the accessibility of remote sites to the extraction products, facilitating the transfer of the products to the extraction fluid (Fahmy et al. 1993).
Table 2. Concentrations of Aromatic Monomers Extracted by Solvents (mg/L)
Furthermore, extracting solvents with high polarity can help to cover active sites in the matrix, thereby preventing the rebinding of extracted products to ILs and water (Langenfeld et al. 1994). The extraction process of products from ILs and water using organic solvents is shown in Fig. 3.
After comparison of the total concentration of the aromatic monomers post-extraction, the efficiency of ethyl acetate was approximately 4 and 5 times higher than that of toluene and tetrachloromethane, respectively. On the contrary, the extraction efficiencies for aromatic aldehydes and ketones of ethyl acetate and dichloromethane were very similar. The difference in extraction efficiency of these two solvents was mainly due to the greater ability of ethyl acetate to extract acidic aromatic monomers.
Fig. 3. Cartoon of the proposed extraction process of products from ILs and water using organic solvents; 1) the debonding process of the products from ILs and water, and 2) the diffusion process of the products into extraction solvents
Effect of Process Conditions on Aromatic Product Extraction
The extraction efficiency of organic solvents (e.g., ethyl acetate) was also affected by temperature and extracting time. As shown in Fig. 4a, temperature had a large effect on the concentration of aromatic monomers. Therefore, the concentration increased with an increase of extraction temperature. The total concentration of guaiacyl and syringyl products increased from 31.8 mg/L to 40.5 mg/L and 89.2 mg/L to 99.1 mg/L, respectively, at a temperature range of 30 °C to 80 °C.
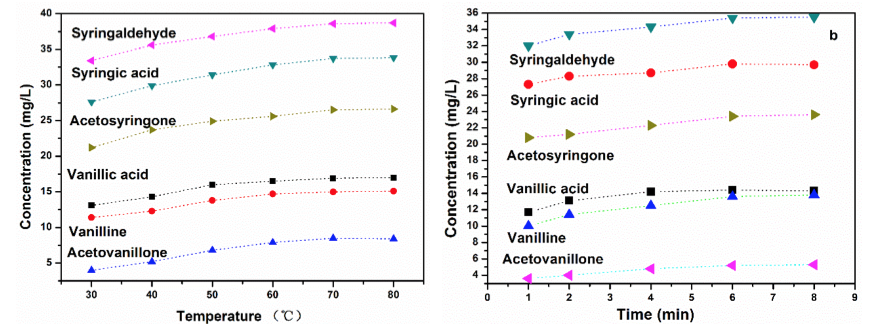
Fig. 4. Effect of the extraction conditions on the product concentrations; a) temperature and b) time
The influence of temperature on extraction could be related to both the equilibrium (solubility) and mass transfer rate (diffusion coefficient) (Hayes 1997). In previous studies, it was found that the rate of solubilization and mass transfer rate increased with increasing extraction temperature (Elbashir et al.2002; Matsuhiro 2006). Unlike solid-liquid extraction, the major advantages of high temperature liquid-liquid extraction (LLE) are the boiling off of solvents and the increased effective contact area between the products in the liquid and extraction of solvent gas.
The effect of extraction time on the efficiency of ethyl acetate concentration was slightly smaller compared to that of extraction temperature, as shown in Fig. 4b. The total concentration of guaiacyl and syringyl products increased from 25.3 mg/L to 33.4 mg/L and 80.1 mg/L to 88.8 mg/L, respectively, when the extraction time was extended from 1 min to 8 min. The extraction time can be reduced dramatically to increase the effective exposed surface area (Zidi et al. 2011) and the extraction can be achieved completely in a short timeframe.
CONCLUSIONS
- The extraction of aromatic monomers from an ionic liquid-based molecular oxygen oxidation system of kraft lignin was significantly improved by adding water due to the changes in partitioning coefficients.
- It was found that the concentration of extractable aromatic monomers increased with an increase of the molar ratio of water to ILs. In other words, the activity coefficients of products in the ILs and organic solvent phases were able to bias the organic extraction.
- Among the seven organic solvents tested, ethyl acetate was the most effective solvent for aromatic monomer extraction.
- Relative to extraction time, increasing temperature had the greatest effect on the concentration of aromatic monomers.
ACKNOWLEDGMENTS
The financial support of the National Natural Science Foundation of China (No. 31170549) is gratefully acknowledged.
REFERENCES CITED
Alexandrou, N., Lawrence, M. J., and Pawliszyn, J. (1992). “Cleanup of complex organic mixtures using supercritical fluids and selective adsorbents,” Analytical Chemistry 64(3), 301-311. DOI: 10.1021/ac00027a011
Argyropoulos, D. S. (2001). Oxidative Delignification Chemistry: Fundamentals and Catalysis, American Chemical Society, Washington, DC.
Binder, J. B., Gray, M. J., White, J. F., Zhang, Z. C., and Holladay, J. E. (2009). “Reactions of lignin model compounds in ionic liquids,” Biomass and Bioenergy 33(9), 1122-1130. DOI: 10.1016/j.biombioe.2009.03.006
Bland, D., Ho, G., and Cohen, W. (1950). “Aromatic aldehydes from the oxidation of some Australian woods and their chromatographic separation,” Australian Journal of Chemistry 3(4), 642-648. DOI: 10.1071/CH9500642
Cammarata, L., Kazarian, S., Salter, P., and Welton, T. (2001). “Molecular states of water in room temperature ionic liquids,” Physical Chemistry Chemical Physics 3(23), 5192-5200. DOI: 10.1039/B106900D
Canongia Lopes, J. N., and Pádua, A. A. (2006). “Nanostructural organization in ionic liquids,” Journal of Physical Chemistry B 110(7), 3330-3335. DOI: 10.1021/jp056006y
Cox, B. J. and Ekerdt, J.G. (2012). “Depolymerization of oak wood lignin under mild conditions using the acidic ionic liquid 1-H-3-methylimidazolium chloride as both solvent and catalyst,” Bioresource Technology 118, 584-588. DOI: 10.1016/j.biortech.2012.05.012
Elbashir, N. O., Al-Zahrani, S. M., and Abdul Mutalib, M. I. (2002). “A method of predicting effective solvent extraction parameters for recycling of used lubricating oils,” Chemical Engineering and Processing 41(1), 765-769. DOI: 10.1016/S0255-2701(02)00006-5
Fahmy, T. M., Paulaitis, M. E., Johnson, D. M., and McNally, M. E. P. (1993). “Modifier effects in the supercritical fluid extraction of solutes from clay, soil, and plant materials,” Analytical Chemistry65(10), 1462-1469. DOI: 10.1021/ac00058a026
Firestone, M. A., Dzielawa, J. A., Zapol, P., Curtiss, L. A., Seifert, S., and Dietz, M. L. (2002). “Lyotropic liquid-crystalline gel formation in a room-temperature ionic liquid,” Langmuir 18(20), 7258-7260. DOI: 10.1021/la0259499
Fu, D., Mazza, G., and Tamaki, Y. (2010). “Lignin extraction from straw by ionic liquids and enzymatic hydrolysis of the cellulosic residues,” Journal of Agricultural and Food Chemistry 58(5), 2915-2922. DOI: 10.1021/jf903616y
Hanke, C., and Lynden-Bell, R. (2003). “A simulation study of water-dialkylimidazolium ionic liquid mixtures,” Journal of Physical Chemistry B 107(39), 10873-10878. DOI: 10.1021/jp034221d
Hayes, D. G. (1997). “Mechanism of protein extraction from the solid state by water-in-oil microemulsions,” Biotechnology and Bioengineering 53(6), 583-593. DOI:10.1002/(SICI)1097-0290(19970320)53:6<583::AID-BIT6>3.0.CO;2-I
Jiang, W., Wang, Y., and Voth, G. A. (2007). “Molecular dynamics simulation of nanostructural organization in ionic liquid/water mixtures,” Journal of Physical Chemistry B 111(18), 4812-4818. DOI: 10.1021/jp067142l
Kim, J. Y., Shin, E. J., Eom, I. Y., Won, K., Kim, Y. H., Choi, D., Choi, I. G., and Choi, J. W. (2011). “Structural features of lignin macromolecules extracted with ionic liquid from poplar wood,” Bioresource Technology 102(19), 9020-9025. DOI: 10.1016/j.biortech.2011.07.081
Kim, T. H. and Lee, Y. Y. (2005). “Pretreatment and fractionation of corn stover by ammonia recycle percolation process,” Bioresource Technology 96(18), 2007-2013. DOI: 10.1016/j.biortech.2005.01.015
Langenfeld, J. J., Hawthorne, S. B., Miller, D. J., and Pawliszyn, J. (1994). “Role of modifiers for analytical-scale supercritical fluid extraction of environmental samples,” Analytical Chemistry 66(6), 909-916. DOI: 10.1021/ac00078a024
Lee, S. H., Doherty, T. V., Linhardt, R. J., and Dordick, J. S. (2009). “Ionic liquid-mediated selective extraction of lignin from wood leading to enhanced enzymatic cellulose hydrolysis,” Biotechnology and Bioengineering 102(5), 1368-1376. DOI: 10.1002/bit.22179
Lloyd, T. A. and Wyman, C. E. (2005). “Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis of the remaining solids,” Bioresource Technology96(18), 1967-1977. DOI: 10.1016/j.biortech.2005.01.011
Lobbes, J. R. M., Fitznar, H. P., and Kattner, G. (1999). “High-performance liquid chromatography of lignin-derived phenols in environmental samples with diode array detection,” Analytical chemistry71(15), 3008-3012.
Matsuhiro, B., Lillo, L. E., Sáenz, C., Urzúa, C. C., and Zárate, O. (2006). “Chemical characterization of the mucilage from fruits of Opuntia ficus indica,” Carbohydrate Polymers 63(2), 263-267. DOI: 10.1016/j.carbpol.2005.08.062
Mosier, N., Hendrickson, R., Ho, N., Sedlak, M., and Ladisch, M. R. (2005). “Optimization of pH controlled liquid hot water pretreatment of corn stover,” Bioresource Technology 96(18), 1986-1993. DOI: 10.1016/j.biortech.2005.01.013
Ragauskas, A. J., Williams, C. K., Davison, B. H., Britovsek, G., Cairney, J., Eckert, C. A., Frederick, W. J., Hallett, J. P., Leak, D. J., and Liotta, C. L. (2006). “The path forward for biofuels and biomaterials,” Science 311(5760), 484-489. DOI: 10.1126/science.1114736
Raynie, D. E. (2000). “Extraction,” in: Encyclopedia of Separation Science, Cooke, M. (ed.), Academic Press, Oxford, New York.
Schröder, U., Wadhawan, J. D., Compton, R. G., Marken, F., Suarez, P. A. Z., Consorti, C. S., de Souza, R. F., and Dupont, J. (2000). “Water-induced accelerated ion diffusion: Voltammetric studies in 1-methyl-3-[2, 6-(S)-dimethylocten-2-yl] imidazolium tetrafluoroborate, 1-butyl-3-methylimidazolium tetrafluoroborate and hexafluorophosphate ionic liquids,” New Journal of Chemistry 24(12), 1009-1015. DOI: 10.1039/B007172M
Villar, J. C., Caperos, A., and Garcia-Ochoa, F. (2001). “Oxidation of hardwood kraft-lignin to phenolic derivatives with oxygen as oxidant,” Journal of Wood Chemistry and Technology 35(3), 245-255. DOI: 10.1007/s002260100089
Wu, B., Liu, Y., Zhang, Y., and Wang, H. (2009). “Probing intermolecular interactions in ionic liquid-water mixtures by near-infrared spectroscopy,” Chemistry – A European Journal 15(28), 6889-6893. DOI: 10.1002/chem.200802742
Xu, G. (1984). Principles of Solvent Extraction, Shanghai Science and Technology Press, Shaghai, China.
Zhang, L., Xu, Z., Wang, Y., and Li, H. (2008). “Prediction of the solvation and structural properties of ionic liquids in water by two-dimensional correlation spectroscopy,” Journal of Physical Chemistry B112(20), 6411-6419. DOI: 10.1021/jp8001349
Zidi, C., Tayeb, R., Boukhili, N., and Dhahbi, M. (2011). “A supported liquid membrane system for efficient extraction of vanillin from aqueous solutions,” Separation and Purification Technology 82, 36-42. DOI:10.1016/j.seppur.2011.08.013
Article submitted: March 11, 2015; Peer review completed: May 8, 2015; Revised version received and accepted: May 13, 2015; Published: May 19, 2015.
DOI: 10.15376/biores.10.3.4099-4109
