Abstract
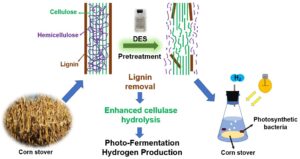
To compare the pretreatment effect of deep eutectic solvents (DESs) on corn stover, three types of DESs (acid, neutral, and alkaline) were used to pretreat corn stover, and their effects on lignin removal were investigated. Then the performance of photo-fermentation hydrogen production (PFHP) using DESs pretreated corn stover was compared and analyzed. The results showed that acid DESs had the best lignin removal efficiency, and the lignin removal percentages of choline chloride/formic acid (ChCl/Fac) and choline chloride/oxalate (ChCl/Oac) were 64.7% and 65.6%, respectively. The alkaline DESs had the second highest removal of lignin, while the neutral DESs had a poor effect on lignin removal. The comparison of hydrogen production indicated that the corn stover pretreated with ChCl/Oac had the best hydrogen production effect. In conclusion, the DESs pretreatment can obviously remove the lignin in corn stover, and then improve the hydrogen production capacity and efficiency of photo-fermentation, which provides a potential pretreatment method for the efficient utilization of straw biomass.
Download PDF
Full Article
Comparative Analysis of Photo-Fermentation Hydrogen Production from Pretreated Corn Stover by Deep Eutectic Solvents
Cunjie Li, Yanwei Lu, Yanyang Liu, Jianzhi Yue, Xiaokai Zhou, and Yanyan Jing *
To compare the pretreatment effect of deep eutectic solvents (DESs) on corn stover, three types of DESs (acid, neutral, and alkaline) were used to pretreat corn stover, and their effects on lignin removal were investigated. Then the performance of photo-fermentation hydrogen production (PFHP) using DESs pretreated corn stover was compared and analyzed. The results showed that acid DESs had the best lignin removal efficiency, and the lignin removal percentages of choline chloride/formic acid (ChCl/Fac) and choline chloride/oxalate (ChCl/Oac) were 64.7% and 65.6%, respectively. The alkaline DESs had the second highest removal of lignin, while the neutral DESs had a poor effect on lignin removal. The comparison of hydrogen production indicated that the corn stover pretreated with ChCl/Oac had the best hydrogen production effect. In conclusion, the DESs pretreatment can obviously remove the lignin in corn stover, and then improve the hydrogen production capacity and efficiency of photo-fermentation, which provides a potential pretreatment method for the efficient utilization of straw biomass.
DOI: 10.15376/biores.19.1.1433-1445
Keywords: Deep eutectic solvents; Corn stover; Pretreatment; Photo-fermentation; Hydrogen production
Contact information: Key Laboratory of New Materials and Facilities for Rural Renewable Energy, Ministry of Agriculture & Rural Affairs, Henan Agricultural University, Zhengzhou 450002, China;
* Corresponding author: jingyanyan123@126.com
GRAPHICAL ABSTRACT
INTRODUCTION
China produces a large amount of straw biomass every year, but the utilisation of straw biomass is very low. To reduce the waste of resources and increase utilisation, it is necessary to develop straw biomass conversion technology. Straw biomass is composed of cellulose, hemicellulose, and lignin, and the structure is very stable through hydrogen bonding and intermolecular force (Zhang et al. 2015). The complex and polymeric structures of straw biomass restrict its hydrolysis, which results in the low substrate degradation rate of straw biomass (Zhang et al. 2016). Therefore, before converting straw biomass into energy products, it is often necessary to interrupt the dense structure of biomass and reduce the crystallinity through pretreatment, to improve degradation and transformation of straw biomass and achieve the effective utilization of resources.
The biohydrogen production process consists of biomass pretreatment prior to product conversion, enzymatic hydrolysis to sugars, and biochemical conversion of sugars to hydrogen energy. Pretreatment is essential to promote the breakdown of cellulosic in biomass to increase fermentable monomer sugar and hydrogen generation (Jing et al. 2022a). Biomass pretreatment methods mainly include physical methods, physicochemical methods, chemical methods, and biological methods (Yu et al. 2019; Huang et al. 2020). Physical pretreatment mainly reduces the size of biomass particles or destroys its structure by mechanical crushing, high-energy radiation and so on, so as to increase the accessibility of enzymes to cellulose. Such methods can be simple to operate, but the energy consumption and the cost of pretreatment equipment are high (Yu et al. 2019). Physicochemical methods such as steam blasting and hydrothermal pretreatment are better than single physical methods, but maintaining high temperature, high pressure, and other conditions often require more energy consumption (Li et al. 2020). Chemical methods involve acid, alkali, and organic solvents, which can effectively disrupt the crystallinity within biomass and facilitate the conversion of carbohydrates into monosaccharides. However, these chemicals can corrode the equipment and also lead to the production of enzymatic inhibitors such as phenols, aldehydes, and acetic or other acids (Huang et al. 2020). Biological methods have the advantages of mild reaction conditions, low energy consumption and no pollution. However, the long pretreatment time and low efficiency limit the wide application of biological methods (Li et al. 2022).
Ionic liquids (ILs) belong to the class of organic solvents. Some of them have excellent properties of removing lignin in pretreatment of straw biomass (Zhao et al. 2018). However, considering its high toxicity and high cost of ILs (Xu et al. 2021), another set of solvent systems that are commonly called deep eutectic solvents (DESs) arose (Jablonsky and Sima 2022). As noted in the cited article, although the reported DESs typically are not true eutectic mixtures, the term “DESs” is still commonly used in the literature. DESs synthesized by a hydrogen bond donor (HBD) and hydrogen bond acceptor (HBA) have similar physical and chemical properties with ILs, including reported lignin solubility (Wang et al. 2022). The effect of choline chloride/oxalate (ChCl/Oac) pretreatment on straw was investigated, and 70% lignin removal rate was reached (Hou et al. 2018). The efficient removal of lignin promoted the glycation effect of cellulase on biomass. Zhang et al. (2016) studied the pretreatment of corn cob with choline chloride/lactic acid (ChCl/LA), which removed 93.1% lignin and obtained nearly 80% glucose. In addition, some DESs have the advantages of simple preparation, cheap price, and recyclability (Roy et al. 2020; Skulcova et al. 2017). Guo et al. (2019) pretreated corn cob with benzyltrimethyl-ammonium chloride /lactic acid (BTMAC/LA) and obtained 90% recovery of DES. The above report demonstrated that the DES pretreatment is an effective method for straw biomass.
In this study, in order to improve the capacity of photo-fermentation hydrogen production (PFHP), three types of DESs with acid HBDs (formic acid, oxalic acid), neutral HBDs (ethylene glycol, 1,4-butylene glycol), and alkaline HBDs (urea, acetamide) were synthesized using choline chloride as HBA, respectively. The effects of DESs on the lignin removal of corn stover were studied, and the performance of hydrogen production from DESs-pretreated corn stover was compared.
EXPERIMENTAL
Materials
The corn stover used in this study came from the experimental field of Henan Agricultural University, China. The corn stover was dried and crushed to the range 0.30 to 0.80 mm for DES pretreatment. The main components of corn stover were tested, among which the content of cellulose, hemicellulose, and lignin were 32.1%, 27.8%, and 22.7%, respectively.
The hydrogen-production bacteria was photosynthetic bacteria HAU-M1 (Henan Agricultural University, China), which consisted of Rhodobacter sphaeroides (9%), Rhodopseudomonas palustris (28%), Rhodospirillum rubrum (27%), Rhodopseudo-monas capsulate (25%), and Rhodobacter capsulatus (11%). The hydrogen-producing medium of HAU-M1 is shown in Table 1 (Jiang et al. 2015; Zhou et al. 2021).
Table 1. The Hydrogen-Producing Medium of HAU-M1
Note: All reagents in the table were dissolved in 1000 mL H2O.
Methods
Synthesis of DESs
The detailed preparation conditions of DESs are shown in Table 2. Two acid HBDs (formic acid and oxalic acid), two neutral HBDs (ethylene glycol, 1,4-butylene glycol), and two alkaline HBDs (urea and acetamide) were selected. Using choline chloride as HBA, HBA, and HBD were mixed according to the corresponding molar ratio, and then the mixture was heated according to the temperature in Table 2. In the process of DESs synthesis, the mixture was stirred with a thermostatic magnetic stirrer (Model B11-2, Shanghai Sile Instrument Co., Ltd., China) until a transparent homogeneous solution was obtained (Ren et al. 2016).
Table 2. Synthesis Conditions of DESs
DES pretreatment of corn stover
50 g corn stover was placed in a beaker of 2 L, and DES was added according to the solid-liquid ratio (stover: DES, g/g) of 1: 20 (Zhang et al. 2016). Then the beaker was placed on the magnetic stirrer at 120 ℃ for 3 h (Hou et al. 2018). Then mixture was then filtered, and the separated corn stover was washed several times with distilled water until neutral. Then, the corn stover of DES pretreatment was dried and crushed to 60 mesh for PFHP.
The chemical compositions of corn stover after pretreatment were determined by the NREL method (Jing et al. 2022a), and the lignin removal rate of corn stover was calculated according to the Eq. 1,
(1)
where WL is lignin removal (%); WR is recovery of corn stover (%); and MR is lignin content of pretreated corn stover (%). M0 is lignin content of untreated corn stover (%).
Hydrogen production of pretreated corn stover
In the PFHP process, 3.75 g of pretreat corn stover and 105 mL of citrate buffer (0.05 M, pH= 4.80) were added into 150 mL conical bottle, and hydrogen-producing medium was also added proportionally. The cellulase purchased from Novozymes (Denmark) with an enzyme activity of 51 FPU/mL was used to hydrolyze corn stover under cellulase loading of 0.15 g/g (enzyme/straw). Then, the pH of the hydrogen production reaction solution was adjusted to 7.0 by 5 M NaOH. Next, 45 mL of HAU-M1 inoculum in the logarithmic growth phase were put into the bioreactors for PFHP. After that, the head-space of the conical bottle was filled with N2 to keep anaerobic environment, and sealed with a rubber plug. The fermentation reactors were placed in a biochemical incubator (Model SPX-250B, Jiangsu Science Analysis Instrument Co., Ltd., China) with a temperature of 30 ℃ and light intensity of 3000 lux. The generated bio-hydrogen was collected by collection bag, and the hydrogen concentration was measured every 12 h. The concentration of hydrogen was determined by the gas chromatograph (Model 6820GC-14B, Agilent, USA). Nitrogen was the carrier gas, the column temperature was 80 ℃, the inlet was 100 ℃, the detector was 150 ℃, the intake volume was 500 μL, and the retention time was 2 min.
The modified Gompertz equation was used to conduct dynamic analysis of the cumulative hydrogen yield of PFHP (Xia et al. 2021). The associated calculation was done with Eq. 2,
(2)
where H(t) is the cumulative hydrogen yield (mL); Hmax is the maximum hydrogen yield potential (mL); t is the time (h); rm is the maximum hydrogen production rate (mL/h); e is the constant 2.718; and λ is the hydrogen production delay period (h).
Parameter detection of fermentation liquid
The pH and oxidation-reduction potential (ORP) of fermentation liquid are important parameters in the process of PFHP. Fermentation liquid of hydrogen production of 2 mL was taken from the reactor every 12 h. Then the pH and ORP were detected by pH meter (Model PHS-25, Mettlertoledo, Switzerland) and ORP meter (Model SX712, Shanghai San-Xin Instrumentation, Inc., China), respectively. And the small molecular fatty acids were analyzed using a high performance liquid chromatography (HPLC) (1260 Infinity, Agilent Technologies, USA).
The reducing sugar concentration of fermentation liquid could show the enzymatic hydrolysis effect of cellulase on corn stover. The concentration of reducing sugar was determined by DNS colorimetry (Jin et al. 2021). The OD value of DNS colorimetry were measured at 540 nm using the visible light spectrophotometer (Model 721, Shanghai Jinghua Technology Instrument Co., Ltd., China), and sugar concentration of fermentation liquid was calculated using standard equations of Eq. 3,
(3)
where Y is the concentration of reducing sugar (g/L); X is the OD value at 540 nm.
RESULTS AND DISCUSSION
Effect of DESs on Lignin Removal from Corn Stover
As shown in Fig. 1, DESs types resulted in significant differences for the removal of lignin from corn stover. The lignin removal percentages of ChCl/Fac and ChCl/Oac pretreated corn stover were 64.73% and 65.60%, respectively. The results showed that the acidic DESs broke the connection structure among lignin, cellulose, and hemicellulose during the pretreatment process, and effectively dissolved the lignin from corn stover. The lignin removal rates of ChCl/U and ChCl/A pretreated corn stover were 35.2% and 47.1% respectively, which were lower than that of the acid DESs. The reason was that this type of DES is weakly alkaline, and the pretreatment process was mild, resulting in a low lignin removal percentage (Zhao et al. 2018). The neutral DESs synthesized by ethylene glycol and 1,4-butylene glycol had the lowest lignin removal ability, and the average lignin removal rate was about 23%. Gou et al. (2018) used ChCl/BG to pretreat xylose residue. They also found that the lignin removal effect was poor, and the viscosity of the DES was high after pretreatment. Zhao et al. (2018) reached the same conclusion that acid or alkaline DESs were helpful to remove lignin, while neutral DESs had a poor effect on lignin removal. And the compositions of DESs pretreated corn stover were shown in Table 3.
Fig. 1. Lignin removal of DESs pretreated corn stover
Table 3. Compositions of DESs Pretreated Corn Stover
Comparison of Hydrogen Production of Pretreated Corn Stover
The cumulative hydrogen yield of corn stover pretreated with different DESs types is shown in Fig. 2a. The hydrogen production capacity of corn stover pretreated with acid DESs were the highest and was much higher than that of neutral and alkaline DESs. The cumulative hydrogen yield of corn stover pretreated with acid DESs was 403 mL (ChCl/Oac) and 398 mL (ChCl/Fac), namely 107 mL/g and 106 mL/g, respectively. It was slightly higher than 104 mL/g hydrogen yield of corn stover pretreated with 0.6% HCl (Wang 2009). The hydrogen yield effect of corn stover pretreated with the neutral DESs was poor, which was consistent with the lignin removal effect (Fig. 1). For DESs composed of alkaline HBDs, they had different effects on hydrogen yield. The cumulative hydrogen yield of corn stover pretreatment with ChCl/A was 250 mL, which was 2.16 times of that with ChCl/U pretreatment (116 mL). The reason for this phenomenon was that the lignin removal percentage of ChCl/A was 47.1% (Fig. 1), and the higher lignin removal promoted the hydrogen production. After DESs pretreatment, the cumulative hydrogen yield of corn stover from high to low was ChCl/Oac > ChCl/Fac > ChCl/A > ChCl/U > ChCl/BG > ChCl/EG > untreated. The results showed that the pretreatment effect of acid DESs were the best, followed by alkaline DESs, and that of neutral DESs were poor (Pan et al. 2017).
Fig. 2. Effects of DESs pretreatment on (a) cumulative hydrogen yield, (b) hydrogen production rate
Figure 2b shows the effects of different DESs pretreatment on the hydrogen production rate. Although DESs types had a great influence on the hydrogen production rate of pretreated stover, the trend of hydrogen production was basically consistent with the change of time. The peak of hydrogen production was concentrated between 12 and 36 h, and the hydrogen production gradually stopped after 48 h. The reason was that the fermentation system had sufficient sugar and nutrients in the initial stage of hydrogen production, and the photosynthetic bacteria also had strong metabolism and hydrogen production capacity. Therefore, the hydrogen production rate was quite high. With the increase of hydrogen production, the nutrients in fermentation liquid decreased. And harmful substances such as small molecular acids produced by the metabolism of photosynthetic bacteria inhibited the activity of bacteria, resulting in a rapid decrease in hydrogen production rate (Yang et al. 2022). The hydrogen production rates of the acid DESs pretreated corn stover were also relatively high, which were 27.4 mL/h (ChCl/Oac) and 22.0 mL/h (ChCl/Fac), respectively.
The modified Gompertz equation was used to fit the PFHP process of corn stover pretreated with DESs, and the fitting parameters obtained are shown in Table 4. The determination coefficients R2 were all greater than 0.96, and the Hmax (maximum hydrogen yield potential) of ChCl/Fac and ChCl/Oac pretreatment corn stover were 398 mL and 403 mL, respectively, which was similar to the experimental results (Fig. 2a). Therefore, the results showed that the kinetic fitting was effective. The λ (hydrogen production delay period) showed that the λ of DESs pretreatment was all less than that at 12 h. And the λ of two alkaline DESs was shorter, indicating that DESs greatly accelerated the hydrogen production process (Yang 2023).
Table 4. Water Demand of Wheat at Different Growth Periods
The above results showed that although the λ of the two acid DESs pretreatment methods was longer than that of other DES pretreatment methods, they had the highest Hmax and rm (hydrogen production), especially the Hmax of the ChCl/Oac pretreatment method was 403 mL. According to the comprehensive comparative analysis, the pretreatment effect of acid DESs was the best, followed by alkaline DESs, and the hydrogen production effect of neutral DESs pretreatment was poor.
Changes of Reducing Sugar in PFHP
The changes of reducing sugar in hydrogen production process with DESs pretreated corn stover are shown in Fig. 3. The concentration of reducing sugar in fermentation liquid increased rapidly within the first 12 h and reached the maximum. A large amount of reducing sugar was produced by cellulolytic hydrolysis, and the consumption of reducing sugar by photosynthetic bacteria was low in the initial stage of hydrogen production (Li et al. 2022). When the fermentation process was at 12 to 24 h, photosynthetic bacteria consumed a large amount of reducing sugar, the hydrogen yield increased rapidly (Fig. 2a), and the reducing sugar concentration in fermentation liquid decreased rapidly. With the increase of fermentation process, photosynthetic bacteria gradually entered the stable stage. And the production and consumption of reducing sugar were basically equal, so the reducing sugar of the fermentation liquid tended to be stable. There was an interesting phenomenon that the reducing sugar concentration of the two acid DESs pretreated test groups showed a slight increase trend after 24 h. This was attributed to the fact that acid DESs penetrated more deeply into the corn stover than others, the stover at the bottom of the reactor was further hydrolyzed by cellulase during the late stage of synchronous saccharification hydrogen production, releasing some reducing sugars again (Zhang et al. 2020).
In the whole process of hydrogen production, the reducing sugar concentration of corn stover pretreated with ChCl/Fac and ChCl/Oac were higher, and the reducing sugar concentration of corn stover pretreated with two acid DESs at 12 h was the highest, which were 2.72 g/L and 1.75 g/L, respectively. The results showed that the pretreatment with acid DESs effectively destroyed the structure of stover and promoted cellulase hydrolysis. The reducing sugar concentration of corn stover pretreated with ChCl/Fac was higher than that of ChCl/Oac, but the hydrogen yield of corn stover pretreated with two acid DESs was almost the same (Fig. 2a). Combined with Fig. 4a, the pH of the fermentation liquid (ChCl/Fac) was 5.31 at 12 h, which was lower than that of the ChCl/Oac, resulting in a large number of small molecular fatty acids in the fermentation liquid (ChCl/Fac pretreated). This inhibited the hydrogen production capacity of photosynthetic bacteria (Yang et al. 2022). The concentrations of reducing sugar in the fermentation liquid pretreated with neutral and alkaline DESs were all lower than 1.0 g/L, because the two types DESs not only dissolved lignin, but they also partially dissolved the cellulose and hemicellulose in the stover, resulting in low cellulolytic efficiency and low reducing sugar content in the fermentation liquid (Li et al. 2020).
Fig. 3. Changes of reducing sugars during PFHP
Changes of pH and Small Molecular Fatty Acids Concentration in PFHP of DESs Pretreatment
The pH and small molecular fatty acids concentrations of fermentation liquid during PFHP process are shown in Fig. 4. In the range of 0 to 12 h, the pH of fermentation liquid decreased rapidly. This was due to the metabolism of photosynthetic bacteria to produce acetic acid, butyric acid, and other small molecular fatty acids, which affected the pH of fermentation liquid (Jing et al. 2022b). Later, with the extension of PFHP, the pH of fermentation liquid pretreated by different types of DESs showed different changes. The pH of hydrogen production under corn stover pretreated by ChCl/Oac and ChCl/Fac continued to decline and tended to be stable, and the pH finally stabilized at about 4.85. The hydrogen-producing fermentation liquid of corn stover pretreated with ChCl/A, ChCl/U, ChCl/EG, and ChCl/BG all showed a trend of slow rise at first and then gradually stable with the increase of time. When hydrogen production ended, the pH of the fermentation liquid was maintained at 6 to 7.
At 0 to 12 h, hydrogen production was in the initial stage, and a large number of reducing sugar produced by cellulase hydrolysis was utilized by photosynthetic bacteria. Then a large number of small molecular fatty acids were produced, resulting in a rapid decline in the pH of the fermentation liquid. With the increase of hydrogen production time, some small molecular fatty acids produced by photosynthetic bacteria metabolism were reused (Zhang et al. 2017). The difference in the production and consumption of small molecular fatty acids caused the trend fluctuation of pH values. For DESs composed of two acid HBDs, there were still many available reducing sugars in the hydrogen production system (Fig. 3). A large amount of small molecular weight fatty acids produced by photosynthetic bacteria caused the pH of fermentation liquid to decrease continuously (Fig. 4b). The lower pH inhibited the activity of the bacteria, resulting in an early end to hydrogen production. In the course of hydrogen production by alkaline and neutral DESs pretreatment, the stover saccharification efficiency was low, which caused photosynthetic bacteria to use small molecular fatty acids to maintain growth and produce hydrogen. Because the consumption of small molecular fatty acids in PFHP, the pH of fermentation liquid showed a slow increasing trend. When the production and consumption of small molecular fatty acids showed a dynamic balance, the pH of the fermentation liquid eventually tended to stabilize (Jing et al. 2022b).
Fig. 4. pH (a) and small molecular fatty acids (b) changes during the process of PFHP
Changes of ORP in PFHP of DESs Pretreatment
The ORP of fermentation liquid is an important index to reflect the vital signs of photosynthetic bacteria. Some of the enzymes necessary for the growth of photosynthetic bacteria can only be activated after being completely reduced, so the ORP needs to be negative in order to grow better for bacteria. Meanwhile, the lower the ORP of fermentation liquid, the stronger the reducibility of bacteria, and the greater the hydrogen production capacity. On the contrary, with higher ORP, hydrogen production capacity was weaker when ORP was higher and the oxidation of bacteria was stronger (Li et al. 2022).
In the process of hydrogen production through pretreatment with different types of DESs, the ORP changes are shown in Fig. 5. The ORP of fermentation liquid showed the same variation, which is a trend of increasing at first and then stabilizing slowly. The ORP values of fermentation liquid under different conditions were all lower than -100 mV between 12 and 36 h, indicating that photosynthetic bacteria had high activity, which was consistent with the change of hydrogen production rate in Fig. 2b. When hydrogen production of corn stover pretreated with two acid DESs was at 12 h, the ORP of fermentation liquid was lower. In particular, the ORP of ChCl/Oac experimental group was -428 mV, indicating that the photosynthetic bacteria in this group had strong growth and hydrogen production activity. From 12 to 24 h, ORP of fermentation liquid began to rise, which was because reducing sugar was consumed by photosynthetic bacteria, and then it decreased rapidly (Fig. 3), and the reducibility of bacteria was decreased. This phenomenon was consistent with the conclusion of Yu’s research (Yu et al. 2017). At the end of hydrogen production process, the activity of photosynthetic bacteria was weakest.
Fig. 5. ORP changes during the process of PFHP
CONCLUSIONS
- In terms of lignin removal efficiency, the removal percentages of lignin in corn stover by different types of deep eutectic solvents (DESs) were as follows: ChCl/Oac > ChCl/Fac > ChCl/A > ChCl/U > ChCl/BG > ChCl/EG > Untreated. The lignin removal efficiency of two acid DESs was about 65%.
- From the point of hydrogen production performance, the hydrogen production of corn stover after pretreatment with ChCl/Oac (ChCl/Fac) was 403 mL (398 mL), and the maximum hydrogen production rates were 27.4 mL/h and 22.0 mL/h, respectively. The alkaline DESs followed and neutral DESs pretreated corn stover resulted in poor hydrogen production.
- The pretreatment effects of different types of DESs on corn stover were compared through photo-fermentation hydrogen production (PFHP) experiments. The results showed that DES pretreatment can effectively remove lignin from straw biomass, improve its hydrogen production capacity, and is a potential pretreatment method for straw biomass.
ACKNOWLEDGMENTS
The authors are grateful for the financial support provided by National Natural Science Foundation of China (Grant No. 52206245).
REFERENCES CITED
Guo, Z., Ling, Z., Wang, C., Zhang, X., and Xu, F. (2018). “Integration of facile deep eutectic solvents pretreatment for enhanced enzymatic hydrolysis and lignin valori-zation from industrial xylose residue,” Bioresource Technology 265, 334-339. DOI: 10.1016/j.biortech.2018.06.027
Guo, Z., Zhang, Q., You, T., Zhang, X., Xu, F., and Wu, Y. (2019). “Short-time deep eutectic solvent pretreatment for enhanced enzymatic saccharification and lignin valorization,” Green Chemistry 21(11), 3099-3108. DOI: 10.1039/C9GC00704K
Hou, X., Li, A., Lin, K., Wang, Y., and Cao, S. (2018). “Insight into the structure-function relationships of deep eutectic solvents during rice straw pretreatment,” Bioresource Technology 249, 261-267. DOI: 10.1016/j.biortech.2017.10.019
Huang, B., Wang, L., Wei, X., Xu, W., Sun, Z., and Li, T. (2020). “Lignocellulose pretreatment by deep eutectic solvents for biobutanol production,” Progress in Chemistry 32(12), 2034-2048. DOI: 10.7536/PC200424
Jablonsky, M., and Sima, J. (2022). “Is it correct to name DESs deep eutectic solvents?” BioResources 17(3), 3880-3882. DOI: 10.15376/biores.17.3.3880-3882
Jiang, D., Han, B., Wang, Y., Wang, S., You, X., and Zhang, Q. (2015). “Characteristic analysis of physiological feature and hydrogen production by HAU-M1 photosynthetic bacteria,” Acta Energiae Solaris Sinica 36(02), 289-294. DOI: 10.3969/j.issn.0254-0096.2015.02.005
Jin, P., Zhang, Q., Lu, C., Li, Y., and Liu, Z. (2021). “Study on photofermentative hydrogen production from Paulownia catalpifolia leaves,” Acta Energiae Solaris Sinica 42(07), 444-449. DOI: 10.19912/j.0254-0096.tynxb.2019-0216
Jing, Y., Li, F., Li, Y., Jiang, D., Lu, C., Zhang, Z., and Zhang, Q. (2022a). “Biohydrogen production by deep eutectic solvent delignification-driven enzymatic hydrolysis and photo-fermentation: Effect of liquid-solid ratio,” Bioresource technology 349, 126867-126867. DOI: 10.1016/j.biortech.2022.126867
Jing, Y., Zhou, X., Li, F., Wang, Z., and Zhang, Q. (2022b). “Effect of pH values on cogon photosynthetic hydrogen production,” Journal of Safety and Environment 22(02), 1013-1018. DOI: 10.13637/j.issn.1009-6094.2020.1792
Lynam, J. G., Kumar, N., and Wong, M. J. (2017). “Deep eutectic solvents’ ability to solubilize lignin, cellulose, and hemicellulose; thermal stability; and density,” Bioresource Technology 238, 684-689. DOI: 10.1016/j.biortech.2017.04.079
Li, L., Wu, Z., Liang, J., and Yu, L. (2020). “Application of deep eutectic solvents in ligno-cellulosic biomass processing,” Journal of Forestry Engineering 5(04), 20-28. DOI: 10.13360/j.issn.2096-1359.201907035
Li, F. (2022). Optimization of Corncob Deep Eutectic Solvent Pretreatment Process and Effect on Photo-fermentation Hydrogen Production, Master’s Thesis, Zhengzhou, China.
Phadtare, S. B., and Shankarling, G. S. (2010). “Halogenation reactions in biodegradable solvent: Efficient bromination of substituted 1-aminoanthra-9,10-quinone in deep eutectic solvent (choline chloride:urea),” Green Chemistry 12(31), 458-462. DOI: 10.1039/b923589b
Pan, M., Zhao, G., Ding, C., Wu, B., Lian, Z., and Lian, H. (2017). “Physicochemical transformation of rice straw after pretreatment with a deep eutectic solvent of choline chloride/urea,” Carbohydrate Polymers 176, 307-314. DOI: 10.1016/j.carbpol.2017.08.088
Ren, H., Chen, C., Wang, Q., Zhao, D., and Guo, S. (2016). “The properties of choline chloride-based deep eutectic solvents and their performance in the dissolution of cellulose,” BioResources 11(2), 5435-5451. DOI: 10.15376/biores.11.2.5435-5451
Roy, R., Rahman, M. S., and Raynie, D. E. (2020). “Recent advances of greener pretreatment technologies of lignocellulose,” Current Research in Green and Sustainable Chemistry 3, 100035. DOI: 10.1016/j.crgsc.2020.100035
Skulcova, A., Majova, V., Sima, J., and Jablonsky, M. (2017). “Mechanical properties of pulp delignified by deep eutectic solvents,” BioResources 12(4), 7479-7486. DOI: 10.15376/biores.12.4.7479-7486
Wang, Y. (2009). Experimental Study on Biological Hydrogen Production by Anaerobic Fermentation of Corn Straw, Master’s Thesis, Xi’an, China.
Wang D., and Liu, Y. (2018). “Fractionation of lignocellulose with deep eutectic solvent (DES),” Journal of Beijing University of Chemical Technology (Natural Science) 45(06), 40-47. DOI: 10.13543/j.bhxbzr.2018.06.007
Wang, Y., Zhang, W., Yang, J., Li, M., Peng, F., and Bian, J. (2022). “Efficient fractionation of woody biomass hemicelluloses using cholinium amino acids-based deep eutectic solvents and their aqueous mixtures,” Bioresource Technology 354, 127139-127139. DOI: 10.1016/j.biortech.2022.127139
Xia, C., Zhang, Q., Zhang, Z., Hu, J., He, C., and Jing, Y. (2021). “Effect of amino acids on photo-fermentative hydrogen production of biomass straw,” Acta Energiae Solaris Sinica 42(05), 488-493. DOI: 10.19912/j.0254-0096.tynxb.2018-1411
Xu, F., Cheng, P., Guo, Z., and Xu, Y. (2021). “Research progress on the fractionation and structural properties of lignin based on deep eutectic solvents,” Journal of Beijing Forestry University 43(04), 158-168. DOI: 10.12171/j.1000-1522.20200410
Yang, J., Jiang, D., Shui, X., Lei, T., Zhang, H., Zhang, Z., Zhang, X., Zhu, S., and Zhang, Q. (2022). “Effect of 5-HMF and furfural additives on bio-hydrogen production by photo-fermentation from giant reed,” Bioresource Technology 347, 126743-126743. DOI: 10.1016/j.biortech.2022.126743
Yang, J. (2023). Experimental Study of The Portable Bio-Hydrogen Production Reactor by Photo-Fermentation, Master’s Thesis, Zhengzhou, China.
Yu, J., Zhao, Y., Zhang, H., Hua, B., Yuan, X., Zhu, W., Wang, X., and Cui, Z. (2017). “Hydrolysis and acidification of agricultural waste in a non-airtight system: Effect of solid content, temperature, and mixing mode,” Waste Management 59, 487-497. DOI: 10.1016/j.wasman.2016.10.019
Yu, Q., Liu, R., Li, K., and Ma, R. (2019). “A review of crop straw pretreatment methods for biogas production by anaerobic digestion in China,” Renewable and Sustainable Energy Reviews 107, 51-58. DOI: 10.1016/j.rser.2019.02.020
Zhang, Q., Li, Y., Jing, Y., Jiang, D., and Zhang, Z. (2015). “Effect of acid and alkaline pretreatment on simultaneous saccharification fermentation for bio-hydrogen production from Platanus orientalis leaves,” Transactions of the Chinese Society for Agricultural Machinery 46(05), 202-207. DOI: 10.6041/j.issn.1000-1298.2015.05. 028
Zhang, C., Xia, S., and Ma, P. (2016). “Facile pretreatment of lignocellulosic biomass using deep eutectic solvents,” Bioresource Technology 219, 261-267. DOI: 10.1016/j.biortech.2016.07.026
Zhang, Q., Liu, H., Hu, J., Zhou, X., Jing, Y., Wang, Y., Zhang, T., and Zhang, Z. (2017). “Effect of phosphate and carbonate on photo-fermentative hydrogen production of biomass straw,” Transactions of the Chinese Society of Agricultural Engineering 33(13), 251-257. DOI: 10.11975/j.issn.1002-6819.2017.13.033
Zhang, Z., Zhang, H., Li, Y., Lu, C., Zhu, S., He, C., Ai, F., and Zhang, Q. (2020). “Investigation of the interaction between lighting and mixing applied during the photo-fermentation biohydrogen production process from agricultural waste,” Bioresource Technology 312, article 123570. DOI: 10.1016/j.biortech.2020.123570
Zulkefli, S., Abdulmalek, E., and Rahman, M. (2017). “Pretreatment of oil palm trunk in deep eutectic solvent and optimization of enzymatic hydrolysis of pretreated oil palm trunk,” Renewable Energy 107, 36-41. DOI: 10.1016/j.renene.2017.01.037
Zhao, Z. (2018). Study on Ionic Liquids and Deep Eutectic Solvents Pretreating Wheat Straw, Master’s Thesis, Beijing University of Chemical Technology, Beijing, China.
Zhao, Z., Chen, X., Ali, M. F., Abdeltawab, A. A., Yakout, S. M., and Yu, G. (2018). “Pretreatment of wheat straw using basic ethanolamine-based deep eutectic solvents for improving enzymatic hydrolysis,” Bioresource Technology 263, 325-333. DOI: 10.1016/j.biortech.2018.05.016
Zhou, N., Jing, Y., Xia, C., Han, M., and Zhang, Q. (2021). “Effect of vitamin B4 on photo-fermentative hydrogen production of straw biomass,” Journal of Thermal Science and Technology 20(05), 495-501. DOI: 10.13738/j.issn.1671-8097.019334
Article submitted: November 10, 2023; Peer review completed: December 9, 2023; Revised version received and accepted: January 4, 2024; Published: January 10, 2024.
DOI: 10.15376/biores.19.1.1433-1445
