Abstract
Hydrolysate pretreatment (HP) uses hot water pre-hydrolysis liquor (HWPL) as partial or full pretreatment medium for biomass. Pentosan dissolution during Eucalyptus HP was studied under holding times between 0 min and 160 min, holding temperatures between 150 °C and 190 °C, a hot water pre-hydrolysate ratio (HWPR) from 20% to 100%, and a fixed liquid to wood ratio of 1:6. Both the pentosan removals in the hydrolysate pretreated solid (HPS) and the hydrolysate pretreatment liquor (HPL) pento-saccharides contents were determined and compared with those of hot water pre-hydrolysis (HWP). When compared to HWP, the HP enhanced pentosan removal from the solid phase, and enriched the saccharides or promoted the in-situ conversion of saccharides into other chemicals in the liquid phase. Pentosan removal in the HPS increased when holding time and temperature were increased. Increasing holding time first increased the pento-saccharide content in the HPL, and then decreased it after reaching the maximum. Elevating the holding temperature increased the pento-saccharide content in the HPL, except for arabino-oligosaccharide. Different HWPR had varying influences on pentosan removal in the HPS and on the saccharides concentration in the HPL. When controlled, HP positively influenced hemicellulose removal from biomass, and increased utilization value of the liquid phase obtained post pretreatment.
Download PDF
Full Article
Research on the Dissolution of Pentosans during Eucalyptus Hydrolysate Pretreatment
Mingshuai Ma,a Ran Liu,a Yanzhu Guo,a Haiming Li,a,* Jinghui Zhou,a,* Haisong Wang,a Jie Lu,a and Shijie Zhang a,b
Hydrolysate pretreatment (HP) uses hot water pre-hydrolysis liquor (HWPL) as partial or full pretreatment medium for biomass. Pentosan dissolution during Eucalyptus HP was studied under holding times between 0 min and 160 min, holding temperatures between 150 °C and 190 °C, a hot water pre-hydrolysate ratio (HWPR) from 20% to 100%, and a fixed liquid to wood ratio of 1:6. Both the pentosan removals in the hydrolysate pretreated solid (HPS) and the hydrolysate pretreatment liquor (HPL) pento-saccharides contents were determined and compared with those of hot water pre-hydrolysis (HWP). When compared to HWP, the HP enhanced pentosan removal from the solid phase, and enriched the saccharides or promoted the in-situ conversion of saccharides into other chemicals in the liquid phase. Pentosan removal in the HPS increased when holding time and temperature were increased. Increasing holding time first increased the pento-saccharide content in the HPL, and then decreased it after reaching the maximum. Elevating the holding temperature increased the pento-saccharide content in the HPL, except for arabino-oligosaccharide. Different HWPR had varying influences on pentosan removal in the HPS and on the saccharides concentration in the HPL. When controlled, HP positively influenced hemicellulose removal from biomass, and increased utilization value of the liquid phase obtained post pretreatment.
Keywords: Eucalyptus; Hydrolysate Pretreatment; Pentose; Pentosan; Xylan; Xylose; Autohydrolysis
Contact information: a: School of Light Industry and Chemical Engineering, Dalian Polytechnic University, Dalian, Liaoning, 116034 China; b: State Key Laboratory of Pulp and Paper Engineering, South China University of Technology, Guangzhou 510640;
* Corresponding authors: 286911185@qq.com; zhoujh@dlpu.edu.cn
INTRODUCTION
Fossil fuel resources have been the main energy and chemical source for humans since the Industrial Revolution. However, the availability of fossil fuel resources on earth is limited. Biomass resources are renewable, in contrast to fossil fuel resources, and sustainable management can be obtained in the long term if the biomass is properly utilized (Amidon and Liu 2009). Plant biomass is mainly composed of cellulose, hemicellulose, and lignin (Agbor et al. 2011). Cellulose is a highly crystallized polymer with glucose as the monomer. Hemicellulose is polymerized from several different monosaccharides such as pentoses (arabinose, xylose, etc.) and hexoses (galactose, glucose, mannose, etc.). Lignin is a three-dimensional natural polymer with building blocks of phenylpropane units joined together through ether bonds and carbon-carbon bonds. The existence of complex linkages, caused by physical or chemical action between those three components, makes plant biomass resistant to the production of chemicals or fuel directly (Yedro et al. 2015).
Traditionally, hemicellulose is partly dissolved in the pulping effluent during the pulping process and burned to produce heat together with lignin, which dramatically hinders its utilization. In recent years, scientists worldwide have paid increasing attention to the extraction of hemicellulose from raw paper-making material through all kinds of pretreatment technologies that occur prior to the pulping and paper-making process. They have also been interested in the application of extracted oligomers in pharmaceutical, food, agriculture, paper-making, and other industries (Jaskari et al. 1998). Hot water pre-hydrolysis (HWP), also well known as autohydrolysis (Yap 1987; Liu et al. 2012) or hydrothermal processing (Garrote et al. 1999a), is another method available to extract hemicellulose from biomass, and is emphasized because of its effectiveness, economic efficiency, and eco-friendliness (Liu et al. 2014).
As an auto-catalyzed hydrolysis technology, HWP employs liquid water of high temperature as a pre-hydrolysis medium. The comparatively high dissociation constant of the medium makes it slightly acidic, which causes the acetyl and uronic acidic groups on hemicellulose to break off and form acetic acid and uronic acid. Those acids increase the acidity of the liquid phase, and they further break down the glycosidic linkages of hemicellulose. The breakdown causes the degradation of high molecular hemicellulose into dissolvable oligosaccharides, and it furthers the hydrolysis of the oligomers into monosaccharides and promotes the conversion of monosaccharides into acids, aldehydes, and other substances (Liu et al. 2013; Cantero et al. 2015; Kaur and Ni 2015). Those substances derived from the removed hemicellulose have great potential values for development. Oligosaccharides can be used as prebiotics, and monomeric sugars can serve as a detoxified carbon source for bioconversion to obtain bioethanol or organic compounds such as lactic acid (Alonso et al. 2011). Furfural can be used in the fields related to foundry, petroleum production, pharmaceuticals, chemical production, plastics, food, agricultural industry and so on (Garcίa-Domίnguez, et al. 2015). Understanding their behavior during particular pretreatment process is a key to make a tailored scheme for further utilization of the pretreatment liquor.
The formation of acids during HWP has drawn the attention of many researchers. Vallejos et al. (2015) pre-hydrolyzed bagasse with hot water between 160 °C and 180 °C and 0 min and 240 min. The resultant pre-hydrolysate contained acetic acid and formic acid, with the content of the former higher than that of the latter. Their content reached a maximum of 6.2% based on bone-dry raw material. Some researchers have suggested that organic acid could speed up the HWP process (Garrote et al. 1999a). Li et al. (2010) investigated the influence of an acetic acid addition on hardwood HWP between 30 min and 120 min at 170 °C. They found that the time needed to obtain the maximum xylose concentration in the pre-hydrolysate was shortened after acetic acid was added to the pre-hydrolysis system, which elucidated that the addition of acetic acid not only promoted the degradation of hemicellulose into xylo-oligosaccharide, but it also facilitated the conversion of xylo-oligosaccharide to xylose (Li et al. 2010). Liu et al. (2013) utilized the pre-hydrolysis liquor generated from hardwood to produce furfural from 20 min to 100 min between 150 °C and 190 °C, and they studied the catalysis effect of acetic acid on furfural production under those conditions. The results showed that the addition of acetic acid increased furfural production and the xylose conversion rate, indicating that the existence of acetic acid increased the decomposition of xylose (Liu et al. 2013). Because the pre-hydrolysate generated during conventional HWP contains organic acids of low molecular, theoretically those acids will catalyze the hydrolysis process when conventional hot water pre-hydrolysate is used as a hydrolysis medium to pretreat plant biomass. This recently invented process is called hydrolysate pretreatment (HP) (Cui and Li 2014; Li et al. 2015).
With HWP as a pre-study, this study investigated the dissolution of pentosans in the HP process to assay the feasibility of this process and discover its potential advantages.
EXPERIMENTAL
Materials
Eucalyptus grandis chips were supplied by Shandong Huatai Papermaking Company, Ltd. (Dongying, China). The chips were milled into powder and then separated by screens of different mesh sizes. The part passing through a 40-mesh and retaining on a 60-mesh screen was used as raw material for the experiment. The arabinan content and xylan content in the raw material was 1.1% and 21.9%, respectively.
Arabinose and xylose that were used as references were both purchased from Sigma-Aldrich Company (Saint Louis, USA), with the purity of both higher than 99%. Sodium acetate was purchased from Dionex Company (Sunnyvale, USA), with purity higher than 99.9%. Sodium hydroxide used as an eluent was purchased from Honeywell-Fluka Company (Seelze, Germany), with a weight percentage of 50% to 52%.
Methods
Hot water pre-hydrolysis
A certain amount of Eucalyptus powder was placed into a reactor, and distilled water was added to ensure a liquid to wood ratio of 6:1. The reactor was sealed and then transferred into an Intelligent Oil Bath (ZKYY-5L, Huayi Instrument Company Limited, Gongyi City, China) with the oil temperature at 40 °C. The reactor was heated to the maximum temperature (i.e. the holding temperature) varied from 150 °C to 190 °C, and then held at the temperature for 0 min to 120 min, i.e. the holding time. Once the holding time had elapsed, the reactor was removed and cooled to room temperature by flushing with water. The mixture in the reactor was removed and the HWPL was separated from the hot water pre-hydrolyzed solid (HWPS) phase through filtration. The HWPL was stored at 4 °C, and the HWPS was further washed with deionized water and air-dried for analysis.
Hydrolysate pretreatment
The HWPL generated from the HWP was mixed with deionized water at a certain ratio (i.e. 20%, 40%, 60%, 80%, or 100%) to prepare the medium for hydrolysate pretreatment (HP). The HP was performed with this prepared medium under otherwise the same conditions as the corresponding HWP from which the medium was obtained. After HP, the resultant mixture was separated into two parts through the same procedure as HWP. The hydrolysate pretreated liquor (HPL) was stored at 4 °C, and the hydrolysate pretreated solid (HPS) was washed thoroughly by deionized water before air-dried for further study.
Pento-saccharide analysis
For the pento-saccharide analysis in the solid material (including both the raw material and pretreated solids), the solids were subjected to two stages of sulfuric acid hydrolysis and then tested via ion chromatography (ICS-5000, Dionex Company, Sunnyvale, USA) (Li et al. 2010; Cui et al. 2013). The pentosan removal was calculated according to Eq. 1,
R = (1 – M1 / M0) × 100% (1)
where R is pentosan removal (%), M1 is the weight of pentosan in the pretreated solid (g), and M0 is the weight of pentosan in the raw material (g).
The monomeric sugar contents in the liquid phase were measured by ion chromatography (Li et al. 2010). The monomeric sugar content in the liquid phase after further hydrolysis by diluted sulfuric acid was also tested. Oligosaccharides in the liquid phase were characterized by the monose difference before and after dilute sulfuric acid hydrolysis (Sluiter et al. 2006; Li et al. 2010).
Calculation of combined severity factor (CSF)
A combined severity factor (CSF) was calculated, following Eq. 2 (Ertas et al. 2014),
CSF=log[t*exp((T-100)/14.75)]-pH (2)
where t is the pretreatment time (min), T is the pretreatment temperature (°C), and 14.75 is an empirical parameter related to temperature and activation energy.
RESULTS AND DISCUSSION
Hot Water Pre-hydrolysis
Pentosan dissolution at different holding times
To prepare the medium for HP, HWP was performed first. The effect of the holding time on the pentosan removal in the HWPS and on the pento-saccharide content in the HWPL was studied at 190 °C.
The results of the arabinan and xylan removal in the HWPS are shown in Fig. 1. When the holding time was 0 min, arabinan removal was 14. 6%. Arabinan had been partly removed from the Eucalyptus powder during the heating stage. The arabinan removal initially increased by extending the holding time, and reached 100.0% at the holding time of 60 min. The removal of xylan was lower than that of arabinan. Only 1.7% of xylose was removed during the heating stage. Xylan removal continued to increase with prolonged holding time, which could be explained by elevated CSF (-1.14, -0.01, 1.42, 1.54, 1.66, 1.80, 1.89, and 1.95 for 20 min, 40 min, 60 min, 80 min, 100 min, 120 min, 140 min, and 160 min, respectively). The xylan removal rate decreased after 60 min, and it almost reached a plateau after 120 min of holding time. 86.1% of xylan removal was obtained at a holding time of 120 min. The maximum xylan removal obtained at a holding time of 160 min, 89.8%, was close to the data reported for the autohydrolysis of Eucalyptus globulus (87.4%) (Garrote et al. 1999b), Eucalyptus urograndis (88.4%), and Eucalyptus grandis (90.4%) (Da Silva Morais et al. 2016).
Hemicellulose is a non-crystalline polymer with a low polymerization degree, a high branching degree, and poor hydrothermal stability. Because xylose comprises the backbone of the molecular chain of Eucalyptus hemicellulose (Agbor et al. 2011) while arabinose is on the branch of the hemicellulose (Garrote et al. 2004), arabinose is more easily dissolved than xylose during HWP. Hemicellulose was divided into “easy to hydrolyze” fractions and “difficult to hydrolyze” fractions, which were determined by the bonds among hemicellulose, cellulose, and lignin (Trajano et al. 2015; Carrasco and Roy 1992; Conner 1984). According to the research done by Garrote et al. (1999b), the susceptible fraction of Eucalyptus globulus, measured by the parameter α was around 84.3%, which was close to 86.2% of xylan removal at 120 min numerically. For the HWP conditions of this experiment, the “easy to hydrolyze” fractions of hemicellulose were dissolved from the raw material. Further extending of holding time caused slight increase of xylan removal, which indicated that a small amount of the “difficult to hydrolyze” part was affected under the conditions.
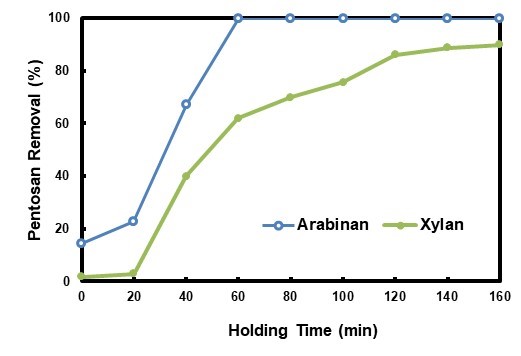
Fig. 1. Pentosan removals in HWPS at different holding times
The arabino-saccharide and xylo-saccharide contents in HWPL at different holding times are shown in Fig. 2. Arabino-saccharides existed in both the monomer and oligomer form in the HWPL. Arabino-oligosaccharide content increased with the extension of the holding time, reaching a maximum at a holding time of 20 min, and then it began to decrease as time increased further. Arabinose had a concentration similar to arabino-oligosaccharide for the first 20 min. However, arabinose continued to increase with further extension of the holding time, and it reached a maximum at a holding time of 60 min. Subsequently, arabinose decreased, which was caused by further degradation of arabinose into furfural or formic acid (Vallejos et al. 2015). The trend of total arabino-saccharides was similar to that of arabinose. Arabino-saccharides contained in the HWPL were mainly in monomer form during the pre-hydrolysis, which was also found by Garrote et al. (1999b).
As can be seen in Fig. 2, xylose, xylo-oligosaccharide, and total xylo-saccharides in the HWPL all initially increased with the extension of the holding time, and then they decreased after reaching the maximum concentration. The maximum concentrations of 6.31 g·L-1, 19.27 g·L-1, and 22.18g·L-1 appeared at the holding times of 100 min, 60 min, and 60 min, respectively. The corresponding holding times for maximum concentrations were all longer than those reported in the literature (Garrote et al. 1999b; Jesús Rangel et al. 2016; Zhuang et al. 2011). The difference could be caused by varied reactor system employed in the experiment (Borrega and Sixta 2015). Xylo-saccharides were also in both monomer form and oligomer form, and the amount of the former was almost always smaller than that of the latter. The later reached its maximum ahead of the former. A similar trend was found in reported data (Garrote et al. 1999b). For the pre-hydrolysis after the holding time of 60 min, the increase in xylose did not compensate for the decrease in xylo-oligosaccharide. This indicated that the hydrolyzation of xylo-oligosaccharide into xylose and the degradation of xylose into acid or aldehyde (Lau et al. 2014; Vallejos et al. 2015) occurred simultaneously, and that the hydrolyzation rate was lower than the degradation rate.
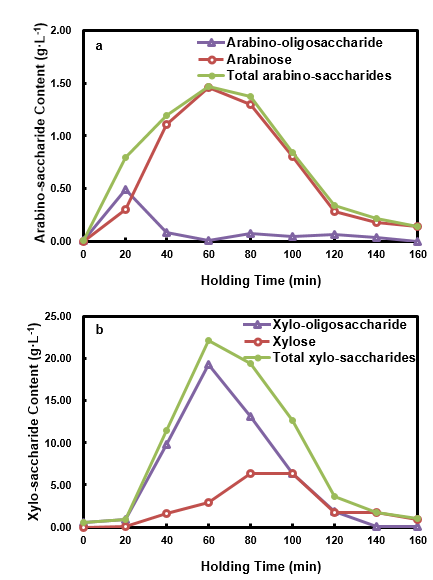
Fig. 2. Arabino-saccharides (a) and xylo-saccharides (b) in HWPL at different holding times
Pentosan dissolution at different holding temperatures
Based on the results of the pentosan dissolution at different holding times, the effects of the holding temperature on HWP were investigated at a fixed holding time of 60 min. The related results are shown in Figs. 3 and 4.
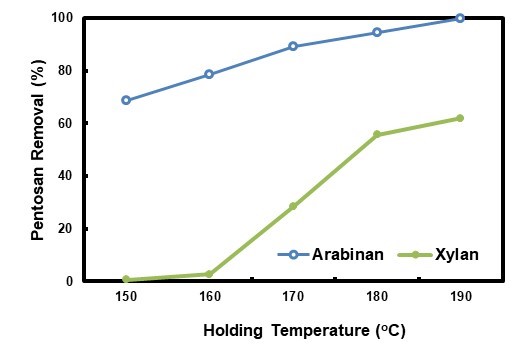
Fig. 3. Pentosan removals in the HWPS at different holding temperatures
The effects of the holding temperature on pentosan removal are shown in Fig. 3. Temperature is one of the most important parameters that affects the dissolution of hemicellulose. The removal of both arabinan and xylan increased with the rise in holding temperature. This is well related to the changes of CSFs, which were -3.42, -2.24, -1.05, 0.54, and 1.42 for 150 °C, 160 °C, 170 °C, 180 °C, and 190 °C, respectively. The arabinan removal increased from 68.8% to 100.0% with an increase in the holding temperature from 150 °C to 190 °C, and the xylan removal increased from 0.7% to 62.0%. Arabinan removal was higher than xylan removal at the same holding temperature. The dissolution rate of arabinan was nearly constant. The dissolution rate of xylan was comparatively low at temperatures less than 160 °C. It increased remarkably at holding temperatures between 160 °C to 180 °C, and then it slowed down with further increase in temperature up to 190 °C. This phenomenon has been explained by the hemicellulose dissolvability and the existence of mass transfer resistance at different temperatures (Vallejos et al. 2015). Research on autohydrolysis of pine showed that the apparent activation energy of arabinan (57.34 kJ∙mol-1) was much smaller than that of xylan (106.03 kJ∙mol-1), which resulted higher removal of arabinan than xylan. The apparent reaction constant at lower temperature is smaller than that at higher temperature, which resulted in increasing of pentosan with elevated temperature (Li et al. 2012).
The arabino-saccharide and xylo-saccharide contents in the HWPL at different holding temperatures are shown in Fig. 4. As can be seen, the arabino-oligosaccharide content in the HWPL first ascended with an increase in holding temperature, and then it descended after reaching its maximum (170 °C). When the temperature was lower than 170 °C, the arabino-oligosaccharide content was higher than the arabinose content. When the temperature was higher than 170 °C, the arabinose content was higher than the arabino-oligosaccharide content. Heat sped up the decomposition of the arabino-oligosaccharide into arabinose. The arabino-oligosaccharide content was almost the same as that of arabinose at 170 °C. The arabinose content and total arabino-saccharide content both increased with a rise in the holding temperature. A similar trend was also found in the literature, i.e. the arabinose content was increased with the temperature elevated from 145 °C to 175 °C (Garrote et al. 1999b).
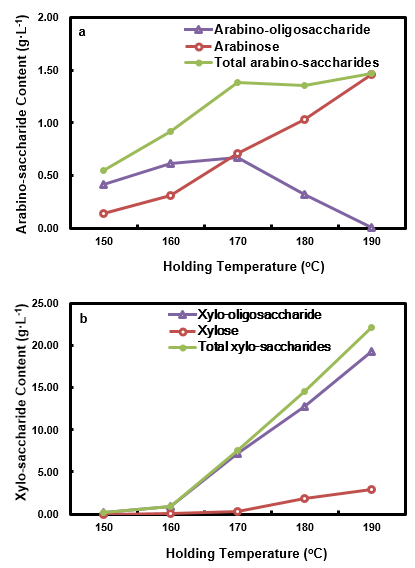
Fig. 4. Arabino-saccharides (a) and xylo-saccharides (b) in the HWPL at different holding temperatures
The contents of xylo-oligosaccharide, xylose, and total xylo-saccharides all increased with a rise in the holding temperature, and the gap between the xylo-oligosaccharide content and the xylose content increased as well. Xylo-oligosaccharide was the major form of xylo-saccharide in the HWPL under the conditions employed. These results were quite different from the data reported in the literature (Garrote et al. 1999b) where both xylo-saccharides and xylose were decomposed greatly. Variation in raw material, reactor system, and other parameters could cause the difference.
Hydrolysate Pretreatment
Pentosan dissolution at different holding times
The hydrolysate pretreatment was performed at different holding times, and the related pentosan removal results are shown in Fig. 5. Arabinan removal increased with extending the holding time during the first 40 min of HP, and it was higher than that of HWP at the same holding time. The arabinan removal had been nearly 100% at the holding time of 40 min, which was approximately 20 min ahead of HWP. Generally, xylan removal was also increased when the holding time was prolonged during HP, with three plateaus shown during the 0 min to 20 min period, 60 min to 100 min period, and 120 min to 160 min period, respectively. This trend was quite different from the results calculated from the data in autohydrolysis-related literature (Garrote et al. 1999b; Da Silva Morais et al. 2016). CSFs, which were -0.83, 0.14, 1.49, 1.62, 1.72, 1.82, 1.90, and 1.95 for 20min, 40 min, 60 min, 80 min, 100 min, 120 min, 140 min, and 160 min, respectively, were not well correlated to the xylan removal. The xylan removal was also higher than that of HWP at the same holding time, which could be explained by comparatively higher CSF of HP. The maximum xylan removal was approximately 91%. The xylan removal could not reach 100% due to the existence of a lignin-carbohydrate compound (Surewicz 1962), and it was hard to hydrolyze part of the hemicellulose (Canettieri et al. 2007).
During autohydrolysis, the hydronium ions generated from acetic acid auto-ionization act as catalysts in the degradation of polysaccharides. Under the operational conditions usually employed in hydrothermal treatments, the formation of hydronium ions from acetic acid is more important than from water (Heitz et al. 1986), the role of water auto-ionization being limited to the initial reaction stages (Garrote et al. 1999a). Acidic substances that can generate hydronium ions play a key role during the dissolution and decomposition of pentosan in the raw material. Usage of acetic acid could increase selectivity of hemicellulose (Tunc et al. 2014) and enhance degradation of hemicellulose in prehydrolysis, thus contributing to the improvement of subsequent stage performance (Allen et al. 1996; Ertas et al. 2014; Li et al. 2015). The utilization of hydrolysate as the pretreatment medium enhanced pentosan removal from the raw material. The acids contained in the HP medium sped up the pentosan dissolution, and together with the acids formed during the pretreatment, they strengthened pentosan removal. Pretreatment time could be shortened by application of HP process in order to obtain the same pentosan removal when compared with autohydrolysis. At the same time, pretreatment effluent was reduced, fresh water usage lowered, heat in it utilized efficiently, and overall energy consumption was possibly saved. Acetic acid might also be interesting as a side product, since it was accumulated in HP process.
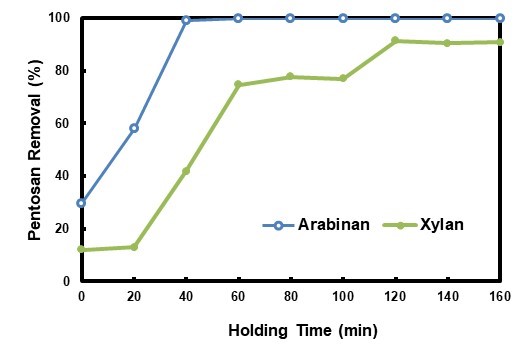
Fig. 5. Pentosan removals in the HPS at different holding times
The effects of holding time on the pento-saccharide content in the HPL are shown in Fig. 6. As seen, the arabinose, arabino-oligosaccharide, and total arabino-saccharides content all increased with increased holding time. The contents then declined after reaching the maximum at different holding times, namely, holding times of 40 min, 20 min, and 60 min, respectively. The content of the arabino-saccharides was higher than those of HWP when the holding times were no greater than 60 min, and they were all lower when the holding time was longer than 80 min. This phenomenon indicated that HP enriched the arabino-saccharide content during the early stages of the pretreatment, and it promoted the degradation of arabino-saccharides (Gao et al. 2013; Vallejos et al. 2015) at later stages of the pretreatment.
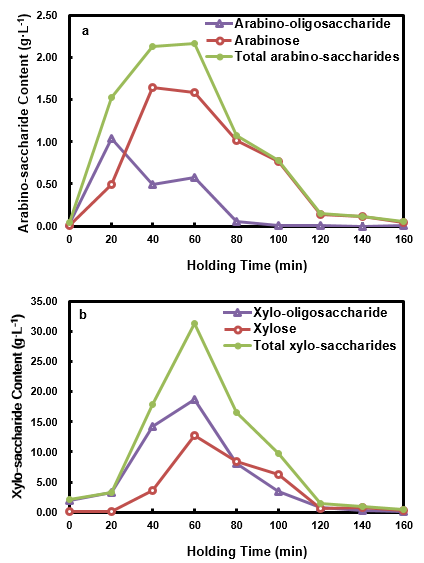
Fig. 6. Arabino-saccharides (a) and xylo-saccharides (b) in the HPL at different holding times
The xylose, xylo-oligosaccharide, and total xylo-saccharides content all initially increased until the maximum was reached at a holding time of 60 min, and then they decreased with the further prolongation of the holding time. When the holding time was less than 60 min, the xylo-saccharide content was greater than in the HWP, which indicated that xylo-saccharide enrichment happened with HP. When the holding time was longer than 100 min, both the total xylo-saccharides and xylose content were smaller than those of HWP. The xylo-oligosaccharide followed a decreasing trend during this period, and the xylan removal from the solid phase was increased by 14.4% when the holding time was extended from 100 min to 120 min. This phenomenon suggested that the dramatic degradation of xylo-saccharides occurred during the later stage of the HP, and that it was possible to convert xylo-saccharides in-situ to other potentially high added-value products, such as acids or aldehydes (Mittal et al. 2009).
Since the HWPL was dilute, the production of value-added materials from it would be challenging (Khazraie et al. 2017). With proper control of holding time (less than 60 min), the absolute quantity of saccharide was improved, which could be favorable to its utilization once suitable strain and process without detoxification were chosen (Kelbert et al. 2016; Romani et al. 2014) or when a proper detoxification process was employed (Castro et al. 2013). Since saccharides already contained in the HP medium, its conversion to furfural and HMF during the pretreatment, would increase furfural and HMF content, and make it more feasible to recover them as side-products.
Pentosan dissolution at different holding temperatures
The hydrolysate pretreatment was performed at different holding temperatures, and the related pentosan removal results are shown in Fig. 7. With the elevation of the holding temperature from 150 °C to 190 °C, arabinan removal increased from 71.1% to 100.0%, and xylan removal from 8.0% to 74.7%, a similar trend to that of HWP found by Tunc and Van Heiningen (2008). The trend related to CSFs well, which were -2.87, -2.14, -0.95, 0.62, and 1.49 for 150 °C, 160 °C, 170 °C, 180 °C, and 190 °C, respectively. With the exception of xylan removal at 170 °C, both the arabinan and xylan removals for HP were higher than those for HWP at the same holding temperatures. Those results further established that HP was beneficial to pentosan dissolution from biomass. Holding temperature for HP could be lowered for the same pentosan removal obtained by autohydrolysis, thus its energy could be saved.
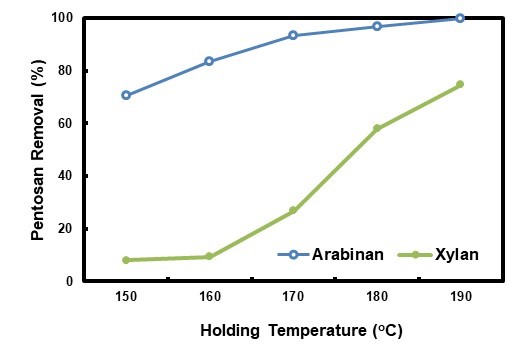
Fig. 7. Pentosan removals in the HPS at different holding temperatures
Variations of pento-saccharide contents in HPL at differing holding temperatures are shown in Fig. 8. As can be seen, the total arabino-saccharides content initially increased when the holding temperature was increased from 150 °C to 170 °C, and then it decreased slightly from 170 °C to 180 °C and increased again from 180 °C to 190 °C. The trend of the total arabino-saccharides was the same as that of HWP, with the exception that HP had a higher level of total arabino-saccharides. The arabino-oligosaccharide content decreased until 180 °C, and then it increased slightly when the holding temperature was further increased to 190 °C.
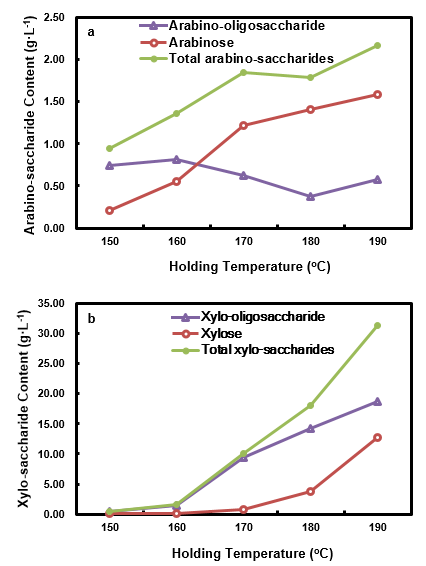
Fig. 8. Arabino-saccharides (a) and xylo-saccharides (b) in the HPL at different holding temperatures
Arabinose content increased continuously with the elevation of the holding temperature. When compared with HWP, the crossing point of the arabinose content curve and arabino-oligosaccharide content curve was advanced, i.e. it happened at a comparatively lower holding temperature, which indicated that higher holding temperatures facilitated the depolymerization of arabinan. The amount of xylose, xylo-oligosaccharide, and total xylo-saccharides all increased when the holding temperature was elevated. Their contents were lower than those of arabino-saccharides at the holding temperature of 150 °C, due to the strong thermal stability of xylan. When compared with HWP, their contents were all at higher levels, except for xylo-oligosaccharide, at holding temperatures no less than 180 °C. The highest total xylo-saccharides content (31.35 g·L-1) was obtained at 190 °C. The major form in the HPL was as an oligomer, although the xylose content dramatically increased at holding temperatures higher than 180 °C. The xylose content was also noticeably greater than that of the HWPL when the holding temperature was higher than 180 °C. These phenomena suggested that the xylo-saccharides in the HPL were enriched when the hydrolysate was used as a pretreatment medium.
Pentosan dissolution at different hot water pre-hydrolysate ratio (HWPR)
The hydrolysate pretreatment was performed at different HWPR, and the related pentosan removal results are shown in Fig. 9. Arabinan was extracted completely under the experimental conditions. The xylan removal initially increased when the HWPR was increased from 20% to 40%, and it reached a maximum at 40%, after which it decreased and then remained at approximately 73% with further increase of the ratio. Utilization of a hot water pre-hydrolysate as a pretreatment medium enhanced xylan removal by 10.2% to 14.7%. The changes of xylan removal did not relate well to CSFs, which were 1.46, 1.47, 1.49, 1.52, and 1.53 for HWPR of 20%, 40%, 60%, 80%, and 100%, respectively. CSF was slight increased with the increase of HWPR.
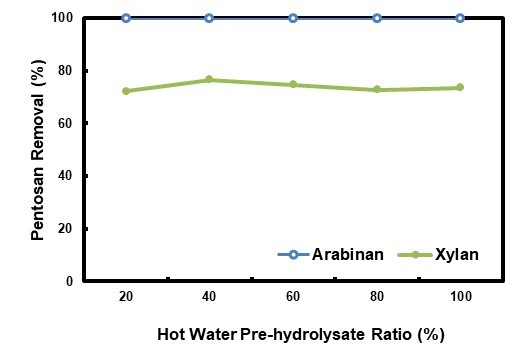
Fig. 9. Pentosan removals in the HPS at different HWPRs
The pento-saccharides content in the HPL is shown in Fig. 10. Both the arabino-oligosaccharide content and the total arabino-saccharides content initially increased and then began to decrease after reaching the maximum at the HWP ratio of 40% and 60%, respectively. The xylo-oligosacccharide content and the total xylo-saccharides content had the same trends as the arabino-saccharides, and they obtained the maximum at the same ratios. Generally, xylose increased with an increased HWPR. When compared to HWP, both the total xylo-saccharides content and total arabino-saccharides content were at a higher level for HPL. It is worth noting that the xylose content increased remarkably for the HPL. This phenomenon illustrated that a high HWPR could speed up the conversion of oligosaccharides into monose, and the optimum enrichment for pentose, pento-oligosaccharides, and total pento-saccharides were obtained at different HWPRs.
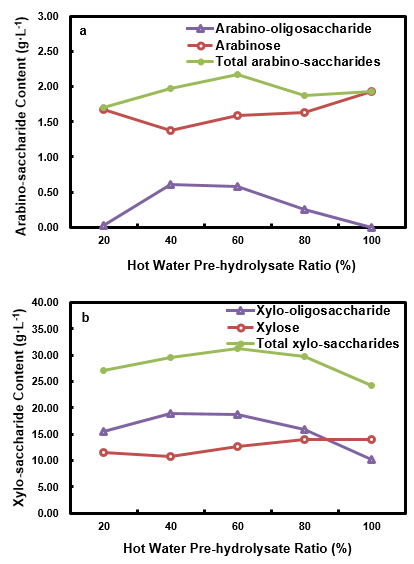
Fig. 10. Arabino-saccharides (a) and xylo-saccharides (b) in the HPL at different HWPRs
Potential application of hydrolysate pretreatment (HP)
The performance of HP and its comparison with hot water pre-hydrolysis provides fundamental support for its potential application in industry. These findings could be applied in practical application through both batch and continuous system. For a batch system, 100% of HP medium can be used if two parallel lines were established, with one of low productivity using HP and the other of high enough productivity using hot water pre-hydrolysis. For a continuous application, liquid phase and solid phase should flow contra to each other. With proper reconstruction, batch digester, horizontal tubular continuous digester, and other types of digester currently utilized in pulp and papermaking industry could be used in HP-based processes.
CONCLUSIONS
- A hydrolysate pretreatment used a pretreatment medium partially or fully composed of HWPL to pretreat biomass. Pento-saccharides were either enriched or converted in-situ to other high added-value chemicals, and pentosan removal from the biomass was enhanced at the same time for HP. Thus, HWPL could benefit the high added-value utilization of extracted hemicellulose, reduce water consumption for the pretreatment, and decrease the potential waste water treatment cost.
- The influences of holding time and holding temperature on HP were similar to those on HWP. The pentosan removal in the HPS was increased by prolonging the holding time, and the pento-saccharide content in the HPL initially increased and then decreased after it reached the maximum at different holding times. If the holding time was not long enough, the pento-saccharides enrichment effect occurred in the HPL. If the holding time was too long, it promoted the in-situ conversion of pento-saccharides in the HPL.
- Pentosan removal in the HPS increased with the elevation of the holding temperatures, with the exception of xylan removal at 170 °C. Pento-saccharides in the HPL generally increased with the elevation of holding temperature, with the exception of arabino-oligosaccharide. When the holding time was 60 min, different holding temperatures favored enrichment of pento-saccharides, with the exception of xylo-oligosaccharides at 190 °C.
- Although the usage of HWPL as a pretreatment medium enhanced pentosan removal in the HPS and enriched total pento-saccharides in the HPL, the changes in the removal and in the saccharide content caused by a variation of the HWPR were minimal compared to the holding time and holding temperature. When the HWPR was raised, both the total pento-saccharides and pento-oligosaccharides in the HPL increased and then decreased after reaching the maximum. The xylose increased remarkably with an increase in HWPR.
ACKNOWLEDGMENTS
The authors are grateful for the support of National Natural Science Foundation of China (31370584), Natural Science Foundation of Liaoning (2015020640), Fundamental Research Fund of Dalian Polytechnic University (2016J001), State Key Laboratory of Pulp and Paper Engineering (201621), and Key Laboratory of Liaoning (LZ2015005).
REFERENCES CITED
Allen, S. G., Kam, L. C., Zemann, A. J., and Antal Jr., M. J. (1996). “Fractionation of sugar cane with hot, compressed, liquid water,” Industrial & Engineering Chemistry Research 35(8), 2709-2715. DOI: 10.1021/ie950594s
Agbor, V. B., Cicek, N., Sparling, R., Berlin, A., and Levin, D. B. (2011). “Biomass pretreatment: Fundamentals toward application,” Biotechnology Advances 29(6), 675-685. DOI: 10.1016/j.biotechadv.2011.05.005
Alonso, J. L., Dominguez, H., Garrote, G., Gonzalez-Munoz, M. J., Gullon, B., Moure, A., Santos, V., Vila, C., and Yanez, R. (2011). “Biorefinery processes for the integral valorization of agroindustrial and forestall wastes,” CyTA-Journal of Food 9(4), 282-289. DOI: 10.1080/19476337.2011.598949
Amidon, T. E., and Liu, S. J. (2009). “Water-based woody biorefinery,” Biotechnology Advances 27(5), 542-550. DOI: 10.1016/j.biotechadv.2009.04.012
Borrega, M., and Sixta, H. (2015). “Water prehydrolysis of birch wood chips and meal in batch and flow-through systems: a comparative evaluation,” Industrial & Engineering Chemistry Research 54(23), 6075-6084. DOI: 10.1021/acs.iecr.5b00908
Canettieri, E. V., Rocha, G. J. M., Carvalho Jr., J. A., and Silva, J. B. A. (2007). “Evaluation of the kinetics of xylose formation from dilute sulfuric acid hydrolysis of forest residues of Eucalyptus grandis,” Industrial & Engineering Chemistry Research 46(7), 1938-1944. DOI: 10.1021/ie0607494
Carrasco, F., and Roy, C. (1992). “Kinetic study of dilute-acid prehydrolysis of xylan-containing biomass,” Wood Science and Technology 26(3), 189-208.
Castro, J. F., Parra, C., Yanez-S, M., Rojas, J., Teixeira Mendonca, R., Baeza, J., and Freer, J. (2013). “Optimal pretreatment of Eucalyptus globulus by hydrothermolysis and alkaline extraction for microbial production of ethanol and xylitol,” Industrial & Engineering Chemistry Research 52(16), 5713-5720. DOI: 10.1021/ie301859x
Cantero, D. A., Tapia, Á. S., Bermejo, M. D., and Cocero, M. J. (2015). “Pressure and temperature effect on cellulose hydrolysis in pressurized water,” Chemical Engineering Journal 276, 145-154. DOI: 10.1016/j.cej.2015.04.076
Conner, A. H. (1984). “Kinetic modeling of hardwood prehydrolysis. Part I: Xylan removal by water prehydrolysis,” Wood and Fiber Science 16(2), 268-277.
Cui, J. L., and Li, H. M. (2014). Research of Circulation-Enhanced Hot-Water Pre-Hydrolysis of Eucalyptus, Master’s Thesis, Dalian Polytechnic University, Dalian, Liaoning, China.
Cui, J. L., Li, H. M., and Bi, J. J. (2013). “Analysis of carbohydrates in paper-making materials by ion chromatography,” Journal of Dalian Institute of Light Industry 32(6), 432-436.
Da Silva Morais, A. P., Sansίgolo, C. A., and Neto M. D. O. (2016). “Effects of autohydrolysis of Eucalyptus urograndis and Eucalyptus grandis on influence of chemical components and crystallinity index,” Bioresource Technology 214, 623-628. DOI: 10.1016/j.biortech.2016.04.124
Ertas, M., Han, Q., and Jameel, H. (2014). “Acid-catalyzed antohydrolysis of wheat straw to improve sugar recovery,” Bioresource Technology 169, 1-8. DOI: 10.1016/j.biortech.2014.06.081
Gao, X. M., Liu, Z. Z., and Zhang, M. H. (2013). “Progress in the production of furfural from biomass,” Chemical Industry and Engineering Progress 32(4), 878-884.
Garcίa-Domίnguez, M. T., Garcίa-Domίnguez, J. C., López, F., and Dίaz, M. J. (2015). “Maximizing furfural concentration from wheat straw and Eucalyptus globulus by nonisothermal autohydrolysis,” Environmental Progress & Sustainable Energy 34(4), 1236-1242. DOI: 10.1002/ep.12099
Garrote, G., Domínguez, H., and Parajo, J. C. (1999a). “Hydrothermal processing of lignocellulosic materials,” Holz Als Roh-und Werkstoff (57), 191-202. DOI: 10.1007/s001070050039
Garrote, G., Domínguez, H., and Parajo, J. C. (1999b). “Mild autohydrolysis: An environmentally friendly technology for xylooligosaccharide production from wood,” Journal of Chemical Technology and Biotechnology 74(11), 1101-1109. DOI: 10.1002/(SICI)1097-4660(199911)74:11<1101::AID-JCTB146>3.0.CO;2-M
Garrote, G., Domínguez, H., and Parajo, J. C. (2004). “Production of substituted oligosaccharides by hydrolytic processing of barley husks,” Industrial & Engineering Chemistry Research 43(7), 1608-1614. DOI: 10.1021/ie0342762
Heitz, M., Carrasco, F., Rubio, M., Chauvette, G., Chornet, E., Jaulin, L., and Overend, R. P. (1986). “Generalized correlations for the aquous liquefaction lignocellulosics,” Canadian Journal of Chemical Engineering (64), 647-650.
Jaskari, J., Kontula, P., Siitonen, A., Jousimies-Somer, H., Mattila-Sandholm, T., and Poutanen, K. (1998). “Oat β-glucan and xylan hydrolysates as selective substrates for Bifidobacterium and Lactobacillus strains,” Applied Microbiology and Biotechnology 49(2), 175-181. DOI: 10.1007/s002530051155
Jesús Rangel, M., Hornus, M., Felissia, F. E., and Area, M. C. (2016). “Hydrothermal treatment of eucalyptus sawdust for a forest biorefinery,” Cellulose Chemistry and Technology 50(5-6), 521-528.
Kaur, I., and Ni, Y. H. (2015). “A process to produce furfural and acetic acid from pre-hydrolysis liquor of kraft based dissolving pulp process,” Separation and Purification Technology 146, 121-126. DOI: 10.1016/j.seppur.2015.03.034
Kelbert, M., Romani, A., Coelho, E., Pereira, F. B., Teixeira, J. A., and Domingues, L. (2016). “Simultaneous saccharification and fermentation of hydrothermal pretreated lignocellulosic biomass: Evaluation of process performance under multiple stress conditions,” Bioenergy Research 9(3), 750-760. DOI: 10.1007/s12155-016-9722-6
Khazraie, T., Zhang, Y. Q., Tarasov, D., Gao, W. J., Price, J., DeMartini, N., Hupa, L., and Fatehi, P. (2017). “A process for producing lignin and volatile compounds from hydrolysis liquor,” Biotechnology for Biofuels 10, article 47. DOI: 10.1186/s13068-017-0729-9
Lau, C. S., Thoma, G. J., Clausen, E. C., and Carrier, D. J. (2014). “Kinetic modeling of xylose oligomer degradation during pretreatment in dilute acid or in water,” Industrial & Engineering Chemistry Research 53(6), 2219-2228. DOI: 10.1021/ie403722d
Li, G., Fu, S. Y., Zhou, A. J., and Zhan, H. Y. (2015). “Improved cellulose yield in the production of dissolving pulp from bamboo using acetic acid in prehydrolysis,” BioResources 10(1), 877-886.
Li, H. M., Guo, H. Z., Ma, M. S., Zhou, J. H., and Han, Y. (2015). “Recycle-enhanced pre-hydrolysis process for lignocellulose biomass,” People’s Republic of China Patent No. CN 105112459.
Li, H. M., Saeed, A., Jahan, M. S., Ni, Y. H., and Van Heiningen, A. (2010). “Hemicellulose removal from hardwood chips in the pre-hydrolysis step of the kraft-based dissolving pulp production process,” Journal of Wood Chemistry and Technology 30(1), 48-60. DOI: 10.1080/02773810903419227
Li, P. P., Lei, Y. C., and Li, S. W. (2012). “Degradation kinetics of main carbohydrates in pine wood during hot water pre-hydrolysis of Kraft-based dissolving pulp production,” Proceeding of the 4th International Conference on Pulping, Papermaking and Biotechnology, China Light Industry Press, 994-999
Liu, C., Liu, N., Qin, M. H., and Fu, Y. J. (2014). “Characteristics of poly-pentose dissolution during hot-water hydrolysis of poplar chip,” Paper and Paper Making 33(5), 10-15. DOI: 10.13472/j.ppm.2014.05.004
Liu, H. T., Hu, H. R., Jahan, M. S., and Ni, Y. H. (2013). “Furfural formation from the pre-hydrolysis liquor of a hardwood kraft-based dissolving pulp production process,” Bioresource Technology 131, 315-320. DOI: 10.1016/j.biortech.2012.12.158
Liu, S. J., Lu, H. F., Hu, R. F., Shupe, A., Lin, L., and Liang, B. (2012). “A sustainable woody biomass biorefinery,” Biotechnology Advances 30(4), 785-810. DOI: 10.1016/j.biotechadv.2012.01.013
Mittal, A., Chatterjee, S., Scott, G. M., and Amidon, T. E. (2009). “Modeling xylan solubilization during autohydrolysis of sugar maple wood meal: Reaction kinetics and mass transfer,” Chemical Engineering Science 64(13), 3031-3041. DOI: 10.1016/j.ces.2009.03.011
Romani, A., Ruiz, H. A., Pereira, F. B., Teixeira, J. A., and Domingues, L. (2014). “Integrated approach for effective bioethanol production using whole slurry from autohydrolyzed Eucalyptus globulus wood at high-solid loadings,” Fuel 135, 482-491. DOI: 10.1016/j.fuel.2014.06.061
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., and Templeton, D. (2006). Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples (NREL/TP-510-42623), U. S. Department of Energy Office of Energy Efficiency & Renewable Energy, National Renewable Energy Laboratory, Golden, CO, USA.
Surewicz, W. (1962). “The sorption of organic components from cooking liquor by cellulose fibres; its relation to the dangerous cooking crest in alkaline pulping (1),” TAPPI Journal 45(7), 570.
Trajano, H. L., Pattathil, S., Tomkins, B. A., Tschaplinski, T. J., Hahn, M. G., Van Berkel, G. J., and Wyman, C. E. (2015). “Xylan hydrolysis in Populus trichocarpa × P. deltoides and model substrates during hydrothermal pretreatment,” Bioresource Technology 179, 202-210. DOI: 10.1016/j.biortech.2014.11.090
Tunc, M. S., and Van Heiningen, A. R. P. (2008). “Hydrothermal dissolution of mixed southern hardwoods,” Holzforschung 62(5), 539-545. DOI: 10.1515/HF.2008.100
Tunc, M. S., Chheda, J., and Van Heiningen, A. (2014). “Pretreatment of hardwood chips via autohydrolysis supported by acetic and formic acid,” Holzforschung 68(4), 401-409. DOI: 10.1515/hf-2013-0102
Vallejos, M. E., Felissia, F. E., Kruyeniski, J., and Area, M. C. (2015). “Kinetic study of the extraction of hemicellulosic carbohydrates from sugarcane bagasse by hot water treatment,” Industrial Crops & Products 67, 1-6. DOI: 10.1016/j.indcrop.2014.12.058
Yap, M. (1987). “Autohydrolysis of tropical hardwoods,” Journal of Wood Chemistry and Technology, 7(3), 343-351. DOI: 10.1080/02773818708085273
Yedro, F. M., Cantero, D. A., Pascual, M., and García-Serna, J. (2015). “Hydrothermal fractionation of woody biomass: Lignin effect on sugars recovery,” Bioresource Technology 191, 124-132. DOI: 10.1016/j.biortech.2015.05.004
Zhuang, X. S., Qi, W., Yuan, Z. H., Yu, Q., Wang Q., Ma, L. L., and Wu, C. Z. (2011). “Study on kinetics of Eucalyptus sawdust pretreated by liquid hot water,” Acta Energiae Solaris Sinica 32(10), 1487-1493.
Article submitted: January 9, 2017; Peer review competed: March 6, 2017; Revised version received: March 23, 2017; Accepted: March 24, 2017; Published: April 3, 2017.
DOI: 10.15376/biores.12.2.3677-3694
