Abstract
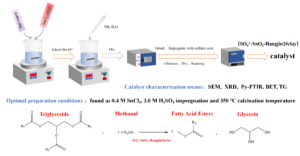
A new sulfate oxide solid acid catalyst SO42-/SnO2-Hangjin2# clay was compounded with Hangjin2#clay, activated by sulfuric acid, as a carrier. In the catalysis of Xanthoceras sorbifolium Bunge, using the yield of oil and methanol synthesis of biodiesel as an index, the effect of SO42-/SnO2-Hangjin2#clay and the preparation conditions on the activity of solid acid were investigated. The variables included in the optimization process were concentration of Sn, impregnation sulfuric acid concentration, and calcination temperature. The optimal conditions were found as 0.4 M SnCl4, 3.0 M H2SO4 impregnation, and 350 °C calcination temperature. The catalyst was examined by scanning electron microscope, X-ray diffraction meter, Fourier transform infrared spectroscopy, surface area, and thermal weight analysis. The results showed that the introduction of Hangjin2#clay in the SO42–-SnO2 solid acid catalyst improves its catalytic activity.
Download PDF
Full Article
Preparation, Characterization, and Catalytic Properties of SO42-/SnO2-Hangjin2#Clay Solid Superacid Catalyst
Lijun Ding,a Jinrui Ni,a Yinan Hao,b,* Bo Hai,a,* and Tegen Ao a
A new sulfate oxide solid acid catalyst SO42-/SnO2-Hangjin2# clay was compounded with Hangjin2#clay, activated by sulfuric acid, as a carrier. In the catalysis of Xanthoceras sorbifolium Bunge, using the yield of oil and methanol synthesis of biodiesel as an index, the effect of SO42-/SnO2–Hangjin2#clay and the preparation conditions on the activity of solid acid were investigated. The variables included in the optimization process were concentration of Sn, impregnation sulfuric acid concentration, and calcination temperature. The optimal conditions were found as 0.4 M SnCl4, 3.0 M H2SO4 impregnation, and 350 °C calcination temperature. The catalyst was examined by scanning electron microscope, X-ray diffraction meter, Fourier transform infrared spectroscopy, surface area, and thermal weight analysis. The results showed that the introduction of Hangjin2#clay in the SO42--SnO2 solid acid catalyst improves its catalytic activity.
DOI: 10.15376/biores.18.3.6194-6203
Keywords: Biodiesel; Hangjin2#Clay; Solid acid catalyst; Xanthoceras sorbifolium Bunge
Contact information: a: College of Science, Inner Mongolia Agricultural University, Hohhot 01001, China; b: College of Materials Science and Art Design, Inner Mongolia Agricultural University, Hohhot 010018, China; *Corresponding author: atgbyr@126.com; haibo_0314@126.com
GRAPHICAL ABSTRACT

INTRODUCTION
The continuous depletion of fossil fuels and the improvement of people’s awareness of environmental protection have accelerated the evolution of renewable energy. Biodiesel is one of the most popular and economically viable alternative fuels. It has the characteristics of environmental protection, regeneration, and reduction of greenhouse gas emissions (Munir et al. 2019; Durak 2020; Cherian et al. 2021; Ding et al. 2021). Biodiesel is produced under mild conditions using homogeneous catalysts (sodium hydroxide and potassium hydroxide). However, there is a difficulty of being separated from the product, producing a large amount of wastewater. Thus, the search for environmentally benign heterogeneous catalysts has driven research on substitutes for current liquid acids and halogen-based solid acids.
Sulfated oxides, such as sulfated zirconia, titania, and iron oxide, exhibit high thermostability, super acidic property, and high catalytic activity (Tanabe et al. 1989; Sohn et al. 2006; Shang et al. 2015; Vijaya et al. 2019; Chen et al. 2020). Solid heterogeneous clay catalyst has been used extensively in biodiesel synthesis to avoid the drawback of the homogenous biodiesel production process (Abdullah et al. 2017; Wang et al. 2019). Hangjin2#clay is a natural clay mineral located in Hangjin Banner, Inner Mongolia. Hanjin2#clay activated by acid has a large specific surface area, good thermal stability, and strong adsorption capacity. It is a catalyst carrier with excellent performance (Ding 2013). Studies have revealed that the amount of B-acid and L-acid centers affect the solid acid catalyst’s function in esterification and transesterification, and that more B-acid centers are advantageous for the transesterification process (Zhang et al. 2022). Tin will increase the catalyst’s acidity by interacting with the sulfuric acid root group to create a stronger b-acid center (Varala et al. 2016). This will enable transesterification to produce biodiesel. This study considers the preparation and characterization of SO42-/SnO2-Hangjin2#clay as a solid acid catalyst for the catalytic synthesis of biodiesel. The catalytic performance of the solid acid catalyst was evaluated by catalyzing Xanthoceras sorbifolium kernel oil to produce biodiesel. It is expected to obtain a solid acid catalyst with excellent performance for producing biodiesel.
EXPERIMENTAL
Materials
The seeds of Xanthoceras sorbifolium Bunge were purchased from Chifeng City, Inner Mongolia. The seeds of X. sorbifolium were dried at 110 ℃ for 4 h and then crushed through a 10-mesh sieve. Hangjin2#clay was purchased from Hangjin Banner, Inner Mongolia.
The clay was pretreated by drying and sieving. Stannic chloride pentahydrate, sulfate acid, hydrochloric acid, anhydrous sodium sulfate, methanol, and ammonia are used for catalyst preparation and synthesis of biodiesel. All chemicals were analytical grade without further purification.
Oil Extraction
Xanthoceras sorbifolium seeds were sieved through a 10-mesh, dried, crushed, and placed in a round-bottom flask. Petroleum ether was added in the solid to liquid ratio of 9:1. Ultrasonic extraction was carried out at 65 ℃ for 50 min with a frequency of 40 kHz. After suction filtration and separation in a sand core funnel, distillation was performed under reduced pressure. The mixture was evaporated under reduced pressure, and the solvent was recovered. Following weighing after drying with anhydrous sodium sulfate (Hao et al. 2011; Ding et al. 2013), the oil yield was calculated using Eq. 1.
(1)
Preparation of Catalysts
First, 10 g of the sample screened by 20 mesh sieve was added into 3.0 M H2SO4 with the liquid-solid ratio of 5:1. The mixture was activated at 90 ℃ for 4 h, centrifugally washed to pH 5 to 6, dried at 110 ℃ for 3 h, and ground to obtain activated clay (Zhao et al. 2004; Wei et al. 2010). The SnCl4·nH2O samples were dissolved, and then 25% ammonia water was added slowly to adjust the pH to 5 to 6. The mixture was mixed with active clay at a ratio of 5:1(mL/g) and refluxed in a water bath at 70 ℃ under agitation for 12 h. After cooling, the sample was washed with water and centrifuged until there was no Cl–, dried at 90 ℃ for 3 h, then crushed with a mortar and prepped for use later.
The powder was soaked in sulfuric acid solution at a solid-liquid ratio of 1:5(g/mL) for 4 h, incubated overnight, centrifuged, dried at 110 ℃ for 3 h, and baked for 3 h to obtain the solid acid catalyst: SO42-/SnO2-Hangjin2#clay. The temperature programmed settings were as follows: heating rate of 2 ℃/min, rising from ambient temperature to the required baking temperature, with baking time of 3 h. The samples were roasted, cooled to room temperature, and placed into the dryer for later use.
Characterization of Catalyst
The specific surface areas (BET) of the prepared catalysts were measured respectively (degassing mode was heating and vacuuming temperature 120 ℃, time 180 min, the adsorbent was N2 saturated vapor pressure 0.924 MPa, environment temperature 20 ℃). X-ray powder diffraction (XRD) patterns of the catalysts were determined by a Bruker diffractometer using Cu Ka radiation. The operating voltage and current were 40 kV and 80 mA. The scanning rate was 2°/min. The surface morphology of the samples was characterized using a scanning electron microscope (SEM). Thermogravimetric analysis (TG) was performed from room temperature to 1000 ℃, at a heating rate of 5°/min, measured in air atmosphere.
Evaluation of Catalytic Activity
First, 1% catalyst (oil-to-weight ratio), Xanthoceras Sorbifolia Bunge oil and methanol (alcohol-oil-to-motorcycle ratio 6:1) were added into an iodine flask and reacted at 90 ℃ for 2 h. After the reaction, the product was transferred to the separation funnel and left overnight, resulting in two layers. The lower layer of glycerol was released, and the upper bio-diesel oil layer was distilled in a vacuum to recover excess methanol. The product was washed with distilled water and dried with anhydrous sodium sulfate to calculate the yield, as follows.
RESULTS AND DISCUSSION
Optimization of Experimental Conditions
Effect of different Sn addition on its catalytic activity
The experiments were performed with the liquid-solid ratio of Sn to soil of 5:1, concentration of impregnated sulfuric acid of 3.0 M, and calcination temperature of 300 °C. The effects of different concentrations of Sn solution on the catalytic activity of the catalyst are exhibited in Table 1. When the concentration of SnCl4 was 0.4 M, its catalytic activity was the highest. Therefore, the concentration of SnCl4 was chosen as 0.4 M.
Table 1. Effect of Sn Concentration on Catalytic Activity of Catalyst
Influence of sulfuric acid impregnation solution concentration
The experiments were performed with the liquid-solid ratio of Sn to soil of 5:1, of 0.4 M SnCl4, and the calcination temperature of 350 ℃. The influence of different sulfuric acid impregnation solution concentration on the catalytic activity of the catalyst is shown in Table 2. When the sulfuric acid concentration was 3.0 M, its catalytic activity was the highest.
Table 2. Effect of Catalytic Activity on Different Acidity
Effect of different calcination temperature on its catalytic activity
The experiments were performed with the liquid-solid ratio of Sn to soil of 5:1, 0.4 M SnCl4, and 3.0 M immersion sulfuric acid. The influence of different calcination temperatures on the catalytic activity of the catalyst is shown in Table 3. When the calcination temperature was 350 °C, catalytic activity was the highest. Therefore, the optimum calcination temperature was judged to be 350 ℃. According to the analysis of the above test results, the optimum preparation conditions of SO42-/SnO2-Hangjin2#clay solid acid catalyst were as follows: 0.4 M SnCl4, liquid to solid ratio of 5:1 (mL/g), 3.0 M impregnation H2SO4 (liquid-solid ratio 5:1), roasting temperature of 350 ℃, and roasting time of 3 h.
Table 3. Effect of Catalytic Activity on the Event Calcination Temperature
Evaluation of catalytic activity of different catalysts
The experiments were performed with 3.0 M sulfuric acid, 0.4 M SnCl4, and calcination temperature of 300 ℃. The catalytic activities of raw ore soil, activated clay, SnO2–Hangjin2#clay, and SO42-/SnO2-Hangjin2#clay were evaluated, as shown in Table 4.
Table 4. Effect of Catalytic Activity on Different Catalysts
Catalyst Characterization
BET surface area analysis of synthesized catalyst
As shown in Table 5, the SO42--Hangjin2#clay surface area increased by nearly 31 m2/g, but its void volume decreased. The reason may be that after adding SnO2 components in SO42-–Hangjin2#clay, it becomes increasingly rich. When impregnated with sulfuric acid, the specific surface area and pore volume decreased. This may be because the composite carrier SnO2-Hangjin2#clay absorbs a large amount of SO42- on its surface. Some original channels are blocked, so the specific surface area and pore volume decrease. However, a large amount of SO42-was adsorbed on the surface formed a large amount of acid center, thereby increasing its catalytic activity.
Table 5. Surface Area, Pore Volume, and Average Aperture of Hangjin2#Clay within Different Substances
Infrared spectral analysis of the synthesized catalyst
The infrared spectra of SO42--Hangjin2#clay, SnO2-Hangjin2#clay and SO42-/SnO2-Hangjin2#clay are shown in Fig. 1. After adding Sn, a small absorbance peak appeared at 1270 cm-1, but the absorbance peaks at 529 cm-1 and 599 cm-1 disappeared, which indicates that SnO2 was introduced into Hangjin2#clay after being activated by sulfuric acid. The Hangjin2#clay effectively increases the combination of Sn and SO42- and increases its catalytic activity.
Fig. 1. The FT-IR spectra of Hangjin2#clay within different substances
X-ray polycrystalline diffraction analysis (XRD) of the resized catalyst
The XRD spectra of Hangjin2#clay, SO42--Hangjin2#clay, SnO2-Hangjin2#clay and SO42-/SnO2-Hangjin2#clay at different calcination temperatures are shown in Figs. 2 and 3. There were characteristic diffraction peaks of SiO2, feldspar, and calcite in the XRD diagram of Hangjin2#clay. The diffraction peaks of silica and calcite in Hangjin2#clay activated by sulfuric acid decreased, and the characteristic peaks of calcium sulfate appeared at 2θ=25.43°, 31.94°, and 49.24°. Hence, CaCO3 was converted into CaSO4 after acid activation. When Sn was added, the characteristic peak of CaSO4 disappeared, and the intensity of SiO2 diffraction peak decreased. The crystallinity decreased and defects increased, leading to increased specific surface area. After impregnation with sulfuric acid, the intensity of SiO2 diffraction peak further decreased, indicating that the crystallinity also decreased. Thus, the combination of SO42- and SnO2 enhanced the catalyst activity.
Fig. 2. XRD pattern of Hangjin2#clay within different substances
The characteristic peaks of SiO2 at 2θ = 20.85° and 26.60° gradually weakened with the increasing calcination temperature. When the calcination temperature was 350 ℃, its strength was the lowest. When the calcination temperature was higher than 350 ℃, the characteristic peak intensity of SiO2 gradually increased.
According to Sagala (2018), when the temperature is high, SO42- adsorbed on the catalyst surface decomposes to SOx. With the decomposition of SO42-, the crystallinity of the catalyst becomes higher, and the active center on the surface decreases continuously. Thus, its catalytic activity decreases, which is consistent with the analysis of the results in Table 4.
Fig. 3. XRD patterns of catalyst prepared at different temperatures
TG analysis of synthesized catalyst
The TG curves of SO42--Hangjin2#clay, SO42-/SnO2-Hangjin2#clay and SO42-/SnO2-Hangjin2#clay with different impregnation H2SO4 concentrations were measured in this work, as shown in Figs. 4 and 5. The TG curves of Hangjin2#clay before and after adding Sn. The weight loss at 20 to 200 ℃ is the main reason for dehydration of the catalyst surface. The weight loss between 400 and 800 ℃ is mainly accounted for by the decomposition of SO42- adsorbed on the catalyst surface. After adding Sn, the weight loss rate of catalyst increased between 400 and 800 ℃. This shows that after the composite carrier was impregnated with an acid of this concentration, the amount of SO42- bound on the catalyst surface was the highest, and the catalytic activity was the strongest. This is consistent with the result analysis in Table 2.
Fig. 4. TG curve of catalysts on Sn in Hangjin2#clay and Hangjin2#clay
Fig. 5. TG curve of catalysts on different acid concentration
SEM analysis of synthesized catalyst
A scanning electron microscope was used to measure SO42--Hangjin2#clay, SnO2-Hangjin2#clay, and SO42-/SnO2-Hangjin2#clay. The results are shown in Fig. 6.
Fig. 6. Scanning electron micrograph of (a) SO42--Hangjin2#clay, (b) SnO2--Hangjin2#clay, and (c) SO42-/SnO2--Hangjin2#clay
The surface of the sample became loose and rough after adding tin. The results show that SnO2 was able to modify the surface of a specimen. The catalysts had a honeycomb structure, which leads to the increase of specific surface area. After further impregnation with sulfuric acid, its surface structure becomes more loose and orderly. The combination of tin and sulfate is good, which immensely improves the catalytic activity of Sn. However, after adding SO42-, the surface was agglomerated and some channels were blocked. The specific surface area decreased, which is consistent with BET and XRD results.
The results demonstrate a strong catalytic effect of Hangjin 2# clay -loaded SO42-/SnO2 on the conversion of Xanthoceras sorbifolium Bunge oil to biodiesel. However, the catalyst still has room for stability improvement. In future studies is important to promote the use of catalysts in industrial production. This can be done by enhancing the activity and stability of the catalyst by incorporating cocatalysts.
CONCLUSIONS
- SO42- /SnO2 was modified by introducing Hangjin2#clay. Solid superacid catalysts were prepared with comparatively higher catalytic performance and satisfactory stability.
- When the concentration of SnCl4 was 0.4 M, the concentration of H2SO4 was 3.0M, and the calcination temperature was 350 °C, the yield of biodiesel prepared with catalytic Xanthoceras sorbifolium Bunge oil was as high as 88.6%.
ACKNOWLEDGMENTS
This work is supported by the Project of Inner Mongolia Science and Technology Department (2019CG018), the Scientific research projects of colleges and universities in Inner Mongolia Autonomous Region(NJZY21346),the Natural Science Foundation of Inner Mongolia Autonomous Region (2015MS0224), asnd the Inner Mongolia Agricultural University doctoral research initiation Fund Project (BJ2014-13).
REFERENCES CITED
Abdullah, S., Hanapi, N., Azid, A., Umar, R., Juahir, H., Khatoon, H., and Endut, A. (2017). “A review of biomass-derived heterogeneous catalyst for a sustainable biodiesel production,” Renewable and Sustainable Energy Reviews 70, 1040-1051. DOI: 10.1016/j.rser.2016.12.008
Cherian, E., Yazhini, D., and Victor, M. (2021). “Production of biodiesel from pork fat using alumina-doped calcium oxide nanocomposite as heterogeneous catalyst,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 43, 1386-1395. DOI: 10.1080/15567036.2019.1637971
Ding, L. J. (2013). Study on the Technology of Producing Biodiesel from Wenguanguo Seed Oil, Inner Mongolia Agricultural University.
Ding, L. J., Wang, X. M., and Wang, X. M. (2013). “Study on the optimization of ultrasonic extraction technology of seed kernel of Xanthoceras sorbifolium by CCD method,” Transactions of the Chinese Society of Agricultural Engineering 29, 202-208. DOI: 10.3969/j.issn.1002-6819.2013.06.025
Ding, L. J., Wang, X. M., Chen, J., Hai, B., and Wang, X. M. (2021). “Optimization of one-step production of biodiesel by Sulfuric acid/Stannic oxide-Hangjin2 clay catalyzed Xanthoceras sorbifolium Bunge kernel,” Energ Source Part A 4, 13.
Durak, H. (2020). “Hydrothermal liquefaction of Glycyrrhiza glabra L. (Liquorice): Effects of catalyst on variety compounds and chromatographic characterization,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 42, 2471-2484. DOI: 10.1080/15567036.2019.1607947
Hao, Y. N., Wang, X. M., and Ding, L. J. (2011). “The research of biodiesel produced by Xanthoceras sorborifolia burge oil,” Journal of Inner Mongolia Agriculturai University 32, 224-229. DOI: CNKI:SUN:NMGM.0.2011-02-051
Munir, M., Saeed, M., Ahmad, M., Waseem, M. Z., Sultana, S., Zafar, M., and Srinivasan, G. R. (2019). “Optimization of novel Lepidium perfoliatum Linn. biodiesel using zirconium-modified montmorillonite clay catalyst,” Energy Sources, Part A: Recovery, Utilization and Environmental Effects 2019, 1-15. DOI: 10.1080/15567036.2019.1691289
Sagala (2018). “The preparation of Yb/TiO2/Hangjin2#clay and in-situ IR study on photocatalytic degradation of benzene,” Spectral Analysis 38, 76-77. DOI: CNKI:SUN:GUAN.0.2018-S1-035
Shang, Y., Jiang, Y., and Gao, J. (2015). “One-step synthesis of peanut shell-derived solid acid for biodiesel production,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 37, 1039-1045. DOI: 10.1080/15567036.2011.603026
Sohn, J. R., Lee, S. H., and Lim, J. S. (2006). “New solid superacid catalyst prepared by doping ZrO2 with Ce and modifying with sulfate and its catalytic activity for acid catalysis,” Catalysis Today 116, 143-150. DOI: 10.1016/j.cattod.2006.01.023
Tanabe, K., Misono, M., Hattori, H., and Ono, Y. (1989). New Solids Acids and Bases: Their Catalytic Properties, Elsevier Science.
Varala, R, Narayana, V, Kulakarni, S. R., Khan, M., Alwarthan, A., and Adil, S. F. (2016). “Sulfated tin oxide (STO) – Structural properties and application in catalysis: A review,” Arabian Journal of Chemistry 9(4), 550-573. DOI: 10.1016/j.arabjc.2016.02.015
Vijaya, K. B., Ramesh, K., and Pandian, S. (2019). “Biodiesel production from tannery waste using a nano catalyst (ferric-manganese doped sulphated zirconia),” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 1-13. DOI: 10.1080/15567036.2019.1639849
Wang, S. X., Shan, R., Wang, Y., Lu, L. L., and Yuan, H. R. (2019). “Synthesis of calcium materials in biochar matrix as a highly stable catalyst for biodiesel production,” Renewable Energy 130, 41-49. DOI: 10.1016/j.renene.2018.06.047
Wei, J. F., Hao, X. Y., and Guo, H. F. (2010). “Synthesis of n-butyl acetate catalyzed by solid super acid SO42-/Hangiin2#clay,” Journal of Zhaoqing University 31, 40-43. DOI: 10.3969/j.issn.1001-8735.2010.04.018
Zhang, H., Chen, L., Li, Y., Hu, Y.-L., Li, H., Xu, C. C., and Yang, S. (2022). “Functionalized organic-inorganic hybrid porous coordination polymers based catalysts for biodiesel production via trans/esterification,” Green Chemistry 24, article 7763. DOI: 10.1039/d2gc02722d
Zhao, R., Wu, Y., and Bao, D. (2004). “Preparation of activated Hangjin2#clay and its bleaching effects on vegetable oil,” China Oils and Fats 29, 19-21. DOI: 10.3321/j.issn:1003-7969.2004.08.005
Article submitted: April 25, 2023; Peer review completed: May 13, 2023; Revised version received: July 21, 2023; Accepted: July 22, 2023; Published: July 26, 2023.
DOI: 10.15376/biores.18.3.6194-6203
