Abstract
Wet-laid forming, which can be regarded as being analogous to conventional papermaking processes but with use of chopped synthetic or staple fibers, continues to draw attention as an advantageous way to prepare advanced nonwoven textile products. This review of the literature considers scientific advances in the field, with emphasis placed on applications involving cellulosic fibers as a significant component of the product. Some primary challenges with respect to wet-laid processing concern the dispersion of the synthetic fibers in aqueous media and methods for avoiding their subsequent entanglement. Both mechanical and chemical strategies have been employed in order to achieve well-formed sheets of high uniformity and binding among the fibers to meet a variety of end-use specifications. The incorporation of cellulosic fibers has been shown to facilitate fiber dispersion and to impart certain beneficial characteristics and properties to wet-laid fabrics. The contrasting attributes of synthetic and cellulosic fibers contribute to some unique challenges during the processing of their mixtures during wet-laid forming.
Download PDF
Full Article
Wet-Laid Nonwovens Manufacture – Chemical Approaches Using Synthetic and Cellulosic Fibers
Martin A. Hubbe,a,* and Alexander A. Koukoulas b
Wet-laid forming, which can be regarded as being analogous to conventional papermaking processes but with use of chopped synthetic or staple fibers, continues to draw attention as an advantageous way to prepare advanced nonwoven textile products. This review of the literature considers scientific advances in the field, with emphasis placed on applications involving cellulosic fibers as a significant component of the product. Some primary challenges with respect to wet-laid processing concern the dispersion of the synthetic fibers in aqueous media and methods for avoiding their subsequent entanglement. Both mechanical and chemical strategies have been employed in order to achieve well-formed sheets of high uniformity and binding among the fibers to meet a variety of end-use specifications. The incorporation of cellulosic fibers has been shown to facilitate fiber dispersion and to impart certain beneficial characteristics and properties to wet-laid fabrics. The contrasting attributes of synthetic and cellulosic fibers contribute to some unique challenges during the processing of their mixtures during wet-laid forming.
Keywords: Chopped synthetic fibers; Staple fibers; Dispersion; Colloidal stability; Entanglement; Cellulosic fibers; Uniformity of formation; Product performance
Contact information: a: Department of Forest Biomaterials, North Carolina State University, Box 8005, Raleigh, NC 27695-8005 USA; b: ANL Consultants LLC, P.O. Box 2981, Savannah, GA 31408-2981 USA;
* Corresponding author: hubbe@ncsu.edu
Contents of Article

This article reviews chemical aspects of the intriguing field of wet-laid nonwoven fabric manufacture. The wet-laid process – in its various forms – makes it possible to achieve cloth-like characteristics without weaving and without use of continuous filaments or threads. It offers high production speeds, great flexibility regarding the blending of different fiber types, and opportunities to achieve uniformity in the product.
There are strong similarities between wet-laid technology and conventional papermaking. According to the Association of Nonwovens Fabrics Industry (INDA), a nonwoven product is a sheet of fibers that are bonded without weaving and without hydrogen bonding being the primary bonding mechanism (Kozak 1991). A key point of differentiation between papermaking and wet-laid nonwoven forming is that wet-laid systems employ a substantial content of fibers having lengths much greater than 3 mm, the typical length of fibers in softwood pulp (Nanko et al. 2005). Also, most nonwoven fibers are made mainly from chopped synthetic fibers or staple fibers, such as glass, polypropylene, or polyester, among others. A wide variety of cellulosic fibers are used in various wet-laid products, with particular scholarly interest having been shown for flax (Fages et al. 2012, 2013; Maity et al. 2014), cotton (Hervey et al. 1975; Farer et al. 1998), regenerated cellulose fibers (Wilke 1989; Johnson 1996; Maity et al. 2014), and wood pulp fibers (Gill et al. 1972; Schon 1977; Yang and Dean 1978a; Inagaki 1979). In wet-laid processing the fibers are initially suspended in an aqueous solution and then formed into a sheet on a moving screen, where the water is removed. To put things into perspective, it is much more common to prepare nonwoven fabric products – especially bulky nonwoven fabrics – without the use of water, using so-called “air-laid” or “dry-formed” processes; such approaches have been reviewed by others (Lichstein 1988; Turbak 1993; Russell 2007). There also have been some useful reviews of various aspects of the wet-laid nonwoven process (Fredericks 1976; Schoffmann 1980; Lichstein 1988; Turbak 1993; Williamson 1993). Table 1 lays out some typical ranges of fiber length, machine width, line speed, and basis weight for some of the most common product types.
Table 1. Typical Fiber Length, Machine Parameters, and Basis Weights for Wet-Laid and Dry-Laid Production Methods (Russell 2007)
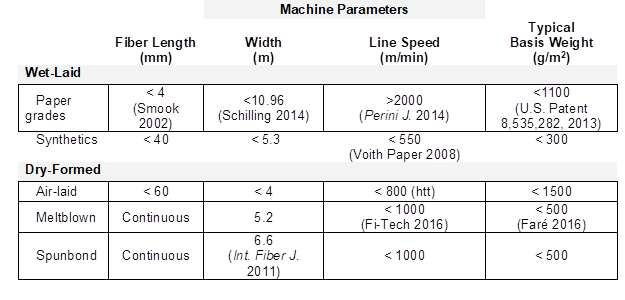
The global nonwovens market, which comprises both dry-formed and wet-laid production methods, represents a $37.4 billion industry (Smithers Apex 2015). Wet-laid products comprise a $534 million global industry with applications in diverse segments including wall covering, filtration, and specialties (Smithers Apex 2014). Starr (1973) noted that in the early 1970s the wet-laid sector constituted about 15% of the nonwovens market. Today, the dry-laid sector is by far the larger market, owing to strong growth in automotive, hygienic applications, disposables, and other markets. For many years production of wet-laid nonwovens has been regarded as relatively stagnant, following a climax of activity during the 1970s (Anon. 2005). The stagnation in new manufacturing capacity since about 1980 has been attributed to environmental concerns – noting the huge volumes of water that the process employs; however it appears that such concerns can be met with new materials and available water treatment technologies (Anon. 2005). Thus, continued growth in wet-laid demand seems likely in coming years, with annual growth of 5.1% projected for wet-laid nonwovens through 2019 (Smithers Apex 2015). Driving this growth is the continued expansion of traditional markets as well as the recent rapid growth in the disposable wipes market (Suominen 2015).
Organization of this Article
To lay some groundwork for a focus on chemical aspects of wet-laid nonwoven processing, this article begins with a brief overview and description of commonly used equipment and processes. Chemical aspects are then considered based generally on the usual sequence of operations. Thus, the subject of wetting is considered, with an emphasis on the use of surfactants as a means to encourage various oleophilic or oil-loving synthetic fibers to become mixed with the water medium. The next topic is dispersion, with emphasis on the use of dispersants to aid in the separation of the synthetic fibers from each other in the presence of agitation. A third class of chemicals, the thickeners, are considered next. These highly viscous hydrophilic or water-loving polymers and related chemicals are intended to repress the premature entanglement of the long fibers. And finally, a series of different approaches to achieving bonding in the wet-laid structure are considered. This is important because, unlike cellulosic fibers, the glass, polypropylene, and other types of fibers commonly used in wet-laid manufacture all lack the combination of conformability and hydrogen bonding ability that allows ordinary paper sheets to develop considerable strength during the drying process even when no bonding agent has been added.
Motivations
Fabric-like attributes; paper-like rate of production
The use of a wet-laid manufacturing strategy is generally easiest to justify in cases where the product bears some similarity to paper. Paper-like characteristics can include having the fibers oriented mainly within the plane of the sheet – though this initial orientation is sometimes later modified by hydroentanglement (White 1992). Because the forming of a paper sheet represents a kind of filtration process, wet-laid processing can provide relatively predictable or adjustable range of pore size – making the approach attractive for the preparation of various filtration media (Ahrens 1982; Bergmann 1989; Hutten 2007). Wet-laid systems allow a great deal of flexibility in the blending of different kinds of fibers, making it possible to optimize products for a wide range of applications (Ahrens 1982; Scholz 1985). In fact, even a biodegradable cover sheet for agriculture, prepared from sludge (Wang et al. 2014) can be regarded as a wet-laid nonwoven product.
The examples given in certain patents suggest that wet-laid forming is being viewed by inventors as a promising way to prepare sheets that resemble ordinary paper in many respects; the distinction that makes such products different from ordinary paper is the fact that some or all of the fibers may be non-cellulosic and considerably longer than those from hardwood or softwood trees (Clitherow et al. 1991). On the other hand, there is a strong motivation for manufacturers to incorporate cellulosic fibers due to their generally much lower cost in comparison to the synthetic alternatives (Gill et al. 1972; Williamson 1993).
The rate of production on a typical paper machine is many times greater than can be achieved in a typical woven textile line (Ahrens 1982; Williamson 1993). Williamson estimated a 1000- to 10,000-fold advantage in production rate. Some limitations or inherent drawbacks to wet-laid forming have been mentioned. For instance, wet-laid products tend not to achieve the levels of softness, conformity, or strength of ordinary cloth (Williamson 1993).
Flexibility in Blending of Fiber Types
A great variety of fiber types, such as glass, polyester, polyamide, and regenerated cellulose, etc., have been employed in wet-laid nonwovens (Scholz 1985). Evans and Pfeiffer (1992) described the development and use of fibrillated acrylic pulp. The high surface area and ease of dispersion in water of this material suggest that it should be regarded as a substitute for cellulosic fibers. Cellulose acetate fibrets are another example of fibrous materials that are relatively easy to disperse in water and that can provide strength advantages relative to ordinary papermaking fibers (Kozak 1991). Kevlar® fibers, based on aromatic polyamide chemistry, likewise can be prepared in a form that is highly fibrillated and easy to disperse (Merriman 1981).
Glass fibers
Chopped glass fibers are among the most studied and employed types in wet-laid applications (Chakrabarti 1979a; Stassen 1983; Fegley 1985; Whichard 1985; Brandon et al. 1988; Latimer and Schultz 1990; Jakush 1991; Brandenburg 1993; Yousfani et al. 2012). According to Chakrabarti (1979a), glass fibers often do not disperse well in water and require surfactants. Stassen (1983) recommended cleaning glass fiber surfaces with strong acid, presumably to remove hydrophobic substances that may have accumulated onto the fiber surfaces during processing, since such substances would tend to impede wetting by water. Brandon et al. (1988) describe the importance of wet-chopping to achieve the required fiber lengths. Miller (1996) describes a practical test to evaluate the quality of dispersion of glass fibers in water.
Polyolefin fibers
Polypropylene and polyethylene, i.e. polyolefins, are other polymer types that are widely used in wet-laid nonwoven manufacture (Floyd 1984; DeJong 1999; Holm and Milding 1999; Malkan 2009; Fages et al. 2012). In unmodified form such fibers are highly hydrophobic. Strategies for dispersing hydrophobic fibers in water will be dealt with in later sections (Cai et al. 2003). Considerable research has been devoted to the use of combinations of cellulosic fibers and polypropylene in wet-laid composites, allowing a range of end products, while also taking advantage of the favorable price and ease of processing of the cellulosic component (DeJong 1999; Holm and Milding 1999; Fages et al. 2012). Alternatively, as described by Butterworth et al. (1978), it is possible to prepare polyethylene fibers that resemble wood pulp in many respects, making it possible to prepare blends with ordinary papermaking stock. The use of polyethylene fibers and/or bicomponent fibers as a meltable binder in wet-laid nonwoven manufacture will be considered later.
Polyester fibers
Innovations in the development and supply of polyester staple fibers, for wet-laid applications, were summarized by Ezaki (1988). Wet-laid applications of such fibers have been described (Gregg 1974; Latimer and Schultz 1990; Robertson 1992; Shiffler 1999; Reese and Cooper 2002). Polyester fibers can be blended in different ratios with glass fibers to achieve a range of properties (Esaki et al. 1989; Latimer and Schultz 1990) for such applications as roofing mats. Polyester micro-fibers can be used for specialty applications that can benefit from small fiber size (Robertson 1992).
Aramid fibers
Nonwovens made from aromatic polyamine or aramid fibers are excellent examples of high-performance materials manufactured using wet-laid methods. These papers are derived from techniques developed in the early 1960’s, wherein staple fibers and fibrids of various polymer types were combined using a papermaking process to produce synthetic paper-like composites of improved strength and thermal resistance (Morgan 1961; Merriman 1981). In such applications the fibrids act as film-like binders made from synthetic polymers, which are placed in a shear field to enhance their surface area. The applied shear field can be generated through shear precipitation or mechanical action, such as refining (Morgan 1961). The specific surface area of fibrids generated by these methods can be as high as 60 m2/kg. Paper comprising synthetic fiber and fibrids can be calendered at elevated pressures and temperatures above the second order transition temperature of the polymer to enhance internal bonding and increase paper strength.
Paper made from aramid fibers were developed by DuPont in the early 1970s (Gross 1973). Aramid fibers, because of their high temperature resistance, are less likely to embrittle during calendaring (Gross 1973). This is especially the case with aromatic polyamide fibers of low modulus. Papers made from aramid fibers are sold under the Kevlar®, Nomax®, and Twaron brands and are used in a variety of industrial applications including personal protection wear and composite structures used in aerospace applications (DuPont 2016a,b). Other applications include electrical insulation paper, printed circuit boards, and as separators for Li-ion batteries (Teijin 2016).
Carbon fibers
Carbon fibers, which are very strong relative to their weight, can be used for specialized applications via wet-laid forming (Saylor 1981). For example, some such fabrics can be impregnated with resin and made into parts for advanced aircraft. Though it is also common to prepare pre-impregnated woven textiles for such applications, the nonwoven fabrication method can allow for higher production rates and the use stiff fiber types that are difficult to weave. Karwa et al. (2012) employed carbon nanofibers for preparation of filtration media.
Regenerated cellulose fibers
Wide ranges of fiber length, denier, and morphology of cellulosic fibers can be provided by various processes of cellulose regeneration, followed by chopping. Viscose, a process that regenerates cellulose from sulfuric acid solution to produce RayonÒ fibers, is widely used for nonwovens (Gregg 1974; Woodings 1979; Floyd 1984; Wilke 1989; Hutten 1995; Maity et al. 2014). Wilke (1989) describes an innovative RayonÒ fiber having a tri-lobal cross-section, which can offer some performance advantages in absorbent products. The related lyocell fibers, which are regenerated from an n-methylmorpholine oxide (NMMO) solution, are also used in wet-laid nonwovens (Johnson 1996). Such products can offer high strength for such products as tea bags, wipes, and electrical papers. Riedel et al. (1996) describe the preparation of lyocell microfibers, which are said to have improved handling and can serve as a bonding agent in blends of other fibers wet-laid nonwoven products.
Flax and hemp
Among the non-wood plant-based fibers, flax has received much attention (DeJong 1999; Kohler and Nebel 2006; Fages et al. 2012, 2013; Maity et al. 2014). Use of hemp fibers has been described by Hutten (1995) and DeJong (1999). In each case the bast fibers are long and strong enough to meet the requirements of demanding end-use performance, and the cellulosic nature is helpful for achieving good fiber dispersion in water. DeJong used a chemimechanical pulping process and advocated using bast fibers from flax or hemp as a replacement for polypropylene or polyethylene fibers on some nonwoven products. Hervey et al. (1975) patented a process of partially acetylating flax fibers as a means to further improve their dispersibility in water.
Cotton
Cotton fiber usage in wet-laid applications requires careful consideration on account of its relatively high fiber length. Ordinary, unchopped cotton fibers have lengths of 22 to 36 mm, whereas cotton linters are about 12 to 15 mm long (Balaji 2015). Farer et al. (1998) described the blending of cotton fibers of different length to achieve suitable dispersion for wet-laid forming. Achieving good dispersion of very long fibers (>18 mm) remains a challenge for typical cotton fibers (Hervey et al. 1975; Farer et al. 1998). Gilmore et al. (1996) found that hydroentanglement technology (to be described later) was very well suited to the bonding of nonwoven sheets comprised of cotton fibers.
Wood pulp
Cellulosic fibers can play some important roles both during the production and usage of wet-laid nonwoven products. During the initial dispersion and forming of mats composed mainly of long synthetic fibers, the addition of wood pulp fibers, which are much shorter, in principle can decrease the tendency for fiber entanglement (Kerekes and Schell 1992, 1995). The substitution of a portion of the fibers with wood pulp fibers can be expected to decrease the entanglement tendency due to crowding among the fibers in suspension. In addition, if cellulosic material becomes adsorbed or draped over the surfaces of the hydrophobic fibers, then the more hydrophilic character of the cellulosic matter can be expected to help compatibilize fibers such as polypropylene with the aqueous medium. From a product standpoint, the cellulosic fibers can contribute to absorbency, a softer feel, bonding, and opacity, among other attributes.
Challenges
A variety of technical, economic, and environmental challenges need to be overcome in order to achieve commercially viable production of wet-laid nonwoven fabrics. In the face of environmental regulations, coupled with the large flows of water within a typical wet-laid process, some people have wondered whether wet-laid nonwoven production would become a thing of the past (Anon. 2005). Relative to conventional paper forming, which typically involves fibers solids levels of about 0.3% to 1.0% just before preparation of the sheet, formation of a nonwoven sheet often requires dilution levels that are 10× greater. As will be described later, excessive crowding of the fibers can be expected to yield clumping (i.e. flocs) and knot formation among the long fibers in the suspension. Due to the high aspect ratios of the chopped synthetic fibers that are typically used in nonwoven manufacture, there is a high potential for unintended entanglement of fibers into “ropes,” which then can become incorporated into the product as blemishes (Ramasubramanian et al. 2008). Measures to decrease the tendency of fibers to prematurely become entangled, such as high levels of dilution, as well as the use of surfactants, dispersants, and thickeners (see later sections), can all be regarded as potential sources of pollution when water is discharged from the production facility. Careful planning, testing, and implementation of water treatment systems will be needed to mitigate such problems.
The capital equipment needed for wet-laid processing can serve as a contributing barrier to entry into the manufacture of wet-laid nonwoven fabrics. It is worth noting that ordinary papermaking equipment is often ill-suited for the manufacture of products that have fibers longer than about 3 mm. For one thing, the slotted screens used in a typical paper mill for removal of large debris from the process can be expected to become clogged rapidly if longer fibers are present. To minimize such problems, wet-laid nonwoven systems generally omit such devices and employ equipment that has been designed especially for processing of relatively long fibers.
Process Overview
Key steps in the process of manufacturing a wet-laid nonwoven sheet have been reviewed by Angelini (1988). Procedures used during stock preparation, the flow of water in the approach system, and the headbox operations all play a critical role.
As noted by Hutten (1995), the amount of dilution water needed for such operations and initial dispersion and for forming of the sheet increases roughly with the square of fiber length. Thus, relatively large tankage and large pumping capacity is required in comparison to conventional papermaking of the same amount of dry mass per unit of time. The design and layout of equipment such as tanks, agitators, and piping, needs to be well planned to avoid vortices and flow restrictions that could promote entanglement of long fibers (Sholz 1985; Hutten 1995).
Forming section
The sheet-forming equipment that is typically used for manufacture of wet-laid nonwoven fabrics differs in some characteristic ways from what is used for ordinary paper or paperboard products. Figure 1 illustrates some essential features of a so-called “inclined-wire Fourdrinier” system. Such devices, as well as some alternative versions of forming devices intended for wet-laid manufacturing, have been described (Saich 1969; Keller and Kroetz 1972; Schoffmann 1980; Scholz and Lasschuit 1981; Ahrens 1982; Fegley 1985; Scholz 1985; Angelini 1988; Dunn and Harke 1991; Schoeffman 1992; Schoffmann and Schwend 1992; Williamson 1993; Hutten 1995, 2007). As shown in Fig. 1, in an incline-wire system the fibers that will comprise the product are deposited onto a moving screen, which is often called the “forming fabric” or “the wire”. Typically such systems are designed so that the velocity of the approach flow approximately corresponds to the velocity of the forming fabric. By such means, the goal is to capture – in two dimensions – the same degree of uniformity that has been achieved in dispersing the fibers in the aqueous medium. Also, as noted by Ahrens (1982), it is possible to achieve an approximately random distribution of fiber orientations within the plane of the sheet (sometimes called a “square sheet”). Vacuum is used to facilitate rapid and controllable dewatering of the web.
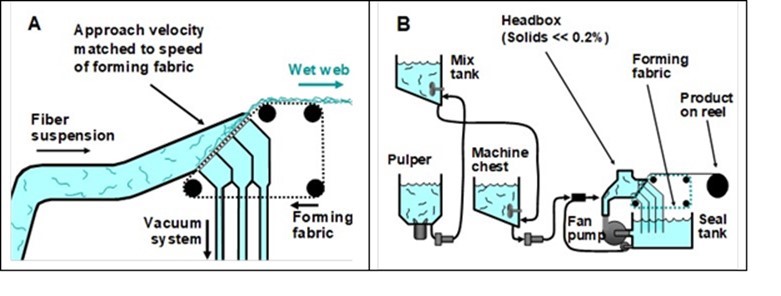
Fig. 1. Schematic diagrams of typical equipment for preparation of wet-laid nonwoven fabrics. A: Inclined-wire forming equipment; B: Possible arrangement of chests and recirculation of process water. Figures redrawn based on concepts presented in US Patent 4,200,488 (1980).
Figure 1(B) shows how the forming section of the machine is integrated into a system. The lower right of the figure shows how water is recirculated via a fan pump so that the fibers can be in a highly diluted state during the forming operation. The system also includes tanks for dispersing the fibers (the pulper), for blending different types of fibers, and for feeding the mixture to the forming section.
As alternatives to the inclined-wire Fourdrinier equipment, the wet-laid sheet also can be formed on a rotating cylinder screen, which has provision for vacuum application from its interior (Gill et al. 1972; Dunn and Harke 1991). A variety of other specialized designs of formers have become available (Schoffmann 1980; Elliot 1987; Dunn and Harke 1991), especially for high-speed production of tissue-like products.
Once it is formed, a typical freshly-formed nonwoven sheet, especially if it does not contain cellulosic fibers, will be very weak. In contrast to plant-derived cellulosic fibers, typical synthetic fibers employed in wet-laid nonwoven manufacture are relatively non-conformable, and as a result the areas of close contact between adjacent fibers in a freshly-made nonwoven sheet will be relatively small. Furthermore, most of the synthetic fibers, with the possible minor exception of glass, lack the ability to form hydrogen bonds among the fibers during the drying process. Therefore, most wet-laid nonwoven operations include treatment of the freshly formed web with a bonding agent and/or the use of meltable or otherwise bondable fibers. The unit operations for strength development in wet-laid nonwovens have been reviewed (Scholz 1985; Parsons 1999).
WETTING
The first step in almost every implementation of wet-laid nonwovens processing is the placement of dry fibers into an aqueous solution. This step is reviewed in detail here due to its critical importance, relative to the quality of the final product. If the fibers happen to be cellulosic (from wood, cotton, flax, hemp, etc.), then the fibers may immediately become surrounded intimately on all sides by water. Such behavior can be attributed to the oxygen-rich surfaces of the fibers. Oxygen, often in the form of –OH groups, provides opportunities for numerous hydrogen bond associations with the surrounding water. Other fibers, such as ordinary polypropylene, polyethylene terephthalate (polyester), nylon, etc., generally lack oxygen or other polar groups on their surfaces and therefore do not interact strongly enough with water to promote easy wetting (Ring et al. 1977). If one places pure polypropylene fibers onto the surface of pure water, the fibers will appear to float. Water will not be able to work its way between the adjacent fibers to allow them to sink or to become dispersed. Because of this situation, treatments to increase fiber wettability can be regarded as among the most important of the chemical strategies affecting the overall success of the wet-laid nonwoven processing (Shiffler 1999).
Contact Angles and Wettability
The wettability of a fiber and its ability to form a dispersion can be interpreted in terms of the contact angle of the aqueous solution with the surface of a monofilament fiber (Rebouillat et al. 1999). Figure 2 (A) defines the angle of contact (drawn through the liquid phase) in the case of surfaces that are either wettable of not wettable by pure water. As shown by the data presented in Part B of the figure, typical glass and cellulosic fibers have very low contact angles with water, and they are regarded as hydrophilic. By contrast, conventional polyethylene, polyester, polyamide, and polytetrafluoroethylene fibers yield water contact angles substantially greater than 90 degrees, and they are called hydrophobic.
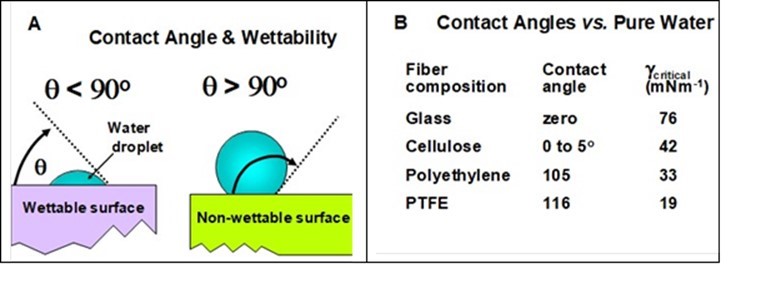
Fig. 2. Wettability of water droplets on smooth surfaces. A: Definition of wettable vs. non-wettable surfaces; B: Water contact angles on selected surfaces. Data are from Lyne (1993).
Capillarity
A hydrophobic type of fiber, when placed on the top of pure water, will give a contact angle greater than 90 degrees. This situation is depicted in Fig. 3(A). An adverse consequence of such a non-wetting tendency is that such fibers will tend to remain at the surface of pure water. This tendency can be compounded by the capillary effect illustrated in Fig. 3(B). Note that a trapped bubble of air at the junction between hydrophobic fibers in water will contribute to holding bunches of semi-dry fibers together in an arrangement akin to a floating raft. Interfacial forces, associated with the boundary between air and the aqueous phase, act to pull the adjacent fibers towards each other. The capillary force can be estimated by multiplying the surface tension of the aqueous solution times the estimated length of interfaces that intersect with the plane of interest.
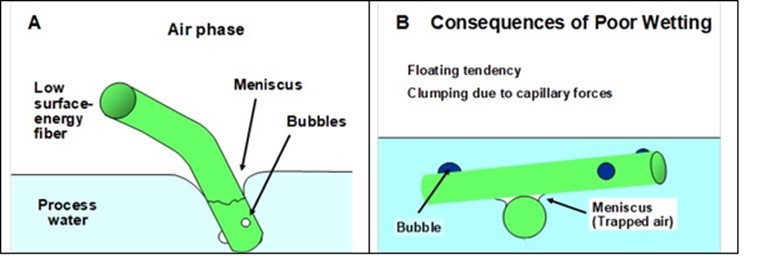
Fig. 3. Schematic illustrations of problems related to the limited wettability by water of synthetic fibers such as polyethylene, polyester, polyamide, etc. A: Meniscus shape and air bubbles at fiber surfaces; B: Role of bubbles in holding hydrophobic fibers together in clumps
Surfactants
The situation described in the preceding paragraph can be overcome by the judicious use of surface-active agents, i.e. surfactants. Briefly stated, a surfactant is a molecule that contains both hydrophilic and hydrophobic moieties. Applications of surfactants in wet-laid nonwoven production have been explained in earlier articles (Stassen 1983; Cai et al. 2003). Surfactants have been shown to help achieve a good distribution of fibers in wet-laid nonwoven processing (Chakrabarti 1979a,b; Whichard 1985; Jakush 1991; Razak 1994; Ramasubramanian et al. 2008). An example of a surfactant widely used in wet-laid nonwoven manufacture is shown in Fig. 4 (A).
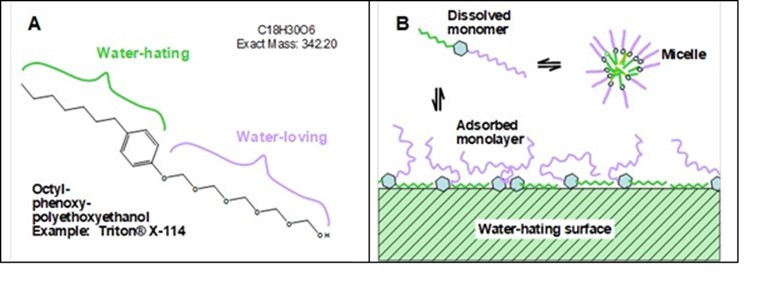
Fig. 4. Example of a surface-active molecule and its role in enhancing wettability. A: Molecular structure of octylphenoxypolyethoxyethanol, a commonly used wetting agent for wet-laid nonwoven fibers; B: Schematic diagram illustrating individually dispersed surfactant molecules, the formation of micelles, and adsorption onto a hydrophobic surface from water
Though in principle the water-loving group can be either ionic (having a fixed charge) or nonionic, it is the nonionic surfactants that are mainly being used as wetting agents in wet-laid nonwoven processing. Relatively short hydrophilic molecular chains having the composition –(CH2CH2O)n – are readily prepared by ring-opening polymerization of ethylene oxide in the presence of acid or base as a catalyst (Harris 1985). A wide range of hydrophobic groups, attached to the hydrophilic group, are in commercial use. Das et al. (2012) described the alternative usage of a cationic surfactant for dispersing of fibers for wet-laid forming.
Figure 4(B) illustrates the manner in which typical surface-active agents interact with the surfaces of hydrophobic solids in an aqueous suspension. As shown, the surfactant molecules can be expected to be present as an equilibrated mixture comprised of individually-dispersed molecules and micelles (Alexandridis and Hatton 1995; Prosser and Franses 2001). The hydrophobic parts of the molecules, represented by zig-zag green tails, tend to self-associate, allowing the hydrophilic portions of the molecule generally to face the aqueous phase. In addition, the surfactant will tend to associate with the hydrophobic solids (Cai et al. 2003), adsorbing in such a way that the hydrophilic parts of the molecules extend into the aqueous phase.
One of the functions of a surfactant (or wetting agent) is to compatibilize the two phases, essentially making the fiber surfaces act as if they had a higher surface free energy and an ability to form hydrogen bonds. Thus, in Fig. 5 one can envision the contact angle as having been transformed into a relatively low value. That situation allows the aqueous fluid (with surfactant present) to surround each fibers. Bundles of fibers are then able to sink into the suspending medium, and air bubbles entrapped within the bundles of chopped synthetic fibers are able to free themselves and rise to the water surface.
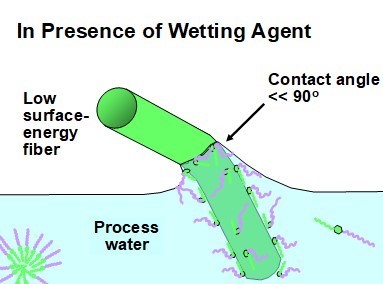
Fig. 5. Schematic illustration of the role of a surface-active agent in facilitating the wetting of a hydrophobic fiber
The selection of the type and dosage of surfactant involves a compromise between achieving wetting and excessive stabilization of foam. The combined use of both surfactants and water-soluble polymers (see later) in the aqueous phase can be regarded as being almost the worst situation if one wishes to avoid the stabilization of the foam bubbles (Karakashev and Grozdanova 2012; Fauser and von Klitzing 2014). Fortunately, by the judicious choice of the surfactant, the stabilization of bubbles can be minimized. Table 2 gives examples of some widely used surfactants. In general terms, the characteristics of a surfactant can be predicted by comparing the lengths or efficiencies of the water-hating groups versus the water-loving group, i.e. the hydrophile-lipophile balance (HLB). Good wetting agents for most wet-laid nonwoven applications will have HLB values in the range of 7 to 9 (Swarup and Schoff 1993).
Table 2. Examples of Surface-active Agents Use to Enhance the Wetting of Hydrophobic Fibers Used in Wet-laid Manufacture

Key: EO = ethylene oxide; PO = Propylene oxide
As illustrated in Fig. 6, it can be advantageous in some situations if at least some of the surfactant is initially on the dry fibers rather than all present in the aqueous phase. The key to understanding Fig. 6 is that several seconds may be required for surfactant molecules to migrate from the bulk of solution to a freshly-created air-water interface. Once at the interface, such molecules contribute to a film pressure, which reduces the value of surface tension at that location. Thus, in Fig. 6(A), if there is little or no surfactant initially present on the fiber surfaces, or alternatively if there is a lot of surfactant already in the aqueous system, then there may be a momentarily greater level of surfactant at the air-water interface toward the outside of the fiber bundles, compared to within them. This situation will tend to force the adjacent fibers towards one another during the critical moments when one is attempting to disperse the fibers in water. By contrast, if there is momentarily a higher amount of surfactant within the fiber assembly (as in Part B of the figure), then the film-pressure of surfactant at the water-air interface will tend to force the fiber bundles apart. Note that the two-dimensional pressure of adsorbed surfactant molecules will act to decrease the surface tension of the water at that location. Over a longer period of time the effects just described will diminish to zero as the amounts of surfactant adsorbed at interfaces throughout the system approach equilibrium.
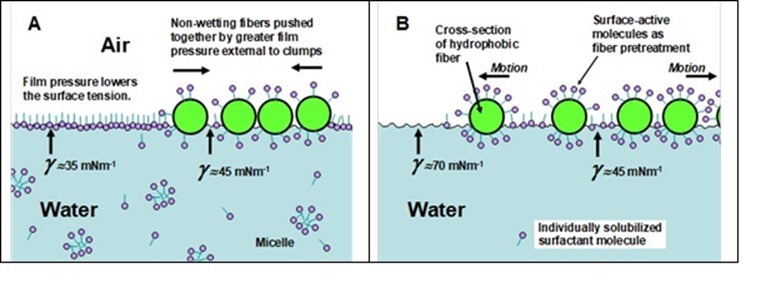
Fig. 6. Schematic illustration showing how (A) a build-up of surface-active molecules in the process water may tend to cause the hard-to-wet fibers to remain as clumps floating on the surface during the critical initial moments of the mixing process, whereas (B) pretreatment of hard-to-wet fibers with surface-active molecules can help push the fiber bundles apart during initial wetting
Foam and Defoamers
In addition to the desirable effects of surfactants in promoting the wetting of various hydrophobic fiber materials, surfactants also can stabilize foam bubbles (Avery-Edwards et al. 1994). Foam can be a serious problem in wet-laid nonwoven forming, especially when there is an effort to reuse the process water multiple times. Figure 7 provides a schematic view of how this happens. Part A of the figure envisions a bank of foam floating on the surface of an aqueous system. If one were able to peer within the microscopic scale of the wall of one of the bubbles, a cross-sectional view might resemble what is shown in Fig. 7(B). Note that the wall of a bubble suspended in air will consist of a bi-layer of surfactant molecules enclosing a thin film of water. Given sufficient time, the liquid in the film will tend to drain towards the bottom of a bubble in response to gravity. If one closely observes the kind of bubbles used at parties for children, a floating bubble often will gradually change colors and then turn colorless before it breaks. The colors can be attributed to the interference with certain wavelengths of light that are close to a specific fraction of the thickness of the bubble walls. The changes in color are consistent with decreases in thickness. The transition to colorlessness occurs when the thickness decreases below the lengths associated with visible light. The tendency to stabilize bubbles, either at the surface of water or when the bubbles are entrained in the solution, depends greatly on the detailed characteristics of the surfactant, in addition to other factors such as temperature and the presence of dissolved macromolecules (Karakashev and Grozdanova 2012).
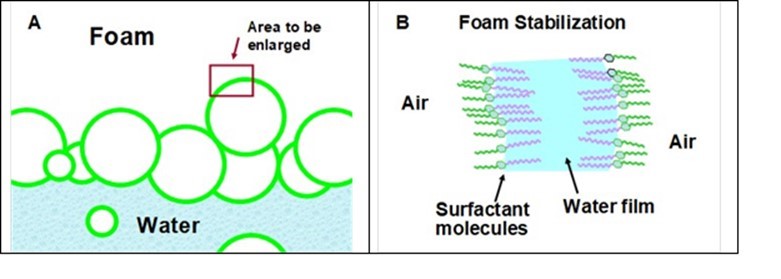
Fig. 7. Schematic illustration of foam bubbles (A) and the manner in which surfactant molecules stabilize foam (B)
Defoamer chemicals are widely employed in wet-laid nonwoven manufacture in order to avoid excessive levels of entrained bubbles and/or floating foam (Twoomey 1987; Jakush 1991; Avery-Edwards et al. 1994). Figure 8 illustrates the action of a typical droplet of an emulsified defoamer product. As shown in this illustration, the droplet diffuses randomly in the suspension until it contacts the walls of a pair of bubbles in the suspension (Karakashev and Grozdanova 2012). Once reaching the interface, the surface-active compound in the defoamer product will tend to spread very rapidly in the plane of the bubble wall. If the defoamer droplet contains hydrophobic particles, such as hydrophobized silica particles, then the rapid spreading of the defoamer’s surfactant at the interface will cause such particles to pierce the bubble walls and allow adjacent bubbles to coalesce. Such action, when repeated multiple times, will transform small bubbles into large bubbles that rise to the surface of the aqueous phase and pop.
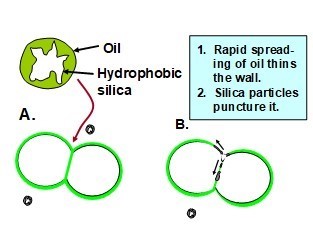
Fig. 8. Schematic illustration (A) of an anti-foam emulsion droplet in the water phase approaching the Plateau border region of a pair of bubbles; and (B) rapid spreading of the water-insoluble surface-active agent within the bubble walls, tearing an opening between the pair of bubbles
While defoamer chemicals can be very helpful for foam management, there can be some downsides, in addition to their cost. Ring et al. (1977) found that unspecified defoaming agents contributed to the flocculation of long fibers. Lickfield and Liu (1993) found that a silicone-based defoamer product interfered with the action of latex binders added later in the process.
DISPERSION OF FIBERS
Once each individual fiber added to the blend chest in a wet-laid processing system has become surrounded on all sides by water, i.e. wetted, the next step is to separate the adjacent fibers from each other. In the case of typical cellulosic fibers, as well as some glass fibers and some fibrillated synthetic fibers (Merriman 1981; Smith 1988a,b; Evans and Pfeiffer 1992), which are generally hydrophilic, such dispersion may occur almost spontaneously as soon as the fibers have been wetted. But in the case of hydrophobic fibers, hydrodynamic shear and the addition of chemical agents known as dispersants are required (Miller 1996).
The kinds of synthetic fibers most often used in wet-laid nonwoven fabrics manufacture are prepared by the chopping of bundles of continuous filaments to produce staple fibers. Thus, even after the wetting of each individual fiber, one still may face a challenge in separating the bundles of fibers (sometimes called “logs”) into separately dispersed fibers (Shiffler 1985, 1986, 1988, 1999; Latifi et al. 2008; Ramasubramanian et al. 2008; Fages et al. 2013). Fiber separation can be especially a problem in the case of crimped fibers (Ring et al. 1997; Reese and Cooper 2002). If efforts to disperse fiber bundles are unsuccessful, then the wet-laid product will appear non-uniform, and its properties may fail to meet expectations. In addition, the mass per unit area of the product may differ from location to location (Keller et al. 2012).
Particular difficulty in dispersing fiber bundles into the components fibers can be encountered if the ends of adjacent cut fibers had become fused together in the course of chopping. The quality of cutting of fibers intended for other usages is often not good enough to meet the demands of wet-laid forming (Bourdon 1971; Brandon et al. 1988; Shiffler 1988, 1999; Williamson 1993; Keith 1994). Fusing of the ends of chopped fiber may happen, for instance, if the temperature of the chopping blades exceeds the melting temperature of the fiber material. Dull chopping equipment can make it more likely that the ends of the fiber bundles become fused and resistant to separation. The critical nature of cut quality tends to raise the costs associated with acquisition of suitable quality fibers for wet-laid nonwoven applications.
Critical Force Concept
Two critical factors in the dispersing of fibers, in preparation for nonwovens processing, are hydrodynamic shear and the use of dispersant chemicals. The topic of hydrodynamic shear will be considered first.
According to Shiffler (1999) the resistance to dispersion of a bundle of chopped fibers can be envisioned as the sum of contributing factors. These factors include adhesive forces between the fibers, capillary effects, inter-fiber friction, and various problems related to the ends of fiber bundles. It follows that there ought to be a certain level of hydrodynamic shear that is just sufficient to cause separation of individual fibers from a typical fiber bundle. Indeed, Shiffler (1985) found that the rate of bundle breakup increased with increasing speed of agitation. Latifi et al. (2008) proposed that for each type of fiber and set of aqueous conditions there is likely to be an optimum level of hydrodynamic shear that is just enough to disperse the fibers. Ideally this can be accomplished without inducing other problems, such as entanglement, which will be considered in a later section. Ramasubramanian et al. (2008) carried out computational fluid dynamics modeling, along with experimental study, to verify and quantify the general concept of fiber dispersion, along with some other concepts. Switzer and Klingenberg (2003) found that shear flow was effective for disrupting fiber bundles, whereas extensional flow resulted in only partial dispersion of fibers, but left many fragments intact. Safavi et al. (2009) found that although the frequency of defects related to fiber entanglement tended to increase with both fiber length and fiber fineness, at constant solids content of the suspension, the speed at which hydrodynamic shear was able to disperse the fibers was also increased with increasing fiber length and fineness.
Figure 9(A) presents an idealized view of the actions of localized hydrodynamic shear flow in bringing about dispersion of bundles of parallel chopped synthetic fibers. The idealized shear flow assumes a steady gradient of velocity with distance perpendicular to the axis of flow.
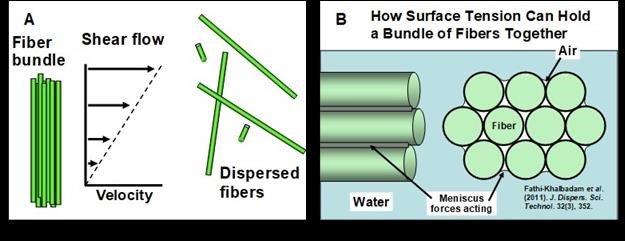
Fig. 9. Schematic illustration of bundles of chopped fibers. A: Hydrodynamic shear forces help separate such bundles by overcoming the mechanical factors, adhesion forces, and capillary forces holding them together; B: Illustration of how surface tension forces tend to hold a bundle of fibers together, especially if they are difficult to wet by water.
In theory, the separation will take place quickly if and when the applied hydrodynamic force exceeds the combined effects of mechanical fusion, attractive forces, capillary effects, and friction. Figure 9(B) illustrates how pockets of air located within a bundle of hydrophobic fibers can be expected to act in holding the bundle together. The attractive force will be approximately proportional to the surface tension of the aqueous phase, as affected by surfactants and other solutes.
Fiber dimensions
As one would expect from the mechanism just described, fiber dispersion has been found to vary a lot between different types and batches of synthetic fibers that have been prepared for wet-laid nonwoven applications. In general, the length of fibers used in wet-laid forming is restricted to about 10 mm or less (Lichstein 1988). The presence of even a minority of excessively long fibers can give rise to a kind of defect known as “dumbbells” or “double-knubs” (Schoeffmann 1992; Shiffler 1988, 1999). Such defects consist of pairs of clumps of fibers joined together by at least one over-length fiber.
Thicker fibers tend to be more difficult initially to disperse (Safavi et al. 2009). Likewise, Fathi-Khalfbadam et al. (2011) found that with increasing fiber diameter it was necessary to apply agitation for a longer time in order to adequately disperse the fibers. This tendency can be attributed to increased stiffness of the thicker fibers.
Clark and Shiffler (1985) proposed that the cross-sectional shape of a fiber can influence its ability to disperse in water. Fibers prepared with a scalloped-oval cross-section were found to disperse in water more readily than similar fibers having a round cross-section. Other properties that can be affected by fiber cross-sectional shape include softness and the wicking of water in absorbent products (Clark and Shiffler 1985; Ezaki 1988; Haile and Phillips 1995).
Capillary forces
If air remains within the fiber bundle, then the hydrodynamic force needed to separate the fibers can be estimated based on the situation illustrated in Fig. 9(B). As shown, one can expect that the surface tension at the air-water interface will be acting to hold the fibers together as a bundle. The difference in pressure tending to hold a bundle of fibers together can be estimated from the relationship,
DP £ 2g/RL (1)
where P is the pressure difference pulling adjacent fibers together, g is the surface tension of the aqueous solution, and RL is the radius of the fiber bundle, which is treated as being cylindrical. Also, the fibers are assumed to be sufficiently hydrophobic such that the contact angle of aqueous solution on the fibers surfaces is 90 degrees or higher.
Capillary forces, as just described, can be expected to oppose separation of fiber bundles in two ways. On the one hand, they can act to prevent outward lifting or peeling of a fiber away from the perimeter of the bundle. In addition, due to friction between the fibers, the inward-directed capillary forces also will tend to resist a sliding mechanism of fiber separation. Ideally, frictional resistance to sliding can be expected to be governed by the product of the capillary force drawing the fiber bundle together and a coefficient of friction (Shiffler 1999).
Though it may be unrealistic to attempt to calculate the precise hydrodynamic conditions needed to separate a bundle of chopped fibers, all of the mechanisms summarized above point to a common theme: There ought to be a threshold of force needed to separate individual fibers from the bundle. Alternatively, a random distribution of resistance to detachment may make it necessary to exceed the highest threshold in the distribution.
Time and flow patterns
The force-threshold concept, as just introduced, suggests that one ought to apply a very short spurt of agitation at high intensity. In practice, however, it has been observed that several minutes of agitation with an impeller may be needed to achieve nearly complete dispersion of fiber bundles in a tank (Shiffler 1988; Latifi et al. 2008; Safavi et al. 2009; Fathi-Khalfbadam et al. 2011). The likely reason for this time dependency can be understood by considering the diagram in Fig. 10(A).
As shown, at any given time only a small fraction of the fibers in the mixing tank can be near to the impeller, which would be the point of greatest hydrodynamic force. In addition, the presence of eddies of turbulent flow introduce an element of chance. With the passage of time it becomes more and more likely that each fiber in the mixture has come close to the impeller. However, as will be discussed later, an excessive amount of time of agitation can be expected to lead to fiber entanglement and to formation of flocs (Das et al. 2012). Thus, one can expect that both the intensity and duration of agitation will need to be optimized in typical cases in order to meet the goals for fiber dispersion in a given application (Shiffler 1988; Richardson et al. 1988; Latifi et al. 2008).
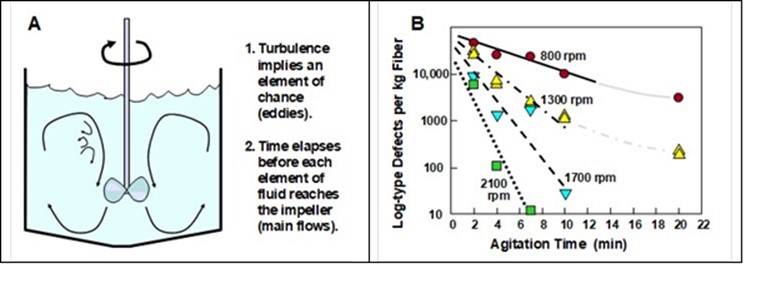
Fig. 10. Effect of time on the dispersion of bundles of chopped fibers. A: Schematic explanation of why time is required for essentially all parts of the mixture to have been exposed to high hydrodynamic shear at the tips of the impeller. B: Data showing effects of agitation speed and mixing time on the numbers of bundle-type defects observed in wet-laid product (replotted from the findings of Shiffler 1988).
The concept just introduced is supported by the data shown in Fig. 10(B), which is replotted from the work of Shiffler (1988). As shown, the frequency of bundle-type defects in a nonwoven product decreased sharply with increasing time of agitation during preparation of the slurries. In addition, the rate of decrease in defects was a found to be a strong function of the speed of agitation. This makes sense because stronger agitation will tend to bring different parts of the suspended material more quickly to the neighborhood of the impeller blades, and also a higher volume of the system, at any one time, will be exposed to shear forces exceeding a threshold level.
Chemical Aspects
One of the most fundamental mechanisms by which solids in a suspension can be kept from agglomerating together involves electrostatic repulsive forces. This charge stabilization mechanism is important, for instance, in the case of glass fibers in aqueous suspension. Glass develops a negative ionic charge at its surface due to dissociation of silanol groups (Berli et al. 2003; Atalay et al. 2014). However, charge-stabilization can be achieved with essentially any kind of fiber, since a negative charge can arise by adsorption of anionic dispersants or adsorption of certain kinds of surface-active agents.
Figure 11 illustrates the manner in which ions of opposite charge, i.e. counter-ions, tend to gather adjacent to a charged surface in aqueous solution. Here we imagine ions such as sodium, potassium, and calcium ions in the solution very close to the surfaces of synthetic fibers that have been stabilized by the adsorption of negatively charged polyelectrolyte molecules. The figure represents only those positively charged ions that are playing the role of balancing the net negative charge of the surface. The positions of such ions are governed by two opposing tendencies: Electrostatic attraction causes the ions to migrate in the direction of the opposite charges at the fiber surfaces. But thermal energy causes the ions to diffuse randomly in all directions. The net effect is that counter-ions will be present as a type of “atmosphere” at the fiber surfaces. In principle, electrostatic repulsion can be expected whenever like-charged double-layers come close enough (generally within a few nanometers) to overlap significantly.
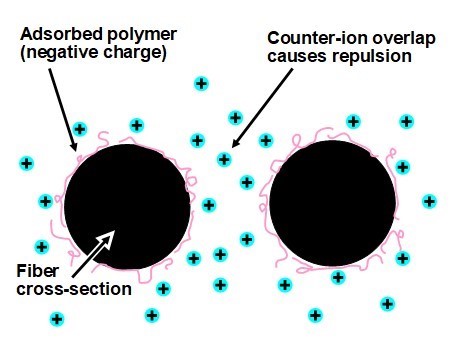
Fig. 11. Schematic illustration of overlap of ionic double-layers, resulting in charge-induced repulsion between fibers immersed in aqueous solution
Dispersants
Chemical-based strategies to promote the dispersal of fibers from each other after wetting has been achieved generally rely on electrostatic forces of repulsion. Addition of dispersants, such as polyacrylates, to the aqueous solution can be quite effective (Jansma and Smith 1993; Miller 1996). In principle, the negatively charged polymer chains adsorb onto fiber surfaces in sufficient amount to impart a strong negative charge. Addition of a dispersant at an excessively high concentration can actually decrease its effectiveness (Huang et al. 1991; Briscoe et al. 1998; Chen et al. 2014). The dosages of dispersing agents need to be optimized, since excess dispersant in the process water can be regarded as a contribution to the pollution load when the water is eventually discharged from the system.
Figure 12 illustrates the structure of a typical polyacrylate dispersant. In this figure some of the carboxyl groups are shown as being protonated, whereas others of them are shown in their dissociated, sodium acrylate form. The ratio between the two forms of carboxyl groups on such a molecule will depend on the pH. When the pH equals the pKa value, which is typically near to 6.8 for this kind of macromolecule, there will be an equal number of negatively charged and uncharged carboxyl functions (Charman et al. 1991). As the pH is increased above that point, the dispersant molecule becomes more fully anionic.
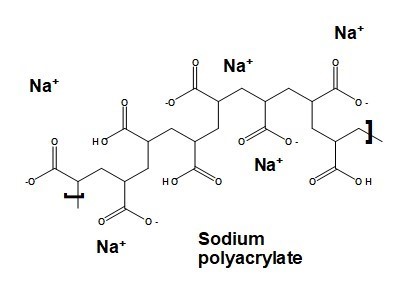
Fig. 12. Typical molecular structure of a sodium polyacrylate dispersing agent
Order of addition
Because conventional wet-laid nonwoven production can include addition of several different additives, such as dry fibers, surfactants, dispersants, and thickeners, among others, it is reasonable to expect that somewhat different results can be achieved depending on the order of addition (Richardson et al. 1988). Brandon et al. (1985) patented a process in which fibers were added to a solution containing only water and a dispersant, for the purpose of maximizing the effectiveness of breakup of bunches of fibers, and then a thickener was added later. Likewise, Jakush (1991) stated that thickener products tend to impede the action of dispersants. Nonetheless, they found that the ratio between the two kinds of additives could be adjusted to achieve more favorable results.
Sizing agent and spin finishing
In light of the challenges just discussed in bringing about the initial dispersion of fibers, many suppliers of chopped synthetic fibers intended for wet-laid processing treat the fiber surfaces in advance to promote dispersibility. The pre-application of surface-active agents was already suggested in Fig. 6 and the related discussion. In addition, lubricants may be applied to the fiber surfaces (Reese and Cooper 2002). The fiber-dispersion-promoting agents listed as background information in the cited patent range from surfactants to block co-polymers having pendant ethylene-oxide chains. Clark and Shiffler (1985), likewise, give citations supporting the use of block copolymers of ethyleneterephthalate and ethylene oxide as sizing agents to promote later dispersion of dry fibers after their delivery to the point of use.
Spin finishing (also referred to as lubrication) is a well-known process for modifying the physical properties of synthetic fibers before they are chopped (McIntyre 1968). Bajaj (1997) has reviewed of spin finishing methods and chemistries and states that it is one of the most important variables influencing the performance, quality and uniformity of processing synthetic fibers. Typical chemistries used in spin finishing include lubricants, emulsifiers, and antistatic agents (EPA 1997). Lubricating agents include animal, vegetable, and minerals oils; fatty acid esters such as methyl oleate and butyl stearate; non-ionic surface active agents such as ethylene oxide adducts of fatty acids or fatty acid esters of higher or polyhydric alcohols; and anionic surface active agents such as alkyl sulfates, alkyl phosphates, and fatty acid salts. Antistatic agents include alkyl phosphates and sulfates, as well as cationic surface active agents such as quaternary ammonium salts (Kawanaka et al. 1974). Water-soluble polymers such as polypropylene glycol and polyalkylene glycol can also be used impart lubricity to the fiber (Dow 2106). Add-on weights of active ingredients in spin finishing are typically less than 5% by weight. Lubricating agents modify fiber-to-fiber friction and are used to minimize falling-out of fibers during the drawing process (Kawanaka et al. 1974). In wet-lay applications, it has been shown that staple fibers spin finished with an emulsion made principally from an ethoxylated castor oil promoted fiber dispersion (Hawkins 1979).
AVOIDANCE OF ROPING
One of the great challenges inherent in wet-laid nonwoven processing is that the same hydrodynamic forces that are essential for initial dispersion of synthetic fibers can also promote fiber entanglement and the formation of ropes (Shiffler 1988; Smith 1992; Ramasubramanian et al. 2008). In particular, vortexes of flow are known to favor rope formation (Shiffler 1986, 1988; Ramasubramanian et al. 2008). Depending on the nature of the fibers, such entanglement may be difficult or impossible to reverse. It has been recommended, therefore, to avoid or suppress vortex flow (Ramasubramanian et al. 2008). Also, it has been recommended to avoid application of shear stress that is in excess of what is needed to disperse the fibers (Das et al. 2012). In principle, high levels or shear, beyond what are needed, will tend to momentarily bend the fibers. Then, as the elasticity of the fibers acts to straighten them again, they may become locked into persistent floc structures that are held together by a combination of elastic forces and friction between the adjacent fibers (Parker 1972; Hubbe 2007).
Crowding Factor Concepts
In principle, entanglement among fibers in suspension can be avoided by providing each fiber with sufficient space so that it can rotate in any direction without coming into contact with any of its neighbors. In practice, it is seldom practical to employ such a high dilution of fibers as implied by that statement. However, the idea of having enough volume so that each fiber can rotate freely without colliding with other fibers has been found useful for predicting the flocculation tendency of different fiber suspensions (Kerekes and Schell 1992, 1995; Williamson 1993; Hutten 1995). Kerekes and Schell (1992, 1995) showed that a crowding factor can be defined as follows,
NC = (2/3) Cv (L / d )2 (2)
where Cv is the volume-based concentration of fiber material in the suspension, L is the fiber length (assumed to be uniform), and d is the fiber diameter. It was predicted that an NC value of 60 was sufficient for there to be an average of three contact points per fiber, allowing the onset of structure formation (Kerekes and Schell 1992).
It follows from Eq. 2 that the required amount of dilution water to keep the value of NC below a defined limit will increase as the square of the ratio of L over d (Hutten 1995). To illustrate how the equation can be used, Fig. 13 considers two examples. In “Case 1” one imagines what would happen if it were possible to reduce the length of each fiber by cutting them in the middle. As indicated in the figure, the net volume of rotation occupied by all of the fibers in the suspension would then be decreased by a factor of four. By contrast, “Case 2” shows that the net volume occupied by the rotating fibers would be doubled if each fiber in the suspension were to be split lengthwise.
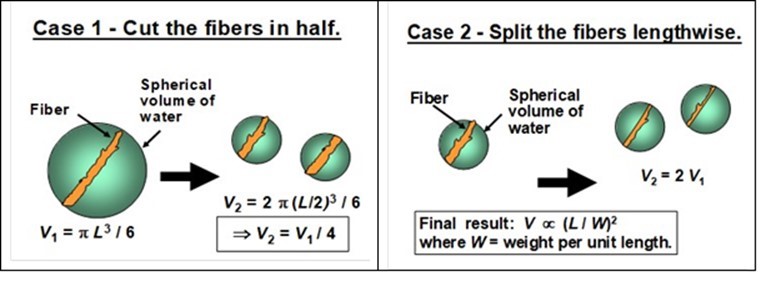
Fig. 13. Illustration of ways in which “cutting” of fibers can be expected to affect the crowding factor, hence affecting the clumping tendency and the required dilution. Case 1: Cutting all the fibers in half would decrease the occupied volume due to rotation of fibers by a factor of four. Case 2: Length-wise splitting of fibers would increase the occupied volume by a factor of two.
Based on the analysis just presented, it follows that one of the most promising ways to decrease the flocculation tendency of a fiber suspension would be to shorten the fibers, assuming that other parameters, including that the volumetric content of fiber material, remains constant. Alternatively, one could utilize coarser fibers of the original length. But when product goals require the usage of long, slender fibers, then high levels of dilution would be essential. Figure 14 gives an example of calculated results corresponding to a crowding number of 100 (Soszynski and Kerekes 1988). Above that number it is assumed that coherent flocs will become common in the fiber suspension. Below that number one expects there to be few persistent flocs. As shown in the figure, the ratio of fiber length to diameter, i.e. the aspect ratio, plays a very large role in determining whether coherent flocs of fibers are likely to be important at a given level of fiber solids.
Though most published literature related to the crowding factor concept has been aimed at conventional papermaking applications, various studies dealing with wet-laid nonwovens have provided support for the concept of fiber crowding (Scholz and Lasschuit 1981; Kinn and Mate 1986; Keith 1994; Ramasubramanian et al. 2008). For instance, Das et al. (2012) showed that nonwoven fabrics made up of shorter and coarser fibers tended to be more uniform in comparison with those prepared with fibers that were longer and finer.
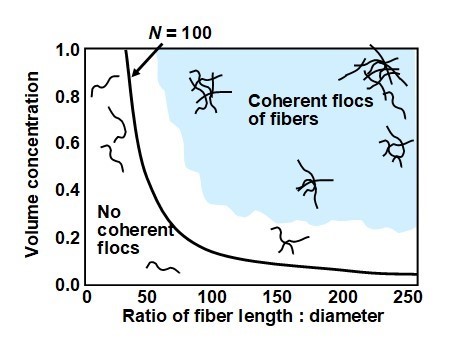
Fig. 14. Effects of fiber volume concentration and aspect ratio on whether or not persistent flocs are present. Data replotted from the work of Soszynski and Kerekes (1988)
Vortex Effects
A vortex can be defined as a cyclone-like twirling motion of fluid surrounding a funnel-shaped core of air. Vortex flow can easily form in stirred tanks, especially if a centrally located impeller draws fluid downward with a high enough rate of rotation. It has been reported that vortex flow can be a cause of rope formation during preparation of fiber suspensions for wet-laid processing (Tafreshi and Pourdeyhimi 2003; Ramasubramanian et al. 2008). This is illustrated schematically in Fig. 15(A). It is possible that such ropes could form by the kind of twisting mechanism suggested by Nanko and associates (Nanko et al. 2006; Hubbe et al. 2009). Tafreshi and Pourdeyhimi (2003) carried out numerical simulations and experimental tests to help clarify the mechanisms underlying such observations.
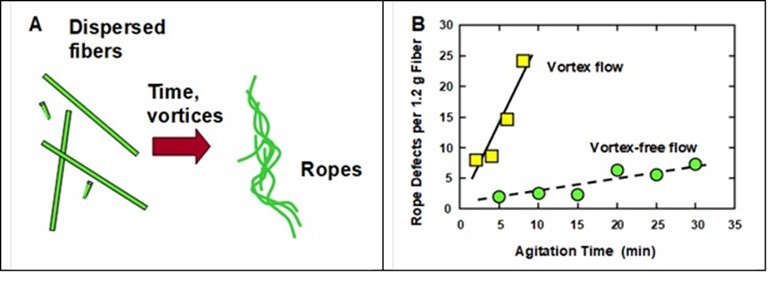
Fig. 15. A: Illustration of the formation of “ropes” due to entanglement of long fibers, especially in cases where vortex flow is present during mixing. B: Effect of agitation time on the frequency of rope-type defects, in the presence or absence of vortex flow. Data replotted from Shiffler (1999)
Evidence of the role of vortex flow in generating rope defects in a wet-laid nonwoven system is presented in Fig. 15(B) (Shiffler 1999). As shown, in the presence of a distinct vortex a rapid increase was observed in the number of rope defects for a given mass of fibers. Stirring in the absence of vortex flow also gave an increase in rope defects with time, but the rate of rope formation was very much smaller.
It has been reported that vortex development can be inhibited by increasing the viscosity of the fluid (Wilharm 1986). While there is no reason to doubt the validity of using thickeners to repress vortex flow, it is worth keeping in mind that flow patterns are highly dependent on the setup of the equipment. A vortex is less likely to develop, at a given speed of rotation, if the axis of rotation of the impeller is placed away from the center of the tank. Ramasubramanian et al. (2008) carried out experiments to determine the relative state of entanglement of fibers collected from different parts of a stirred tank that was provided with baffles. Samples were collected onto screens dipped quickly into different locations of a stirred tank. Defects related to entanglement were 43% more frequent in the region behind the baffles, where vortex flow was present, in comparison with the center of the tank.
Thickeners
Many studies have shown that one of the most effective ways to reduce the tendency of long fibers to form ropes and fiber bundles is to add very-high-mass soluble polymers, i.e. thickeners, to the aqueous medium (Wasser 1978; Wilharm 1986, 1990; Wilharm et al. 1987; Richardson and Smith 1989; Smith 1992; Miller 1996; Nedwick and Greenblatt 1996; Farer et al. 1998). Further evidence of the effectiveness of thickeners in making wet-laid products more uniform is provided in patents (Brandon et al. 1980a,b, 1985; Richardson et al. 1990). One of these patents specifies ranges of viscosity that should be achieved in the solution (Brandon et al. 1980a). As summarized in the subsections below, thickening agents can have a variety of chemical compositions. Some are ionically charged – usually anionic. But a common feature is that they are highly water-soluble and capable of increasing the solution viscosity. Acrylamide copolymers, carboxymethylcellulose (CMC), and various natural gums have been considered as thickeners for formation of sheets from long fibers.
For centuries practitioners of certain handcraft papermaking traditions have employed natural gums, including slime from the roots of Hibiscus manihot. These gums have been used to prepare high-quality paper from relatively long bast fibers (Swanson 1950; Huber and Weigl 1970; Seibert and Bähr 1970; Hubbe and Bowden 2009). Among natural gums, deacetylated karaya gum has been found to be especially effective as a formation aid (Hervey et al. 1975). Dissolved CMC has also been shown to be effective as a thickener (Giri et al. 2000). The cited authors showed that CMC remained effective even in the presence of moderate concentrations of salt ions.
In modern times various acrylamide copolymers have emerged as the most widely used agents to suppress fiber entanglement in wet-laid manufacture (Wasser 1978; Smith 1986; Wilharm 1986; Richardson et al. 1988; Lee and Lindström 1989; Farer et al. 1998). Richardson et al. (1988) showed that polymer ionic charge, molecular mass, and especially that dosage all had important effects.
Figure 16 represents the chemical composition and swelling tendency of a typical thickener macromolecule. In some important aspects, the chemistry of such a thickener molecule is similar to that of the dispersant molecule represented earlier in Fig. 12. Key differences are that a typical thickener can contain about 50 to 95% of neutral, unsubstituted acrylamide monomer units and the molecular mass can reach up to 20 million Daltons (Lee and Lindström 1989). As shown in Part A of the figure, the molecular extension in solution is expected to be highly pH-dependent. The net effect will be an increased viscosity with increasing pH in a range of about 4 to 8, as shown in Part B of the figure.
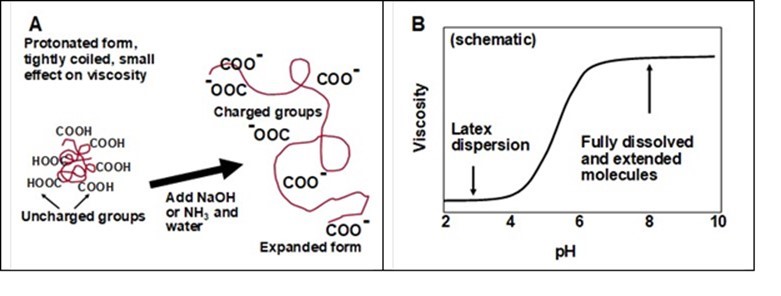
Fig. 16. Effect of pH on the macromolecular chain extension (A) and viscosity (B) of a solution of anionic high-mass polyelectrolyte (thickener)
It has been reported that the effectiveness of thickening agents can be decreased markedly in the presence of salt, with particularly adverse effects attributable to divalent cations such as Ca2+ (Wilharm et al. 1987; Charman et al. 1991; Shiffler 1999). Such effects are attributable to the kind of conformational change illustrated in Fig. 17. A condensed conformation of the macromolecule will express itself as a much lower contribution to the viscosity of a solution. Also, there will be a reduced ability of the solution to resist the formation of ropes in an agitated suspension of long fibers.
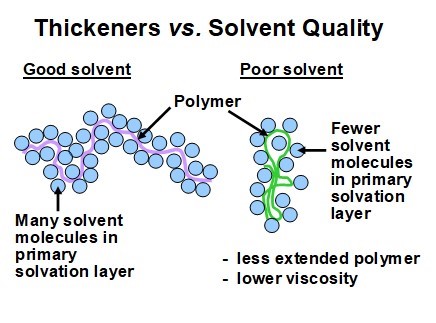
Fig. 17. Schematic diagram illustrating the expected effect of salt addition on the conformation of a very-high-mass anionic polyelectrolyte (thickener)
Figure 18 compares the effects of Na+ and Ca2+ ions with respect to solution viscosity (Part A) and the degree of fiber flocculation (Part B) (Shiffler 1999). A greater adverse effect of the divalent cation is apparent.
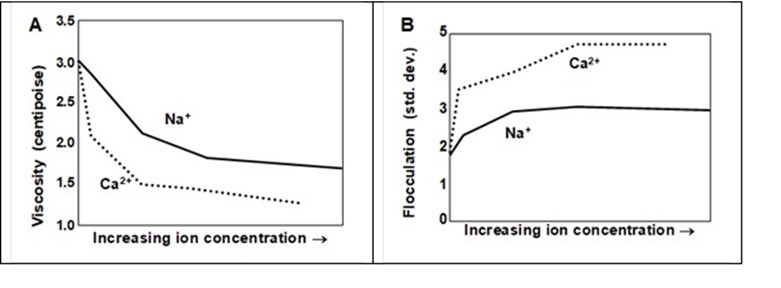
Fig. 18. Effect of monovalent and divalent ion concentration on the viscosity of the solution (A) in a long-fibers suspension stabilized by anionic very-high-mass polyelectrolyte, and (B) the corresponding degree of flocculation
Associative thickeners
One strategy to boost the effectiveness of thickeners is to modify the copolymerization so that some long-chain alkyl (hydrophobic) groups are included, along with anionic or neutral hydrophilic acrylamide units. Such products, called associative thickeners, have been shown to be effective for inhibiting the entanglement of long fibers (Tse et al. 1989; Nedwick and Greenblatt 1996). Because such products have a surface-active character, it may be unnecessary or even unhelpful to separately add surfactants to achieve initial wetting of the fibers (Tse et al. 1989).
Figure 19 (Part A) redrawn from Rohm & Haas product literature shows some examples of how the viscosity of an aqueous solution was increased with increasing concentration of different macromolecular thickening agents.
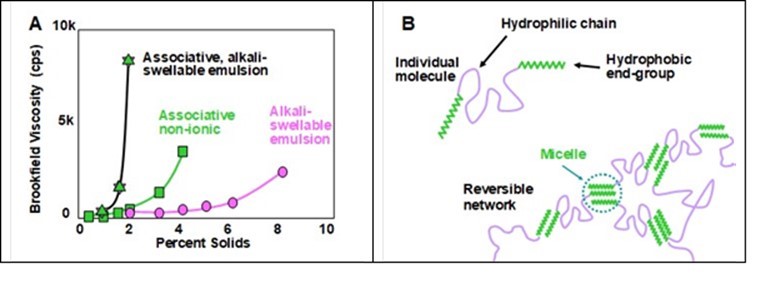 Fig. 19. Associative thickener effects. A: Example in which an anionic associative thickener achieved a higher viscosity at lower solids compared to a non-ionic associative thickener and an ordinary alkali-swellable emulsion; B: Principle by which transient cross-linking among molecules of an associative thickener induce high viscosity
Fig. 19. Associative thickener effects. A: Example in which an anionic associative thickener achieved a higher viscosity at lower solids compared to a non-ionic associative thickener and an ordinary alkali-swellable emulsion; B: Principle by which transient cross-linking among molecules of an associative thickener induce high viscosity
Notably, in the cases shown, a specific associative, alkali-swellable thickener outperformed a non-ionic associative thickener and an ordinary alkali-swellable emulsion product. Part B of the figure provides a mechanistic explanation. As shown, the hydrophobic groups of the thickener molecules are able to form transient cross-link points, which make the molecules behave like they are part of a huge interconnecting network.
Nonionic thickeners
All of the thickeners discussed so far have been ionically charged. Such products have an inherent tendency to be adversely affected by ions – especially multivalent ions of opposite charge – in the aqueous solution (Shiffler 1999). One strategy to avoid such problems is to employ a non-ionic polymer thickener such as polyethylene oxide (Seibert and Bähr 1970; Ashikaga 1972). Seibert and Bähr (1970) found that PEO was more effective than many other prospective polymer solutions for the preparation of handmade paper from long fibers. The work by Ashikaga (1972) showed that PEO use tended to make the fiber mat sticky, and it was necessary to use a release agent to be able to separate the product from dryer can surfaces.
Formation-aid Mechanisms
There are at least three concepts that have been invoked in order to explain the ability of very-high-mass water-soluble polymers to repress the tendency for entanglement of fibers. These will be briefly stated, and then explained:
- The thickener increases the bulk viscosity of the solution enough to inhibit the formation of a vortex.
- The thickener adsorbs onto fibers and provides steric stabilization, thus reducing inter-fiber friction to a very low level and letting the fibers slide easily past each other.
- The thickener macromolecules dissolved within the solution phase increase the solution’s extensional viscosity, which results in a lubrication effect between fibers during agitation.
Suppression of vortex formation
The hypothesis based on the suppression of vortex flow is consistent with the earlier-cited evidence indicating that vortex flow is involved in the creation of rope defects in nonwoven products. Increased viscosity is known to reduce the tendency for a vortex to form (Wilharm 1986; Scargiali et al. 2013). However, the present literature search did not reveal a specific study showing a case where addition of thickener was reported to change the flow regime in a tank being used for wet-laid nonwovens processing.
Adsorption and steric stabilization
If fibers in a suspension are somehow prevented from coming close together, then it makes sense that friction between the fibers can be avoided and that there might be less entanglement as a consequence. It is well known that solid surfaces in aqueous solution can be held apart from each other by a steric stabilization mechanism (Sato 1993; Dickinson 2003). Huber and Weigl (1970) proposed that such a mechanism can account for the effect of thickeners in improving the formation uniformity of wet-laid nonwovens.
As a further contributing factor, the negative charge of adsorbed CMC chains will give rise to an electrostatic component of repulsive force between such fibers (Huber and Weigl 1970). Steric stabilization happens when essentially all the surfaces are coated with protruding loops and tails of water-loving polymer or long-chain surfactant molecules. Such a situation is illustrated in Fig. 20.
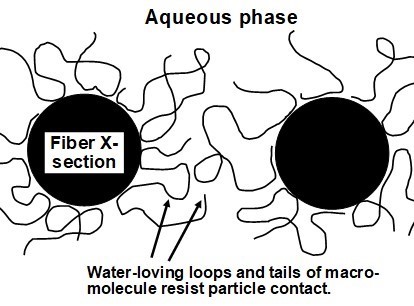
Fig. 20. Concept of steric stabilization, in which repulsion between fibers in a suspension is achieved by adsorption of either polymers or block surfactants that have extended water-loving chains extending into the aqueous solution
Zauscher and Klingenberg (2000) showed that such effects could be achieved by adsorption of carboxymethyl cellulose (CMC) onto cellulosic surfaces. A similar mechanism of adsorption and steric stabilization also may account for improvements in sheet uniformity when very-high-mass anionic polyelectrolytes are being used as “formation aids” when preparing sheets from suspensions of long fibers (Giri et al. 2000). Thus, Giri et al. (2000) proposed that the amount of adsorbed CMC was the critical factor in their work related to dispersion of fibers. The cited authors observed that the uniformity of dispersion of the fiber slurries increases with increasing molecular weight of the CMC. Also, it appears that the effect requires there to be sufficient addition of thickener to at least coat essentially all of the external surface area of fibers with an extended monolayer of the additive (Sato 1993). Important improvements in mat uniformity have been reported in some cases at relatively low levels of thickener addition – perhaps not sufficient to significantly increase the bulk viscosity of the solution (Giri et al. 2000).
An adsorption-based mechanism also fits well with established practices of “sizing” of fibers intended for wet-laid practices (Hawkins 1979). Such treatment implies placement of various soluble polymers, or other substances, onto the fiber surfaces, so that when the dried fibers are later placed into aqueous solution they will behave differently during processing.
Extensional viscosity
Lee and Lindström (1989) found that the effectiveness of very-high-mass anionic acrylamide copolymers in improving the uniformity of long-fibered handsheets was strongly increased with increases in both dosage and molecular mass. Some of their key results are shown in Fig. 21 (Part A). Both of those factors are consistent with a central role of the bulk viscosity of the solution. However, rather than the shear viscosity, which is much more commonly evaluated, the cited authors proposed that the anti-entanglement capability of very-high-mass polyelectrolytes is due to their effects on extensional viscosity.
The definition and importance of extensional viscosity has been reviewed by Petrie (2006). To evaluate this quantity, liquid at a known pressure is forced through a contracting orifice and the flow rate is quantified. Studies have shown that high-mass polyelectrolytes generally have a much higher relative effect on extensional viscosity compared to their effects on shear viscosity (Gupta et al. 2000; Larson 2005). The sensitivity of extensional viscosity to dissolved polymers has been attributed to the polymers’ resistance to deformation or stretching (Larson 2005). Indeed, as illustrated in Fig. 21(B), a linear polyelectrolyte will tend to become stretched out as it passes into and through a sufficiently long zone of contracting flow (Hsieh et al. 2005; May and Moore 2013). In cases where the solution phase contains a sufficient concentration of very-mass-mass soluble polymer, it has been proposed that the molecules delay the close approach of fiber surfaces toward each other. The result is a lubrication effect that does not depend on any of the polymer having become adsorbed onto the fiber surfaces.
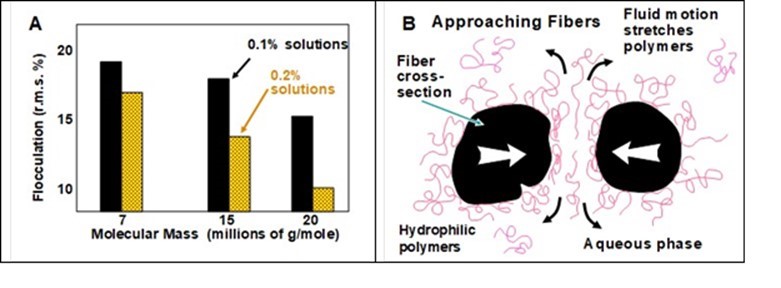
Fig. 21. Effects of very-high-mass anionic acrylamide copolymer on formation uniformity. A: Effects of molecular mass and dosage. Figure redrawn from the results of Lee and Lindström (1989), B: Explanation of the effect based on extensional flow
As shown in Fig. 22(A), the relative importance of extensional viscosity in the presence of high-mass soluble polymers will depend on the nominal rate of the local flow event. Thus, Cai (1992) predicted a ratio of as high in 160 when comparing the apparent extensional viscosity vs. the apparent shear viscosity in such systems at a relatively high nominal rate. This ratio also has been called Trouton’s factor (Larson 2005).
Figure 22(B) represents the kinds of conformational changes likely to be induced in the semi-random coils of a long linear polymer in solution when experiencing the two kinds of local flow regimes. The stretching effect of extensional flow has been shown by the breakage of polyelectrolyte chains exposed to such regimes (Nguyen and Kausch 1992).
The importance of extensional flow relative to the dispersion of long fibers in a suspension is also consistent with the tendency of such flow events in stretching of bunches of entangled fibers (Higashitani et al. 1989; Li and Ödberg 1997). Thus, Kerekes (1983) found that fiber flocs passing into the entrance to a tube often were stretched to the point of becoming dispersed.
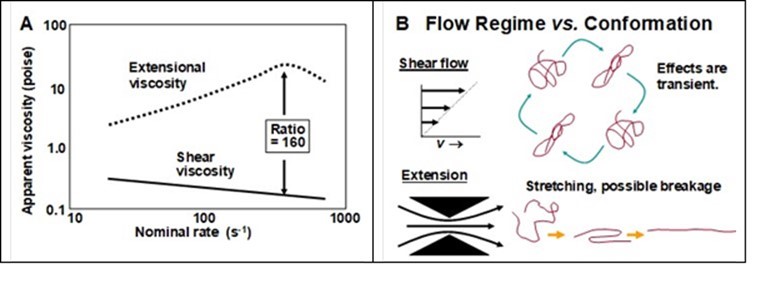
Fig. 22. Relative effects of very-high-mass polyelectrolytes on two aspects of viscosity – extensional vs. shear. A: Apparent viscosity (depending on measurement method) vs. nominal rate of extension or shear. Figure redrawn form the data of Cai (1992). B: Schematic diagram of expected effects of two types of idealized flow on macromolecular conformation
Which of the mechanisms just described best accounts for the main effects in a given system of wet-laid nonwoven processing probably depends on how serious is the need to resist fiber entanglement. When preparing ordinary paper from wood-derived pulp fibers, it is best to avoid high amounts of very-high-mass polymers, since such polymers, due to the increased bulk viscosity, can be expected to lower the rate of water release during the forming process. Papermakers commonly use very-high-mass polyelectrolytes, but only at the rather low addition levels suitable to impart a bridging mechanism of flocculation, thus helping to retain fine particles in the sheet during its formation (Hubbe et al. 2009). The pronounced slowing of dewatering rates from paper sheets when such polyelectrolytes are being used at the higher concentrations sufficient to improve formation uniformity can probably be attributed to the extensional viscosity effects mentioned earlier (Lee and Lindström 1989).
In cases where entanglement problems are relatively minor, it would make sense to strive for effects that can be achieved at a relatively low level of polymer addition, probably relying on adsorption of the polymer molecules to lower friction between the fibers. Such an approach is consistent with the use of associative thickeners, which are water-soluble high polymers having pendant hydrophobic groups (Fonnum et al. 1993; Zhang 2001). Such pendant groups, in addition to contributing to the viscosity of the solution, can also be expected to promote efficient adsorption onto fiber surfaces.
For environmental reasons, the use of highly viscous aqueous solutions is probably best limited to the preparation of wet-laid nonwoven sheets from the most challenging types of fibers in terms of fiber length, hydrophobic character, and entanglement tendency. In such cases, it is necessary to remove the thickener molecules from any wastewater streams before water is discharged from the facility. In principle it is possible to precipitate essentially all of an anionic charged thickener out of solution and onto a particulate filter-aid material such as diatomaceous earth. This can be done by adding a sufficient amount of a high-charge cationic substance in order to neutralize the system charge (Sansalone and Kim 2008; Billuri et al. 2015). The point of charge neutralization can be detected by use of the streaming current method (Barron et al. 1994; Hubbe and Chen 2004) or with a dye indicator system (Rice and Roeraade 2003). The resulting neutral polyelectrolyte complexes, together with the filter aid, will settle and can be collected mechanically from a wastewater clarifier unit. Although water pollution can be largely avoided by the strategy just outlined, one still needs to be concerned about industrial practices that are wasteful of materials, some of which are non-renewable petroleum-derived polymers. Although the non-ionic polyethylene oxide (PEO) has been used in the past as a thickener for wet-laid nonwoven processing (Seibert and Bähr 1970; Ashikaga 1972), PEO cannot be removed from solution by the process just described. Therefore, unless a means has been found to remove PEO from wastewater, its use as a thickener is not recommended.
Cellulosic Fiber Effects
Researchers have suggested that incorporation of a portion of cellulosic fibers along with the chopped synthetic fibers can make it easier to disperse and process hydrophobic fibers within wet-laid fiber suspensions (Ramasubramanian et al. 2008). Though such blends have been reported in research studies (Schon 1977; Yang and Dean 1978a,b; Kozak 1991), there is a need for research to better understand potential advantages related to fiber dispersion and processing.
Blending vs. crowding
One possible contributing factor to the improvement in dispersion of fiber suspensions, upon addition of cellulosic fibers to a long-fibered mixture, is the lower aspect ratio of typical wood-pulp fibers. For instance, Farer et al. (1998) recommended the addition of a substantial proportion of 3-mm softwood kraft fibers to enable the processing of 14-mm cotton fibers. Such a strategy can be rationalized in terms of the crowding factor concepts described earlier. Schoeffmann (1992) described a similar strategy in which relatively short polyester fibers appeared to play the role of “spacers” in a suspension of longer fibers, thus decreasing the frequency of dumbbell-type defects. Holm and Milding (1999) patented a process in which wood-pulp fibers were used in combination with long, hydrophilic plant fibers.
Refining of the cellulosic component
Cellulosic fibers can be modified in various ways to improve the processing or end-use attributes of wet-laid nonwovens. For instance, pulp refining can be used to render the cellulosic fibers more compliant (Holm and Milding 1999). It makes sense that the refined cellulosic pulp fibers, being fibrillated and ribbon-shaped, would be better able to drape over the surfaces of longer synthetic fibers, thus serving as a hydrophilic lubricating layer and facilitating good dispersion. Further research work will be needed to show whether or not such a mechanism is important.
Foam Forming
As an alternative to the use of aqueous solutions as the suspending and transporting medium for wet-laid fibers, it is also possible to employ a surfactant-stabilized foam (Radvan and Gatward 1972; Ring et al. 1977). Foam forming was first developed in the early 1970s using surfactants as viscosity modifiers to create a thixotropic mixture of fiber and aqueous foam (Gatward and Radvan 1973). A properly formulated foam reputedly can achieve good dispersion of the fibers. The patent by Ring et al. (1977) describes the entrainment of air into aqueous solution containing surfactant and thickening agent. This was then used as the suspending medium for wet-laid processing.
As one might expect, the product formed in the presence of foam tends to be bulky but not high in strength (Radvan and Gatward 1972). For example, tissue grades produced with form forming were found to exhibit 30% higher bulk than those formed using conventional methods (Gatward and Radvan 1973). It was later shown that foam forming in combination with multi-ply construction could recover strength loss and create a high-bulk product (Awofeso and Harper 1992). It should be noted that foams also are widely used for application of binders to an existing wet-laid mat (Parsons 1999), a topic that will be considered in the next section.
STRENGTH DEVELOPMENT
Achieving sufficient inter-fiber bonding within a typical all-synthetic wet-laid nonwoven product can be a challenge for two main reasons. First, unlike the types of cellulosic fibers that are widely used in papermaking, the synthetic fibers generally display insufficient conformability of their surfaces and insufficient hydrogen bonding ability to become bound together upon simple drying of the sheet. Second, the presence of relatively high concentrations of thickener in the aqueous solution used in forming the sheet precludes the type of retention aid program that is used in the vast majority of paper machine systems employing only cellulosic fibers (Hubbe et al. 2009). This makes it difficult to achieve efficient retention of latex of other binder materials added to the fiber slurry. Briefly stated, if one were to inject cationic polyelectrolytes into a typical wet-laid nonwoven system, as is often done during ordinary papermaking, then one would run the risk of destroying the progress one has made in dispersing the fibers and inhibiting their entanglement.
As was stated in the Introduction, several different approaches have been used to impart inter-fiber binding to wet-laid nonwovens products. To summarize, those approaches can be listed as (a) saturation (dipping, foam application, or spraying the fiber web), (b) the use of meltable fiber (including bi-component fibers), (c) the use of semi-soluble fibers, (d) addition of binders to the wet end of the operation (perhaps assisted by pre-flocculation, polyelectrolyte complexes, etc.), and (e) blending cellulosic fibers into the composition at a suitable level. In addition, cohesion of wet-laid fabrics also can be achieved by hydroentanglement, which will be considered first.
Hydroentanglement
Hydroentanglement or spunlacing is commonly used to mechanically bond fibers in nonwovens and to impart improved physical properties, such as soft handle and drapability (Kamath et al. 2004). Hydroentanglement in wet-laid production is achieved by subjecting a freshly-formed mat of fibers to numerous tiny jets of water so that the fibers knot about one another (Evans and Shambelan 1971; Nakamae and Shima 1990; White 1992; Riedel et al. 1996; Holm and Milding 1999; Maity et al. 2014). The method is analogous to needling of fibers on dry mats, which is used to improve mechanical properties through enhanced fiber-to-fiber bonding. Probably the earliest example of a hydroentangling process is the work of Kalwaites and Guerin, describing the use of water jets to achieve fiber locking and entanglement on dry-formed nonwovens (Kalwaites 1958; Guerin and Jeandron 1965). In wet-laid forming, it was well known that fibers within a wet sheet can be reoriented to improve mechanical properties. For example, shaking the forming section of the machine to redistribute fiber and improve formation was used as early as the 1920’s (Aldrich 1926). Similarly, water jets sprayed through the forming fabric and carrier felts have been used to disrupt fiber distribution and improve cross-direction strength (Kalwaites 1973). However, the earliest use of the direct use of water jets impinging on a wet-formed sheet is attributed to the work of Evans in the mid-1960’s (Evans 1970). According to White (1992), it is typical to employ about 12 water jets per cm, with each such jet having a diameter of about 0.1 mm. The little jets change the orientation of some of the fibers that make up the sheet, such that some of them become at least partly oriented in the thickness direction (Gilmore et al. 1996). Friction among the thus-entangled fibers is often enough to provide adequate strength to the sheet even in the absence of chemical bonding agents. Viazmensky et al. (1991) were among the first to demonstrate the benefits of hydroentanglement on wood-based composites. They showed how toughness in polyester fiber-wood pulp composites could be increased by 200% using hydroentanglement. Additionally, their process used relatively low pressure water jets, requiring 300 to 2000% less input energy. According to Riedel et al. (1996), hydroentanglement can be regarded as the processing method of choice for wet-laid nonwoven production in general. Holm and Milding (1999) in their patent disclosed the advantageous use of a dispersing agent (75% bis(hydrogenated tallow alkyl) dimethyl ammonium chloride and 25% propyleneglycol) to help the fibers slide past each other in response to the impinging jets of water. Dever (1993) described favorable results from ultrasonic bonding; it appears likely that hydroentanglement may account for at least some of the observed effects.
Binder Application to the Web
Binder chemicals such as latex can be applied in various ways (Scholz 1985; Williamson 1993; Hutten 1995; Parsons 1999). As illustrated in Fig. 23, these can include saturating of the freshly-formed fiber mat, spraying (Chakrabarti 1979a; Parsons 1999; Williams 1999), foam application (Parsons 1999; Williams 1999), and use of a size press nip (Williamson 1993; Hutten 2007).
Fig. 23. Schematic views of alternative schemes for saturation of a freshly-formed nonwoven fabric with latex binder. A: saturation by immersion in a bath; B: spay application; C: foam application; D: size press application
In addition to the “pond” type of size press illustrated in part D of the figure, it is also possible to meter solution to be applied to the front and back surfaces (Hutten 2007). It is also possible to “print” regions of bonding onto a nonwoven mat just after its formation, followed by curing (Parsons 1999; Williams 1999). Also it is possible to rely upon the action of meltable or partially soluble fibers to develop bonding during the hot conditions of drying the product. It is less common to attempt the addition of non-fibrous bonding chemicals to the suspension of fibers before preparation of a nonwoven mat. All of these options will be considered here, with emphasis on chemical aspects. The range of available binder application technologies, emphasizing web application, has been reviewed (Parsons 1999).
Saturation
Various strategies that can be lumped under the category of “saturation” make it possible to apply binder after the wet-laid fiber structure has been prepared. Due to their content of relatively long fibers, such webs typically have sufficient strength to remain intact on a moving screen while being pulled through a dipping bath, a foam application, or being exposed to spray, or passing through a size-press nip, etc.
In a typical saturation operation, the freshly-formed sheet, after vacuum application, is directed into a bath that contains a latex suspension. For instance, a rotating roll, around which the web is passing, may be lowered into the bath at the start of the operation. Parsons (1999) gives typical ranges of composition as 10 to 25% binder solids in the saturating bath, leading to between 20 and 60% of binder, by mass, in the final product. In addition to the binder, the bath can include a wetting agent, a defoamer, and sometimes an agent to induce curing of the binder, i.e. a catalyst.
According to Gill et al. (1972), saturation of the fiber mat with a bonding agent such as latex has been found to achieve substantially greater effectiveness in comparison to adding the same amount of bonding agent to the water suspension before forming the sheet. Such findings can be rationalized based on two key aspects of a typical wet-laid forming operation. First, it is typical to have a very high ratio of aqueous solution to solids in such a mixture, making it inherently challenging to attempt to retain fine solids on the fiber surfaces during sheet formation. Second, as already discussed, it is typical to include both dispersants and thickeners in the solution, and these substances will tend to disperse latex particles or other bonding agents, rather than to retain them on fiber surfaces. According to Hutten (1995), some manufacturers practice “dry-end saturation” in which the web is dried, then subjected to binder treatment, then dried again.
One of the challenges associated with most saturation systems is that the binder often consists of small, round particles, usually latex. For instance, the binder may have diameters less than about 1 mm (Oelkers and Sweeney 1988; Ozaki 2011). Even if such particles are retained on the web of fibers, they will not necessarily be in locations where they are able to connect adjacent fibers together. Also, due to the excess of negatively charged thickening agent still present, negatively charged latex particles (which are the most common) may tend to remain in the solution phase rather than become deposited onto the fiber structure.
Binder types
Latex
Latex products, usually comprised of copolymer particles prepared from monomers such as styrene, butadiene, and crosslinking agents, have been widely used as binders for wet-laid nonwoven fabrics (Seifer and Kline 1977; Distefano 1985; Tischer et al. 1989; Dever 1993; Williams 1999; Kumar et al. 2006). A general review of the technology is provided by Distefano (1985), who proposed that some of the applied latex, but not all of it, tends to migrate toward fiber junctions during drying, as a result of capillary action. Finley and Mlynar (1999) describe the dilution of latex formulations, as well as the addition of processing aids in preparation for usage. Belokurova et al. (2007) describe a radiation-induced polymerization of latex and claim that it can offer improved properties of nonwoven fabrics. Barlett et al. (1981) describe the use of thermoplastic resins in wet-laid nonwoven production.
Reactive-cure resins
Curing-type resin systems also have been widely used for binding of nonwoven products. Urea-formaldehyde systems can provide water-resistant bonding at moderate cost, so they have been widely used (Whichard 1985). Young (1996) and Jackson (1997) describe the use of waterborne epoxy resins, which generally are nonionic and compatible with latex products. Wallace (1999) describes the use of crosslinking thermo-set resins such as a melamine-formaldehyde product, to increase durability.
Salt-sensitive
Wet-wipe products intended for baby-care can be prepared by an innovative bonding system in which a solution of a salt is used to induce precipitation of a highly carboxylated binder (Wang et al. 1999; Farwaha et al. 2012). After usage, if such a product is flushed, the concentration of salt becomes sufficiently diluted such that the mixture is resolubilized and the fibers are redispersed. Wang et al. (1999) patented a system in which divalent ions such as Ca2+ or Mg2+ were used as a more effective way to suppress solubility of the resin and induce the temporary bonding in the wet state. Given the carboxylated character of thickening agents already present in many wet-laid nonwoven systems, this kind of approach appears to be well matched with common wet-laid processing practices.
Sizing of synthetic fiber surfaces with binder
A patent by Gleich et al. (2007) provides an example of a way in which binder can be applied to the surfaces of synthetic fibers at the point of their manufacture. In principle this is done while the fibers are still in the form of continuous filaments, such that they can be exposed to a solution of binder resins, lubricants, and other agents before being dried and chopped. Though this approach makes sense in terms of strength development and other end-use performance issues, one would need to carefully evaluate such sized products, in each case, to make sure that the sizing treatment did not adversely affect the process of dispersal of the fibers in preparation for wet-laid processing.
Meltable Fibers
Low-density polyethylene is an example of a fiber-forming synthetic polymer that melts at about 115 °C (Tomiyoka 1977). By including such fibers as a minor component in a mixture of higher-melting fibers such as polypropylene or nylon, one can achieve bonding by drying the web at an intermediate temperature (Mehta et al. 1995; Tadokoro et al. 1999; Goettmann et al. 2001; Yeang et al. 2012; Wang et al. 2013). If the different polymers are sufficiently similar in chemical composition, then one can expect a degree of interpenetration of macromolecular chains at the phase boundaries (Voyutskii 1963), giving rise to adhesive strength that can approach the cohesive strength of the weaker plastic material.
In principle, meltable binder fibers offer the advantages of having sufficient length to bridge efficiently between the main structural fibers of the web. In addition, nearly 100% of the binder fibers, depending on their lengths, can be expected to be retained during formation of the web. Goettmann et al. (2001) patented a further variation of such a process in which a mat composition incorporating meltable fibers was dried below the melting point, rendering it more easily recyclable. Users of such a dried product then have the option of later inducing enhanced bonding, either generally or locally, by the application of heat. Thus, Michielsen et al. (2006) reviewed technology to achieve point bonding in nonwoven fabrics containing meltable binder fibers. Yeang et al. (2012), by contrast, patented the use of a biodegradable low-melting poly-lactic acid binder fiber. Smith (1988a,b) reported the use of cellulose acetate fibrets, which were found to display a thermoplastic bonding capability.
In an effort to achieve a combination of good bonding and dimensional stability, various authors have reported the use of co-extruded bi-component fibers (Tomiyoka 1977; Madsen 1984; Ezaki 1988; Nielsen and Davies 1992; Nielsen and Johnson 1992; Doh et al. 2013; Fages et al. 2013). These are designed such that only one of the components melts during heat-drying of the web.
Semisoluble Fibers
Closely related to the use of meltable fibers, it is also possible to employ fibers that have limited or substantial solubility in water. For instance, Doh et al. (2013) reported the use of carboxymethylcellulose fibers, the solubilization of which was temporarily suppressed by the use of a water-alcohol mixture during formation of the web. Related technology is described by Ohta et al. (2015).
Polyvinyl alcohol (PVOH) fibers also have been shown to provide bonding in a wet-laid nonwoven process, in which high-temperature drying is used as a curing step (Ashikaga 1972; Inagaki 1979; Scholz 1985; Fages et al. 2013). PVOH fibers appear to provide bonding by the softening of their semi-soluble surfaces, followed by resolidification during drying of the fiber mat. As noted by Ashikaga (1972), it may be necessary to employ a release agent to avoid excessive sticking of such a product to drier can surfaces. Jung et al. (2005) described the preparation of nano-sized PVOH fibers by electrospinning and their use in making nonwoven mats.
Wet-end Binders
As already mentioned, the relatively small particles of latex tend to be inefficiently retained in a nonwoven sheet when added to a fiber slurry (Gill et al. 1972). It follows that some of the potential ways to overcome this low retention efficiency are based on increasing the effective size of the binder particles.
Pre-flocced nanoparticles
Nanofibers might potentially play a role in the retention of bonding agents within a nonwoven sheet. Karwa et al. (2012) found that carbon nanofibers could be retained in a wet-laid process. The nanofibers were preflocculated with cationic polyacrylamide before addition to the fiber suspension.
In principle, latex particles could be incorporated into such agglomerates, which then, depending on the size of the nanofibers, might likely be retained by filtration in the wet-laid mat. Kohler and Nebel (2006) took the opposite approach of adding cellulose nanofibers and then impregnating the nanocellulose sheet with thermosetting resins, as described in a previous section.
Pre-flocced binders
In principle, the effective size of binder particles can be increased by agglomerating them together. If this is done using a very-high mass cationic acrylamide copolymer (a retention aid), then such agglomerates might persist even after the system has become surrounded by an excess of thickener macromolecules, as mentioned before.
Even after latex particles have been agglomerated together, it is still doubtful that they would be large enough to be efficiently filtered out of solution by the fiber mat. So the answer may lie in co-agglomeration of latex with highly fibrillated cellulose fiber or nanofibers, yielding a structure that will be efficiently retained and be capable of bridging between adjacent synthetic fibers in the wet-laid web. A search of the literature, in preparation for the present article, did not reveal any significant research of this type.
Polyelectrolyte complexes
In the case of glass fibers, some varieties of which are readily dispersed in water even in the absence of dispersants, it has been shown that paper-like inter-fiber bonding strength can be achieved by in-situ formation of polyelectrolyte complexes (PECs) (Hubbe 2005). Sheets formed from such fibers in the absence of such treatment had absolutely no strength, such that the fibers could be dispersed into the air just by blowing onto the dried sheet. Similarly, mere addition of either positively or negatively charged polymers in solution was ineffective in imparting strength. As shown in Fig. 24, a sequential addition of carboxymethyl cellulose (CMC) (anionic) and poly-(diallyldimethylammonium chloride) (cationic) was able to achieve paper-like strength, especially when the ratio of polyelectrolytes was within about 30% of a charge-balanced condition.
Fig. 24. Importance of in-situ mixing procedure to achieve high increases in bonding strength among glass fibers by use of sequential addition of cationic and anionic polyelectrolytes. Data from Hubbe (2014); copyright held by author
Whether such a PEC system, as just described, could be used in a system already stabilized by anionic thickener macromolecules remains uncertain. Addition of a stoichiometric amount of cationic polymer to balance the charge might have the unfortunate effect of causing overall flocculation and entanglement of a long-fibered suspension, leading to poor uniformity of the mat. If the amount of cationic polyelectrolyte is below the stoichiometric amount needed for neutralization, then the polyelectrolyte complex would likely not be retained effectively. Thus, though the described in-situ complexation strategy has been found to perform very well in the laboratory as a bonding agent for glass microfibers (Hubbe 2005, 2014), there are some unsolved issues that would need to be addressed before applying such a system to practical wet-laid nonwovens applications.
Blending with Cellulosic Fibers
Even in products that contain a major proportion of wood-derived fibers, the term “nonwovens” seems appropriate when the blend contains a portion of synthetic fibers that are too long to be employed in a typical paper machine system. For instance, such fibers would tend to clog or become rejected in the screening systems used just before the approach to the forming section of a conventional paper machine. Given the fact that cellulosic pulp fibers from wood, sugarcane bagasse, wheat straw, and the like are being used on a very large scale for ordinary papermaking, in addition to their widespread usage also in wet-laid processing, it makes sense to consider them as a way to contribute to bonding of the wet-laid products made predominantly from chopped synthetic fibers. In principle, cellulosic fibers can contribute substantial hydrogen bonding within a blended wet-laid sheet, such that substantial dry-strength can be achieved even without addition of a separate binder (Hubbe 2006). Favorable results, for such usage of cellulosic fibers, have been reported (Schon 1977; Yang and Dean 1978a,b; Kozak 1991). Yang and Dean (1978a,b) showed that the relatively long wood-derived fibers obtained by the kraft pulping of Douglas fir and redwood fibers were promising in combination with synthetic fibers for the development of strength. Johnson and Noegi (1990) patented the use of bacterial cellulose as a binder in wet-laid nonwovens, claiming that it could serve as a total replacement for latex.
Wet-strength chemistry
Another potential advantage of adding cellulosic fibers to a blend, in the preparation of a nonwoven product, is that one then may be able to take advantage of using the kind of wet-strength chemistry that is in most common use within the paper industry (Devore and Fischer 1993; Espy 1995). Such additives, due to their cationic charge, adsorb readily onto cellulose fiber surfaces. Curing, involving mainly self-crosslinking of the copolymer, takes place during drying of the paper. Schon (1977) employed such a wet-strength agent in a wet-laid sheet that contained unbeaten bleached softwood pulp. To compensate for the relatively low inter-fiber bonding in a bulky sheet prepared from such unrefined fibers, the bonding was supplemented by saturating the sheets with a latex binder.
Cationic latex
Since wet-end addition of strength-enhancing agents is used routinely in conventional papermaking with wood-derived fibers (Hubbe 2006), the methods typical of such applications will be considered first. In particular, there appear to be opportunities to employ traditional bonding agents, common to ordinary papermaking, when cellulosic fibers make up a substantial proportion of the fiber blend. Typical agents for ordinary paper products include cationic starch and cationic acrylamide copolymers that can be added to the cellulosic fiber suspension. The generally negative net charge of untreated cellulosic fiber surfaces (Herrington and Petzold 1992) provides a favorable situation for adsorption of the positively charged polyelectrolytes. During drying of a typical paper web, the cationic starch or other bonding agent can participate in hydrogen bond formation that connects the solid surfaces together, typically exceeding the strength achievable in the absence of the additive. Ulyatt (1976) proposed the use of cationic latex products in order to achieve better retention onto fibers, with emphasis on rayon and wood pulp.
A major hurdle when seeking to employ a conventional papermaking approach to dry-strength development among the cellulosic fiber component is an inherent incompatibility of such treatments in the presence of a high concentration of anionic polyelectrolytes, i.e. the thickening agents. Instead of adsorbing onto the fiber surfaces, cationic polyelectrolytes such as cationic starch would be expected to form polyelectrolyte complexes with any anionic thickener macromolecules present in the solution (Dautzenberg 1997). As already noted, there may be opportunities to employ a polyelectrolyte complexation strategy is some cases (Hubbe 2005; Arboleda et al. 2014; Hubbe 2014; Lee et al. 2015), but such an approach has not been widely considered and can hardly be regarded as a conventional papermaking approach.
Based on previous work, the adsorption of cationic starch and related chemicals onto cellulosic fibers tends to be highly influenced by the charge environment, at least during the time periods associated with the formation of a paper sheet (Shirazi et al. 2003). Thus one can envision a process in which cellulosic fibers are initially treated with an optimized level of cationic starch or related dry-strength agent, and then the fiber suspension is added to a mixture where there is a sufficiently large excess of anionic thickening agent in solution – possibly also with the presence of some synthetic fibers that may be part of the formulation. There is reason to expect there to be local interaction between the anionic macromolecules of thickening agents and the pre-adsorbed (cationic) dry-strength agent (Dautzenberg 1997), and past work suggests that the resulting surface-bound polyelectrolyte complexes will be quite effective in the promotion of dry strength (Hubbe 2005). Tests would need to be conducted to verify or optimize the continued retention of the cationic starch or other strength-enhancing polymer onto the fiber surface even in the presence of an excess of anionic polymers in solution.
It has not been clearly demonstrated whether or not latex particles can be retained on the cellulosic components in a mixed natural-synthetic fiber blend, using the strategy just described. Yang and Dean (1978b,c) described the usage of latex binder along with wood pulp fibers in sheets made mainly from chopped synthetic fibers. Past work suggests that cationic polyelectrolytes can become desorbed relatively easily from the surfaces of typical latex particles (Eriksson and Alm 1993). Thus, even if the latex particles initially had been anchored onto cellulosic fibers by means of a cationic acrylamide retention aid (Hubbe et al. 2009), such particles might end up individually suspended in the bulk solution once the fibers have become surrounded by negatively charged polyelectrolytes, i.e. the dispersants and thickeners. One way to overcome such a problem may lie in the use of cationic latex products (Ulyatt 1976; Alince 1977). Such products are prepared with incorporation of amine groups, which render a positive charge when immersed in water. Alternatively, Harding (1986) employed cationic cellulose fibers and was able to retain ordinary anionic latex particles on their surfaces during a wet forming process. Persistent adsorption of latex particles onto the cellulosic fibers may also be enhanced by mechanical refining, such that cellulosic nanofibrils at the fiber surface become draped over latex particles. Indeed, microfibrillated cellulose fibers (Siro and Plackett 2010; Sandquist 2013), which are achieved by extensive refining, may offer opportunities as a co-binder material in combination with cationic latex particles.
Cationic latex products were already mentioned in the context of systems in which a binder is added to the fiber suspension, an approach that ordinarily makes sense in the absence of anionic thickening agents in the aqueous solution. But when papermaking fibers are being used, their hydrophilic character and moderate length and aspect ratio make it feasible to directly add cationic latex to a slurry of the cellulosic fibers. The latex can be expected to adhere pretty well to the fibers due to opposite charges (Alince 1977; Alince et al. 1978; Alince 1999; Alince et al. 2000) and due to fibrillation of the fibers, which increases their effective surface area and makes them more compliant (Paavilainen 1993; Dasgupta 1994; Hubbe 2006). Alince (1999) showed that, in addition to providing bonding, latexes can be used as a way to increase the hydrophobic character of sheets formed from cellulosic pulp.
Curing Processes
Except in the case of wet-wipe products (Wang et al. 1999; Farwaha et al. 2012), essentially all wet-laid nonwoven products are delivered to the customer in dried form. Curing conditions and their effects have been found to be important, for instance, when using low-melting binder fibers (Tomiyoka 1977; Clark and Shiffler 1985; Ezaki 1988; Latimer and Schultz 1990; Mehta et al. 1995). Fages et al. (2012) found that by hot-press molding it was possible to achieve bonding in a wet-laid structure with a relatively low amount of polypropylene binder fibers. Notably, their work was carried out with a majority of flax fibers. As noted by Alince (1999), the application of heat during drying of a latex-saturated mat of fibers can induce coalesce of the latex into a film, which can at least partly cover the surfaces of the fibers and bind them together. By contrast, thermoset binders in a nonwoven sheet require heat to complete a reaction in order to achieve strong, water-resistant bonding (Butterworth et al. 1980).
Although drying is often the last step in forming a wet-laid nonwoven product, there are some exceptions to this rule. For instance, the dry product can be subjected to calendering, embossing, and specialized procedures to improve the soft feel of nonwoven products (Gill et al. 1972).
One way to enhance softness is by a creping operation, i.e. the material is doctored off of a rotating roll (Fredericks 1976; Wang et al. 1999). The creping induces microscopic corrugations into the material, rendering it bulkier and more stretchable. As a related approach, Reese and Cooper (2002) described various heat treatments induced by passing a nonwoven mat containing a heat-setting binder component between heated rollers.
SUMMARY AND FUTURE PROSPECTS
Based on the results of studies and patents considered in this review article, though interest in wet-laid nonwoven manufacturing may have reached a peak in the 1970s and 1980s, there continues to be progress in technical aspects of the field. Also, there is a continuing need for improvement in fiber quality and novel attributes than can allow wet-laid products to displace textiles or conventional paper products in a range of applications. Despite progress being made in a variety of practical issues, the fundamentals of fiber wetting, fiber dispersion, and repression of fiber entanglement are in need of research attention to address various open questions mentioned in the course of this article. The complex interactions between different water-soluble components imply that there will be a continuing need for research related to different binding strategies. In addition, there will be a need for research related to the treatment of process water from wet-laid nonwovens facilities – especially in light of the use of water-soluble dispersants, thickeners, defoamers, sizing agents desorbed from synthetic fibers, and various surfactants and binders in the process water.
There is a need for more study of the economic factors related to the manufacture of wet-laid nonwovens. As was noted earlier, it has been predicted that such manufacture is on the threshold of a period of growth at a 5.1% annual rate (Smithers Apex 2015). Such growth is consistent with the known characteristics of the technology and its great variety of potential product attributes. Based on the types of products currently made using wet-laid forming, it is possible to anticipate growth in such product classes as hygiene, upholstery, filtration, wipes, roofing, coverings, and automotive (Koukoulas 2016). The extent of this growth cannot be precisely known, since it will depend on future market conditions and individual investment decisions. On the other hand, the unique combinations of high strength, uniformity of porosity, relatively dense sheets, and great flexibility of composition with blends of different fibers can be expected to provide a stable market segment of nonwoven products that will continue to employ wet-laid forming as a main manufacturing route. There is a need for further research related to characteristics such as pore size distribution (Bagherzadeh et al. 2013), which can be regarded as a strong-point of wet-laid nonwovens technology.
Regarding increased usage of cellulosic fibers in wet-laid nonwovens, it seems that the prospects are quite positive, especially in their expanded use in the disposable and flushable wipe market segments. It is clear from the cited work that cellulosic fibers, including microfibrillated cellulose, are highly compatible with wet-laid processing. The renewable and eco-friendly nature of cellulosic fibers can be expected to attract cautious support from regulatory agencies in the coming years. Also, the testing of cellulosic fibers to facilitate uniformity of fiber dispersion and to help inhibit entanglement of long fibers in the mixture will tend to be attractive to manufacturers of wet-laid nonwoven products over the long run. At the limit where wet-laid nonwovens merge with conventional papermaking technology, i.e. when the content of synthetic fibers is relatively low, the costs of material, the production rates, and eco-friendly processing suggest a bright future for paper-like products that contain chopped synthetic fibers as a means to achieve enhanced sheet properties.
REFERENCES CITED
Ahrens, R. A. (1982). “Wet-laid forming of nonwoven fabrics,” TAPPI Nonwovens Symp., Myrtle Beach, Notes, pp. 39-40.
Aldrich, A. (1926). “Papermachine frameless type,” U.S. Patent 1,595,593.
Alexandridis, P., and Hatton, T. A. (1995). “Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block-copolymer surfactants in aqueous-solutions and at interfaces – Thermodynamics, structure, dynamics, and modeling,” Colloids Surf. A 96, 1-46. DOI: 10.1016/0927-7757(94)03028-X
Alince, B. (1977). “Performance of cationic latex as a wet-end additive,” Tappi J. 60(12), 133-136.
Alince, B. (1999). “Cationic latex as a multifunctional papermaking wet-end additive,” Tappi J. 82(3), 175-187.
Alince, B., Arnoldova, P., and Frolik, R. (2000). “Cationic latex: Colloidal behavior and interaction with anionic pulp fibers,” J. Appl. Polym. Sci. 76(11), 1677-1682. DOI: 10.1002/(SICI)1097-4628(20000613)76:11<1677::AID-APP9>3.0.CO;2-7
Alince, B., Robertson, A. A., and Inoue, M. (1978). “Deposition of cationic styrene and styrene-butadiene latex particles on cellulose fibers,” J. Colloid Interface Sci. 65(1), 98-107. DOI: 10.1016/0021-9797(78)90262-X
Angelini, P. J. (1988). “Fiber to web process – Wet lay,” INDA-TEC Int. Nonwovens Fabrics Conf., Book of Papers, Ft. Lauderdale, pp. 49-62.
Anon. (2005). “Wet nonwoven technology – Nonwovens on the way out?”, (“Wasserstrahlverwirbelung zur Vliesverfestigung und zu vielem mehr,”) AVR Allgemeiner Vliesstoff-Report (6), p. 12.
Arboleda, J. C., Niemi, N., Kumpunen, J., Lucia, L. A., and Rojas, O. J. (2014). “Soy protein-based polyelectrolyte complexes as biobased wood fiber dry strength agents,” ACS Sustainable Chem. Eng. 2(10), 2267-2274. DOI: 10.1021/5c500399d
Ashikaga, T. (1972). “Manufacture of wet-laid nonwovens by application of polyvinyl alcohol fibers,” Proc. IUPAC/EUCEPA Symp. Man-Made Polymers Pmkg, Helsinki, pp. 187-192.
Atalay, S., Ma, Y., and Qian, S. Z. (2014). “Analytical model for charge properties of silica particles,” J. Colloid Interface Sci. 425, 128-130. DOI: 10.1016/j.jcis.2014.03.044
Avery-Edwards, D., Elms, R., and Buckingham, A. (1994). “Silicone antifoams for nonwoven applications,” Proc. TAPPI 1994 Nonwovens Conf., 111-115.
Awofeso, A. O., and Harper, F. D. (1992). “Soft, high bulk foam-formed stratified tissue and method for making same,” U.S. Patent 5,164,045.
Bagherzadeh, R., Najar, S. S., Latifi, M, Tehran, M. A., and Kong, L. X. (2013). “A theoretical analysis and prediction of pore size and pore size distribution in electrospun multilayer nanofibrous materials,” J. Biomed. Mater Res. Part A 101A: 2107-2117.
Bajaj, P. (1997). “Spin finishes for manufactured fibres,” in: Manufactured Fibre Technology, Gupta, V. B., and Kothari, V. K. (eds.), Chapter 7, pp. 139-169.
Balaji (2015). Balaji Cotton Linter, “Cotton linter: Introductions,” website: https://sites.google.com/site/cottonlinters/
Barlett, C. A., Kenned, D. J., and Wallace, P. D. (1981). “Custom designing and development of resins for the nonwovens industry,” INDA Tech. Symp. Nonwovens 9, Atlanta, pp. 33-42.
Barron, W., Murray, B. S., Scales, P. J., Healy, T. W., Dixon, D. R., and Pascoe, M. (1994). “The streaming current detector: A comparison with conventional electrokinetic techniques,” Colloids Surf. A Physicochem. Eng. Aspects 88, 129-139. DOI: 10.1016/0927-7757(94)02824-9
Belokurova, G. B., Malyukova, E. B., Bobylev, A. P., Fomin, V. N., Gorchakova, V. M., and Polikarpov, V. V. (2007). “Manufacture of polymer composite materials using copolymeric latexes of different nature,” Fibre Chem. 39(3), 185-188. DOI: 10.1007/s10692-007-0036-5
Bergmann, L. (1989). “Nonwovens for filtration media,” Tappi J. 72(1), 77-79.
Berli, C. L. A., Piaggio, M. V., and Deiber, J. A. (2003). “Modeling the zeta potential of silica capillaries in relation to the background electrolyte composition,” Electrophoresis 24(10), 1587-1595. DOI: 10.1002/elps.200305371
Billuri, M., Bonner, J. S., Fuller, C. B., and Islam, M. S. (2015). “Impact of natural cationic polymers on charge and clarification of microalgae suspensions,” Environ. Eng. Sci. 32(3), 212-221. DOI: 10.1089/ees.2014.0301
Bourdon, R. P. (1971). “Cutting synthetic fibers for use on paper machines,” Tappi 54(7), 1103-1104.
Brandenburg, K. L. (1993). “Critical process variables in the white water system that affect glass fiber dispersion,” Proc. 1993 Nonwovens Conf., TAPPI Press, Atlanta, pp. 45-49.
Brandon, R. E., Dale, R. S., and Householder, K. A. (1988). “Utilization of glass fibers in nonwoven fabrics,” INDA-TEC Int. Nonwovens Fabrics Conf., Ft. Lauderdale, pp. 447-458.
Brandon, R. E., Davis, C. J., Ring, M., and Swenson, R. S. (1980a). “Viscous dispersion for forming wet-laid, non-woven fabrics,” U.S. Pat. 4,049,491
Brandon, R. E., Davis, C. J., Ring, M., and Swenson, R. S. (1980b). “Viscous dispersion for forming wet-laid, non-woven fabrics,” U.S. Pat. 4,200,488.
Brandon, R. E., Davis, C. J., Ring, M., and Swenson, R. S. (1985). “Wet-laid non-woven fabrics,” US Pat. 4,512,849, International Paper.
Briscoe, B. J., Khan, A. U., and Luckham, P. F. (1998). “Optimising the dispersion on an alumina suspension using commercial polyvalent electrolyte dispersants,” J. Eur. Ceramic Soc. 18(14). 2141-2147. DOI: 10.1016/S0955-2219(98)00147-2
Butterworth, G. A. M., Elias, R. T., and Miller, W. D. (1978). “Fibrous material and method of making the same,” US Pat. 4,129,132.
Butterworth, G. A. M., Elias, R. T., Miller, W. D., and Shepherd, R. C. (1980). “Fibrous material and method of making the same using thermoplastic synthetic wood pulp fibers,” US Pat. 4,425,126
Cai, B., Zheng, Q.-K., Li, R. X., and Wu, D.-C. (2003). “Adsorption mechanism of surfactants on nonwoven fabrics,” J. Appl. Polymer Sci. 89(12), 3210-3215. DOI: 10.1002/app.12428
Cai. (1992). Center for Interfacial Engineering, redrawn figure.
Chakrabarti, P. M. (1979a). “Method of increasing the strength of wet glass fiber mats made by the wet-laid process,” US Pat. 4,178,203.
Chakrabarti, P. M. (1979b). “Glass fiber dispersions for making uniform glass fiber mats by the wet-laid process,” US Pat. 4,179,331.
Charman, W. N., Christy, D. P., Guenin, E. P, and Monkhouse, D. C. (1991). “Interaction between calcium, a model divalent cationic, and a range of poly(acrylic acid) resins as a function of solution pH,” Drug Devel. Indust. Pharmacy 17(2), 271-280. DOI: 10.3109/03639049109043824
Chen, Q., Chen, Z. F., Li, C. D., Wu, W. P., Boafo, F. E., Li, B. B. (2014). “Effect of dispersants on dispersion of glassfiber suspensions,” Asian J. Chem. 26(16), 5100-5104.
Clark, J. T., and Shiffler, D. A. (1985). “Synthetic water-dispersible fiber,” US Pat. 4,707,407.
Clitherow, B. M., Dean, T. W. R., Gascoigne, J. A., and Van Issum, B. E. (1991). “Filler compositions and their use in papermaking,” US Pat. 5,017,268.
Das, D., Butola, B. S., and Renuka, S. (2012). “An investigation into fiber dispersion behavior in water with reference to wet-lay nonwoven technology,” J. Disper. Sci. Technol. 33(8), 1225-1232. DOI: 10.1080/01932691.2011.590437
Dasgupta, S. (1994). “Mechanism of paper tensile-strength development due to pulp beating,” Tappi J. 77(6), 158-166.
Dautzenberg, H. (1997). “Polyelectrolyte complex formation in highly aggregating systems. 1. Effect of salt: Polyelectrolyte complex formation in the presence of NaCl,” Macromol. 30(25), 7810-7815. DOI: 10.1021/ma970803f
DeJong, E. (1999). “Towards industrial applications of bast fiber pulps,” Pulp Paper Can. 100(9), 19-22.
Dever, M (1993). “Comparison between acrylic latex bonding and ultrasonic bonding of a polyolefin nonwoven,” Tappi J. 76(4), 181-189.
Devore, D. I., and Fischer, S. A. (1993). “Wet-strength mechanism of polyaminoamide-epichlorohydrin resins,” Tappi J. 76(8), 121-128.
Dickinson, E. (2003). “Hydrocolloids at interfaces and the influence on the properties of dispersed systems,” Food Hydrocolloids 17(1), 25-39. DOI: 10.1016/S0268-005X(01)00120-5
Distefano, F. V. (1985). “Chemical bonding of nonwoven webs,” in: TAPPI Notes: 1985 Nonwovens Binders: Additives Chemistry and Use Seminar, TAPPI Press, Atlanta, pp. 49-54.
Doh, S. J., Lee, J. Y., Lim, D. Y., and Im, J. N. (2013). “Manufacturing and analyses of wet-laid nonwoven consisting of carboxymethyl cellulose fibers,” Fibers and Polymers 14(12), 2176-2184. DOI: 10.1007/s12221-013-2176-y
Dow (2016). “Polypropylene glycols and copolymers North America – Fiber and textile processing,” http://www.dow.com/polyglycols/ppgc/na/apps/fiber.htm
Dunn, M. P., and Harke, J. F. (1991). “Evolution and development of headbox systems for specialty nonwoven formers,” TAPPI Nonwoven Conf. Proc., Marco Island, FL, pp. 263-272.
Dupont (2016a). “NOMEX®: a family of insulation materials,” http://www2.dupont.com/ReliatranV3/en_RU/products/Nomex/More/Nomex_family.html
Dupont (2016b). “Honeycomb composite made lighter and stronger,” http://www.dupont.com/products-and-services/fabrics-fibers-nonwovens/fibers/uses-and-applications/honeycomb-composites.html
Elliot, I. T. (1987). “Rotonier concept for wet-laid nonwovens,” TAPPI Nonwovens Conf. Proc., Orlando, pp. 179-182.
EPA (1997). “The synthetic fiber industry,” in: Industrial Process Profiles for Environmental Use, U.S. Environmental Protection Agency, Chapter 11.
Eriksson, L., and Alm, B. (1993). “Effects of polyelectrolyte characteristics and flocculation conditions on fine particle floc properties,” Nordic Pulp Paper Res. J. 8(1), 153-159. DOI: 10.3183/NPPRJ-1993-08-01-p153-159
Esaki, T., Okazaki, M., and Sonetaka, T. (1989). “High-bulky polyester paper produced by wet-laid method,” Kinoshi Kenkyu Kaishi (28), 35-39.
Espy, H. H. (1995). “The mechanism of wet-strength development in paper – A review,” Tappi J. 78(4), 90-99.
Evans, F. J. (1970). “Aperture tanglelaced nonwoven textile fabric,” U.S. Patent 3,498,874.
Evans, F. J., and Shambelan, C. (1971). “Water-dispersible nonwoven fabric,” US Pat. 3,563,241.
Evans, R. E., and Pfeiffer, R. E. (1992). “A new generation of fibrillated acrylic fiber for high performance non-wovens and specialty composites,” in: Fibers and Forming for Nonwovens, TAPPI Press.
Ezaki, T. (1988). “Polyester staple fibers for nonwoven materials,” Kinoshi Kenkyu Kaishi (27), 10-18.
Fages, E., Cano, M. A., Gironés, S., Boronat, T., Fenollar, O., and Balart, R. (2013). “The use of wet-laid techniques to obtain flax nonwovens with different thermoplastic binding fibers for technical insulation applications,” Textile Res. J. 83(4), 426-437. DOI: 10.1177/0040517512454183
Fages, E., Gironés, S., Sánchez-Nacher, L., Garcia-Sanoguera, D., and Balart, R. (2012). “Use of wet-laid techniques to form flax-polypropylene nonwovens as base substrates for eco-friendly composites by using hot-press molding,” Polymer Composites 33(2), 253-261. DOI: 10.1002/pc.22147
Faré (2016). “Spunbond and meltblown systems,” http://www.farespa.com/spunbound.html
Farer, R., Batra, S., and Gilmore, T. (1998). “Processing cotton and cotton/pulp blends for wet-laid nonwovens using novel dispersion technologies,” 1998 Nonwovens Conf.: Proceedings, TAPPI Press, Atlanta, pp. 133-136.
Farwaha, R., Pauls, Sr., S. P., Mumick, P. S., and Walker, J. L. (2012). “Method of making salt-sensitive binders and nonwoven webs,” US Pat. 8,232,345.
Fathi-Khalfbadam, S., Latifi, M., Sheikhzadeh-Najar, S., and Towhidkhah, F. (2011). “Analysis and simulation of fiber dispersion in water using a theoretical analogous model,” J. Disper. Sci. Technol. 32(3), 352-358. DOI: 10.1080/01932691003659833
Fauser, H., and von Klitzing, R. (2014). “Effect of polyelectrolytes on (de)stability of liquid foam films,” Soft Matter 10(36), 6903-6916. DOI: 10.1039/c4sm01241k
Fegley, N. L. (1985). “Advanced specialty nonwovens via the wet-laid process,” TAPPI Nonwovens Symp. Proc., Myrtle Beach, pp. 9-20.
Finley, M. J., and Mlynar, M. (1999). “Auxiliaries and additives for resin bonded nonwoven webs,” TAPPI Nonwovens Conf. Proc., New Orleans, TAPPI Press, Atlanta, pp. 23-28.
Fi-Tech (2016). “REICOFIL SMS composite lines – Fulfilling quality standards in the hygiene and medical industries,” http://www.fi-tech.com/View.aspx?page=nonwovencomposite/smsnonwovenplants
Floyd, K. L. (1984). “Developments in nonwoven technology,” EDANA INDEX 84 Congr., Geneva, Session 1, Paper 1, 17 pp.
Fonnum, G., Bakke, J., and Hansen, F. K. (1993). “Associative thickeners .1. Synthesis, rheology and aggregation behavior,” Colloid Polym. Sci. 271(4), 380-389. DOI: 10.1007/BF00657419
Fredericks, P. (1976). “Parameters in the manufacture of wet-laid fabrics,” in: Mod. Nonwovens Technol., Ward, D. T. (ed.), UMIST Conf. 3, pp. 129-140.
Gatward, A., and Radvan, B. (1973). “Method and apparatus for forming a non-woven fibrous web from a foamed fiber furnish,” U.S. Patent 3,716,449.
Gill, R. A., Drennen, T. J., Sweeney, E. J., and Mlynar, L. (1972). “Effect of fiber, pulp, and binder variables on characteristics of wet-laid nonwovens,” Tappi J. 55(5), 761-768.
Gilmore, T. F., Morton, G. P., and Timble, N. B. (1996). “Hydroentangled unbleached cotton,” Proc. TAPPI 1996 Nonwovens Conf., TAPPI Press, Altanta, pp. 129-133.
Giri, M., Simonsen, J., and Rochefort, W. E. (2000). “Dispersion of pulp slurries using carboxymethylcelluose,” Tappi J. 83(10), 58. (Online posting)
Gleich, K. F., Betts, L. C., and Loren (2007). “Sizing composition, useful in wet, sized glass fibers and for making fibrous nonwoven mats or fibrous preform, comprises a liquid and solids comprising binder resins, silanes and lubricants,” Eur. Pat. EP1770072-A1
Gregg, R. L. Jr. (1974). “Fibers for nonwoven fabrics,” TAPPI Testing/Paper Synt. Conf., Boston, pp. 139-141.
Goettmann, J. A., Angelini, P. J., Monroe, S. H., and Boylan, J. R. (2001). “Recyclable polymeric synthetic paper and method for its manufacture,” US Pat. 6,171,443.
Gross, G. (1973). “Synthetic paper structures of aromatic polyamides,” US Pat. 3,756,908.
Guerin, J. A., and Jeandron, H. T. (1965). “Method of forming hydraullically loomed fibrous material,” U.S. Patent 3,214,819.
Gupta, R. K., Nguyen, D. A., and Sridhar, T. (2000). “Extensional viscosity of dilute polystyrene solutions: Effects of concentration and molecular weight,” Phys. Fluids 12(6), 1296-1318. DOI: 10.1063/1.870383
Haile, W. A., and Phillips, B. M. (1995). “Deep-grooved polyester fiber for wet-lay [nonwoven] application,” Tappi J. 78(9), 139-142.
Harding, M. J. (1986). “Latex treated cationic cellulose product and method for its preparation,” US Pat. 4,584,357.
Harris, J. M. (1985). “Laboratory synthesis of polyethylene-glycol derivatives,” J. Macromol. Sci. – Rev. Macromol. Chem. Phys. C25(3), 325-373. DOI: 10.1080/07366578508081960
Hawkins, R. L. (1979). “Staple fiber, finish therefor and process for use of same,” US Pat. 4,137,181 A.
Herrington, T. M., and Petzold, J. C. (1992). “An investigation into the nature of charge on the surface of papermaking woodpulps. 2. Analysis of potentiometric titration data,” Colloids Surf. 64, 109-118. DOI: 10.1016/0166-6622(92)80089-K
Hervey, L. R. B., Starr, J. R., and Tschirch, R. P. (1975). “Wet-lay formation of nonwovens from native cellulose fibers,” Can. Pat. 968, 915.
Higashitani, K., Inada, N., and Ochi, T. (1989). “Floc breakup in contraction flow to orifice,” presented at AICHE Ann. Mtg., San Francisco.
Holm, U., and Milding, E. (1999). “Nonwoven material containing a mixture of pulp fibres and long hydrophilic plant fibres and a method of producing the nonwoven material,” US Pat. 5,958,186.
Hsieh, C. C., Park, S. J., and Larson, R. G. (2005). “Brownian dynamics modeling of flow-induced birefringence and chain scission in dilute polymer solutions in a planar cross-slot flow,” Macromol. 38(4), 1456-1468. DOI: 10.1021/ma0491255
Huang, Y. C., Fowkes, F. M., Sanders, N. D., and Lloyd, T. B. (1991). “The impact of surface chemistry on particle electrostatic charging and viscoelasticity of precipitated calcium carbonate slurries,” Proc. TAPPI 1991 Coating Conf., TAPPI Press, Atlanta, pp. 123-129.
Hubbe, M. A. (2005). “Dry-strength development by polyelectrolyte complex deposition onto non-bonding glass fibers,” J. Pulp Paper Sci. 31(4), 159-166.
Hubbe, M. A. (2006). “Bonding between cellulosic fibers in the absence and presence of dry-strength agents – A review,” BioResources 1(2), 281-318. DOI: 10.15376/biores.1.2.281-318
Hubbe, M. A. (2007). “Flocculation and redispersion of cellulosic fiber suspensions: A review of effects of hydrodynamic shear and polyelectrolytes,” BioResources 2(2), 296-331. DOI: 10.15376/biores.2.2.296-331
Hubbe, M. A. (2014). “Prospects for maintaining strength of paper and paperboard products while using less forest resources: A review,” BioResources 9(1), 1634-1763. DOI: 10.15376/biores.9.1.1634-1763
Hubbe, M. A., and Bowden, C. (2009). “Handmade paper: A review of its history, craft, and science,” BioResources 4(4), 1736-1792. DOI: 10.15376/biores.4.4.1736-1792
Hubbe, M. A., and Chen, J. (2004). “Charge-related measurements – A reappraisal. Part 1: Streaming current,” Paper Technol. 45(8), 17-23.
Hubbe, M. A., Nanko, H., and McNeal, M. R. (2009). “Retention aid polymer interactions with cellulosic surfaces and suspensions: A Review,” BioResources 4(2), 850-906. DOI: 10.15376/biores.4.2.850-906
Huber, O., and Weigl, J. (1970). “Das Japan-Papier und seine Rätsel,” Das Papier 24(10A), 823-833.
Hutten, I. M. (1995). “The concerns of forming wet-lay nonwovens for long fibers,” Proc. TAPPI 1995 Nonwovens Conf., 161-172.
Hutten, I. M. (2007). Handbook of Non-Woven Filter Media, Elsevier, Amsterdam. ISBN-13: 978-1-85617-441-1; ISBN-10: 1-8561-7441-7
Inagaki, H. (1979). “Studies on wet-laid nonwoven fabrics. 1. ‘Vinylon’ polyvinyl alcohol fiber, Nylon fiber or wood pup bonding with polyvinyl alcohol fiber type binder,” Kobe Joshi Daigaku Kiyo (Bull Kobe Women’s College), (8), 81-95.
International Fiber Journal (2011). “ANDRITZ Perfojet receives three orders from Chinese customers,” http://www.fiberjournal.com/andritz-perfojet-receives-three-orders-from-chinese-customers/
Jackson, R. J. (1997). “Formulating binder systems for nonwovens with epoxy resin waterborne dispersions,” TAPPI 1997 Nonwovens Conf., Memphis, TN, pp. 215-221.
Jakush, E. A. (1991). “Dispersants in wet-laid glass mat: Theory and practice,” TAPPI Nonwovens Conf. Proc., Marco Island, FL, pp. 163-172.
Jansma, R. H., and Smith, J. H. (1993). “Water-soluble dispersant which aids in the dispersion of polyester fibers during the preparation of a wet-laid nonwoven fiber mat,” US Pat. 5,209,823, Nalco Chem. Co.
Johnson, D. C., and Noegi, A. N. (1990). “Nonwoven fabric-like product using a bacterial cellulose binder and method for its preparation,” US Pat. 4,919,753, Weyerhaeuser Co.
Johnson, P. (1996). “Courtaulds [Fibers’] Lyocell: A cellulosic fiber for special[ty] papers and nonwovens,” 1996 Nonwovens Conf. Proc., TAPPI Press, Altanta, pp. 245-248.
Jung, Y. H., Kim, H. Y., Lee, D. R., Park, S. Y., and Khil, M. S. (2005). “Characterization of PVOH nonwoven mats prepared from surfactant-polymer system via electrospinning,” Macromol. Res. 13(5), 385-390. DOI: 10.1007/BF03218470
Kalwaites, F. (1958). “Method of and apparatus for producing nonwoven product,” U.S. Patent 2,862,251.
Kalwaites, F. (1973). “Method for producing a rearranged fabric having improved cross-strength,” U.S. Patent 3,747,161.
Kamath, M. G., Dahiya, A., and Hegde, R. R. (2004). “Spunlace (hydroentanglement),” http://www.engr.utk.edu/mse/Textiles/Spunlace.htm
Karakashev, S. I., and Grozdanova, M. V. (2012). “Foams and antifoams,” Advan. Colloid Interface Sci. 176-177, 1-17. DOI: 10.1016/j.cis.2012.04.001
Karwa, A. N., Barron, T. J., Davis, V. A., and Tatarchuk, B. J. (2012). “A novel nano-nonwoven fabric with three-dimensionally dispersed nanofibers: Entrapment of carbon nanofibers within nonwovens using the wet-lay process,” Nanotech. 23(18), article 185601. DOI: 10.1088/0957-4484/23/18/185601
Kawanaka, H., Katabe, K., and Kameda, S. (1974). “Fiber-lubricant composition,” U.S. Patent 3,788,888.
Keith, J. M. (1994). “Dispersion of synthetic fiber for wet-lay nonwovens,” Tappi 77(6), 207-210.
Keller, D. S., Branca, D. L., and Kwon, O. (2012). “Characterization of nonwoven structures by spatial partitioning of local thickness and mass density,” J. Mater. Sci. 47(1), 208-226. DOI: 10.1007/s10853-011-5788-x
Keller, M. B., and Kroetz, K. C. (1972). “Nonwoven forming with the wet-laid process,” Pulp and Paper 46(8), 50-54. ISSN 00334081
Kerekes, R. J. (1983). “Pulp floc behavior in entry flow to constrictions,” Tappi 66(1), 88-91.
Kerekes, R. J., and Schell, C. J. (1992). “Characterization of fiber flocculation regimes by a crowding factor,” J. Pulp Paper Sci. 18(1), J32-J38.
Kerekes, R. J., and Schell, C. J. (1995). “Effects of fiber length and coarseness on pulp flocculation,” Tappi J. 78(2), 133-139.
Kinn, L. L., and Mate, Z. (1986). “Fiber length – fiber surface area relationships in wet-laid polyester nonwovens,” Nonwovens Ind. 17(10), 88, 90, 94, 96, 98, 100.
Kohler, R., and Nebel, K. (2006). “Cellulose nanocomposites: Towards high performance composite materials,” Macromol. Sympos. 244, 97-106. DOI: 10.1002/masy.200651209
Koukoulas, A. (2016). “Wet-laid forming: Where do we go from here?” RISE: Research, Innovation & Science for Engineering Fabrics Conf., New Orleans, 26 Jan.
Kozak, S. M. (1991). “Use of specialty cellulose fibers and modified cellulose fibers in nonwovens,” TAPPI Nonwovens Conf. Proc., Marco Island, FL, pp. 37-48.
Kumar, M. N. S., Siddaramaiah, Jagannath, J. H., and Bawa, A. S. (2006). “Thermal and morphological studies of acrylonitrile-butadiene (NBR) latex reinforced jute nonwoven fabric composites,” Polymers Polym. Comps. 14(4), 391-402.
Larson, R. G. (2005). “The rheology of dilute solutions of flexible polymers: Progress and problems,” J. Rheol. 49(1), 1-70. DOI: 10.1122/1.1835336
Latifi, M., Tafreshi, H. V., and Pourdeyhimi, B. (2008). “A note on an optical method to evaluate fiber dispersion in wet-laid nonwoven process,” Textile Res. J. 78(6), 518-523. DOI: 10.1177/0040517507081910
Latimer, J. J., and Schultz, B. J. (1990). “Design of polyester and glass/polyester wet-laid mats – Laboratory and pilot studies,” TAPPI Nonwovens Conf. Proc., Marco Island, pp. 349-354.
Lee, P. F. W., and Lindström, T. (1989). “Effects of high molecular mass anionic polymers on paper sheet formation,” Nordic Pulp Paper Res. J. 4(2), 61-70. DOI: 10.3183/NPPRJ-1989-04-02-p061-070
Lee, S. H., Lee, H. L., and Youn, H. J. (2015). “Adsorption of xylan onto cellulose fibers pretreated with cationic polyelectrolyte and its effect on paper properties,” BioResources 10(1), 851-865.
Li, T.-Q., and Ödberg, L. (1997). “Studies of flocculation in cellulose fiber suspensions by NMR imaging,” J. Pulp Paper Sci. 23(8), J401-J405.
Lichstein, B. M. (1988). The Nonwovens Handbook, INDA, Association of the Nonwovens Fabrics Industry, 105 pp.
Lickfield, G. C., and Liu, M. (1993). “Effects of a silicone antifoam on a latex binder’s wetting and adhesion,” Proc. TAPPI 1993 Nonwovens Conf., TAPPI Press, Atlanta, pp. 325-329.
Lyne, M. B. (1993). “On the interaction of liquids with paper under dynamic conditions,” in: Products of Papermaking, Baker (ed.), Pira, 1993, Vol. 2, p. 885-911.
Madsen, L. O. (1984). “Use of bicomponent fibers for nonwoven bonding,” EDANA INDEX 84 Congr., Geneva, Session 2, Paper 3, 11 pp.
Maity, S., Gon, D. P., and Paul, P. (2014). “A review of flax nonwovens: Manufacturing, properties, and applications,” J. Nat. Fibers 11(4), 365-390. 10.1080/15440478.2013.861781
Malkan, S. R. (2009). “Improving the use of polyolefins in nonwovens,” Polyolefin Fibres: Industrial and Medical Applications, 82, 288-315.
May, P. A., and Moore, J. S. (2013). “Polymer mechanochemistry: Techniques to generate molecular force via elongational flows,” Chem. Soc. Revs. 42(18), 7497-7506. DOI: 10.1039/c2cs35463b
McIntyre, J. E. (1968). “Man-made fibers, manufacture,” Encyclopedia of Polymer Science and Technology, Vol. 8, Mark, H. F. (ed.), Wiley, New York, pp. 374-404.
Mehta, H. R., Singh, T. M., and Meyer, J. E. (1995). “Wet-lad nonwoven fabric and method for making same,” US Pat. 5,415,738, Evanite Fiber Corp, 11 pp.
Merriman, E. A. (1981). “KevlarÒ aramid pulp for paper making,” Seminar Notes: 1981 Nonwoven Fibers and Binders, TAPPI Press, Atlanta, 5-9.
Michielsen, S., Pourdeyhimi, B., and Desai, P. (2006). “Review of thermally point-bonded nonwovens: Materials, processes, and properties,” J. Appl. Polym. Sci. 99(5), 2489-2496. DOI: 10.1002/app.22858
Miller, E. E. (1996). “A simple test for dispersion of wet chop fiberglass in water,” Proc. TAPPI 1996 Nonwovens Conf., 205-209.
Morgan, P. W. (1961). “Synthetic polymer fibrid paper,” U.S. Patent 2,999,788.
Nakamae, K., and Shima, T. (1990). “High strength wet-laid nonwoven fabric and process for producing same,” US Pat. 4,891,262.
Nanko, H., Button, A., and Hillman, D. (2005). The World of Market Pulp, Swann, C. E. (ed.), WOMP LLC, Appleton, WI, USA.
Nanko, H., McNeal, M., and Pan, S. (2006). “Understanding wet-end polymer performance through visualization of macromolecular events by transmission electron microscopy,” Pan Pacific Conf. – Advances in Pulp and Paper Sciences and Technologies, Seoul, Korea, 1-18.
Nedwick, P., and Greenblatt, G. (1996). “An overview of associative and alkali-swellable rheology modifiers,” Proc. TAPPI 1996 Nonwovens Conf., 205-209.
Nguyen, T. Q., and Kausch, H.-H. (1992). “Chain extension and degradation in convergent flow,” Polymer 33(12), 2611-2621. DOI: 10.1016/0032-3861(92)91145-R
Nielsen, S. F., and Davies, B. L. (1992). “Wet-laid bonded fibrous web containing bicomponent fibers including LLDPE,” US Pat. 5,167,765, Hoechst-Celanese Corp.
Nielsen, S. F., and Johnson, C. E. (1992). “Wet-laid bonded fibrous web,” US Pat. 5,167,764, Hoechst-Celanese Corp.
Oelkers, J. M., and Sweeney, E. J. (1988). “Latex binders and binding techniques for disposable nonwovens,” Tappi J. 71(8), 69-76.
Ohta, S., Nishiyama, T., Sakoda, M., Machioka, K., Fuke, M., Ichimura, S., Inagaki, F., Shimizu, A., Hasegawa, K., Kokudo, N., Kaneko, M., Yatomi, Y., and Ito, T. (2015). “Development of carboxymethyl cellulose nonwoven sheet as a novel hemostatic agent,” Journal of Bioscience and Bioengineering 119(6), 718-723. DOI: 10.1016/j.jbiosc.2014.10.026
Ozaki, Y. (2011). “Application of confocal laser scanning microscopy (CLSM) for observing adhesives in paper,” J. Adhes. Sci. Technol. 25(6-7), 723-741. DOI: 10.1163/016942410X525902
Paavilainen, L. (1993). “Conformability, flexibility and collapsibility of sulfate pulp fibers,” Paperi Puu 75(9-10), 689-702.
Parker, J. D. (1972). The Sheet Forming Process, TAPPI STAP Ser. 9, TAPPI Presss, New York.
Parsons, J. (1999). “Chemical binder application technology,” TAPPI Nonwovens Conf. Proc., New Orleans, pp. 13-21.
Perini Journal (2014). “Andritz quietly builds full tissue machine lineup,” Perini J. 26, articles not numbered; http://www.perinijournal.com/Items/en-US/Articoli/PJL-26/Andritz-quietly-builds-full-tissue-machine-lineup
Petrie, C. J. S. (2006). “Extensional viscosity: A critical discussion,” J. Non-Newtonian Fluid Mechan. 137, 15-23. DOI: 10.1016/j.jnnfm.2006.01.011
Prosser, A. J., and Franses, E. I. (2001). “Adsorption and surface tension of ionic surfactants at the air-water interface: Review and evaluation of equilibrium models,” Colloids Surf. A. 178, 1-40. DOI: 10.1016/S0927-7757(00)00706-8
Radvan, B., and Gatward, A. P. J. (1972). “Formation of wet-laid webs by a foaming process,” Tappi J. 55(5), 748-751.
Ramasubramanian, M. K., Shiffler, D. A., and Jayachandran, A. (2008). “A computational fluid dynamics modeling and experimental study of the mixing process for the dispersion of the synthetic fibers in wet-lay forming,” J. Eng. Fiber Fabrics 3(1), 11-19; Reprinted in: TAPPI J. 9(3), 6-13.
Razak, S. (1994). “Novel amine alkoxylate surfactants as glass fiber dispersants for the manufacture of uniform glass fiber mat,” Proc. TAPPI 1994 Nonwovens Conf., 107-110.
Rebouillat, S., Letellier, B., and Steffenino, B. (1999). “Wettability of single fibres – Beyond the contact angle approach,” Intl. J. Adhes. Adhesives 19(4), 303-314. DOI: 10.1016/S0143-7496(99)00006-8
Reese, G. P., and Cooper, G. W. (2002). “Low shrinkage, uncrimped short-cut fibers for use in wet laid non-woven products and method for making same,” US Pat. 6,472,066.
Rice, M., and Roeraade, J. (2003). “Continuous filtration and titration apparatus for real time monitoring of polyelectrolyte concentration and cationic demand of a paper furnish,” Nordic Pulp Paper Res. J. 18(1), 95-107. DOI: 10.3183/NPPRJ-2003-18-01-p095-107
Richardson, P. F., Dunn, S. N., and Romberger, J. A. (1990). “Acrylamide-2-acrylamido-2-methylpropatesulfonic acid polymers as formation aids in wet-laid nonwovens production,” US Pat. 4,948, 464, Nalco Chem. Co.
Richardson, P. F., Dunn, S. N., Wilharm, M. T., and Nielson, S. F. (1988). “Effect of acrylamide copolymers on wet-laid sheets produced from high L/D ratio polyester fibers,” TAPPI Nonwovens Conf. Proc., Nashville, pp. 57-64.
Richardson, P. F., and Smith, J. H. (1989). “Techniques for laboratory evaluation of dispersants and formation aids use in the wet-laid process,” Tappi J. 72(7), 155-158.
Riedel, B., Knobelsdorf, C., Mieck, K.-P., Seyfarth, E., and Taeger, E. (1996). “New ways to produce cellulosic microfiber nonwovens,” Chemical Fibers International 46(6), 454-455.
Ring, M., Godsay, M. P., Swenson, R. S., and Kent, J. N. (1977). “Method for forming wet-laid nonwoven fibers,” US. Pat. 4,007,083.
Robertson, J. S. (1992). “Wet-laid PET polyester microfiber handsheets,” Nonwovens Conf. Proc., Marco Island, FL, pp. 47-50.
Russell, S. J. (2007). Handbook of Nonwovens, CRC Press, Boca Raton, FL, USA.
Saich, A. M. (1969). “Formation of wet-laid synthetic fiber webs using an inclined wire machine,” Chem. Ind. (London) (3), 68-74.
Safavi, A., Fathi, S., Babaei, M. R., Mansoori, Z., and Latifi, M. (2009). “Experimental and numerical analysis of fiber characteristics effects on fiber dispersion for wet-laid nonwoven,” Fibers and Polymers 10(2), 231-236. DOI: 10.1007/s12221-009-0231-5
Sandquist, D. (2013). “New horizons for microfibrillated cellulose,” APPITA J. 66(2), 156-162.
Sansalone, J. J., and Kim, J. Y. (2008). “Suspended particle destabilization in retained urban stormwater as a function of coagulant dosage and redox conditions,” Water Res. 42(4-5), 909-922. DOI: 10.1016/j.watres.2007.08.037
Sato, T. (1993). “Stability of dispersion,” J. Coatings Technol. 65(825), 113-121.
Saylor, D. K. (1981). “Carbon fibers for nonwoven products and related applications,” Seminar notes: 1981 Nonwoven Fibers & Binders, TAPPI Press, Atlanta, pp. 27-33.
Scargiali, F., Busciglio, A., Grisafi, F., Tamburini, A., Micale, G., and Brucato, A. (2013). “Power consumption in uncovered unbaffled stirred tanks: Influence of the viscosity and flow regime,” Indust. Eng. Chem. Res. 52, 14998-15005. DOI: 10.1021/ie402466w
Schilling, D. R. (2014). “World’s largest papermachine: Automated, efficient & sustainabile,” Industry Tap, http://www.industrytap.com/worlds-largest-paper-machine-automated-efficient-sustainable/18732
Schoffmann, E. A. (1980). “Advancement in producing nonwovens by the wet-process method,” INDA Tech. Symp. Nonwoven Technol. 8, Kissimmee, FL, pp. 104-120.
Schoeffmann, E. (1992). “Laboratory Fourdrinier for nonwovens,” in: Fibers and Forming for Nonwovens, Coleman, M. J. (ed.), TAPPI Press, pp. 358-373.
Schoffmann, E., and Schwend, F. (1992). “Meeting trend developments in wet forming,” in: Fibers and Forming for Nonwovens, Coleman, M. J. (ed.), TAPPI Press, pp. 327-331.
Scholz, B. (1985). “Web process,” in: Non-Woven Bonded Fabrics, Lünenschloss, J., and Albrecht, W. (eds.), Ellis Horwood, Ltd., Chichester, Chapter 2, “Manufacturing processes for non-woven bonded fabrics,” Part 2, pp. 317-352.
Scholz, B., and Lasschuit, I. (1981). “Process considerations of the hydroformer wet end system,” Seminar notes: 1981 Nonwoven Fibers & Binders, TAPPI Press, Atlanta, pp. 11-19.
Schon, F. (1977). “Effect of polyacrylate binder on wet-laid nonwovens,” INDA Tech. Symp. Nonwoven Technol., Washington, DE, 5.
Seibert, H., and Bähr, K. (1970). “Die Japanische Handpapiermacherei,” Das Papier 24(6), 331-335.
Seifer, M. I., and Kline, P. J. (1977). “Acrylic latex low-cost binder systems – Effect on tensile properties of nonwoven webs,” Tappi 60(6), 103-105.
Shiffler, D. A. (1985). “Characterizing the dispersion kinetics of synthetic fibers in water,” Tappi 68(8), 99-91.
Shiffler, D. A. (1986). “Fiber containing defects in wet-lay nonwoven fabrics,” Seminar notes: 1986 Synthetic Fibers for Wet System and Thermal Bonding Applications, TAPPI Press, Atlanta, pp. 3-7.
Shiffler, D. A. (1988). “Defective fibers in wet-lay nonwoven fabrics,” Tappi 71(6), 117-121.
Shiffler, D. A. (1999). “Designing fibers for wet lay nonwovens, from a polyester perspective,” TAPPI Nonwovens Conf. Proc., New Orleans, pp. 191-202.
Shirazi, M., van de Ven, T. G. M., and Garnier, G. (2003). “Adsorption of modified starches on pulp fibers,” Langmuir 19(26), 10835-10842. DOI: 10.1021/la035064c
Siro, I., and Plackett, D. (2010). “Microfibrillated cellulose and new nanocomposite materials: A review,” Cellulose 17(3), 459-494. DOI: 10.1007/s10570-010-9405-y
Smith, J. E. (1988a). “Fibrets entrap and entangle to improve specialty industrial applications,” Nonwovens World 3(6), 40-42.
Smith, J. E. (1988b). “Cellulose acetate fibrets: Fibrillated pulp with high surface area,” Tappi J. 71(12), 185-187.
Smith, J. H. (1992). “Using a formation analyzer to evaluate the performance of additives used in the wet-laid process,” TAPPI J. 75(5), 121-123.
Smith, W. J. (1986). “Polyacrylamides as viscosity modifiers for the wet-laid nonwovens industry,” TAPPI Synt. Fibers Sem. Notes, Boston, pp. 43-47.
Smithers Apex, The Future of Wetlaid Nonwovens to 2019, November 2014.
Smithers Apex, The Future of Global Nonwoven Markets to 2020, May 2015.
Smook, G. (2002). Handbook for Pulp & Paper Technologists, Angus Wilde Publ., Vancouver.
Soszynski, R. M., and Kerekes, R. J. (1988). “Elastic interlocking of nylon fibers suspended in liquid. (1). Nature of cohesion among fibers. (2). Process of interlocking,” Nordic Pulp Paper Res. J. 3(4), 172-184. DOI: 10.3183/NPPRJ-1988-03-04-p172-179
Starr, J. (1973). “Commercial opportunities in wet-laid long-fibered nonwoven products,” Paper Technol. Ind. 14(5), 275-278.
Stassen, W. N. (1983). “Dispersing glass fibers in the web process,” Proc. 1983 Nonwovens Symp., TAPPI Press, Atlanta, pp. 7-12.
Suominen (2015). “Suominen confirms it invests in a new production line at its Bethune plant, focusing on nonwovens for household, industrial and flushable wipes,” http://www.suominen.fi/en/media/news/suominen-confirms-it-invests-in-a-new-production-line-at-its-bethune-plant-focusing-on-nonwovens-for-household-industrial-and-flushable-wipes-2015-05-27-06-30-00/
Swanson, J. W. (1950). “The effects of natural beater additives on papermaking fibers,” Tappi 33(9), 451-462.
Swarup, S., and Schoff, C. K. (1993). “A survey of surfactants in coatings technology,” Prog. Organic Coatings 23(1), 1-22. DOI: 10.1016/0033-0655(93)80002-R
Switzer, L. H., and Klingenberg, D. J. (2003). “Simulations of fiber dispersion in linear flow fields,” Nordic Pulp Paper Res. J. 18(2), 141-144. DOI: 10.3183/NPPRJ-2003-18-02-p141-144
Tadokoro, Y., Uesaka, M., Takata, Y., and Goto, F. (1999). “Wet-laid nonwoven fabric for battery separator, its production method and sealed type secondary battery,” US Pat. 5,888,916.
Tafreshi, H. V., and Pourdeyhimi, B. (2003). “Role of baffles on flow fields inside wet-lay mixing tanks and their potential influence on fiber dispersion,” Textile Res. J. 73(7), 575-582. DOI: 10.1177/004051750307300703
Teijin (2016). “Twaron paper,” http://www.teijinaramid.com/aramids/twaron/twaron-paper/
Tischer, L. A., Chou, T., and Seletzky, P. D. (1989). “Binder selection from glass, polyester, and blended wet-lay nonwovens,” Tappi J. 72(4), 155-158.
Tomiyoka, S. (1977). “New type conjugate fibers,” Ann. Tech. Assocn. Man-Made Fiber Paper, Japan, pp. 30-33.
Tse, S. H., Hollenberg, D. H., Martin, R. L., and Manning, J. H. (1989). “Manufacture of wet-laid nonwoven webs,” US Pat. 4,822,452, James River Corp.
Turbak, A. F. (ed.) (1993). Nonwovens: Theory, Process, Performance, and Testing, TAPPI Press, Atlanta.
Twoomey, L. F. (1987). “Foam generation and control in wet-laid nonwoven systems,” TAPPI J. 70(9), 87-90.
Ulyatt, J. M. (1976). “Developments in binders for nonwoven fabrics,” in: Mod. Nonwovens Technol., Ward, D. T. (ed.), UMIST Conf. 3, pp. 44-51.
Viazmensky, H. Richard, C. E., and Williamson, J. E. (1991). “Water entanglement process and product,” U.S. Patent 5,009,747.
Voith Paper (2008). “The largest glass fiber mat machine in the world,” Voith Paper “twogether” (Special issue 1), p. 39, http://www.voith.com/en/twogether-article-twogethers200812-en-39.pdf
Voyutskii, S. S. (1963). Autohesion and Adhesion of High Polymers, Interscience Publ., New York.
Wallace, P. D. (1999). “Crosslinker resins in nonwoven binder systems,” TAPPI Nonwoven’s Conf. Proc., New Orleans, pp. 29-37.
Wang, C. G., Chen, M. L., Jiang, Z. H., Zhang, S. Y., Wu, H., Wang, X., Pei, Y. W., and Liu, C. Q. (2014). “Biodegradable paper sheeting as agricultural covering with incorporation of bamboo pulp sludge,” BioResources 9(3), 4128-4137. DOI: 10.15376/biores.9.3.4128-4137
Wang, K. Y., Demeny, L. M., Pomplun, W. S., Mumick, P. S., Anderson, R. L., and Merker, J. F. (1999). “Dispersible nonwoven fabric and method of making same,” US Pat. 5,935,880.
Wang, Y. K., Wang, X. W., Jian, H., and Jin, L. (2013). “Wet-laid nonwoven preparation a separator for MH-Ni battery,” Intl. J. Electrochem. Sci. 8(7), 9287-9297.
Wasser, R. B. (1978). “Formation aids for paper,” Tappi 61(11), 115-118.
Whichard, M. C. (1985). “Method of making glass fiber mat,” US Pat. 4,542,068.
White, C. F. (1992). “Hydroentanglement technology applied to wet formed and other precursor webs,” in: Fibers and Forming for Nonwovens, Coleman, M. J. (ed.), TAPPI Press, 439-449.
Wilharm, M. T. (1986). “Formation aids for wet-laid nonwovens – An overview,” TAPPI Nonwovens Conf. Proc., Atlanta, pp. 157-158.
Wilharm, M. T. (1990). “White-water chemicals enhance formation of wet-laid glass,” Nonwovens World 5(7), 30-32.
Wilharm, M. T., Novak, R. W., and Savino, C. A. (1987). “Effect of water chemistry on formation aids in the wet-laid process,” TAPPI Nonwovens Conf. Proc., Orlando, pp. 101-104.
Wilke, A. G. (1989). “New viscose-Rayon fiber for nonwovens,” INDA-Tec Int. Nonwoven Fabrics Conf., Philadelphia, pp. 499-515.
Williams, M. M. (1999). “Chemical binders for nonwoven fabrics,” Proc. TAPPI 1999 Nonwovens Conf., TAPPI Press, Atlanta.
Williamson, J. E. (1993). “Wet-laid system,” in: Nonwovens – Theory, Process, Performance, & Testing, TAPPI Press, Atlanta, Chapter 6, pp. 139-154.
Woodings, C. R. (1979). “Development of viscose Rayon for nonwoven applications,” TAPPI Seminar Notes Nonwoven Fibers, Myrtle Beach, SC, pp. 15-28.
Yang, C., and Dean, J. (1978a). “Effect of long-fibered wood pulp species on latex-saturated wet-laid nonwovens,” Paper Trade J. 162(14), 42-44.
Yang, C., and Dean, J. (1978b). “Wood pulps for wet-laid nonwovens,” INDA Tech. Symp. Nonwoven Technol., Atlanta, 6, pp. 34-51.
Yang, C. H. C., and Dean, J. H. (1978c). “Redwood fibers in latex-saturated wet-laid nonwovens,” TAPPI Paper Synt. Conf. Proc., Orlando, pp. 19-25.
Yeang, B. J., Choi, Y. O., and Nam, I. W. (2012). “Preparation of biodegradable low melting binder fibers for nonwoven composites,” 8th Asian-Australasian Conference on Composite Materials 2012, ACCM 2012 – Composites: Enabling Tomorrow’s Industry Today, pp. 447-450.
Young, G. C. (1996). “Waterborne epoxy resin systems for use as binders in nonwovens and textiles,” Proc. TAPPI 1996 Nonwovens Conf., 171-183.
Yousfani, S. H. S., Gong, R. H., and Porat, I. (2012). “Manufacturing of fibreglass nonwoven webs using a paper making method and study of fibre orientation in these webs,” Fib. Tex. Eastern Eur. 20(2), 61-67.
Zauscher, S., and Klingenberg, D. J. (2000). “Surface and friction forces between cellulose surfaces measured with colloidal probe microscopy,” Nordic Pulp Paper Res. J. 15(5), 459-468. DOI: 10.3183/NPPRJ-2000-15-05-p459-468
Zhang, L. M. (2001). “Cellulosic associative thickeners,” Carbohydrate Polymers 45(1), 1-10. DOI: 10.1016/S0144-8617(00)00276-9
Article submitted: November 18, 2015; Peer review completed: February 13, 2016; Revisions accepted: February 23, 2016; Published: March 9, 2016.
DOI: 10.15376/biores.11.2.Hubbe
