Abstract
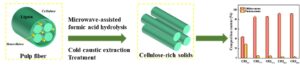
Effective separation of cellulose and hemicellulose from lignocellulosic biomass is an essential step for creating high-value products. In this study, a modified treatment process was proposed for cellulose purification via microwave-assisted formic acid catalytic hydrolysis followed by cold caustic extraction. The sugar content in the extract was determined using UV spectrophotometer and dual-wavelength visible spectrophotometry. Combined microwave-assisted formic acid (M-FA) with cold caustic extraction (CCE) treatments achieved rapid separation and removal of hemicelluloses from waste hardwood pulp fibers. The hemicelluloses content decreased from 28.6% to 2.3%, and the lignin content changed from 27.8% to 6.1%, which resulted in a maximal cellulose content of 91.5% under the optimal M-FA/CCE treatment conditions. In addition, the crystallinity index of pulp fibers increased from 54.3% to 67.1%, and the initial decomposition temperature decreased from 335.4 to 270.2 °C with the decrease of hemicellulose and lignin content. The modified process provided a sustainable and effective method for hemicellulose separation and lignin removal from cellulosic fibers.
Download PDF
Full Article
Microwave-assisted Formic Acid/Cold Caustic Extraction for Separation of Cellulose and Hemicellulose from Biomass
Longxiao Zhu,a Kangzhe Liang,a Min Wang,b Tao Xing,a,* Chunxia Chen,c,* and Quanliang Wang a,*
Effective separation of cellulose and hemicellulose from lignocellulosic biomass is an essential step for creating high-value products. In this study, a modified treatment process was proposed for cellulose purification via microwave-assisted formic acid catalytic hydrolysis followed by cold caustic extraction. The sugar content in the extract was determined using UV spectrophotometer and dual-wavelength visible spectrophotometry. Combined microwave-assisted formic acid (M-FA) with cold caustic extraction (CCE) treatments achieved rapid separation and removal of hemicelluloses from waste hardwood pulp fibers. The hemicelluloses content decreased from 28.6% to 2.3%, and the lignin content changed from 27.8% to 6.1%, which resulted in a maximal cellulose content of 91.5% under the optimal M-FA/CCE treatment conditions. In addition, the crystallinity index of pulp fibers increased from 54.3% to 67.1%, and the initial decomposition temperature decreased from 335.4 to 270.2 °C with the decrease of hemicellulose and lignin content. The modified process provided a sustainable and effective method for hemicellulose separation and lignin removal from cellulosic fibers.
DOI: 10.15376/biores.18.4.6896-6912
Keywords: Microwave-assisted formic acid; Cold caustic extraction; High-purity cellulose; Hemicelluloses separation; Cellulosic fibers
Contact information: a: College of Mechanical and Electrical Engineering, Northeast Forestry University, Harbin 150040, China; b: Material Science and Engineering College, Northeast Forestry University, Harbin 150040, China; c: College of Chemistry, Chemical Engineering and Resource Utilization, Northeast Forestry University, Harbin 150040, China;
* Corresponding authors: 13244658175@126.com; quanliangwang@nefu.edu.cn; ccx1759@163.com
GRAPHIC ABSTRACT

INTRODUCTION
Lignocellulosic biomass meets the needs of green and sustainable development due to its abundance, renewability, and biodegradability; thus, it is regarded as a powerful alternative to petroleum-based products (Dai et al. 2020). The biomass is mainly composed of cellulose (30 to 50 wt%), hemicelluloses (20 to 35 wt%), and lignin (15 to 30 wt%), and it is produced with an annual yield of about 180 billion tons worldwide (Awoyale and Lokhat 2021; Wang et al. 2023). The cellulose, hemicellulose, and its derivatives are processed into gels, membranes, chemicals, etc. They are widely applied in biomedical, electronic components, energy and other fields (Qin et al. 2023; Wan Azelee et al. 2023). Dissolving pulp (more than 92% cellulose) is a high-purity cellulose material, which serves as the source to manufacture cellulose products, including rayon, lyocell, and nanocellulose materials (Wang et al. 2021). Lignin and its derivatives are converted into various additives, which can perform well in areas including building, UV blocking, and bacteriostasis (Moreno and Sipponen 2020; Feng et al. 2022; Xu et al. 2022; Yu et al. 2023). However, the cell wall of natural biomass materials is complex and hard. Cellulose is connected with lignin and hemicellulose to form a typical reinforced concrete-like structure, which limits the comprehensive utilization of lignocellulosic biomass (Figueiredo et al. 2018). Thus, the separation technology of cellulose and hemicellulose has always been a hot topic in the comprehensive utilization of lignocellulosic biomass.
To separate hemicellulose and extract cellulose from lignocellulose biomass, physical, chemical, and biological pretreatment methods are used, e.g., ultrasonic, ball-milling, chemical reagents, and enzyme treatment (Rastogi and Shrivastava 2017; Alam et al. 2020; Sun et al. 2020). Chemical methods are widely used. The traditional hemicellulose extraction employs an alkaline method followed by ethanol precipitation. However, the yield of hemicellulose is low because of the large amount of residual lignin (Sporck et al. 2017). The extraction method of alkaline hemicellulose has been improved, including delignification with sodium chlorite acid, and then extraction to obtain high hemicellulose yield (Chem et al. 2022). However, due to the high difficulty, cost, and environmental hazards of operation, most current methods are only used in laboratories, and are difficult to use in factories (Wang et al. 2018; Li et al. 2021). The combined treatment method overcomes the shortcomings of a single treatment (Yuan et al. 2017). Microwave-assisted formic acid catalyzed hydrolysis can effectively remove hemicellulose and open fiber structure. Then alkali is easier to penetrate into the fiber during low alkali 4% CCE treatment, which is conducive to the removal of residual hemicellulose. Therefore, it is of great significance to develop a combined treatment method to separate hemicellulose and extract cellulose.
Microwave-assisted pretreatment has been applied to the separation of biomass components (Kan et al. 2018). Microwave-assisted pretreatment has the advantages of reducing energy consumption, obtaining high product yield, reducing the formation of by-products, and ease of operation (Davila et al. 2019; Wei et al. 2019). Liu et al. (2018) reported that microwave-assisted treatment promotes catalytic reaction and increases the removal and separation of hemicellulose by damaging the structure of wood fiber. Camani et al. (2020) evaluated the effect of the microwave-assisted method on eucalyptus waste liquid by experiments and obtained the best microwave-assisted condition in sodium hydroxide pretreatment. In addition, acid as a catalyst allows effective and selective depolymerization of hemicellulose while reducing the hornification of fibers and opening the structures of fiber cells (Liu et al. 2021). As the catalyst, formic acid exhibits stable physical properties almost like water, as well as reusability (Wang et al. 2019). High-efficiency separation of hemicellulose and cellulose is achieved by short-time microwave-assisted formic acid treatment. After acid pretreatment, a low concentration of alkali is added to react with biomass, which promotes the inward penetration and diffusion of alkali, and the removal of degraded hemicelluloses (Wang et al. 2021). However, the combination of microwave-assisted formic acid (M-FA) and cold caustic extraction (CCE) for the separation and removal of hemicellulose has not been reported.
The separation of cellulose and hemicellulose from lignocellulosic biomass is always a focus of biomass comprehensive utilization. Most separation technologies are limited because of high cost, high energy consumption, low yield, and low purity. In this work, a combined M-FA/CCE treatment process was developed to obtain high-purity cellulose and reduce energy consumption. Microwave-assisted formic acid treatment was used to catalyze hemicellulose degradation and open the fiber structure to obtain the initial catalytic hydrolysis pulp. A low-concentration cold caustic treatment was used to remove the degraded hemicellulose to obtain cellulose-rich pulp. The effects of different concentrations of formic acid and alkali were investigated on cellulose pulp yield. The changes in pulp and filtrate components were analyzed during formic acid and caustic soda treatments. The results indicate that cellulose content in CRS88/4 pulp (91.5%) was close to the purity requirement of dissolving pulp, which can be a suitable source for making dissolving pulp products. Moreover, the pulp fibers before and after various treatments were characterized by X-ray diffraction (XRD) analysis and thermogravimetric analysis (TGA).
EXPERIMENTAL
Poplar residues were harvested from Baihe Forestry Bureau (Jilin, China). The poplar fibers (43.6% cellulose, 28.6% hemicelluloses, and 27.8% lignin) were prepared by chemi-thermomechanical pulping (CTMP) and mechanical refining treatment. The pulp fibers were put in a polyethylene bag and stored in a low-temperature dry environment for use. The formic acid used was purchased from Sinopharm Chemical Reagent Co., Ltd (Beijing, China). Other chemical reagents were purchased from Tianli Chemical Reagent Co., Ltd (Tianjin, China). All chemicals were used as received without any purification, which was of analytical grade.
Microwave-assisted Formic Acid (M-FA) Treatments
The M-FA treatments were used to separate hemicellulose. First, 3 g of absolutely dry fibers and 120 mL formic acid (72% and 88%) were placed in an Erlenmeyer flask (250 mL), shaken well, and heated using a microwave oven (Midea, EG720KG3-NR1). A temperature sensor (FLANK8855, Shenzhen Frank Instrument Company, China) was placed in the formic acid solution for monitoring the temperature. The Erlenmeyer flask was placed at the center of the microwave and heated at 90 °C for 4 h. Then, the Erlenmeyer flask was removed from the microwave oven and cooled to room temperature. The solids were separated from the liquid hydrolysate by vacuum filtration. After that, the solids were washed with distilled water to neutral for the next step. The filtrate was collected for analysis. Microwave-assisted formic acid (72% and 88%) treatments were defined as 72% M-FA and 88% M-FA in the following text.
Cold Caustic Extraction (CCE)
After microwave-assisted formic acid (M-FA) treatments, the collected solids were removed from residual lignin by cold caustic extraction (CCE) treatment. The cellulose-rich solids (CRS) and 45 mL caustic soda (4% and 8%) were added into an Erlenmeyer flask (250 mL). After uniform mixing, the liquid mixture was heated in a water bath at 25 °C for 30 min. Finally, the CRS was separated from the liquid mixture by vacuum filtration. The CRS was washed with distilled water to neutral for further measurement and analysis. The filtrate was collected for analysis. The cold caustic extraction (4% and 8%) treatments were defined as 4% CCE and 8% CCE in the following text.
Determination of Cellulose-rich Solids (CRS) Yield
The original poplar pulp is composed of cellulose, lignin, hemicellulose, and small amounts of other substances, accounted for 43.6%, 28.6% and 27.8%, respectively. The effect of microwave-assisted formic acid/cold alkali treatments on pulp yield was evaluated by comparing the yield of CRS after treatments with the original poplar pulp. The CRS was placed in a clean and dry weighing bottle. Then, the weighing bottle was put into the oven to dry at 105 °C for 4 h. The weighing bottle was put into a dryer to cool for 30 min before being weighed. The CRS yield was evaluated based on Eq. (1),
(1)
where C is the quality of CRS after drying, and M0 is the quality of original dry pulp.
Determination of Pulp Composition
The contents of lignin, hemicellulose and cellulose in CRS were determined by the sodium chlorite method, volumetric method, and indirect method, respectively. The changes in lignin, hemicellulose, and cellulose content in CRS were evaluated by comparison with the original pulp.
The holocellulose content in the CRS, which refers to all cellulose and hemicellulose in plant fiber materials, was determined according to the GB/T 2677.10 (1995). The holocellulose content was evaluated based on Eq. 2.
(2)
where is the quantity of holocellulose after drying, and C is the quantity of CRS after drying.
Due to the chemical components of pulp being mainly holocellulose and lignin, the lignin content in CRS was determined using Eq. 3.
(3)
where is the quantity of holocellulose after drying, and is the quantity of CRS after drying.
Pentosans are the main component of hemicelluloses. Thus, the hemicellulose content was expressed as the pentosan content. The pentosan content in pulp was determined according to the GB/T 2677.9 (1994). The cellulose content of microwave-assisted formic acid and cold alkali treatment was calculated using the following Eq. (4).
Cellulose content (%) =Holocellulose content (%) -Hemicellulose content (%) (4)
Characterization of the Pulp
The characterization of the pulp was analyzed by X-ray diffraction (XRD) and thermogravimetric analysis (TGA). The TGA analysis was performed via a thermogravimetric analyzer (TGA; NETZSCH STA 449 F5/F3 Jupiter) at a heating rate of 10 °C/min. The XRD analysis was performed on an X-ray diffractometer (*/X, Pert3 Powedr, Panaco, Netherlands, China) with the Ni-filtered Cu Kαradiation (λ= 0.15406 nm) at 52 kV and 60 mA in the range of 2θ from 5° to 50° at a rate of 5°/min. The crystallinity index (CrI) was calculated according to the conventional peak intensity method according to Eq. (5). (Segal et al. 1959),
(5)
where I200 is the intensity of the crystalline portion at about 2θ= 22.60° and Iam is the intensity of the amorphous portion at about 2θ = 18.00°.
The average width of the crystallite obtained from (hkl) diffraction was determined as follows (Chen et al. 2009):
(6)
where k is the Scherrer constant (0.9), λ is the X-rays wavelength (0.154 nm), β is the half-band width in radians, and θ is the diffraction angle at the highest peak.
Determination of Carbohydrate Composition
The sugar content in hemicellulose extract was determined according to a UV spectrophotometer and dual-wavelength visible spectrophotometry (Gehmayr and Sixta 2012). In brief, a three-step procedure was conducted: 1) According to the maximum absorbance of xylose and total sugar, the first wavelength for measuring xylose and the second wavelength for measuring total sugar were determined: 2) The absorbance values of xylose and total sugar in different concentrations were determined at characteristic wavelengths, respectively. Further, the fitting lines were plotted and the standard curve equation was obtained by analysis. 3) Six kinds of extract samples were evaluated by means of the same steps. Based on the standard curve equation, the concentration of xylose, total sugar and glucose were calculated by Eqs. (7 to 9),
(7)
(8)
(9)
where Y1 is the absorbance value of the sample at the first wavelength, Y2 is the absorbance of the sample at the second wavelength, and K1 and K2 are the slopes. Based on the determined monosaccharide content, the cellulose (glucose) and hemicellulose (xylose) yields of the different extracts can be calculated.
Determination of Lignin Yield
The precipitated acid-insoluble lignin and acid-soluble lignin contents were determined by acid precipitation and national standard UV spectrophotometry, respectively.
Based on the insolubility of lignin in strong inorganic acid, the acid-insoluble lignin content was directly determined by treating the sample with a certain concentration of sulphuric acid. The acid-insoluble lignin yield was calculated according to Eq. 10,
(10)
where is the weight of acid-insoluble lignin from the extract solution and is the total weight of lignin from the original pulp.
The chemical structure of acid-soluble lignin is similar to that of acid-insoluble lignin, and its basic structure is p-hydroxybenzoic acid and syringaldehyde (Wang et al. 2021). The acid-soluble lignin yield was calculated according to Eq. 11,
(11)
where L2 is the weight of acid-soluble lignin from the extract solution, and T is the total weight of lignin from the original pulp.
RESULTS AND DISCUSSION
Process of M-FA and CCE Treatments for the Cellulose-Rich Solids
The complex cell wall structure of lignocellulosic fiber is composed of cellulose, hemicellulose, and lignin, which limits the comprehensive utilization of lignocellulosic biomass. In this work, a modified approach combining microwave-assisted formic acid (M-FA) and cold alkali extraction (CCE) is reported to convert a pulp fiber into cellulose-rich solids (Fig. 1). The M-FA pretreatment achieved the effective removal of the hemicellulose and lignin and partially opened the fiber structure. The microwave irradiation was able to quickly and fully heat up the fibers (≤ 100 °C) and reduce energy consumption. The pulp fibers could be heated up from the core to the surface by the microwave heating, which led to an abrupt increase in temperature and pressure inside the pulp fibers. Thus, the compact structure of fibers could be broken. The formic acid agent was recycled for reutilization without additional inorganic acids. For the CCE process, the alkali solution needs to wet the pulp fibers and gradually penetrate the fibers. However, the hemicelluloses removal was difficult due to the powerful inter-molecular and intra-molecular hydrogen bonds. Moreover, the fibril aggregate size increased with the increase in sodium hydroxide concentration (Dou and Tang 2017). After full penetration of the formic acid, fibers can promote the inward penetration and diffusion of alkali during CCE treatment. The swelling of fiber was increased with the increase in absorbing NaOH. Thus, combining M-FA and CCE treatment was found to be an effective approach to a pulp fiber conversion into cellulose-rich solids.
Fig. 1. Illustration of microwave-assisted formic acid and cold caustic extraction treatment for hemicelluloses removal from pulp fiber
Effect of M-FA and CCE Treatments on the Yield of Cellulose-Rich Solids
The pulp fiber was treated by microwave-assisted formic acid/cold alkali process to achieve the effective separation of cellulose and hemicelluloses. The yield of cellulose-rich solids (CRS) is shown in Table 1. The yield of CRS was 64.8% with the treatment of 72% M-FA and 4% CCE. The yield of CRS decreased to 62.1% with the increase of CCE concentration from 4% to 8%. Similarly, the yield of CRS decreased to 61.5% as the M-FA concentration increased from 72% to 88%. As the concentrations of M-FA and CCE increases simultaneously, the yield of CRS may decrease to the minimum. In fact, the yield of CRS decreased to a minimum of 60.8% with the treatment of 88% M-FA and 8% CCE. This indicates that the high concentration of M-FA and CCE can lead to a decrease in the yield of CRS. The degree of random chain breakage of carbohydrates evidently was affected by the concentration of M-FA during acid hydrolysis (Liu et al. 2022). The content of hemicellulose and lignin in CRS decreased with the increase of M-FA and CCE concentration, which led to a decrease in CRS yield. However, a high concentration of M-FA was favorable to the hydrolysis of hemicellulose from the lignocellulose structure and the decomposition of lignin.
Table 1. Cellulose-rich Solids Yield after M-FA and CCE Treatments
Effect of M-FA and CCE Treatments on Compositions of Cellulose-rich Solids
The effect of M-FA and CCE treatments was analyzed for compositions of cellulose-rich solids, as shown in Fig. 2. The cellulose-rich solids were mainly composed of cellulose with a small amount of lignin and hemicellulose. The content of cellulose was increased while the content of pentosan was reduced using high concentrations of M-FA and CCE for treatment (Fig. 2a).
Fig. 2. (a) Contents of cellulose and pentosan, and (b) contents of holocellulose and lignin of pulp fibers under different M-FA and CCE treatments
A certain concentration of M-FA (72% or 88%) was used, such that the cellulose content increased from 84.9% to 85.5% (72% M-FA) or from 91.5% to 91.7% (88% M-FA) due to the increase in CCE concentration. Using a certain concentration of CCE (4% or 8%), the cellulose content increased from 84.9% to 91.5% (4% CCE) or from 85.5% to 91.7% (8% CCE) due to the increase in M-FA concentration. Similarly, when a certain concentration of M-FA (72% or 88%) was used, and the pentosan content decreased from 4.4% to 4.2% (72% M-FA) or from 2.4% to 2.3% (88% M-FA) due to the increase in CCE concentration. Using a certain concentration of CCE (4% or 8%) decreased the pentosan content from 4.4% to 2.4% (4% CCE) or from 4.2% to 2.3% (8% CCE) due to the increase in M-FA concentration. This can be explained by the high concentration of M-FA being more conducive to the hydrolysis of pulp fibers and the opening of fiber structure. It could promote the inward penetration of alkali and the removal of hemicellulose and lignin, thus increasing the cellulose content in the final product even at a low alkali concentration (4% CCE). Changes in CCE concentration have little impact on the increase in cellulose content (only 0.6% and 0.2%) and the decrease in pentosan content (only 0.2% and 0.1%). However, changes in M-FA concentration have an obvious impact on the increase in cellulose content (6.6% and 6.2%) and the decrease in pentosan content (2% and 1.9%). Thus, high-purity cellulose was obtained while hemicellulose was efficiently separated by using 88% M-FA and CCE. Moreover, high alkaline extraction will lead to a decrease of fiber in accessibility and reactivity due to the removal of hemicellulose and the serious microfibril aggregation caused by the crystal transformation (Gehmayr and Sixta 2012; Wang et al. 2021). Thus, 88% M-FA/4% CCE could be selected for optimal treatment conditions, which could not only obtain high-purity cellulose but also save chemicals.
As shown in Fig. 2b, after M-FA/CCE pretreatment, the holocellulose content was increased, whereas the lignin content was obviously decreased after pretreatment with M-FA/CCE. The holocellulose contents were increased to 89.2% in 72% M-FA/4% CCE treated pulp and increased to 89.74 % in 72% M-FA/8% CCE treated pulp compared to the original pulp fibers (72.2%). The higher concentration of 88% M-FA/ (4% or 8%) CCE could further increase the holocellulose content to 93.9%. This indicated that a high concentration of 88% M-FA and 4% CCE treatment could increase the holocellulose content. In contrast, the lignin content was decreased with increasing concentrations of M-FA and CCE. The lignin contents were 10.8% and 6.1% using 72% or 88% M-FA/4% CCE pretreatment, and under 72% or 88% M-FA/8% CCE conditions, it was 10.3% and 6.0%, respectively. This was attributed to the massive cleavage of α-aryl ether and aryl glyceride β ether bonds, leading to the dissolution of numerous lignin macromolecules in high-concentration M-FA treatment. Compared to 8% CCE, the lignin content of 4% CCE extraction was increased by 0.5 % (72% M-FA) and 0.1% (88% M-FA), which indicated that the use of 4% CCE in industrial production was more cost-effective. Meanwhile, 88% M-FA/4% CCE was beneficial for lignin macromolecular cracking in M-FA/CCE process. A large number of lignin-hemicellulose complexes present in the outer layer of the fiber cell wall were easily extracted, hydrolyzed, and dissolved by formic acid. Compared to 4% CCE, the pentosan content of 8% CCE extraction was only decreased by 0.12 % (72% M-FA) and 0.1% (88% M-FA). Therefore, the effect of M-FA was obvious in the separation of lignin from cellulose, and 4% CCE was suitable for the removal of hemicellulose. The optimal process conditions for cellulose extraction from poplar lignocellulose are 88% M-FA/4% CCE according to cellulose extraction efficiency and green environmental protection.
XRD Analysis of Cellulose-Rich Solids
The crystallinity of the original pulp fibers and M-FE/CCE treated cellulose-rich solids were analyzed by XRD measurement. As shown in Fig. 3, all samples showed three characteristic reflections at 2θ = 14.8°, 16.0° and 22.5°, corresponding to the (1-10), (110), and (200) crystallographic planes of cellulose I, respectively. This indicated that the M-FA/CCE process did not change the type of cellulose crystal (Yunke et al. 2022). The diffraction angle and microcrystalline size (Lhkl) of the crystal plane exhibited different changes due to the tension exerted by amorphous substances on the crystal structure of cellulose. The reactive surface area of the fibrous crystal region was decreased with the increase in the cross-sectional area (A) of microcrystals, which led to a decrease in the accessibility of cellulose. The change may be attributed to the phenomenon of co-crystallization. Meanwhile, the crystallinity value of the fiber processed with M-FA/CCE increased from 54.3% to 67.1%, which was due to the removal of lignin and the extraction of amorphous hemicellulose in cellulose. It can be seen that the crystallinity value of the pulp sample process with 8% CCE decreased from 67.1% to 63% compared with that processed with 4% CCE. Due to the regular aggregation of element fibers in fibers that were disturbed under CCE, there was a decrease in crystallinity. A low crystallinity index indicates a large proportion of amorphous regions, indicating high accessibility of fibers (Dou and Tang 2017; Wang et al. 2021). Thus, the 88% M-FA/4% CCE process was more suitable in terms of fiber accessibility and crystallinity.
Fig. 3. XRD spectra of cellulose-rich solids
Table 2. Analyses of Cellulose-Rich Solids Structure with Different Treatment Conditions
Thermal Analysis of Cellulose-rich Solids
The thermal stabilities of the original pulp fiber samples pretreated by the M-FA/CCE process are presented as TG and DTG curves, respectively, in Fig. 4. As shown in Fig. 4a, the pulp processed by the four methods all presented similar weight loss stages. The slight weight loss from 25 to 100 ℃ was the first thermal decomposition weight loss stage, which was due to the evaporation of water. The weight loss of pulp after four treatments were similar, indicating that the moisture absorption rate was the same. There was almost no weight loss of the original pulp from 100 to 180 ℃, while the weight loss of the pulp samples treated with M-FA from 100 to 180 ℃ was not obvious. From 180 to 360 ℃, the weight of the original pulp was greatly lost, which was attributed to the thermal depolymerization of hemicellulose and cellulose. After M-FA/CCE was processed, the weight of the pulp began to decrease sharply at 180 ℃ until the temperature reached about 330 ℃. As shown in Fig. 4b, the characteristic peaks of the pulp after processing were similar, indicating that the chemical reactions in the thermal decomposition process were similar. However, the intensity of characteristic peaks was different because of the different chemical compositions of the pulp after processing. The thermal stability was evaluated by the onset decomposition temperature (Ton). The Ton of raw pulp, Pulp72/4, Pulp88/4 and Pulp88/8 were 335.4, 244.4, 270.2, and 233.8 ℃, respectively. Compared with the original pulp, the Ton decreased after M-FA/CCE processing, indicating that the thermal stability of the pulp after that processing was lower than that of the original pulp. This was due to the reduction in the lignin content of the treated pulp, which weakens its thermal stability. With the removal of hemicellulose and lignin, the residue yield was increased at 490 ℃. It is worth noting that the Ton of Pulp88/4 was higher than the Ton of Pulp72/4 and Pulp88/8. This finding could be attributed to the high cellulose content and high crystallinity of Pulp88/4 compared to the other two samples.
Fig. 4. (a) TGA and (b) DTG curves of cellulose-rich solids
Feasibility Analysis of Standard Curve for Measuring Sugar Content in Solution
The first wavelength (555 nm) for measuring xylose and the second wavelength (410 nm) for measuring total sugar were determined according to the maximum absorbance of xylose and total sugar (Table 3). Moreover, the fitting lines were plotted, and the standard curve equation was obtained by analysis (Fig. 5a and b). As shown in Table 3, the maximum absorption value of xylose can be obtained at a wavelength of 555 nm, while glucose was almost zero here. Therefore, the wavelength used for xylose measurement was determined to be 555 nm in this work. The report showed that the ε2/ε2′ reached the minimum (Chi et al. 2010). Thus, 410 nm was selected as the second wavelength determined by referring to the method and spectrum of this experiment. As shown in Fig. 4a, it can be seen that the standard curve equation of xylose was y=0.6254x+0.059 (R²=0.9963), where y was the absorbance value at 555 nm and x was the xylose concentration (mmol/L). The equation of total sugar was y=0.1086x-0.0138 (R²=0.9736), where x was the total sugar concentration (mmol/L) at 410 nm in Fig. 4b. Moreover, the absorption of hexose (mainly glucose) at 555 nm was lower than that of pentosan (mainly xylose). In broadleaf trees, the pentosan is the main component of the extract; thus the absorption of hexose at this wavelength can be ignored (Jie et al. 2016). And the linear correlation coefficient of two standard curve equations were 0.995 and 0.965, indicating good linearity. Therefore, it was feasible to measure sugar concentration using the above two standard equations.
Table 3. Wavelength Absorbance of 1 mmol/L and 2 mmol/L xylose and 1 mmol/L and 2 mmol/L Glucose
Fig. 5. (a) Absorbance value of xylose at 555 nm, and (b) total sugar at 410 nm
Effect of M-FA Treatment on the Filtrate Compositions
The main components in the filtrate were xylose, glucose, acid-soluble lignin, and acid-insoluble lignin after the M-FA treatment process with different concentrations of formic acid (72% and 88%), as shown in Fig. 6. Part 6a shows that the contents of recoverable xylose and glucose in the filtrate were increased by a high concentration of M-FA treatment. The xylose and glucose contents were obtained after 72% M-FA treatment at levels of 3.2% and 13.4%, respectively. Moreover, the contents of xylose and glucose increased to 3.4% and 16.3% after 88% M-FA treatment. It can be seen that the recoverable xylose content of 88% M-FA (3.4%) was higher than that of 72% M-FA (3.2%). This indicated that a higher concentration of 88% M-FA was easier to penetrate the interior of fiber cell walls, which facilitates the dissolution and removal of hemicellulose. It is worth noting that the glucose content increased. As shown in Fig. 6b, most lignin was removed from the pulp during the M-FA treatment. The contents of acid-soluble lignin and acid-insoluble lignin were obtained, which were 16.2% and 0.84% after 72% M-FA treatment respectively. The contents of acid-soluble lignin and acid-insoluble lignin increased to 22.8% and 0.8% after 88% M-FA treatment. During the process of catalytic hydrolysis with 88% M-FA, the yield of total lignin was the highest, which was 23.6% (22.8% + 0.8%), which was obviously higher than that of 17.0% (16.2% + 0.8%) generated by 72% M-FA treatment. Therefore, a high content of lignin in the filtrate can be obtained by selecting 88% M-FA.
Fig. 6. (a) Carbohydrate content and (b) lignin yield in the filtrates after M-FA treatments
Effect of M-FA and CCE Treatments on the Filtrate Compositions
The carbohydrate content and acid-soluble lignin yield in filtrates are shown in Fig 7. As shown in Fig. 7a, the hemicellulose and cellulose content in the filtrate were changed after M-FA and CCE treatment. The xylose content was measured after 72% and 88% M-FA/4% CCE treatments; the amounts were 1.2% and 0.6%, respectively. Correspondingly, the glucose content was 6.2% and 5.5%. Moreover, the contents of xylose increased to 1.8% and 1.6% after 72% and 88% M-FA/8% CCE treatment. Similarly, the glucose content increased to 6.7% and 6.4%, respectively. It can be seen the xylose contents of Filtrate72/8 (1.8%) and Filtrate88/8 (1.6%) were higher than those of Filtrate72/4 (1.2%) and Filtrate88/4 (0.6%). It was attractive that the high concentration 8% CCE treatment showed a higher hemicellulose removal rate compared with the 4% CCE treatment. However, the loss of cellulose was increased. Therefore, 4% CCE treatment was selected in the microwave-assisted formic acid and cold alkali treatment process. As shown in Fig. 7b, the lignin was further removed from the pulp during the CCE treatment. A small amount of lignin, (mainly acid-soluble lignin) was obtained in addition to the total sugar in the filtrate after M-FA/CCE treatments. The acid-soluble lignin content was measured after 72% and 88% M-FA/4% CCE treatments, which were 0.26% and 0.18%, respectively. Meanwhile, the acid-soluble lignin content increased to 0.28% and 0.21% after M-FA 72% and 88% M-FA/8% CCE treatment. It can be seen that the acid-soluble lignin content increased slightly from 0.26% (Filtrate72/4) to 0.28% (Filtrate72/8) and from 0.18% (Filtrate88/4) to 0.21% (Filtrate88/8) as the M-FA concentration increased, which indicated that lignin in the filtrate was mainly obtained in the M-FA treatment process. It was concluded that a high-concentration cold alkali treatment could further improve the lignin yield in the filtrate.
The sugar and lignin contents were measured in the filtrate after M-FA/CCE treatments. A small amount of lignin, (mainly acid-soluble lignin) was obtained in addition to the total sugar in the filtrate after M-FA/CCE treatments. The glucose content exhibited the lowest value of 5.5% in the Filtrate88/4, indicating less loss of cellulose. The presence of lignin is known to have an inhibitory effect on the saccharification of raw materials in enzymatic saccharification. A large amount of cellulase loses its hydrolysis ability due to the presence of lignin, resulting in an increase in enzyme usage. Thus, there was the lowest acid-soluble lignin content of 0.17% in the Filtrate88/4, which was beneficial for the reutilization of glucose in the filtrate. Further in-depth research is needed on the recovery and reuse of fiber components in the filtrate.
Fig. 7. (a) Carbohydrate content, and (b) acid-soluble lignin yield in filtrates after M-FA and CCE treatments
CONCLUSIONS
- A green combined treatment process was presented to separate cellulose from other components of biomass. The process consisted of microwave-assisted formic acid (M-FA) and cold caustic extraction (CCE). The pulp fiber was converted into cellulose-rich solids via the process with a maximum cellulose content of 91.7% (fulfilling the requirements for high purity of dissolving pulp) and a maximum yield of 64.8%. The effect of M-FA was obvious in the separation of lignin from cellulose, and 4% CCE was suitable for the removal of hemicellulose.
- The optimal process conditions for cellulose extraction from poplar pulp were 88% M-FA/4% CCE according to cellulose extraction efficiency, energy saving, and green environmental protection. The cellulose content in pulp fiber increased from 43.6% to 91.5%, while the hemicellulose and lignin content decreased from 28.6% to 2.3% and 27.8% to 6.1%, respectively.
- Ultraviolet (UV) spectrophotometer and dual-wavelength visible spectrophotometry were used to determine the sugar content in hemicellulose extract, and the method of feasibility was verified. The glucose and xylose contents in the filtrate were 5.5%. and 0.6%. The lignin in the filtrate was mainly obtained in the M-FA treatment process.
- The crystallinity and thermal stability were measured by X-Ray diffraction (XRD) and thermogravimetric analysis (TGA) after the 88% M-FA/4% CCE process-treated pulp fibers. The crystallinity index and cross-sectional area of pulp fiber were increased from 54.3% to 67.1% and from 11.48 nm2 to 14.88 nm2, respectively. The initial decomposition temperature of pulp fiber decreased from 335.4 ℃ to 270.2 ℃ with the decrease of hemicellulose and lignin content.
ACKNOWLEDGMENTS
The authors are grateful for the support of the Natural Science Foundation of Heilongjiang Province (YQ2023C025) and the Fundamental Research Funds for the Central Universities (2572021BL02).
REFERENCES CITED
Alam, A., Wang, Y., Liu, F., Kang, H., Tang, S.-w., Wang, Y., Cai, Q., Wang, H., Peng, H., Li, Q., et al. (2020). “Modeling of optimal green liquor pretreatment for enhanced biomass saccharification and delignification by distinct alteration of wall polymer features and biomass porosity in Miscanthus,” Renewable Energy 159, 1128-1138. DOI: 10.1016/j.renene.2020.06.013
Awoyale, A. A., and Lokhat, D. (2021). “Experimental determination of the effects of pretreatment on selected Nigerian lignocellulosic biomass in bioethanol production,” Scientific Reports 11(1), article 557. DOI: 10.1038/s41598-020-78105-8
Camani, P. H., Anholon, B. F., Toder, R. R., and Rosa, D. S. (2020). “Microwave-assisted pretreatment of eucalyptus waste to obtain cellulose fibers,” Cellulose 27(7), 3591-3609. DOI: 10.1007/s10570-020-03019-7
Chem, M., Tanifuji, K., Utami, S. P., Putra, A. S., Ohi, H., and Nakagawa-izumi, A. (2022). “Development of dissolving pulp from Phyllostachys pubescens stem by prehydrolysis soda cooking with 2-methylanthraquinone,” Industrial Crops and Products 178, article 114570. DOI: 10.1016/j.indcrop.2022.114570
Chen, Y., Wang, Y., Wan, J., and Ma, Y. (2009). “Crystal and pore structure of wheat straw cellulose fiber during recycling,” Cellulose 17(2), 329-338. DOI: 10.1007/s10570-009-9368-z
Chi, C.-C., Zhang, Z., Chai, X.-S., and Ge, W.-W. (2010). “Rapid determination of sugar content in hemicellulose extract by dual wavelength visible spectrometry,” Spectroscopy and Spectral Analysis 04, 1084-1087. DOI: 10.3964/j.issn.1000-0593(2010)04-1084-04
Dai, L., Wang, Y., Zou, X., Chen, Z., Liu, H., and Ni, Y. (2020). “Ultrasensitive physical, bio, and chemical sensors derived from 1-, 2-, and 3-D nanocellulosic materials,” Small 16(13), article e1906567. DOI: 10.1002/smll.201906567
Davila, I., Remon, J., Gullon, P., Labidi, J., and Budarin, V. (2019). “Production and characterization of lignin and cellulose fractions obtained from pretreated vine shoots by microwave assisted alkali treatment,” Bioresource Technology 289, article 121726. DOI: 10.1016/j.biortech.2019.121726
Dou, X., and Tang, Y. (2017). “The influence of cold caustic extraction on the purity, accessibility and reactivity of dissolving-grade pulp,” ChemistrySelect 2(35), 11462-11468. DOI: 10.1002/slct.201701551
Feng, C., Zhu, J., Hou, Y., Qin, C., Chen, W., Nong, Y., Liao, Z., Liang, C., Bian, H., and Yao, S. (2022). “Effect of temperature on simultaneous separation and extraction of hemicellulose using p-toluenesulfonic acid treatment at atmospheric pressure,” Bioresource Technology 348, article 126793. DOI: 10.1016/j.biortech.2022.126793
Figueiredo, P., Lintinen, K., Hirvonen, J. T., Kostiainen, M. A., and Santos, H. A. (2018). “Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications,” Progress in Materials Science 93, 233-269. DOI: 10.1016/j.pmatsci.2017.12.001
GB/T 2677.9 (1994). “Fibrous raw material – Determination of pentosan,” Standardization Administration of China, Beijing, China.
GB/T 2677.10 (1995). “Fibrous raw material – Determination of holocellulose,” Standardization Administration of China, Beijing, China.
Gehmayr, V., and Sixta, H. (2012). “Pulp properties and their influence on enzymatic degradability,” Biomacromolecules 13(3), 645-651. DOI: 10.1021/bm201784u
Jie, Z., Fengjuan, G., and Yuxiao, W. (2016). “Improved dual wavelength method for determining glucose and xylose content in binary mixed systems,” Anhui Agricultural Science 03, 9-12. DOI: 10.13989/j.cnki.0517-6611.2016.03.004
Kan, X., Zhang, J., Tong, Y. W., and Wang, C.-H. (2018). “Overall evaluation of microwave-assisted alkali pretreatment for enhancement of biomethane production from brewers’ spent grain,” Energy Conversion and Management 158, 315-326. DOI: 10.1016/j.enconman.2017.12.088
Li, J., Liu, Z., Feng, C., Liu, X., Qin, F., Liang, C., Bian, H., Qin, C., and Yao, S. (2021). “Green, efficient extraction of bamboo hemicellulose using freeze-thaw assisted alkali treatment,” Bioresource Technology 333, article 125107. DOI: 10.1016/j.biortech.2021.125107
Liu, B., Liu, L., Deng, B., Huang, C., Zhu, J., Liang, L., He, X., Wei, Y., Qin, C., Liang, C. et al. (2022). “Application and prospect of organic acid pretreatment in lignocellulosic biomass separation: A review,” International Journal of Biological Macromolecules 222, 1400-1413. DOI: 10.1016/j.ijbiomac.2022.09.270
Liu, Q., He, W. Q., Aguedo, M., Xia, X., Bai, W. B., Dong, Y. Y., Song, J. Q., Richel, A., and Goffin, D. (2021). “Microwave-assisted alkali hydrolysis for cellulose isolation from wheat straw: Influence of reaction conditions and non-thermal effects of microwave,” Carbohydrate Polymers 253, article 117170. DOI: 10.1016/j.carbpol.2020.117170
Liu, Y., Sun, B., Zheng, X., Yu, L., and Li, J. (2018). “Integrated microwave and alkaline treatment for the separation between hemicelluloses and cellulose from cellulosic fibers,” Bioresource Technology 247, 859-863. DOI: 10.1016/j.biortech.2017.08.059
Moreno, A., and Sipponen, M. H. (2020). “Lignin-based smart materials: a roadmap to processing and synthesis for current and future applications,” Materials Horizons 7(9), 2237-2257. DOI: 10.1039/d0mh00798f
Qin, Y., Zhang, W., Liu, Y., Zhao, J., Yuan, J., Chi, M., Meng, X., Du, G., Cai, C., Wang, S., et al. (2023). “Cellulosic gel-based triboelectric nanogenerators for energy harvesting and emerging applications,” Nano Energy 106, article 108079. DOI: 10.1016/j.nanoen.2022.108079
Rastogi, M., and Shrivastava, S. (2017). “Recent advances in second generation bioethanol production: An insight to pretreatment, saccharification and fermentation processes,” Renewable and Sustainable Energy Reviews 80, 330-340. DOI: 10.1016/j.rser.2017.05.225.
Segal, L., Creely, J. J., Martin, Jr. A. E., and Conrad, C. M. (1959). “An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer,” Text. Res. J. 29, 786-794. DOI: 10.1177/004051755902901003
Sporck, D., Reinoso, F. A. M., Rencoret, J., Gutierrez, A., Del Rio, J. C., Ferraz, A., and Milagres, A. M. F. (2017). “Xylan extraction from pretreated sugarcane bagasse using alkaline and enzymatic approaches,” Biotechnology for Biofuels 10, article 296. DOI: 10.1186/s13068-017-0981-z
Sun, D., Sun, S. C., Wang, B., Sun, S. F., Shi, Q., Zheng, L., Wang, S. F., Liu, S. J., Li, M. F., Cao, X. F., et al. (2020). “Effect of various pretreatments on improving cellulose enzymatic digestibility of tobacco stalk and the structural features of co-produced hemicelluloses,” Bioresource Technology 297, article 122471. DOI: 10.1016/j.biortech.2019.122471
Wan Azelee, N. I., Mahdi, H. I., Cheng, Y.-S., Nordin, N., Illias, R. M., Rahman, R. A., Shaarani, S. M., Bhatt, P., Yadav, S., Chang, S. W., et al. (2023). “Biomass degradation: Challenges and strategies in extraction and fractionation of hemicellulose,” Fuel 339, article 126982. DOI: 10.1016/j.fuel.2022.126982
Wang, Q., Xiao, S., Shi, S. Q., and Cai, L. (2019). “Microwave-assisted formic acid extraction for high-purity cellulose production,” Cellulose 26(10), 5913-5924. DOI: 10.1007/s10570-019-02516-8
Wang, S., Cheng, A., Liu, F., Zhang, J., Xia, T., Zeng, X., Fan, W., and Zhang, Y. (2023). “Catalytic conversion network for lignocellulosic biomass valorization: A panoramic view,” Industrial Chemistry & Materials. DOI: 10.1039/D2IM00054G
Wang, X., Duan, C., Feng, X., Qin, X., Wang, W., Wang, J., Xu, Y., and Ni, Y. (2021). “Combining phosphotungstic acid pretreatment with mild alkaline extraction for selective separation of hemicelluloses from hardwood kraft pulp,” Separation and Purification Technology 266, article 118562. DOI: 10.1016/j.seppur.2021.118562
Wang, X., Zhang, C., Lin, Q., Cheng, B., Kong, F., Li, H., and Ren, J. (2018). “Solid acid-induced hydrothermal treatment of bagasse for production of furfural and levulinic acid by a two-step process,” Industrial Crops and Products 123, 118-127. DOI: 10.1016/j.indcrop.2018.06.064
Wei, W., Zhang, H., and Jin, Y. (2019). “Comparison of microwave-assisted zinc chloride hydrate and alkali pretreatments for enhancing eucalyptus enzymatic saccharification,” Energy Conversion and Management 186, 42-50. DOI: 10.1016/j.enconman.2019.02.054
Xu, Y., Zhang, X., Wang, G., Zhang, X., Luo, J., Li, J., Shi, S. Q., Li, J., and Gao, Q. (2022). “Preparation of a strong soy protein adhesive with mildew proof, flame-retardant, and electromagnetic shielding properties via constructing nanophase-reinforced organic–inorganic hybrid structure,” Chemical Engineering Journal 447, article 137536. DOI: 10.1016/j.cej.2022.137536
Yu, X., Yang, B., Zhu, W., Deng, T., Pu, Y., Ragauskas, A., and Wang, H. (2023). “Towards functionalized lignin and its derivatives for high-value material applications,” Industrial Crops and Products 200, article 116824. DOI: 10.1016/j.indcrop.2023.116824
Yuan, Z., Wen, Y., Kapu, N. S., Beatson, R., and Mark Martinez, D. (2017). “A biorefinery scheme to fractionate bamboo into high-grade dissolving pulp and ethanol,” Biotechnology for Biofuels 10, 38. DOI: 10.1186/s13068-017-0723-2
Yunke, L., Zhenxin, L., Yutong, Z., Qirui, Y., and Erni1, M. (2022). “Water-induced effects of matrix in wood cell wall on cellulose crystalline structure,” Journal of Beijing Forestry University 44(12), 121-131. DOI: 10.12171/j.1000−1522.20220150
Article submitted: May 25, 2023; Peer review completed: July 8, 2023; Revised version received and accepted: July 23, 2023; Published: August 8, 2023.
DOI: 10.15376/biores.18.4.6896-6912
