Abstract
The pulp and paper (P&P) industry worldwide has achieved substantial progress in treating both process water and wastewater, thus limiting the discharge of pollutants to receiving waters. This review covers a variety of wastewater treatment methods, which provide P&P companies with cost-effective ways to limit the release of biological or chemical oxygen demand, toxicity, solids, color, and other indicators of pollutant load. Conventional wastewater treatment systems, often comprising primary clarification followed by activated sludge processes, have been widely implemented in the P&P industry. Higher levels of pollutant removal can be achieved by supplementary treatments, which can include anaerobic biological stages, advanced oxidation processes, bioreactors, and membrane filtration technologies. Improvements in the performance of wastewater treatment operations often can be achieved by effective measurement technologies and by strategic addition of agents including coagulants, flocculants, filter aids, and optimized fungal or bacterial cultures. In addition, P&P mills can implement upstream process changes, including dissolved-air-flotation (DAF) systems, filtration save-alls, and kidney-like operations to purify process waters, thus reducing the load of pollutants and the volume of effluent being discharged to end-of-pipe wastewater treatment plants.
Download PDF
Full Article
Wastewater Treatment and Reclamation: A Review of Pulp and Paper Industry Practices and Opportunities
Martin A. Hubbe,*,a Jeremy R. Metts,a,b Daphne Hermosilla,c M. Angeles Blanco,d Laleh Yerushalmi,e Fariborz Haghighat,e Petra Lindholm-Lehto,f Zahra Khodaparast,g Mohammadreza Kamali,h and Allan Elliott i
The pulp and paper (P&P) industry worldwide has achieved substantial progress in treating both process water and wastewater, thus limiting the discharge of pollutants to receiving waters. This review covers a variety of wastewater treatment methods, which provide P&P companies with cost-effective ways to limit the release of biological or chemical oxygen demand, toxicity, solids, color, and other indicators of pollutant load. Conventional wastewater treatment systems, often comprising primary clarification followed by activated sludge processes, have been widely implemented in the P&P industry. Higher levels of pollutant removal can be achieved by supplementary treatments, which can include anaerobic biological stages, advanced oxidation processes, bioreactors, and membrane filtration technologies. Improvements in the performance of wastewater treatment operations often can be achieved by effective measurement technologies and by strategic addition of agents including coagulants, flocculants, filter aids, and optimized fungal or bacterial cultures. In addition, P&P mills can implement upstream process changes, including dissolved-air-flotation (DAF) systems, filtration save-alls, and kidney-like operations to purify process waters, thus reducing the load of pollutants and the volume of effluent being discharged to end-of-pipe wastewater treatment plants.
Keywords: Wastewater treatment; Pulp and paper manufacturing; Advanced oxidation; Membrane technologies; Clarification; Activated sludge
Contact information: a: North Carolina State University, Dept. of Forest Biomaterials, Campus Box 8005, Raleigh, NC 29695-8005; b: WestRock Company, Water and Waste Treatment, 600 S 8th St, Fernandina Beach, FL 32034; c: Department of Agricultural and Forestry Engineering, University of Valladolid, Campus Duques de Soria, E-42004 Soria, Spain; d: Complutense University of Madrid, Department of Chemical Engineering, Ingn. Quim, Facultad de Ciencias Químicas, Ciudad Universitaria s/n,S-N, E-28040 Madrid, Spain; e: Concordia University, Dept. Bldg. Civil & Environm. Engn., 1455 Maisonneuve Blvd, West Montreal, PQ H3G 1M8, Canada; f: University of Jyväskylä, Dept. Chem., Box 35, FI-40014 Jyväskylä, Finland; g: Department of Biology, Center for Environmental and Marine Studies, CESAM, University of Aveiro, 3810-193 Aveiro, Portugal; h: Department of Environment and Planning, Center for Environmental and Marine Studies, CESAM, Aveiro & Department of Materials and Ceramics, Institute of Materials, CICECO, University of Aveiro, 3810-193 Aveiro, Portugal; i: FPInnovations, 570 St. Jean Blvd., Pointe Claire, PQ H9R 3J9, Canada; * Corresponding author: hubbe@ncsu.edu
INTRODUCTION
The pulp and paper (P&P) industry occupies a challenging position with respect to the natural environment. On the positive side, the industry is based on the usage of renewable, photosynthetic resources. On the other hand, the industry discharges huge quantities of aqueous effluents. Large volumes (up to 70 m3) of wastewater are generated for each metric ton of paper produced, depending on the nature of raw material, the type of finished product, and the extent of water reuse (Rintala and Puhakka 1994; Latorre et al. 2007). The P&P industry uses about 70% of its massive water intake as process water. Reducing water consumption, by increasing internal water recirculation after the implementation of internal cleaning processes, saves money and also decreases the use of scarce environmental resources. The industry has reduced water consumption over the past 20 years by nearly a half and over the past 30 years by an impressive 95% per tonne of paper (Blanco et al. 2004). Since the main unit operations associated with pulping and papermaking are carried out in aqueous media, the application of various chemical additives can considerably alter the properties of the produced effluents, making it harmful for the receiving environments. The total closure of the water circuits is limited by the accumulation of contaminants in the process water, which can give rise to corrosion, deposits, and odors and alter the runnability of the paper machine and the quality of the final product. If further closure is required, then the effluent has to be extensively treated so that it can be used again in the process.
The main pollutants discharged to water streams nowadays are solids and organic matter. In general, pulp and paper mill effluents contain a complex mixture of various classes of organic compounds, such as degradation products of carbohydrates, lignin, and extractives (Uğurlu et al. 2008; Uğurlu and Karaoğlu 2009). Polluting effluents are formed in wood preparation, pulping, pulp washing, screening, paper machine, and coating operations, and especially in bleaching operations (Ali and Shreekrishnan 2001; Pokhrel and Viraraghavan 2004; Savant et al. 2006). Clearly, the properties of wastewater from various process stages depend on the type of process and raw material, the recirculation of the effluent, and the amount of water used (Pokhrel and Viraraghavan 2004). The effluents contain high values of biochemical oxygen demand (BOD), chemical oxygen demand (COD), and chlorinated chemicals that are collectively termed as absorbable organic halides (AOX). While AOX content is generally proportional to the chlorine consumption in bleaching (Savant et al. 2006), this emission has been reduced by over 80% since 1990 (Friere et al. 2003). The ratio of BOD to COD is a particularly useful quantity because it represents the fraction of organic compounds in the effluent that are easy to degrade (McCubbin and Folke 1993). Chemical pulping processes have been reported to generate more than 40% of poorly biodegradable organics within the total organic matter of the effluent (Dahlman et al. 1995).
If the wastewater were to be discharged without treatment, then the adverse effects would include depletion of dissolved oxygen, toxic effects on fish and other aquatic organisms, and unacceptable changes to color, turbidity, temperature, and solids content of the receiving waters. Issues related to toxicity have been the focus of much attention (Walden and Howard 1981; Owens 1991; Kamali and Khodaparst 2015). For the protection of the environment, and also to satisfy legal requirements, it is necessary for the industry to remove harmful materials from wastewater before it is discharged to the environment. For example, in the US the establishment of “cluster rules” for regulation of discharges from P&P facilities (Vice et al. 1996; Swann 1998; Vice and Carroll 1998) provided major incentive for reductions in discharged pollutants. In China part of the solution to the same issues has been to replace older manufacturing facilities with new capacity that is better suited to the minimization of wastewater impacts (Zhang et al. 2012).
In Canada, the discharge of wastewaters from pulp and paper mills into water frequented by fish is controlled by the Pulp and Paper Effluent Regulations (PPER). These regulations aim at protecting water quality that sustains fish, fish habitat and the use of fisheries resources. The PPER set limits on the amounts of total suspended solids (TSS) and biochemical oxygen demand (BOD), and prohibit the discharge of effluents that display acute lethality to fish (Environment and Climate Change Canada 2016). Secondary treatment for the biological break down of biodegradable material and toxic components, resulting in reductions in biochemical oxygen demand, toxicity and total suspended solids, became common by 1996 following the establishment of current regulatory limits in 1992. Environmental regulation of pulp and paper mills also includes the Pulp and Paper Mill Effluent Chlorinated Dioxins and Furans Regulations, issued under the Canadian Environmental Protection Act (CEPA), to control the level of dioxin and furan in the effluent of mills using a chlorine bleaching process. Additionally, Pulp and Paper Mill Defoamer and Wood Chip Regulations, also issued under the CEPA, govern the use of defoamers containing dibenzofuran or dibenzo-para-dioxin and wood chips from wood that was treated with polychlorinated phenols (Environmental Compliance Insider 2010).
In Europe, the Integrated Pollution Prevention and Control Directive sets permit conditions based on the use of best available technologies published in the BREF documents (BREF 2015), and voluntary agreements through the Confederation of European Paper Industries (CEPI) further enhanced the sustainability behavior.
Although the focus of this article is on the treatment of wastewater, it is important to keep in mind that purification of the effluent water is not the only goal. A wider goal is to achieve a sustainable P&P production in which environmental performance and competitiveness go hand in hand to supply a wide range of useful products. For efficient water use and treatment, a sustainable integrated water management is motivated by multiple drivers including: legislation; protection against water-related risks in water stress regions; limits in sensitive water bodies; cost of water; business opportunities; general expectation; proudness of the company; and corporate image, etc. Within this approach the concept of water-fit-for-use is becoming increasingly important; therefore attention will be paid in this review to which approaches tend to be cost-effective and sufficient to meet the different water quality demands.
Much of the content of the present article can be regarded as an overview of “best practices” in terms of wastewater and process water treatment technologies that have been implemented in the P&P industry. The article aims to provide explanations not only of practices that have become widely used in the industry, but also of practices that hold the potential of greater effectiveness of wastewater treatment in the future within the P&P industry. Ideally, wastewater treatment operations ought to be regarded as highly reliable, highly effective, cost-competitive, and evidence of good stewardship on the part of industry leaders.
The subject of treating the effluent and process water from P&P manufacturing facilities has been considered earlier, with emphasis in a number of key areas. The toxicity of such wastewater and its potential effects on the environment have been reviewed by Ali and Sreekrishnan (2001). Review articles by Pokhrel and Viraraghavan (2004), Zhao et al. (2014), and Kamali and Khodaparast (2015) provide background for different types of treatment facilities. Bajpai and Bajpai (1994) reviewed strategies for the removal of color from P&P mill wastewater. Various articles have focused on the performance and “best available technology” for treating P&P mill wastewater in different regions (Lescot and Jappinen 1994; Hammar and Rydholm 1972; Gehm 1973; Rajvaidya and Markandey 1998; European Commission 2001; Demel et al. 2003; Tiku et al. 2007; Menezes et al. 2010; Zhu et al. 2012; BREF 2015). Regarding advanced oxidation systems applicable as a primary or a tertiary treatment, the review by Bautista et al. (2008) gives excellent descriptions of Fenton (iron/ hydrogen peroxide) wastewater treatment systems, though P&P applications are not considered. Hermosilla et al. (2015) reviewed the implementation and development of advanced oxidation systems implemented for P&P mill effluents. Buyukkamaci and Koken (2010) focus on the economic aspects of different wastewater treatment options available for P&P mill effluent. Various authors have considered options of treating the process water so that it can be reused within the normal operations of papermaking (Tenno and Paulapuro 1999; Verenich et al. 2000; Webb 2002; Nuortila-Jokinen et al. 2004; Hubbe 2007c; Ordóñez et al. 2010; Mauchauffee et al. 2012; Saif et al. 2013). Wider review articles and books dealing with general aspects of wastewater treatment have appeared (Scott and Ollis 1995; Thompson et al. 2001; Pokhrel and Viraraghavan 2004; Singh et al. 2012; Riffat 2013; Ranade 2014; Spellman 2014; Zhao et al. 2014; Hopcroft 2015; Blanco et al. 2016; Fatta-Kassinos et al. 2016). Finally, Ince et al. (2011) reviewed strategies that can be implemented in the P&P industry to prevent pollution.
Criteria of Successful Treatment
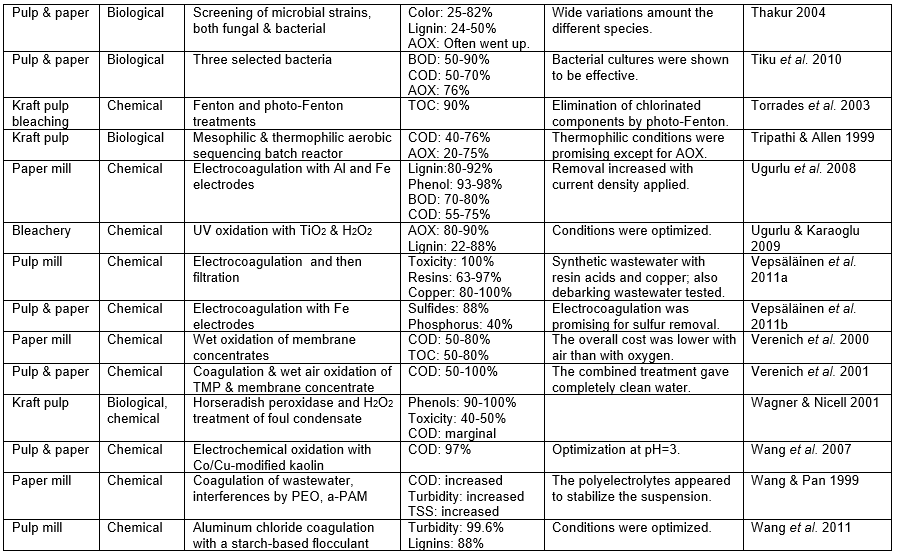
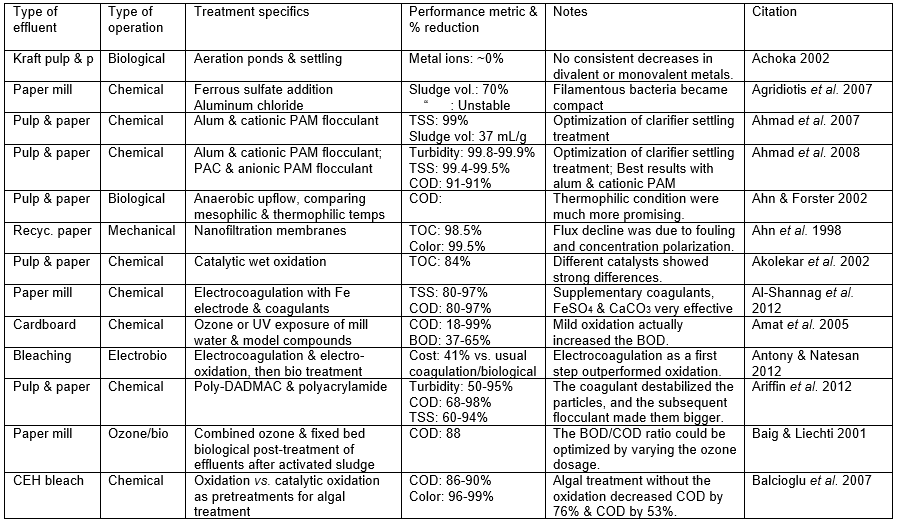
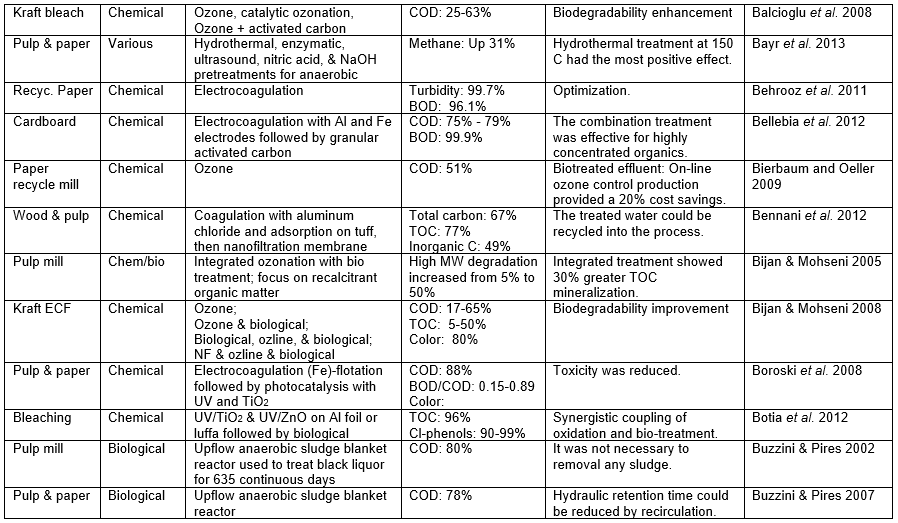
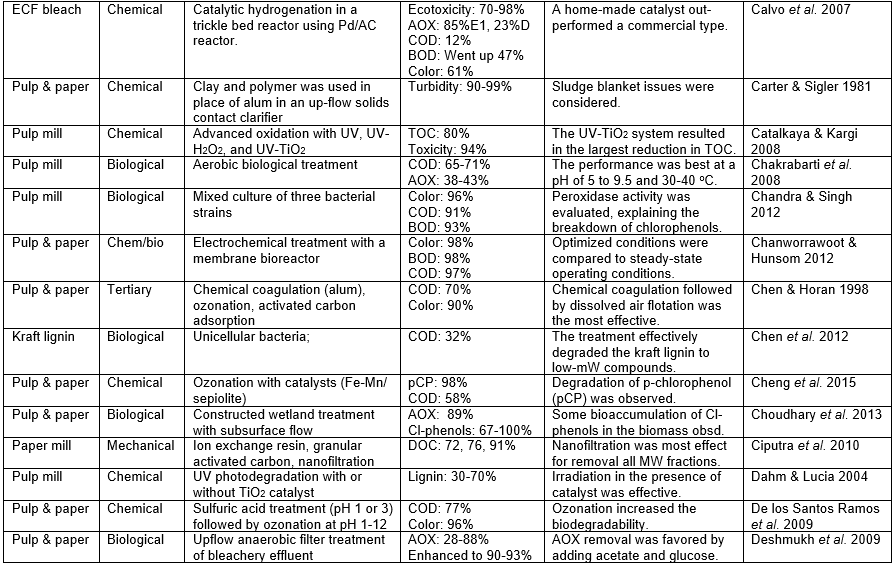
Those who set out to remove pollutants from the wastewater of P&P manufacturing facilities face a variety of challenges, the difficulty of which will depend a lot on the nature of the wastewater that needs to be treated. The challenge also will depend on the required purity to be achieved in the treated water. To begin with, any successful wastewater management system needs to clearly define water demands and water qualities for different uses in order to optimize water reuse based on the principle of water-fit-for-use, where water is treated only to the required quality. Second, the final effluent must be treated to accomplish the discharge limits. Table A in the appendix of this article lists the percentage removal values reported in a wide range of studies devoted to the treatment of P&P wastewater. In addition, it is important to achieve stable and reliable operations of both the pulp/papermaking process and the wastewater treatment system.
Settling or flotation rate, speed of processing
The rate of separation of wastewater solids in a waste treatment plant can limit the throughput of settling or flotation units. On the other hand, if methods can be developed and optimized to accelerate separation, then the size and capital costs of the plant might be reduced. Various researchers have shown that separation rates can be sped up by chemical treatments (Leitz 1993; Al-Jasser 2009). Furthermore, the use of some hybrid chemicals can promote the removal of a higher percentage of organic matter or specific inorganic material (e.g. silica) with the solids (Miranda et al. 2015). The operating conditions of the biological treatment plant can affect settling rates (Avella et al. 2011). Elliott and Mahmood (2012) found that biological sludge digestion could be optimized to speed up the process of handling the sludge.
Quantity of sludge
In recent years there has been increasing realization that, in addition to removing pollutants from the wastewater, it is important to minimize the amount of solid waste sludge that is generated as a byproduct of the treatment operations (Leitz 1993; Tripathi and Allen 1999; Alvares et al. 2001; Mahmood and Elliott 2006; Chanworrawoot and Hunsom 2012). One way to reduce sludge is to place increased emphasis on anaerobic biological treatment as an early step in the treatment program (Ashrafi et al. 2015; Kamali and Khodaparast 2015; Ordóñez et al. 2010). Alternatively, researchers have shown that thermophilic (higher temperature) biological treatment systems can achieve better efficiency and lower sludge amounts even under aerated conditions (LaPara and Alleman 1999; Skouteris et al. 2012). Also, by carrying out the treatment in stages, much of the initial sludge volume can be consumed by other types of organisms living within different zones within wastewater treatment operations (Lee and Welander 1996). Optimum sludge dewatering is also important, as well as the maximum removal of dissolved and colloidal material to keep the water circuit relatively clean. In conjunction with the development of technologies to minimize sludge production, avenues have been explored to utilize the generated sludge for beneficial purposes as opposed to just bearing expenses for treatment and removal of waste (Elliott and Mahmood 2005).
Greenhouse gas emissions
While the main goal of wastewater treatment is to remove pollutants from wastewater, one of the secondary goals, as we look to the future, is to minimize the production of other pollutants, such as gaseous emissions. Ashrafi et al. (2015) emphasized impacts of different wastewater-treatment options on greenhouse gas (GHG) emissions. In addition to the evolution of CO2, methane, and other GHG components from the waste materials, one also needs to be concerned about GHGs associated with the usage of electrical energy during the treatment of wastewater (Baig and Liechti 2001; Ashrafi et al. 2015). For instance, though improved efficiencies and treatment rates often can be achieved with modern reactors designed for biological wastewater treatment, such operations sometimes may require more electrical energy in comparison to conventional treatment systems (Castillo and Vivas 1996; Buzzini and Pires 2002; El-Ashtoukhy et al. 2009). According to Kamali and Khodaparast (2015), a goal of saving electrical energy costs may be responsible for a more widespread use of mesophilic (medium temperature) rather than thermophilic (higher temperature) conditions in biological treatment plants, even though the latter have been reported to achieve higher performance in many cases. In fact, some of the generated effluents already have a relatively high temperature, so they can already be handled in the thermophilic range without further chilling the effluent. Anaerobic wastewater treatment operations are often regarded favorably by environmental advocates because they actively generate methane that can be retrieved and used in place of other combustible fuels (Maat and Habets 1987; Tabatabaei et al. 2010; Saha et al. 2011; Meyer and Edwards 2014; Sanusi and Menzes 2014).
GHG emissions by on-site and off-site processes in a typical industrial wastewater treatment plant that used aerobic, anaerobic, and hybrid anaerobic/aerobic treatment processes were studied by Bani Shahabadi et al. (2009, 2010) and Yerushalmi et al. (2011). Yerushalmi et al. (2013) and Ashrafi et al. (2013, 2014, 2015) estimated GHG emission and energy consumption by wastewater treatment plants of the P&P industry and determined the contribution of individual processes to the on-site and off-site GHG emissions. On-site GHG emissions are due to liquid and solid treatment processes as well as biogas and fossil fuel combustion for energy generation. Off-site GHG emissions are related to the production of electricity for plant, production, and transportation of fuel and chemicals for on-site use, degradation of remaining constituents in the effluent of wastewater treatment plant, as well as transportation and disposal of solids. The on-site biological treatment processes were shown to make the highest contribution to GHG emissions in the aerobic treatment system, while the higher usage of chemicals in anaerobic and hybrid treatment systems resulted in higher GHG emission from material production and transportation in those treatment systems. The recovery of biogas, generated during anaerobic treatment processes or anaerobic sludge digestion, and its reuse as fuel were shown to have a remarkable impact on GHG emissions and reduction of the overall emissions. Biogas recovery and reuse as fuel can cover the total energy needs of the treatment plants for aeration, heating, and electricity, considerably reducing the net GHG emissions in the treatment plants. Heating of an anaerobic digester was identified as a major energy-demanding process, suggesting that solid digestion at lower temperatures should be exercised to reduce the associated GHG emissions.
Based on the results of studies published by the above investigators, the manufacturing of material for on-site consumption should use methods that generate lower amounts of GHGs, thus reducing upstream GHG emissions attributed to the wastewater treatment plant. Electricity and fossil fuels should also be generated and handled by more efficient methods in order to decrease the overall GHG emissions of treatment plants.
Another strategy for GHG emissions reduction is to use alternative nutrient removal processes such as the anaerobic Anammox process (Greenfield and Batstone 2005), which removes nitrogen with a lower consumption of energy and lower carbon use. In this process, the reduced aeration energy consumption reduces GHG production related to energy demands of the treatment plant, while the extra available carbon can be converted to methane via anaerobic processes and be used as a source of energy for on-site consumption, further reducing GHG emissions. The use of hybrid anaerobic/aerobic systems for wastewater treatment under optimized operating conditions was shown to be the most appropriate option for pulp-and-paper industry to obtain a satisfactory treatment performance, reduce GHG emission and energy costs, and meet environmental regulations.
Operating costs justified
Closely related to the topic of energy usage is the topic of operating costs. Hammar and Rydholm (1972), when reviewing the state of the art of wastewater treatment in the 1970s, complained about the relatively high cost of treatments capable of removing biodegradable organic pollutants. Although aerated biological treatment has long been regarded as a highly reliable and predominant approach utilized within the P&P industry, the pumping of the air represents one of the major operating costs of such systems (Maat and Habets 1987). Advanced oxidation systems, which have the potential to remove some of the most challenging toxic or highly colored components from P&P mill effluents, also have been criticized for their high operating costs (Heinzle et al. 1992; Byukkamaci and Koken 2010). Membrane treatments are also very efficient, and they are becoming more popular due to substantial reductions in membrane prices.
Quantification of Wastewater Load
Before considering different approaches used for the minimization or removal of contaminants from wastewater, the subsections below address some of the most common criteria for assessing the quality of treated water (McKeown and Gellman 1976; Owens 1991). Standard methods for the most widely used tests have been established in the US by the American Public Health Association (APHA 2001, 2005). In Europe, the corresponding standards are in the EU Directive 91/271/EEC of the Environmental Protection Agency.
Oxygen demand
From an environmental standpoint, biodegradable substances, which are quantified in terms of the biological oxygen demand (BOD) test, are generally regarded with favor. However, if such materials were to be discharged into rivers, lakes, and estuaries at excessive levels, the resulting biological metabolism would sometimes be sufficient to consume all or most of the available dissolved oxygen, leading to the death of fish and other aquatic life (Taylor et al. 1996; Chhonkar et al. 2000). Such impacts are often quantified by measuring the BOD. This quantity is evaluated by sealing a volume of water to be tested with a known amount of gaseous oxygen; the level of oxygen gas in the container is measured five days later (BOD5 test), after microbes have had a chance to reproduce and to consume the decomposable organic materials (APHA 2016, Standard Method 5210). As can become evident from inspection of the contents of Table A (see Appendix), the BOD test is among the most frequently used methods to characterize untreated and treated wastewaters.
It has been recently shown that microbial consortia that have become acclimated to the degradation of lignin-related compounds can reduce the BOD levels in the pulp-and-paper wastewaters below levels that are normally achievable by using indigenous microorganisms (Ordaz-Diaz et al. 2014). BOD levels also have been found to correlate with the pulping yield and with the color in the treated wastewater from P&P mill facilities (Bajpai and Bajpai 1994).
The oxidizable matter in a wastewater also can be measured by the chemical oxygen demand (COD) test (APHA 2001). The COD test takes advantage of the relatively rapid reaction of potassium dichromate with the oxidizable materials in the water sample. The results from BOD and COD tests will be different if the wastewater sample contains difficult-to-biodegrade oxidizable compounds or toxic materials that inhibit biological activities. Thus the ratio of BOD to COD is often reported as a way to quantify the relative recalcitrance of organic compounds in wastewater (Yeber et al. 1999b; Baig and Liechti 2001; Kreetachat et al. 2007; Ghaly et al. 2011). This value, which is often in a range between about 0.05 and 0.5 for P&P wastewaters, will help to determine what treatment strategy is appropriate.
Color
The color of an organic chemical compound, i.e. its ability to absorb visible light, generally requires a sufficiently long sequence of single and double carbon-carbon bonds, i.e. a conjugated structure (Adachi and Nagao 2001). The lignin component of wood is highly susceptible to becoming strongly colored due to its high content of aromatic rings, in addition to other unsaturated structures (Sarkanen 1971). Thus, although natural lignin present in native wood generally has a light color, deep coloration develops during kraft pulping. Indeed, the appearance of “black liquor,” the spent alkaline solution left over after kraft pulping, can be attributed to such compounds. As noted by Bajpai and Bajpai (1994), highly colored compounds in typical pulping and bleaching wastewaters often are resistant to biodegradation and removal during treatment operations. The sources and types of colored compounds that develop during pulping and papermaking have been reviewed (NCASI 2011). Measures to remove color from the effluent of P&P mills have been reviewed and reported (Bajpai and Bajpai 1994; Garg and Tripathi 2011; Garg et al. 2012). Langergraber et al. (2004) discussed the evaluation of color in such wastewater by instrumental methods.
Total suspended solids (TSS) and turbidity
Suspended matter in wastewater has the potential to obstruct the passage of light and interfere with the respiration of aquatic organisms. These contaminants are commonly quantified as the total suspended solids (TSS) and turbidity. The TSS values are determined by weighing the solid matter filtered from a water sample onto a tared piece of standard filter paper composed of glass fibers (APHA 2016, Standard Method 2540). Some of the most effective measures for reducing TSS are separation of solids by sedimentation, flotation, or membrane techniques. Treatment of the raw wastewater with coagulants and flocculants, at optimized dosages, can promote the speed and completeness of such separations.
Toxicity
Toxic compounds may be present in wastewaters of pulping facilities, but they are not common in papermaking processes. They are a matter of concern (Lugowski 1991; Owens 1991; Ali and Sreekrishnan 2001) not only because of their toxic effects on the environment (Mahmood-Khan et al. 2013), but also they may disrupt the beneficial biological activities during wastewater treatment operations (Freire et al. 2000; Liu et al. 2011). Discharged effluents having toxic content can damage the reproductivity of aquatic species (Costigan et al. 2012; Waye et al. 2014). According to Magnus et al. (2000) long-chain fatty acids, generated during pulping operations, constitute one of the primary sources of toxicity coming from pulping operations. Chlorinated organic compounds, and dioxin in particular, have been noted for their toxic and often carcinogenic effects (Safe 1990; Wiegand et al. 2006). The presence of toxic substances (mainly AOX and dioxins) in effluents from pulp bleaching has been reduced by 95% down to a level of ≤0.1 KgAOX·t-1 of pulp since 1990, mainly thanks to the replacement of chlorine gas by elemental-chlorine-free and total-chlorine-free chemicals in bleaching processes (Aspapel 2011; CEPI 2013). Kostamo and Kukkonen (2003) reported that secondary wastewater treatment with activated sludge can be effective for the removal of toxicity.
Recalcitrant, persistent compounds
Organic compounds that resist biodegradation are of great concern because they can remain in the wastewater even after it has been treated by widely used aerobic biological processes that employ activated sludge (Gergov 1988; Scott and Ollis 1995; Magnus et al. 2000; Contreras Lopez 2003) or with anaerobic biological wastewater treatment (Korczak et al. 1991; Sierraalvarez et al. 1991). Eriksson and Kolar (1985) employed isotopic labeling to explore the mechanisms involved in the biodegradation of such compounds. Efforts to eliminate such compounds by alternative treatment methods such as advanced oxidation processes (AOPs) have been widely reported (Rodriguez et al. 1999; Pokhrel and Viraraghavan 2004; Eskelinen et al. 2010; Merayo et al. 2013; Lindholm-Lehto et al. 2015). AOPs can be employed either as pretreatment to increase the biodegradability of the organic solids or as post-treatment to remove the remaining organic content. Inoculation of wastewater treatment systems with lignin-degrading fungal species has shown promise in breaking down the recalcitrant compounds and reducing the toxicity levels (Pellinen and Joyce 1990; Thakur 2004), in spite of the inherent issues arising from such methods such as low tolerance of the fungal strains for variations in pH.
Sulfur content
Sulfur-containing compounds in aqueous wastewaters are considered a problem because of possible action of sulfate-reducing bacteria in the environment. Anaerobic conditions can lead to the generation of toxic H2S gas, as well as other sulfur-containing compounds that contribute to unpleasant odors (Smet et al. 1998; Devai and DeLaune 1999; Goyer and Lavoie 2001). The removal of sulfur from pulp-and-paper wastewaters will be discussed in a later section (Lens et al. 1998; Janssen et al. 2009).
Salt, electrical conductivity
Inorganic salts and ionic compounds such as sodium chloride in industrial wastewaters can significantly increase the salinity and electrical conductivity of the receiving waters (Gehm 1973; Achoka 2002). Upset conditions in a P&P mill, causing the release of alkaline pulping liquors, can be detected by conductivity measurements (Kemeny and Banerjee 1997). Sulfates may also be limited by regulation or contract when treated effluents are discharged.
PULP AND PAPER WASTEWATER
The closure degree of P&P mill water circuits depends on the type of product. While water circuits can be highly closed for brown grades (Pietschker 1996; Gubelt et al. 2000), a total closure without the application of advanced treatment processes is not practical for chemical pulping processes and for manufacturing of white paper grades. Because the nature of different waste streams from P&P operations has been reviewed in other publications (Gehm 1973; Costa et al. 1979; Ashafi et al. 2015; Kamali and Khodaparast 2015; Blanco et al. 2016), such subjects will be treated only briefly here.
Sources and Characteristics of P&P Wastewater
The first point worth emphasizing is that wastewater characteristics are highly variable, especially when comparing one pulp or papermaking operation to another. Much of the observed variation can be attributed to the different characteristics of wastewater streams emerging from specific kinds of pulping, bleaching, or papermaking processes (Gehm 1973; Hubbe 2007b; Hossain and Ismail 2015; Kamali and Khodaparast 2015). Also, the nature of the effluent will be dependent on how much fresh water is being utilized in the process. Figure 1 provides a snapshot of the categories or “streams” of wastewater mentioned in subsequent paragraphs.
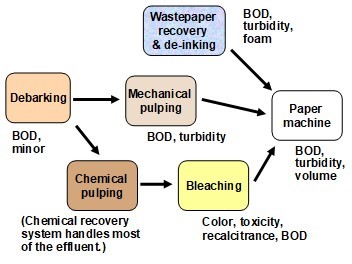
Fig. 1. Main categories of contaminated water within a typical pulp and paper manufacturing facility, with an indication of important potential environmental impacts
Debarking
Following the sequence of the manufacturing process, one of the first streams of contaminated water is associated with soaking of logs and removal of bark (Gehm 1973; Vepsäläinen et al. 2011). Because mainly mechanical processes are involved in bark removal, the levels of contamination resulting from debarking tend to be relatively low in comparison to other wastewater streams. For example, Vepsäläinen et al. (2011) showed that such wastewater could be effectively treated by electro-coagulation. Furthermore, dry debarking allows reducing wastewater production and potentially making it possible to obtain more energy from the bark in a power boiler (BREF 2015).
Mechanical pulping
The type of virgin pulp most often used in the production of newsprint and high-circulation magazine papers is obtained by mechanically separating the fibers in wood. Though this is another mainly mechanical process, the amount of energy expended per unit mass of generated pulp is high, and that may explain why there is significant release of soluble and particulate materials into the water phase (Stephenson and Duff 1996). Mechanical pulping is often carried out at elevated temperatures, i.e. thermomechanical pulping (TMP), which enables softening of the lignin between the fibers, making it possible to preserve a much greater proportion of full-length fibers during the separation process. The heating is probably responsible for increased release of material into the water phase (Rintala and Puhakka 1994; Stephenson and Duff 1996; Kortekaas et al. 1998; Willfor et al. 2003), though the literature search did not reveal data to support such an expectation. The peroxide-bleaching of thermomechanical pulp (BCTMP) is known to convert hemicellulose components of TMP into negatively charged carboxylate species, which readily enter into solution and can contribute to the pollutant load (Sundberg et al. 2000; Puro et al. 2010; Hubbe et al. 2012b; Miao et al. 2013). In general, however, high-yield pulps contribute relatively low levels of COD to the effluent.
Kraft pulping
Because kraft pulping and optional subsequent bleaching of wood-based and non-wood cellulosic pulps can involve solubilization of about 30 to 60% of the solid mass, depending on the manufacturer’s objectives (Biermann 1996), it is easy to understand why such operations have the potential to produce highly contaminated wastewaters. The leftover liquid after a kraft cooking process (the black liquor) is rich in lignin byproducts, as well as alkali, sodium sulfate, and the soaps of resin acids and fatty acids, among other contaminants (Vishtal and Kraslawski 2011; Lehto and Alén 2015).
Under ideal circumstances a very high percentage of the solubilized matter from alkaline pulping is circulated back to the chemical recovery system, diverting the potential contaminants away from the wastewater treatment system (Biermann 1996). But when the fiber source contains a high content of silica, as is the case for many annual plant types of biomass, the chemical recovery can be very difficult due to the deposition of inorganic scale on the equipment (Deniz et al. 2004). In addition, conventional bleaching technologies, based on chlorine dioxide, sodium hypochlorite, and related chemicals, yield wastewaters that would be excessively corrosive to the equipment used for chemical recovery, hence discouraging the recovery of pulping chemicals from such wastewater streams. The condensate collected during evaporative concentration of spent pulping liquor is a notable source of toxicity and odor, which needs to be treated (Wagner and Nicell 2001; Tielbaard et al. 2002).
Pollutants from various stages of bleaching of kraft pulps are among the most difficult to remove from wastewater (Prat et al. 1988; Kemeny and Banerjee 1997; Vidal et al. 1997; Yeber et al. 1999b; Kansal et al. 2008; Uğurlu and Karaoğlu 2009; Quezada et al. 2014; Larsson et al. 2015). Many of these compounds may have some adverse effects on the receiving media, such as formation of slime and scum, toxic effects to the exposed living organisms, and thermal impacts (Ali and Sreekrishnan 2001; Catalkaya and Kargi 2008; Garg and Tripathi 2011) or they are highly colored (Bajpai and Bajpai 1994).
Sulfite pulping process
The production of sulfite pulp is presently much smaller than the production of kraft pulp because of generally worse strength properties. However, sulfite pulp does provide better properties in terms of some specialty pulp applications. Different sulfite processes, such as acid (bi)sulfite, magnefite, or neutral sulfite, may mainly be applied by just changing the pH and the type of base that is used in the process. The sulfite cooking process uses aqueous sulfur dioxide (SO2) and a base of magnesium, calcium, sodium or ammonium. Chemical and energy recovery, as well as water use, will be affected by the selected base type. Magnesium sulfite pulping is the dominating process in Europe (≈11 mills), and there is another mill using sodium base, because both types allow chemical recovery. Lignosulfonates, sugars, and other substances that are generated within the cooking liquor might further be used as raw materials for the production of different chemical products. As noted by Rintala and Puhakka (1994), it is a high priority to remove toxicity from the evaporator condensate in a sulfite plant’s recovery cycle. Most of these sulfite pulp mills operate biological treatment plants to purify wastewater generated in different processes (washing losses, effluents from the bleach plant, and condensate from the evaporation plant, mainly) (BREF 2015).
Paper recycling operations
When fibers are recovered from recycled papers, a wider spectrum of contaminants will be present due to the variability in the nature of the used paper materials, their contamination during paper’s use and recovery, and the additives that are used for dispersing the fibers, removing ink, and bleaching (Muhamad et al. 2012a). Such waters are characterized by a high organic dissolved load, which typically has an anionic character (Miranda et al. 2009a).
In the past, deinking wastes were regarded as posing challenges during their clarification (Gehm 1973). Difficulties in gravitational separation of solids from deinking operations might be attributable to the dispersants that are widely used to separate the ink particles from the fibers. In this case, adequate coagulants and flocculants are required to destabilize the colloidal material and promote the aggregation of particles for their removal by sedimentation or dissolved air flotation (DAF). The past challenges during the clarification of de-inking waters generally have been solved, and these days it is possible to treat and reuse the effluents by membrane procedures, although silicic ions have to be removed to increase the recovery in reverse osmosis (RO) units (Ordóñez et al. 2011). Though the focus here is on the water-borne pollutants, it is important to bear in mind that a deinking process can result in the generation of large amounts of solid wastes; it has been estimated that about 70% of solid wastes, mainly in the form of sludge, are generated by de-inking operations (Monte et al. 2009).
Papermaking operations
The paper machine itself, in some respects, can be regarded as having the cleanest process water circuit in the plant. Excess water from the forming and press sections of the paper machine is clarified by filtration (i.e. a save-all operation) or by DAF and reused in the papermaking process. When high quality water is required, for example in high-pressure showers, a further treatment such as ultrafiltration (UF) can be used.
On the other hand, the process water in a paper machine system, i.e. the “white water”, can contain a variety of additives, including mineral filler products such as calcium carbonate, clays, and titanium dioxide. Sizing agents, such as rosin products, alkenylsuccinic anhydride, or alkylketene dimer, are typically added to the fiber slurry in the form of emulsions, and not all of this is retained in the paper product. In the case of colored papers, though they are usually present at low amounts, dyes are important in terms of wastewater treatment on account of their high visibility.
Substantial pollutant loads can be expected whenever certain additives to the paper machine wet end are poorly retained in the product (Bilitewski et al. 2012). Starch products, which are rendered soluble in water by cooking operations, are commonly added to paper furnish at levels as high as 1% of the product mass (Howard and Jowsey 1989; Formento et al. 1994). Most of that wet-end-added starch is of the cationic form, and its positive charge favors a relatively high efficiency of retention onto the negatively charged fibers (Roberts et al. 1987; Howard and Jowsey 1989). By contrast, the surface-applied starch (“size-press starch”) is typically uncharged “pearl” starch, which does not have any electrostatic attraction to the fiber surfaces. The size press starch can be routinely added at amounts several times larger than what is practical at the wet end of the paper machine. Thus, when defective paper, i.e. “dry-end broke”, is repulped and sent back to the paper machine, a high proportion of the surface-applied starch may end up in the aqueous phase, such that it later can be discharged to the wastewater treatment plant.
Recalcitrant Organic Compounds in Pulp and Paper Mill Wastewaters
Pulp and paper mill effluents contain a variety of recalcitrant materials, such as lignosulfonic acids, chlorinated resin acids, chlorinated phenols, dioxins, and chlorinated hydrocarbons (Kumara Swamy et al. 2012; Singh and Srivastava 2014). Although in most cases the toxicity is low, effluents from pulp bleaching are characterized by high strength of COD (1000 to 7000 mg L-1), low biodegradability ratio (BOD5/ COD) ranging from 0.02 to 0.07, and a moderate strength of suspended solids (500 to 2000 mg L-1) (Ramos et al. 2009; Eskelinen et al. 2012). Compounds, especially those containing chlorine (measured by the parameter AOX) are recalcitrant because they contain chemical structures uncommon in nature, such as the carbon-chlorine bond (Jokela et al. 1993; Mounteer et al. 2007a). It is widely reported that high molecular weight (HMW > 1 kDa) organic matter in bleaching effluents is more recalcitrant to biological treatment than low molecular weight (LMW < 1 kDa) organic matter (Dahlman et al. 1995; Savant et al. 2006). Dissolved lignin and its degradation products, hemicelluloses, resin acids, fatty acids, diterpene alcohols, juvaniones, tannins, and phenols (Pokhrel and Viraraghavan 2004) are responsible for the dark color and toxicity of the effluent (Malaviya and Rathore 2007; Chopra and Singh 2012).
Lignin and its derivatives
Among the main biomass components, lignin is the most difficult to degrade by biological means (Kumar et al. 2010; Pu et al. 2015). It is a three-dimensional amorphous polyphenolic polymer that is primarily biosynthesized from three typical types of phenylpropanoid precursors: coniferyl, sinapyl, and p-coumaryl alcohol (Thakur 2004). Once incorporated into the lignin polymer, these monomers form guaiacyl, syringyl, and p-hydroxyphenyl lignin subunits. The lignin macromolecule is primarily linked via ether bonds and carbon-carbon bonds among phenylpropanoid units (Samuel et al. 2014).
Lignin is degraded during pulp and paper production to a variety of high, medium, and low molecular weight chlorinated and non-chlorinated fractions (McKague 1981). In particular, the HMW lignin compounds are not effectively degraded during conventional effluent treatment, and the majority of such compounds in wastewater may be discharged into receiving waters (Hyötyläinen and Knuutinen 1993). Lignin and its derivatives are recalcitrant and highly toxic compounds responsible for the high BOD and COD values of effluents as well as the dark brown color of pulp effluents formed during pulping (Wong et al. 2006). In pulp bleaching, chlorine reacts with lignin, its derivatives, and other organic matter present in the pulp, forming highly toxic and recalcitrant compounds, such as chlorinated lignosulfonic acids, chlorinated resin acids, chlorinated phenols, guaiacols, catechols, benzaldehydes, vanillins, syringo-vanillins, and chloropropioguaiacols (Knuutinen 1982; Kringstad and Lindström 1984; Thakur 2004). Especially in acidic media, lignin molecules tend to undergo self-condensation, and they subsequently show resistance to degradation (Ali and Sreekrishnan 2001). Chlorolignins mostly end up in the effluent of the alkaline extraction stage, which is a major source of AOX, COD, BOD, and color (Sun et al. 1989; Ali and Shreekrishnan 2001).
Chlorinated organic compounds
In pulp and paper mill effluents, hundreds of chlorinated organic compounds have been identified, including chlorinated hydrocarbons, phenols, catechols, guaiacols, furans, dioxins, syringyl lignin, and vanillins (Suntio et al. 1988; Freire et al. 2003). Organochlorine compounds are usually biologically persistent, recalcitrant, and highly toxic to the environment (Baig and Liechti 2001; Thompson et al. 2001). They have been detected especially in water and sediments in the vicinity of pulp and paper mill effluents (Virkki et al. 1994; Munawar et al. 2000; Lacorte et al. 2003). Organochlorine compounds are widespread and are found even in relatively pristine environments (Abrahamsson and Klick 1991). The recalcitrance of an individual compound towards biological processes is related to the number and position of the halogen substituents (Naumann 1999). Additionally, enantioselectivity and chiral discrimination of optically active compounds can have an influence on the degree of their accumulation (Reich et al. 1999; Vetter and Maruya 2000). Isomers, diastereomers, and enantiomers can have varying toxicity (Willett et al. 1998; Carr et al. 1999).
Chlorinated phenols are synthetic compounds, typically formed by hydrolysis of chlorobenzenes and in pulp bleaching (Kringstad and Lindström 1984). Chlorophenols may also be formed as by-products in paper production, cooking processes, or distillation of wood (Paasivirta et al. 1983, 1988; Öberg et al. 1989). Monomeric chlorophenols include 19 compounds of mono-, di-, tri-, and tetrachloroisomers and pentachlorophenol. Generally, they are crystalline solids at room temperature, excluding the liquid orthochlorophenol (Ahlborg 1977).
In the bleaching operations following kraft wood pulping, HMW and LMW chlorinated organic compounds are formed, the latter comprising of about 250 smaller chlorinated compounds (Rantio 1997). In pulp bleaching, chlorophenolics are present both in free (hexane extractable) and bound (extractable with strong alkali) forms and bound to dissolved organic matter and particles (Paasivirta et al. 1992). For example, chlorophenols, guaiacols, and catechols are formed in high quantities (Suntio et al. 1988; Koistinen et al. 1990), guaiacols and catechols being chemically bound to chlorolignin (Schechter et al. 1990). They can be methylated by microorganisms to more persistent and lipophilic forms as chloroanisoles, veratroles, or aromatic chloroethers, which are persistent derivatives of chlorophenolics. In addition, during the cooking and chlorine bleaching, chlorinated cymenes, (methyl-(methylethyl)benzenes, CYMS) and cymenenes (methyl-(methylethenyl)-benzenes, CYMD) are formed (Kuokkanen 1989; Rantio 1997). Chlorinated cymenes were first identified in the effluents of kraft pulp and sulfite mills in the late 70s, and dechlorinated cymene compounds have been found both from kraft and sulfite mills and chlorinated compounds from kraft pulp mills (Bjørseth et al. 1977). Residues of CYMS and CYMD have been found in fish and mussels in the recipient water bodies (Paasivirta et al. 1984; Herve 1991), and they can be employed as indicators of the exposure of biota to effluents from pulp bleaching (Rantio 1997).
Polychlorinated dibenzo-p-dioxins (PCDD) and polychlorinated dibenzofurans (PCDF) are two classes of tricyclic, aromatic, and lipophilic compounds that contain some of the most toxic chemical substances ever known (Kringstad and Lindström 1984). PCDD and PCDF are formed as side products in chlorophenol production and in thermic reactions, such as combustions and chlorinations but also in pulp and paper production (Alcock and Jones 1996; Kim et al. 2002). In pulp production, the PCDD and PCDF are mostly formed by condensation of polychlorinated phenoxyphenols in pulping and by chlorination of dibenzofuran and dibenzo-p-dioxin in pulp bleaching (Hrutfiord and Negri 1992; Luthe and Berry 1996; Yunker et al. 2002).
Generally, a mixture of different PCDD and PCDF is formed, but isomers with chlorine atoms in the 2, 3, 7, and 8 positions (7 PCDD and 10 PCDF) are the most toxic to organisms, with 2,3,7,8 tetrachlorodibenzo-p-dioxin, as shown in Fig. 2, being the most toxic (Malisch 2000; Fueno et al. 2002). PCDD and PCDF are acute or chronic toxins, recalcitrant and persistent in nature due to their lipophilic properties and resistance to biological and chemical degradation (Vallejo et al. 2015). This leads to biomagnification along food chains and various degrees of toxic effects (Van den Berg et al. 2006). They have been classified as priority pollutants by the United States Environmental Protection Agency (USEPA 1998) and ‘dirty dozen’ group of persistent organic pollutants (POPs) identified by United Nations Environment Program (UNEP 1995). Dioxins are named as ‘known human carcinogens’ by the World Health Organization (WHO 1997).
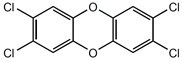
Fig. 2. Chemical structure of 2,3,7,8 tetrachlorodibenzo-p-dioxin
PCDD and PCDF have been detected, e.g. in bleach plant effluents, bleached paper products, sediments of the receiving waters, and fish living in the recipient water bodies (Rappe et al. 1989; Clement et al. 1989; Lacorte et al. 2003). The elimination of dibenzofuran and dibenzo-p-dioxin from defoamer products, the exclusion of chlorophenol-contaminated wood chips, and the introduction of chlorine dioxide bleaching have dramatically decreased the production of PCDD and PCDF in the P&P effluents (Hagen et al. 1997; Yunker et al. 2002).
Other chlorinated and non-chlorinated substances that are released from pulp mills include planar aromatic compounds, which can be characterized as alkylated polychlorodibenzofurans, alkyl polychlorobiphenyls, and alkyl polychlorophenanthrenes (Paasivirta 1988; Wiberg et al. 1989). Their levels are orders of magnitude higher than those of dioxins and furans in pulp mill effluents, several nanograms per gram in dry weight (Paasivirta 1991). Polychlorinated dibenzothiophenes (PCDTs) are sulfur analogues of polychlorinated dibenzofurans (PCDFs) and have structural resemblance to PCDDs and PCDFs (Sinkkonen 1997; Koivisto 2001). In addition, other compounds may have dioxin-like toxicity, including the non-chlorinated ones, such as indocarbazoles, benzanthracenes, and benzoflavones (Paasivirta 1988).
During the last decades, there has been a drastic decrease in the use of molecular chlorine as a bleaching agent, and it has been replaced with chlorine dioxide (Elementary Chlorine Free, ECF), molecular oxygen, peroxide, or ozone (Totally Chlorine Free, TCF), which has led to a drastic decrease in AOX, PCDD, and PCDF (Shimp and Owens 1993; Rantio 1997). There are several modifications made to reduce the generation of chlorinated organic compounds from bleach plant effluents by employing modified strategies. A key strategy involves removing more lignin i.e., by extended delignification, reducing the kappa number of unbleached pulp. Further strategies involve modifying the conventional bleaching process to ECF and TCF bleaching (Kinstrey 1993). This reduction of chlorine content in the effluents has made it possible to improve the closure of the water circuits system of the mill and to recycle the bleach plant effluent back to the mill’s chemical recovery system. The reduction of the generation of organic substances in bleaching stages has been possible thanks to previously increasing delignification efficiency, modifying cooking, additional oxygen stages, spill collection systems, a more efficient washing, and stripping and reusing condensates. The installation of external treatment plants of different designs has been another successful contributing factor to decreasing AOX and unchlorinated toxic organic compounds emissions to receiving water (BREF 2015). In Canada as a result of federal regulations, the quality of pulp and paper effluent released directly to the environment has progressively improved. In 2013, 96.2%, 99.9%, and 99.8% of effluent samples met regulatory requirements for toxicity tests on fish, BOD, and TSS, respectively (Environment and Climate Change Canada, 2016).
Non-chlorinated recalcitrant compounds
Wood extractives are hydrophobic components that are soluble in neutral solvents. They are composed of e.g. resin acids, fatty acids, sterols, diterpene alcohols, and tannins (Holmbom 1999; Lacorte et al. 2003). Resin acids, as shown in Fig. 3, are tricyclic diterpenoids and natural constituents of conifer wood. Fatty acids exist as free fatty acids and neutral esterified fatty acids in triglycerides and steryl esters (Ekman and Holmbom 2000; Björklund Jansson and Nilvebrant 2009). Generally, fatty acids include palmitic, linolenic, linoleic, and oleic acids, while resin acids include abietic, neoabietic, levopimaric, palustic, and dehydroabietic acids (Back and Ekman 2000). Resin acids are released in high amounts during pulping and paper production, especially in mechanical pulp production (Johnsen et al. 1993). Resin acids are very resistant to chemical degradation due to their stable tricyclic structure (Dethlefs and Stan 1996). They are thought to be the main contributors to the toxicity of the pulp mill effluents (Makris and Banerjee 2002; Rigol et al. 2004) and have been reported to end up in sediments of the receiving water bodies (Dethlefs and Stan 1996; Rämänen et al. 2010).
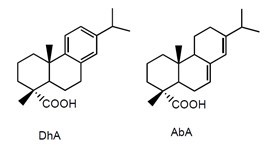
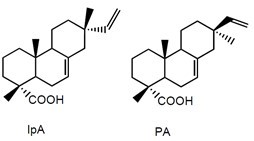
Fig. 3. Chemical structures of typical resin acids that may be released from softwood species during pulping (DhA = dehydroabietic acid; AbA = abietic acid; IpA = isopimaric acid; PA = pimaric acid)
In addition to substances originating in the wood, there may be situations in which P&P wastewater contains toxins from other sources. Many of the organic substances produced by the chemical industry are toxic or resistant to biological treatment (Lapertot et al. 2006; Muñoz and Guieysee 2006). Chelating agents, such as DTPA and EDTA (Fig. 4), are relatively large organic molecules that are able to bind metals (Tana and Lehtinen 1996). They are increasingly used with peroxide and ozone bleaching of wood pulp and discharged with spent bleach liquors. Chelating agents have been reported to either totally resist degradation or to undergo slow biodegradation (Hinck et al. 1997). They have been found e.g. in marine sediment, but so far no direct harmful effects in the environment have been reported (Sprague et al. 1991; Puustinen and Uotila 1994). However, it has been suggested that chelating agents can prevent the sedimentation of metals close to the point of discharge, leading to the spread of metals over a large area in low concentrations (Tana and Lehtinen 1996).
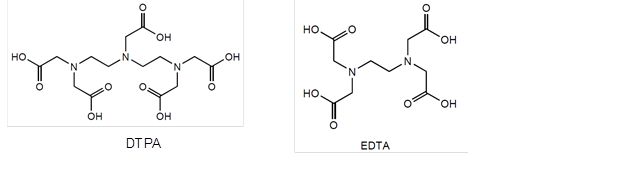
Fig. 4. Molecular structures of two widely-used chelating agents (DTPA = diethylenetriaminepentaacetic acid; EDTA = ethylenediaminetetraacetic acid)
Toxicity of Recalcitrant Compounds
The discharged pollutants of the pulp and paper mill effluents affect both aquatic and terrestrial organisms (Pokhrel and Viraraghavan 2004). Several studies have reported toxic effects on fish species due to pulp mill effluents, including respiratory stress, liver damage, mutagenic, and genotoxic effects (Owens et al. 1994; Johnsen et al. 1998; Leppänen and Oikari 1999; Schnell et al. 2000b; Rana et al. 2004). Sublethal effects and changes in plankton population have been reported (Yen et al. 1996; Baruah 1997), but also delayed sexual maturity, changes in reproduction, smaller gonads, and altered productivity of aquatic invertebrates and fish (Munkittrick et al. 1997; Karels et al. 1999). Additionally, decreased number of juveniles, physiological and skin diseases, and changes in communities, population structure, and in growth rates of fish have been observed (Sepúlveda et al. 2002). Furthermore, effluents from pulp bleaching impair the quality of fish and fish flesh in the receiving waters (Herve 1991; Redenbach 1997).
Increase in the amount of toxic substances in water kills zooplankton and fish, affecting the terrestrial ecosystem (Burton et al. 1983). Other problems may occur due to failure of the treatment processes employed to treat the pulp and paper mill effluent. This can result in the release of suspended solids and nutrients, such as nitrogen and phosphorus, which can lead to eutrophication of the receiving water bodies and oxygen depletion (Thompson et al. 2001). Endocrine disruptors may be also present in the wastewater (Balabanic et al. 2012), though relatively little is known about potential endocrine disruption effects of paper mill effluents on aquatic organisms. For example, plant sterols (phytosterols) may act as disrupters of the hormonal and biochemical systems of aquatic organisms (Kostamo and Kukkonen 2003). Pulp mill effluents are known to contain varying amounts of phytosterols, which have the structure similar to that of the steroid hormones of vertebrates (Lehtinen et al. 1999). In addition, transformation products of sterols can have androgenic (Stahlschmidt-Allner et al. 1997) and hermaphroditic effects on fish near sewage treatment plants (Zachrazewski et al. 1995; Lacorte et al. 2003).
Chlorinated compounds
Chlorinated phenolics and chlorinated lignin derivatives are a group of chemicals contributing greatly to the toxicity of pulp and paper mill effluents (Walden and Howard 1981). Multiple adverse health effects have been linked to organochlorine compounds, such as endocrine disruption (growth retardation, thyroid dysfunction, decreased fertility and feminization or masculinization of biota) and impaired liver function in fish exposed to these effluents (Oikari and Nakari 1982; Munkittrick et al. 1998; Lacorte et al. 2003). Chlorinated phenols, catechols, and guaiacols are known to cause mutagenesis in mammalian cells (Hattula and Knuutinen 1985). Two examples of such molecules are shown in Fig. 5. Additionally, lignin and its derivatives are toxic to aquatic organisms and animals (Priha and Talka 1986).
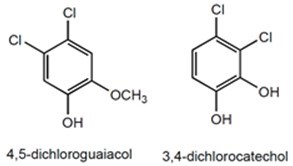
Fig. 5. Molecular structures of two chlorinated phenols
Even though chlorinated phenolics represent less than 2% of the organically bound chlorine in bleaching effluents, they are large contributors to effluent toxicity, carcinogenicity, and mutagenicity (Savant et al. 2006; Kukkola et al. 2011). The majority of organochlorinated compounds present in pulp and paper mill effluents are HMW chlorolignins (>1 kDa). These compounds are likely to be biologically inactive and have a small contribution to the toxicity, mutagenicity, and BOD of pulp mill effluents (Hileman 1993; Ali and Shreekrishnan 2001). On the other hand, LMW compounds (<1 kDa) are the main contributors to mutagenicity and bioaccumulation because they can penetrate cell membranes (Heimberger et al. 1988; Sun et al. 1989). They can bioaccumulate in the aquatic food chain in the body fat of animals in higher trophic levels (Renberg et al. 1980; Ali and Shreekrishnan 2001). However, there are indications that also derivatives of HMW lignin exhibit toxicity (Pessala et al. 2010).
Acute toxicity of chlorinated phenolics increases with the degree of chlorination, the more distant position of chlorine atom relative to the phenolic hydroxyl group, and with chlorophenol lipophilicity (Loehr and Krishnamoorthy 1988; Sierra‑Alvarez et al. 1994). Chlorinated phenolics with the chlorine at position 2 are less toxic than the other chlorophenols, while toxicity increases as a chlorine atom is substituted at 3, 4, and 5 positions (Saito et al. 1991; Czaplicka 2004). The bioaccumulation potential increases with the number of chlorine substituents on the phenolic ring. Phenol and most of the chlorophenolic compounds can induce mutations in certain bacteria and yeasts (Smith and Novak 1987). Chlorophenols are less readily biodegradable than phenol, and their rate of biodegradation decreases with increasing number of chlorine substituents on the aromatic ring (Banerjee et al. 1984). It has been suggested that position of the substituent has an effect on the degradability; the order for chlorophenols was found to be ortho > meta > para during anaerobic conditions in aquifer sediments and in digested sludge (Boyd and Shelton 1984; Genthner et al. 1989; Annachhatre and Gheewala 1996).
Dioxins are found to bioaccumulate in fish downstream of pulp and paper mills, while chlorophenolic compounds, coplanar polychlorinated biphenyls (PCBs) isomers, polychlorinated naphthalenes (PCNs), polychloroanthracenes, tetrachloroazo- and azoxybenzenes, and PCDEs are acutely toxic but not very prone to bioaccumulation (Paasivirta 1988; Howie et al. 1990; Frakes et al. 1993). However, chlorinated hydrocarbons (e.g. hexachlorocyclohexanes, residues of DDT (dichlorodiphenyl-trichloroethane), chlordanes, PCNs, and PCBs) have shown high bioaccumulation rates and biomagnification at higher trophic levels (Paasivirta 1988; Herve et al. 1988; Paasivirta and Rantio 1991; Koistinen 1992).
Extractives
Wood extractives, e.g. resin and fatty acids, sterols, and diterpene alcohols account for a large part of toxicity in various paper mill effluent streams (Lacorte et al. 2003). In particular, the toxicity of resin acids has been studied extensively for decades (e.g. Oikari et al. 1984; Meriläinen et al. 2007). The most commonly monitored resin acids in aqueous pulping effluents include abietic acid, dehydroabietic acid, neoabietic acid, pimaric acid, isopimaric acid, sandaracopimaric acid, levopimaric acid, and palustric acid, among which isopimaric acid is the most toxic (Wilson et al. 1996). Additionally, retene (7-isopropyl-1-methylphenanthrene) is a naturally formed polycyclic aromatic hydrocarbon causing teratogenicity in fish larvae. Retene is formed in surface sediments contaminated by resin acids from pulp mill effluents (Oikari et al. 2002).
The toxicity of tannins to several enzymes has been well established (Korczak et al. 1991; Sierra-Alvarez et al. 1994). Methanogenic toxicity of tannins depends on the degree of polymerization (Field et al. 1988). HMW tannin polymers and humic acids exhibit low toxicity because their size limits their ability to penetrate into the bacterial cells. The highest toxicity is found in oligomeric tannins due to their ability to form strong hydrogen bonds with proteins (Field et al. 1989; Sierra-Alvarez et al. 1994). Condensed tannins from spruce bark are toxic, not only to methanogens at concentrations present in the paper mill wastewaters, but also to aquatic organisms, such as fish (Field et al. 1988; Temmink et al. 1989). In addition, unsaturated fatty acids (16-C and 18-C) of softwood, such as oleic acids, linoleic acid, and linolenic acid are also a source of toxicity to fish, especially salmonoids (Voss and Rapsomatiotis 1985). Long chain fatty acids (LCFA) have been shown to inhibit methanogenic bacteria (Koster and Cramer 1987). This makes the anaerobic treatment of wastewaters relatively troublesome since methanogenic bacteria play a crucial role in the anaerobic wastewater treatment.
Biological Processes – Susceptibility to Enzymatic Breakdown
Microorganisms with lignolytic properties can degrade contaminants in pulp and paper mill effluents containing toxic and recalcitrant compounds (Thakur 2004). Lignolytic microorganisms include fungi, actinomycetes, yeast, bacteria, and algae, all of which can produce enzymes responsible for lignocellulose degradation (Bajpai and Bajpai 1994; Annachatre and Gheewala 1996). Enzymatic treatment can be applied as a single step or with other physical and chemical methods, as extensively reviewed by Chen et al. (2010), Saritha et al. (2012), and Barakat et al. (2014).
Fungal enzymes, collectively termed as ligninases or lignin-modifying enzymes (LMEs), can degrade lignin into simple sugars and starch (Bocchini et al. 2005; Dashtban et al. 2010; Chopra and Singh 2012). Ligninases can be classified as phenol oxidases (laccase) or heme peroxidases (Martínez et al. 2005). The latter is further subdivided as lignin peroxidases, manganese peroxidases, and versatile peroxidases (Singh and Shrivastava 2014). Depending on the species of fungi, one or more of the lignin-modifying enzymes can be secreted. For example, white-rot fungi (e.g. P. chrysosporium) are able to degrade lignin efficiently using a combination of extracellular ligninolytic enzymes, organic acids, mediators, and accessory enzymes (Singh and Shrivastava 2014). Even though lignin peroxidase is able to oxidize the non-phenolic part of lignin (which forms 80 to 90% of the lignin composition), it is absent from many lignin-degrading fungi (Wang et al. 2008a). Enzymes able to perform dechlorination are generally termed as dehalogenases (Savant et al. 2006). These enzymes are able to degrade a variety of environmentally persistent pollutants, such as chlorinated aromatic compounds, heterocyclic aromatic hydrocarbon, and various dyes (Singh and Shrivastava 2014).
A broad range of fungi degrade lignin to varying degree (Pokhrel and Viraraghavan 2004). They include soil fungi (Fusarium sp.), soft rot fungi (Populaspora, Chaetomium sp.), pseudo soft rot fungi (Hyoxylen, Xylaria), lignin degrading fungi (Callybia, Mycena), white rot fungi (Trametes, Phanerochaete), and brown rot fungi (Gleophyllium, Poria). They can also degrade modified lignin and their derivatives found in effluent but also COD, AOX, and color from pulp mill effluents (Pokhrel and Viraraghavan 2004). White-rot fungi, particularly P. chrysosporium and C. versicolor, can degrade refractory material efficiently and reduce color, lignin, and COD (Bajpai and Bajpai 1994). However, only a few strains of fungi can reduce chlorinated aromatic compounds (Bajpai and Bajpai 1997; Singh 2007). White-rot basidiomycetes, such as Coriolus versicolor (Wang et al. 2008a), P. chrysosporium, and T. versicolor (Moreno et al. 2003) have been found to be the most efficient lignin-degrading microorganisms.
In nature, lignin is degraded during wood decay mainly by basidiomycetes white-rot fungi, of which many attack lignin, hemicellulose, and cellulose simultaneously, e.g. Trametes versicolor (Tanaka et al. 1999) or Heterobasidium annosum (Daniel et al. 1998), whereas some other white-rot fungi preferentially work on lignin in a selective manner, e.g. Physisporinus rivulosus (Hilden et al. 2007) and Dichomitus squalens (Fackler et al. 2006).
Enzymatic treatment is a safe and ecofriendly process (Kim et al. 2002), but a long residence time is required (up to 10‑14 days), and high costs of enzymes limit its commercial applications (Pu et al. 2015). Furthermore, selectivity of enzymes can be low and also carbohydrates are partially consumed by microorganisms. So far, enzymatic treatment of pulp and paper mill effluent generally has not been successful due to a lack of suitable organisms, various recalcitrant compounds, and poor process optimization for industrial-scale treatment.
Recalcitrant compounds and their treatment
Approximately half of the organic matter in typical pulp and paper mill effluent is recalcitrant to biodegradation by both anaerobic and aerobic bacteria (Ortega-Clemente et al. 2009). For example, wood resin, tannins, and chlorinated phenolics are toxic to methanogenic bacteria (Rintala and Puhakka 1994; Vidal et al. 2001). However, certain bacterial strains are quite effective in breaking down recalcitrant organic phenolic compounds and their derivatives and can also reduce the color of pulp mill effluent to some extent (Bajpai and Bajpai 1997). Anaerobic bacteria able to degrade chlorinated organics are classified as alkyl dehalogenators and aryl dehalogenators (Mohn and Tiedje 1992). The alkyl dehalogenators include physiologically diverse groups of strict anaerobes and facultative anaerobes, such as methanogens, species of Clostridium, Acetobacterium woodii, and Shewanella (Savant et al. 2006). These organisms mainly dechlorinate LMW compounds, such as chloroform, tetrachloroethene, dichloromethane, trichloroethane, and trichloroethene. Aryl dehalogenators mainly belong to proteobacteria and genera, such as Desulfitobacterium and Desulfobacter.
Anaerobic bacteria are believed to use chlorinated organic compounds as a source of carbon and energy, as a cometabolite, or as an electron acceptor depending on the strain of bacteria (Holliger et al. 1998). Thus they are better suited to reductively dehalogenate highly chlorinated phenolics, while aerobic biological systems are suitable for less halogenated phenolics (Neilson 1990; Latorre et al. 2007). A sequence of anaerobic treatment followed by aerobic treatment permits the degradation of compounds that are non-degradable anaerobically (Rintala and Puhakka 1994).
Mixed microbial communities can overcome the problems faced by monocultures in the environment, such as nutritional limitations and toxic substrates (Thakur 1995; Kim et al. 2002). For example, a mixture of various bacteria can degrade phenolic compounds due to their varying structures and toxicity. In addition, two or more microorganisms can be applied sequentially, one carrying out the initial catabolic reactions and another completing the rest of the metabolic pathway to mineralize the organic compounds. Bicyclic aromatics such as chlorinated biphenyls, chlorinated dibenzofurans, and naphthalene sulfonates have been treated sequentially, resulting a reduction in color, lignin, COD, and phenol (Pokhrel and Viraraghavan 2004; Singh and Thakur 2006).
Susceptibility to oxidation
In the natural environment, many organic refractory compounds can be degraded, either biologically or by photochemical reactions, and further transformed by evaporation or adsorption (Czaplicka 2004). Photochemical processes include photodissociation, photosubstitution, photooxidation, and photoreduction. In the aquatic environment, photodegradation occurs only in the surface layer and is faster in summer than in winter, especially in the northern latitudes (Kawaguchi 1992a). The properties of chlorophenols are favorable to sorption and accumulation, especially into bottom sediment and suspended matter (Brusseau and Rao 1991; Peuravuori et al. 2002; Czaplicka 2004). Chlorophenols can undergo reactions of auto-oxidation and catalysis, especially on surfaces made of clay and silica. They can evaporate, with the rate depending on vapour pressure and water solubility, but under typical environmental conditions evaporation plays an insignificant role (Piwoni et al. 1989; Czaplicka 2004).
OVERVIEW OF WASTEWATER TREATMENT OPTIONS
Before reviewing advances in wastewater treatment technologies related to the P&P industry, an overview is presented of conventional approaches. These approaches address not only the treatment of such effluent, but also reducing its amount by implementing strategic changes to the operations within P&P mills. Wastewater treatment systems have commonly been understood to function as “end-of-pipe” solutions to environmental issues, whereas the least expensive way to treat polluted water may be to produce less of it in the first place (Stratton et al. 2004; Hossain and Ismail 2015). Unit operations within P&P mill water treatment systems have been reported before and can be mentioned here (European Commission 2001; Demel et al. 2003). Blanco et al. (2016) have recently reviewed the state of the art on water reuse.
Pulp Mill Operations and Wastewater
A process known as counter-current washing has been widely used within the P&P industry to effectively separate used cooking liquors from the cellulose fibers while minimizing the volume of rinse water (Hammar and Rydholm 1972; Ala-Kaila et al. 2005). Such systems are set up with a series of washing operations, such that the cleanest water is used to wash the cleanest pulp. The rinse water is directed backwards through the series of washers until the most contaminated rinse water enters the chemical recovery system.
In modern pulp and paper making facilities it is still common to employ screen cylinders (i.e. “deckers”) which rotate slowly while partially submerged in a vat filled with pulp suspension (Fig. 6). A mat of pulp collects on the outer surface of the cylinder, as filtrate is removed by gravity from the space within the cylinder. Wash water is applied above the wet mat of fibers to displace the contaminated water with cleaner water. Lindau et al. (2007) demonstrated that higher efficiency can be achieved in such systems by improving the uniformity of the pulp pad. The counter-current principle can easily be applied to such systems by employing the filtrate from the final stage of washing as the rinse water for the next preceding stage, and so-on for the whole series of deckers. Such systems have been considered theoretically (Potucek 2003, 2005). Counter-current washing is also used in recovered paper mills. In such mills, water is used for cleaning operations and separated from the pulp through disk filters and pressed in such a way as to segregate filtrate streams having different levels of contamination. The separated waters then can be recirculated in different process loops, such as showers. The most contaminated water is circulated back to the pulper.
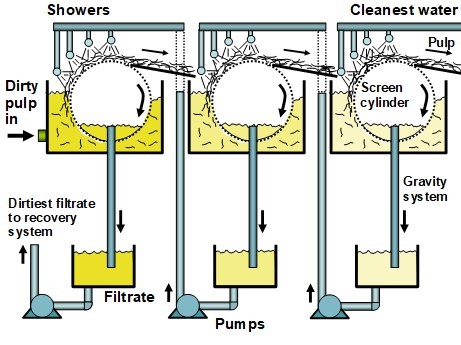
Fig. 6. Schematic of a series of three decker-type pulp washers, with counter-current washing
Continuous washing equipment has been installed in many modern pulp mills, especially those that employ continuous kraft digesting equipment, as a means to achieve more favorable washing efficiency and to minimize the amount of concentrated liquor that needs to be evaporated during the chemical recovery process (Richter 1966; Edwards and Rydin 1976; Lee 1979, 1984; Gullichsen 1999). In addition to incorporating the principle of counter-current washing, some additional gains in displacement efficiency have been achieved in such installations by avoiding the dense compaction of pulp within the pulp washing tower. Because the rinse water flows quite slowly from where it is injected towards a cylindrical screen in the tower, there is sufficient time for contaminants to diffuse from the fibers and become almost equilibrated with the rinse water at each point. Thus, higher overall efficiency can be achieved in comparison to systems that rapidly displace the contaminated water from a wet mat of pulp (Richter 1966; Potucek 2005; Lindau et al. 2007). By operating under pressure, some such designs make it possible to utilize very hot rinse water, while at the same time avoiding the generation of foam bubbles (Gullichsen 1999).
Oxygen delignification
Oxygen bleaching (often called extended delignification) does not involve corrosive chloride ions. Therefore, the wash water can be combined with the brown-stock wash liquor and directed back towards the chemical recovery system. This process reduces the emission of organic substances to the wastewater and the amount of chemicals to be added, which makes such an operation suitable for systems with high levels of water reuse (BREF 2015). In addition, research suggests that effluent from oxygen bleaching stages is highly biodegradable (Vidal et al. 1997). Furthermore, this stage will reduce the load of bleaching plant pollutants entering the biological wastewater treatment system. Hence, such a process can reduce the loading of organic material that otherwise would be released from the pulp during bleaching of a kraft pulp (Hammar and Rydholm 1972; Stratton et al. 2004). Oxygen delignification tends to be less selective than some other options, such as using chlorine dioxide. As a consequence, extensive oxygen delignification tends to break down the cellulose macromolecules, lowering the average molecular mass, and eventually weakening the material. Therefore, magnesium salts (typically MgSO4) are usually added, with the aim of preserving the strength of pulp.
Paper Mill Operations and Wastewater
Paper machine systems use large volumes of water. Nevertheless, there are many opportunities either to limit the amounts of substances dissolved in the water or to reuse the process water multiple times in the mill.
Separation of loops
A good strategy for sustainable water use is the separation of process water in several loops (1 to 4) to keep the water around the paper machine as clean as possible. Stock from a more contaminated process loop is transferred to the following one at high consistency to minimize the transfer of contaminant. Kappen and Wildered (2002) have developed a method to determine the efficiency of this approach based on the COD content at different stages of the process.
Increased retention of fines and polymeric materials
The basic strategy for efficiently retaining polymeric and fine particulate matter in the fiber web during the manufacturing of paper can be summarized in the two words coagulation and flocculation (Bratby 2006; Hubbe and Rojas 2008; Nawaz et al 2014). It is well known that the negative charge associated with cellulosic materials, especially the hemicellulose and extractives components, tends to favor a well-dispersed system. The colloidal matter from the natural plant materials tends to coat the free surfaces with a layer of negative charges (Jaycock and Pearson 1976). So one of the first steps towards getting the finely divided and polymeric materials to be retained in the wet web of paper often involves a partial or almost complete neutralization of the net surface charges in the system (Strazdins 1989; Hubbe and Rojas 2008). This can be done by adding such agents as aluminum sulfate, polyaluminum chloride, or highly charged cationic polymers. With the advent and greater usage of streaming current devices in paper mills, it is possible to carry out the (partial) neutralization with greater repeatability, leading to more predictable outcomes in terms of fine-particle retention, dewatering rates, and paper properties. Such equipment also can be applied in wastewater treatment (Dentel and Kingery 1989; Dentel 1995).
The reason that partial neutralization of net surface charges is often preferred, rather than aiming for a fully neutralized system (i.e. zero zeta potential) is to allow for the efficient action of another charged additive, the flocculant, which is called a “retention aid” by papermakers. By leaving a small proportion of the original negative charge at the surfaces of the fibers, a cationic acrylamide copolymer of very high molecular mass is able to find suitable points of adsorption in order to be effective (Hubbe et al. 2012b). Retention aids have been shown to function by a bridging mechanism, such that the polyelectrolyte is able to join the solids together efficiently, especially in the presence of agitation (Gregory 1973; Hubbe et al. 2009). The mechanisms of coagulation and flocculation are worth emphasizing here, since essentially the same steps are also often involved in the primary or secondary clarification of P&P effluents. On the paper machine an effective combined usage of coagulation and flocculation will tend to gather most of the polymeric and finely divided matter onto the fiber surfaces, such that relatively little solid material leaves the system with the excess water from the process. Also, it has been shown that any retention aid polymer that enters the wastewater treatment system is likely to end up in the sludge rather than remaining dissolved in the treated water (Pelzer 2008).
Save-all systems
The volumetric flow rate of effluent can have a major effect on the retention time of a wastewater treatment system; therefore it is very important to review the systems that papermakers employ to reduce the volume of wastewater. In the early days of mechanized papermaking using a continuous mesh screen, any excess water was sent directly to the sewer (Hunter 1947; Hills 1988). Fine particles that had not been retained in the wet web of paper ended up in the outfall from the mill. To improve process yield and to maintain better community relationships, unit operations called save-alls were developed and implemented (Stevens 1975; Perrault 1993). As noted by Milliken (2006), a well-operating save-all system can save both energy and water costs.
Two types of save-all systems are common. The most prominent system, especially in recent installations, is based on screening. Disk screen systems generally work by collecting fine matter on a mat of “sweetener fibers” that initially collect on the screen surfaces, while the clarified process water flows through (Stevens 1975; Perrault 1993; Milliken 2006). As shown in Fig. 7 (redrawn based on the design described by Milliken 2006), such equipment employs pairs of filter disks arranged in a sandwich form, such that the solids collect on the outside of the pair, and filtrate is collected from the interior space. By careful design and operation of such a system, operators are able to collect filtered process water having two levels of clarity. Only the clearest water, having the lowest content of solid particles, is discharged to the wastewater treatment plant. Such filtered water also may be further cleaned and then used in such applications as showers within the papermaking process. Karvonen et al. (1999) showed that the performance of a save-all system can be enhanced by the use of retention aid treatment of the incoming process water and sweetener fiber. Recent developments in save-all design and operation have been reported (Shukla 2012). The other common type of save-all device is the flotation save-all (Rebarber 1998; Teerikangas 2001; Nasser 2003).
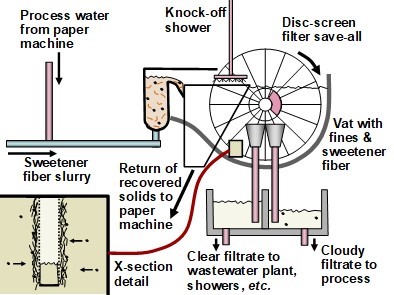
Fig. 7. Schematic drawing of a disk filter save-all system (redrawn based on illustrations by Milliken 2006)
Water circuit closure
Process water reuse is limited by the accumulation of dissolved matter that may affect the process and the final product quality. The optimum closure mainly depends on the nature of the final product. Various paper mills and operators of pilot paper machine systems have either evaluated or implemented the complete elimination of wastewater (Alexander and Dobbins 1977; Heller et al. 1979; Pietschker 1996). Wastewater-free operation is common in systems in which recycled paperboard products are produced, while generally it is not feasible in pulping operations and white grades. Alternatively, wastewater treatment schemes closely related to those that will be featured in later sections of this review article can, in principle, be implemented within the paper mill’s water cycle (Tenno and Paulapuro 1999; Verenich et al. 2000; Webb 2002; Nuortila-Jokinen 2004; Hubbe 2007c; Mauchauffee et al. 2012; Singh et al. 2012; Saif et al. 2013). Another approach, which ends up as having the same net effect, has been to employ the final effluent from wastewater treatment as the intake for the papermaking system (Ordóñez et al. 2010). A water reduction monograph was prepared detailing many in-mill strategies to minimize the demand for fresh water for many areas of the papermaking process (PAPRICAN 2001).
Basic Wastewater Treatment Operations
All wastewater treatment systems need to accomplish three objectives: They need to remove suspended particles from the wastewater, they need to remove solubilized pollutants, and they need to eliminate acute toxicity. Conventionally this is done in two steps, starting with gravity separation, i.e. primary treatment, with the use of a clarifier or a set of clarifiers. Flotation may be used in place of gravity separation in some cases. The second step is biological wastewater treatment, i.e. “secondary treatment”. The general principles and operations of conventional and state of the art wastewater treatment technologies, with emphasis on P&P mill applications, have been reviewed (Gehm 1973; Scott and Ollis 1995; Kamali and Khodaparast 2015).
Primary treatment: Clarification by settling
Gravity-based separation systems are dependent not only on a difference in density between the contaminant and the liquid medium, but they also require that the solid particles be large enough to settle at a fast enough rate. The latter objective is typically achieved by agglomerating the particles together, noting that larger particles of the same general type generally settle more rapidly (Leitz 1993; Matko et al. 1996; Fernandes et al. 2014).
Although settling can be achieved in a simple unagitated “pond” or “stabilization basin” (Gehm 1973), it is generally preferred to employ a circular-shaped clarification basin, equipped with a slowly-rotating skimmer and raking device (Lugowski 1991; Grijspeerdt et al. 1996; Albertson and Wilson 1997). The skimmer picks up foam and any lighter-than-water material that has floated to the surface. The raking device, operating at the floor of the basin, pushes settled sludge into a collection system. Hynninen (1998) describes common designs of clarifier equipment, some of which employ baffles to regulate the direction of flow or create laminar conditions as wastewater moves through the unit. As might be expected, primary sludge from papermaking facilities tends to have a high content of fine cellulosic matter and minerals such as calcium carbonate or clays (Mahmood and Elliott 2006).
Dissolved air flotation units
In addition to settling, flotation can be found as an alternative for some P&P facilities to clarify mill effluent. This method is commonly referred to as a dissolved air flotation (DAF) system. As illustrated in Fig. 8, such devices inject a pressurized stream of air-saturated water at the base of a shallow chest containing the process water from the paper mill. Upon reduction of pressure, as the injected water is released into the chest, tiny bubbles of air come out of solution and begin to rise. The rising bubbles tend to carry along any solid particles to which they adhere. The solids then are able to be skimmed from the surface of the water.
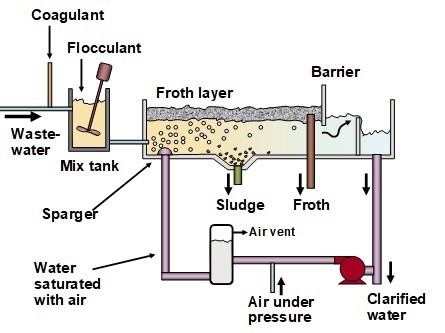
Fig. 8. Schematic diagram of a dissolved air flotation clarifier unit in operation
DAF units have been found to be cost effective for treatment of large water flows transporting a wide range of solids content (300 to 5000 mg·L-1) (Ackermann et al. 2000). In fact, it is possible to implement up to five DAF units (first loop, second loop, paper machine loop, sludge treatment, and effluent treatment) in recycled paper mills; which may efficiently remove 80 to 98% of the suspended solids, as well as a wide variety of contaminants such as ink particles and lipophilic extractives. Furthermore, it is possible to remove finely dispersed and colloidal organic particles (>0.2 μm) using appropriate coagulant and flocculant aids. On the other hand, there is a limit of about 20%, based on the COD, for the reduction of organics by use of traditional coagulants (Miranda el al 2009b), but greater reductions can be achieved with hybrid coagulants (Miranda et al. 2015). Finally, sludge from DAF units may be jointly treated in some mills with the sludge coming out from the biological wastewater treatment plant.
Secondary treatment: Activated Sludge
The most common system to remove organic material from wastewater, with 100 years of tradition, involves aeration and the recirculation of a portion of the sludge back to the intake of the system, i.e. an activated sludge system (Liver et al. 1993; Pere et al. 1993; Scott and Ollis 1995; Nakamura et al. 1997; Novak et al. 1998; Sarlin et al. 1999; Thompson et al. 2001; Diez et al. 2002; Kostamo and Kukkonen 2003; Mahmood and Elliott 2006; Agridiotis et al. 2007; Chakrabari et al. 2008; Elliott and Mahmood 2012; Kaluža et al. 2014). Figure 9 provides a schematic illustration of a clarifier unit that would follow such treatment.
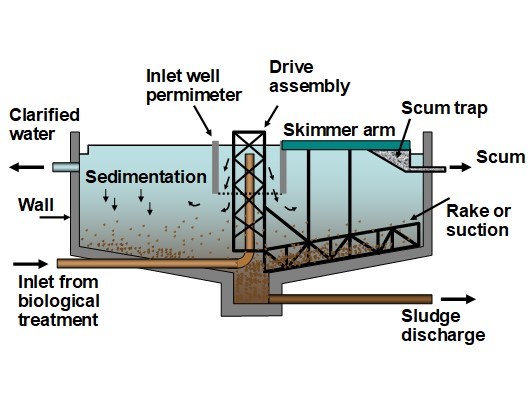
Fig. 9. Schematic illustration of a clarifier, as usually employed after a typical aerated biological system
When further efficiency is needed or there is not sufficient space for a gravity-based clarifier, a membrane bioreactor (MBR) or other type of bioreactor with a mixed or moving bed can be used. MBRs are able to operate in a submerged design, thus requiring low trans-membrane pressure (TMP) working values, which minimize fouling effects. Moreover, as microfiltration (MF) or ultrafiltration (UF) membranes are installed to separate sludge, MBR technology will not experience problems associated with filamentous bulking. Furthermore, incorporating membrane treatment into biological processing can allow the reactors to run with a higher dried solid concentration (8 to 15 g•L-1) than conventional activated sludge (3-5 g•L-1), therefore producing less biological sludge and improving the degradation of organics. These properties can make it possible to reduce hydraulic retention times and/or volumes needed to carry out effective biological treatment (Judd 2006).
Sludge thickening
Secondary sludge, generated during biological treatment operations, is primarily composed of biological cells and their decomposition products. As a consequence, such sludge is notoriously difficult to extract water from. Each biological cell can be regarded as being like a little water balloon. To compensate for the poor dewaterability, it is common practice to blend the secondary sludge with primary sludge, which is composed of fibrous materials, achieving a mixture that is more amenable to dewatering (Elliott and Mahmood 2005). A variety of methods have been employed to raise the solids content of secondary sludge, rendering it more suitable for transportation and beneficial use or landfilling (Gehm 1973; McKeown 1979; Mahmood and Elliott 2006). These include the effect of the raking or scraping of sludge at the floor of a clarifier, various pressing devices, and centrifugation. One of the most popular and effective devices for decreasing the water content of sludge is the belt press (Nichols et al. 2003). Screw presses also can be found at many P&P facilities and generally provide a dryer final sludge cake (Dorica et al. 1999).
Polishing treatments
To reach the discharge requirements, a wide variety of treatment options have been listed as possible polishing or “tertiary” treatments (Gehm 1973; Chen and Horan 1998; Rajvaidya and Markandey 1998; Kamali and Khodaparast 2015). In particular, the colored, hard-to-biodegrade compounds resulting from delignification and bleaching processes in P&P mills often survive the first two stages of wastewater treatment. As tertiary treatments for such water, authors have described approaches such as coagulation, adsorption onto activated carbon (Chen and Horan 1998; Rajvaidya and Markandey 1998), advanced oxidation systems (Helbe 1999; Lucas et al. 2012), membrane technologies (Quezada et al. 2014), and electrocoagulation (Zodi et al. 2011). Such options will be discussed in more detail in later sections of this article.
ADVANCES IN PRIMARY CLARIFICATION
Although much of the following section deals with primary treatment, several of the key principles, such as the fundamentals of settling, also can be applied to enhance the secondary clarification of biological treated effluent in secondary clarifiers.
Stokes Settling
Rates of sedimentation in a gravity field can be estimated based on the simplifying assumption that the suspended particles or agglomerates of particles can be modeled as perfect spheres, making it possible to employ the Stokes equation (Lamb 1994),
![]() (1)
(1)
where V is the terminal velocity of settling, rp is the density of the particulate matter (which needs to account for any fluid within agglomerated solids), rf is the density of the fluid, m is the dynamic viscosity of the fluid, g is the gravitational acceleration, and R is the particle radius (modeled as a sphere). As shown by Eq. 1, if one models suspended matter as a variety of spheres or spherical clusters, then the rate of settling can be expected to depend on two factors – the average equivalent diameter and the effective density. One of the challenges in clarification of wastewater is the fact that the agglomerated matter is likely to have a lower effective density than that of the component particles that make up a cluster of particles. That is because the agglomerate is likely to function as an assembly of the solid matter plus pockets of water. This implies that an ideal coagulating agent or process ought not only to increase the effective size of the units of suspended matter, but it also ought to produce relatively dense clusters.
Colloidal Mechanisms
The use of gravity settling systems or DAF units as a first step in the treatment of water is highly dependent on whether or not the suspended particles in the mixture have a tendency to stick together during random collisions. The ability of particles to remain suspended without forming agglomerates is called colloidal stability. Factors affecting colloidal stability have been discussed in several articles dealing with the treatment of wastewater from P&P facilities (Leitz 1993; Novak et al. 1998; Wang and Pan 1999; Huang and Pan 2002; Opedal et al. 2011; Nawaz et al. 2014). Mikkelsen et al. (1996) showed that the colloidal stability of activated sludge solids had large effects on the floc size of sludge particles.
Colloidal destabilization
One of the key factors affecting the colloidal stability in wastewater is the surface charge of particles. Most biological materials bear a negative electrical charge, which can be attributed to the dissociation of carboxylic acid groups (Herrington and Petzold 1992; Stenius and Laine 1994; Laine 1997). Repulsive forces develop as these like-charged surfaces approach each other at distances less than a few nanometers (Hiemenz and Rajagopalan 1997). The most common strategy for destabilization of colloidal suspensions involves the addition of ionic materials having an opposite charge and high valence. The dosage of such additives is important; an overdose of a strongly positively charged additive may reverse the charge of the suspended solids, leading to a restabilized system (Agridiotis et al. 2007).
As illustrated in Fig. 10, chemical-based clarification may be regarded as a three-step process.
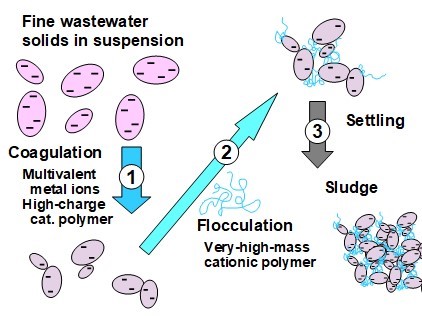
Fig. 10. Schematic representation of three key steps (coagulation, flocculation, and settling or flotation) in a chemical program to enhance separation of finely-suspended solids in either primary clarification or in clarification following a biological wastewater treatment stage
In the first step, as recommended by the downward arrow, the system is treated with a coagulant, such as aluminum sulfate (papermaker’s alum) or a high-charge cationic polymer (e.g. poly-dimethylamine-epichlorohydrin). As suggested in the figure, such treatment decreases the negative character of the surfaces of the suspended particles, allowing them to come together and stick, due mainly to the London dispersion component of van der Waals forces (Hiemenz and Rajagopalan 1997).
Inorganic Coagulants
Positively charged multivalent ions are widely used to neutralize negatively charged particles of cellulosic matter. According to an empirically-based relationship known as the Shulze-Hardy rule (Schulze 1882; Hardy 1900), the ability of an oppositely charged ion to bring about coagulation is related to the inverse sixth power of its valence. In other words, the concentrations of different cations to bring about coagulation are according to the following relationship (Eq. 2),
CNa+ = 26 * CCa2+ = 36 * CAl3+ (2)
where the C terms represent the relative concentration of the different ions to bring about the same degree of coagulation of a given suspension. The ratio of concentrations represented by the series shown in Eq. 2 is 1 : 1/64 : 1/729. It follows that more highly charged cations can be hugely more effective in coagulation than lower-charged ions such as Na+ and Ca2+.
Aluminum sulfate
Aluminum sulfate, which is often called “alum,” is a widely used coagulant for wastewater treatment. The use of alum for coagulation and settling of suspended solids in the wastewater from P&P facilities has been frequently reported (Rudolfs and Amberg 1953; Carter and Sigler 1981; Stephenson and Duff 1996; Ganjidoust et al. 1997; Chen and Horan 1998; Rohella et al. 2001; Mahesh et al. 2006; Agridiotis et al. 2007; Ahmad et al. 2007, 2008; Žarković et al. 2011; Simonič and Vnučec 2012; Nawaz et al. 2014).
Although the hydrated Al3+ ion almost certainly is prominent in highly acidic solutions of aluminum sulfate, research has shown that alum’s remarkable ability to coagulate suspensions of negatively charged particles can be attributed to oligomeric species comprised of several aluminums in combination with OH– ligands (Akitt et al. 1972a,b; Arnson and Stratton 1983; Bottero and Fiessinger 1989; Exall and vanLoon 2003; Bi et al. 2004; Zhao et al. 2008). The ratio between the amounts of Al and OH– in the system has been found to be critical in the performance of the coagulant species (Strazdins 1986, 1989; Öhman et al. 1997). An especially stable and well-studied aluminum oligomer has the formula AlVI12(H2O)24AlIVO4(H2O)127+ (Akitt et al. 1972a; Bottero and Fiessinger 1989).
Figure 11 represents the partial hydroxylation process by which trivalent aluminum ions, present in highly acidic aqueous solution, can be transformed to this advantageous species under suitable conditions of hydroxylation. This ion has been shown to be remarkably effective for the destabilization of colloidal suspensions, including wastewater (Exall and vanLoon 2003; Zhao et al. 2008).
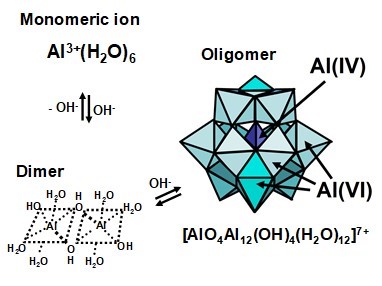
Fig. 11. Representation of the monomeric ionic species of aluminum, in contrast with the highly symmetric, high-valence oligomeric species that appears to play a key role in effective coagulation processes
Polyaluminum chloride (PAC)
The coagulating agent poly-aluminum chloride (PAC), which contains partially hydroxylated aluminum, can be regarded as a ready-to-deliver recipe containing some of the most effective ionic species for coagulation (Jiang and Graham 1997; Lartiges et al. 1997). Usage of PAC and related products for the treatment of P&P wastewater has been reported (Stephenson and Duff 1996; Wang and Pan 1999; Nandy et al. 2002; Agridiotis et al. 2007; Ahmad et al. 2008; Renault 2009; Wang et al. 2011; Bennani et al. 2012; Dhakhwa et al. 2012; Simonič and Vnučec 2012). Alternative products such as polyaluminum nitrate sulfate (PANS) are also being used (Latour et al. 2013; Miranda et al. 2015). An advantage of PANS is that it minimizes the input of chloride, which can be corrosive to equipment.
Iron products
Iron salts such as ferric chloride and ferrous sulfate are often used as coagulants for wastewater treatment (Nandy et al. 2002; Agridiotis et al. 2007; Dhakhwa et al. 2012). The ion Fe3+ ion is similar to Al3+ in terms of valence and its strong tendency to complex with OH– ions. The fact that iron compounds are highly colored generally precludes their usage in papermaking applications, but for water treatment they can offer a less expensive alternative compared to aluminum salts in some cases.
Massive lime (CaO)
The so-called “massive lime” strategy for the treatment of wastewater from P&P mills takes advantage of the fact that large amounts of calcium oxide are routinely combined with water (i.e. “slaked”) and then converted to calcium carbonate during the process of regenerating the pulping chemicals that are used for kraft cooking of the fibers (Gehm 1973; Rajvaidya and Markandey 1998; Eskelinen et al. 2010). The following recipe for this process is provided by Rajvaidya and Markandey (1998): The CaO generated in the calcining operation of a kraft pulp mill needs to be mixed with water (slaked) to yield Ca(OH)2. Instead of mixing the CaO with fresh water, in the massive lime process it is instead combined with wastewater, and the resulting Ca(OH)2 is allowed to settle. The sludge then is used in the causticizing process to prepare the kraft pulping liquor (white liquor). Because the organic solids are returned to the chemical recovery system for kraft pulping, most of them eventually become incinerated, with the production of steam, which is used in the manufacturing process.
pH
It is also technically possible to neutralize the surface charge of biological materials by lowering the pH of the solution. By reducing the pH to about 2, essentially all of the carboxyl groups present will be protonated, giving a neutral charge and encouraging coagulation. However, such an approach is generally regarded as impractical in full-scale operations, and there are few mentions of this strategy in the literature (Pere et al. 1993; de los Santos Ramos et al. 2009). For example, de los Santos Ramos et al. (2009) lowered the pH to 1 or 3 in order to precipitate lignin from the solution before ozone treatment.
Organic Coagulants
Highly cationic organic polymers are often preferred over inorganic coagulants because of the potential for forming denser or larger agglomerates that settle more rapidly (Ghosh et al. 1985; Leitz 1993; Aguilar et al. 2003; Al-Jasser 2009; Ariffin et al. 2012). Several investigators have described the usage of such agents in the treatment of wastewater from P&P facilities (Ganjidoust et al. 1997; Pere et al. 1993; Rohella et al. 2001; Al-Jasser 2009; Razali et al. 2011). Ariffin et al. (2012) found that poly-diallyldimethylammonium chloride (poly-DADMAC) was effective for coagulation of suspended matter into small flocs. Razali et al. (2011) found that the effectiveness of poly-DADMAC as a coagulant for P&P mill wastewater increased with molecular mass. It seems likely that the observed effect of molecular mass was related to a “charged patch” mechanism in which the polyelectrolyte adsorption created a contrast between covered areas having positive charge and uncovered areas having a negative charge. Such conditions have been shown to bring about more effective coagulation at low dosage than mere charge neutralization, especially if the generated charged patches are large enough (Sandell and Luner 1974; Goossens and Luner 1976; Pfau et al. 1999; Horn 2001).
There has been much interest in the use chitosan as a coagulating agent, in light of the fact that it is the only cationic polymer that can be easily isolated from a natural product. Chitosan is made from chitin, which is found in the shells of shrimp and crabs. The chitin is deacetylated to form chitosan by treatment with NaOH. The following authors have demonstrated its effectiveness for coagulating P&P wastewater (Ganjidoust et al. 1997; Rodrigues et al. 2008; Renault et al. 2009; Wang et al. 2009). Rodrigues et al. (2008) reported that the chitosan treatment favored settling and the formation of a dense sludge. Miranda et al. (2013) showed the efficiency of chitosans for the treatment of process water by DAF.
Hybrid Coagulants
Polyaluminum chlorides (PACs), as well as polyaluminum nitrate sulfate (PANS), have been extensively used in the last decades for wastewater treatment in the paper industry (Pernitsky and Edzwald 2006; Miranda et al. 2009b, 2015). These products can be modified with organic polymers to create inorganic-organic hybrids or composite coagulants, which improve the coagulation performance of the inorganic salts alone due to the synergy between the individual components (Lee et al. 2012; Latour et al. 2015). The organic coagulants offer the advantage of lower dosages, broader pH operating ranges, and smaller sludge production (de Nardi et al. 2008; Miranda et al. 2009b). Although these organic polymers are more expensive, considerable savings on coagulant dosage can be made when they are combined with inorganic salts for the same removal performance (Bolto and Gregory 2007; Miranda et al. 2009b; Latour et al. 2013).
Flocculants
The main function of flocculants, which consist of very-high-mass polyelectrolytes, usually having a cationic charge, is to collect small agglomerates into large agglomerates.
According to Ghosh et al. (1985), the agglomerates formed by means of flocculants tend to be stronger and more shear-resistant than agglomerates brought about just by charge neutralization. Early researchers studying how such polyelectrolytes work in bringing about agglomeration coined the term “bridging,” since it was possible to destabilize suspensions even when there was a net surface charge preventing the particles from coming close to each other (La Mer and Healy 1963). With their very long chains, flocculants are able to overcome such barriers by adsorbing on two particles simultaneously. Such polyelectrolytes have become widely used in clarification of wastewater from P&P mills (Wang and Pan 1999; Wong et al. 2006; Ahmad et al. 2007, 2008; Žarković et al. 2011; Ariffin et al. 2012; Simonič and Vnučec 2012).
Wastewater treatment operations commonly use sequential combinations of treatment with a coagulant, then with a cationic flocculant of very high mass but low charge density (Ghosh et al. 1985; Ahmad et al. 2007, 2008; Wang et al. 2011; Žarković et al. 2011; Ariffin et al. 2012; Simonič and Vnučec 2012). The idea is to employ a suitable amount of a relatively inexpensive coagulant to reduce the surface charge of the suspended matter. The flocculants, which are more expensive, would be wasted if they came into contact with an excess of negative charges in the system. In some systems it can be very effective to “sensitize” the suspension with the addition of a sufficient amount of cationic coagulant, then use an anionic flocculant to bring about precipitation of large agglomerates (Wang and Pan 1999). Miranda et al. (2008, 2009c) investigated the effect of flocculant charge density and of new chemicals on the removal of contaminants by DAF. By contrast, nonionic polyacrylamide, which does not have any electrostatic attraction to either type of charged sites on wastewater solids, has been found to be ineffective for the treatment of wastewater from a P&P mill (Ganjidoust et al. 1997).
Enhanced Clarification
Dosage optimization
Because the composition of wastewater tends to be highly variable, it follows that the optimum treatment level of a coagulant is likely to change over time. One common way to deal with such a situation is to equip the plant operator with a laboratory and a set of matched stirrers in a matched set of beakers. The operator then treats the contents of several beakers with a different level of coagulant and finds out which of the treatment levels bring about the most effective coagulation and settling. Follow-up tests might be done if the best treatment level appeared to be outside of the tested range. A further challenge faced by operators of wastewater treatment plants is that the coagulant by itself sometimes is insufficient to bring about a distinct change in the appearance of the mixture; rather, the desired rapid settling and large flocs might occur only upon addition of the flocculant, and then only if the coagulant had been added within a narrow optimum window of treatment level.
The situation just described can be addressed by use of a charge demand titration (Hubbe et al. 2012b). The charge demand corresponds to the amount of a strongly charged polyelectrolyte that needs to be added to reach a charge-neutral condition of the surfaces of the suspended matter. One of the most popular ways to sense the neutralized conditions, thus facilitating the needed titrations with polyelectrolytes, is by means of a streaming current detector (Gerdes 1966; Barron et al. 1994; Chen et al. 2003). The use of streaming current instruments to enable the addition of optimal amounts of flocculant to wastewater, thus ensuring reliable removal of solids, has been considered in various articles (Dentel and Kingery 1989; Dentel 1995; Mahmood and Elliot 2007; Yap et al. 2012; Al Momani and Ormeci 2014; Ratnaweera and Fettig 2015).
Focused beam reflectance probe measurements have also been demonstrated as being very useful to monitor and optimize flocculation processes. Flocculation performance can be monitored on-line, allowing a continuous assessment and insights into flocculation mechanisms (Rasteiro et al. 2008; Blanco et al. 2002a,b). This approach has been used to optimize dissolved air flotation systems for pulp and paper mills (Negro et al. 2005; Saarimaa et al. 2006a,b; Miranda et al. 2008, 2009b, 2013).
Particulate coagulation aids
Minerals such as clay and calcium carbonate, which are much denser than water, have been considered as a way to increase the rate of settling in a wastewater treatment operation. For example, Carter and Sigler (1981) employed clay in combination with poly-DADMAC as a coagulant to treat paper mill wastewater. The “massive lime” treatment mentioned earlier might be regarded as essentially the same kind of approach. Diatomite and other solid materials are often added as “filter aids” during the clarification of wastewater with the goal of increasing the rate of press dewatering of the resulting sludge (Zhao et al. 1995; El-Safey et al. 2005). Any of these mineral products can be expected to increase the density of agglomerated matter in the suspension, thus increasing the rate of settling.
In addition to mineral products, modified lignin has been found to be effective in promoting the settling of proteins in wastewater (Wang et al. 2014). Kraft lignin that had been irradiated with gamma rays was found to be somewhat more effective in wastewater from an enzyme production facility. The benefit was correlated with a drop in phenolic hydroxyl content and an increased water solubility of the lignin.
Electrocoagulation
As an alternative to the direct addition of coagulating chemicals, similar effects can be obtained by an electrochemical method (Mollah et al. 2001; Chen 2004). Electrocoagulation uses the oxidation of either an aluminum or an iron electrode, which functions as the anode in contact with the water to be treated (Mollah et al. 2001; Emamjomeh and Sivakumar 2009). Figure 12, based on a system studied by Pérez–Sicairos et al. (2011), illustrates an example of such a system. Several authors have described experiments in which electrocoagulation was used to clarify wastewater from P&P mills (Ugurlu et al. 2008; Khansorthong and Hunsom 2009; Behrooz et al. 2011; Katal and Pahlavanzadeh 2011; Vepsäläinen et al. 2011a,b; Zodi et al. 2011; Al-Shannag et al. 2012; Antony and Natesan 2012; Bellebia et al. 2012; Chanworrawoot and Hunsom 2012; Lewis et al. 2013; Shankar et al. 2013). All of the cited studies reported positive results. There was no clear indication of markedly different results relative to conventional coagulation by means of chemical additives.
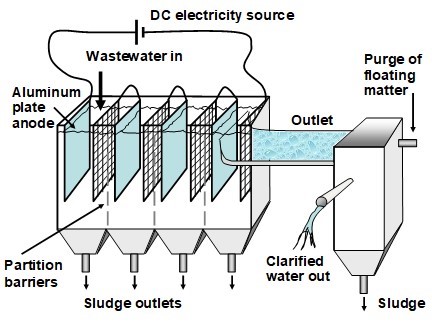
Fig. 12. Schematic illustration of an electrocoagulation system in which an aluminum electrode serves as the anode, releasing soluble ionic species of aluminum (redrawn based on a system studied by Pérez–Sicairos et al. (2011)
Because the end result of electrocoagulation is very similar what can be achieved by the direct addition of inorganic coagulants such as alum or iron salts, it is worth considering whether there are any potential advantages of employing a system that requires the installation of electrodes, which need frequent replacement, in addition to a system for supplying a controlled voltage between the electrodes. According to Mollah et al. (2001), electrocoagulation can avoid the introduction of excess ions to the treated water. In a well-designed system, the metal ions produced at the anode eventually associate with OH– ions, leading to the generation of charge-neutral sediment. Moreover, unlike the use of alum or ferric chloride, there is no addition of chloride or sulfate ions to the water. Avoidance of sulfate ions can be important, both to avoid problems related to sulfate reducing bacteria (Roest et al. 2005; Janssen et al. 2009) and because sulfate ions have been shown to suppress the availability of coagulating species such as Al3+ (Akitt et al. 1972). There is also a possibility that by in-situ generation of the metal cations, within the medium that is being treated, the metal ionic species might be different initially from what can be achieved during addition of an inorganic coagulant. According to Mollah et al. (2001), another key reason why some operators prefer electrocoagulation is because it has a reputation of yielding lower amounts of sludge.
A key limitation of the electrocoagulation method is that the solution to be treated needs to have a relatively high electrical conductivity. Chen (2004) described the optimization of conditions for electrocoagulation of a variety of wastewaters and noted that an optimum range of current density may minimize the wastage of energy, the heating of the wastewater, and the fouling of the cathode surfaces. Best results can be expected with relatively salty effluents or solutions with pH values several units away from the neutral point (such as those from ECF bleaching effluents).
Electrode type
Several studies have considered the use of iron electrodes, aluminum electrodes, or a comparison of both to bring about destabilization of P&P mill wastewater. The following authors considered only the use of iron electrodes: Khansorthong and Hunsom 2009; Vepsalainen et al. 2011b; Al-Shannag et al. 2012. Al-Shannag et al. (2012) found that the results could be enhanced by carrying out the treatment in the presence of iron sulfate or calcium carbonate additives. Vepsalainen et al. (2011b) found that the ferrous ions produced by such treatment were very effective for the removal of sulfide ions from pulp mill effluent.
Iron and aluminum electrodes have been compared in several studies involving wastewater from P&P mills. Boroski et al. (2008) and Uğurlu et al. (2008) found fairly similar performance, whereas Zodi et al. (2011) obtained more favorable results with the iron electrodes. Katal and Pahlavanzadeh (2011) found positive results with either kind of electrode over a wide range of pH (5 to 7), making it feasible to treat the paper mill wastewater without the adjustment of pH. Bellebia et al. (2012) found that an aluminum electrode system had a lower optimum pH (5.3) compared to an iron system (pH=7.2).
Coagulants used with electrocoagulation systems
A few studies have shown benefits of combined treatments with electrocoagulation in the presence of conventional coagulating agents such as alum or iron compounds (Mahesh et al. 2006; Al-Shanning et al. 2012). For instance, Mahesh et al. (2006) found that treatment with aluminum sulfate after electrochemical degradation of paper mill wastewater contributed to further reduction in oxygen demand and improved settlability. Al-Shanning et al. (2012) employed either iron sulfate or calcium carbonate, both of which substantially increased the efficiency of removal of chemical oxygen demand (COD) components from the water. By contrast, Khansorthong and Hunsom (2009) did not find any benefit of polyelectrolyte addition during an electrocoagulation process, with wastewater from a P&P facility.
Dissolved air flotation used with electrocoagulation systems
Dissolved air flotation (DAF) often has been incorporated into electrocoagulation (Boroski et al. 2008; Emamjomeh and Sivakumar 2009). According to Mollah et al. (2001), microbubbles, presumably hydrogen gas (Chen 2004) formed at the cathode of an electrocoagulation system, can contribute to the flotation of suspended particles. According to Chen (2009) the hydrogen bubbles formed in a typical electrocoagulation unit are smaller (usually 15 to 45 mm) in comparison to a typical DAF unit (usually 50 to 70 mm bubble size).
Conventional DAF units have a variety of applications in separating solids from water. Such equipment also is widely used, in combination with surfactants, to remove hydrophobic ink particles from aqueous mixtures during the deinking of recycled printing papers (Ashley and Heindel 1999; Heindel 1999).
DAF systems rely upon the fact that increasing amounts of gas can be made to become solubilized in water by the application of pressure (Thompson et al. 2001; Edzwald 2010). Then, if the pressure is suddenly reduced, the dissolved gas quickly forms itself into small bubbles, just as it does when a shaken soda can is opened. When this takes place in the presence of suspended particles, the rising bubbles collide with particles, which then can be lifted to the water surface and collected there. Such separations have been shown to be enhanced by addition of coagulants (Meyssami and Kasaeian 2005). Furthermore, Han et al. (2006) observed the highest flotation efficiency following treatment with coagulant, resulted in an opposite sign of effective charge of the surfaces of bubbles and solid particles.
ADVANCED OXIDATION SYSTEMS
An inherent difficulty in the treatment of wastewater from kraft pulping and the bleaching of kraft pulps, is the resistance of lignin-related species to biodegradation (Lindholm-Lehto et al. 2015). This problem has been often addressed by advanced oxidation processes (AOPs) (Esplugas et al. 1994; Masten and Davies 1994; Hörsch et al. 2003; Oller et al. 2011; Merayo et al. 2013; Hermosilla et al. 2015). AOPs can be regarded as an enabling step before biological treatment of wastewater from P&P facilities because they may increase biodegradability as well as decompose bio-refractory organic substances (Oller et al. 2011; Merayo et al. 2013). The efficiency of subsequent biological treatment stages can be increased (Kamali and Khodaparast 2015). Whether they are used as a pretreatment or for the polishing of water after biological treatment, AOPs can reduce organic refractory and toxic compounds from pulp and paper mill effluents (Catalkaya et al. 2003; Hsueh et al. 2005). AOPs include ozone, various oxidizing species used in combination with catalysts or UV light, and the Fenton method, which is a catalytic oxidation method based on electron transfer between H2O2 and metal ions (Fe2+) serving as homogeneous catalysts (Ince et al. 1997; Catalkaya and Kargi 2007).
Ozonation
Ozone is a powerful oxidant and has been studied since the 1970s (Bauman and Lutz 1974; Rodríguez et al. 1998). It is a highly energetic form of oxygen that has the capability of breaking down many hard-to-biodegrade organic compounds found in pulping and bleaching wastewaters in two ways, directly reacting with dissolved substances, and indirectly producing hydroxyl radicals; the reaction is improved under basic pH conditions, without the need to apply ultraviolet light, use a catalyst, or add iron compounds (Rice 1997; Alvares et al. 2001; Pokhrel and Viraraghavan 2004; Tripathi et al. 2011; Hermosilla et al. 2015). There have been many investigations devoted to the treatment of P&P wastewater using ozone by itself (Prat et al. 1988; Heinzle et al. 1992; Chen and Horan 1998; Rodrígues et al. 1988; Heble et al. 1999; Yeber et al. 1999b; Freire et al. 2000; Amat 2005b; Kreetachat et al. 2007; Wang et al. 2008b; de los Santos Ramos 2009; Kishimoto et al. 2010; Merayo et al. 2013; Cheng et al. 2015; Hermosilla et al. 2015). Enhanced biodegradation of contaminants owing to the inclusion of an ozone pre-treatment of industrial wastewaters has been reported (Yeber et al. 1999b; Heinzle et al. 1992; Wang et al. 2008b; de los Santos Ramos 2009; Tripathi et al. 2011).
Ozone use as a post-treatment after biodegradation was found to be favorable by Merayo et al. (2013). The review article by Alvares et al. (2001) advocates the “partial” treatment with ozone as a pretreatment for biological wastewater treatment; such an approach has the potential to minimize the high costs generally associated with ozone use. Heble et al. (1999) found best results when using two stages of ozonation and an intermediate biodegradation stage. Baig and Liechti (2001) reported that the alternate combination of bio-filters after ozonation stages (O3+bio-filter+O3+bio-filter) improved the removal of the COD and reduced the overall cost of the treatment. One of the successes of ozonation for the treatment of real wastewater from pulp and paper mills, whether biologically pre-treated or not, is their capability to produce an effective degradation of toxic lignin products and chlorophenolic compounds and the reduction of color (Hermosilla et al. 2015).
Ozone reacts with colored and halogenated organic compounds; it improves biodegradability of recalcitrant compounds by degrading and transforming HMW compounds into smaller and more harmless fractions (Masten and Davies 1994; Zhou and Smith 1997). However, it is still an emerging method for wastewater treatment, even though it is widely used in drinking water preparation (Zhou and Smith 1997; Freire et al. 2001). By ozonation, even 80 to 90% reductions in color have been reported (Catalkaya and Kargi 2007; Meza et al. 2011). The efficiency of ozonation depends on the process operating conditions and the quality of wastewater. Furthermore, it is a rather expensive process and has been shown to be more efficient in decoloration than in COD removal (Pokhrel and Viraraghavan 2004; Lei and Li 2014). Ozonation has successfully been used to oxidize chemicals in pulp and paper mill effluents, such as eugenol, cathecol, vanillin, guaiacol, syringaldehyde, phenol, chlorinated phenols, and cinnamic acid derivatives (Amat et al. 2005b; Fontanier et al. 2005a). In addition, ozone oxidation of resin acids has been shown to be relatively effective (Korhonen and Tuhkanen 2000; Ledakowicz et al. 2006).
The combination of ozone and hydrogen peroxide (O3/H2O2) has also been proposed to remove refractory organic chemicals, but hydrogen peroxide leads to faster ozone degradation and higher O3 consumption (Masten and Davies 1994; Gogate and Pandit 2004a,b; Amat et al. 2005b; Mounteer et al. 2007b).
Although ozone treatments generally have been carried out without the assistance of a catalyst, a recent study by Cheng et al. (2015) showed a benefit of using a heterogeneous Fe-Mn sepiolite catalyst in combination with the ozone treatment of wastewater from papermaking processes. By enhancing decomposition of organic matter, catalytic ozonation has been found to lower the amount of ozone required (Fontanier et al. 2005b). The almost complete mineralization of compounds, including phenol, chlorinated phenols, guaiacol, vanillin, catechol, and syringaldehyde, has been achieved by using cobalt as the active metal deposited on a mineral catalytic support (Fontanier et al. 2005b). Other combinations, e.g. peroxymonosulfate salt (2KHSO5:KHSO4: K2SO4) and ozone led to a reduction of color and COD (79 % and 14 %) but a negligible TOC removal (Joss et al. 2007). The CO32- ion negatively affected the ozone oxidation, manifesting a radical scavenging effect under neutral and basic conditions (Barndõk et al. 2012).
Balcioglu et al. (2007) proposed the treatment of bleaching effluents from a pulp mill by ozone assisted by granulated activated carbon (GAC) as catalyst. COD removal improvement was 23% relative to direct ozonation and 15% in comparison to standalone GAC. Ko et al. (2009) also found similar results. In addition, the reduction of COD of a bleaching wastewater from a kraft pulp and paper mill was increased by more than 25% by the addition of 5 mM Fe2+ or Mn2+ in comparison to its ozone treatment (Balcioglu and Moral 2008).
Ozonation is the most frequently employed AOP in combination with biological treatment processes (Bijan and Mohseni 2005; Balcioğlu et al. 2006). Pre-ozonation before biological treatment can prevent process failure and improve the purification efficiency by decreasing BOD and COD load (Tuhkanen et al. 1997; Thompson et al. 2001). Another option is to use ozone after biological treatment (Hostachy et al. 1997; Chow et al. 1999). Secondary reactions of ozone with the by-products are in some cases unwanted, creating new compounds, such as ketones, organic acids, and aldehydes of differing toxicity (Hostachy et al. 1997; Latorre et al. 2007).
Fenton and photo-Fenton processes
The Fenton method and its variants are discussed by Bautista et al. (2008). Such processes make use of the catalytic reaction between hydrogen peroxide and ferric or ferrous ions in solution, giving rise to the HO· species with high oxidation potential, i.e. the hydroxyl radical. The latter is believed to be the key reagent in breaking down hard-to-biodegrade compounds related to lignin. The main reactions involved in a typical Fenton treatment are as shown below (Hermosilla et al. 2009).
Fe2+ + H2O2 ® Fe3+ + OH– + OH× k1 = 70.0 M-1×s-1 (3)
Fe3+ + H2O2 ® Fe2+ + OH×2 + H+ k2 = <<< k1 (4)
H2O2 + OH× ® OH×2 + H2O k3 = 3.3×107 M-1×s-1 (5)
Fe2+ + OH× ® Fe3+ + OH– k4 = 3.2×108 M-1×s-1 (6)
Fe3+ + HO×2 ® Fe2+ + O2H+ k5 = < 2.0×103 M-1×s-1 (7)
Fe2+ + HO×2 + H+ ® Fe3+ + H2O2 k6 = 1.20×106 M-1×s-1 (8)
HO×2 + HO×2 ® H2O2 + O2 k7 = 8.3×105 M-1×s-1 (9)
HO×2 + H2O2 ® OH× + H2O + O2 k8 = 3.0 M-1×s-1 (10)
Figure 13 provides a molecular view of one of the key reactions, which is a subsequent step in which Fenton species take part in degradation of an aromatic compound.
Fig. 13. Fenton reaction sequence leading to decomposition of phenol, as an example
Reactions taking place between biomass components (shown as “R”) and the Fenton reagents include the following:
HO× + RH ® H2O + R× (11)
R× + O2 ® RO×2 ® Products (12)
The conventional Fenton process has been tested in the pulp and paper industry at laboratory scale to polish different mill effluents (from kraft pulp to recovered paper mills) reporting good oxidation removal efficiencies (COD removal > 60%; Hermosilla et al. 2015), although the partially-oxidized composition of the treated wastewater did not show a reduction of toxicity to Artemia salina in comparison with the untreated effluent (Tambosi et al. 2006).
Figure 14 outlines the process sequence in a conventional Fenton system for wastewater treatment.
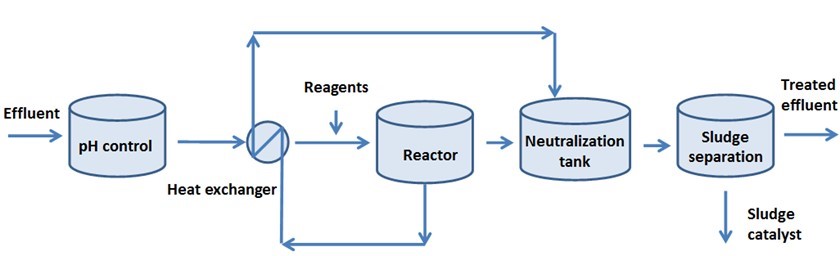
Fig. 14. Schematic of a conventional Fenton wastewater treatment system
Torrades et al. (2003) and Hermosilla et al. (2012a) showed that the Fenton reaction was more efficient when combined with ultraviolet irradiation (i.e. “photo-Fenton) when used for the treatment of pollutants in the retentate from a reverse osmosis membrane separation of contaminated water in a recovered paper mill. Similar results were reported by Jamil et al. (2011). Moreover, Merayo et al. (2016) reported good efficiency for the heterogeneous photo-Fenton treatment of lignin using zero valent iron microspheres as catalyst instead of ferrous iron.
Lucas et al. (2012) and Fernandes et al. (2014) reported effective treatment of pulp mill wastewater with a solar-Fenton process. In such systems daylight illumination is used to assist the process, significantly reducing the energy cost associated to UV-light application. Rodriguez et al. (1999) found that the decomposition of chlorophenols (which are often present in pulp bleaching wastewaters) was promoted by the addition of various dihydroxybenzoic acid or benzene species in combination with Fenton treatment.
Parilti and Akten (2011) showed the effectiveness of a hybrid system for the treatment of pulp mill wastewaters; they combined a Fenton system (ferric ions) with TiO2 as a catalyst. The main mechanism involving the application of such a catalyst is the formation of reactive oxygen barriers under which superoxide (·O2−) is produced as a result of the reduction of molecular oxygen (O2) (Turrens 2003). Such species are very reactive towards the pollutants in P&P mill wastewater.
According to Hermosilla et al. (2015), although Fenton processes seem to perform well under laboratory settings, they are not often implemented at industrial scale in comparison to ozone treatment, probably due to there being much more experience in the implementation of ozone processes. Other factors include the more frequent availability of ozone treatments for bleaching stages in paper mills, as well as some disadvantages of Fenton processes application, such as the production of iron sludge and the requirement of acidic conditions for optimal performance. These drawbacks may be overcome by using heterogeneous catalysts or by applying these treatments at neutral pH (Merayo et al. 2016; Hermosilla et al. 2012a). Beltrán-Heredia et al. (2001) tested the use of sequential treatments with Fenton reagants and ozone.
Biodegradability improvement is generally difficult to demonstrate after the application of these highly oxidizing processes, since they are not selective and also remove biodegradable material. On the other hand, their use after a biological treatment, where almost all the biodegradable fraction has already been removed, has been reported to be very efficient, achieving over 90% removal of the COD present in the effluent after the biological treatment (Hermosilla et al. 2015).
UV-catalysis (with TiO2 or peroxide)
Ultraviolet radiation alone is, in general, less effective than other AOPs, but its efficiency may be increased greatly when it is used in combination with other AOPs (e.g. UV-ozone) or in combination with H2O2 or certain catalysts (metal salts and semiconductors) (Hermosilla et al. 2015). Due to its strong absorption in the short wavelength range, UV irradiation has the potential to break down aromatic-based compounds such as lignin byproducts. This has been demonstrated, for instance, by Amat et al. (2005b), who used model compounds to represent the recalcitrant components of pulp mill wastewater. In that study the UV irradiation increased the breakdown of the model compounds under fixed conditions of ozonation. Similar results were reported earlier by Yeber et al. (1999b) in a study of bleachery wastewater and in the review article by Rice (1997). Dahm and Lucia (2004) demonstrated the breakdown of lignin and showed that the catalyst (TiO2) had to be present to ensure effectiveness of the process. Prat et al. (1988) found that combinations of UV light and H2O2, in the absence of catalyst, necessitated long treatment times and had low efficiency for the treatment of bleachery waters. Jamil et al. (2011) studied the treatment of effluent from a board paper mill by different photocatalytic processes, achieving a COD removal efficiency of ≈80% by the photo-Fenton treatment; as well as the ≈11 and 33% respectively when applying UV and UV/H2O2. In fact, 4-chlorophenol and phenol have been reported to be efficiently degraded by UV/H2O2 (Rueda-Márquez et al. 2015). In addition, Ahmed et al. (2009) found good efficiencies in the UV/H2O2 treatment of a Tunisian pulp and paper mill effluent, suggesting that the degradation of lignin derivatives and tannins leads to generation of aromatic intermediates that further undergo oxidative ring opening, producing aliphatic carboxylic acids, just as it has previously been discussed for other AOPs application. In final treatment steps, carboxylic acids may mineralize into CO2 and H2O.
The efficacy of ultraviolet light in the presence of catalysts had been demonstrated (Rodrigues et al. 2008; Bu et al. 2014). The catalyst is usually a semiconductor material, of which TiO2 particles are the most commonly applied. These methods have been shown to be effective in breaking down organic compounds derived from lignin in the wastewater from pulping and papermaking processes (Catalkaya and Kargi 2008; Merayo et al. 2013, 2016). However, Hermosilla et al. (2012a) found such a system to be less effective than photo-Fenton treatment in the case considered. Various authors have shown that such systems can be made even more effective by the addition of hydrogen peroxide (Boroski et al. 2008; Catalkaya and Kargi 2008; Ugurlu and Karaoglu 2009; Ghaly et al. 2011; Kumar et al. 2011).
Botia et al. (2012) compared TiO2 and ZnO catalysts in the presence of UV light for the treatment of pulp bleachery wastewater. The photocatalytic system resulted in very efficient removal of chemical oxygen demand (COD) with either kind of catalyst after the biological treatment with an aerated film reactor. The photocatalytic system by itself, without the biological treatment, gave rise to a high level of oxygen demand, however. Kansal et al. (2008), who studied the treatment of bleach plant effluents, found better results when using a TiO2 system rather than a ZnO system. In particular, it has been reported that photocatalytic treatments developed with a supported catalyst showed similar results as when added in suspension, but the supported type required a longer reaction time to produce the same results (Yeber et al. 1999a, 2000); although the supported system avoids the need for a recovery step. In addition, Yuan et al. (2007) showed that it was possible to attach TiO2 particles securely to activated carbon fibers; such particles were found to be effective, while loaded onto such a support, in the treatment of paper mill wastewater under UV irradiation.
As a pretreatment before biological treatment, Duran et al. (1994) showed that irradiation in the presence of ZnO markedly enhanced the effectiveness of a specific type of fungus in decolorization of the first alkaline extraction stage (E1) of kraft pulp bleaching effluent. Likewise, Goel et al. (2010) showed that such an integrated treatment was very effective for the treatment of 4-chlorophenol in water. Ruas et al. (2012) reported that the combination of H2O2 with UV irradiation was helpful as a pretreatment before anaerobic digestion to remove chlorinated organics from bleached kraft mill wastewater effluent. Jamil et al. (2011) reported 0.25, 0.45, and 0.7 increases of the BOD5/COD ratio for the treatment of an effluent from a board paper mill by UV, UV/H2O2, and photoFenton processes, respectively; as well as a significant destruction of aromatic chlorinated compounds in all the trialed photocatalytic processes. Moraes et al. (2006) achieved good efficiencies (75% color removal and an increase of mineralization of the 140% with respect to the previous biological treatment) in the application of photocatalysis as post-biological treatment of black liquor. In addition, a mineralization increase of the 45% was obtained for the pre-biological treatment of kraft effluent by photocatalysis (Moraes et al. 2006). Notably, Merayo et al. (2013) did not find any significant benefit of photocatalysis either before or after biological treatment, whereas ozone was found to be quite effective as a post-treatment.
The use of solar light instead of UV radiation has also been tested, leading to good results. Amat et al. (2005a) assessed its application to the treatment of effluents from the board industry, achieving about a 40% COD removal at the pre-industrial level and a 50% in laboratory trials.
Wet oxidation with O2
Oxygen is a much less reactive oxidant than ozone, but it can be made to react with organic compounds in wastewater by application of heat and pressure, i.e. by implementation of wet oxidation conditions (Bhargava et al. 2006). The effectiveness of such systems in treating wastewater from P&P mills has been reported (Verenich et al. 2000, 2001; Pintar et al. 2001; Akolekar et al. 2002; Garg et al. 2005, 2007; Dhakhwa et al. 2012). Verenich et al. (2000, 2001) showed that wet oxidation improved the effectiveness of a subsequent biodegradation stage of treatment. Others have demonstrated the ability of catalysts to promote wet oxidation (Verenich et al. 2000; Pintar et al. 2001; Akolekar et al. 2002; Garg et al. 2005). Bhargava et al. (2006) noted that wet oxidation treatments without the use of a catalyst can yield recalcitrant compounds having carboxylic acid functions. Pintar et al. (2001) found a good correlation between the catalytic effects and the surface areas of the solid catalysts used. Garg et al. (2005) showed that a variety of catalysts were able to promote removal of color and oxygen demand from P&P effluent at temperatures between 20 and 95 °C. By contrast, Dhakhwa et al. (2012) found the best results when using a non-catalyzed wet oxidation treatment.
Insights into the mechanism were revealed by Garg et al. (2007), who found that the pH of the solution decreased initially and then decreased in the course of wet oxidation of synthetic P&P mill effluent. This sequence was attributed to the initial formation of carboxylic acids, presumably through breakage of ester bonds, followed by the decomposition of those acids.
Herney-Ramirez et al. (2011) investigated the degradation of Acid Orange 7, which is a typical dye used in the paper industry, by a heterogeneous catalytic wet hydrogen peroxide process that used a pillared saponite as support for the impregnation with Fe (16% w:w). This catalytic process completely degraded the dye in less than 4 h of treatment. On the other hand, Baurotian et al. (2013) assessed the use of wet oxidation as pre-treatment of anaerobic biological treatment of sludge from paper mills, achieving more than a 90% destruction of extractive compounds after 20 min of wet oxidation performance. The 95.7% of phenolics, 98.6% fatty acids, 99.8% resin acids, and the 100% of phytosterols were degraded in 120 min. Moreover, acetic acid concentration significantly increased after this pre-treatment, thus making the pre-treated sludge more suitable for anaerobic digestion.
Electro-oxidation
The term “electro-oxidation” is used when voltages and other factors in an electrode system are optimized for in-situ production of reactive, oxidizing species (El-Ashtoukhy et al. 2009), in contrast to the generation of Al3+, Fe3+, or Fe2+ in the case of electrocoagulation (Mollah et al. 2001; Chen 2004). Organic compounds will therefore directly be degraded on the electrodes, and different oxidative radicals will be produced by electrolysis, including the hydroxyl radical, sulfate, and chlorine. The main factors affecting this process are: current density, electrode material, reaction time, and the characteristics of the wastewater (Hermosilla et al. 2015). According to Chen (2009), such direct oxidation takes place by a surface interaction between the organic compound and an adsorbed hydroxyl radical species. Alternatively, metal ions having a higher oxidation state, e.g. Fe3+, may serve as mediators for the transformation of the organic compounds.
Electro-oxidation has been examined by several research groups in the treatment of wastewater from P&P facilities (Mahesh et al. 2006; Wang et al. 2007; El-Ashtoukhy et al. 2009; Särkkä et al. 2009; Soloman et al. 2009; Kishimoto et al. 2010; Antony and Natesan 2012; Qu et al. 2012; Krishna et al. 2014; Lindholm-Lehto et al. 2015) even using rice straw as raw material to produce paper pulp (El-Ashtoukhy et al. 2009). Patel et al. (2008) evaluated the removal of pentachlorophenol, as well as the treatment of pulp bleaching effluent from a bamboo-based mill, by an electrochemical process, reporting the removal of 10 mg L−1 of pentachlorophenol in less than 10 minutes when performing such treatment. Eskelinen et al. (2010) aimed the treatment of some wood extractives and achieving 51%, 76%, and 83% concentration removals of 12 mg/L abietic acid, 11.8 mg/L oleic acid, and 0.6 mg/L β-sitosterol, respectively. Particular value-added products, such as vanillin and syringaldehyde, were identified in the electro-oxidized reaction media of acid bisulfite pulping effluents (Moodley et al. 2011). Dominguez-Ramos et al. (2008) obtained positive results for the treatment of lignosulfonate, which may be regarded as a component of effluent from sulfite pulping. Särkkä et al. (2009) used an electrochemical treatment to oxidize sulfides present in paper mill wastewater. Krishna et al. (2014) used the term “bioelectrochemical treatment” to describe a system in which wastewater was treated within a reactor that was set up to generate electrical current. The electrode that was identified as the cathode was at the air-water interface, and that part of the cell was separated from the rest of the aqueous system with a selectively permeable membrane. The authors reported that not only did the system outperform parallel systems carried out under anaerobic conditions without an electrochemical set-up, but also the decomposition of organic matter was greater. Loraine and Huchler (2006) reviewed the use of a variety of electrochemical approaches that have been used in Holland for treatment of P&P mill waters. Wang et al. (2007) presented evidence that a modified kaolin additive had a catalytic effect on the electro-oxidation process.
Other authors have reported the enhancement of biodegradability in the solution after its electro-oxidation treatment (Soloman et al. 2009; Antony and Natesan 2012). Particularly, anodic oxidation enhanced the biodegradability of 2,4-dichlorophenol solutions and it is a promising alternative for the degradation of chlorophenols (Chu et al. 2010). Qu et al. (2012) reported the improvement of COD removal efficiency after an anaerobic membrane bioreactor stage, suggesting that this integrated technology may produce a more suitable effluent for subsequent reuse and ultimate systems closure.
Finally, the electro-Fenton treatment, i.e. the combination of Fenton process with electrochemical oxidation, might also be an interesting alternative to improve treatment efficiency. Selvabharathi and Kanmani (2010) reported a 90% COD removal during the application of electro-Fenton to a biologically-treated newsprint paper industry effluent.
Reduction
Since the colored nature of many organic compounds can be attributed to conjugated carbon-carbon double bonds (repeated double-single-double, etc. sequences), it is logical that decolorization of pulp mill wastewater might be achieved by chemical reduction. Such an approach has been demonstrated (Calvo et al. 2007; Ghoreishi and Haghighi 2007). For instance, Calvo et al. (2007) used catalytic hydrogenation to detoxify and decolorize kraft bleaching effluents. Ghoreishi and Haghighi (2007) used the strong reducing agent sodium borohydride to decolorize P&P mill wastewater. This was followed by aerated biodegradation in a second reactor. Given the rarity of studies of this nature, this would seem to be a prime field for future research.
FILTRATION, PACKED BED, AND BIOREACTOR SYSTEMS
Various kinds of filtration procedures are used at different stages of wastewater treatment, but there has been particular interest in their usage in connection with so-called “bioreactors,” as internal or external configurations (Kamali et al. 2016). Such systems are designed to speed up biological decomposition processes in an efficient way. A later section will consider some further applications of filtration technologies when they are employed as a final polishing step before discharge of treated water.
Filtration Options
When paper mill operators are looking for ways to save money and increase the overall yield, much attention can be paid to save-all systems. The final effluent leaving from a paper machine system may be carrying away solid material that could become part of the product, rather than part of the sludge from water treatment. The most commonly used save-all systems are essentially screen systems, and they might be regarded as a first step in wastewater treatment. There is potential to minimize solids losses at that point in the paper machine system by such steps as proper maintenance of the save-all equipment, attention to providing a steady amount of suitable sweetener fiber, and the use of an effective retention aid system (Hubbe et al. 2009). Though it has been shown that retention aid can be fed directly to the intake of the save-all itself (Milliken 2006), it is often regarded as more practical just to apply such chemicals before forming the paper sheet in an effort to achieve a suitably high first-pass retention, leading to less load on the save-all operation.
Membrane filtration
When the goal is to mechanically separate contaminants smaller than about 100 mm in diameter from a suspension, then membrane technology can be a promising approach. In particular, membrane systems tend to use less energy relative to evaporation of water or centrifugation. Various authors have provided overviews of membrane-related treatments of wastewater from the P&P industry (Gehm 1973; Nuortila-Jokinen 2004; Hubbe 2007c). Different membrane products are rated according to their pore sizes, which also determine what entities (particles, molecules, etc.) they are able to effectively exclude. Membrane systems have been used by some P&P facilities to meet government regulations regarding effluent discharge. The Irving Pulp and Paper in New Brunswick, which is a 950 t/d facility, has been in operation for 15 years without the need for a biological wastewater treatment system (Hodgson et al. 1998) due to membrane usage.
Some of the most commonly mentioned types of membrane filtration are indicated in Table 1 (Khulbe et al. 2008).
Table 1. Classification of Membrane Filtrations Systems
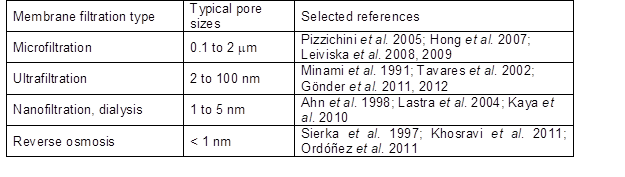
Starting from the top of Table 1, the coarsest membranes, i.e. microfiltration membranes, appear to have been mainly considered as pre-filtration membranes upstream of membranes having finer pores for wastewater applications (Le-Clech et al. 2006). Another usage has been as a means to separate and analyze different size classes of solids within P&P mill wastewaters (Hong et al. 2007; Nataraj et al. 2007; Leiviska et al. 2008, 2009). Nataraj et al. (2007) combined microfiltration with electrodialysis to purify paper industry wastewater. Sierka et al. (1997) and Ordóñez et al. (2011) showed that microfiltration or ultrafiltration steps could serve as useful pretreatments before reverse osmosis membrane separations.
Ultrafiltration (UF) membranes have been more widely used for the treatment of P&P industry wastewaters. One of the key considerations is to employ a membrane having sufficiently fine pore distribution to exclude the passage of biological cells and viruses. The following studies have employed ultrafiltration to separate clarified water from the solids in P&P mill process waters or effluent (Minami et al. 1991; Zaidi et al. 1992; Ragona and Hall 1998; Huuhilo et al. 2002; Tavares et al. 2002; Komesvarakul et al. 2003; Nuortila-Jokinen et al. 2004; Mänttäri et al. 2008; Ordonez et al. 2010; Puro et al. 2010; Gönder et al. 2011, 2012; Simonič and Vnučec 2012; Singh et al. 2012; Quezada et al. 2014). Notably, Tavares et al. (2002) were able to demonstrate the removal of metals from such effluent. Iron, magnesium, and calcium compounds were complexed by addition of polyelectrolytes and then retained during membrane clarification of the water. Ragona and Hall (1998) ran an ultrafiltration system in parallel with a bioreactor (see page 8000) and obtained good results with both systems.
Nanofiltration (NF) can be considered when the goal is to exclude large molecules from the permeate. For example, Lastra et al. (2004) were able to separate metal complexes from pulp mill wastewater by nanofiltration. Other researchers have found nanofiltration to be effective for the removal of color and hard-to-degrade organic compounds in such effluents (Ahn et al. 1998; Huukilo et al. 2002; Mänttäri and Nyström 2007; Gönder et al. 2011; Khosravi et al. 2011; Sheldon et al. 2012). Laitinen et al. (2001) found that the performance of membrane processes could be improved by an optimized program of backflushing.
Reverse osmosis (RO) membranes, which are the “tightest” membranes available, are designed to allow the passage of water but to exclude most other molecules, including salt ions. High pressures are required to force water molecules through such membranes, often in opposition to what would be expected from salt concentration gradients and the resulting osmotic pressure. They have been used in some P&P industry applications where the goal is to desalinate the water, in addition to removing other contaminants (Zhang et al. 2009; Khosravi et al. 2011; Li and Zhang 2011; Sheldon et al. 2012; Saif et al. 2013; Ordóñez et al. 2013). For instance, Khosravi et al. (2011) demonstrated the use of an RO system in removing color and oxygen demand from P&P mill effluent. The permeate flux was low, but the resulting water quality was distinctly higher than what was achieved in parallel tests involving nanofiltration. Li and Zhang (2011) found that the RO process was facilitated by pretreating the raw wastewater with a flocculant blend. Ordóñez et al. (2010) found the possibility of reusing the effluent of a recovered newsprint paper mill as fresh water (i.e. in high pressure showers) by reverse osmosis. Recovery, and therefore efficiency were limited by the presence of silica. As a consequence, several studies have been devoted to achieving the successful removal of silica as a necessary pre-treatment of membrane operation within the pulp and paper industry (Hermosilla et al. 2012b; Latour et al. 2013, 2014a-c, 2015). Results showed that the level of silica can be lowered by water softening treatment or by flocculation and separation.
When choosing among the options just presented, a compromise often needs to be reached between the ability to exclude passage of substances and the rate of flux (Quezada et al. 2014). The main drawback is that membrane filters tend to become fouled (see next subsection), as indicated by a decrease in the flux at a constant pressure. For instance, Kallioinen et al. (2005, 2006) found that the ratio of decrease in membrane permeability was related in complex ways to the chemical nature of the process water from P&P facilities. Filtration technologies merely separate, but do not change any of the components of the mixture. This is important especially in the case of highly polluted effluents. Other steps are often necessary to degrade the solid matter concentrates after being separated from the effluents.
Membrane Operational Issues
Clogging, fouling
The plugging or blocking of pores, i.e. fouling, has been a main preoccupation of those who have considered or used membrane technologies for the treatment of various types of wastewater (Puro et al. 2010). Some review articles have focused on strategies to minimize or delay fouling (Le-Clech et al. 2006; Meng et al. 2009).
Studies conducted under constant applied pressure have found an initial rapid decrease in membrane flux, followed by a more gradual decrease (Le-Clech et al. 2006). These findings suggest an initial rapid build-up of a “cake” at the membrane surfaces, followed by more gradual changes, such as either filling in of the cake structure or gradual occlusion of pores within the membrane itself. These features are illustrated in Fig. 15, which is based on concepts presented by Meng et al. (2009).
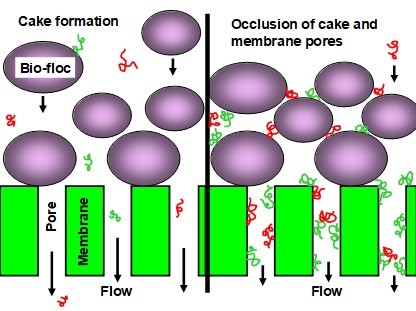
Fig. 15. Concepts of cake formation on membranes (left) and progressive occlusion of membranes (right) that are used for the filtration of wastewater. In this schematic illustration the green coiled lines represent colloidal organic matter having a generally hydrophobic nature; the red coiled lines represent negatively charged colloidal matter.
Flow strategies
Studies have shown that fouling and reduced permeability of membranes may develop more rapidly with the increase of applied pressure or flow rate (Le-Clech et al. 2006). In particular, particulate matter appears to get trapped at membrane surfaces when the flow rates are too high, whereas polymeric substances can accumulate onto membrane surfaces even in the absence of flow. Such results have suggested that membrane filtration operations ought to be conducted with optimized flow rates that are less than a critical flux value. Also, a two-phase “bubbly” flow has been found to minimize or slow the rate of fouling in some cases (Le-Clech et al. 2006). Strategies such as surface functionalization (e.g., attachment of some other materials to the surface of the membranes) are increasingly under development to resist the fouling of the membranes. Electrical treatment of the membrane is another possible way to minimize fouling (Kamali et al. 2016).
Pretreatments
The fouling of membranes often can be substantially reduced by pre-treating the contaminated water upstream of the membrane units. Nuortila-Jokinen et al. (2004) listed the following pretreatment strategies as being promising for maintaining the flux through membranes when treating P&P mill wastewaters: chemical treatment (i.e. coagulation), ozonation, and biological treatment. Such strategies may prevent fine suspended matter from clogging the membrane pores or eroding the active membrane layer. However, few authors have reported favorable results regarding membrane performance after such treatments of P&P mill effluent (Li and Zhang 2011). Even Simonič and Vnuceč (2012) and Bennani et al. (2012), who compared the performance of coagulation and ultrafiltration systems in parallel, did not run any tests of the two approaches in sequence.
A variety of other pretreatment strategies have been employed in an attempt to minimize the fouling of membranes. For instance, Gönder et al. (2011) optimized the pH, temperature, applied pressures, and a volume reduction factor in order to minimize flux reduction through a nanofiltration membrane. The pH of the treated wastewater was found to have the biggest effect, and a pH of 10 resulted in the minimum rate of loss of flux. Related work by Kallioinen et al. (2006) showed that although it may be possible to correlate permeability reduction to the values of adjustable variables, such relationships can be completely changed in the course of biological treatment of the wastewater. Thus, those authors were surprised to observe a case in which biological treatment failed to follow their predictions of improved membrane permeability behavior. A possible explanation is that there was flux loss due to silica deposition (Ordóñez et al. 2011). Kamali and Khodaparast (2015) cited various studies in which enzymatic treatments tended to improve the flux through membranes used for treating the effluent from P&P mills.
An alternative approach for the prevention of fouling and clogging of membranes is the generation and use of microbubbles, as discussed by Takahashi et al. (2003), Agarwal et al. (2012), and Yerushalmi (2014). Microbubbles are produced when gas-saturated water passes through the membranes. In this approach, the water is mixed with a gas, e.g. air or ozone, and will go through a de-gassing stage for the removal of non-dissolved gases before passing through the membranes. During the passage of gas-saturated water through the membranes, the transmembrane pressure gradient transforms the dissolved gases into microbubbles. The microbubbles contribute to the prevention of fouling and continuous cleaning of membranes by several mechanisms including scrubbing and self-collapse. Microbubbles benefit from a large surface area, high gas-dissolving capability, and enhanced mass transfer efficiency. In addition, they have the tendency of shrinking under water due to the surface tension and dissolution of gas in the surrounding water. According to the Young-Laplace equation (Takahashi et al. 2003; Agarwal et al. 2012), the shrinking of microbubbles leads to the progressive increase in their internal pressure until they collapse. The high pressure spot created at the final stage of microbubbles collapse produces pressure waves that will be distributed in the vicinity of a collapsing bubble and will promote the detachment of deposits from the membranes. As reported by Agarwal et al. (2012), microbubbling is more efficient than chemical cleaning with NaOCl solution for the removal of extracellular polymeric matrix of biofilms. Furthermore, collapsing microbubbles have been shown to generate free radicals (Takahashi et al. 2003; Agarwal et al. 2012), which react rapidly and non-selectively with the chemicals in the water and cause their rapid degradation. The strong oxidation effects of free radicals will further degrade the recalcitrant compounds and will remove them from the water.
Bioreactor Designs Incorporating Filters
Membrane bioreactor concept
A membrane bioreactor (MBR) can replace an independent settling-clarification stage with a membrane separation stage (Le-Clech et al. 2006). Yamamoto et al. (1989) were apparently the first to submerge a membrane filter assembly right within the reaction vessel where biodegradation of pollutants in wastewater was taking place. Both hollow fiber and flat sheet membrane assemblies have been used in bioreactors for wastewater treatment (Le-Clech et al. 2006). However, for the treatment of P&P mill effluents, polyvinylidine fluoride (PVDF) is the dominant polymer used so far (Kamali et al. 2016). Membrane bioreactors have become commonly used in the treatment of P&P mill effluents (Pellinen and Joyce 1990).
Figure 16 illustrates two simplified designs of MBR systems based on the textbook by Park et al. (2015). Figure 16A corresponds generally to the approach used by Yamamoto et al. (1989), which has been widely applied. Figure 16B shows a general design that offers more active recirculation of activated sludge within the bioreactor system. According to Le-Clech et al. (2006) typical membrane pore sizes employed in membrane bioreactors are in the range 50 to 400 nm (corresponding to the upper end of UF up to the midrange of MF).
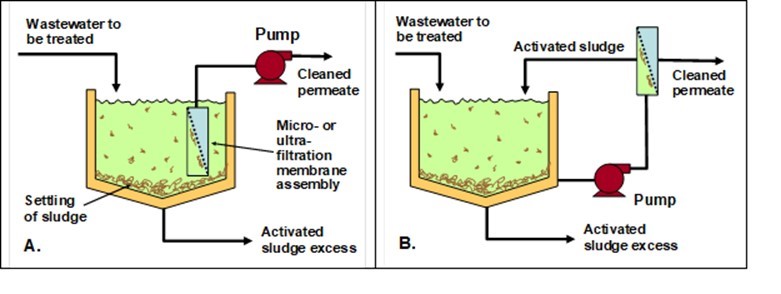
Fig. 16. Conceptual views of two types of bioreactor
The attachment of bacterial or fungal cells to solids surfaces or sludge particles within a bioreactor appears to play an important role in enabling such a system to metabolize various organic chemicals. In other words, a bio-film composed of living cells is present within the reactor (Rusten et al. 1994; Lee and Welander 1996; Helble et al. 1999; Baig and Liechti 2001; Huuhilo et al. 2002; Wu et al. 2005; Muhamad et al. 2012a,b, 2013; Osman et al. 2013; Kamali and Khodaparast 2015). Biofilms anchored to suitable support surfaces or activated sludge within biological treatment systems allow the microorganisms to become acclimated to the wastewater and to diversify their populations in such a way as to be more capable of degrading the diverse organic compounds present in that source of wastewater. According to Kamali and Khodaparast (2015), the biofilm surfaces provide a combination of adsorption and biodegradation; thus, hard-to-degrade compounds can be removed from the water phase, and the enzymatic breakdown may take place at least partly within the immobilized biofilm. There are a number of such processes currently in operation at P&P mills (Malmqvist et al. 2004; Rankin et al. 2007). At these facilities the biofilm/activated sludge (BAS) system is used. Benefits of the system include reduced nutrient demand, less excess secondary sludge generated, and a reduced footprint for the operating equipment.
Fixed bed (film) reactor
In fixed bed systems for wastewater treatment, the bioreactor is packed with a porous material, often granulated solids (Minami et al. 1991; Welander et al. 1997; Marwaha et al. 1998; Helble et al. 1999; Baig and Liechti 2001; Tziotzios et al. 2005). For example, Nakamura et al. 1997) used polyurethane foam as the support medium for immobilization of activated sludge. Osman et al. (2013) studied the use of activated carbon as a support. Rajeshwari et al. (2000), in their review article, mention the use of polyvinyl chloride particles, ground rock, and ceramic rings as supports for biofilm in reactors used for wastewater treatment. In other wastewater applications, Apilánez et al. (1998) compared diatomite, sand, and activated carbon surfaces; they reported the best results with the activated carbon.
Fluidized bed
Bioreactor designs that rely on packed bed systems often suffer from clogging or channeling problems (Maat and Habets 1987; Rusten et al. 1994). Such problems can be overcome by the use of fluidized bed systems in which the upward flow of wastewater and/or recirculated treated water causes support particles to remain suspended in the fluid phase (Rajeshwari et al. 2000; Hubbe 2007c). The flow system can be optimized to provide continual mixing of the suspended media. For instance, as suggested in Fig. 17, the circulation may be achieved as part of the aeration system. Applications of fluidized bed bioreactors have been reported for P&P effluent treatment (Haggblom and Salkinojasalonen 1991; Heinzle et al. 1992; Rusten et al. 1994). According to Maat and Habets (1987) the energy required to maintain fluidization of such systems probably accounts for why they are not more widely used.
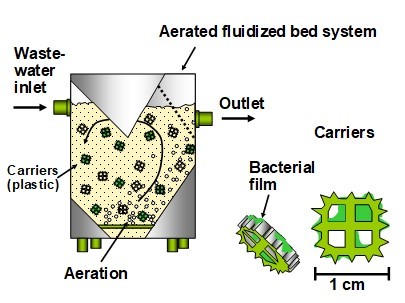
Fig. 17. Schematic view of a fluidized bed system that employs plastic carriers, with agitation provided by aeration
Upflow sludge blanket
Upflow sludge blanket systems operate similarly to the fluidized bed concept just discussed, except that no support media are used, other than the sludge itself, and the material has a tendency to form itself into a contiguous blanket rather than being continually mixed. Although mixing sounds like a great concept, from a design standpoint, the sludge blanket concept has the potential to allow different organisms to thrive in different layers of the sludge, thus achieving a better overall effect (Roest et al. 2005). Upflow sludge blanket reactors have been reported for the treatment of P&P mill effluent (Lettinga et al. 1980; Maat and Habets 1987; Kortekaas et al. 1998; Rajeshwari et al. 2000; Ahn and Forester 2002; Buzzini and Pires 2002, 2007; Roest et al. 2005; Bhunia and Ghangrekar 2008; Buyukkamaci and Koken 2010). Carter and Sigler (1981) describe a system in which a blanket of sludge helps to filter the effluent water from an otherwise conventional biological treatment. Rajeshwari et al. (2000) noted that upflow sludge blanket systems may have a relatively long start-up time due to the need for formation of a new sludge blanket after any long interruption in the operation.
Bio-disk systems
By utilization of a slowly rotating disc it is possible to achieve a more active control of biological interactions during wastewater treatment (Apilánez et al. 1998). Such systems only rarely have been reported for treating P&P industry effluents (Castillo and Vivas 1996; Hynninen 1998).
ADVANCES IN AEROBIC TREATMENT WITH ACTIVATED SLUDGE
Mechanism of Action
Aerobic biological treatment of wastewater is widely practiced in P&P mills (Ghem 1973; Costa et al. 1974; Gergov et al. 1988; LaPara and Alleman 1999; Schnell et al. 2000a; Thompson et al. 2001; Kostamo and Kukkonen 2003; Chakrabarti et al. 2008; Kamali and Khodaparast 2015). One of the main objectives of aerated biological treatment is to reduce the BOD levels of the treated wastewater. As has been noted (Ghem 1973), such treatments are typically less effective for the removal of color, which would require the breakdown of recalcitrant compounds (Graves and Joyce 1994).
The mechanism of aerobic biological treatment of wastewater can be understood in terms of the key steps of biosorption, metabolism, and bioflocculation. Adsorption means that compounds present in the wastewater can come into contact with either the living microbes or with the enzymes that they produce. Metabolism involves a series of biochemical reactions by which microorganisms, through use of their enzymes and organelles, transform the contaminants into smaller molecules or completely mineralize them to carbon dioxide and water (Dilek et al. 1999; Tarlan et al. 2002a,b; Park et al. 2015). The term bioflocculation implies that the extracellular exudates from the microbes may play an important role in the agglomeration of the biological material, with the formation of settlable sludge. Avella et al. (2011) characterized such extracellular matter in a wastewater treatment system by means of gel permeation chromatography.
Aerobic treatment of P&P industry wastewater can be limited by the presence of inhibitory or toxic matter in the wastewater. Achoka (2002) found that the amounts of various metal ions in treatment wastewater could be increased in the process of aerated treatment. As can be seen from various published data summarized in Table A, it is common for aerated biological treatment to remove far less than 100% of BOD from effluent.
Recirculation
The concept of returning a portion of the sludge from aerated biological treatment back to the intake of the process has been utilized for a long time (Rudolfs and Amberg 1953). Such a system is illustrated schematically in Fig. 18. The recirculation process increases the solids retention time (SRT), which plays an important role in improving the stability of operation and the overall removal efficiency of contaminants. Moreover, the return activated sludge (RAS) provides diversity in the microorganisms. The incoming effluent is continually inoculated with microbes that have become acclimatized with the prevailing conditions; thus activated sludge tends to speed up the process of biodegradation and to allow the process to proceed in a more stable manner. Also, the recirculation of microbes can allow for the development of a more optimized community of bacteria and other organisms, resulting in a system that is better able to endure the shocks inherent in changing processing conditions. It has been shown that a consortium of different organisms may provide for a more comprehensive decomposition (Chakrabarti et al. 2008; Chandra and Singh 2012; Ordaz- Díaz et al. 2014). Hynninen (1998) provides a discussion of how operators of activated sludge treatment plants can optimize their systems by monitoring and controlling such factors as the sludge load, the sludge’s dry weight per unit volume, the settling properties of the sludge, the ratio of COD to activated sludge amount, the dissolved oxygen concentration, the number of times biosludge is recirculated on average, the sludge age, and other such factors.
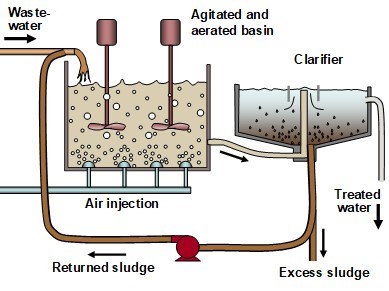
Fig. 18. Schematic illustration of an activated sludge wastewater treatment system, equipped with an aerated basin, a clarifier, and return of a portion of the settled sludge from the clarifier
Sludge activation treatment
Researchers have reported that activated sludge systems can benefit from various treatments of the sludge before it is returned to an aeration basin. For example, Mahmood and Elliott (2006) describe how ozone treatment of returned sludge has the potential to render the organic compounds in it more susceptible to biodegradation; such treatment can lower the overall production of sludge from the process. Chu et al. (2009) provided a comprehensive review of the status of ozonation of activated sludge including its addition via the sludge recirculation line. This well proven technology can reduce the sludge yield of an activated sludge system between 45 and 100% depending on the point of application and the dosage applied. Additional benefits of ozonation of recirculated activated sludge include improved settleability and denitrification. A dosage range of 0.03 to 0.05 gO3/g TSS is suggested. Drawbacks of the technology include its high capital and operational costs. The technology has come to market and is being implemented in full-scale facilities. Praxair has patented the Lyso TM Process, which is their marketed technology.
Aeration
The addition of air (or oxygen) is an essential step in the aerobic treatment of wastewater. Aeration is commonly implemented by fountains of spray or by use of diffusers to create bubbles of air in lagoons or more formally constructed wastewater containment basins. Such systems have been widely studied for the treatment of P&P industry effluents (Costa et al. 1974; Graves and Joyce 1994; Welander et al. 1997; Schnell et al. 2000a; Ordaz-Díaz et al. 2016).
According to Mahmood and Elliott (2006), a venturi system can be employed in order to more efficiently disperse air into the wastewater and to increase its dissolution rate. Garcia-Ochoa and Gomez (2009) reviewed oxygen transfer rates in bioreactors, showing the importance of flow conditions, surface areas, and other aspects of reactor design and running conditions. Based on the literature review, there appears to be a need for more study of ways to ensure an adequate degree of aeration to avoid anaerobic conditions and to optimize the process in P&P effluent treatment.
Temperature
Because different bacterial populations need different temperatures for optimal growth and proliferation, the adjustment of temperature can be very important relative to the results of biological treatment. Thus, Chakrabarti et al. (2008) cautioned that P&P mill effluent often needs to be allowed to cool down before being subjected to biological treatment. On the other hand, it can be advantageous to maintain conditions that favor the proliferation of thermophilic microbes, those that can thrive at temperature levels between 45 and 60 °C (Tripathi and Allen 1999; Suvilampi et al. 2001). LaPara and Alleman (1999) reported that substrate utilization rates can be three to ten times higher when using thermophilic, compared to mesophilic temperatures. Tripathi and Allen (1999) found that thermophilic conditions were more favorable for the decomposition of long-chain fatty acids, which otherwise can be hard to break down. Aerated biotreatment under thermophilic conditions has been shown to be well suited to the use of fixed bed bioreactors (Huuhilo et al. 2002; Simstich et al. 2012).
According to Suvilampi et al. (2001) the P&P industry has been slow to adopt thermophilic conditions of biological treatment, perhaps due to a perception that the high temperatures will suppress the performance of activated sludge processing. Also, it has been reported that thermophilic bacteria do not aggregate readily (LaPara and Alleman 1999), which can be unfavorable in terms of sludge clarification. However, it has been shown that the sludge from a mesophilic process can be relatively rapidly re-acclimated to thermophilic conditions, leading to efficiencies that are at least as high as those achieved under mesophilic conditions (Suvilampi et al. 2001).
Retention Time
The residence time of bacterial matter in a reactor is clearly a key variable. Time is required for such processes as cell division, enzyme production, and metabolism. The following studies have considered the effects of the retention time of solids on the outcome of treating P&P mill wastewaters (Diez et al. 2002; Pougatch et al. 2007). By extending the aeration time, a greater degree of biodegradation can be achieved (Buyukkamaci and Koken 2010). Extending the time of aeration also can be expected to reduce the final amount of sludge produced, which can be attributed to a greater completion of the metabolic processes (Mahmood and Elliot 2006). The same authors noted that a longer aeration time also promotes the development of a broader range of organisms, including protozoa and rotifers, which consume the bacterial matter in the sludge.
Figure 19 compares the relative sizes of some typical organisms involved with biodegradation. Ordaz-Díaz et al. (2016) studied the acclimation of bacterial populations during long-term treatment in an aerated lagoon. Such populations were found to adapt to changes in aeration and other operating parameters.
Operational Issues of Aerobic Stages
Filamentous bacteria
Various practical issues confront the operators of aerated wastewater treatment systems. Depending on the design and operational conditions in a wastewater treatment plant, the bacterial culture may develop either as singly dispersed, attached, or filamentous forms.
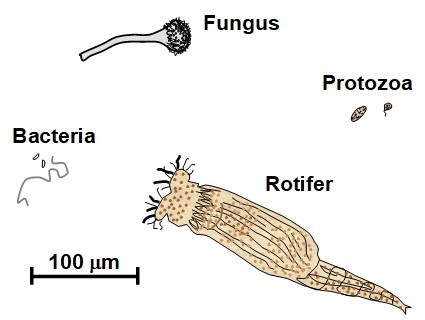
Fig. 19. Relative sizes of typical kinds of organisms involved in the biodegradation of pulp and paper mill effluents
Filamentous bacteria can be regarded as an integral component within a biological waste treatment system (Richard 2003). From a qualitative perspective, it is desirable to maintain a controlled balance of filamentous bacteria within a biological system. Operational issues become apparent when proportional amounts of filamentous bacteria are either too great, or deficient. A lack thereof can create issues with turbidity in effluent streams (Sezgin et al. 1978) due to less desirable ‘pin-floc’ formations. At increased proportions within the biomass, filamentous bacteria give rise to what is known as bulking sludge. This type of unfavorable sludge condition decreases settling velocities and reduces compaction of sludge in sedimentation tanks (Thompson et al. 2001), though such effects can be at least partly addressed by the use of coagulants (Agridiotis et al. 2007). Factors that contribute to the occurrence of bulking sludge development can be linked to poor aeration, extended periods of lower food/mass ratios (Cingolani et al. 1994), as well as deficient nutrient concentrations of nitrogen and phosphorous (Richard and Cummins 1997). In a multi-site microbial survey, research found the development of Thiorix bacteria to be the most predominant form of filamentous existing in bulk settling circumstances (Bergeron and Pelletier 2004). This operational concern is warranted by the elevated risk of increased COD in final effluent streams (Wanner 1994), or potential loss in treatment capacity due to reductions in recirculation volumes (Thompson et al. 2001).
pH
Favorable pH conditions are critical to the optimization of a successful secondary wastewater treatment system. Typically, a permit requirement holds operations accountable to specific pH conditions in both pre- and post-treatment of wastewater. In aerobic systems, pH adjustments are made in order to reach maximal process efficiency. This is due, in part, to the adaptive preference of floc-forming species of bacteria and their ability to flourish in these conditions. Ghanizadeh and Sarrafpour (2001) demonstrated that enhanced settling can be brought forth by increases in pH from 5 to 9 in sludge treatment systems. Alkalinity also serves as a buffer to resist abrupt swings in pH, therefore allowing for a greater degree of process stability with regards to influent and effluent pH.
Bio-augmentation
The term bio-augmentation refers to the addition of selected populations of viable bacterial, algal, or fungal cells to enhance the performance of biological processes. Various researchers have reported improvements in biological treatment owing to the addition of selected bacterial cultures (Chandra and Singh 2012; Chen et al. 2012; Garg et al. 2012; Hossain and Ismail 2015). For instance, Garg et al. (2012) showed that dechlorination and detoxification of bleach plant effluent components could be enhanced by the addition of Pseudomonas putida, which is a Gram-negative bacterium. Matafonova et al. (2006) isolated and cultured a bacterium from an aeration pond at a P&P mill and demonstrated that the pure strain was effective for the degradation of 2,4-dichlorophenol, which may serve as a model compound for pulp bleaching effluents. Tiku et al. (2010) found favorable results with three kinds of bacteria for the reduction of BOD, toxicity, color, and related factors of pulp mill wastewaters.
Others researchers showed favorable results when using mixed cultures of bacteria (Chakrabarti et al. 2008; Chandra and Singh 2012; Ordaz-Díaz et al. 2014). The consortia of bacteria present in a properly operating wastewater treatment plant may be accompanied by protozoa, fungi, and rotifers (Chakrabarti et al. 2008). Such a combination can be expected to allow the system to deal effectively with a wide variety of pollutants, which are likely to shift from hour to hour.
Various authors have found favorable effects when using white-rot fungal species to treat pulp mill wastewater and bleach plant effluents (Eriksson and Kolar 1985; Pellinen and Joyce 1990; Duran et al. 1994; Gökçay and Dilek 1994; Wolfaardt 1994; Marwaha et al. 1998; Saxena and Gupta 1998; Taseli and Gökçay 1999; Sakurai et al. 2001; Pokhrel and Viraraghavan 2004; Thakur 2004; Wu et al. 2005; Malaviya and Rathore 2007; Freitas et al. 2009; Singhal and Thakur 2009; Garg and Tripathi 2011; Kakahi et al. 2011; Liu et al. 2011; Souza et al. 2014; Hossain and Ismail 2015; Kamali and Khodaparast 2015). Eriksson and Kolar (1985) observed much more favorable effects from the addition of a white-rot fungal strain, in comparison to two bacterial mixtures; the fungus promoted more rapid degradation of chlorolignins. Thakur (2004) evaluated eight fungal species and three bacterial isolates and found all of them capable of degrading absorbable organic halides (AOX) in P&P mill effluent. Kakahi et al. (2011) reviewed the use of fungal treatments to augment wastewater treatment in the P&P industry and noted that fungal treatment has been effective for color removal. The strong enhancements in biodegradation, decolorization, and removal of toxicity observed generally in the studies cited above are consistent with the role of white-rot fungi in degrading the lignin component of wood by the release of specialized enzymes such as laccase and peroxidases (Duran et al. 1994; Freitas et al. 2009; Souza et al. 2014).
Algae can be defined as a diverse primitive group of eukaryotes that receive energy by photosynthesis. Since many wastewater treatment operations are exposed to sunlight, the use of algal treatment has been considered for wastewater from P&P mills. A number of researchers have shown encouraging results for such treatment (Dilek et al. 1999; Tarlan et al. 2002a,b; Hossain and Ismail 2015). Dilek et al. (1999) demonstrated that color removal was mainly a result of metabolism, rather than biosorption. On the other hand, Tarlan et al. (2002a) found that decolorization of P&P mill effluent was attributable to a combination of metabolism and conversion of chromophoric compounds to colorless compounds.
Mahmood and Elliott (2006) consider that bioaugmentation is a promising approach for the reduction of sludge amounts resulting from aerobic treatment of wastewaters. Presumably, some of these favorable results can be attributed to the release of enzymes capable of breaking down some of the hard-to-biodegrade and possibly toxic organic compounds present in the wastewater (Chandra and Singh 2012; Chen et al. 2012).
Nutrient Supplementation
Because biodegradation is dependent on life processes, certain nutrients are required to be present (Simstich et al. 2012). Depending on the inputs to a pulp and paper process, from which the wastewater is derived, it may be necessary to supply some macro- (mainly phosphorous) and micronutrients to properly operate biological treatment systems. Nutrient limitations can hinder effective waste treatment within biological systems. This may be countered by the addition or reduction of nitrogen and phosphorous to the system, yet issues can arise if supplementation is not managed correctly (Cingolani et al. 1994). Similar to the necessary presence and operational control of filamentous bacteria, a homeostatic balance is needed for nutrient supplementation. Insufficient nutrient addition can promote performance decline in the treatment. This is indicative in the decline of metabolic function, which can be symptomatic of increased dissolved oxygen concentrations in aerobic monitoring systems. Just as nutrient deficiency can be troublesome to operations, a nutrient surplus can also be unfavorable. Nutrient overabundance can create issues such as excessive foam, secondary scum, increased BOD and TSS load within the outgoing effluent, and the potential for eutrophication (Ekholm et al. 2007; Kenny 2010). The optimization of nutrient addition not only serves as effective quality control; it also offers the important benefit of operational cost reduction. Thus, Wu et al. (2005) found benefits of treating the system with ammonium tartrate. Likewise, Garg et al. (2012) found that supplementation of the wastewater with yeast extract, a source of nitrogen, promoted microbial decolorization of P&P mill wastewater.
The addition of glucose, used as an alternative energy source by microorganisms, has been shown to increase the rate and extent of biodegradation (Gökçay and Dilek 1994; Wu et al. 2005; Garg et al. 2012). However, such treatment may not always be advantageous in terms of the overall results of treatment. According to Mahmood and Elliott (2006) it can be advantageous to keep the nutrient level low. Such an approach can favor the production of polysaccharide-producing bacteria, which then can be consumed by higher level organisms, leading to less final sludge in specially designed treatment systems.
Enzyme Treatment
The specific enzymes responsible for observed degradation of pollutants have been isolated or identified in some studies (Owens 1991; Duran et al. 1994; Freitas et al. 2009; Chandra and Singh 2012; Chen et al. 2012; Souza et al. 2014). It has been shown that direct addition of those enzymes to P&P mill effluent can facilitate the degradation of lignin species (Hakulinen 1988; Wagner and Nicell 2001; Fernandez-Fernandez et al. 2013; Hossain and Ismail 2015; Kurnik et al. 2015). Ko and Fan (2010) showed the feasibility of a two-step process in which laccase enzyme was used to polymerize lignin-based compounds and then the mixture was separated using membranes with a range of different pores sizes.
Immobilization
The use of pure enzymes in the treatment of wastewaters requires their extraction and purification. The costs of such extraction and purification often prohibits the use of isolated enzymes in full-scale applications. Hence, it is generally preferred to use bacterial or fungal cells to grow and to release the enzymes in situ. One possible motivation for isolation and use of pure enzymes might be related to the possibility of immobilization (Duran and Esposito 2000). As noted by Fernandez-Fernandez et al. (2013), immobilization of enzymes can improve their storage stability and prolong their effectiveness during wastewater treatment. On the other hand, it is also possible to immobilize the fungal organisms on a suitable support and generate the needed enzymes in-situ (Marwaha et al. 1998; Taseli and Gökçay 1999; Wesenberg et al. 2003; Wu et al. 2005; Malaviya and Rathore 2007). Given the relatively low number of studies in this area, further work of this type seems warranted.
Aerobic Bioreactors
The use of bioreactor equipment, including MBRs, may be justified for aerobic treatment of P&P mill effluents for various reasons, such as avoiding the space and capital expense of building conventional settling basins or clarifier equipment or making it possible to process wastewater in a smaller space (Kamali and Khodaparast 2015). The feasibility of such systems has been evaluated, with generally favorable results reported (Helbe et al. 1999; Taseli and Gökçay 1999; Magnus et al. 2000; Tziotzios et al. 2005; Muhamad et al. 2012a,b, 2013; Mahmood-Khan and Hall 2013).
Aerobic trickling filter
In an effort to enhance the performance and stability of bioreactors for the treatment of P&P mill wastewaters, various authors have introduced solid support media onto which bacterial or fungal cells can attach themselves (Haggblom and Salkinojasalonen 1991; Wang et al. 2008b; Muhamad et al. 2012a,b; 2013). Haggblom and Salkinojasalonen (1991) demonstrated the effectiveness of a “trickling filter” for degradation of chlorinated organic compounds from kraft pulp bleaching. Taseli and Gökçay (1999) showed that a system with packed glass wool could be used for over a year in the treatment of pulp mill effluents. Huuhilo et al. (2002) demonstrated the effectiveness of a “suspended carrier” concept for internal treatment of P&P mill wastewaters, which were then returned to the mill process streams. Rusten et al. (1994) employed freely-suspended plastic elements that provided surfaces for biofilms to form, while the system was kept well mixed in an agitated tank. Wang et al. (2008b) employed clay beds in a packed column “biofilter” system for evaluation of the effect of ozonation on subsequent biodegradation. Muhamad et al. (2012a,b, 2013) and Osman et al. (2013) evaluated systems in which granulated activated carbon was used as the support medium.
Noting that a solid support can enhance biodegradation, various authors have employed the activated sludge itself as a kind of bio-filter for treatment of P&P mill wastewater (Tziotzios et al. 2005; Souza et al. 2014). Tziotzios et al. (2005) found that a packed bed system with gravel was more tolerant of high phenol concentrations and achieved higher removal rates in comparison to a stirred, aerated reactor without packing materials.
Although the use of support material for the formation of biofilms mainly has been implemented in compact bioreactor installations and lab-scale studies, Welander et al. (1997) showed that a similar approach can be applied in an aerated lagoon. The use of support materials encourages the establishment of biofilms. Much better results, using the support materials, were obtained in a pilot-scale implementation, in comparison to parallel evaluations in an industrial-scale wastewater treatment plant, indicating that further study may be needed.
The use of a rotating solid support medium within a biodegradation vat reactor was reported by Pellinen and Joyce (1990), Rovel et al. (1994), and Hynninen (1998). According to Pellinen and Joyce (1990) such a system appeared to provide inadequate contact time, and the system needed to be cleaned out every three to four weeks when treating concentrated effluent from P&P mills.
Aerobic membrane reactor
As was discussed earlier, the incorporation of a membrane into the equipment design, immersed into or following the biological treatment stage, makes it possible to remove purified water from the mixture while concentrating the sludge and permitting adequate residence time of the decomposing biomaterials within a relatively small space (Smith et al. 1969; Gander et al. 2000). Such designs have been evaluated for treatment of P&P mill wastes (Helbe et al. 1999; Tenno and Paulapuro 1999; Magnus et al. 2000; Huuhilo et al. 2002; Mahmood and Elliott 2006; Zhang et al. 2009; Qu et al. 2012; Simstich et al. 2012; Mahmood-Khan and Hall 2013). Tenno and Paulapuro (1999) developed a mathematical model for their bioreactor and showed that the throughput could be increased by increasing the solids level. Qu et al. (2012) reported that cake formation on the membrane surfaces was the primary mode of membrane fouling during the aerobic treatment of P&P mill wastewater.
Staged biological treatment
Because attached-growth bioreactors are designed to occupy considerably less space than a conventional wastewater treatment operation of similar capacity, it sometimes can make sense to think about using two or more stages of treatment sequentially. Thakur (2004), who studied biodegradation by immobilized bacterial or fungal cells in packed columns, found superior results when the wastewater was passed sequentially through two columns. Mahmood and Elliott (2006), in their review article, noted that sequential treatment often results in less sludge production in comparison with a conventional aerated wastewater treatment operation.
Several authors have noted that the inclusion of higher-level organisms such as protozoa and rotifers, along with the bacterial and fungal cells in a biological treatment operation, will reduce the amount of final generated sludge (Lee and Welander 1996; Chakrabarti et al. 2008). Provision of a second stage of treatment, where the sludge is at a later stage of maturity, has been found to favor the action of predator species that feed upon the bacterial material in the sludge (Lee and Welander 1996; Mahmood and Elliott 2006). It is also likely that different trophic levels of organisms may inhabit the suspended sludge growth vs. the attached growth in the same reactor system (Mahmood and Elliott 2006). The process is termed the low sludge production (LSP) process.
Sludge Post-treatment
Before leaving the subject of aerated biological wastewater treatment, it is important to note a key area of developing technologies related to post-treatment of biological sludge (Pere et al. 1993; Mahmood and Elliott 2006). Key concerns are to minimize the amount of sludge and to increase its solids content, thus reducing the cost and various problems associated with its transportation and disposal. Thus, Pere et al. (1993) showed that the amount of sludge could be reduced by oxidative conditioning (Fenton’s reagent), which facilitated dewatering in a filter press. Pervaiz and Sain (2011) employed a different approach and used sludge from a paper mill wastewater treatment plant as a source of proteins, which were successfully demonstrated as a potential wood adhesive. In many countries such biological sludge can be used in agriculture. In recovered paper mills, sludge from biological treatment stages can be combined with deinking sludge and treated by gravity tables and presses (Hermosilla et al. 2010). This dried sludge can be revalorized, for example within the ceramic and cement industries.
A bottleneck of many aerobic treatment systems involves the time, effort, and expense of dewatering biological sludge. The conventional method is to combine the difficult-to-dewater biological sludge with the relatively easy to dewater primary sludge. Specifically designed polymers are then added and mixed with the combined sludge, and the mixture is thickened prior to pressing using either a centrifuge, a belt press, or a screw press. An in-depth review of the dewatering practices can be found in the work of Dorica et al. (1999). Up 50% of the operating costs of a wastewater treatment system can be associated with sludge management (Mahmood and Elliott 2007). As much as 70% of the cost can be attributed to the demand for dewatering chemicals. Mahmood and Elliott (2007) provided a novel method for reducing the demand for expensive dewatering chemicals by applying a low-cost acid to the secondary sludge prior to dewatering.
ADVANCES IN ANAEROBIC TREATMENT
The term anaerobic implies a process of biodegradation in which no air or oxygen is supplied. The ensuing biological processes are analogous to fermentation. Rajeshwari et al. (2000) provided a general review of anaerobic digestion as a stage in wastewater treatment operations. Also there have been review articles devoted to the treatment of P&P mill effluents using anaerobic conditions (Graves and Joyce 1994; Rintala and Puhakka 1994; Savant et al. 2006; Meyer and Edwards 2014; Kamali and Khodaparast 2015). Many other authors have reported evaluations of factors affecting the anaerobic treatment of P&P mill wastewaters (Sierraalvarez et al. 1991; Korczak et al. 1991; Vidal et al. 1997; Kortekaas et al. 1998; Bengtsson et al. 2008a; Ruas et al. 2012; Krishna et al. 2014; Larsson et al. 2015).
A plus side of anaerobic treatment is that one does not need to pay for the pumping of air into the system (Maat and Habets 1987), and, according to many reports, the amounts of sludge produced are generally less than in conventional aerated biological treatment systems (Ashrafi et al. 2015; Kamali and Khodaparast 2015). Anaerobic processes produce gases such as methane, requiring their collection and safe disposal. Nevertheless, the retrieving and reuse of biogases such as methane and hydrogen as a source of energy during full-scale treatment operations can provide substantial economic benefits to the treatment plants. Such methane and hydrogen either can be sold or they can be burnt for the generation of heat (Tabatabaei et al. 2010).
Principles of Anaerobic Treatment
In chemical terms, the process of anaerobic digestion in a typical wastewater treatment operation can be broken down into the steps of hydrolysis, acid formation, acetogenesis (with the production of hydrogen and carbon dioxide), and methanogenesis (Maat and Habets 1987; Kamali et al. 2016). These terms are diagrammed as a process schematic in Fig. 20. Due to the value of methane as a fuel, and perhaps also the importance of avoiding its release into the atmosphere (Weiland 2010), much research has been devoted to its generation during anaerobic treatment of P&P mill wastewater (Maat and Habets 1987; Korczak et al. 1991; Minami et al. 1991; Sierraalvarez et al. 1991; Vidal et al. 1997; Kortekaas et al. 1998; Ahn and Forster 2002; Buzzini and Pires 2007; Yilmaz et al. 2008; Tabatabaei et al. 2010; Lin et al. 2011; Saha et al. 2011; Elliott and Mahmood 2012; Bayr et al. 2013; Ekstrand et al. 2013; Hagelqvist 2013; Hassan et al. 2014; Meyer and Edwards 2014; Larsson et al. 2015).
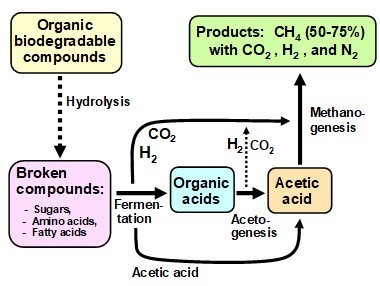
Fig. 20. Schematic diagram of main chemical and metabolic steps involved in the anaerobic decomposition of biodegradable organic compounds
Other byproducts
In addition to fuel-type products such as methane, some researchers have explored the feasibility of generating more complex and potentially more valuable byproducts (Laycock et al. 2014). For instance, Bengtsson et al. (2008a,b) isolated polyhydroxyalkanoates (PHA), which were attributed to the biotransformation of fatty acids in P&P mill effluents. Acetate, propionate, and butyrate compounds also were obtained in that study.
Bioethanol production from cellulosic materials has been widely studied (Alvira et al. 2010). Because the cellulosic material in the wastewater from a P&P facility may already have been delignified and otherwise broken down, such wastewater has been considered as a raw material source for ethanol production (Lin et al. 2012; Liu and Shonnard 2014). Two inherent problems that would need to be faced, to make such production viable, involve the expected variability of the effluent water to be treated and the relatively dilute nature of cellulosic matter in typical wastewater.
Sulfur reducing bacteria
An inherent problem associated with anaerobic processes is the likelihood of reducing sulfate ions to other compounds such as sulfides or H2S (Lens 1998; Janssen et al. 2009). Such compounds are generated through the action of sulfate-reducing bacteria (Roest et al. 2005; Janssen et al. 2009). The reduced sulfur compounds can interfere with the generation of methane (Minami et al. 1991). H2S is regarded as the most toxic form of the sulfide species towards microbial communities responsible for the production of methane. In addition, the concrete corrosion in full-scale reactors is another drawback of the activity of the sulfate reducing bacteria (Barton and Fauque 2009). One practical way to deal with the problem is to process the water or sludge through further aerated stages of treatment (Lens 1998; Janssen et al. 2009). Combination treatments aimed at the removal of sulfur-containing compounds from the system will be considered later in this article.
Operating Conditions of Anaerobic Treatment
Temperature
The temperature of the mixture has a big influence on anaerobic treatment. In particular, various studies have shown the potential for higher rates of biodegradation at higher temperatures, favoring the growth and activity of thermophilic rather than mesophilic organisms (Ahn and Forster 2002; Tartakovsky et al. 2003; Yilmaz et al. 2008). Tartakovsky et al. (2003) found that thermophilic conditions were more effective in the breakdown of lignin-related compounds in the mixture. Yilmaz et al. (2008) found more effective removal of the soluble components of biological oxygen demand while operating an anaerobic system in the higher temperature range. Ahn and Forster (2002) found that lowering of the biological oxygen demand took place progressively under thermophilic conditions, whereas lower temperature treatment did not show any reduction in oxygen demand with increasing hydraulic retention time. Jeison et al. (2008) showed that thermophilic anaerobic reactors may treat higher contents of organic substances than mesophilic ones. Moreover, reactors working in the thermophilic range managed to work at higher volumetric loading rates than AnMBRs operating within the mesophilic range. Saha et al. (2011) found that when thermophilic conditions were employed during anaerobic treatment, there was little benefit of pretreating the sludge by any of the methods that they considered.
Supplementation
The term “supplementation” will be used here to mean that something is being added to wastewater before subjecting it to anaerobic treatment. Savant et al. (2006) reviewed the action of specific micro-organisms in the degradation of absorbable organic halides, which are a product of the bleaching of kraft pulps. The cited authors make the point that such micro-organisms need nutrients to be in certain ranges in order to thrive and be effective in breaking down the organic matter.
Deshmukh et al. (2009) found that the addition of glucose or acetate before anaerobic treatment of P&P mill wastewater has potential to promote the breakdown of absorbable organic halides (AOXs). The observed effects were attributed to the additives serving as electron donors in an enzymatic process.
Because anaerobic digestion is a biological process, it is critical that the mixture contains a sufficient level of biologically available nitrogen. Kamali and Khodaparast (2015) in their review emphasized the importance of this variable and the need to optimize the ratio of nitrogen to carbon in anaerobic treatment of P&P mill effluents. However, there appears to be a lack of published research in this area.
Meyer and Edwards (2014) recommended co-digestion of different wastewater streams from paper mills as a way to avoid disruptions and to diminish toxic effects. Co-digestion also can be considered for the further treatment of sludge generated from the treatment of P&P mill wastewaters. Such an approach can achieve a favorable balance between nitrogen and carbon, as required for biological growth. Hagelqvist (2013) co-digested secondary sludge from a P&P mill together with municipal sludge. The forest-industry sludge was able to replace up to 50% of the municipal sludge while maintaining the same level of methane production. Lin et al. (2011, 2012) co-digested P&P sludge with monosodium glutamate (MSG) waste liquor. A high efficiency of methane generation was observed.
Reactor Systems for Anaerobic Treatment
Anaerobic baffled reactors, anaerobic filters, upflow anaerobic sludge bed reactors, and anaerobic membrane bioreactors are the main anaerobic reactors applied so far for the treatment of P&P mill wastewaters (Kamali et al. 2016). The main idea for the development of such high-rate reactors has been to decrease the hydraulic retention time, making it possible to treat higher amounts of wastewater in a given time.
As in the case of aerobic systems, a variety of reactor designs have been considered in efforts to promote anaerobic biodegradation and to reduce the footprint of the treatment plant. One of the underlying principles is to enhance the convective contact between water and films of biomass. For instance, Minami et al. (1991) employed a fixed bed of pumice stone so that the wastewater would pass over the surfaces of adsorbed biofilms under anaerobic conditions.
Jackson-Moss et al. (1992) employed activated carbon as a substrate for a bioreactor for bleach plant effluent and anaerobic conditions. Rajeshwari et al. (2000), in their review article, mention the use of activated carbon, polyvinyl chloride, crushed rock, and ceramic rings as packing materials that can be used for microbial immobilization during anaerobic treatment of wastewater. In addition to the formation of biofilm on the support medium, sludge aggregates become trapped between the packing elements of such systems, which may be beneficial to the overall process. Ruas et al. (2012) employed an immobilized biomass system for pretreatment of bleached kraft mill wastewater before an advanced oxidation system. Zain et al. (2013) and Hassan et al. (2014) employed a baffled reactor in which the wastewater was forced to flow up and down repeatedly as it passed through the system.
Anaerobic upflow filters
Upflow filter systems have been reported for anaerobic treatment of P&P mill wastewaters (Ahn and Forster 2002; Deshmukh et al. 2009). For instance, Deshmukh et al. (2009) employed a column in which the effluent was slowly pumped up through a column filled with plastic pall rings (38 mm x 38 mm) of skeletal form. The main disadvantage of systems using attached biomass growth appears to be an increased occasion for clogging (Maat and Habets 1987). Additional measures may be required to minimize clogging, thus increasing the cost of the treatment process.
As was mentioned earlier in the context of aerobic treatment, a blanket of sludge in principle can serve as a support medium for microbial immobilization. Such an arrangement is illustrated in Fig. 21. In such systems the wastewater to be treated is introduced below a layer of sludge and allowed to percolate up through that layer (Maat and Habets 1987; Hynninen 1998). The flow velocity can be as high as 2 to 3 m/h in some high velocity upflow systems (Rajeshwari et al. 2000).
Under anaerobic conditions, where the production of sludge is expected to be less (Ashrafi et al. 2015; Kamali and Khodaparast 2015), the same approach nevertheless has been evaluated (Lettinga et al. 1980; Habets and Knelissen 1985; Maat and Habets 1987; Rajeshwari et al. 2000; Buzzini and Pires 2002; Roest et al. 2005; Bhunia and Ghangrekar 2008; Buyukkamaci and Koken 2010; Sheldon et al. 2012). For instance, Bhunia and Ghangrekar (2008) found that such a system could be optimized by controlling the organic loading rate (OLR) to the system and by inoculating the system with thick sludge. Buzzini and Pires (2002) reported stable operation of an anaerobic reactor, with low maintenance and operational costs. Later work by the same group showed advantages of recirculating some of the sludge from treatment of P&P mill wastewater (Buzzini and Pires 2007).
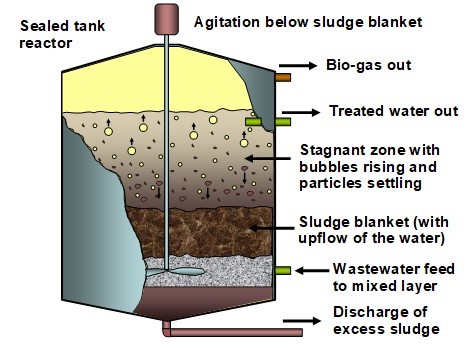
Fig. 21. Schematic of an anaerobic wastewater treatment with a sealed reactor, agitation at the level where wastewater is fed to the system, an upflow sludge blanket, and a stagnant liquid zone in which sludge particles settle and the generated bio-gas rises to the gaseous headspace
Anaerobic fluidized bed
Fluidized bed systems operating under anaerobic conditions have been used for wastewater treatment. The support media in such systems, if present, are sized such that they can be suspended by agitation and the rising of pumped wastewater that is being treated. The bed is generally composed of granular anaerobic sludge. Rajeshwari (2002) pointed out that the firm attachment of the biological matter to the support surfaces also can be critical to the operation. Various researchers have evaluated such systems for the treatment of P&P mill wastewaters (Haggblom and Salkinojasalonen 1991; Rusten et al. 1994; Huang et al. 2015). Huang et al. (2015) fluidized the sludge particles themselves as a relatively uniform mixture.
Unlike aerobic systems, in which aeration serves as a means to mix the liquid by the rise of bubbles, anaerobic systems use mechanical agitation, e.g. an impeller, to provide adequate mixing of liquid. Rusten et al. (1994) considered using such a system for P&P industry wastewaters. Maat and Habets (1987) criticized such systems as having relatively large energy demands to maintain fluidized conditions.
Anaerobic membrane bioreactor
Several researchers have evaluated the use of membrane bioreactors operating under anaerobic conditions in which ultrafiltration or nanofiltration is used to extract clarified water, while pollutants are concentrated in the sludge, which is digested under anaerobic conditions (Liao et al. 2006; Skouteris et al. 2012; Lin et al. 2013; Ashrafi et al. 2015). However, a search of the literature did not reveal publications of such equipment being used for anaerobic treatment of P&P industry wastes. This could be due to the relatively low accumulation of sludge often observed under anaerobic conditions. Alternatively, this could be due to the efficient results achieved by other anaerobic reactors in the pulp and paper industry, such as UASB and EGSB (Ordóñez et al. 2010), which do not need the use of energy for the separation of the sludge.
Pretreatments of Sludge before Anaerobic Digestion
The pre-treatment of sludge, generated during the treatment of pulp-and-paper wastewaters, has often been applied in an effort to increase the efficiency of subsequent digestion process (Saha et al. 2011; Elliott and Mahmood 2012; Bayr et al. 2013; Meyer and Edwards 2014). The anaerobic digestion of sludge is discussed by the following authors (Mahmood and Elliott 2006; Elliott and Mahmood 2012; Hagelqvist 2013; Kaluža et al. 2014; Meyer and Edwards 2014). The rupturing of P&P secondary sludge by a rapid release in pressure was found to be an effective method to render the sludge more amendable for subsequent anaerobic treatment (Stephenson et al. 2007). A drawback of many of these systems is the energy costs of operation.
Ultrasonic treatment of secondary sludge was considered by Saha et al. (2011) and Bayr et al. (2013). Saha et al. (2011) found ultrasonication to be effective for dispersing the sludge and breaking down the solids. However, the quantity of soluble compounds that resisted subsequent anaerobic treatment actually increased. Bayr et al. (2013) observed only a minor increase in methane yield during subsequent anaerobic treatment following ultrasonication. The ultrasonic treatment is generally expected to disrupt biological cells in the sludge by lysis, which might have enabled more effective degradation. Shaw and Lee (2008) showed that ultrasonication was able to decrease the color and turbidity of final effluent from a kraft pulp and paper mill.
Hydrothermal treatment, consisting of either 70 °C heating for 40 min or 150 °C heating for 10 min, was evaluated by Bayr et al. (2013). The higher temperature pretreatment was found to be among the most effective strategies that they considered. The amount of sludge was reduced and methane yield was increased. Enzymatic treatment with a cellulose-decomposing cocktail was found to be effective, especially in combination with the hydrothermal treatment (Bayr et al. 2013).
Saha et al. (2011) found microwave pretreatment to be effective for secondary sludge from a pulp mill, enabling more effective action of an anaerobic treatment stage. Specific methane yields were increased by 90% compared to controls. Ozone has also been tested for the pre-treatment of sludge, increasing its biodegradation rate (Mahmood and Elliott 2006).
One of the systems considered by the Japan Sewage Works Agency for sludge reduction involves the ozonation of return sludge before it enters the aeration basin solubilizing bacterial cells (Murakami 1998). Chu et al. (1999) reported a process called BIOLEADER, which uses these principles, and it was applied to pulp and paper wastewater treatment systems. Degremont Inc. has also developed a process called the Biolysis, which has been shown to reduce sludge yield by as much as the 80% (Rewcastle et al. 2004).
Considering that both sonochemical treatment and microwave irradiation have the potential to provide a considerable output energy to the system, for instance through the phenomenon of ultrasonic cavitation in the case of ultrasonic irradiation, more studies are required to identify the optimum conditions for such methods and to maximize their efficiency when used for the (pre-) treatment of pulp and paper mill sludge.
COMBINATION TREATMENTS
Sequences that Make Sense
With the goal of achieving more efficient and more comprehensive treatment of P&P mill effluents in the coming years, one of the most effective approaches can be expected to be the combining of more than one treatment stage, taking advantage of positive features of each step. As noted by Kamali and Khodaparast (2015), such approaches have the potential to achieve better overall environmental benefits and/or lower overall costs of operation. Mauchauffee et al. (2012) reviewed such approaches relative to treatment of effluents from a broad range of industries.
The selection of appropriate treatment processes and the sequence of operation is a challenging issue that confronts the engineers during the design of future wastewater treatment systems. Ordóñez et al. (2010) investigated a sequence involving gravity-based clarification, anaerobic biological treatment, aerobic biological treatment, ultrafiltration, and finally reverse osmosis. Such a sequence makes sense because the gravity-based clarification removes a lot of solids at low cost, avoiding the need to subject the removed solid matter to further stages of treatment. The anaerobic stage can be justified because it reduces the amount of BOD, without generating a lot of sludge byproduct. The follow-up aerobic treatment will degrade the byproducts of anaerobic treatment and will further decrease the BOD and undesirable byproducts of anaerobic treatment, such as sulfides and the final treatment of organic matter. The ultrafiltration ensures that there is no significant carry-over of biological cells from the biotreatment stages, while avoiding the cost of building a clarifier to begin the thickening process of the final sludge, ensuring adequate water quality for reverse osmosis. And finally, the reverse osmosis stage can be regarded as an option in cases where a high purity, desalinated water might be required for a specific usage. Such options will be considered in somewhat more detail in the subsections that follow.
Coagulation Followed by Other Processes
Coagulant followed by flocculant
As was noted earlier in this article, the settling of solids during gravity-based clarification can be enhanced by the addition of highly cationic agents that neutralize the predominantly negative surface charge of typical wastewater solids (Stephenson and Duff 1996; Ariffin et al. 2012; Simonič and Vnučec 2012). Some published strategies will be discussed in which such coagulation was used as an initial stage in two-stage treatments.
The combination treatment in which a high-charge or high-valence cationic agent (the coagulant) is followed by a very-high-mass polyelectrolyte (the flocculant) is worth mentioning again at this point due to its central role in many treatment plant operations. Ahmad et al. (2008) described a typical example in which either aluminum sulfate (alum) or poly-aluminum chloride (PAC) served as the coagulant, and then they added either a positively or negatively charged acrylamide copolymer as the flocculant. Highly efficient removal of suspended solids and reduction in oxygen demand were observed, and a low settling time was achieved.
Coagulation, then flotation
Coagulation also can be used in combination with a subsequent flotation stage. This is essentially what happens in an electrocoagulation-flotation system (Chen 2004; Han et al. 2006; Boroski et al. 2008; Emamjomeh and Sivakumar 2009). In the cited work by Boroski et al. (2008), Al3+ or iron (ferric or ferrous ions) were formed at an anode, and bubbles of hydrogen gas, formed at the cathode, caused agglomerated materials to rise and to form a froth. In principle, uncharged substances in water tend to be less hydrophilic in comparison with substances bearing an ionic charge. Thus, the neutralization of charges, brought about by addition of a coagulant, can be favorable for the attachment of air bubbles (which can be regarded as hydrophobic) with the agglomerated matter. The effectiveness of such electrocoagulation-flotation systems for the treatment of P&P mill effluents has been demonstrated (Chen and Horan 1998; Boroski et al. 2008).
The same benefits also can be achieved, in principle, by the direct addition of coagulating agents, followed by dissolved air flotation (DAF). Meyssami and Kasaeian (2005) showed that the addition of coagulants such as chitosan, alum, and ferric chloride, followed by flotation, could achieve high levels of water clarification. Yap et al. (2012) found that it was possible to optimize the surface charge of a system before DAF clarification by means of streaming current tests; in the cited work the neutralization was achieved either by the addition of alum or by lowering the pH with acid.
Electrocoagulation, then adsorption or photodegradation
Bellebia et al. (2012) showed that electrocoagulation could be used in combination with adsorption of the precipitated matter onto granular activated carbon. The combination was found to be effective for the removal of oxygen demand from paper mill wastewaters. Laboratory work showed that electrocoagulation also can be used as a precursor to photocatalytic degradation (Boroski et al. 2008). P&P mill effluent was coagulated using iron electrodes and subsequently treated with UV light in the presence of TiO2. The combined treatment was more effective for reducing the color of the treated wastewater.
Coagulation, then membrane separation
There are several logical explanations by which to justify the idea of using a sequence of coagulation followed by membrane filtration. On the one hand, membranes achieve separation by preventing the passage of particles above a specified size. Coagulation gathers colloidal or dissolved matter together into particles and tends to increase their size by means of continued agglomeration. A further issue is that membrane separation methods continually face difficulties related to the occlusion of their pores; by coagulating the solids into larger particulates, it is then less likely that the solids will be able to get into the fine pores or to form a dense, impermeable cake layer on the membrane surface. Hong et al. (2007) showed that the ultrafiltration flux could be enhanced by pretreating pulp mill wastewater with alum or ferric chloride. The flux decline rates were greatly reduced, in addition to reducing the oxygen demand and color in the permeate. Li and Zhang (2011) showed good results when using a composite flocculant ahead of a reverse osmosis membrane.
Bennani et al. (2012) demonstrated a further refinement of the approach just described by coagulating the wastewater solids, using aluminum chloride, so that it adsorbed onto the surface of clinoptilolite tuff, which is a natural zeolite having a high absorptive capacity. The treatment enhanced the performance of a subsequent filtration process with a reverse osmosis membrane.
Advanced Oxidation followed by Other Processes
Although advanced oxidation processes such as ozonation, Fenton oxidation systems, or UV irradiation in the presence of a catalyst have exhibited quite effective degradation of various aromatic organic compounds, they do not offer a full treatment option because the associated cost may be too high. Sometimes costs can be reduced by the combination of AOPs with conventional treatment processes, which are often less expensive. However, some advanced oxidation processes are not yet sufficiently mature to be applied in full-scale applications. Eskelinen et al. (2010) suggested integrating advanced oxidation with physico-chemical and biological treatments in order to maximize the treatment efficiency (Merayo et al. 2013). The hypothesis here is that one can break down the non-biodegradable compounds in the content of P&P mill wastewater (e.g., AOXs from bleaching sequences) and make these compounds ready for the subsequent biological treatments.
Biological treatment after AOP
The combination of advanced oxidation followed by biological treatment has received attention from many research teams (Heinzle et al. 1992; Pere et al. 1993; Nakamura et al. 1997; Rice 1997; Helble et al. 1999; Yeber et al. 1999b; Alvares et al. 2001; Baig and Liechti 2001; Bijan and Mohseni 2005; Balcioglu et al. 2007; Wang et al. 2008b; Soloman et al. 2009; Buyukkamci and Koken 2010; Goel et al. 2010; Oller et al. 2011; Tripathi et al. 2011; Antony and Natesan 2012; Hermosilla et al. 2012a; Lucas et al. 2012; Merayo et al. 2013). Essentially all of these researchers presented evidence, either direct or collected from other sources, of more complete or more rapid biodegradation following the oxidative treatment. The overall finding supports the view that those compounds in pulp and bleaching effluents that are recalcitrant to enzymatic degradation by micro-organisms are nevertheless susceptible to degradation by sufficiently strong oxidizing species such as the OH· radical, which has been shown to play a central role in such systems (Bautisa et al. 2008). However, the need for the catalysts that can be activated without illumination (e.g., UV irradiation) is also an urgent need to reduce the treatment cost and to enhance the application of such systems in full-scale treatment plants. Duran et al. (1994) reported good results from UV irradiation in the presence of a catalyst followed by fungal treatment.
The concept of partial oxidation before biological treatment was reviewed by Alvares et al. (2001). According to the concept, a sufficient and effective overall treatment can be achieved without fully oxidizing the organic substances during the initial treatment. Based on results presented in the cited work, it was concluded that over 90% transformation to oxidized species was needed in order to ensure the best overall results following the biological treatment.
Various studies, involving a sequence of advanced oxidative treatment followed by biological treatment, have helped to shed light on the mechanisms. Bijan and Mohseni (2005) showed that ozonation decreased the molecular mass of recalcitrant organic matter in pulp mill effluent, and it was suggested that the smaller molecules would be more biodegradable. Goel et al. (2010) reported that although chlorophenol could be broken down eventually by extended biotreatment, the time required could be decreased by almost a factor of three by photo-oxidation pretreatment. Because the combined treatments often remove toxicity effects (Yeber et al. 1999b; Rodriguez et al. 1999; Freire et al. 2000; Balcioglu et al. 2007; Catalkaya and Kargi 2008), it seems likely that some of the benefit of pretreatment could be related to higher viability of microorganisms during a subsequent biological phase of treatment. On the other hand, Alvares et al. (2001), in their review article, cited evidence of the development of toxic species following certain advanced oxidation treatments.
Reduction followed by Aeration
As mentioned earlier, there has been scant attention to the possibility of reductively treating paper mill wastes, even though such treatment has been shown to be very effective for decreasing the color of lignin-derived chromophores in wastewater (Ghoreichi and Haghighi 2007). The cited authors employed an aerated biological treatment as a follow-up stage to reductive treatment. The oxidative conditions of the biological treatment substantially reduced the oxygen demand, and also a low level of suspended solids was achieved by the combined treatment.
Anaerobic followed by Aerobic Treatment
Many researchers have evaluated the use of an anaerobic biological treatment stage followed by an aerobic stage for the treatment of P&P mill effluents (Haggblom and Salkinojasalonen 1991; Graves and Joyce 1994; Rusten et al. 1994; Demel et al. 2003; Zhang et al. 2009; Ordóñez et al. 2010; Sheldon et al. 2012). Marwaha et al. 1998) showed the feasibility of aerobic treatment of the sludge obtained from anaerobic digestion of black liquor. Review articles considering sequential anaerobic and aerobic biological wastewater treatment technology have been presented (Maat and Habets 1987; Suvilampi et al. 2001; Pokhrel and Viraraghavan 2004; Buyukkamaci and Koken 2010; Katahi et al. 2011; Ashrafi et al. 2015). In many instances, the primary reason for the aerobic stage is to remove residual organic matter from the anaerobically treated mill effluent. This sequential combination has some other important advantages. The anaerobic treatment reduces the total production of sludge, and also it produces biogas that could be used for energy production in the mill. On the other hand, anaerobic treatment cannot remove all the organic substances, and some inorganic compounds will be decreased. This means that, after an anaerobic stage, the final aerobic stage may be more effective in the removal of organics. Such an approach may be helpful in meeting legislated discharge levels. In other cases, the combination of these treatments with membrane filtration may be used to further polish the effluent for a possible reuse as a replacement for fresh water in the mill. However, Kortekaas et al. (1998) reported some undesirable effects of sequential anaerobic and aerobic treatment of hemp thermomechanical pulping wastewater; the aerobic stage resulted in increases in both color and the molecular weight of residual lignin.
The combination of both anaerobic and aerobic treatments can reduce the organic load and toxicity of a paper mill whitewater (Latorre et al. 2007). Singh and Thakur (2006) employed anaerobic treatment and subsequently fungus (Paecilomyces sp.) and bacterial strain (Microbrevis luteum) separately in two steps (Singh and Thakur 2006). Reduction of AOX during anaerobic treatment indicated the slow degradation of toxic compounds and elimination of chlorinated compounds present in the effluent. On the other hand, batch post-treatment of anaerobically-treated wastewater using L. edodes was able to remove COD, color, and ligninoids from the anaerobic effluent (Estrada-Vázquez et al. 1998). An aerobic reactor packed with T. versicolor immobilized on wood cubes fed with the anaerobic effluent is one of the few applications with extended performance (3 months) with no need of glucose supplement or other expensive carbohydrate, leading to the reduction of color, ligninoids, and COD by 68%, 52%, and 32% in 30 days (Ortega-Clemente et al. 2009).
Sulfur conversion
Several researchers have investigated a combined treatment, starting with anaerobic treatment of P&P mill wastewater, as a means to remove sulfur. This is important because sulfur compounds may be at high concentrations in effluents from paper mills, and it may be necessary to remove them from the effluent before their discharge. Sulfur compounds have the potential to be transformed into hydrogen sulfide if the discharged water subsequently encounters anaerobic conditions (Lens et al. 1998). According to Minami et al. (1991), sulfur compounds can inhibit anaerobic biological treatment, so it is advisable to strip the H2S gas and purge it from the system. According to Janssen et al. (2009), sulfur was substantially removed from the biogas after an anaerobic stage of treatment. To avoid the corrosive conditions inherent in a conventional gas sweetening process with NaOH scrubbing, the hydrogen sulfide gas was first dissolved into water in the form of hydrogen sulfite and then it was oxidized to elemental sulfur under a controlled level of aeration in a biological treatment stage. Residual reduced sulfur compounds still present in the water after the anaerobic stage were oxidized by a subsequent conventional aerobic biological wastewater treatment. Another alternative proposed by researchers is to convert sulfide to elemental sulfur at short hydraulic retention times by applying a biological post-treatment to anaerobic effluents (Buisman et al. 1988, 1990). In addition, Särkkä et al. (2009) demonstrated the electrochemical oxidation of sulfides from anaerobic wastewater treatment. Alternatively, Vepsalainen et al. (2011b) showed that ferrous ions could be used to precipitate iron sulfide from such solutions, which is currently the most common method for sulfide precipitation (Bajpai 2000). The drawbacks of this method are the cost of iron salts and the accumulation of FeS in the reactor, which may be responsible for low contents of active biomass in the suspended solids fraction, and may also increase total sludge production.
Another option is a two-stage anaerobic process, where sulfur is reduced to hydrogen sulfide and removed in a first stage (Bajpai 2000); this creates better conditions for the activity of sulfate reducing bacteria, so that sulfate removal will be improved. In a second stage, better conditions for the activity of methanogenic bacteria activity are set, which improves the removal of the organic load. Furthermore, another alternative to remove sulfurous compounds based on the same process consists of using an anaerobic filter reactor functioning with downflow conditions. Such a system enables the physical separation of sulfur-reducing bacteria in the upper portion of the reactor’s fixed media; whereas methanogenic bacteria will grow in the lower section. The produced biogas in the lower section of the reactor tends to strip out hydrogen sulfide, so it can work as a two-stage reactor (Bajpai 2000).
Anaerobic treatment followed by UV H2O2
The treated water after an anaerobic biological treatment stage commonly benefits from an oxidative treatment. Hence, as an alternative to aerobic biological treatment, chemical oxidants can be considered. Such a post-treatment process for treatment of kraft pulp bleaching effluent after anaerobic biotreatment was demonstrated by Ruas et al. (2012).
Aerobic Treatment Combinations
Combined treatment processes can address some problems often encountered during aerobic biological treatment processes such as the presence of recalcitrant compounds in the solution, difficulties in the settling of sludge, and the generation of a large volume of sludge.
Staged biotreatment with bacterial, predator steps
It may be counterintuitive to think that a promising way to address some of the issues just mentioned may be to follow aerobic treatment with another stage of aerobic treatment, but several researchers have proposed just such an approach. As was mentioned earlier, in another context, this can be called “staged” treatment (Lee and Welander 1996; Chakrabarti et al. 2008). As noted in the cited work, the system needs to be designed in such a way that active biological organisms are allowed to establish themselves on suitable support surfaces within the second stage of treatment. Predator species (protozoa and metazoa, including rotifers) in the second stage of treatment can proliferate under such conditions and feed upon the bacterial matter that is allowed to proliferate in the absence of predation in the first stage of treatment. Lee and Welander (1996) showed substantial reductions in both suspended solids and sludge solids after the second stage.
Aerobic treatment followed by coagulation
Given the widespread use of coagulants and flocculants in wastewater treatment, it is surprising that few publications have addressed the impact of using such additives in conjunction with aerobic wastewater treatment of P&P mill effluents. Aerobic treatment of effluent is generally regarded as a necessary step to achieve cost-effective compliance with local government regulations, but chemical additives may be needed to achieve adequate clarification after the aerobic stage. Hodgson et al. (1998) documented the use of aluminum sulfate as the coagulant to remove residual contaminants before its discharge into environmentally sensitive receiving waters. Agridiotis et al. (2007) showed that the addition of ferrous sulfate during secondary treatment of paper mill wastewater changed the bacterial matter from a filamentous form to a compact, more rapidly settling form. Aluminum chloride initially showed some promising results, but the sludge quality deteriorated; a likely explanation for the latter observations is that the system may have become overdosed with cationic charge, causing redispersal of the solid matter (Strazdins 1989).
For the treatment of secondary sludge, Pere et al. (1993) studied the effect of the residual iron in the content of wastewater when Fenton oxidation was applied as a pre-treatment method. They found that the dewatering was improved after advanced oxidation, using Fenton’s reagent. Though it is likely that some of the improvement in dewatering was due to the oxidation itself, the cited authors also noted a partial neutralization of the negative charge of the flocs, consistent with the presence of the iron ions. Finally, Newis et al. (2013) found that electrocoagulation was effective either as a pretreatment or as a post-treatment for aerobic biological treatment of sulfite pulping wastewater.
Ultrasonic treatments to enhance biodegradation
Ultrasonication has been applied to facilitate the removal of chlorine-based organic compounds from effluents (Pham et al. 2009). Efforts have been made to use not only sonication, but also hybrid technologies that couple ultrasound with hydrogen peroxide or ozone towards the destruction of pollutants associated with industrial waste water (Mason 2007; Gogate 2008). Acoustic cavitation produces hydroxyl radicals that are expected to oxidize organic species, lowering COD, and bleaching organic chromophores (Eskelinen et al. 2010). Sonication of pulp and paper effluent can bleach chromophores and reduce turbidity (Shaw and Lee 2009). However, the decrease of COD remains low, possibly due to scavenging of hydroxyl radicals by bicarbonate and sulfate ions present in the effluent. Additionally, Fenton-like oxidation has been used in combination with ultrasonic irradiation to breakdown refractory compounds from bleaching effluents (Vilve et al. 2009). Ultrasonic treatment as a pre- or post-oxidation combined with biodegradation of paper mill effluent has been reported to decrease toxicity and enhance biodegradability (Gonze et al. 2003).
POLISHING TREATMENTS
The term “polishing” implies a step to finish up a wastewater treatment process, ideally helping to assure that the discharged water will reliably meet the specifications and to comply with stringent environmental regulations. In earlier publications the term “tertiary treatment” often has been applied as a general label for all such treatment operations (Chen and Horan 1998). One of the priorities is that such a treatment must not add anything objectionable, such as a precipitate, and it ought not result in extremes of pH, which would remain in the outfall. One is aiming to end up with water that would be clear, odorless, and compatible with aquatic life.
Membrane Polishing Filtration
Many researchers have evaluated membrane filtration as a final step before discharge of the treated effluent from P&P making facilities (Huuhilo et al. 2002; Pokhrel and Viraraghavan 2004; Mänttäri et al. 2008; Gönder et al. 2011), or using that water in place of fresh water in mill operations (Ciputra et al. 2010; Ordóñez et al. 2010; Benani et al. 2012). Ciputra et al. (2010) described nanofiltration as a “broad spectrum” purification method, meaning that it is able to remove a very wide range of contaminants, all in one shot.
Among the various available membrane types, ultrafiltration membranes having pores in the range of about 2 to 100 nm (see Table 1) have been mentioned the most often for the final step in cleaning of paper industry wastewaters (Huuhilo et al. 2002; Pokhrel and Viraraghavan 2004; Mänttäri et al. 2008; Ordóñez et al. 2010). Such membranes have sufficiently fine porosity to exclude not only biological cells but also large molecules, such as some of the lignin decomposition products. At the same time, the flux through an ultrafiltration membrane will tend to be much higher than that of the next finer classes of membrane, the nanofiltration and reverse osmosis membranes, at a given transmembrane pressure.
Nanofiltration membranes, having a pore size range of about 1 to 5 nm, have been reported to outperform ultrafiltration membranes for the exclusion of organic contaminants (Huuhilo et al. 2002). Furthermore, Ciputra et al. (2010) found that nanofiltration was generally superior to the use of an ion exchange resin or activated carbon adsorption for comprehensive polishing of recycling paper mill effluent.
Going one step further, reverse osmosis membranes, which have pore sizes generally less than 1 nm, have shown good capability in cases where there is a need to exclude even small ions (Ordóñez et al. 2010, 2011; Li and Zhang 2011). Thus, the permeate can be used as intake water for a paper mill, making it possible to run the operation with close to zero liquid effluent (Pizzichini et al. 2005; Khosravi et al. 2011; Hermosilla et al. 2012a; Saif et al. 2013). Sierka et al. (1997), Zhang et al. (2009), and Bennani et al. (2012) evaluated systems in which nanofiltration was used as a preliminary step before reverse osmosis separation of paper industry wastewater, reporting almost complete removal of solutes. Mattari and Nystrom (2007) found that a nanofiltration membrane offered higher flux than a reverse osmosis membrane, but it was not as effective for the exclusion of monovalent ions such as chloride.
Treatment of Retentate from Membrane Filtration
Organic content of membrane retentate
The treatment and disposal of the retentate is an important problem in membrane filtration processes. As most treatment systems for P&P mill wastewaters will include at least one biological treatment stage; the retentate from membrane filtration can be recycled back to the intake of such a stage (Mahmood and Elliott 2006), but the presence of salts may inhibit the biological stage. Recognizing the recalcitrant nature of the organic compounds likely to be present in such retentate, Mänttäry et al. (2008) showed the effectiveness of ozone treatment before returning the stream to biological treatment. Hermosilla et al. (2012a) treated the membrane retentate with advanced oxidation systems including Fenton oxidation and UV-assisted oxidation, achieving the reduction of the concentration of organics to values meeting legislation requirements. It is possible to remove essentially all of the organics when performing the photo-Fenton process.
Salt removal from membrane retentate
Even after removal of most of the organic matter from retentate, high concentrations of inorganic ions may render the water unsuitable for re-use or for discharge to the environment. Ways to overcome such problems have been reviewed (Curcio and Drioli 2005; Kim 2011; Pérez-González et al. 2012). For instance, solar evaporation and deep-well injection of brines are often regarded as low-cost options for their disposal (Kim 2011). Evaporation technology to recover fresh water from salty or brackish water has become quite advanced (Woldai 2016). Alternatively, it may be advantageous to remove inorganic matter by crystallization, which traditionally has been carried out by evaporation and cooling. Membrane technology is providing opportunities to carry out such evaporation and crystallization with less use of space and energy (Lawson and Lloyd 1997; Kim 2011; Pérez-González et al. 2012). In addition, by suitable feeding of chemical agents, there are opportunities to selectively precipitate such compounds as CaSO4 (gypsum) or CaCO3 (Curcio and Drioli 2005). However, because of the complex and variable nature of waste streams from pulp and paper facilities, there will be challenges to overcome in order to adopt methods that have become established in the desalination industry.
Adsorption
The need for high applied pressures generally can be avoided by use of a media filtration process that takes advantage of adsorption of the contaminant on solids having a suitably high surface area and affinity (Chen and Horan 1998; Pokhrel and Viraraghavan 2004; Ciputra et al. 2010). Due to their very high surface area and generally hydrophobic nature, activated carbon products would be expected to be quite effective for removal of colored or hard-to-decompose aromatic compounds still present in water after other treatment stages. Several studies have shown the effectiveness of activated carbon for polishing of P&P mill wastewaters (Chen and Horan 1998; Ciputra et al. 2010). Activated carbons, as well as other adsorbent products derived from cellulosic materials, have been found to be effective for removal of metal ions, dyes, and other organic compounds from waters (Hubbe et al. 2011; 2012a; 2014). Ciputra et al. (2010) found that both ion exchange resins and activated carbon were effective for adsorptive removal of higher-mass fractions of dissolved organic matter present in paper mill wastewaters. One likely complication of such approaches is that, depending on the level of organic matter still present in the water, the surfaces might yet again become coated with biofilms; in other words what was intended as a final adsorption step might morph into another aerobic bioreactor stage at the end of the treatment cycle.
Ozone as a Polishing Treatment
Ozone treatment has a number of features that tend to recommend it as a final polishing treatment. Consisting of only a reactive form of oxygen, it is not expected to produce any objectionable residue having long-term stability. It generally does not induce the formation of precipitates or sludge. It can disinfect water and remove color (Hermosilla et al. 2015). Also it can convert any remnant substance from anaerobic treatment into suitable oxidized forms that are less likely to contribute to smells or depletion of oxygen in the receiving waters; or to contribute to the final polishing of the effluent after biological treatment. Moreover, its implementation is easy, and its presence is usual in the bleaching operations of pulp and paper mills. Thus, several authors have specifically recommended ozonation as a polishing treatment for P&P industry effluents before their discharge (Chen and Horan 1998; Baig and Liechti 2001; Pokhrel and Viraraghavan 2004; de los Santos Ramos et al. 2009). Oxidation by ozone as a stand-alone technology could be considered as uneconomical (Bijan and Mohseni 2008) due to the usual high volume of effluents in pulp and paper mills. Also, there may be a high content of organics and biodegradable compounds in the solution that can be removed cost-effectively from the effluent by biological technologies before a final treatment by ozone.
The efficiency of ozonation has mainly been attributed to the effective degradation of non-biodegradable compounds, such as toxic lignin by-products and chlorophenolic compounds (Hermosilla et al. 2015). A previous pre-treatment of easily biodegradable organic compounds can increase the efficiency of the treatment because the oxidants are then used for the oxidation of non-biodegradable compounds, and not for the treatment of organics that can be more inexpensively treated by biological treatment. Furthermore, if ozone is applied to increase biodegradability by breaking down the biorefractory compounds that are remaining in the effluent, a final bio-treatment (e.g. biofilters) may increase the overall treatment efficiency up to an 80% of COD reduction (Mobius and Helble 2004), as well as it may consequently reduce the overall treatment cost. An average of 20 to 25% COD removal enhancement has been reported for the ozone post-treatment of biologically-treated effluents in comparison to performing such an AOP as a pre-treatment step (Hermosilla et al. 2015). In fact, the implementation of on-line control systems for the treatment of biologically treated effluents by ozone provided a 20% cost saving per year (Bierbaum and Oeller 2009). This technology has also been included in the BREF document as a post-biological treatment alternative for bio-recalcitrant organic load persisting in effluents of pulp and paper mills aiming to meet the quality standards for discharge (BREF 2015).
Another very interesting treatment alternative is the application of membrane treatments instead of biological processes, or the combination of both, as in MBRs, avoiding the unnecessary oxidation of low molecular weight or biodegradable compounds (Bijan and Mohseni 2008; Mänttäri et al. 2008). Complementarily, and as it has previously been described, a second bio-treatment stage may be developed to remove turbidity, color, and further COD (Schlichter et al. 2003; Bijan and Mohseni 2008; Mänttäri et al. 2008). In addition, Balcioglu et al. (2007) considered improving algal treatment with ozone pre-treatment. This approach mainly enhanced color removal, but it also significantly reduced COD and UV280. Furthermore, the residence time of algal treatment was reduced from 8 to 5 days. Beneventi et al. (2009), reported the increase of efficiency of an ozone flotation system, achieving more than 20% COD removal in the flotation stage. Moreover, the use of pectinases, hemicellulases, cellulases, and lignolytic enzymes may be an option for the removal of persistent compounds after this treatment, being also an effective solution for deinking (Pala et al. 2004; Kamali et al. 2015). Finally, other different synergic options combining ozone with other treatments have been studied for increasing the overall treatment efficiency of ozonation as a polishing stage, such as the combined application with electrolysis (Kishimoto et al. 2010; Kamali et al. 2015).
Although ozone is probably the most applied AOP at the industrial scale for the final treatment of pulp and paper effluents, as well as being the reference technology in the BREF document (BREF 2015), there are other AOPs that have been tested, mainly at laboratory scale, for the final treatment of pulp and paper mills effluents. These are conventional Fenton (Hermosilla et al. 2015; Lucas et al. 2012; Balabaniç et al. 2012; Sevimli 2005; Catalkaya and Kargi 2007; Kazmi and Thul 2007), photo-Fenton (Catalkaya and Kargi 2007; Balabaniç et al. 2012), solar photo-Fenton (Gomathi and Kanmani 2006; Lucas et al. 2012; Hermosilla et al. 2015), electro-Fenton (Selvabharathi and Kanmani 2010), photocatalysis (Balabaniç et al. 2012), solar TiO2 photocatalysis (Merayo et al. 2013; Gomathi and Kanmani 2006), or photocatalysis + H2O2 (Catalkaya and Kargi 2008). In short, the pretreatment of the effluents by a biological stage can lead to an approximate 20% increase in the COD removal efficiency of the AOP treatment (Hermosilla et al. 2015).
In addition, information about the wastewater composition is not sufficient to establish the optimum operating conditions for these treatment processes. Rather, it is recommended to perform laboratory trials to determine specific operating conditions and the expected efficiencies of these treatments for each effluent (Hermosilla et al. 2015).
Bioaccumulation
Final lagoons and constructed wetlands
Once the final treated effluent is discharged from the wastewater treatment plant, it can be expected to mingle with the waters already present in the environment. That being the case, it makes logical sense to wonder whether there might be an advantage to letting such mingling to an ecosystem take place for a while before the final discharge beyond the property of the P&P mill facility. In other words, is there value of establishing or maintaining a wetland through which the treated effluent passes before joining the receiving waters?
In principle, as water flows through a marsh or other plant-filled wetland, various chemical components can be taken up by the plants, i.e. a process of bio-accumulation (Sakurai et al. 2001). Choudhary et al. (2013) found evidence of such bioaccumulation in the case of chlorinated organics as P&P mill wastewater flowed through a constructed wetland. There was substantial removal of absorbable organic halides from the water. On the other hand, the same organic matter may have the potential to persist in the environment and to become passed up the food chain (Contreras Lopez 2003). Thus, bioaccumulation can be regarded as a less favorable outcome than biodegradation of the organic compounds from P&P mill effluents.
In a constructed wetland one can envision the flow gradually moving horizontally below the water surface, i.e. sub-surface flow (Vymazal 2009; Choudhary et al. 2013). Vymazal (2009) describes the use of such designed lagoon systems for treatment of wastewaters from a wide variety of sources. Knight (2004) reported on a comprehensive study of the use of constructed wetlands for treatment of P&P mill wastewaters on behalf of the National Council for Air and Stream Improvement (NCASI). Although the study found many benefits of wetland strategies, there was no indication of reductions in the concentrations of dissolved solids, conductivity, or color. Hence, constructed wetlands were not regarded as being cost-effective relative to other means of treating P&P mill wastewaters.
SUMMARY COMMENTS
Use Nature as a Guide
An important benchmark against which to compare the quality of treated water, after a P&P operation, consists of assessing the quality of water upstream of that operation. Such intake water can be expected to contain a variety of contaminants including humic acids (Hubbe 2007a). In principle the discharged water ought to be clean, in important respects, relative to the quality of the water that was borrowed from the environment.
Visions for Future Implementations
To envision what might be good options for future green-field implementations or retrofitting of existing systems for P&P mills, a series of two figures will be considered. The first of these figures will show just the “bare bones” of a very traditional system entailing a save-all operation, primary clarification, and secondary aerobic biological treatment. The second figure will introduce a number of additional optional stages that seem to follow from findings that have been discussed in the course of this article.
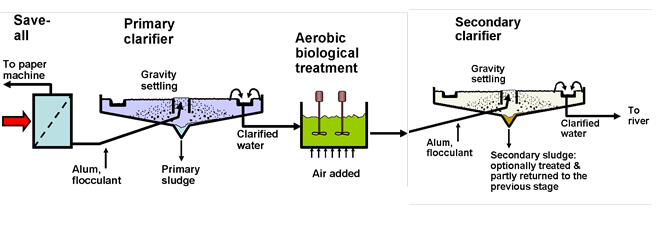
Fig. 22. Schematic diagram of a traditional, simple wastewater treatment plant for the pulp and paper industry
The traditional system, as shown in Fig. 22, may in fact be an ideal design in cases where it can consistently meet the effluent quality requirements at a suitably low cost of operations. This is particularly the case when the costs of construction of clarifiers and other equipment already have been incurred.
In Fig. 22, some factors that are especially important for primary treatment are coagulant dosage, flocculant dosage, and pH. Likewise, some factors that are especially important for aerobic treatment include aeration rate, temperature, retention time, nutrients, pH, active sludge recycling, and sludge age.
The cost of building and operating save-all systems for paper machines can be justified by improvements in the yield of a papermaking operation (Milliken 2006). The fine, solid matter present in excess process water discharged from papermaking ought to be returned efficiently to the process so that most of it becomes incorporated into the paper product. Furthermore, the fines fraction of a papermaking furnish can be expected to contain a disproportionate amount of the adsorbed additives, such as sizing agents and strength agents (Marton 1980a,b). Thus, if fines are allowed to pass into the wastewater treatment system, then the company is losing the potential value of those additives.
One might think that in some cases it can be reasonable to simply skip the primary clarification of paper machine effluent and immediately commence with biological treatment. But the problem with that option is that the sludge from primary treatment is usually much easier to handle. It tends to be dense and compact, especially if the source is a paper mill that employs a mineral filler such as calcium carbonate or clay (Mahmood and Elliott 2006). It might be a mistake to wait and have to handle such solids as part of the secondary sludge, which is characteristically much more difficult to dewater. Still, given the cost and space requirements of primary clarification, there is no assurance that this treatment stage will be included in future wastewater treatment plants for P&P mills.
None of the work cited in this review questioned the need for at least one biological treatment stage to treat wastewaters from pulp or paper mills. The large quantity of biodegradable material, such as hemicellulose, extractives, starches, and their decomposition products present in these wastewaters dictates the need for the inclusion of a biological process during the treatment operation. Biodegradation processes tend to be cost-effective relative to the available alternatives, especially for dealing with the most easily degradable compounds present.
Though Fig. 22 does not explicitly show a separate clarification stage after the biological treatment stage, the use of a separate clarifier can be regarded as a standard practice in most facilities at present.
One might argue that Fig. 22 really ought to include a “tertiary treatment” stage, since such stages have been widely mentioned in the literature for many years (Chen and Horan 1998; Rajvaidya and Markandey 1998; Helbe 1999; Gragnon and 2002). However, there has been no widespread consensus regarding the necessity to employ such a tertiary stage, nor has there been any widespread consensus of one system that is best for most P&P mill facilities.
Figure 23 portrays a hypothetical treatment plant that incorporates a number of optional steps, consistent with findings discussed in the course of this article. In Fig. 23, the save-all options would include disk screen systems and dissolved air flotation (DAF). The oxidation options would include ozone, Fenton processes, UV illumination with or without catalysts such as TiO2 and possibly supplemented by H2O2, as well as electro-oxidation. The membrane options would include ultrafiltration, nanofiltration, and reverse osmosis.
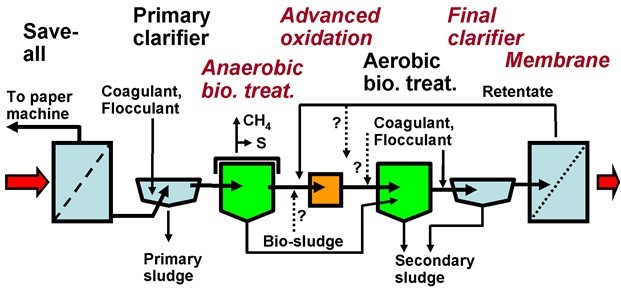
Fig. 23. Schematic diagram showing options for enhancements of wastewater treatment plants for the pulp and paper industry
A key item that appears in Fig. 23 but not in Fig. 22 is an anaerobic treatment stage. The reasons for suggesting the inclusion of such stages in future implementations and improvements of wastewater treatment plants for P&P mills can be summarized as follows: low operating costs, relatively low sludge production, production of valuable methane gas, and substantial reductions of biological oxygen demand. But the capital cost of a new anaerobic treatment plant requires careful consideration, in light of the fact that the operation needs to be covered, and there must be a system for collection of methane and other reduced gases produced. Note also that Fig. 23 depicts the use of a scrubber system and associated sub-processes to extract sulfur-containing gases.
An advanced oxidation stage has been drawn into Fig. 23 as a further option to consider, if it is needed. In Fig. 23 such a stage is placed after the anaerobic stage, where the strategy would be to avoid using up valuable chemicals in the treatment of either easily biodegradable materials or that portion of such compounds that would be carried away as sludge. One of the open questions, however, is whether to target the soluble portion or to oxidize the entire discharge from the anaerobic biological treatment, thereby facilitating an overall reduction in the amount of sludge as a byproduct from the treatment operations. Additionally, an advanced oxidation system could be placed after the aerobic biological treatment as a polishing step. The answer is likely to depend on what option is most cost-effective. Other open questions are whether increasing the biodegradability of the organic matter, by means of a further biological stage (e.g. biofilters) or performing further wastewater treatment, will allow a production facility to meet their legislated limits.
Aerobic treatment is still included in Fig. 23, despite the option of using anaerobic treatment, due to the expected need to oxidize the biodegradable compounds that the anaerobic stage is not able to polish. The aerobic treatment also will oxidize any sulfides and sulfur-containing compounds remaining in the water phase. In some cases it might make sense to consider an ozonation or high-pressure oxygen stage, instead, but even in such cases, an aerated biological treatment stage would ordinarily be regarded as needed to economically cut down the release of biological oxygen demand.
As a final treatment stage, though Fig. 23 depicts the symbol of a membrane filter, it also can make sense to employ coagulative and flocculative settling of final water, or to ozonate the final water. Alternatively, as also shown in the figure, flocculation can be added as a feature during the settling of the sludge during clarification after the aerated biological treatment stage. Thus, many options present themselves, depending on specific needs.
Although Fig. 23 can be regarded as a scheme that follows from the concepts discussed in this review article, there are many alternative designs that can be considered for the effective treatment of pulp-and-paper wastewaters. Much research is needed in a variety of disciplines including biology, microbiology, chemistry, and chemical and mechanical engineering to ensure the removal of recalcitrant pollutants that exist at very low concentrations in wastewaters emerging from P&P mills, especially those that resist removal by traditional treatment processes. These efforts will ensure minimal impact of the effluents on the quality of downstream waters and will contribute to the protection of the environment and aquatic life.
Degradation products and legislative requirements
In Europe, “The Best Available Techniques (BAT) Reference Document for the Production of Pulp, Paper, and Board,” which is included in the Industrial Emissions Directive 2010/75/EU (Integrated Pollution Prevention and Control), is the reference document determining BAT-associated emission levels for direct wastewater discharge from the different types of pulp and paper mills to receiving water (BREF 2015). In short, the yearly average emissions reported in this document go up to 30 kg/ADt of COD, 1.5 kg/ADt of total suspended solids, 0.3 kg/ADt of total nitrogen, 0.11 kg/ADt of total phosphorus, and 0.2 kg/ADt of adsorbable organically bound halogens. The higher values listed in the document generally are associated with bleached kraft and sulfite pulp mills.
Directive 2008/105/EC, on environmental quality standards in the field of water policy, includes a list of priority substances in its Annex I. According to the BREF (2015), none of these listed priority substances are used within pulp and papermaking processes in Europe. Some of these substances (e.g. dichloromethane, hexachlorocyclohexane, di(2-ethylhexyl)phthalate, nonylphenols, octylphenols, pentachlorophenol, or some other polyaromatic hydrocarbons) can be found in the emissions from waste water treatment plants, as it has previously been described in the “Pulp and Paper Wastewater” section of this review, or they can be introduced in the production process included in imported pulps or contained in upstream abstracted surface water that will be used in the papermaking process itself. Also, some of these compounds of concern can be produced in unit processes in pulp and paper manufacture, especially in bleaching operations. Further research related to such releases and their treatment will be required in the future.
ACKNOWLEDGMENTS
The authors wish to acknowledge the following volunteers who made suggestions regarding the improvement of this article: Juan José Villaverde (University of Aveiro, Portugal; INIA, DTEVPF, Plant Protect Prod Unit, Madrid, Spain), Alawi Sulaiman (Tropical Agro-Biomass Research Group, Universiti Teknologi MARA, Selangor, Malaysia), Félix J. Ferdnández-Gómez (Universidad de Castilla-La Manca, Spain), and Mark Rueber (WestRock, USA).
REFERENCES CITED
Abrahamsson, K., and Klick, S. (1991). “Degradation of halogenated phenols in anoxic marine sediments,” Mar. Pollut. Bull. 22(5), 227‑233. DOI: 10.1016/0025-326X(91)90915-F
Achoka, J. D. (2002). “The efficiency of oxidation ponds at the kraft pulp and paper mill at Webuye in Kenya,” Water Research 36(5), 1203-1212. DOI:10.1080/02772248.2011.610498
Ackermann, C., Gottsching, L., and Pakarinen, H. (2000). “Papermaking potential of recycled fiber,” in: Recycled Fiber and Deinking, L. Göttsching and H. Pakarinen (eds.), Papermaking Science and Technology series, Book 7, Fapet Oy, Jyvaskyla, Finland, pp. 359-439.
Adachi, M., and Nagao, Y. (2001). “Design of near-infrared dyes based on pi-conjugation system extension 2. Theoretical elucidation of framework extended derivatives of perylene chromophore,” Chem. Mater. 13(2), 662-669. DOI: 10.1021/cm000857v
Agarwal, A., Xu, H., Ng W. J., and Liu, Y. (2012). “Biofilm detachment by self-collapsing air microbubbles: A potential chemical-free cleaning technology for membrane biofouling,” Journal of Materials Chemistry 22, 2203-2207. DOI: 10.1039/C1JM14439A
Agridiotis, V., Forster, C. F., and Carliell-Marquet, C. (2007). “Addition of Al and Fe salts during treatment of paper mill effluents to improve activated sludge settlement characteristics,” Bioresour. Technol. 98(15), 2926-2934. DOI: 10.1016/j.biortech.2006.10.004
Aguilar, M. I., Sáez, J., Lloréns, M., Soler, A., and Ortuño, J. F. (2003). “Microscopic observation of particle reduction in slaughterhouse wastewater by coagulation-flocculation using ferric sulphate as coagulant and different coagulant aids,” Water Res. 37, 2233-2241. DOI: 10.1016/S0043-1354(02)00525-0
Ahlborg, U. G. (1977). “Metabolism of chlorophenols: Studies on dechlorination in mammals,” Rapport från Statens Naturvårdsverk, Solna, Sweden.
Ahmad, A. L., Wong, S. S., Teng, T. T., and Zuhairi, A. (2007). “Optimization of coagulation-flocculation process for pulp and paper mill effluent by response surface methodological analysis,” J. Hazard. Mater. 145(1-2), 162-168. DOI: 10.1016/j.jhazmat.2006.11.008
Ahmad, A. L., Wong, S. S., Teng, T. T., and Zuhairi, A. (2008). “Improvement of alum and PACl coagulation by polyacrylamides (PAMs) for the treatment of pulp and paper mill wastewater,” Chem. Eng. J. 137, 510-517. DOI: 10.1016/j.cej.2007.03.088
Ahmed, B., Mohamed, H., Limem, E., and Nasr, B. (2009). “Degradation and mineralization of organic pollutants contained in actual pulp and paper mill wastewaters by a UV/H2O2 process,” Ind. Eng. Chem. Res. 48, 3370-3379. DOI: 10.1021/ie801755u
Ahn, J. H., and Forster, C. F. (2002). “A comparison of mesophilic and thermophilic anaerobic upflow filters treating paper-pulp-liquors,” Process Biochem. 38(2), 257-262. Article Number: PII S0032-9592(02)00088-2
Ahn, K.-H., Cha, H.-Y., Yeom, I.-T., and Song, K.-G. (1998). “Application of nanofiltration for recycling of paper regeneration wastewater and characterization of filtration resistance,” Desalination 119, 169-176. DOI: 10.1016/S0011-9164(98)00141-6
Akitt, J. W., Greenwood, N. N., Khandelwal, B. L., and Lester, G. D. (1972a). “27Al nuclear magnetic resonance studies of the hydrolysis and polymerization of the hexa-aquo-aluminum(III) cation,” J. Chem. Soc. Dalton Trans. 1972(5), 604-610. DOI: 10.1039/dt9720000604
Akitt, J. W., Greenwood, N. N., and Khandelwal, B. L. (1972b). “Aluminum-27 nuclear magnetic resonance studies of sulphato-complexes of the hexa-aquo aluminum ion,” J. Chem. Soc. Dalton Trans. 1972(12), 1226-1229. DOI: 10.1039/dt9720001226
Akolekar, D. B., Bhargava, S. K., Shirgoankar, I., and Prasad, J. (2002). “Catalytic wet oxidation: An environmental solution for organic pollutant removal from paper and pulp industrial waste liquor,” Appl. Catal. A – General 236(1-2), 255-262. DOI: 10.1016/S0926-860X(02)00292-2
Ala-Kaila, K., Poukka, O., and Tervola, P. (2005). “Challenging the traditional counter-current water circulation system. Case: Washing of soap in a kraft pulp fibreline,” Can. J. Chem. Eng. 83(4), 784-790. DOI: 10.1002/cjce.5450830420
Albertson, O. E., and Wilson, J. P. (1997). “Clarifier design concept – Larger is better,” J. Environ. Eng. – ASCE 123(11), 1159-1161. DOI: 10.1061/(ASCE)0733-9372(1997)123:11(1159)
Alcock, R.E., and Jones, K.C. (1996). “Dioxins in the environment: A review of trend data,” Environ. Sci. Technol. 30(11), 3133‑3143. DOI: 10.1021/es960306z
Alexander, S. D., and Dobbins, R. J. (1977). “The buildup of dissolved electrolytes in a closed paper mill system,” Tappi 60(12), 117-120.
Ali, M., and Sreekrishnan, T. R. (2001). “Aquatic toxicity from pulp and paper mill effluents: A review,” Adv. Environ. Res. 5(2), 175-196. DOI: 10.1016/S1093-0191(00)00055-1
Al-Jasser, A. O. (2009). “Enhancement of sludge settling with chemical additives,” Water Environ. Res. 81(9), 849-857. DOI: 10.2175/106143008X390799
Al Momani, F. A., and Ormeci, B. (2014). “Optimization of polymer dose based on residual polymer concentration in dewatering supernatant,” Water Air Soil Pollution 225(11), Article no. 2154. DOI: 10.1007/s11270-014-2154-z
Al-Shannag, M., Lafi, W., Bani-Melhem, K., Gharagheer, F., and Dhaimat, O. (2012). “Reduction of COD and TSS from paper industries wastewater using electro-coagulation and chemical coagulation,” Separ. Sci. Technol. 47(5), 700-708. DOI: 10.1080/01496395.2011.634474
Alvira, P., Tomas-Pejo, E., Ballesteros, M., and Negro, M. J. (2010). “Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review,” Bioresour. Technol. 101(13), 4851-4861. DOI: 10.1016/j.biortech.2009.11.093
Alvares, A. B. C., Diaper, C., and Parsons, S. A. (2001). “Partial oxidation by ozone to remove recalcitrance from wastewaters – A review,” Environ. Technol. 22(4), 409-427. DOI: 10.1080/09593332208618273
Amat, A. M., Arques, A., López, F., and Miranda, M. A. (2005a). “Solar photo-catalysis to remove paper mill wastewater pollutants,” Sol Energy 79, 393-401. DOI: 10.1016/j.solener.2005.02.021
Amat, A. M., Arques, A., Miranda, M. A., and López, F. (2005b). “Use of ozone and/or UV in the treatment of effluents from board paper industry,” Chemosphere 60(8), 1111-1117. DOI: 10.1016/j.chemosphere.2004.12.062
Annachhatre, A. P., and Gheewala, S. H. (1996). “Biodegradation of chlorinated phenolic compounds,” Biotechnol. Adv. 14(1), 35‑56. DOI: 10.1016/0734-9750(96)00002-X
Antony, S. P., and Natesan, B. (2012). “Optimization of integrated electro-bio process for bleaching effluent treatment,” Indus. Eng. Chem. Res. 51(24), 8211-8221. DOI: 10.1021/ie3009633
APHA (2001, 2005). Standard Methods for the Examination of Water and Wastewater, American Public Health Association, Washington, DC. DOI:10.1029/2002JC001375; downloaded March 2016: http://secure.apha.org/imis/ItemDetail?iProductCode=978-087553-0130&Category=BK&WebsiteKey=0b8c9ed4-d1c5-4381-9c20-fa84b9f2e4a0
APHA (2016). Standard Methods for the Examination of Water and Wastewater, American Public Health Association, Washington, DC, 22nd Edition.
Apilánez, I., Gutiérrez, A., and Díaz, M. (1998). “Effect of surface materials on initial biofilm development,” Bioresour. Technol. 66(3), 225-230. DOI: 10.1016/S0960-8524(98)00052-2
Ariffin, A., Razali, M. A. A., and Ahmad, Z. (2012). “PolyDADMAC and polyacrylamide as a hybrid flocculation system in the treatment of pulp and paper mills waste water,” Chem. Engin. J. 179, 107-111. DOI: 10.1016/j.cej.2011.10.067
Arnson, T. R., and Stratton, R. A. (1983). “The adsorption of complex aluminum species by cellulosic fibers,” Tappi J. 66(12), 72-75.
Ashley, C. R., and Heindel, T. J. (1999). “Visualizing air bubbles in pulp suspensions common to recycling operations,” Proc. TAPPI 99 Proceedings, TAPPI Press, Atlanta, pp. 617-632.
Ashrafi, O., Yerushalmi, L., and Haghighat, F. (2013). “Greenhouse gas emission by wastewater treatment plants of the pulp and paper industry – Modeling and simulation,” Internatl. J. Greenhouse Gas Contl. 17, 462-472. DOI: 10.1016/j.ijggc.2013.06.006
Ashrafi, O., Yerushalmi, L., and Haghighat, F. (2014). “Greenhouse gas emission and energy consumption in wastewater treatment plants: impact of operating parameters,” CLEAN – Soil, Air, Water 42(3), 207-220. DOI: 10.1002/clen.201200158
Ashrafi, O., Yerushalmi, L., and Haghighat, F. (2015). “Wastewater treatment in the pulp-and-paper industry: A review of treatment processes and the associated greenhouse gas emission,” J. Environ. Manag. 158, 146-157. DOI: 10.1016/j.jenvman.2015.05.010
Aspapel (2011). “Sustainability report,” Spanish Association of Pulp, Paper and Board Producers.
Avella, A. C., Görner, T., Yvon, J., Chappe, P., Guinot-Thomas, P., and de Donato, P. (2011). “A combined approach for a better understanding of wastewater treatment plants operation: Statistical analysis of monitoring database and sludge physico-chemical characterization,” Water Res. 45(3), 981-992. DOI: 10.1016/j.watres.2010.09.028
Back, E. L., and Ekman, R. (2000). “Definitions of wood resin and its components,” in: Pitch Control, Wood Resin and Deresination, E. L. Back and L. H. Allen (eds.), TAPPI Press, Atlanta.
Baig, S., and Liechti, P. A. (2001). “Ozone treatment for biorefractory COD removal,” Water Sci. Technol. 43(2), 197-204.
Bajpai, P. (2000). Treatment of Pulp and Paper Mill Effluents with Anaerobic Technology, Pira International, Surrey, UK.
Bajpai, P., and Bajpai, P. K. (1994). “Biological color removal of pulp and paper-mill wastewaters,” J. Biotechnol. 33(3), 211-220. DOI: 10.1016/0168-1656(94)90069-8
Bajpai P, and Bajpai P. K. (1997). “Reduction of organochlorine compounds in bleach plant effluents,” in: Advances in Biochemical Engineering and Biotechnology, E. L. Eriksson (ed.), Springer-Verlag, Berlin. DOI: 10.1007/bfb0102076
Balabanic, D., Hermosilla, D., Merayo, N., Klemencic, A. K., and Blanco, A. (2012). “Comparison of different wastewater treatments for removal of selected endocrine disruptors from paper mill wastewaters,” J. Environ. Sci. Health. Pt. A – Toxic/Haraz. Subst. Environ. Eng. 47(10), 1350-1363. DOI: 10.1080/10934529.2012.672301
Balcioğlu, I. A., Sarac, C., Kivilcimdan, C., and Tarlan, E. (2006). “Application of ozonation and biotreatment for forest industry wastewater,” Ozone‑Sci. Eng. 28(26), 431‑436. DOI: 10.1080/01919510600987685
Balcioglu, I. A., Tarlan, E., Kivilcimdan, C., and Sacan, M. T. (2007). “Merits of ozonation and catalytic ozonation pre-treatment in the algal treatment of pulp and paper mill effluents,” J. Environ. Manag. 85, 918-926. DOI: 10.1016/j.jenvman.2006.10.020
Balcioglu, I. A., and Moral, C. K. (2008). “Homogeneous and heterogeneous catalytic ozonation of pulp bleaching effluent,” J. Adv. Oxidation Technol. 11(3), 543-550.
Banerjee, S., Howard, P. H., Rosenberg, A. M., Dombrowski, A. E., Sikka, H., and Tullis, D. L. (1984). “Development of a general kinetic model for biodegradation and its application to chlorophenols and related compounds,” Environ. Sci. Technol. 18(6), 416‑422. DOI: 10.1021/es00124a005
Bani Shahabadi, M., Yerushalmi, L., and Haghighat, F. (2009). “Impact of process design on greenhouse gas (GHG) generation by wastewater treatment plants,” Wat. Res. 43(10), 2679-2687. DOI: 10.1016/j.watres.2009.02.040
Bani Shahabadi, M., Yerushalmi, L., and Haghighat, F. (2010). “Estimation of greenhouse gas generation in wastewater treatment plants–model development and application,” Chemosphere 7, 1085-1092. DOI: 10.1016/j.chemosphere.2009.12.044
Barakat, A., Mayer-Laigle, C., Solhy, A., Arancon, R. A. D., de Vries, H., and Luque, R. (2014). “Mechanical pretreatments of lignocellulosic biomass: Towards facile and environmentally sound technologies for biofuels production,” RSC Adv. 4(89), 48109‑48127. DOI: 10.1039/c4ra07568d
Barndõk, H., Hermosilla, D., Cortijo, L., Negro, C., and Blanco, A. (2012). “Assessing the effect of inorganic anions on TiO2-photocatalysis and ozone oxidation treatment efficiencies,” J. Adv. Oxid. Technol. 15(1), 125‑132.
Barron, W., Murray, B. S., Scales, P. J., Healy, T. W., Dixon, D. R., and Pascoe, M. (1994). “The streaming current detector: A comparison with conventional electrokinetic techniques,” Colloids Surf. A.: Physicochem. Eng. Aspects 88, 129-139. DOI: 10.1016/0927-7757(94)02824-9
Barton, L. L., and Fauque, G. D. (2009). “Biochemistry, physiology and biotechnology of sulfate-reducing bacteria,” Adv. Appl. Microbiol. 68, 41-98. DOI: 10.1016/S0065-2164(09)01202-7
Baruah, B. K. (1997). “Effect of paper mill effluent on plankton population of wetland,” Environ. Ecol. 15(4), 770‑777.
Bauman, H. D., and Lutz, L. R. (1974). “Ozonation of kraft mill effluent,” Tappi J. 57(5), 116-119.
Baroutian, S., Robinson, M., Smit, A.-M., Wijeyekoon, S., and Gapes, D. (2013). “Transformation and removal of wood extractives from pulp mill sludge using wet oxidation and thermal hydrolysis,” Bioresource Technology 146, 294-300. DOI: 10.1016/j.biortech.2013.07.098
Bautista, P., Mohedano, A. F., Casas, J. A., Zazo, J. A., and Rodriguez, J. J. (2008). “Review. An overview of the application of Fenton oxidation to industrial wastewaters treatment,” J. Chem. Technol. Biotechnol. 83(10), 1323-1338. DOI: 10.1002/jctb.1988
Bayr, S., Kaparaju, P., and Rintala, J. (2013). “Screening pretreatment methods to enhance thermophilic anaerobic digestion of pulp and paper mill wastewater treatment secondary sludge,” Chem. Eng. J. 223, 479-486. DOI: 10.1016/j.cej.2013.02.119
Behrooz, R., Abadi, H. H., and Mohebby, B. (2011). “Turbidity and BOD removal of a paper recycling mill effluent by electro-coagulation technique,” Desalination Water Treat. 28, 65-68. DOI: 10.5004/dwt.2011.2202
Bellebia, S., Kacha, S., Bouyakoub, A. Z., and Derriche, Z. (2012). “Experimental investigation of chemical oxygen demand and turbidity removal from cardboard paper mill effluents using combined electrocoagulation and adsorption processes,” Environ. Prog. Sustain. Energy 31(3), 361-370. DOI: 10.1002/ep.10556
Beltrán-Heredia, J., Torregrosa, J., García, J., Domínguez, J. R., and Tierno, J. C. (2001). “Degradation of olive mill wastewater by the combination of Fenton’s reagent and ozonation processes with an aerobic biological treatment,” Water Sci. Technol. 44(5), 103‑108.
Beneventi, D., Almeida, F., Marlin, N., Curtil, D., Salgueiro, L., and Aurousseau, M. (2009). “Hydrodynamics and recovered papers deinking in an ozone flotation column,” Chem. Eng. Process. Process Intensif. 48, 1517-1526. DOI: 10.1016/j.cep.2009.10.007
Bengtsson, S., Hallquist, J., Werker, A., and Welander, T. (2008a). “Acidogenic fermentation of industrial wastewaters: Effects of chemostat retention time and pH on volatile fatty acids production,” Biochem. Eng. J. 40(3), 492-499. DOI: 10.1016/j.bej.2008.02.004
Bengtsson, S., Werker, A., Christensson, M., and Welander, T. (2008b). “Production of polyhydroxyalkanoates by activated sludge treating a paper mill wastewater,” Bioresour. Technol. 99(3), 509-516. DOI: 10.1016/j.biortech.2007.01.020
Bennani, Y., Kosutic, K., Drazevic, E., and Rozic, M. (2012). “Wastewater from wood and pulp industry treated by combination of coagulation, adsorption on modified clinoptilolite tuff and membrane processes,” Environ. Technol. 33(10), 1159-1166. DOI: 10.1080/09593330.2011.610828
Bergeron, J., and Pelletier, C. (2004). “Occurrence and significance of filamentous bacteria in pulp and paper activated sludge systems,” Water Sci. Technol. 50(3) 39-48.
Bhargava, S. K., Tardio, J., Prasad, J., Foger, K., Akolekar, D. B., and Grocott, S. C. (2006). “Wet oxidation and catalytic wet oxidation,” Indust. Eng. Chem. Res. 45(4), 1221-1258. DOI: 10.1021/ie051059n
Bhunia, P., and Ghangrekar, M. M. (2008). “Effects of cationic polymer on performance of UASB reactors treating low strength wastewater,” Bioresour. Techol. 99, 350-358. DOI: 10.1016/j.biortech.2006.12.014
Bi, S. P., Wang, C., Cao, Q., and Zhang, C. H. (2004). “Studies on the mechanism of hydrolysis and polymerization of aluminum salts in aqueous solution: Correlations between the “Core-links” model and “Cage-like” Keggin-Al13 model,” Coordination Chem. Rev. 248, 441-455. DOI: 10.1016/j.ccr.2003.11.001
Bierbaum, S., and Oeller, H. J. (2009). “Cost savings in the ozone treatment of paper mill effluents achieved by a closed-loop ozone control system,” Ozone Sci. Eng. 31, 454-460. DOI: 10.1080/01919510903323141
Biermann, C. J. (1996). Handbook of Pulping and Papermaking, 2nd Ed., Academic Press, San Diego, 754 pp.
Bijan, L., and Mohseni, M. (2005). “Integrated ozone and biotreatment of pulp mill effluent and changes in biodegradability and molecular weight distribution of organic compounds,” Water Res. 39(16), 3763-3772. DOI: 10.1016/j.watres.2005.07.018
Bijan, L., and Mohseni, M. (2008). “Novel membrane pretreatment to increase the efficiency of ozonation-biooxidation,” Environ. Eng. Sci. 25, 229-237. DOI: 10.1089/ees.2006.0264
Bilitewski, B., Darbra, R. M., and Barcelo, D. (eds.). (2012). Global Risk-based Management of Chemical Additives I: Production, Usage and Environmental Occurrence, Springer, London, New York. DOI: 10.1007/978-3-642-24876-4
Björklund Jansson, M., and Nilvebrant, N.-O. (2009). “Wood extractives,” in: Pulp and Paper Chemistry and Technology, Wood Chemistry and Biotechnology. M. Ek, G. Gellerstedt, and G. Henriksson (eds.), Walter de Gruyter GmbH & Co. KG, Berlin.
Bjørseth, A., Lunde, G., and Gjøs, N. (1977). “Analysis of organochlorine compounds in effluents from bleacheries by neutron activation analysis and gas chromatography/mass spectrometry,” Acta Chem. Scand. B31(9), 797‑801. DOI: 10.3891/acta.chem.scand.31b-0797
Blanco, A., del la Fuente, E., Negro, C., Monte, M. C., and Tijero, J. (2002a). “Focused beam reflectant measurement as a tool to measure flocculation,” TAPPI J. 1(10), 14-20.
Blanco, A., Fuente, E., Negro, C., and Tijero, J. (2002b). “Flocculation monitoring: Focused beam reflectance measurement as a measurement tool,” Can. J. Chem. Eng. 80(4), 734-740. DOI: 10.1002/cjce.5450800403
Blanco, A., Hermosilla, D., and Negro, C. (2016). “Water reuse within the paper industry,” in: The Handbook of Environmental Chemistry, Vol. 44, Fatta-Kassinos, D., Dionysiou, D. E., and Kümmerer, K. (eds.), Springer, Switzerland, pp. 213-238. ISBN: 978-3-319-23891-3 (Print) 978-3-319-23892-0 (Online)
Blanco, A., Negro, C., Monte, C., Fuente, E., and Tijero, J. (2004). “The challenges of sustainable papermaking,” Environ. Sci. Technol. 38(21), 414A-420A. DOI: 10.1021/es040654y
Bocchini, D. A., Oliveira, O. M. M. F., Gomes, E. F., and Silva, R. D. (2005). “Use of sugarcane bagasse and grass hydrolysates as carbon sources for xylanase production by Bacillus circulans D1 in submerged fermentation,” Process Biochem. 40(12), 3653‑3659. DOI: 10.1016/j.procbio.2005.02.021
Bolto, B., and Gregory, J. (2007). “Organic polyelectrolytes in water treatment,” Water Res. 41(11), 2301-2324. DOI: 10.1016/j.watres.2007.03.012
Boroski, M., Rodrigues, A. C., Garcia, J. C., Gerola, A. P., Nozaki, J., and Hioka, N. (2008). “The effect of operational parameters on electrocoagulation – flotation process followed by photocatalysis applied to the decontamination of water effluents from cellulose and paper factories,” J. Hazard. Mater. 160(1), 135-141. DOI: 10.1016/j.jhazmat.2008.02.094
Botia, D. C., Rodríguez, M. S., and Sarria, V. M. (2012). “Evaluation of UV/TiO2 and UV/ZnO photocatalytic systems coupled to a biological process for the treatment of bleaching pulp mill effluent,” Chemosphere 89(6), 732-736. DOI: 10.1016/j.chemosphere.2012.06.046
Bottero, J.-Y., and Fiessinger, F. (1989). “Aluminum chemistry in aqueous solution,” Nordic Pulp Paper Res. J. 4(2), 81-89. DOI: 10.3183/NPPRJ-1989-04-02-p081-089
Boyd, S. A., and Shelton, D. R. (1984). “Anaerobic biodegradation of chlorophenols in fresh and acclimated sludge,” Appl. Environ. Microbiol. 47(2), 272‑277.
Bratby, J. (2006). Coagulation and Flocculation in Water and Wastewater Treatment, 2nd Ed., IWA Publ., London, 407 pp.
BREF (2015). “Best available techniques (BAT) reference document for the production of pulp, paper and board,” JRC Science and Policy Reports, Industrial Emissions Directive 2010/75/EU, Integrated Pollution Prevention and Control, Suhr, M., Klein, B., Kourti, I., Gonzalo, M. R., Santonja, C. C., Roudier, S., and Sancho, L. D., contributors, Uploaded March 2016, eippcb.jrc.ec.europa.eu/reference/BREF/PP_revised_BREF_2015.pdf
Brusseau, M. L., and Rao, P. S. C. (1991). “Influence of sorbate structure on nonequilibrium sorption of organic compounds,” Environ. Sci. Technol. 25(8), 1501‑1506. DOI: 10.1021/es00020a022
Bu, Y. H., Siu, J., Zhou, K. N.., and Yu, K. (2014). “Application of photocatalytic oxidation technology in the treatment of wastewater from papermaking mills,” Advan. Mater. Res. 1030-1032, 296-300. DOI: 10.4028/www.scientific.net/AMR.1030-1032.296
Burton, D. T., Hall, L. W. M., Klauda, R. J., and Margrey, S. L. (1983). “Effects of treated bleached kraft mill effluent on eggs and prolarvae of striped bass (Morone saxatilis),” Water Resour. Bull. 19(6), 869‑879. DOI: 10.1111/j.1752-1688.1983.tb05935.x
Buyukkamaci, N., and Koken, E. (2010). “Economic evaluation of alternative wastewater treatment plant options for pulp and paper industry,” Science Total Environ. 408(24), 6070-6078. DOI: 10.1016/j.scitotenv.2010.08.045
Buzzini, A. P., and Pires, E. C. (2002). “Cellulose pulp mill effluent treatment in an upflow anaerobic sludge blanket reactor,” Proc. Biochem. 38(5), 707-713. DOI: 10.1016/S0032-9592(02)00190-5
Buzzini, A. P., and Pires, E. C. (2007). “Evaluation of an upflow anaerobic sludge blanket reactor with partial recirculation of effluent used to treat wastewaters from pulp and paper plants,” Bioresour. Technol. 98(9), 1838-1848. DOI: 10.1016/j.biortech.2006.06.030
Calvo, L., Gilarranz, M. A., Casas, J. A., Mohedano, A. F., and Rodríguez, J. J. (2007). “Detoxification of kraft pulp ECF bleaching effluents by catalytic hydrotreatment,” Water Res. 41(4), 915-923. DOI: 10.1016/j.watres.2006.11.018
Carr, R. L., Couch, T. A., Liu, J., Coats, J. R., and Chambers, J. E. (1999). “The interaction of chlorinated alicyclic insecticides with brain GABA(A) receptors in channel catfish (Ictalurus punctatus),” J. Toxicol. Environ. Heath A, 56(8), 543‑553. DOI: 10.1080/009841099157881
Carter, C. N., and Sigler, R. G. (1981). “Clay/polymer vs. alum treatment in a solids contact clarifier for mill process water,” Tappi 64(9), 135-139.
Castillo, F., and Vivas, F. (1996). “Treatment of paper mill wastewater with a Biodisk system,” Acotepac 29, 17-23. [Spanish]
Catalkaya, E., Bali, U., and Sengul, F. (2003). “Photochemical degradation and mineralization of 4‑chlorophenol,” Environ. Sci. Pollut. Res. 10(2), 113‑120. DOI: 10.1065/espr2002.10.135
Catalkaya, E. C., and Kargi, F. (2007). “Color, TOC and AOX removals from pulp mill effluent by advanced oxidation processes: A comparative study,” J. Hazard. Mater. 139(2), 244‑253. DOI: 10.1016/j.jhazmat.2006.06.023
Catalkaya, E. C., and Kargi, F. (2008). “Advanced oxidation treatment of pulp mill effluent for TOC and toxicity removals,” J. Environ. Manag. 87(3), 396-404. DOI: 10.1016/j.jenvman.2007.01.016
CEPI (2013). “European Paper Industry – Advancing the economy,” Sustainability report 2013, Confederation of European Paper Industries.
Chakrabarti, S. K., Gupta, S., Kaur, A., Karn, S., Sharma, K. D., and Varadhan, R. (2008). “Biological treatment of pulp mill wastewater – Effects of pH and temperature of the influent on the microbial ecology and reactor performance,” IPPTA 20(1), 123-132.
Chandra, R., and Singh, R. (2012). “Decolourisation and detoxification of rayon grade pulp and paper mill effluent by mixed bacterial culture isolated from pulp paper mill effluent polluted site,” Biochemical Engineering Journal 61, 49-58. DOI:10.1016/j.bej.2011.12.004
Chanworrawoot, K., and Hunsom, M. (2012). “Treatment of wastewater from pulp and paper mill industry by electrochemical methods in membrane reactor,” J. Environ. Manag. 113, 399-406. DOI: 10.1016/j.jenvman.2012.09.021
Chen, G. H. (2004). “Electrochemical technologies in wastewater treatment,” Separ. Purif. Technol. 38(1), 11-41. DOI: 10.1016/j.seppur.2003.10.006
Chen, J., Heitmann, J. A., and Hubbe, M. A. (2003). “Dependency of polyelectrolyte complex stoichiometry on the order of addition. 1. Effect of salt concentration during streaming current titrations with strong poly-acid and poly-base,” Colloids Surf. A 223(1-3), 215-230. DOI: 10.1016/S0927-7757(03)00222-X
Chen, S., Zhang, X., Singh, D., Yu, H., and Yang, X. (2010). “Biological pretreatment of lignocellulosics: Potential, progress and challenges,” Biofuels 1(1), 177‑199. DOI: 10.4155/bfs.09.13
Chen, W., and Horan, N. J. (1998). “The treatment of a high strength pulp and paper mill effluent for wastewater re-use – III) Tertiary treatment options for pulp and paper mill wastewater to achieve effluent recycle,” Environ. Technol. 19(2), 173-182. DOI: 10.1080/09593331908616669
Chen, Y. H., Chai, L. Y., Zhu, Y. H., Yang, Z. H., Zheng, Y., and Zhang, H. (2012). “Biodegradation of kraft lignin by a bacterial strain Comamonas sp B-9 isolated from eroded bamboo slips,” J. Appl. Microbiol. 112(5), 900-906. DOI: 10.1111/j.1365-2672.2012.05275.x
Cheng, Z., Yang, R., Wang, B., and Yang, F. (2015). “Chlorophenol degradation in papermaking wastewater through a heterogeneous ozonation process catalyzed by Fe-Mn/sepiolite,” BioResources 10(3), 5503-5514. DOI: 10.15376/biores.10.3.
Chhonkar, P. K., Datta, S. P., Joshi, H. C., and Pathak, H. (2000). “Impact of industrial effluents on soil health and agriculture – Indian experience: Part I – Distillery and paper mill effluents,” J. Sci. Indus. Res. 59(5), 350-361.
Chopra, A. K., and Singh, P. P. (2012). “Removal of color, COD and lignin from pulp and paper mill effluent by Phanerochaete chrysosporium and Aspergillus fumigatus,” J. Chem. Pharm. Res. 4(10), 4522‑4532.
Choudhary, A. K., Kumar, S., and Sharma, C. (2013). “Removal of chlorophenolics from pulp and paper mill wastewater through constructed wetland,” Water Environ. Res. 85(1), 54-62. DOI: 10.2175/106143012X13415215907419
Chow, S., Obermajer, G., and Van Shaik, G. (1999). “HPLC evaluation of resin acid degradation with ozone treatment of pulp mill effluent,” Wood Sci. Technol. 33(1), 55‑62. DOI: 10.1007/s002260050098
Chu, K., Ishiuka, S., and Shibata, M. (1999). “New activated sludge process without excess-sludge production,” Kami Pa Gikyoshi 53(10), 81-86. DOI: 10.2524/jtappij.53.1339
Chu, L., Yan, S., Xing, X., Sun, X., and Jurcik, B. (2009). “Progress and perspectives of sludge ozonation as a powerful pretreatment method for minimization of excess sludge production,” Water Research 43(7), 1811-1822. DOI: 10.1016/j.watres.2009.02.012
Chu, Y.-Y., Wang, W.-J., and Wang, M. (2010). “Anodic oxidation process for the degradation of 2,4-dichlorophenol in aqueous solution and the enhancement of biodegradability,” J. Hazard. Mater. 180, 247-252. DOI: 10.1016/j.jhazmat.2010.04.021
Cingolani, L., Ciccarelli, E., Cossignani, M., Tornari, Q., and Scarlata, V. (1994). “Management of papermill wastes: The role of filamentous microorganisms as indicators,” Water Science and Technology 29(7), 185-188.
Ciputra, S., Antony, A., Phillips, R., Richardson, D., and Leslie, G. (2010). “Comparison of treatment options for removal of recalcitrant dissolved organic matter from paper mill effluent,” Chemosphere 81(1), 86-91. DOI: 10.1016/j.chemosphere.2010.06.060
Clement, R., Tashiro, C., Suter, S., Reiner, E., and Holliger, D. (1989). “Chlorinated dibenzo-p-dioxins (CDDs) and dibenzofurans (CDFs) in effluents and sludges from pulp and paper mills,” Chemosphere 18(1‑6), 1189‑1196. DOI: 10.1016/0045-6535(89)90254-3
Contreras Lopez, M. C. (2003). “Determination of potentially bioaccumulating complex mixtures of organochlorine compounds in wastewater: A review,” Environ. Intl. 28(8), 751-759. DOI: 10.1016/s0160-4120(02)00120-4
Costa, H., McKeown, J. J., and Blosser, R. O. (1979). “Critical review of studies on characterization, fate and impact of residual solids of biological treatment origin,” TAPPI J. 62(10), 41-46.
Costa, H., McKeown, J. J., Buckley, D. B., Gove, G., and Carpenter, W. L. (1974). “Review of turbidity removal associated with biological treatment of paper mill waste waters,” NCASI Stream Improvement Tech. Bull., No. 274, 55 pp.
Costigan, S. L., Werner, J., Ouellet, J. D., Hill, L. G., and Law, R. D. (2012). “Expression profiling and gene ontology analysis in fathead minnow (Pimephales promelas) liver following exposure to pulp and paper mill effluents,” Aquat. Toxicol. 122-123, 44-55. DOI: 10.1016/j.aquatox.2012.05.011
Curcio, E., and Drioli, E. (2005). “Membrane distillation and related operations – A review,” Separation & Purification Reviews 34(1), 35-86. DOI: 10.1081/SPM-2000054951
Czaplicka, M. (2004). “Sources and transformations of chlorophenols in the natural environment,” Sci. Total Environ. 322(1‑3), 21‑39. DOI: 10.1016/j.scitotenv.2003.09.015
Dahlman, O. B., Reimann, A. K., Strömberg, L. M., and Mörck, R. E. (1995). “High molecular weight effluents materials from modern ECF and TCF bleaching,” Tappi J. 78(12), 99‑109.
Dahm, A., and Lucia, L. A. (2004). “Titanium dioxide catalyzed photodegradation of lignin in industrial effluents,” Indust. Eng. Chem. Res. 43, 7996-8000. DOI: 10.1021/ie0498302
Daniel, G., Asiegbu, F., and Johansson, M. (1998). “The saprotrophic wood-degrading abilities of Heterobasidium annosum intersterility groups P and S,” Mycol. Res. 102(8), 991‑997. DOI: 10.1017/S0953756297005935
Dashtban, M., Schraft, H., Syed, T. A., and Qin, W. (2010). “Fungal biodegradation and enzymatic modification of lignin,” Int. J. Biochem. Mol. Biol. 1(1), 36-50.
de los Santos Ramos, W., Poznyak, T., Chairez, I., and Córdova, I. (2009). “Remediation of lignin and its derivatives from pulp and paper industry wastewater by the combination of chemical precipitation and ozonation,” J. Hazard. Mater. 169, 428-434. DOI: 10.1016/j.jhazmat.2009.03.152
Demel, I., Schmid, F., Bobek, B., and Hamm, U. (2003). “Benchmarking von Abwasserreiningungsanlagen – Processvergleich und Effizienzsteigerung,” das Papier (ipwonline.de), 39-44.
de Nardi, I. R., Fuzi, T. P., and Del Nery, V. (2008). “Performance evaluation and operating strategies of dissolved-air flotation system treating poultry slaughterhouse wastewater,” Resources, Conservation and Recycling 52(3), 533-544. DOI: 10.1016/j.resconrec.2007.06.005
Deniz, I., Kirci, H., and Ates, S. (2004). “Optimisation of wheat straw Triticum durum kraft pulping,” Indust. Crops Prod. 19(3), 237-243. DOI: 10.1016/j.indcrop.2003.10.011
Dentel, S. K. (1995). “Use of the streaming current detector in coagulation monitoring and control,” J. Water SRT – Aqua 44(2), 70-79.
Dentel, S. K., and Kingery, K. M. (1989). “Using streaming current detectors in water treatment,” J. AWWA 81(3), 85-94.
Deshmukh, N. S., Lapsiya, K. L., Savant, D. V., Chiplonkar, S. A., Yeole, T. Y., Dhakephalkar, P. K., and Ranade, D. R. (2009). “Upflow anaerobic filter for the degradation of adsorbable organic halides (AOX) from bleach composite wastewater of pulp and paper industry,” Chemosphere 75(9), 1179-1185. DOI: 10.1016/j.chemosphere.2009.02.042
Dethlefs, F., and Stan, H.-J. (1996). “Determination of resin acids in pulp mill EOP bleaching process effluent,” Fresinius´ J. Anal. Chem. 356(6), 403‑410. DOI: 10.1007/s0021663560403
Devai, I., and DeLaune, R. D. (1999). “Emission of reduced malodorous sulfur gases from wastewater treatment plants,” Water Environ. Res. 71(2), 203-208. DOI: 10.2175/106143098X121842
Dhakhwa, S., Bandyopadhyay, S., Majozi, T., and Garg, A. (2012). “Efficacy of chemical oxidation and coagulation for COD and color reduction from pulp mill effluent,” J. Environ. Eng. – ASCE 138(12), 1194-1199. DOI: 10.1061/(ASCE)EE.1943-7870.0000611
Diez, M. C., Castillo, G., Aguilar, L., Vidal, G., and Mora, M. L. (2002). “Operational factors and nutrient effects on activated sludge treatment of Pinus radiata kraft mill wastewater,” Bioresour. Technol. 83(2), 131-138. DOI: 10.1016/S0960-8524(01)00204-8
Dilek, F. B., Taplamacioglu, H. M., and Tarlan, E. (1999). “Colour and AOX removal from pulping effluents by algae,” Appl. Microbiol. Biotechnol. 52(4), 585-591. DOI: 10.1007/s002530051564
Dominguez-Ramos, A., Aldaco, R., and Irabien, A. (2008). “Electrochemical oxidation of lignosulfonate: Total organic carbon oxidation kinetics,” Indust. Eng. Chem. Res. 47(24), 9848-9853. DOI: 10.1021/ie801109c
Dorica, J., Harland, R., and Kovacs, T. (1999). “Sludge dewatering practices at Canadian pulp and paper mills,” Pulp and Paper Canada 100(5) 19-22.
Duran, N., and Esposito, E. (2000). “Potential applications of oxidative enzymes and phenoloxidase-like compounds in wastewater and soil treatment: A review,” Appl. Catal. B – Environ. 28(2), 83-99. DOI: 10.1016/S0926-3373(00)00168-5
Durán, N., Esposito, E., Innocentini-Mei, L. H., and Canhos, V. P. (1994). “A new alternative process for kraft E1 effluent treatment,” Biodegradation 5(1), 13-19. DOI.10.1007/BF00695209
Edwards, L., and Rydin, S. (1976). “Washing of pulps. 5. Dispersion model for washing of pup in mill continuous diffusers,” Svenk Papperstidning 79(11), 354-358.
Edzwald, J. K. (2010). “Dissolved air flotation and me,” Water Res. 44(7), 2077-2106. DOI: 10.1016/j.watres.2009.12.040
Ekholm, P., Jouttijarvi, T., Priha, M., Rita, H., and Nurmesniemi, H. (2007). “Determining algal-available phosphorus in pulp and paper mill effluents: Algal assays vs. routine phosphorus analysis,” Environ. Pollut. 145(3), 715-722. DOI: 10.1016/j.envpol.2006.06.001
Ekman, R., and Holmbom, B. (2000). “The chemistry of wood resin,” in: Pitch Control, Wood Resin and Deresination, E. L. Back and L. H. Allen (eds.), TAPPI Press, Atlanta.
Ekstrand, E., Larsson, M., Truong, X., Cardell, L., Borgström, Y., Björn, A., Ejlertsson, J., Svensson, B. H., Nilsson, F., and Karlsson, A. (2013). “Methane potentials of the Swedish pulp and paper industry – A screening of wastewater effluents,” Appl. Energy 112, 507-517.
El-Ashtoukhy, E.-S. Z., Amin, N. K., and Abdelwahab, O. (2009). “Treatment of paper mill effluents in a batch-stirred electrochemical tank reactor,” Chem. Eng. J. 146(2), 205-210. DOI: 10.1016/j.cej.2008.05.037
Elliott, A., and Mahmood, T. (2005). “Survey benchmarkings generation, management of solid residues,” Pulp Paper 79(12), 49-55.
Elliott, A., and Mahmood, T. (2012). “Comparison of mechanical pretreatment methods for the enhancement of anaerobic digestion of pulp and paper waste activated sludge,” Water Environ. Res. 84(6), 497-505. DOI: 10.2175/106143012X13347678384602
Emamjomeh, M. M., and Sivakumar, M. (2009). “Review of pollutants removed by electrocoagulation and electrocoagulation/flotation processes,” J. Environ. Manag. 90(5), 1663-1679. DOI: 10.1016/j.jenvman.2008.12.011
Environmental Compliance Insider (2010). “Environmental regulation of pulp & paper mills,” Environmental Compliance Insider, June 28, 2010. Available at: environmentalcomplianceinsider.com/test/ta/environmental-regulation-of-pulp-paper-mills
Environment and Climate Change Canada (2016). “Canadian environmental sustainability indicators: Managing pulp and paper effluent quality in Canada, Environment and Climate Change Canada. Available at: www.ec.gc.ca/indicateurs-indicators/default.asp?lang=en&n=E20C2E23-1.
Eriksson, K.-E., and Kolar, M.-C. (1985). “Microbial degradation of chlorolignins,” Environ. Sci. Technol. 19(11), 1086-1089. DOI: 10.1021/es00141a011
Eskelinen, K., Särkkä, H., Kurniawan, T. A., and Sillanpää, M. E. T. (2010). “Removal of recalcitrant contaminants from bleaching effluents in pulp and paper mills using ultrasonic irradiation and Fenton-like oxidation, electrochemical treatment, and/or chemical precipitation: A comparative study,” Desalination 255, 179-187. DOI: 10.1016/j.desal.2009.12.024
Estrada-Vázquez, C., Hernández-Vera, R., Magaña-Plaza, I., Hernández-González, A., Poggi-Varaldo, H. M., and Fernández-Villagómez, G. (1998). “Biological post-treatment of anaerobically-treated black liquor spills from kraft pulp-mill,” in: Proceedings of the 52nd Industrial Waste Conference, Purdue University, R. F. Wukasch (ed.), Ann Arbor Press Inc., Michigan.
European Commission (2001). “Integrated Pollution Prevention and Control (IPPC) – Reference document on best available techniques in the pulp and paper industry,” Retrieved from: 〈http://eippcb.jrc.ec.europa.eu/reference/BREF/sa_bref_0505.pdf〉.
Exall, K. N., and vanLoon, G. W. (2003). “Effects of raw water conditions on solution-state aluminum speciation during coagulant dilution,” Water Res. 37(14), 3341-3350. DOI: 10.1016/S0043-1354(03)00229-X
Fackler, K., Gradinger, C., Hinterstoisser, B., Messner, K., and Schwanninger, M. (2006), “Lignin degradation by white rot fungi on spruce wood shavings during short-time solid-state fermentations monitored by near infrared spectroscopy,” Enzym. Microb. Technol. 39(7), 1476‑1483. DOI: 10.1016/j.enzmictec.2006.03.043
Fatta-Kassinos, D., Dionysiou, D. E., and Kümmerer, K. (2016). The Handbook of Environmental Chemistry, Vol. 44. ISBN: 978-3-319-23891-3 (Print) 978-3-319-23892-0 (Online)
Fernandes, L., Lucas, M. S., Maldonado, M. I., Oller, I., and Sampaio, A. (2014). “Treatment of pulp mill wastewater by Cryptococcus podzolicus and solar photo-Fenton: A case study,” Chem. Eng. J. 245, 158-165. DOI: 10.1016/j.cej.2014.02.043
Fernandez-Fernandez, M., Sanromán, M. A., and Moldes, D. (2013). “Recent developments and applications of immobilized laccase,” Biotechnol. Advan. 31(8), 1808-1825. DOI: 10.1016/j.biotechadv.2012.02.013
Field, J. A., Kortekaas, S., and Lettinga, G. (1989). “The tannin theory of methanogenic toxicity,” Biol. Waste 29(4), 241‑262. DOI: 10.1016/0269-7483(89)90016-5
Field, J. A., Leyendeckers, M. J. H., Sierra-Alvarez, R., Lettinga, G., and Habets, L. H. A. (1988). “The methanogenic toxicity of bark tannins and the anaerobic biodegradability of watersoluble bark matter,” Water Sci. Technol. 20(1), 219‑240.
Fontanier, V., Albet, J., Baig, S., and Molinier, J. (2005a). “Simulation of pulp mill wastewater recycling after tertiary treatment,” Environ. Technol. 26(12), 1335‑1344. 10.1080/09593332608618610
Fontanier, V., Baig, S., Albet, J., and Molinier, J. (2005b). “Comparison of conventional and catalytic ozonation for the treatment of pulp mill wastewater,” Environ. Eng. Sci. 22(5), 127‑137. DOI: 10.1089/ees.2005.22.127
Formento, J. C., Maximino, M. G., Mina, L. R., Srayh, M. I., and Martinez, M. J. (1994). “Cationic starch in the wet end: Its contribution to interfibre bonding,” APPITA 47(4), 305-308.
Frakes, R. A., Zeeman, C. Q. T., and Mower, B. (1993). “Bioaccumulation of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) by fish downstream of pulp and paper mills in Maine,” Ecotox. Environ. Safe. 25(2), 244‑252. DOI: 10.1006/eesa.1993.1023
Freire, C. S. R., Silvestre, A. J. D., and Neto, C. P. (2003). “Carbohydrate derived chlorinated compounds in ECF bleaching of hardwood pulps: Formation, degradation and contribution to AOX in a bleached kraft pulp mill,” Environ. Sci. Technol. 37(4), 811‑814. DOI: 10.1021/es0200847
Freire, R. S., Kubota, L.T., and Durán, N. (2001). “Remediation and toxicity removal from kraft E1 paper mill effluent by ozonation,” Environ. Technol. 22(8), 897‑904. DOI: 10.1080/09593332208618224
Freire, R. S., Kunz, A., and Duran, N. (2000). “Some chemical and toxicological aspects about paper mill effluent treatment with ozone,” Environ. Technol. 21(6), 717-721. DOI: 10.1080/09593332108618088
Freitas, A. C., Ferreira, F., Costa, A. M., Pereira, R., Antunes, S. C., Gonçalves, F., Rocha-Santos, T. A. P., Diniz, M. S., Castro, L., Peres, I., and Duarte, A. C. (2009). “Biological treatment of the effluent from a bleached kraft pulp mill using basidiomycete and zygomycete fungi,” Sci. Total Environ. 407(10), 3282-3289. DOI: 10.1016/j.scitotenv.2009.01.054
Fueno, H., Tanaka, K., and Sugawa, S. (2002). “Theoretical study of the dechlorination reaction pathways of octachlorodibenzo-p-dioxin,” Chemosphere 48(8), 771‑778. DOI: 10.1016/s0045-6535(02)00141-8
Gander, M., Jefferson, B., and Judd, S. (2000). “Aerobic MBRs for domestic wastewater treatment: A review with cost considerations,” Separ. Purif. Technol. 18(2), 119-130. DOI: 10.1016/S1383-5866(99)00056-8
Ganjidoust, H., Tatsumi, K., Yamagishi, T., and Gholian, R. N. (1997). “Effect of synthetic and natural coagulant on lignin removal from pulp and paper wastewater,” Water Sci. Tech. 35(2-3), 291-296. DOI: 10.1016/S0273-1223(96)00943-2
Garcia-Ochoa, F., and Gomez, E. (2009). “Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview,” Biotechnol. Advan. 27(2), 153-176. DOI: 10.1016/j.biotechadv.2008.10.006
Garg, A., Mishra, I. M., and Chand, S. (2005). “Thermochemical precipitation as a pretreatment step for the chemical oxygen demand and color removal from pulp and paper mill effluent,” Ind. Eng. Chem. Res. 44(7), 2016-2026. DOI: 10.1021/ie048990a
Garg, A., Mishra, I. M., and Chand, S. (2007). “Catalytic wet oxidation of the pretreated synthetic pulp and papermill effluent under moderate conditions,” Chemosphere 66(9), 1799-1805. DOI: 10.1016/j.chemosphere.2006.07.038
Garg, S. K., and Tripathi, M. (2011). “Strategies for decolorization and detoxification of pulp and paper mill effluent,” in: Reviews of Environmental Contamination and Toxicology, Vol. 212, Whitacre, D. M. (ed.), pp. 113-136. DOI: 10.1007/978-1-4419-8453-1_4
Garg, S. K., Tripathi, M., Kumar, S., Singh, S. K., and Singh, S. K. (2012). “Microbial dechlorination of chloroorganics and simultaneous decolorization of pulp-paper mill effluent by Pseudomonas putida MTCC 10510 augmentation,” Environ. Monitoring Assess. 184(9), 5533-5544. DOI: 10.1007/s10661-011-2359-1
Gehm, H. (1973). State-of-the-art Review of Pulp and Paper Waste Treatment, Envir. Protection Technol. Ser. EPA-R2-73-184, 267 pp.
Genthner, B. R. S., Price II, W. A., and Pritchard, P. H. (1989). “Anaerobic degradation of chloroaromatic compounds in aquatic sediments under a variety of enrichment conditions,” Appl. Environ. Microbiol. 55(6), 1466‑1471.
Gerdes, W. F. (1966). “A new instrument – The streaming current detector,” 12th Natl. ISA Analysis Instrumentation Symp., Houston, TX, 11-13, May, pp. 181-198.
Gergov, M., Priha, M., Talka, E., Valttila, O, Kangas, A., and Kukkonen, K. (1988). “Chlorinated organic compounds in effluent treatment at kraft mills,” Tappi J. 71(12), 175-184.
Ghaly, M. Y., Jamil, T. S., El-Seesy, I. E., Souaya, E. R., and Nasr, R. A. (2011). “Treatment of highly polluted paper mill wastewater by solar photocatalytic oxidation with synthesized nano TiO2,” Chem. Eng. J. 168(1), 446-454. DOI: 10.1016/j.cej.2011.01.028
Ghanizadeh, G., and Sarrafpour, R. (2001). “The effects of temperature and pH on settleability of activated sludge flocs,” Iran. J. Publ. Health 30(3-4), 139-142.
Ghoreishi, S. M., and Haghighi, M. R. (2007). “Chromophores removal in pulp and paper mill effluent via hydrogenation-biological batch reactors,” Chem. Eng. J. 127, 59-70. DOI: 10.1016/j.cej.2006.09.022
Ghosh, M. M., Cox, C. D., and Prakash, T. M. (1985). “Polyelectrolyte selection for water treatment,” J. Amer. Water Works Assoc. 77(3), 67-73.
Goel, M., Chovelon, J.-M., Ferronato, C., Bayard, R., and Sreekrishnan, T. R. (2010). “The remediation of wastewater containing 4-chlorophenol using integrated photo-catalytic and biological treatment,” J. Photochem. Photobiol. B – Biol. 98(1), 1-6. DOI: 10.1016/j.jphotobiol.2009.09.006
Gogate, P. R. (2008). “Treatment of wastewater streams containing phenolic compounds using hybrid techniques based on cavitation: A review of the current status and the way forward,” Ultrason. Sonochem. 15(1), 1‑15. DOI: 10.1016/j.ultsonch.2007.04.007
Gogate, P. R., and Pandit, A. B. (2004). “A review of imperative technologies for wastewater treatment. I: Oxidation technologies at ambient conditions,” Adv. Environ. Res. 8, 501-551. DOI: 10.1016/S1093-0191(03)00032-7
Gogate, P. R., and Pandit, A. B. (2004). “A review of imperative technologies for wastewater treatment. II: Hybrid methods,” Adv. Environ. Res. 8, 553-597. DOI: 10.1016/S1093-0191(03)00031-5
Gökçay, C. F., and Dilek, F. B. (1994). “Treatment of effluents from hemp-based pulp and paper industry. II. Biological treatability of pulping effluents,” Water Science and Technology 29(9), 161-163.
Gomathi, G., and Kanmani, S. (2006). “Tertiary treatment of pulp and paper industry wastewater by solar photocatalysis and photofenton,” J. Inst. Public Health Eng. 2006-07, 5-10.
Gönder, Z. B., Arayici, S., and Barles, H. (2011). “Advanced treatment of pulp and paper mill wastewater by nanofiltration process: Effects of operating conditions on membrane fouling,” Separ. Purif. Technol. 76(3), 292-302. DOI: 10.1016/j.seppur.2010.10.018
Gönder, Z. B., Arayici, S., and Barlas, H. (2012). “Treatment of pulp and paper mill wastewater using ultrafiltration process: Optimization of the fouling and rejections,” Indust. Eng. Chem. Res. 51(17), 6184-6195. DOI: 10.1021/ie2024504
Gonze, E., Commenges, N., Gonthier, Y., and Bernis, A. (2003). “High frequency ultrasound as a pre- or post-oxidation for paper mill wastewaters and landfill leachate treatment,” J. Chem. Eng. 92(1‑3), 215‑225. DOI: 10.1016/s1385-8947(02)00258-9
Goossens, J. W. S., and Luner, P. (1976). “Flocculation of microcrystalline cellulose suspensions with cationic polymers – Effect of agitation,” Tappi 59(2), 89-94.
Goyer, N., and Lavoie, J. (2001). “Emissions of chemical compounds and bioaerosols during the secondary treatment of paper mill effluents,” AIHAJ 62(3), 330-341. DOI: 10.1202/0002-8894(2001)062<0330:EOCCAB>2.0.CO;2
Graves, J. W., and Joyce, T. W. (1994). “Critical review of the ability of biological treatment systems to remove chlorinated organics discharged by the paper industry,” Water SA 20(2), 155-160.
Greenfield, P. F., and Batstone, D. J. (2005). “Anaerobic digestion: Impact of future greenhouse gases mitigation policies on methane generation and usage,” Water Sci. Technol. 52, 39-47.
Gregory, J. (1973). “Rates of flocculation of latex particles by cationic polymers,” J. Colloid Interface Sci. 42(2), 448-456. DOI: 10.1016/0021-9797(73)90311-1
Grijspeerdt, K., Bogaert, H., and Verstraete, W. (1996). “Design and verification of a model secondary clarifier for activated sludge,” J. Chem. Technol. Biotech. 67(4), 404-412. DOI: 10.1002/(SICI)1097-4660(199612)67:4<404::AID-JCTB592>3.0.CO;2-M
Gubelt, G., Lumpe, C., and Joore, L. (2000). “Towards zero liquid effluents at Niederauer Muhle. The validation of two novel separation technologies,” Paper Technology (UK) 41(8), 41-48.
Gullichsen, J. (1999). “Fiber line operations,” in: J. Gullichsen and C.-J. Fogelholm (eds.), Chemical Pulping, Book series: Paper Science and Technology, Book 6A, Fapet Oy, Helsinki, pp. A17-A243 (see p. A114).
Habets, L. H. A., and Knelissen, J. H. (1985). “Application of the UASB reactor for anaerobic treatment of paper and board mill effluent,” Water Sci. Technol. 17(1), 61-75.
Hagelqvist, A. (2013). “Batchwise mesophilic anaerobic co-digestion of secondary sludge from pulp and paper industry and municipal sewage sludge,” Waste Manage. 33(4), 820-824. DOI: 10.1016/j.wasman.2012.11.002
Hagen, M. E., Colodey, A. G., Knapp, W. D., and Samis, S. C. (1997). “Environmental response to decreased dioxin loadings from British Columbia coastal pulp mills,” Chemosphere 34(5), 1221‑1229. DOI: 10.1016/S0045-6535(97)00420-7
Haggblom, M., and Salkinojasalonen, M. (1991). “Biodegradability of chlorinated organic-compounds in pulp bleaching effluents,” Water Sci. Technol. 24(3-4), 161-170.
Hakulinen, R. (1988). “Use of enzymes for waste water treatment in the pulp and paper industry – A new possibility,” Water Sci. Technol. 20(1), 251-262.
Hammar, B., and Rydholm, S. (1972). “Measures taken against water pollution in the kraft pulp and paper industry,” Pure Appl. Chem. 29, 263-280. DOI: 10.1351/pac197229010263
Han, M. Y., Kim, M. K., and Ahn, H. J. (2006). “Effects of surface charge, micro-bubble size and particle size on removal efficiency of electro-flotation,” Water Sci. Technol. 53(7), 127-132. DOI: 10.2166/wst.2006.216
Hardy, W. B. (1900). “On the conditions which determine the stability of irreversible hydrosols,” Proc. Royal Soc. London 66, 110-125. DOI: 10.1098/rspl.1899.0081
Hassan, S. R., Zwain, H. M., Zaman, N. Q., and Dahlan, I. (2014). “Recycled paper mill effluent treatment in a modified anaerobic baffled reactor: start-up and steady-state performance,” Environ. Technol. 35(3), 294-299. DOI: 10.1080/09593330.2013.827222
Hattula, M.-L., and Knuutinen, J. (1985). “Mutagenesis of mammalian cells in culture by chlorophenols, chlorocatechols and chloroguaiacols,” Chemosphere 14(10), 1617‑1625. DOI: 10.1016/0045-6535(85)90015-3
Heimberger, S. A., Blevins, D. S., Bostwick, J. H., Donnini, G. P. (1988). “Kraft bleach mill plant effluents: Recent developments aimed at decreasing their environmental impact, Part 1,” Tappi J. 71(10), 51‑59.
Heindel, T. J. (1999). “Fundamentals of flotation deinking,” TAPPI J. 82(3), 115-124.
Heinzle, E., Geiger, F., Fahmy, M., and Kut, O. M. (1992). “Integrated ozonation biotreatment of pulp bleaching effluents containing chlorinated phenolic-compounds,” Biotechnol. Prog. 8(1), 67-77. DOI: 10.1021/bp00013a010
Helble, A., Schalyer, W., Liechti, P. A., Jenny, R., and Christian, M. H. (1999). “Advanced effluent treatment in the pulp and paper industry with a combined process of ozonation and fixed bed biofilm reactors,” Water Science and Technology 40(11-12), 343-350. DOI:10.1016/S0273-1223(99)00737-4
Heller, P., Scott, W. E., and Springer, A. M. (1979). “Potential operational problems under conditions of complete water reuse. Assessment using a model paper machine,” Tappi 62(12), 79-84.
Hermosilla, D., Cortijo, M., and Huang, C. P. (2009). “Optimizing the treatment of landfill leachate by conventional Fenton and photo-Fenton processes,” Sci. Total Environ. 407(11), 3473-3481. DOI: 10.1016/j.scitotenv.2009.02.009
Hermosilla, D., Merayo, N., Gascó, A., and Blanco, Á. (2015). “The application of advanced oxidation technologies to the treatment of effluents from the pulp and paper industry: A review,” Environ. Sci. Pollution Res. 22(1), 168-191. DOI: 10.1007/s11356-014-3516-1
Hermosilla, D., Merayo, N., Ordoñez, R., and Blanco, Á. (2012a). “Optimization of conventional Fenton and ultraviolet-assisted oxidation processes for the treatment of reverse osmosis retentate from a paper mill,” Waste Manag. 32(6), 1236-1243. DOI: 10.1016/j.wasman.2011.12.011
Hermosilla, D., Ordoñez, R., Blanco, L., Fuente, E., and Blanco, A. (2012b). “pH and particle structure effects on silica removal by coagulation,” Chem. Eng. Technol. 35, 1-10. DOI: 10.1002/ceat.201100527
Hermosilla, D., San Pío, I., and Blanco, A. (2010). “Towards sustainable water use in the paper industry,” in: Proceedings of The Sixth International Conference on Sustainable Water Environment: Water Infrastructures in Time of Climate Change, University of Delaware, Huang (ed.), University of Delaware, Newark, DE, USA.
Herney-Ramirez, J., Silva, A. M. T., Vicente, M. A., Costa, C. A., and Madeira, L. M. (2011). “Degradation of Acid Orange 7 using a saponite-based catalyst in wet hydrogen peroxide oxidation: Kinetic study with the Fermi’s equation,” Applied Catalysis B: Environmental 101, 197-205. DOI: 10.1016/j.apcatb.2010.09.020
Herrington, T. M., and Petzold, J. C. (1992). “An investigation into the nature of charge on the surface of papermaking woodpulps. 2. Analysis of potentiometric titration data,” Colloids Surf. 64(2), 109-118. DOI: 10.1016/0166-6622(92)80089-K
Herve, S. (1991). “Mussel incubation method for monitoring organochlorine compounds in freshwater recipients of pulp and paper industry,” PhD thesis, University of Jyväskylä.
Herve, S., Heinonen, P., Paukku, R., Knuutila, M., Koistinen, J., and Paasivirta, J. (1988). “Mussel incubation method for monitoring organochlorine pollutants in watercourses. Four-year application in Finland,” Chemosphere 17(10), 1945‑1961. DOI: 10.1016/0045-6535(88)90006-9
Hiemenz, P. C., and Rajagopalan, R. (1997). Principles of Colloid and Surface Science, 3rd Ed., Dekker, New York.
Hilden, K., Hakala, T. K., Maijala, P., Lundell, T. K., and Hatakka, A. (2007). “Novel thermotolerant laccases produced by the white-rot fungus Physisporinus rivulosus,” Appl. Microbiol. Biotechnol. 77(2), 301‑309. DOI: 10.1007/s00253-007-1155-x
Hileman, B. (1993). “Concerns broaden over chloride and chlorinated hydrocarbons,” Chem. Eng. News 71(16), 11‑20. DOI: 10.1021/cen-v071n016.p011
Hills, R. L. (1988). Papermaking in Britain 1488-1988, The Athlone Press, London and Atlantic Highlands, NJ, 249 pp.
Hinck, M. L., Ferguson, J., and Puhakka, J. (1997). “Resistance of EDTA and DTPA to aerobic biodegradation,” Water Sci. Technol. 35(1‑2), 25‑31.
Hodgson, A., Hitzroth, A., Premdas, P., Hodson, P., and Duff, S. (1998). “Effect of tertiary coagulation and flocculation treatment on effluent quality from a bleached kraft mill,” Tappi J. 81(2), 166-172.
Holliger, C., Wolfarth, G., and Diekert, G. (1998). “Reductive dehalogenation in the energy metabolism of anaerobic bacteria,” FEMS Microbiol. Rev. 22(5), 383‑398. DOI: 10.1111/j.1574-6976.1998.tb00377.x
Holmbom, B. (1999). “Extractives,” in: Analytical Methods in Wood Chemistry, Pulping, and Papermaking, E. Sjöström, and R. Alén (eds.), Springer, Berlin. DOI: 10.1007/978-3-662-03898-7_5
Hong, Y. S., Zhou, H., and Zytner, R. G. (2007). “Combining ultrafiltration process with coagulation pretreatment for pulp mill wastewater treatment,” Environ. Technol. 28(9), 995-1006. DOI: 10.1080/09593332808618860
Horn, D. (2001). “Exploring the nanoworld of interfaces and their functions during paper manufacturing and upgrading,” Wochenbl. Papierfabr. 129(23/24), 1589-1596.
Hopcroft, F. J. (2015). Wastewater Treatment Concepts and Practices, Momentum Press, 212 pp.
Hörsch, P., Speck, A., and Frimmel, F. H. (2003). “Combined advanced oxidation and biodegradation of industrial effluents from the production of stilbene-based fluorescent whitening agents,” Water Res. 37(11), 2748‑2756. DOI: 10.1016/S0043-1354(03)00061-7
Hossain, K., and Ismail, N. (2015). “Bioremediation and detoxification of pulp and paper mill effluent: A review,” Res. J. Environ. Toxicol. 9(3), 113-134. DOI:10.3923/rjet.2015.113.134
Hostachy, J.-C., Lenon, G., Pisicchio, J.-L., Coste, C., and Legay, C. (1997). “Reduction of pulp and paper mill pollution by ozone treatment,” Water Sci. Technol. 35(2‑3), 261‑268.
Howard, R. C., and Jowsey, C. J. (1989). “The effect of cationic starch on the tensile strength of paper,” J. Pulp Paper Sci. 15(6), J225-J229.
Howie, L., Dickerson, R., Davis, D., and Safe, S. (1990). “Immunosuppressive and mono-oxygenase induction activities of polychlorinated diphenyl ether congeners in C57BL/6N mice: Quantitative structure-activity relationships,” Toxicol. Appl. Pharmacol. 105(2), 254‑263. DOI: 10.1016/0041-008X(90)90187-Y
Hrutfiord, B. F., and Negri, A. R. (1992). “Dioxin sources and mechanisms during pulp bleaching,” Chemosphere 25(1-2), 53‑56. DOI: 10.1016/0045-6535(92)90478-a
Hsueh, C. L., Huang, Y. H., Wang, C. C., and Chen, C. Y. (2005). “Degradation of azo dyes using low iron concentration of Fenton and Fenton-like system,” Chemosphere 58(10), 1409‑1414. DOI: 10.1016/j.chemosphere.2004.09.091
Huang, C., and Pan, J. R. (2002). “Coagulation approach to water treatment,” Encyclopedia of Surface and Colloid Science Vol. 1, 1049-1064.
Huang, R., Mei, Z., Long, Y., Xiong, X., Wang, J., Guo, T., Luo, T., and Long, E. (2015). “Impact of optimized flow pattern on pollutant removal and biogas production rate using wastewater anaerobic fermentation,” BioResources 10(3), 4826-4842. DOI: 10.15376/biores.10.3.4826-4842
Hubbe, M. A. (2007a). “Water and papermaking. 1. Fresh water components,” Paper Technol. 48(1), 18-24.
Hubbe, M. A. (2007b). “Water and papermaking. 2. White water components,” Paper Technol. 48(2), 31-40.
Hubbe, M. A. (2007c). “Water and papermaking. 3. Measures to clean up process water,” Paper Technol. 48(3), 23-30.
Hubbe, M. A., Beck, K. R., O’Neal, W. G., and Sharma, Y. C. (2012a). “Cellulosic substrates for removal of pollutants from aqueous systems: A review. 2. Dyes,” BioResources 7(2), 2592-2687. DOI: 10.15376/biores.7.2.2592-2687
Hubbe, M. A., Hasan, S. H., and Ducoste, J. J. (2011). “Cellulosic substrates for removal of pollutants from aqueous systems: A review. 1. Metals,” BioResources 6(2), 2161-2287. DOI: 10.15376/biores.6.2.2161-2287
Hubbe, M. A., Nanko, H., and McNeal, M. R. (2009). “Retention aid polymer interactions with cellulosic surfaces and suspensions: A Review,” BioResources 4(2), 850-906. DOI: 10.15376/biores.4.2.850-906
Hubbe, M. A., Park, J., and Park, S. (2014). “Cellulosic substrates for removal of pollutants from aqueous systems: A review. Part 4. Dissolved petrochemical compounds,” BioResources 9(4), 7782-7925. DOI: 10.15376/biores.9.4.
Hubbe, M. A., and Rojas, O. J. (2008). “Colloidal stability and aggregation of lignocellulosic materials in aqueous suspension: A review,” BioResources 3(4), 1419-1491. DOI: 10.15376/biores.3.4.1419-1491
Hubbe, M. A., Sundberg, A., Mocchiutti, P., Ni, Y., and Pelton, R. (2012b). “Dissolved and colloidal substances (DCS) and the charge demand of papermaking process waters and suspensions: A review,” BioResources 7(4), 6109-6193. DOI: 10.15376/biores.7.4.6109-6193
Hunter, D. (1974). Papermaking. The History and Technique of an Ancient Craft, Dover Publ., New York, 611 pp.
Huuhilo, T., Suvilampi, J., Puro, L., Rintala, J., Mänttäri, M., Nuortila-Jokinen, J., and Nystrom, M. (2002). “Internal treatment of pulp and paper mill process waters with a high temperature aerobic biofilm process combined with ultrafiltration and/or nanofiltration,” Paperi ja Puu 84(1), 50-53.
Hynninen, P. (1998). “Effluent treatment,” in: Environmental Control, Part 19 of Papermaking Science and Technology series, P. Hynninen (ed.), Fapet Oy, Helsinki, Ch. 6, pp. 57-93.
Hyötyläinen, J., and Knuutinen, J. (1993). “Chemical degradation products of lignin and humic substances. Part 1: Synthesis structure verification and gas chromatographic separation of chlorinated vanillins and syringaldehydes,” Chemosphere 26(10), 1843‑1858. DOI: 10.1016/0045-6535(93)90078-j
Ince, B. K., Ince, O., and Cetecioglu, Z. (2011). Pollution Prevention in the Pulp and Paper Industries, INTECH Open Access Publisher.
Ince, N. H., Stefan, M. I., and Bolton, J. R. (1997). “UV/H2O2 degradation and toxicity reduction of textile azo dyes remazol black B, A case study,” J. Adv. Oxid. Technol. 2(3), 442‑448.
Jackson-Moss, C. A., Maree, J. P., and Wotton, S. C. (1992). “Treatment of bleach plant effluent with the biological granular activated carbon process,” Water Sci. Technol. 26, 427-434.
Jamil, T. S., Ghaly, M. Y., El-Seesy, I. E., Souaya, E. R., and Nasr, R. A. (2011). “A comparative study among different photochemical oxidation processes to enhance the biodegradability of paper mill wastewater,” J. Hazard. Mater. 185(1), 353-358. DOI: 10.1016/j.jhazmat.2010.09.041
Janssen, A. J. H., Lens, P. N. L., Stams, A. J. M., Plugge, C. M., Sorokin, D. Y., Muyzer, G., Dijkman, H., Van Zessen, E., Luimes, P., and Buisman, C. J. N. (2009). “Application of bacteria involved in the biological sulfur cycle for paper mill effluent purification,” Sci. Total Environ. 407(4), 1333-1343. DOI: 10.1016/j.scitotenv.2008.09.054
Jaycock, M. J., and Pearson, J. L. (1976a). “A study of the retention of pigment during paper formation,” J. Colloid Interface Sci. 55(1), 181-190. DOI: 10.1016/0021-9797(76)90024-2
Jeison, D., Van Betuw, W., and Van Lier, J. B. (2008). “Feasibility of anaerobic membrane bioreactors for the treatment of wastewaters with particulate organic matter,” Sep. Sci. Technol. 43, 3417-3431. DOI: 10.1080/01496390802221659
Jiang, J.-Q., and Graham, N. J. D. (1997). “Pre-polymerised inorganic coagulants for treating water and waste water,” Chem. Ind. 10, 389-391.
Johnsen, K., Tana, J., and Lehtinen, K.-J. (1993). “Physiological responses in rainbow trout exposed to total mill effluents from an integrated newsprint mill,” The 18th International Mechanical Pulping Conference, June 15‑17, Norway, Oslo.
Johnsen, K., Tana, J., Lehtinen, K.-J., Stuthridge, T., Mattsson, K., Hemming, J., and Carlberg, G. E. (1998). “Experimental field exposure of brown trout to river receiving effluent from an integrated newsprint mill,” Ecotoxicol. Environ. Safe. 40(3), 184‑193. DOI: 10.1006/eesa.1998.1683
Jokela, J. K., Laine, M., Ed, M., and Salkinoja-Salonen, M. (1993). “Effect of biological treatment on halogenated organics in bleached kraft pulp mill effluents studied by molecular weight distribution analysis,” Environ. Sci. Technol. 27(3), 547‑557. DOI: 10.1021/es00040a014
Joss, E. N., McGrouther, K. G., and Slade, A. H. (2007). “Comparison of the efficacy of oxidative processes and flocculation for the removal of colour from Eop effluent,” Water Sci. Technol. 55(6), 57‑64. DOI: 10.2166/wst.2007.212
Judd, S. (2006). The MBR Book: Principles and Applications of Membrane Bioreactors in Water and Wastewater Treatment, Elsevier, Oxford, UK.
Kakahi, F. B., Kulkarni, S., and Pathade, G. R. (2011). “A review on the role of microorganisms in treatment of paper and pulp industry effluent,” Nature Environ. Pollution Technol. 10(3), 361-368.
Kallioinen, M., Huuhilo, T., Reinikainen, S. P., Nuortila-Jokinen, J., and Mänttäri, M. (2006). “Examination of membrane performance with multivariate methods: A case study within a pulp and paper mill filtration application,” Chemometrics Intel. Lab. Sys. 84, 98-105. DOI: 10.1016/j.chemolab.2006.04.015
Kallioinen, M., Reinikainen, S.-P., Nuortila-Jokinen, J., Mänttäri, M., Sutela, T., and Nurminen, P. (2005). “Chemometrical approach in studies of membrane capacity in pulp and paper mill application,” Desalination 175(1), 87-95. DOI: 10.1016/j.desal.2004.11.004
Kaluža, L., Šuštaršič, M., Rutar, V., and Zupančič, G. D. (2014). “The re-use of waste-activated sludge as part of a “zero-sludge” strategy for wastewater treatments in the pulp and paper industry,” Bioresour. Technol. 151, 137-143. DOI: 10.1016/j.biortech.2013.10.041
Kamali, M., Gameiro, T., Costa, M. E., and Capela, I. (2016). “Anaerobic digestion of pulp and paper wastes – An overview of developments and improvement opportunities,” Chem. Eng. J., in press, accepted for publication. DOI: 10.1016/j.cej.2016.03.119
Kamali, M., and Khodaparast, Z. (2015). “Review on recent developments on pulp and paper mill wastewater treatment,” Ecotoxicol. Environ. Safety 114, 326-342. DOI: 10.1016/j.ecoenv.2014.05.005
Kansal, S. K., Singh, M., and Sud, D. (2008). “Effluent quality at kraft/soda agro-based paper mills and its treatment using a heterogeneous photocatalytic system,” Desalination 228, 183-190. DOI: 10.1016/j.desal.2007.10.007
Kappen, J., and Wilderer, P. A. (2002). “Key parameter methodology for increased water recovery in the pulp and paper industry,” in: Water Recycling and Resource Recovery in Industries: Analysis Technologies and Implementation, Lens, P., Pol, L. H., Winderer, P., and Asano, T. (eds.), IWA Publishing, London, UK, pp. 229-251.
Karels, A., Soimasuo, M., and Oikari, A. (1999). “Effects of pulp and paper mill effluents on reproduction, bile conjugates and liver MFO (mixed function oxygenase) activity in fish at Southern Lake Saimaa, Finland,” Water Sci. Technol. 40(11‑12), 109‑114.
Karvonen, V., Leskinen, A., and Sillanpaa, M. (1999). “Filtration effects of retention chemicals in save-all disc filter in news production,” TAPPI 99 Proceedings, TAPPI Press, Atlanta, pp. 1543-1552.
Katal, R., and Pahlavanzadeh, H. (2011). “Influence of different combinations of aluminum and iron electrode on electrocoagulation efficiency: Application to the treatment of paper mill wastewater,” Desalination 265, 199-205. DOI: 10.1016/j.desal.2010.07.052
Kawaguchi, H. (1992). “Photolysis of 2-chlorophenol in natural waters,” J. Contam. Hydrol. 9(1‑2), 105‑114. DOI: 10.1016/0169-7722(92)90053-H
Kaya, Y., Gönder, Z. B., Vergili, I., and Barlas, H. (2010). “The effect of transmembrane pressure and pH on treatment of paper machine process waters by using a two-step nanofiltration process: Flux decline analysis,” Desalination 250(1), 150-157. DOI: 10.1016/j.desal.2009.06.034
Kemeny, T. E., and Banerjee, S. (1997). “Relationships among effluent constituents in bleached kraft pulp mills,” Water Res. 31(7), 1589-1594. DOI: 10.1016/S0043-1354(96)00397-1
Kenny, R. (2010). “Nutrient optimization for pulp and paper wastewater treatment plants – An opportunity for major cost savings,” Pulp and Paper-Canada 111(2), 20-24.
Khansorthong, S., and Hunsom, M. (2009). “Remediation of wastewater from pulp and paper mill industry by the electrochemical technique,” Chem. Eng. J. 151, 228-234. DOI: 10.1016/j.cej.2009.02.038
Khosravi, M., Gholikandi, G. B., Bali, A. S., Riahi, R., and Tashaouei, H. R. (2011). “Membrane process design for the reduction of wastewater color of the Mazandaran pulp-paper industry, Iran,” Water Resour. Manag. 25(12), 2989-3004. DOI: 10.1007/s11269-011-9794-1
Khulbe, K. C., Feng, C. Y., and Matsuura, T. (2008). Synthetic Polymer Membranes. Characterization by Atomic Force Microscopy, Springer, Berlin, 197 pp.
Kim, D. H. (2011). “A review of desalting process techniques and economic analysis of the recovery of salts from retentates,” Desalination 270, 1-8. DOI: 10.1016/j.sesal.2010.12.041
Kim, J.-H., Oh, K.-K., Lee, S.-T., Kim, S.-W., and Hong, S.-I. (2002). “Biodegradation of phenol and chlorophenols with defined mixed culture in shake-flasks and a packed bed reactor,” Process Biochem. 37(12), 1367‑1373. DOI: 10.1016/S0032-9592(02)00007-9
Kinstrey, R. B. (1993). “An overview of strategies for reducing the environmental impact of bleach plant effluents,” Tappi J. 76(3), 105‑113.
Kishimoto, N., Nakagawa, T., Okada, H., and Mizutani, H. (2010). “Treatment of paper and pulp mill wastewater by ozonation combined with electrolysis,” J. Water Environ. Technol. 8, 99-109. DOI: 10.2965/jwet.2010.99
Knight, R. L. (2004). “Use of constructed wetland effluent treatment system in the pulp and paper industry,” NCASI Technical Bulletin, No. 876.
Knuutinen, J. (1982). “Analysis of chlorinated guaiacols in spent bleach liquor from a pulp mill,” J. Chromatogr. A 248(2), 289‑295. DOI: 10.1016/S0021-9673(00)87280-5
Ko, C.-H., and Fan, C. (2010). “Enhanced chemical oxygen demand removal and flux reduction in pulp and paper wastewater treatment using laccase-polymerized membrane filtration,” J. Hazard. Mater. 181, 763-770. DOI: 10.1016/j.jhazmat.2010.05.079
Ko, C.-H., Hsieh, P.-H., Chang, M.-W., Chern, J.-M., Chiang, S.-M., and Tzeng, C.-J. (2009). “Kinetics of pulp mill effluent treatment by ozone-based processes,” J. Hazard. Mater. 168, 875-881. DOI: 10.1016/j.jhazmat.2009.02.111
Komesvarakul, N., Scamehorn, J. F., and Gecol, H. (2003). “Purification of phenolic-laden wastewater from the pulp and paper industry by using colloid-enhanced ultrafiltration,” Separ. Sci. Technol. 38(11), 2465-2501. DOI: 10.1081/SS-120022283
Koistinen, J. (1992). “Alkyl polychlorobibenzyls and planar aromatic chlorocompounds in pulp mill products, effluents, sludges and exposed biota,” Chemosphere 24(5), 559‑573. DOI: 10.1016/0045-6535(92)90212-a
Koistinen, J., Paasivirta, J., and Särkkä, J. (1990). “Organic chlorine compounds in lake sediments. IV. Dioxins, furans and related chloroaromatic compounds,” Chemosphere 21(12), 1371‑1379. DOI: 10.1016/0045-6535(90)90041-Q
Koivisto, J. (2001). “Structural analysis of selected polychlorinated persistent organic pollutants (POPs) and related compounds,” PhD thesis, University of Jyväskylä.
Korczak, M. K., Koziarski, S., and Komorowska, B. (1991). “Anaerobic treatment of pulp-mill effluents,” Water Sci. Technol. 24(7), 203-206.
Korhonen, S., and Tuhkanen, T. (2000). “Effects of ozone on resin acids in thermomechanical pulp and paper mill circulation waters,” Ozone‑Sci. Eng. 22(6), 575‑584. DOI: 10.1080/01919510009408800
Kortekaas, S., Sijngaarde, R. R., Clompt, J.-W., Lettinga, G., and Field, J. A. (1998). “Anaerobic treatment of hemp thermomechanical pulping wastewater,” Water Res. 32(11), 3362-3370. DOI: 10.1016/S0043-1354(98)00120-1
Kostamo, A., and Kukkonen, J. V. K. (2003). “Removal of resin acids and sterols from pulp mill effluents by activated sludge treatment,” Water Res. 37(12), 2813-2820. DOI: 10.1016/S0043-1354(03)00108-8
Koster, I. W., and Cramer, A. (1987). “Inhibition of methanogenesis from acetate in granular sludge by long chain fatty acids,” Appl. Environ. Microbiol. 53(2), 403‑409.
Kreetachat, T., Damrongsri, M., Punsuwon, V., Vaithanomsat, P., Chiemchaisri, C., and Chomsurin, C. (2007). “Effects of ozonation process on lignin-derived compounds in pulp and paper mill effluents,” J. Hazard. Mater. 142, 250-257. DOI: 10.1016/j.jhazmat.2006.08.011
Kringstad, K. P., and Lindström, K. (1984). “Spent liquors from pulp bleaching,” Environ. Sci. Technol. 18(8), 236A‑248A.
Krishna, K. V., Sarkar, O., and Mohan, S. V. (2014). “Bioelectrochemical treatment of paper and pulp wastewater in comparison with anaerobic process: Integrating chemical coagulation with simultaneous power production,” Bioresour. Technol. 174, 142-151. DOI: 10.1016/j.biortech.2014.09.141
Kukkola, J., Knuutinen, J., Paasivirta, J., Herve, S., Pessala, P., and Schultz, E. (2011). “Size-exclusion chromatographic study of ECF and TCF softwood kraft pulp bleaching liquors,” Environ. Sci. Pollut. Res. 18(7), 1049‑1056. DOI: 10.1007/s11356-011-0556-7
Kumar, A., Dhall, P., and Kumar, R. (2010). “Redefining BOD:COD ratio of pulp mill industrial wastewaters in BOD analysis by formulating a specific microbial seed,” Int. Biodeterior. Biodegrad. 64(3), 197‑202. DOI: 10.1016/j.ibiod.2010.01.005
Kumar, P., Kumar, S., Bhardwaj, N. K., and Choudhary, A. K. (2011). “Optimization of process parameters for the photocatalytic treatment of paper mill wastewater,” Environ. Eng. Manag. J. 10(5), 595-601.
Kumara Swamy, N., Singh, P., and Sarethy, I. P. (2012). “Color and phenols removal from paper mill effluent by sequential treatment using ferric chloride and Pseudomonas putida,” Int. J. Pharm. Biol. Sci. 3(2), 380‑392.
Kuokkanen, T. (1989). “Chlorocymenes and chlorocymenenes: Persistent chlorocompounds in spent bleach liquors of kraft pulp mills,” PhD thesis, University of Jyväskylä.
Kurnik, K., Treder, K., Skorupa-Klaput, M., Tretyn, A., and Tyburski, J. (2015). “Removal of phenol from synthetic and industrial wastewater by potato pulp peroxidases,” Water Air Soil Pollution 226(8), Article number 254. DOI: 10.1007/s11270-015-2517-0
Lacorte, S., Latorre, A., Barceló, D., Rigol, A., Malmqvist, A., and Welander, T. (2003). “Organic compounds in paper-mill process waters and effluents,” Trends Anal. Chem. 22(10), 725‑737. DOI: 10.1016/S0165-9936(03)01009-4
Laine, J. (1997). “Effect of ECF and TEC bleaching on the charge properties of kraft pulp,” Paperi Puu 79(8), 551-559.
Laitinen, N., Luonsi, A., Levanen, E., and Nystrom, M. (2001). “Effect of backflushing conditions on ultrafiltration of board industry wastewaters with ceramic membranes,” Separ. Purif. Technol. 25, 323-331. DOI: 10.1016/S1383-5866(01)00059-4
Lamb, H. (1994). Hydrodynamics, Sixth Ed., Cambridge Univ. Press, UK.
La Mer, V. K., and Healy, T. W. (1963). “Adsorption-flocculation reactions of macro-molecules at the solid-liquid interface,” Rev. Pure Appl. Chem. 13(Sept.), 112-133.
Langergraber, G., Fleischmann, N., Hofstaedter, F., and Weingartner, A. (2004). “Monitoring of a paper mill wastewater treatment plant using UV/VIS spectroscopy,” Water Sci. Technol. 49(1), 9-14.
LaPara, T. M., and Alleman, J. E. (1999). “Thermophilic aerobic biological wastewater treatment,” Water Res. 33(4), 895-908. DOI: 10.1016/S0043-1354(98)00282-6
Lapertot, M., Pulgarín, C., Fernández-Ibañez, P., Maldonado, M. I., Pérez-Estrada, L., Oller, I., Gernjak, W., and Malato, S. (2006). “Enhancing biodegradability of priority substances (pesticides) by solar photo-Fenton,” Water Res. 40(5), 1086‑1094. DOI: 10.1016/j.watres.2006.01.002
Larsson, M., Truong, X. B., Bjorn, A., Ejlertsson, J., Bastviken, D., Svensson, B. H., and Karlsson, A. (2015). “Anaerobic digestion of alkaline bleaching wastewater from a kraft pulp and paper mill using UASB technique,” Environ. Technol. 36(12), 1489-1498. DOI: 10.1080/09593330.2014.994042
Lartiges, B. W., Bottero, J. Y., Derrendinger, L. S., Humbert, B., Tekely, P., and Suty, H. (1997). “Flocculation of colloidal silica with hydrolyzed aluminum: An 27Al solid state NMR investigation,” Langmuir 13(2), 147-152. DOI: 10.1021/la951029x
Lastra, A., Gomez, D., Romero, J., Francisco, J. L., Luque, S., and Alvarez, J. R. (2004). “Removal of metal complexes by nanofiltration in a TCF pulp mill: Technical and economic feasibility,” J. Membrane Sci. 242, 97-105. DOI: 10.1016/j.memsci.2004.05.012
Latorre, A., Malmqvist, A., Lacorte, S., Welander, T., and Barceló, D. (2007). “Evaluation of the treatment efficiencies of paper mill whitewaters in terms of organic composition and toxicity,” Environ. Pollut. 147(3), 648‑655. DOI: 10.1016/j.envpol.2006.09.015
Latour, I., Miranda, R., and Blanco, A. (2013). “Silica removal from newsprint mill effluents with aluminum salts,” Chem. Eng. J. 230(15), 522-531. DOI: 10.1016/j.cej.2013.06.039
Latour, I., Miranda, R., and Blanco, A. (2014a). “Silica removal in industrial effluents with high silica content and low hardness,” Environ. Sci. Pollut. Res. 21 (16), 9832-9842. DOI: 10.1007/s11356-014-2906-8
Latour, I., Miranda, R., and Blanco, A. (2014b). “Silica removal with sparingly soluble magnesium compounds. Part I,” Sep. Purif. Technol. 138, 210-218. DOI: 10.1016/j.seppur.2014.10.016
Latour, I., Miranda, R., and Blanco, A. (2014c). “Silica removal with sparingly soluble magnesium compounds. Part II,” Sep. Purif. Technol. 149, 331-338. DOI: 10.1016/j.seppur.2015.05.037
Latour, I., Miranda, R., Carceller, R., and Blanco, A. (2015). “Efficiency of polyaluminum nitrate sulfate–polyamine hybrid coagulants for silica removal,” Desalination and Water Treatment, pp.1-12. Published online. DOI:
10.1080/19443994.2015.1091992
Lawson, K. W., and Lloyd, D. R. (1997). “Membrane distillation,” J. Membrane Sci. 124, 1-25. DOI: 10.1016/S0376-7388(96)00236-0
Laycock, G., Pratp, S., Halley, P, Werker, A., and Lant, P. (2014). “Biodegradable polymers from pulp and paper wastewater streams – A critical review,” APPITA J. 67(4), 309-315.
Le-Clech, P., Chen, V., and Fane, T. A. G. (2006). “Fouling in membrane bioreactors used in wastewater treatment,” J. Membrane Sci. 284, 17-53. DOI: 10.1016/j.memsci.2006.08.019
Ledakowicz, S., Michniewicz, M., Jagiella, A., Stufka-Olczyk, J., and Martynelis, M. (2006). “Elimination of resin acids by advanced oxidation processes and their impact on subsequent biodegradation,” Water Res. 40(18), 3439‑3446. DOI: 10.1016/j.watres.2006.06.038
Lee, N. M., and Welander, T. (1996). “Reducing sludge production in aerobic wastewater treatment through manipulation of the ecosystem,” Water Res. 30(8), 1781-1790. DOI: 10.1016/0043-1354(96)00059-0
Lee, K. E., Morad, N., Teng, T. T., and Poh, B. T. (2012). “Development, characterization and the application of hybrid materials in coagulation/flocculation of wastewater: A review,” Chem. Eng. J. 203, 370-386. DOI: 10.1016/j.cej.2012.06.109
Lee, P. F. (1979). “Optimizing the displacement washing of pads of wood pulp fibers,” Tappi 62(9), 75-78.
Lee, P. F. (1984). “Channeling and displacement washing of wood pulp fiber pads,” Tappi J. 67(11), 100-103.
Lehtinen, K.-J., Mattson, K., Tana, J., Engström, C., Lerche, O., and Hemming, J. (1999). “Effects of wood-related sterols on the reproduction, egg survival, and offspring of brown trout (Salmo trutta lacustris L.),” Ecotoxicol. Environ. Safe. 42(1), 40‑49. DOI: 10.1006/eesa.1998.1724
Lehto, J., and Alén, R. (2015). “Organic materials in black liquors of soda-AQ pulping of hot-water-extracted birch (Betula pendula) sawdust,” Holzforschung 69(3), 257-264. DOI: 10.1515/hf-2014-0094
Lei, L., and Li, Y. (2014). “Effect of ozonation on recalcitrant chemical oxygen demand (COD), color and biodegradability of hardwood kraft pulp (KP) bleaching effluent,” BioResources 9(1), 1236‑1245. DOI: 10.15376/biores.9.1.1236-1245
Leitz, C. R. (1993). “Chemical allies in water, wastewater and sludge dewatering applications; Selection and determination of overall treatment economics,” presented at Tri-State Seminar On-the-River, sponsored by The Nevada WPCF, the California WPCF, the Arizona WPCF, and California-Nevada AWWA.
Leiviska, T., Nurmesniemi, H., Poykio, R., Ramo, J., Kuokkanen, T., and Pellinen, J. (2008). “Effect of biological wastewater treatment on the molecular weight distribution of soluble organic compounds and on the reduction of BOD, COD and P in pulp and paper mill effluent,” Water Res. 42(14), 3952-3960. DOI: 10.1016/j.watres.2008.06.016
Leiviska, T., Ramo, J., Nurmesniemi, H., Poykio, R., and Kuokkanen, T. (2009). “Size fractionation of wood extractives, lignin and trace elements in pulp and paper mill wastewater before and after biological treatment,” Water Res. 43(13), 3199-3206. DOI: 10.1016/j.watres.2009.04.051
Lens, P. N. L., Visser, A., Janssen, A. J. H., Pol, L. W. H., and Lettinga, G. (1998). “Biotechnological treatment of sulfate-rich wastewaters,” Crit. Rev. Environ. Sci. Technol. 28(1), 41-88. DOI: 10.1080/10643389891254160
Leppänen, H., and Oikari, A. (1999). “Occurrence of retene and resin acids in sediments and fish bile from lake receiving pulp and a paper mill effluents,” Environ. Toxicol. Chem. 18(7), 1498‑1505. DOI: 10.1002/etc.5620180723
Lescot, J. C., and Jappinen, H. (1994). “Effluent treatment in pulp and paper mills: Present technology and development trends,” APPITA Journal 47(4), 330-332.
Lettinga, G., van Velsen, A. F. M., Hobma, S. W., de Zeeuw, W., and Klapwijk, A. (1980). “Use of the upflow anaerobic sludge blanket (UASB) reactor concept for biological wastewater treatment, especially for anaerobic treatment,” Biotech. & Bioenerg. 22(6), 699-734. DOI: 10.1002/bit.260220402
Lewis, R., Nothrop, S., Chow, C. W. K., Everson, A., and Leeuwen, J. A. Van. (2013). “Color formation from pre and post-coagulation treatment of Pinus radiata sulfite pulp mill wastewater using nutrient limited aerated stabilization basins,” Sep. Purif. Technol. 114, 1-10. DOI: 10.1016/j.seppur.2013.04.022
Li, S., and Zhang, X. (2011). “The study of PAFSSB on RO pre-treatment in pulp and paper wastewater,” Proc. Environ. Sci. 8, 4-10. DOI: 10.1016/j.proenv.2011.10.003
Liao, B.-Q., Kraemer, J. T., and Bagley, D. M. (2006). “Anaerobic membrane bioreactors: Applications and research directions,” Crit. Rev. Environ. Sci. Technol. 36(6), 489-530. DOI: 10.1080/10643380600678146
Lin, H. J., Peng, W., Zhang, M. J., Chen, J. R., Hong, H. C., and Zhang, Y. (2013). “A review on anaerobic membrane bioreactors: Applications, membrane fouling and future perspectives,” Desalination 314, 169-188. DOI: 10.1016/j.desal.2013.01.019
Lin, Y., Wang, D., Li, Q., and Xiao, M. (2011). “Mesophilic batch anaerobic co-digestion of pulp and paper sludge and monosodium glutamate waste liquor for methane production in a bench-scale digester,” Bioresour. Technol. 102(4), 3673-3678. DOI: 10.1016/j.biortech.2010.10.114
Lin, Y., Wang, D., and Wang, T. (2012). “Ethanol production from pulp & paper sludge and monosodium glutamate waste liquor by simultaneous saccharification and fermentation in batch condition,” Chem. Eng. J. 191, 31-37. DOI: 10.1016/j.cej.2011.09.040
Lindau, J., Sedin, P., and Theliander, H. (2007). “Investigating the influence of bed structure on pulp displacement washing using an in-situ measurement technique,” J. Pulp Paper Sci. 33(4), 189-198.
Lindholm-Lehto, P. C., Knuutinen, J. S., Ahkola, H. S. J., and Herve, S. H. (2015). “Refractory organic pollutants and toxicity in pulp and paper mill wastewaters,” Environ. Sci. Pollution Res. 22(9), 6473-6499. DOI: 10.1007/s11356-015-4163-x
Liu, J. F., and Shonnard, D. R. (2014). “Life cycle carbon footprint of ethanol and potassium acetate produced from a forest product wastewater stream by a co-located biorefinery,” ACS Sustain. Chem. Eng. 2(8), 1951-1958. DOI: 10.1021/sc500256y
Liu, T., Hu, H., He, Z., and Ni, Y. (2011). “Treatment of poplar alkaline peroxide mechanical pulping (APMP) effluent with Aspergillus niger,” Bioresour. Technol. 102(15), 7361-7365. DOI: 10.1016/j.biortech.2011.04.043
Liver, S. F., Miyamoto, H. K., and Black, S. A. (1993). “Bench-scale testing of activated sludge treatment for a TMP wastewater,” Water Pollut. Res. J. Can. 28(3), 571-596.
Loehr, R. C. and Krishnamoorthy, R. (1988). “Terrestrial bioaccumulation potential of phenolic compounds,” Hazard. Waste Hazard. 5(2), 109‑119. DOI: 10.1089/hwm.1988.5.109
Loraine, A., and Huchler, P. E. (2006). “Non-chemical water treatment systems: Histories, principles and literature review,” Paper Technol. 47(4), 23-34.
Lucas, M. S., Peres, J. A., Amor, C., Prieto-Rodríguez, L., Maldonado, M. I., and Malato, S. (2012). “Tertiary treatment of pulp mill wastewater by solar photo-Fenton,” J. Hazard Mater. 225-226, 173-181. DOI: 10.1016/j.jhazmat.2012.05.013
Lugowski, A. (1991). “Review of pulp- and paper-mill wastewater treatment,” Celul. Papel 7(12), 19-26.
Luthe, C. E., and Berry, R. M. (1996). “The role of dibenzo-p-dioxin and dibenzofuran precursors in the formation of tetrachlorinated dibenzo-p-dioxins/-furans during bleaching,” Chemosphere 32(5), 881‑891. DOI: 10.1016/0045-6535(96)00022-7
Maat, D. Z., and Habets, L. H. A. (1987). “Upflow anaerobic sludge blanket wastewater treatment system: Technological review,” Pulp Paper Canada 88(11), 60-64.
Magnus, E., Hoel, H., and Carlberg, G. E. (2000). “Treatment of an NSSC effluent in a biological high-efficiency compact reactor,” TAPPI Journal 83(1), 149-156.
Mahesh, S., Prasad, B., Mall, I. D., and Mishra, I. M. (2006). “Electrochemical degradation of pulp and paper mill wastewater. Part 1. COD and color removal,” Indust. Eng. Chem. Res. 45(8), 2830-2839. DOI: 10.1021/ie0514096
Mahmood, T., and Elliott, A. (2006). “A review of secondary sludge reduction technologies for the pulp and paper industry,” Water Res. 40(11), 2093-2112. DOI: 10.1016/j.watres.2006.04.001
Mahmood, T., and Elliot, A. (2007). “Use of acid preconditioning for enhanced dewatering of wastewater treatment sludges from the pulp and paper industry,” Water Environ. Res. 79(2), 168-176. DOI: 10.2175/106143006X111970
Mahmood-Khan, Z., and Hall, E. R. (2013). “Biological removal of phyto-sterols in pulp mill effluents,” J. Environ. Manag. 131, 407-414. DOI: 10.1016/j.jenvman.2013.09.031
Makris, S. P., and Banerjee, S. (2002). “Fate of resin acids in pulp mill secondary treatment systems,” Water Res. 36(11), 2878‑2882. DOI: 10.1016/S0043-1354(01)00491-2
Malaviya, P., and Rathore, V. S. (2007). “Bioremediation of pulp and paper mill effluent by a novel fungal consortium isolated from polluted soil,” Bioresour. Technol. 98(18), 3647-3651. DOI: 10.1016/j.biortech.2006.11.021
Malisch, R. (2000). “Increase of the PCDD/F-contamination of milk, butter and meat samples by use of contaminated citrus pulp,” Chemosphere 40(9‑11), 1041‑1053. DOI: 10.1016/s0045-6535(99)00352-5
Malmqvist, A., Berggren, B., Sjölin, C., Welander, T. Heuts, L., Fransén, A., and Ling, D. (2004). “Full scale implementation of the nutrient limited BAS process at Södra Cell Värö,” Water Science and Technology 50(3), 123-130.
Mänttäri, M., Kuosa, M., Kallas, J., and Nyström, M. (2008). “Membrane filtration and ozone treatment of biologically treated effluents from the pulp and paper industry,” J. Membr. Sci. 309, 112-119. DOI: 10.1016/j.memsci.2007.10.019
Mänttäri, M., and Nyström, M. (2007). “Membrane filtration for tertiary treatment of biologically treated effluents from the pulp and paper industry,” Water Sci. Technol. 55(6), 99-107. DOI: 10.2166/wst.2007.217
Martínez, A. T., Speranza, M., Ruiz-Dueñas, F. J., Ferreira, P., Camarero, S., Guillén, F., Martínez, M. J., Gutiérrez, A., and del Río, J. C. (2005). “Biodegradation of lignocellulosics: Microbial, chemical, and enzymatic aspects of the fungal attack of lignin,” Int. Microbiol. 8(3), 195‑204.
Marton, J. (1980a). “The role of surface chemistry in fines – alum interactions,” Tappi 63(2), 121-125.
Marton, J. (1980b). “The role of surface chemistry in fines – cationic starch interactions,” Tappi 63(4), 87-91.
Marwaha, S. S., Grover, R., Prakash, C., Kennedy, J. F. (1998). “Continuous biobleaching of black liquor from the pulp and paper industry using an immobilized cell system,” J. Chem. Technol. Biotechnol. 73(3), 292-296. DOI: 10.1002/(SICI)1097-4660(1998110)73:3<292::AID-JCTB950>3.3.CO;2-3
Mason, T. J. (2007). “Sonochemistry and the environment – Providing a “green” link between chemistry, physics and engineering,” Ultrason. Sonochem. 14(4), 476‑483. DOI: 10.1016/j.ultsonch.2006.10.008
Masten, S. J., and Davies, S. H. R. (1994). “The use of ozonation to degrade organic contaminants in wastewaters,” Environ. Sci. Technol. 28(4), 180A‑185A.
Matafonova, G., Shirapova, G., Zimmer, C., Giffhorn, F., Batoev, V., and Kohring, G.-W. (2006). “Degradation of 2,4-dichlorophenol by Bacillus sp. isolated from an aeration pond in the Baikalsk pulp and paper mill (Russia),” Int. Biodeterior. Biodegrad. 58(3-4), 209-212. DOI: 10.1016/j.ibiod.2006.06.024
Matko, T., Fawcett, N., Sharp, A., and Stephenson, T. (1996). “Recent progress in the numerical modelling of wastewater sedimentation tanks,” Process Safety Environ. Protection 74(B4), 245-258. DOI: 10.1205/095758296528590
Mauchauffee, S., Denieul, M. P., and Coste, M. (2012). “Industrial wastewater re-use: Closure of water cycle in the main water consuming industries – The example of paper mills,” Environ. Technol. 33(19), 2257-2262. DOI: 10.1080/09593330.2012.728734
McCubbin, N., and Folke, J. N. (1993). “A review of literature on pulp and paper mill effluent characteristics in the peace and Athabasca river basins,” in: Norther River Basins Study Project Report No. 15, N. McCubbin Consultants Inc., Edmonton.
McKague, A. B. (1981). “Phenolic constituents in pulp mill process streams,” J. Chromatogr. 208(2), 287‑293. DOI: 10.1016/S0021-9673(00)81940-8
McKeown, J. J. (1979). “Sludge dewatering and disposal practices in the U.S. paper industry,” TAPPI Ann. Mtg. Proc. (New York), pp. 165-172.
McKeown, J. J., and Gellman, I. (1976). “Characterizing effluent variability from paper industry wastewater treatment processes employing biological oxidation,” Prog. Water Technol. 8(1), 147-163.
Meng, F. G., Chae, S. R., Drews, A., Kraume, M., Shin, H.-S., and Yang, F. L. (2009). “Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material,” Water Res. 43(5), 1486-1512. DOI: 10.1016/j.watres.2008.12.044
Menezes, G. B., Golkar, T., and Moo-Young, H. H. (2010). “Pulp and paper mill effluents management,” Water Eviron. Res. 82(10), 1525-1531.
Merayo, N., Hermosilla, D., Blanco, L., Cortijo, L., and Blanco, A. (2013). “Assessing the application of advanced oxidation processes, and their combination with biological treatment, to effluents from pulp and paper industry,” J. Hazard. Mater. 262, 420-427. DOI: 10.1016/j.jhazmat.2013.09.005
Merayo, N., Hermosilla, D., Jefferson, B., and Blanco, A. (2016). “Alkalinity influence on photo-assisted processes efficiency and catalyst behavior,” Chem. Eng. Technol. 39(1), 4-9. DOI: 10.1002/ceat.201400320
Meriläinen, P. R., Krasnov, A., and Oikari, A. (2007). “Time- and concentration-dependent metabolic and genomic responses to exposure to resin acids in brown trout (Salmo trutta m. lacustris),” Environ. Toxicol. Chem. 26(9), 1827‑1835. DOI: 10.1897/06-521R.1
Meyer, T., and Edwards, E. A. (2014). “Anaerobic digestion of pulp and paper mill wastewater and sludge,” Water Res. 65, 321-349. DOI: 10.1016/j.watres.2014.07.022
Meyssami, B., and Kasaeian, A. B. (2005). “Use of coagulants in treatment of olive oil wastewater model solutions by induced air flotation,” Bioresour. Technol. 96(3), 303-307. DOI: 10.1016/j.biortech.2004.04.014
Meza, P. R., Felissia, F. E., and Area, M. C. (2011). “Reduction of the recalcitrant COD of high yield pulp mills effluents by AOP. Part 1. Combination of ozone and activate sludge,” BioResources 6(2), 1053‑1068. DOI: 10.15376/biores.6.2.1053-1068
Miao, Q., Huang, L. L., and Chen, L. H. (2013). “Advances in the control of dissolved and colloidal substances present in papermaking processes: A brief review,” BioResources 8(1), 1431-1455. DOI: 10.15376/biores.8.1.1431-1455
Milliken, J. (2006). “Energy and water savings from the paper machine saveall,” Proc. TAPPI 2006 Papermakers Conf., Paper no. 19-1.
Minami, K., Okamura, K., Ogawa, S., and Naritomi, T. (1991). “Continuous anaerobic treatment of waste-water from a kraft pulp mill,” J. Ferment. Bioeng. 71(4), 270-274. DOI: 10.1016/0922-338X(91)90280-T
Miranda, R., Blanco, A., Fuente, E. D. L., and Negro, C. (2008). “Separation of contaminants from deinking process water by dissolved air flotation: Effect of flocculant charge density,” Separation Science and Technology 43(14), 3732-3754. DOI: 10.1080/01496390802286587
Miranda, R., Blanco, A., and Negro, C. (2009a). “Accumulation of dissolved and colloidal material in papermaking – Application to simulation,” Chem. Eng. J. 148(2-3), 358-393. DOI: 10.1016/j.cej.2008.09.014
Miranda, R., Latour, I., Hörsken, A., Jarabo, R., and Blanco, A. (2015). “Enhanced silica removal by polyamine- and polyacrylamide-polyaluminum hybrid coagulants,” Chem. Eng. Technol. 38(11), 2045-2053. DOI: 10.1002/ceat.201400604
Miranda, R., Negro, C., and Blanco, A. (2009b). “Internal treatment of process waters in paper production by dissolved air flotation with newly developed chemicals. 1. Laboratory tests,” Ind. Eng. Chem. Res. 48(4), 2199-2205. DOI: 10.1021/ie801047h
Miranda, R., Negro, C., and Blanco, A. (2009c). “Internal treatment of process waters in paper production by dissolved air flotation with newly developed chemicals. 2. Field trials,” Industrial & Engineering Chemistry Research 48(7), 3672-3677. DOI: 10.1021/ie801826m
Miranda, R., Nicu, R., Latour, I., Lupei, M., Bobu, E., and Blanco, A. (2013). “Efficiency of chitosans for the treatment of papermaking process water by dissolved air flotation,” Chemical Engineering Journal 231, 304-313. DOI: 10.1016/j.cej.2013.07.033
Mobius, C. H., and Helble, A. (2004). “Combined ozonation and biofilm treatment for reuse of paper mill wastewaters,” Water Sci. Technol. 49, 319-323.
Mohn, W. W., and Tiedje, J. M. (1992). “Microbial reductive dehalogenation,” Microbiol. Rev. 56(3), 482‑507.
Mollah, M. Y. A., Schennach, R., Parga, J. R., and Cocke, D. L. (2001). “Electrocoagulation (EC) – Science and applications,” J. Hazard. Mater. B 84(1), 29-41. DOI: 10.1016/S0304-3894(01)00176-5
Monte, M. C., Fuente, E., Blanco, A., and Negro, C. (2009). “Waste management from pulp and paper production in the European Union,” Waste Manage. 29(1), 293-308. DOI: 10.1016/j.wasman.2008.02.002
Moodley, B., Brookes, H. C., and Mulholland, D.A. (2011). “The electro-oxidation of lignin in Sappi Saiccor dissolving pulp effluent,” Water SA, 37, 33-44.
Moraes, S. G., Duran, N., and Freire, R. S. (2006). “Remediation of kraft E1 and black liquor effluents by biological and chemical processes,” Environ. Chem. Lett. 4, 87-91. DOI: 10.1007/s10311-006-0039-0
Mounteer, A. H., Souza, L. C., and Silva, C. M. (2007a). “Potential for enhancement of aerobic biological removal of recalcitrant organic matter in bleached pulp mill effluents,” Environ. Technol. 28(2), 157‑164. DOI: 10.1080/09593332808618775
Mounteer, A. H., Pereira, R. O., Morais, A. A., Ruas, D. B., Silveira, D. S. A., Viana, D. B., and Medeiros, R. C. (2007b). “Advanced oxidation of bleached eucalypt kraft pulp mill effluent,” Water Sci. Technol. 55(6), 109‑116. DOI: 10.2166/wst.2007.218
Muhamad, M. H., Abdullah, S. R. S., Mohamad, A. B., Rahman, R. A., and Kadhum, A. A. H. (2012a). “Kinetic evaluation and process performance of a pilot GAC-SBBR system treating recycled paper industry wastewater,” Environ. Eng. Manag. J. 11(4), 829-839.
Muhamad, M. H., Abdullah, S. R. S., Mohamad, A. B., Rahman, R. A., and Kadhum, A. A. H. (2012b). “Performance evaluation of a granular activated carbon-sequencing batch biofilm reactor pilot plant system used in treating real wastewater from recycled paper industry,” Environ. Technol. 33(8), 915-926. DOI: 10.1080/09593330.2011.602434
Muhamad, M. H., Sheikh Abdullah, S. R., Mohamad, A. B., Abdul Rahman, R., and Hasan Kadhum, A. A. (2013). “Application of response surface methodology (RSM) for optimisation of COD, NH3-N and 2,4-DCP removal from recycled paper waste-water in a pilot-scale granular activated carbon sequencing batch biofilm reactor (GAC-SBBR),” J. Environmental Manag. 121, 179-190. DOI: 10.1016/j.jenvman.2013.02.016
Munawar, M., Munawar, I. F., Sergeant, D., and Wenghofer, C. (2000). “A preliminary bioassessment of Lake Baikal sediment toxicity in the vicinity of a pulp and paper mill,” Aquatic Ecosyst. Health Manage. 3(2), 249‑257. DOI: 10.1016/s1463-4988(00)00024-5
Munkittrick, K. R., McMaster, M. E., McCarthy, L. H., Servos, M. R., and Van Der Kraak, G. J. (1998). “An overview of recent studies on the potential of pulp‐mill effluents to alter reproductive parameters in fish,” Toxicol. J. Env. Health 1(4), 347‑371. DOI: 10.1080/10937409809524558
Munkittrick, K. R., Servos, M. R., Carey, J. H., and Van Der Kraak, G. J. (1997). “Environmental impacts of pulp and paper wastewater: Evidence for a reduction in environmental effects at North American pulp mills since 1992,” Water Sci. Technol. 35(2‑3), 329‑338. DOI: 10.1016/s0273-1223(96)00948-1
Muñoz, R., and Guieysee, B. (2006). “Algal–bacterial processes for the treatment of hazardous contaminants: A review,” Water Res. 40(15), 2799‑2815. DOI: 10.1016/j.watres.2006.06.011
Murakami, T. (1998). “Recent advances in sludge treatment techniques,” Water Qual. Int. 9/10, 23-24.
Nakamura, Y., Sawada, T., Kobayshi, F., and Godliving, M. (1997). “Microbial treatment of kraft pulp wastewater pretreated with ozone,” Water Science and Technology 35(2-3), 277-282. DOI:10.1016/S0273-1223(96)00941-9
Nandy, T., Vyas, R. D. Shastry, S., and Kaul, S. N. (2002). “Optimization of coagulants for pretreatment of printing ink wastewater,” Environ. Eng. Sci. 19(1), 1-7. DOI: 10.1089/109287502753590197
Nassar, M. M. (2003). “Studies on internal and external water treatment at a paper and cardboard factory,” J. Chem. Technol. Biotech. 78(5), 572-576. DOI: 10.1002/jctb.812
Nataraj, S. K., Sridhar, S., Shaikha, I. N., Reddy, D. S., and Aminabhavi, T. M. (2007). “Membrane-based microfiltration/electrodialysis hybrid process for the treatment of paper industry wastewater,” Separ. Purif. Technol. 57(1), 185-192. DOI: 10.1016/j.seppur.2007.03.014
Naumann, K. (1999). “Influence of chlorine substituents on biological activity of chemicals,” J. Prakt. Chem. 341(5), 417‑435. DOI: 10.1002/(SICI)1521-3897(199907)341:5<417::AID-PRAC417>3.0.CO;2-A
Nawaz, A., Ahmed, Z., Shahbaz, A., Khan, Z., and Javed, M. (2014). “Coagulation-flocculation for lignin removal from wastewater – A review,” Water Sci. Technol. 69(8), 1589-1597. DOI: 10.2166/wst.2013.768
NCASI (2011). “A review of color, color measurement, and color formation in pulp and paper mill sewers and wastewater treatment plants,” NCASI Techn. Bull. 981, 1-85.
Negro, C., Blanco, A., Saarimaa, V., and Tijero, J. (2005). “Optimization of pitch removal by dissolved air flotation in a eucalyptus kraft mill,” Separation Science and Technology 40(5), 1129-1143. DOI: 10.1081/SS-200048173
Neilson, A. H. (1990). “Biodegradation of halogenated organic compounds,” J. Appl. Bacteriol. 69(4), 445‑470. DOI: 10.1111/j.1365-2672.1990.tb01536.x
Nichols, B., Wood, C., and Wachter, D. (2003). “Filter press optimization treating a coating wastewater,” TAPPI Proc. Environ. Conf. Exhib., Portland, OR, pp. 126-132/
Novak, J. T., Love, N. G., Smith, M. L., and Wheeler, E. R. (1998). “The effect of cationic salt addition on the settling and dewatering properties of an industrial activated sludge,” Water Environ. Res. 70(5), 984-996. DOI: 10.2175/106143098X123318
Nuortila-Jokinen, J., Mänttäri, M., Huuhilo, T., Kallioinen, M., and Nyström, M. (2004). “Water circuit closure with membrane technology in the pulp and paper industry,” Water Sci. Technol. 50(3), 217-227.
Öberg, T., Warman, K., and Bergström, J. (1989). “Production of chlorinated aromatics in the post-combustion zone and boiler,” Chemosphere 19(1‑6), 317‑322. DOI: 10.1016/0045-6535(89)90330-5
Öhman, L.-O., Wågberg, L., Malmgren, K., and Tjernström, Å. (1997). “Adsorption of aluminum(III) on cellulosic fibers in neutral to alkaline solutions – Influence of charge and size of the particles formed,” J. Pulp Paper Sci. 23(10), J467-J474.
Oikari, A., Ånäs, E., Kruzynski, G., and Holmbom, B. (1984). “Free and conjugated resin acids in the bile of rainbow trout, Salmo gairdneri,” Bull. Environ. Contam. Toxicol. 33(1), 233‑240. DOI: 10.1007/BF01625536
Oikari, A., Fragoso, N., Leppänen, H., Chan, T., and Hodson, P. V. (2002). “Bioavailability to juvenile rainbow trout (Oncorynchus myciss) of retene and other mixed-function oxygenase-active compounds from sediments,” Environ. Toxicol. Chem. 21(1), 121‑128. DOI: 10.1002/etc.5620210118
Oikari, A. O. J., and Nakari, T. (1982). “Kraft pulp mill effluent components cause liver dysfunction in trout,” Bull. Environ. Contam. Toxicol. 28(3), 266‑270. DOI: 10.1007/BF01608505
Oller, I., Malato, S., and Sánchez-Pérez, J. A. (2011). “Combination of advanced oxidation processes and biological treatments for wastewater decontamination – A review,” Sci. Total Environ. 409(20), 4141-4166. DOI: 10.1016/j.scitotenv.2010.08.061
Opedal, M. T., Stenius, P., and Johansson, L. (2011). “Review: Colloidal stability and removal of extractives from process water in thermomechanical pulping,” Nordic Pulp Paper Res. J. 26(3), 248-257. DOI: 10.3183/NPPRJ-2011-26-03-p248-257
Ordaz-Díaz, L. A., Rojas-Contreras, J. A., Rutiaga-Quiñones, O. M., Moreno-Jiménez, M. R., Alatriste-Mondragón, F., and Valle-Cervantes, S. (2014). “Microorganism degradation efficiency in BOD analysis formulating a specific microbial consortium in a pulp and paper mill effluent,” BioResources 9(4), 7189-7197. DOI: 10.15376/biores.9.4.7189-7197
Ordaz-Díaz, L. A., Valle-Cervantes, S., Rojas-Contreras, J. A., Rodríguez-Flores, F. J., and Bailón-Salas, A. M. (2016). “Optimization of a microbial formulation acclimated for pilot-scale biodegradation of paper mill effluent,” BioResources 11(1), 1071-1079.
Ordóñez, R., Hermosilla, D., San Pio, I., and Blanco, A. (2010). “Replacement of fresh water use by final effluent recovery in a highly optimized 100% recovered paper mill,” Water Sci. Technol. 62(7), 1694-1703. DOI: 10.2166/wst.2010.933
Ordóñez, R., Hermosilla, D., San Pio, I., and Blanco, A. (2011). “Evaluation of MF and UF as pretreatments prior to RO applied to reclaim municipal wastewater for freshwater substitution in a paper mill: A practical experience,” Chem. Eng. J. 166(1), 88-98. DOI: 10.1016/j.cej.2010.10.016
Ortega-Clemente, A., Caffarel-Méndez, S., Ponce-Noyola, M. T., Barrera-Córtes, J., and Poggi-Varaldo, H. M. (2009). “Fungal post-treatment of pulp mill effluents for the removal of recalcitrant pollutants,” Bioresource Technol. 100(6), 1885‑1894. DOI: 10.1016/j.biortech.2007.07.059
Osman, W. H. W., Abdullah, S. R. S., Mohamad, A. B., Kadhum, A. A. H., and Rahman, R. A. (2013). “Simultaneous removal of AOX and COD from real recycled paper wastewater using GAC-SBBR,” J. Environ. Manage. 121, 80-86. DOI: 10.1016/j.jenvman.2013.02.005
Owens, J. W. (1991). “The hazard assessment of pulp and paper effluents in the aquatic environment – A review,” Environ. Toxicol. Chem. 10(12), 1511-1540. DOI: 10.1002/etc.5620101202
Owens, J. W., Swanson, S. M., and Birkholz, D. A. (1994). “Environmental monitoring of bleached kraft pulp mill chlorophenolic compounds in a Northern Canadian River system,” Chemosphere 29(1), 89‑109. DOI: 10.1016/0045-6535(94)90093-0
Paasivirta, J. (1988). “Organochlorine compounds in the environment,” Water Sci. Technol. 20(2), 119‑129.
Paasivirta, J. (1991). Chemical Ecotoxicology, L366, Lewis Publishers Inc., Chelsea, Mic.
Paasivirta, J., Heinola, K., Humppi, T., Karjalainen, A., Knuutinen, J., Mäntykoski, K., Paukku, R., Piilola, T., Surma-Aho, K., Tarhanen, J., Welling, L., and Vihonen, H. (1984). “Polychlorinated phenols, guaiacols and catechols in environment,” Chemosphere 14(5), 469‑491. DOI: 10.1016/0045-6535(85)90241-3
Paasivirta, J., and Rantio, T. (1991). “Chloroterpenes and other organochlorines in Baltic, Finnish and Arctic wildlife,” Chemosphere 22(1‑2), 47‑55. DOI: 10.1016/0045-6535(91)90263-d
Paasivirta, J., Särkkä, J., Surma-Aho, K., Humppi, T., Kuokkanen, T., and Marttinen, M. (1983). “Food chain enrichment of organochlorine compounds and mercury in clean and polluted lakes of Finland,” Chemosphere 12(2), 239‑252. DOI: 10.1016/0045-6535(83)90167-4
Paasivirta, J., Tenhola, H., Palm, H., and Lammi, R. (1992). “Free and bound chlorophenols in kraft pulp bleaching effluents,” Chemosphere 24(9), 1253‑1258. DOI: 10.1016/0045-6535(92)90051-r
Pala, H., Mota, M., and Gama, F. M. (2004). “Enzymatic versus chemical deinking of non- impact ink printed paper,” J. Biotechnol. 108, 79-89. DOI: 10.1016/j.jbiotec.2003.10.016
PAPRICAN (2001). “Water Use Reduction in the Pulp and Paper Industry,” 2nd Edition, Pulp and Paper Research Institute of Canada, Sandwell Inc., NLK Consultants, AGRA Simons (Firm) Publisher, PAPTAC, 172 pp.
Parilti, N. B., and Akten, D. (2011). “Optimization of TiO2/Fe(III)/solar UV conditions for the removal of organic contaminants in pulp mill effluents,” Desalination 265, 37-42. DOI: 10.1016/j.desal.2010.07.027
Park, H.-D., Chang, I.-S., and Lee, K.-J. (2015). Principles of Membrane Bioreactors for Wastewater Treatment, Taylor and Francis, Boca Raton, FL, USA.
Pellinen, J., and Joyce, T. W. (1990). “White-rot fungi for treatment of pulp- and paper-industry waste water,” TAPPI Environ. Conf. Proc. (Seattle), pp. 1-13.
Pelzer, R. (2008). “Polyacrylamides (PAM) as retention aids,” in: Chemical Additives for the Production of Pulp & Paper, Edited by Zellcheming Technical Committees, “Chemical Additives (CHAD)”, Deutscher Fachverlag, Frankfurt am Main, ISBN 978-3-86641-120-3, pp. 139-151.
Pere, J., Alen, R., Viikari, L., and Eriksson, L. (1993). “Characterization and dewatering of activated-sludge from the pulp and paper-industry,” Water Sci. Technol. 28(1), 193-201.
Pérez-González, A., Urtiaga, A. M., Ibáñez, R., and Ortiz, I. (2012). “State of the art and review on the treatment technologies of water reverse osmosis concentrates,” Water Res. 46, 267-283. DOI: 10.1016/j.watres.2011.10.046
Pérez–Sicairos, S., Morales-Cuevas, J. B., Félix–Navarro, R. M., and Hernández–Calderón, O. M. (2011). “Evaluation of the electro-coagulation process of the removal of turbidity of river water, wastewater and pond water,” Revista Mexicana de Ingeniería Química 10(1), 79-91. ISSN 1665-2738
Pernitsky, D. J., and Edzwald, J. K. (2006). “Selection of alum and polyaluminum coagulants. Principles and applications,” J. Water Supply Res. Technol. – AQUA 55(2), 121-141. DOI: 10.2166/aqua.2006.062
Perrault, R. R. (1993). “Evaluating the performance of paper-machine save-alls,” Tappi J. 76(12), 189-192.
Pervaiz, M., and Sain, M. (2011). “Protein extraction from secondary sludge of paper mill wastewater and its utilization as a wood adhesive,” BioResources 6(2), 961-970.
Pessala, P., Schultz, E., Kukkola, J., Nakari, T., Knuutinen, J., Herve, S., and Paasivirta, J. (2010). “Biological effects of high molecular weight lignin derivatives,” Ecotoxicol. Environ. Safe. 73(7), 1641‑1645. DOI: 10.1016/j.ecoenv.2010.02.004
Peuravuori, J., Paaso, N., and Pihlaja, K. (2002). “Sorption behavior of some chlorophenols in lake aquatic humic matter,” Talanta 56(3), 523‑538. DOI: 10.1016/S0039-9140(01)00579-3
Pfau, A., Schrepp, W., and Horn, D. (1999). “Detection of a single molecule adsorption structure of poly(ethylenimine) macromolecules by AFM,” Langmuir 15(9), 3219-3225. DOI: 10.1021/la9808925
Pham, T. D., Shrestha, R. A., and Sillanpää, M. (2009). “Electrokinetic and ultrasonic treatment of kaoline contaminated by POPs,” Sep. Sci. Technol. 44(10), 2410‑2420. DOI: 10.1080/01496390902983760
Pietschker, D. A. (1996). “100% closed water system – What to expect,” Proc. TAPPI 1996 Papermakers Conf., pp. 523-530.
Pintar, A., Besson, M., and Gallezot, P. (2001). “Catalytic wet air oxidation of kraft bleaching plant effluents in the presence of titania and zirconia supported ruthenium,” Appl. Catal. B – Environ. 30, 123-139. DOI: 10.1016/S0926-3373(00)00228-9
Piwoni, M. D., Wilson, J. T., and Walters, D. M. (1989). “Behavior of organic pollutants during rapid-infiltration of wastewater into soil,” Hazard. Waste Hazard. Mater. 3(1), 43‑55. DOI: 10.1089/hwm.1986.3.43
Pizzichini, A., Russo, C., and Di Meo, C. (2005). “Purification of pulp and paper wastewater, with membrane technology, for water reuse in a closed loop,” Desalination 178, 351-359. DOI: 10.1016/j.desal.2004.11.045
Pokhrel, D., and Viraraghavan, T. (2004). “Treatment of pulp and paper mill wastewater – A review,” Science Total Environ. 333, 37-58. DOI: 10.1016/j.scitotenv.2004.05.017
Potucek, F. (2003). “Displacement and leaching – Two principal mechanisms of displacement washing of pulp,” Cellulose Chemistry and Technology 37, 141-152.
Potucek, F. (2005). “Chemical engineering view of the pulp washing,” Papir a Celuloza 60(4), 114-117.
Pougatch, K., Salcudean, M., Gartshore, I., and Pagoria, P. (2007). “Computational modelling of large aerated lagoon hydraulics,” Water Research 41(10), 2109-2116. DOI:10.1016/j.watres.2007.02.019
Prat, C., Vicente, M., and Esplugas, S. (1988). “Treatment of bleaching waters in the paper-industry by hydrogen-peroxide and ultraviolet-radiation,” Water Res. 22(6), 663-668. DOI: 10.1016/0043-1354(88)90176-5
Prat, C., Vicente, M., and Spuglas, S. (1989). “Ozonation of bleaching waters of the paper industry,” Water Res. 23(1), 51‑55. DOI: 10.1016/0043-1354(89)90060-2
Priha, M. H., and Talka, E. T. (1986). “Biological activity of bleached kraft mill effluent BKME fractions and process streams,” Pulp Paper Can. 87(12), 447‑449.
Pu, Y., Hu, F., Huang, F., and Ragauskas, A. J. (2015). “Lignin structural alterations in thermochemical pretreatments with limited delignification,” Bioenerg. Res. 8(3), 992‑1003. DOI: 10.1007/s12155-015-9655-5
Puro, L., Kallioinen, M., Mänttäri, M., Natarajan, G., Cameron, D. C., and Nyström, M. (2010). “Performance of RC and PES ultrafiltration membranes in filtration of pulp mill process waters,” Desalination 264(3), 249-255. DOI: 10.1016/j.desal.2010.06.034
Puustinen, J., and Uotila, J. (1994). “Environmental effects of ECF- and TCF-bleached pulp mill effluents. Part IV Occurrence of complexing agents in model ecosystems and in recipients,” in: M. Verta (ed.), Happikemikaalien Käyttöön Perustuvan Massan Valkaisun Ympäristövaikutuksia (in Finnish), Publications of the Water Environment Administration – Series A 189.
Qu, X., Gao, W. J., Han, M. N., Chen, A., and Liao, B. Q. (2012). “Integrated thermophilic submerged aerobic membrane bioreactor and electrochemical oxidation for pulp and paper effluent treatment – Towards system closure,” Bioresour. Technol. 116, 1-8. DOI: 10.1016/j.biortech.2012.04.045
Quezada, R., Silva, C. M., Passos Rezende, A. A., Nilsson, L., and Manfredi, M. (2014). “Membrane treatment of the bleaching plant (EPO) filtrate of a kraft pulp mill,” Water Sci. Technol. 70(5), 843-850. DOI: 10.2166/wst.2014.304
Ragona, C. S. F., and Hall, E. R. (1998). “Parallel operation of ultrafiltration and aerobic membrane bioreactor treatment systems for mechanical newsprint mill whitewater at 55 degrees C,” Water Sci. Technol. 38(4-5), 307-314. DOI: 10.1016/S0273-1223(98)00513-7
Rajeshwari, K. V., Balakrishnan, M., Kansal, A., Lata, K., and Kishore, V. V. N. (2000). “State of the art of anaerobic digestion technology for industrial wastewater treatment,” Renewable and Sustainable Energy Reviews 4(2), 135-156. DOI:10.1016/S1364-0321(99)00014-3
Rajvaidya, N., and Markandey, D. K. (1998). “Treatment of pulp and paper industrial effluent,” in: Advances in Environmental Science and Technology series, Vol. 7, A. P. H. Publishing, New Delhi, India, 113 pp.
Rämänen, H., Lassila, H., Lensu, A., Lahti, M., and Oikari, A. (2010). “Dissolution and spatial distribution of resin acids and retene in sediments contaminated by pulp and paper industry,” J. Soil Sediments 10(3), 349‑358. DOI: 10.1007/s11368-009-0179-5
Ramos, W. S., Poznyak, T., Chairez, I., and Córdova, I. (2009). “Remediation of lignin and its derivatives from pulp and paper industry wastewater by the combination of chemical precipitation and ozonation,” J. Hazard. Mater. 169(1‑3), 428‑434.
Rana, T., Gupta, S., Kumar, D., Sharma, S., Rana, M., Rathore, V. S., and Pereira, B. M. J. (2004). “Toxic effects of pulp and paper-mill effluents on male reproductive organs and some systemic parameters in rats,” Environ. Toxicol. Pharm. 18(1), 1‑7. DOI: 10.1016/j.etap.2004.04.005
Ranade, V. (2014). Industrial Wastewater Treatment, Recycling and Reuse, Oxford, UK.
Rankin, A., Van Aert, M., Welander, T., and Malmqvist, Ǻ. (2007). “Low sludge yield biofilm activated sludge (BAS) upgrade-Quesnel River Pulp,” Tappi 6(5), 17-22.
Rantio, T. (1997). “Chlorohydrocarbons in pulp mill effluents and their fate in the environment,” PhD thesis, University of Jyväskylä.
Rappe, C., Swanson, S., Glas, B., Kringstad, K., de Sousa, F., and Abe, Z. (1989). “On the formation of PCDDs and PCDFs in the bleaching of pulp,” Pulp Pap. Canada 90(8), T273‑T278.
Rasteiro, M. G., Garcia, F. A. P., Ferreria, P., Blanco, A., Negro, C., and Antunes, E. (2008). “The use of LDS as a tool to evaluation flocculation mechanisms,” Chem. Eng. Processing 47(8), 1323-1332. DOI: 10.1016/j.cep.2007.04.009
Ratnaweera, H., and Fettig, J. (2015). “State of the art of online monitoring and control of the coagulation process,” Water 7(11), 6574-6597. DOI: 10.3390/w7116574
Razali, M. A. A., Ahmad, Z., Ahmand, M. S. B., and Ariffin, A. (2011). “Treatment of pulp and paper mill wastewater with various molecular weight of polyDADMAC induced flocculation,” Chem. Eng. J. 166, 529-535. DOI: 10.1016/j.cej.2010.11.011
Rebarber, E. (1998). “Flotation save-all cell operations,” Tappi J. 81(5), 97-99.
Redenbach, A.E. (1997). “Sensory evaluation of fish exposed to pulp and paper mill effluent: A case study of method used for environmental effects monitoring,” Water Sci. Technol. 35(2‑3), 357‑363.
Reich, S., Jiminez, B., Marsili, L., Hernández, L. M., Schurig, V., and González, M. J. (1999). “Congener specific determination and enantiomeric ratios of chiral polychlorinated biphenyls in striped dolphins (Stenella coeruleoalba) from the Mediterranean Sea,” Environ. Sci. Technol. 33(11), 1787‑1793. DOI: 10.1021/es9807385
Renault, F., Sancey, B., Charles, J., Morin-crini, N., Badot, P.-M., Winterton, P., and Crini, G. (2009). “Chitosan flocculation of cardboard-mill secondary biological wastewater,” Chem. Eng. J. 155(3), 775-783. DOI: 10.1016/j.cej.2009.09.023
Renberg, L., Svanberg, O., Bengtsson, B. E., and Sundström, G. (1980). “Chlorinated guaiacols and catechols bioaccumulation potential in bleaks and reproductive and toxic effect on the harpacticoid Nitocra spinipes,” Chemosphere 9(3), 143‑150. DOI: 10.1016/0045-6535(80)90085-5
Rewcastle, M., Lebrun, T., Perrin, D., Antonelli, A., Taylor, T., and Churchley, J. (2004). “Full-scale trials of Degremont’s Biolysis® ‘O’ sludge minimization process on an activated sludge plant in the UK,” in: Proceedings of The Ninth European Biosolids and Wastes Conference, Wakefield, UK.
Rice, R. G. (1997). “Applications of ozone for industrial wastewater treatment – A review,” Ozone-Sci. Eng. 18(6), 477-515. DOI: 10.1080/01919512.1997.10382859
Richard, M. (2003). “Activated sludge microbiology problems and their control,” Presented at the 20th Annual USEPA National Operator Training Conference.
Richard, M., and Cummins, C. K. (1997). “Results of phosphorous minimization studies for pulp and paper mill activated sludge systems located around the Great Lakes,” Proceeding of the Tappi International Environmental Conference, Minneapolis, Minnesota, May 5-7; TAPPI Press, Atlanta, Georgia, pp. 1003-1006.
Richter, J. (1966). “Pulp washing by continuous diffusion,” Tappi 49(6), A48-49.
Riffat, R. (2013). Fundamentals of Wastewater Treatment and Engineering, CRC Press, Boca Raton, FL, USA.
Rigol, A., Latorre, A., Lacorte, S., and Barceló, D. (2004). “Bioluminescence inhibition assays for toxicity screening of wood extractives and biocides in paper mill process waters,” Environ. Toxicol. Chem. 23(2), 339‑347. ROI: 10.1897/02-632
Rintala, J. A., and Puhakka, J. A. (1994). “Anaerobic treatment in pulp and paper mill waste management: Review,” Bioresour. Technol. 47(1), 1-18. DOI: 10.1016/0960-8524(94)90022-1
Roberts, J. C., Au, C. O., Clay, G. A., and Lough, C. (1987). “A study of the effect of cationic starch on dry strength and formation using C14 labelling,” J. Pulp Paper Sci. 13(1), J1-J5.
Rodrigues, A. C., Boroski, M., Shimada, N. S., Garcia, J. C., Nozaki, J., and Hioka, N. (2008). “Treatment of paper pulp and paper mill wastewater by coagulation-flocculation followed by heterogeneous photocatalysis,” J. Photochem. Photobiol. A – Chem. 194(1), 1-10. DOI: 10.1016/j.jphotochem.2007.07.007
Rodriguez, J., Contreras, D., Parra, C., Freer, J., Baeza, J., and Duran, N. (1999). “Pulp mill effluent treatment by Fenton-type reactions catalyzed by iron complexes,” Water Sci. Technol. 40(11-12), 351-355. DOI: 10.1016/S0273-1223(99)00738-6
Rodrígues, J., Fuentes, S., Freer, J., Mansilla, H.-D., Ferraz, A., and Baeza, J. (1998). “Response to ozonation of different cellulose pulp bleaching effluents,” Environ. Technol. 19(1), 75-81. DOI: 10.1080/09593331908616657
Roest, K., Heilig, H. G. H. J., Smidt, H., de Vos, W. M., Stams, A. J. M., and Akkermans, A. D. L. (2005). “Community analysis of a full-scale anaerobic bioreactor treating paper mill wastewater,” Systematic Appl. Microbiol. 28(2), 175-185. DOI: 10.1016/j.syapm.2004.10.006
Rohella, R. S., Choudhury, S., Manthan, M., and Murthy, J. S. (2001). “Removal of color and turbidity in pulp and paper mill effluents using polyelectrolytes,” Indian Journal of Environment Health 43(4), 159-163.
Rovel, J. M., Trudel, J. P., Lavallée, P., and Schroeter, I. (1994). “Paper mill effluent treatment using biofiltration,” Water Science and Technology 29(10-11), 217-222.
Ruas, D. B., Chaparro, T. R., and Pires, E. C. (2012). “Advanced oxidation process H2O2/UV combined with anaerobic digestion to remove chlorinated organics from bleached kraft pulp mill wastewater,” Revista Facultad Ingen. – Univ. Antioquia 63, 43-54.
Rudolfs, W., and Amberg, H. R. (1953). “White water treatment: V. Aeration with nonflocculent growths,” Sewage and Industrial Wastes 25(1), 70-78.
Rueda-Márquez, J. J., Sillanpää, M., Pocostales, P., Acevedo, A., and Manzano, M. A. (2015) “Post-treatment of biologically treated wastewater containing organic contaminants using a sequence of H2O2 based advanced oxidation processes: Photolysis and catalytic wet oxidation” Water Res. 71(15), 85-96. DOI: 10.1016/j.watres.2014.12.054
Rusten, B., Mattsson, E., Roch-Due, A., and Westrum, T. (1994). “Treatment of pulp and paper industry wastewaters in novel moving bed biofilm reactors,” Water Science and Technology 30(3), 161-171.
Saarimaa, V., Sundberg, A., Holmbom, B., Blanco, A., Fuente, E., and Negro, C. (2006a). “Monitoring of dissolved air flotation by focused beam reflectance measurement,” Ind. Eng. Chem. Res. 45, 7256-7263. DOI: 10.1021/ie060250+
Saarimaa, V., Sundberg, A., Holmbom, B., Blanco, A., Negro, C., and Fuente, E. (2006b). “Purification of peroxide-bleached TMP water by dissolved air flotation,” Tappi J. 5(5), 15-21.
Safe, S. (1990). “Polychlorinated-biphenyls (PCBS), dibenzo-para-dioxins (PCDDS), dibenzofurans (PCDS), and related compounds – Environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFS),” Crit. Rev. Toxic. 21(1), 51-88. DOI: 10.3109/10408449009089873
Saha, M., Eskicioglu, C., and Marin, J. (2011). “Microwave, ultrasonic and chemo-mechanical pretreatments for enhancing methane potential of pulp mill wastewater treatment sludge,” Bioresour. Technol. 102(17), 7815-7826. DOI: 10.1016/j.biortech.2011.06.053
Saif, Y., Almansoori, A., and Elkamel, A. (2013). “Wastewater minimization in pulp and paper industries through energy-efficient reverse-osmosis membrane processes,” Chem. Eng. Technol. 35(3), 419-425. DOI: 10.1002/ceat.201200445
Saito, H., Sudo, M., Shigeoka, T., and Yamauchi, F. (1991). “In vitro cytotoxicity of chlorophenols to goldfish GF-scale (GSF) cells and quantitative structure-activity relationships,” Environ. Toxicol. Chem. 10(2), 235‑241. DOI: 10.1002/etc.5620100212
Sakurai, A., Yamomoto, T., Makabe, A., Kinoshita, S., and Sakakibara, M. (2001). “Removal of lignin in a liquid system by an isolated fungus,” Journal of Chemical Technology and Biotechnology 77(1), 9-14. DOI: 10.1002/jctb.519
Samuel, R., Pu, Y., Jiang, N., Fu, C., Wang, Z. Y., and Ragauskas, A. (2014). “Structural characterization of lignin in wild-type versus COMT down-regulated switchgrass,” Front. Energy Res. 1(14), 1‑9. DOI: 10.3389/fenrg.2013.00014
Sandell, L. S., and Luner, P. (1974). “Flocculation of microcrystalline cellulose with cationic ionene polymers,” J. Appl. Polymer Sci. 18(7), 2075-2083. DOI: 10.1002/app.1974.070180716
Sanusi, O., and Menzes, G. B. (2014). “Pulp and paper mill effluents management,” Water Environ. Res. 86(10), 1535-1544. DOI: 10.2175/106143014X14031280668137
Saritha, M., Arora, A., and Lata (2012). “Biological pretreatment of lignocellulosic substrates for enhanced delignification and enzyme digestibility,” Indian J. Microbiol. 52(2), 122‑130. DOI: 10.1007/s12088-011-0199-x
Särkkä, H., Kuhmonen, K., Vepsäläinen, M., Pulliainen, M., Selin, J., Rantala, P., Kukkamäki, E., and Sillanpää, M. (2009). “Electrochemical oxidation of sulphides in paper mill wastewater by using mixed oxide anodes,” Environ. Technol. 30(9), 885-892. Article Number: PII 913697971, DOI: 10.1080/09593330902927651
Sarkanen, K. V. (1971). Lignins: Occurrence, Formation, Structure and Reactions, Wiley-Interscience, New York, 916 pp.
Sarlin, T., Halttunen, S., Vuoriranta, P., and Puhakka, J. (1999). “Effects of chemical spills on activated sludge treatment performance in pulp and paper mills,” Water Science & Technology 40 (11-12) 319-325. DOI: 10.1016/S0273-1223(99)00734-9
Savant, D. V., Abdul-Rahman, R., and Ranade, D. R. (2006). “Anaerobic degradation of adsorbable organic halides (AOX) from pulp and paper industry wastewater,” Bioresour. Technol. 97(9), 1092-1104. DOI: 10.1016/j.biortech.2004.12.013
Saxena, N., and Gupta, R. K. (1998). “Decolourization and delignification of pulp and paper mill effluent by white rot fungi,” Indian Journal of Experimental Biology 36(10), 1049-1051.
Schechter, A., Fürst, P., Fürst, C., Groebel, W., Kolesnikov, S., Savchenkov, M., Beim, A., Boldonov, E., Trubitsun, E., and Vlasov B. (1990). “Levels of dioxins, dibenzofurans and other chlorinated xenobiotics in human milk from the Soviet Union,” Chemosphere 20(7‑9), 927‑934. DOI: 10.1016/0045-6535(90)90202-5
Schnell, A., Steel, P., Melcer, H., Hodson, P. V., and Carey, J. H. (2000a). “Enhanced biological treatment of bleached kraft mill effluents: I. Removal of chlorinated organic compounds and toxicity,” Water Research 34(2), 493-500. DOI:10.1016/S0043-1354(99)00161-X
Schnell, A., Steel, P., Melcer, H., Hodson, P. V., and Carey, J. H. (2000b). “Enhanced biological treatment of bleached kraft mill effluents: II. Reduction of mixed function oxygenase (MFO) induction in fish,” Water Res. 34(2), 501‑509. DOI: 10.1016/S0043-1354(99)00161-X
Schulze, H. (1882). J. Prakt. Chem. 25, 431-452. DOI: 10.1002/prac.18820250142
Scott, J. P., and Ollis, D. F. (1995). “Integration of chemical and biological oxidation processes for water treatment: Review and recommendations,” Environ. Prog. 14(2), 88-103. DOI: 10.1002/ep.670140212
Selvabharathi, G., and Kanmani, S. (2010). “Tertiary treatment of pulp and paper industrial wastewater by electro-Fenton process,” J. Environ. Sci. Eng. 52, 103-106.
Sepúlveda, M. S., Jonson, W. E., Higman, J. C., Denslow, N. D., Schoeb, T. R., and Gross, T. S. (2002). “An evaluation of biomarkers of reproductive function and potential contaminant effects in Florida largemouth bass (Micropterus salmoides floridanus) sampled from the St. Johns River,” Sci. Total Environ. 289(1), 133‑144. DOI: 10.1016/S0048-9697(01)01029-4
Sezgin, M., Jenkins, D., and Parker, D. S. (1978). “A unified theory of filamentous activated sludge bulking,” Journal of Water Pollution Control Federation 50(2), 362-381.
Shaw, L. E., and Lee, D. (2009). “Sonication of pulp and paper effluent,” Ultrason. Sonochem. 16(3), 321‑324. DOI: 10.1016/j.ultsonch.2008.10.011
Shimp, R. J., and Owens, J. W. (1993). “Pulp and paper technologies and improvements in environmental emissions to aquatic environments,” Toxicol. Environ. Chem. 40(1‑4), 213‑233.
Shankar, R., Singh, L., Mondal, P., and Chand, S. (2013). “Removal of lignin from wastewater through electro-coagulation,” World J. Environ. Eng. 1, 16-20.
Sheldon, M. S., Zeelie, P. J., and Edwards, W. (2012). “Treatment of paper mill effluent using an anaerobic/aerobic hybrid side-stream membrane bioreactor,” Water Sci. Technol. 65(7), 1265-1272. DOI: 10.2166/wst.2012.007
Shukla, B. (2012). “Benefits of paper mill white water recovery using backwash filtration system,” APPITA J. 65(1), 44-47.
Sierka, R. A., Cooper, S. P., and Pagoria, P. S. (1997). “Ultrafiltration and reverse osmosis treatment of an acid stage wastewater,” Water Sci. Technol. 35(2-3), 155-161. DOI: 10.1016/S0273-1223(96)00927-4
Sierraalvarez, R., Kortekaas, S., Vaneckert, M., and Lettinga, G. (1991). “The anaerobic biodegradability and methanogenic toxicity of pulping wastewaters,” Water Sci. Technol. 24(3-4), 113-125.
Simonič, M., and Vnučec, D. (2012). “Coagulation and UF treatment of pulp and paper mill wastewater in comparison,” Central Eur. J. Chem. 10(1), 127-136. DOI: 10.2478/s11532-011-0121-8
Simstich, B., Beimfohr, C., and Horn, H. (2012). “Lab scale experiments using a submerged MBR under thermophilic aerobic conditions for the treatment of paper mill deinking wastewater,” Bioresour. Technol. 112, 11-15. DOI: 10.1016/j.biortech.2012.04.029
Singh, P. (2007). “Sequential anaerobic and aerobic treatment of pulp and paper mill effluent in pilot scale bioreactor,” J. Environ. Biol. 28(1), 77‑82.
Singh, P., and Singh, A. (2004). “Physiochemical characteristics of the distillery effluent and its chemical treatment,” J. Nature Sci. Technol. 3(2), 205‑208.
Singh, P., and Srivastava, A. (2014). “Enzymatic colour removal of pulp and paper mill effluent by different fungal strains,” Int. J. Pharm. Bio. Sci. 5(3), (B) 773‑783.
Singh, P., and Thakur, I. S. (2006). “Colour removal of anaerobically treated pulp and paper mill effluent by microorganisms in two steps bioreactor,” Bioresource Technol. 97(2), 218‑223. DOI: 10.1016/j.biortech.2005.02.022
Singh, S. K., Kraemer, M., and Trebouet, D. (2012). “Studies on treatment of a thermo-mechanical process effluent from paper industry using ultrafiltration for water reuse,” Desalination Water Treat. 49, 208-217. DOI: 10.1080/19443994.2012.719320
Singhal, A., and Thakur, I. S. (2009). “Decolourization and detoxification of pulp and paper mill effluent by Emericella nidulans var. nidulans,” Biochem. Eng. J. 171, 619-625. DOI: 10.1016/j.jhazmat.2009.06.041
Sinkkonen, S. (1997). “PCDTs in the environment,” Chemosphere 34(12), 2585‑2594. DOI: 10.1016/s0045-6535(97)00101-x
Skouteris, G., Hermosilla, D., López, P., Negro, C., and Blanco, A. (2012). “Anaerobic membrane bioreactors for wastewater treatment: A review,” Chem. Eng. J. 198-199, 138-148. DOI: 10.1016/j.cej.2012.05.070
Smet, E., Lens, P., and van Langenhove, H. (1998). “Treatment of waste gases contaminated with odorous sulfur compounds,” Crit. Rev. Environ. Sci. Technol. 28(1), 89-117. DOI: 10.1080/10643389891254179
Smith, C. V., Gregorio, D. D., and Talcott, R. M. (1969). “The use of ultrafiltration membranes for activated sludge separation,” Proc. 24th Annual Purdue Industrial Waste Conf., Lafayette, IN, pp. 1300-1310.
Smith, J. A., and Novak, J. T. (1987). “Biodegradation of chlorinated phenols in subsurface soils,” Water Air Soil Poll. 33(1), 29-42. DOI: 10.1007/BF00191375
Soloman, P. A., Basha, C. A., Velan, M., Balasubramanian, N., and Marimuthu, P. (2009). “Augmentation of biodegradability of pulp and paper industry wastewater by electrochemical pre-treatment and optimization by RSM,” Sep. Purif. Technol. 69(1), 109-117. DOI: 10.1016/j.seppur.2009.07.002
Souza, E. S., Souza, J. V. B., Silva, F. T., and Paiva, T. C. B. (2014). “Treatment of an ECF bleaching effluent with white-rot fungi in an air-lift bioreactor,” Environ. Earth Sci. 72(4), 1289-1294. DOI: 10.1007/s12665-014-3048-5
Spellman, F. R. (2014). Handbook of Water and Wastewater Treatment Plant Operations, CRC Press, Boca Raton, FL, USA.
Sprague, J. B., Bonsor, N., and McCubbin, N. (1991). “Effluents from non-kraft pulp and paper mills in Ontario,” Technical Advisory Committee Pulp and Paper Sector of MISA, Ministry of the Environment, Canada, Toronto.
Stahlschmidt-Allner, P., Allner, B., Römbke, J., and Knacker, T. (1997). “Endocrine disrupters in the aquatic environment,” Environ. Sci. Pollut. Res. 4(3), 155‑162. DOI: 10.1007/BF02986325
Stenius, P., and Laine, J. (1994). “Studies on cellulose surfaces by titration and ESCA,” Appl. Surf. Sci. 75, 213-219. DOI: 10.1016/0169-4332(94)90161-9
Stevens, W. V. (1975). “Disk savaeall in secondary fiber systems,” Tappi 58(4), 88-89.
Stephenson, R. J., and Duff, S. J. B. (1996). “Coagulation and precipitation of a mechanical pulping effluent. 1. Removal of carbon, colour and turbidity,” Water Res. 30(4), 781-792. DOI: 10.1016/0043-1354(95)00213-8
Stephenson, R. J., Laliberte, S., Hoy, P. M., and Britch, D. (2007). “Full scale and laboratory scale results from the trial of MicroSludge at the Joint Water Pollution Control Plant at Los Angeles County,” Water Practice 1(4), 739-745. DOI: 10.2175/193317707X243391
Stratton, S. C., Gleadow, P. L., and Johnson, A P. (2004). “Pulp mill process closure: A review of global technology developments and mill experiences in the 1990s,” Water Sci. Technol. 50(3), 183-194.
Strazdins, E. (1986). “The chemistry of alum in papermaking,” Tappi J. 69(4), 111-114.
Strazdins, E. (1989). “Theoretical and practical aspects of alum use in papermaking,” Nordic Pulp Paper Res. J. 4(2), 128-134. DOI: 10.3183/NPPRJ-1989-04-02-p128-134
Sun, Y. B., Joyce, T. W., and Chang, H. M. (1989). “Dechlorination and decolorization of high molecular weight chlorolignins from bleach plant effluents by an oxidation process,” Tappi J. Sept, 209‑213.
Sundberg, A., Pranovich, A., and Holmbom, B. (2000). “Distribution of anionic groups in TMP suspensions,” J. Wood Chem. Technol. 20(1), 71-92. DOI: 10.1080/02773810009349625
Suntio, L. R., Shiu, W. Y., and Mackay, D. A. (1988). “A review of the nature and properties of the chemicals present in pulp and paper mill effluents,” Chemosphere 17(7), 1249‑1290. DOI: 10.1016/0045-6535(88)90080-X
Suvilampi, J., Lepisto, R., and Rintala, J. (2001). “Biological treatment of pulp and paper mill process and wastewaters under thermophilic conditions – A review,” Paperi ja Puu 83(4), 320-325.
Swann, C. E. (1998). “Water chemistry: Dealing with a cluster of rules,” PIMA’s Papermaker 80(10), 30-33.
Tabatabaei, M., Rahim, R. A., Abdullah, N., Wright, A.-D. G., Shirai, Y., Sakai, K., Sulaiman, A., and Hassan, M. A. (2010). “Importance of the methanogenic archaea populations in anaerobic wastewater treatments,” Process Biochem. 45(8), 1214-1225. DOI: 10.1016/j.procbio.2010.05.017
Takahashi, M., Kawamura, T., Yamamoto, Y., Ohnari, H., Himuro, S., and Shakutsui, H. (2003). “Effect of shrinking micro-bubble on gas hydrate formation,” Journal of Physical Chemistry B 107, 2171-2173. DOI: 10.1021/jp022210z
Tambosi, J. L., Di Domenico. M., Schirmer, W. N., Jose, H. J., and Moreira, R. (2006). “Treatment of paper and pulp wastewater and removal of odorous compounds by a Fenton-like process at the pilot scale,” J. Chem. Technol. Biotechnol. 81, 1426-1432. DOI: 10.1002/jctb.1583
Tana, J., and Lehtinen, K.-J. (1996). “The aquatic environmental impact of pulping and bleaching operations –an overview,” The Finnish Environment Institute, Edita, Finland, Helsinki.
Tanaka, H., Itakura, S., and Enoki, A. (1999). “Hydroxyl radical generation by an extracellular low-molecular -weight substance and phenol oxidase activity during wood degradation by the white-rot basidiomycete Trametes versicolor,” J. Biotechnol. 75(1), 57‑70. DOI: 10.1016/s0168-1656(99)00138-8
Tarlan, E., Dilek, F. B., and Yetis, U. (2002a). “Effectiveness of algae in the treatment of a wood-based pulp and paper industry wastewater,” Bioresour. Technol. 84(1), 1-5. DOI: 10.1016/S0960-8524(02)00029-9
Tarlan, E., Yetis, Ü., and Dilek, F. B. (2002b). “Algal treatment of pulp and paper industry wastewaters in SBR systems,” Water Sci. Technol. 45(12), 151-158.
Tartakovsky, B., Cimpoia, T., Petti, L, Lo, A., Liard, A., and Guiot, S. R. (2003). “Biodegradation of spent pulping liquor lignins under mesophilic and thermophilic anaerobic conditions,” TAPPI J. 2(4), 26-32.
Taseli, B., and Gökçay, C. F. (1999). “Biological treatment of paper pulping effluents by using a fungal reactor,” Water Science and Technology 40(11-12), 93-99. DOI:10.1016/S0273-1223(99)00705-2
Tavares, C. R., Vieira, M., Petrus, J. C. C., Bortoletto, E. C., and Ceravollo, F. (2002). “Ultrafiltration/complexation process for metal removal from pulp and paper industry wastewater,” Desalination 144, 261-265. Article no. PII S0011-9164(02)00325-9. DOI: 10.1016/S0011-9164(02)00325-9
Taylor, B. R., Goudey, J. S., Carmichael, N. B. (1996). “Toxicity of aspen wood leachate to aquatic life: Laboratory studies,” Environ. Tox. Chem. 15(2), 150-159. DOI: 10.1897/1551-5028(1996)015<0150:TOAWLT>2.3.CO;2
Teerikangas, E. (2001). “Controlling solid separation with new flotation techniques,” Water 21(Feb.), 55-61.
Temmink, J. H. M., Field, J. A., Haastrecht, J. C., and van Merkelbach, R. C. M. (1989). “Acute and sub-acute toxicity of bark tannins in carp (Cyprinus carpio L.),” Water Res. 23(3), 341‑344. DOI: 10.1016/0043-1354(89)90100-0
Tenno, R., and Paulapuro, H. (1999). “Removal of dissolved organic compounds from paper machine whitewater by membrane bioreactors: A comparative analysis,” Control Eng. Pract. 7(9), 1085-1099. DOI: 10.1016/S0967-0661(99)00079-9
Thakur, I. S. (1995). “Structural and functional characterization of a stable, 4 chlorosalicylic acid degrading, bacterial community in a chemostat,” World J. Microbial. Biotechnol. 11(6), 643‑645. DOI: 10.1007/BF00361007
Thakur, I. S. (2004). “Screening and identification of microbial strains for removal of colour and adsorbable organic halogens in pulp and paper mill effluent,” Process Biochem. 39(11), 1693-1699. DOI: 10.1016/S0032-9592(03)00303-0
Thompson, G., Swain, J., Kay, M., and Forster, C. F. (2001). “The treatment of pulp and paper mill effluent: A review,” Bioresource Technology 77(3), 275-286. DOI:10.1016/S0960-8524(00)00060-2
Tielbaard, M., Wilson, T, Feldbaumer, E., and Driessen, W. (2002). “Full-scale anaerobic treatment experiences with pulp mill evaporator condensates,” Proc. TAPPI Environmental Conf., TAPPI Press, Atlanta.
Tiku, D. K., Kumar, A., Chaturvedi, R., Makhijani S. D., Manoharan, A., and Kumar, R. (2010). “Holistic bioremediation of pulp mill effluents using autochthonous bacteria,” Int. Biodeter. Biodegrad. 64(3), 173-183. DOI: 10.1016/j.ibiod.2010.01.001
Tiku, D. K., Kumar, A., Sawhney, S., Singh, V. P., and Kumar, R. (2007). “Effectiveness of treatment technologies for wastewater pollution generated by Indian pulp mills,” Environ. Monit. Assess. 132, 453-466. DOI: 10.1007/s10661-006-9548-3
Torrades, F., Pérez, M., Mansilla, H. D., and Peral, J. (2003). “Experimental design of Fenton and photo-Fenton reactions for the treatment of cellulose bleaching effluents,” Chemosphere 53(10), 1211‑1220. DOI: 10.1016/s0045-6535(03)00579-4
Tripathi, C. S., and Allen, D. G. (1999). “Comparison of mesophilic and thermophilic aerobic biological treatment in sequencing batch reactors treating bleached kraft pulp mill effluent,” Water Res. 33(3), 836-846. DOI: 10.1016/S0043-1354(98)00260-7
Tripathi, S., Pathak, V., Tripathi, D. M., and Tripathi, B. D. (2011). “Application of ozone based treatments of secondary effluents,” Bioresour. Technol. 102(3), 2481-2486. DOI: 10.1016/j.biortech.2010.11.028
Tuhkanen, T., Naukkarinen, M., Blackburn, S., and Tanskanen, H. (1997). “Ozonation of pulp mill effluent prior to activated sludge treatment,” Environ. Technol. 18(10), 1045‑1051. DOI: 10.1080/09593331808616624
Turrens, J. F. (2003). “Mitochondrial formation of reactive oxygen species,” J. Physiol. (Lond.) 552 (Pt 2), 335-344. DOI:10.1113/jphysiol.2003.049478
Tziotzios, G., Teliou, M., Kaltsouni, V., Lyberatos, G., and Vayenas, D. V. (2005). “Biological phenol removal using suspended growth and packed bed reactors,” Biochem. Eng. J. 26(1), 65-71. DOI: 10.1016/j.bej.2005.06.006
Uğurlu, M., Gürses, A., Doğar, Ç., and Yalçin, M. (2008). “The removal of lignin and phenol from paper mill effluents by electrocoagulation,” J. Environ. Manag. 87(3), 420-428. DOI: 10.1016/j.jenvman.2007.01.007
Uğurlu, M., and Karaoğlu, M. H. (2009). “Removal of AOX, total nitrogen and chlorinated lignin from bleached kraft mill effluents by UV oxidation in the presence of hydrogen peroxide utilizing TiO2 as photocatalyst,” Environ. Sci. Pollution Res. 16(3), 265-273. DOI: 10.1007/s11356-008-0044-x
UNEP (1995). UNEP/GC, United Nations Environment Programme, 18-32.
USEPA (1998). Clean Water Action Plan: Restoring and Protecting America’s Waters, US Environmental Protection Agency and U.S. Department of Agriculture, EPA-840-R-98-001, Washington.
Vallejo, M., Fernández-Castro, P., San Román, M. F., and Ortiz, I. (2015). “Assessment of PCDD/Fs formation in the Fenton oxidation of 2-chlorophenol: Influence of the iron dose applied,” Chemosphere 137, 135‑141. DOI: 10.1016/j.chemosphere.2015.06.056
Van den Berg, M., Birnbaum, L. S., Denison, M., De Vito, M., Farland, W., Feeley, M., Fiedler, H., Hakansson, H., Hanberg, A., Haws, L., Rose, M., Safe, S., Schrenk, D., Tohyama, C., Tritscher, A., Tuomisto, J., Tysklind, M., Walker, N., and Peterson, R. E. (2006). “The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds,” Toxicol. Sci. 93(2), 223‑241. DOI: 10.1093/toxsci/kfl055
Vepsäläinen, M., Kivisaari, H., Pulliainen, M., Oikari, A., and Sillanpää, M. (2011a). “Removal of toxic pollutants from pulp mill effluents by electrocoagulation,” Sep. Purif. Technol. 81(2), 141-150. DOI: 10.1016/j.seppur.2011.07.017
Vepsäläinen, M., Selin, J., Rantala, P., Pulliainen, M., Särkkä, H., Kuhmonen, K., Bhatnagar, A., and Sillanpaa, M. (2011b). “Precipitation of dissolved sulphide in pulp and paper mill wastewater by electrocoagulation,” Environ. Technol. 32(12), 1393-1400. DOI: 10.1080/09593330.2010.536790
Verenich, S., Laari, A., and Kallas, J. (2000). “Wet oxidation of concentrated wastewaters of paper mills for water cycle closing,” Waste Manag. 20(4), 287-293. DOI: 10.1016/S0956-053X(99)00308-6
Verenich, S., Laari, A., and Kallas, J. (2001). “Combination of coagulation and catalytic wet oxidation for the treatment of pulp and paper mill effluents,” Water Science and Technology 44(5), 145-152.
Vetter, W., and Maruya, K. A. (2000). “Congener and enantioselective analysis of toxaphene in sediment and food web of a contaminated estuarine wetland,” Environ. Sci. Technol. 34(9), 1627‑1635. DOI: 10.1021/es991175f
Vice, K., and Carroll, R. (1998). “The Cluster rule: A summary of phase 1,” TAPPI J. 81(2), 91-98.
Vice, K., Trepte, R., Johnson, T., and Carroll, B. (1996). “EPA announces framework for Cluster Rule effluent limitations guidelines,” TAPPI J. 79(12), 42-46.
Vidal, G., Soto, M., Field, J., Mendez, P. R., and Lema, J. H. (1997). “Anaerobic biodegradability and toxicity of wastewaters from chlorine and chlorine-free bleaching of eucalyptus kraft pulps,” Water Res. 31(10), 2487-2494. DOI: 10.1016/S0043-1354(97)00113-9
Vidal, G., Videla, S., and Diez, M. C., (2001). “Molecular weight distribution of Pinus radiata kraft mill wastewater treated by anaerobic digestion,” Bioresource Technol. 77(2), 183‑191. DOI: 10.1016/S0960-8524(00)00141-3
Vilve, M., Hirvonen, A., and Sillanpää, M. (2009). “Effects of reaction conditions on nuclear laundry water treatment in Fenton process, J. Hazard. Mater. 164(2‑3), 1468‑1473. DOI: 10.1016/j.jhazmat.2008.09.058
Virkki, L., Knuutinen, J., and Hyötyläinen, J. (1994). “Characterization of chlorolignins in bleached kraft pulp mill effluents using elemental analysis and fingerprinting by CuO oxidation and HPLC,” Int. J. Environ. Anal. Chem. 56(2), 133‑147. DOI: 10.1080/03067319408039801
Vishtal, A., and Kraslawski, A. (2011). “Challenges in industrial applications of technical lignins,” BioResources 6(3), 3547-3568.
Voss, R. H., and Rapsomatiotis, A. (1985). “An improved solvent-extraction based procedure for the gas chromatographic analysis of resin and fatty acids in pulp mill effluents,” J. Chromatogr. A 346, 205‑214. DOI: 10.1016/S0021-9673(00)90506-5
Vymazal, J. (2009). “The use constructed wetlands with horizontal sub-surface flow for various types of wastewater,” Ecological Eng. 35(1), 1-17. DOI: 10.1016/j.ecoleng.2008.08.016.
Walden, C. C., and Howard, T. E. (1981). “Toxicity of pulp and paper mill effluents – Review,” Pulp Paper Canada 82(4), 115-116, 118, 121, 123-124.
Wagner, M., and Nicell, J. A. (2001). “Treatment of a foul condensate from kraft pulping with horseradish peroxidase and hydrogen peroxide,” Water Res. 35(2), 485-495. DOI: 10.1016/S0043-1354(00)00276-1
Wang, B., Gu, L., and Ma, H. Z. (2007). “Electrochemical oxidation of pulp and paper making wastewater assisted by transition metal modified kaolin,” J. Hazard. Mater. 143, 198-205. DOI: 10.1016/j.jhazmat.2006.09.013
Wang, I. C., and Pan, T. T. (1999). “Interference of some papermaking chemical additives in the coagulation of wastewater,” Taiwan Journal of Forest Science 14(4), 367-384.
Wang, J. P., Chen, Y. Z., Wang, Y., Yuan, S. J., and Yu, H. Q. (2011). “Optimization of the coagulation-flocculation process for pulp mill wastewater treatment using a combination of uniform design and response surface methodology,” Water Res. 45(17), 5633-5640. DOI: 10.1016/j.watres.2011.08.023
Wang, J. P., Chen, Y. Z., Yuan, S. J., Sheng, G. P. and Yu, H. Q. (2009). “Synthesis and characterization of a novel cationic chitosan-based flocculant with a high water-solubility for pulp mill wastewater treatment,” Water Research 43(20), 5267-5275. DOI: 0.1016/j.watres.2009.08.040
Wang, K. Q., Chen, J.-P., Chen, L., Wu, X.-F., Su, X. J., Amartey, S., and Qin, W. (2014). “Isolation and irradiation-modification of lignin specimens from black liquor and evaluation of their effects on wastewater purification,” BioResources 9(4), 6476-6489. DOI: 10.15376/biores.9.4.6476-6489
Wang, L., Yan, W., Chen, J., Huang, F., and Gao, P. (2008a). “Function of the iron-binding chelator produced by Coriolus versicolor in lignin biodegradation,” Sci. China C. Life Sci. 51(3), 214-221. DOI: 10.1007/s11427-008-0033-9
Wang, S. T., Ma, J., Liu, B. C., Jiang, Y. F., and Zhang, H. Y. (2008b). “Degradation characteristics of secondary effluent of domestic wastewater by combined process of ozonation and biofiltration,” J. Hazard Mater. 150(1), 109-114. DOI: 10.1016/j.jhazmat.2007.04.092
Wanner, J. (1994). Activated Sludge Bulking and Foaming Control, Technomic Publishing Co., Basel, Switzerland, p. 327.
Waye, A., Annal, M., Tang, A., Picard, G., Harnois, F., Guerrero-Analco, J. A., Saleem, A., Hewitt, L. M., Milestone, C. B., Maclatchy, D. L., Trudeau, V. L., and Arnason, J. T. (2014). “Canadian boreal pulp and paper feedstocks contain neuroactive substances that interact in vitro with GABA and dopaminergic systems in the brain,” Sci. Total Environ. 468–469, 315-325. DOI: 10.1016/j.scitotenv.2013.08.040
Webb, L. (2002). “Kidney technology brings success,” Pulp Paper Int. 48(4) 28-32.
Weiland, P. (2010). “Biogas production: Current state and perspectives,” Appl. Microbiol. Biotech. 85(4), 849-860. DOI: 10.1007/s00253-009-2246-7
Welander, T., Lofqvist, A., and Selmer, A. (1997). “Upgrading aerated lagoons at pulp and paper mills,” Water Science and Technology 35(2-3), 117-122. DOI:10.1016/S0273-1223(96)00922-5
Wesenberg, D., Kyriakides, I., and Agathos, S. N. (2003). “White-rot fungi and their enzymes for the treatment of industrial dye effluents,” Biotechnol. Advan. 22, 161-187. DOI: 10.1016/j.biotechadv.2003.08.011
WHO (1997). in: IARC Monographs on the Evaluation of Carcinogenic Risk to Humans, Vol. 69, International Agency for Research on Cancer, World Health Organization, Lyon, France.
Wiberg, K., Lundström, K., Glas, B., and Rappe, C. (1989). “PCDDs and PCDFs in consumers´ paper products,” Chemosphere, 19(1‑6), 735‑740. DOI: 10.1016/0045-6535(89)90400-1
Wiegand, P., Unwin, J., Cook, D., and Ebert, E. (2006). “A review of contemporary topic on dioxin and pulp and paper industry effluents and wastewater residuals,” NCASI Special Report, No. 06-08, pp. 1-79.
Willett, K. L., Ulrich, E. M., and Hites, R. A. (1998). “Differential toxicity and environmental fates of hexachlorocyclohexane isomers,” Environ. Sci. Technol. 32(15), 2197‑2207. DOI: 10.1021/es9708530
Willfor, S., Sjoholm, R., Laine, C., Roslund, M., Hemming, J., and Holmbom, B. (2003). “Characterisation of water-soluble galactoglucomannans from Norway spruce wood and thermomechanical pulp,” Carbohyd. Polym. 52(2), 175-187. DOI: 10.1016/S0144-8617(02)00288-6
Wilson, A. E. J., Moore, E. R. B., and Mohn, W. W. (1996). “Isolation and characterization of isopimaric acid degrading bacteria from sequencing batch reactor,” Appl. Environ. Microbiol. 62(9), 3146‑3151.
Woldai, A. (2016). Multi-stage Fash Desalination: Modeling, Simulation, and Adaptive Control, CRC Press, Boca Raton, FL, USA, 322 pp.
Wolfaardt, F. (1994). “Treatment of bleach-plant effluents by lignin-degrading fungi: Review,” Paper Southern Africa 14(3), 8, 10.
Wong, S. S., Teng, T. T., Ahmad, A. L., Zuhairi, A., and Najafpour, G. (2006). “Treatment of pulp and paper mill wastewater by polyacrylamide (PAM) in polymer induced flocculation,” J. Hazard. Mater. B 135, 378-388. DOI: 10.1016/j.jhazmat.2005.11.076
Wu, J., Xiao, Y.-Z., and Yu, H.-Q. (2005). “Degradation of lignin in pulp mill wastewaters by white-rot fungi on biofilm,” Bioresour. Technol. 96(12), 1357-1363. DOI: 10.1016/j.biortech.2004.11.019
Yamamoto, K., Hiasa, M., Mahmood, T., and Matsuo, T. (1989). “Direct solid-liquid separation using hollow fiber membrane in an activated sludge aeration tank,” Water Sci. Technol. 21, 43-54.
Yap, R., Holmes, M., Peirson, W., Whittaker, M., Stuetz, R., Jefferson, B., and Henderson, R. (2012). “Optimising dissolved air flotation/filtration treatment of algae-laden lagoon effluent using surface charge: A Bolivar treatment plant case study,” Water Sci. Technol. 66(8), 1684-1690. DOI: 10.2166/wst.2012.373
Yeber, M. C., Rodríguez, J., Baeza, J., Freer, J., Zaror, C., Durán, N., and Mansilla, H. D. (1999a). “Toxicity abatement and biodegradability enhancement of pulp mill bleaching effluent by advanced chemical oxidation,” Water Sci. Technol. 40, 337-342. DOI: 10.1016/S0273-1223(99)00736-2
Yeber, M. C., Rodríguez, J., Freer, J., Baeza, J., Durán, N., and Mansilla, H. D. (1999b). “Advanced oxidation of a pulp mill bleaching wastewater,” Chemosphere 39(10), 1679-1688. DOI: 10.1016/S0045-6535(99)00068-5
Yeber, M. C., Rodríguez, J., Freer, J., Duran, N., and Mansilla, H. D. (2000). “Photocatalytic degradation of cellulose bleaching effluent by supported TiO2 and ZnO,” Chemosphere 41, 1193-1197. DOI: 10.1016/S0045-6535(99)00551-2
Yen, N. T., Oanh, N. T. K., Reutergard, L. B., Wise, D. L., and Lan, L. T. T. (1996). “An integrated waste survey and environmental effects of COGIDO, a bleached pulp and paper mill in Vietnam on the receiving water body,” Resour. Conserv. Recy. 18(1‑4), 161‑173.
Yerushalmi, L. (2014). “Drinking water treatment using the combined ozonation and membrane ultrafiltration processes,” Proceedings of the “Ozone: Proven Solution to Emerging Challenges” conference, International Ozone Association-Pan America Group, August 23-27, Montreal, Canada.
Yerushalmi, L., Bani Shahabadi, M., and Haghighat, F. (2011). “Effect of process parameters on greenhouse gas generation by wastewater treatment plants,” Wat. Environ. Res. 83(5), 440-449. DOI: 10.2175/106143010X12851009156321
Yerushalmi, L., Ashrafi, O., and Haghighat, F. (2013). “Reductions in greenhouse gas (GHG) generation and energy consumption in wastewater treatment plants,” Wat. Sci. Technol. 67(5), 1159-1164. DOI: 10.2166/wst.2013.681
Yilmaz, T., Yuceer, A., and Basibuyuk, M. (2008). “A comparison of the performance of mesophilic and thermophilic anaerobic filters treating papermill wastewater,” Bioresour. Technol. 99(1), 156-163. DOI: 10.1016/j.biortech.2006.11.038
Yuan, R. S., Guan, R. B., Liu, P., and Zheng, J. T. (2007). “Photocatalytic treatment of wastewater from paper mill by TiO2 loaded on activated carbon fibers,” Colloids Surf. A – Physicochem. Eng. Aspects 293, 80-86. DOI: 10.1016/j.colsurfa.2006.07.010
Yunker, M. B., Cretney, W. J., and Ikonomou, M. G. (2002). “Assessment of chlorinated dibenzo-p-dioxin and dibenzofuran trends in sediment and crab hepatopancreas from pulp mill and harbor sites using multivariate- and index-based approaches,” Environ. Sci. Technol. 36(9), 1869‑1878. DOI: 10.1021/es0112893
Zaidi, A., Buisson, H., Sourirajan, S., and Wood, H. (1992). “Ultra-filtration and nano-filtration in advanced effluent treatment schemes for pollution control in the pulp and paper industry,” Water Sci. Technol. 25(10), 263-276.
Žarković, D. B., Todorović, Z. N., and Rajaković, L. V. (2011). “Simple and cost-effective measures for the improvement of paper mill effluent treatment – A case study,” J. Cleaner Prodn. 19(6-7), 764-774. DOI: 10.1016/j.jclepro.2010.11.015
Zhang, C., Chen, J., and Wen, Z. (2012). “Alternative policy assessment for water pollution control in China’s pulp and paper industry,” Resour. Conserv. Recycl. 66, 15-26. DOI: 10.1016/j.resconrec.2012.06.004
Zhang, Y. Z., Ma, C. M., Ye, F., Kong, Y., and Li, H. (2009). “The treatment of wastewater of paper mill with integrated membrane process,” Desalination 236, 349-356. DOI: 10.1016/j.desal.2007.10.086
Zhao, H., Hu, C. Z., Liu, H. J., Zhao, X., and Zu, J. H. (2008). “Role of aluminum speciation in the removal of disinfection byproduct precursors by a coagulation process,” Environ. Sci. Technol. 42(15), 5752-5758. DOI: 10.1021/es8006035
Zhao, L. J., Zhang, Y. F., Hong, J. F., and Tu, W. W. (2014). “Papermaking wastewater treatment – A brief review,” Advan. Mater. Res. 926-930, 4276-4279. DOI: 10.4028/www.scientific.net/AMR.926-930.4276
Zhao, Y., Xi, D., and Chen, S. (1995). “Diatomaceous silica filter aid filtration for the effective separation of colloidal Cr(OH)-3 precipitate from tanning wastewater,” J. Environ. Sci. 7(2), 151-156.
Zhou, H., and Smith, D. W. (1997). “Process parameter development for ozonation of Kraft pulp mill effluents,” Water Sci. Technol. 35(2‑3), 251‑259.
Zhu, X. L., Wang, J., Jiang, Y. L., Cheng, Y. J., Chen, F., and Ding, S. B. (2012). “Feasibility study on satisfying standard of water pollutants for pulp and paper industry,” Appl. Mech. Mater. 178–181, 637-640. DOI: 10.4028/www.scientific.net/AMM.178-181.637
Zodi, S., Louvet, J. N., Michon, C., Potier, O., Pons, M.-N., Lapicque, F., and Leclerc, J. P. (2011). “Electrocoagulation as a tertiary treatment for paper mill wastewater: Removal of non-biodegradable organic pollution and arsenic,” Separ. Purif. Technol. 81(1), 62-68. DOI: 10.1016/j.seppur.2011.07.002
DOI: 10.15376/biores.11.3.Hubbe
Table A. Wastewater Treatment Systems and their Performance for Pulp and Paper Mill Effluents