Abstract
Download PDF
Full Article
Lignification Markers of the Tracheid Walls of Scots Pine (Pinus sylvestris (L.)) in Various Forms of Dead Bark
Tomasz Jelonek,a,* Witold Pazdrowski,a Arkadiusz Tomczak,a and Magdalena Arasimowicz-Jelonek b
This study attempted to define the shaping of the quotient of fresh-needled twig mass and fresh conifer needle mass to the lignin content (MFT/LC) in the tracheid walls of the circumferential zone of trunks (MFN/LC) of pines with various forms of dead bark, which were called lignification markers. In the experiment, the researched trees had varying forms of dead bark, including ropy bark (G), scaly bark (L), and shell-type bark (M). The research material came from pine timber forests aged between 89 years to 91 years, located in Northern Poland. A tree tissue chemical analysis encompassed a zone of mature sapwood, i.e., the last ten annual growth rings of diameter increment located at the height of 1.30 m (diameter at breast height-DBH). The acquired results pointed to the fact that pines with dead bark in the ropy form possessed statistically higher values of the analyzed markers (MFT/LC and MFN/LC) than the trees with scaly and shell-type bark. The variances ascertained in the course of the experiments of both markers in the Scots pine (Pinus sylvestris (L.)) are possibly connected to the physiological, physical, and structural conditioning of water transportation, with mineral salts in the stem of the trees.
Keywords: Dead bark form; Lignin; Cell wall; Conifer needles; Diameter at breast height; Scots pine
Contact information: a: Poznan University of Life Science, Department of Forest Utilisation Poznań, Poland; b: Adam Mickiewicz University of Poznan, Department of Plant Ecophysiology Umultowska 89, 61-614 Poznań, Poland; *Corresponding author: tomasz.jelonek@up.poznan.pl
INTRODUCTION
Plants must photosynthesize, transport water, grow, propagate, as well as carry static dynamic loads to survive in a terrestrial environment (Gardiner et al. 2016). The tree structure, structure of its particular elements (root, trunk, and crown), and the structure and properties of the tree tissue must be responsible for the functional premise of both the conduction system and the biomechanical system. That is why there are numerous connections and dependences between the different characteristics of a tree or wood (Wąsik 2010), particularly between the biometric characteristics determining the size and shape of the crown or of the trunk, which has an influence on the stability of a tree (Kroon et al. 2008; Jelonek et al. 2012; Cantiani and Chiavetta 2015; Tomczak et al. 2015; Motallebi and Kangur 2016). Previous literature on the subject devotes a lot of space to experiments that aim to determine the relation between the growth conditions and the quality of the raw wood (Jelonek et al. 2009a). Searching for characteristics, on the basis of which it is easy to determine various properties of tree tissue, can further enrich the already possessed knowledge with new markers to judge the quality of raw wood, depending on its purpose for further processing.
Xylogenesis is a process of change composed of many parts that lead to the creation of secondary tree tissue. An interdependence network that is based on positive or negative impacts of growth mechanisms regulates each step of the process (Larson 1964; Wareing et al. 1964; Wodzicki 1964, 1965; Balatinecz and Kennedy 1967; Little and Savidge 1987; Little and Pharis 1995). From among the wide range of regulators, phytohormones, mainly auxins and cytokinins, play a major role in the growth and development process of plants, including woody plants (Uggla et al. 1996; Kende and Zeewart 1997; Woodward and Bartel 2005; Kramer et al. 2008). These regulators affect certain proteins as well as genes. Consequently, they enforce a course of specific changes that, in turn, lead to the creation of tree tissue (Mellerowicz et al. 2001; Hellgren et al. 2004; Mellerowicz and Sundberg 2008).
The changes in the number of basic components of the tissue wall can change vastly within one species. The changes depend on the growth, development conditions, genetic conditions, and the tree’s age (Fengel and Wegener 1989; Hon and Shiraishi 1991; Lewin and Goldstein 1991).
Lignin is a characteristic component of woody cell walls and has a considerable structural significance, but most importantly, it gives the walls stiffness and strength (Boudet et al. 1995; Abreu et al. 1999). Lignin appears in all of the layers of the cell wall, i.e., the primary and secondary cell wall, and in the middle lamella (Boerjan et al. 2003; Üner et al. 2011; Antonova et al. 2014; Kim and Daniel 2014; Tsuyama and Takabe 2014). The lignification process of plant cells is closely connected with the synthesis of lignin, and its accumulation in the plant cell walls ranges from 16% to 30% according to Pereira et al. (2003), from 25% to 30% of dry mass according to Miidla (1989), or from 18% to 36% according to Sarkanen and Ludwig (1971). The increasing amount of the lignin content in the tree tissue raises the resistance of the cell wall to deformation and ensures its mechanical stability (Boudet et al. 1995; Abreu et al. 1999). Moreover, lignin facilitates the transportation of water and hinders the degradation of polysaccharides in the cell walls. Their activity can be described as antiseptic towards the pathogenic factors (Monties 1989; Hatfield and Vermerris 2001).
Lignin is a three-dimensional polymer of phenylpropane derivatives. It contributes to the decreasing (limiting) the swelling of the walls for the anatomical elements of wood and to the increase in resistance to microorganism activity (Austin and Ballaré 2010; Shmulsky and Jones 2011). The lignin polymerizes among the polysaccharide components of the wall. It appears after the growth of the cell ends, or after a given part of the cell wall’s growth ends. In dead cells, such as tracheids and vessels, the impregnation of the walls with lignin secures the polysaccharides of the wall against partial hydrolysis (Freudenberg 1959; Schubert 1965). The phylogenetic origin of plants determines different proportions of the three basic units in lignin, i.e., syringic, coniferyl, and p-coumaryl alcohol (Gibbs 1958; Boerjan et al. 2003; Vanholme et al. 2010). The lignin of the Scots pine (Pinus sylvestris (L.)) is composed of coniferyl units with a slight admixture of p-coumaryl and syringic units (De Stevens and Nord 1952). A common precursor of all derivatives of phenylpropane is shikimic acid (Neish 1964). The shikimic acid appears in small quantities as one of the first products of photosynthesis in Scots pine (Hasegawa 1962). By adding the radioactive shikimic acid to the callus tissue culture of Eastern white pine (Pinus strobus), Hasegawa et al. (1960) ascertained an efficient incorporation of this compound into lignin.
In this paper, an attempt to delineate the lignification markers on the basis of the proportion of fresh-needled twigs and fresh conifer needles mass to the lignin content in the tracheid walls (of the circumferential zone) in stems of pines with various forms of dead bark is reported.
EXPERIMENTAL
Materials
There are four sample areas containing Scots pine (Pinus sylvestris (L.)) that were found within the natural distribution borders of this productive forest species in Europe. All of the research areas were located in the Tuchola Forest Division (53º 36‘N 17º 51‘E) in the territories of Northern Poland, which belongs administratively to the Regional Directorate of State Forests in Toruń. The research encompassed 36 samples of trees aged between 89 years to 91 years, growing in the optimal habitat environment for P. sylvestris (mixed fresh coniferous forest and fresh coniferous forest).
A sample surface, measuring one hectare, was set up in each tree stand. Measurements were taken for the diameter at breast height (DBH) of all the pines in each tree stand. At the same time, the trees were separated into three groups based on their type of dead bark, i.e., ropy bark (G), shell-type bark (M), and scaly bark (L) (Fig. 1).
All of the trees’ heights were measured according to the frequency in the established 2-cm thickness levels in each of the pine groups, characterized by a different form of dead bark. The measurements of the sample trees were determined on the basis of their thickness class according to the dendrometric method (Van Laar and Akça 2007). During the selection process of the pines, their biosocial position was taken into account. The positioning was determined according to Kraft’s trees classification system (Kraft 1884). The categories of the main crop that were analyzed in the paper include predominant trees, dominant trees, and codominant trees.

Fig. 1. Form of dead bark: scaly bark (L), ropy bark form (G), and shell-type bark (M)
Nine sample trees were cut down from each research surface, and three from each of the established forms of dead bark, for a total of 36 sample trees.
The research material was cut into a disc shape from the diameter at breast height from each sample tree. For each of the acquired trees, the mass of freshly-needled twigs and the mass of fresh conifer needles were determined by directly weighing them. The acquired discs served as a way of determining the sapwood surface area (Sa) as well as of determining the early wood surface area in the sapwood zone (Esa).
Methods
The materials for lignin determination in the tracheid walls were acquired from the last ten diameter increments, involving the mature wood zone of the circumferential part of the stem’s sapwood. The measurements of the sapwood surface area (Sa), as well as the early wood surface area in the sapwood zone (Esa), were taken on four radii of the disc by the means of a Preisser Digi-Met caliper (Helios-Preisser, Germany) and “Grube Comm” computer software (Grube Comm 1.0, Bispingen, Germany). The early wood width measurement was taken in every single annual growth ring in the sapwood zone. The surface area of the early wood in the circumferential zone of the stem (sapwood) was determined as a total area of all annual rings.
The fresh conifer needles mass (Nmass) of the sample trees as well as the sapwood area (Sa) and the early wood surface area in the sapwood zone were acquired. With this information, a relative conductive surface area of the researched pines was determined. It was acquired by dividing the sapwood area and the early wood area of the tracheids in this zone of the stem’s cross-section by the fresh conifer needles mass:
![]() (1)
(1)
![]() (2)
(2)
The lignin content (LC, mg/g of the dry mass) was marked quantitatively by a spectrophotometric method (three times) according to Doster and Bostock with certain modifications (Doster and Bostock 1988). At first, the wood from the last 10 annual rings was treated twice for 48 h with methanol, using a proportion of 1 mL of methanol per 1 g of tree tissue. Afterwards, it was dried and 20 mg of the dry tree tissue was collected from each variant. Subsequently, it was mixed with 5 mL of 2 N HCl and 0.5 mL of thioglycolic acid (Sigma-Aldrich, Warsaw, Poland). The samples were incubated for 4 h at a temperature of 95 °C. They were then centrifuged at 3000 g for 20 min. The acquired residue was rinsed twice with deionized water and incubated with 5 mL of 0.5 N NaOH for 18 h at room temperature. After it was centrifuged at 15,000 g, a NaOH extract was collected, and the residue was rinsed with 4 mL of deionized water and then centrifuged again. The acquired supernatant was joined with the NaOH extract and then acidified with 1 mL of concentrated HCl. Subsequently, the solution was left for the night at a temperature of 5 °C. After centrifuging (15,000 g), the acquired, residue was dissolved in 5 mL of 0.5 N NaOH, and then it was centrifuged again (15,000 g). The absorbance of the acquired residue was measured with a 280-nm wavelength by using a UV-1202 spectrophotometer (Shimadzu, Japan). The lignin content was expressed in terms of relative absorbance units.
The masses of fresh-needled twigs and fresh conifer needles, and the lignin content in the tracheid walls from the last ten diameter increments from the circumferential zone of the stems of the researched pines were prepared. With this information, the quotient of the fresh-needled twigs mass through the lignin content in the tracheid walls was determined. The quotient of the fresh conifer needles mass to the lignin content in the tracheid walls from the last ten annual growth rings was also determined.
The basic statistical characteristics of the analyzed variables were determined in this work. An analysis of the variations was performed as well as a Tukey NIR test (Tukey 1949). The acquired empirical material was analyzed via statistical mathematical methods with the use of the STATISTICA 12.0 (Cracow, Poland) software.
RESULTS AND DISCUSSION
It was found that types of dead bark could be used to determine wood characteristics. The lignification markers, i.e., the quotient of the fresh-needled twigs mass and the fresh conifer needles mass by the lignin content in the tracheid walls of the circumferential zone of the stems (MFT/LC and MFN/LC) of the P. sylvestris, had clearly varied values dependent on the form of dead bark.
The growth and increment of the tree tissue of woody plants, including wood production, are conditioned mainly by the retention of balance between the conductive surface of the trunk or of the stem, and the transpiration and assimilation surface in specific (optimal) habitat conditions. It was attempted to express the relative conductive surface specified by the quotient of the sapwood surface and the tracheid surface of the early wood area in the sapwood zone by the fresh conifer needles mass (Sa/Nmass in mm²/kg, Esa/Nmass in mm²/kg) of the pines with varied forms of dead bark. The acquired results pointed to a diversification of the relative conductive surface of the Scots pine depending on the form of dead bark.
The measurements and their distribution shape for the location and dispersion for the relative conductive surface area (Sa/Nmass and Esa/Nmass) of P. sylvestris with varied dead bark forms are presented in Table 1, and in Figs. 2 and 3. The relative conductive surface area of the stems is described by the quotient of the sapwood surface (Sa) by the fresh conifer needles mass (Nmass). It is also described by the quotient of the surface area of early wood tracheids in the sapwood zone (Esa) by the fresh conifer needles mass (Nmass), which differentiated according to the dead bark form. In both cases, determining the relative conductive surface area of the pine with shell-type and scaly bark demonstrated the highest values of both markers, compared to the trees with ropy bark. It was demonstrated by the mean values of both markers presented in Table 1, as well as the ones presented in graphical form in Figs. 2 and 3.
Table 1. Statistical Characteristics of the Relative Conductive Surface (Sa/Nmass; Esa/Nmass) of Pines with Various Forms of Dead Bark
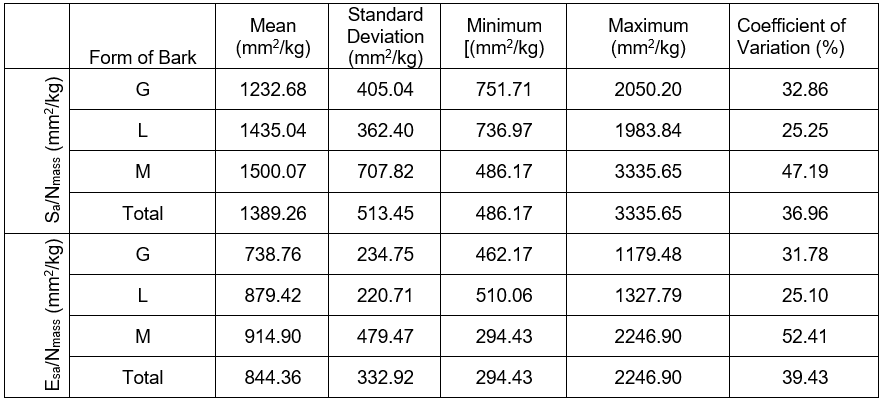
*Note: ropy bark (G), shell-type bark (M), and scaly bark (L)
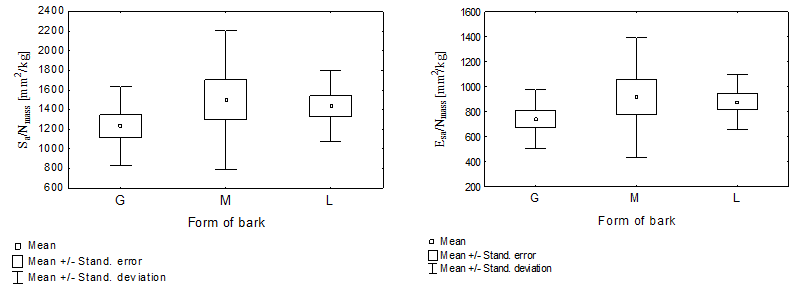
Fig. 2. The relative conductive surface (Sa/Nmass) Fig. 3. The relative conductive surface (Esa/Nmass)
depending on the dead bark form depending on the dead bark form
The minimum and maximum values, as well as the standard deviation of the relative conductive surface areas were determined in two ways, which clearly point to a large variability of the researched markers. Furthermore, a particularly significant (p < 0.05) variability was determined in pines with the shell-type bark (Table 1, Figs. 2 and 3). The analysis of variance did not determine the presence of a statistically significant differentiation between the mean values of the conductive surfaces of the pines with different forms of dead bark. A large variability in the scope of a given form of dead bark was possibly the decisive factor, rather than the variability between the dead bark forms. It was particularly visible in the case of the pines with the shell-type dead bark (Figs. 2 and 3).
The trees with ropy bark (G) in both cases possessed the lowest value of the analyzed marker. However, the pines with scaly bark (L) and shell-type bark (M) had a higher value. This meant that in the trees with ropy bark on 1 kg of the fresh conifer needles mass, there was a markedly smaller conductive surface of sapwood (1232.69 mm²) and the conductive surface of the early wood tracheids in the sapwood zone (738.76 mm²) than in the pines with scaly or shell-type bark. In the case of the former, the values of the discussed markers equaled (1435.04 mm²) and (879.42 mm²), respectively, while in the case of the latter, they equaled (1500.07 mm²) as well as (914.90 mm²).
This regularity was probably connected with a differentiation of assimilation in terms of its intensity. Furthermore, it was also closely connected with the progress of the transpiration of pines with different forms of dead bark. The trees with scaly bark (L) and shell-type bark (M) possibly had a higher level of intensity of creating organic matter through photosynthesis, compared to the pines with ropy bark (G). The trees characterized by ropy bark in similar habitat conditions and of similar age possessed a larger diameter at breast height, a higher mass of fresh needles, as well as a higher mass of fresh-needled twigs, i.e., a larger crown than in the pines with scaly bark and shell-type bark (Pazdrowski et al. 2016). According to the research conducted by Lemke (1996), the pines with small crowns produced biomass more energetically than the trees with large crowns. This was more than likely the result of shadowing of the lower section of the crown. To be more precise, its assimilative apparatus (the needles) were deprived of the access to light and for this reason were produced less efficiently. Larger crowns were characterized by a larger number of leaves remaining in the shadow, which resulted in reduced assimilation energy compared to the trees with smaller crowns (Dengler 1937). According to Lemke (1966), the ratio of the mean size increment of the trees’ volume in Kraft’s biosocial classes 1 through 3 amounted to 3:2:1, respectively. However, similar dependences in the case of the sessile oak were determined by Mayer (1958) in his research.
The statistical characteristic of the quotient of the fresh-needled twigs mass by the lignin content in the tracheid walls of the circumferential zone (MFT/LC), and the quotient of the fresh conifer needles mass by the lignin content in the tracheid walls in the circumferential zone of the stem (MFN/LC) are presented in Table 2 and in Figs. 4 and 5. The mean value of the marker (MFT/LC) remained in the range from 128.4 to 215.9 g/mg/g. The quotient of the fresh-needled twigs mass by the lignin content ranged from 85.8 to 138.6 g/mg/g, where the fresh conifer needles mass was referred to the lignin content in the walls of the last ten annual rings in the circumference of the stems. For the MFT/LC, the standard error remained in the range from 14.5 to 21.0 g/mg/g, while for MFN/LC it was ranged from 9.2 to 13.0 g/mg/g. The standard deviation in the first case was between 50.2 and 72.8 g/mg/g, while in the second case it ranged from 31.8 to 45.0 g/mg/g, as shown in Table 2.
Table 2. Statistical Characteristic of the Lignification Markers in Pines with Various Forms of Dead Bark
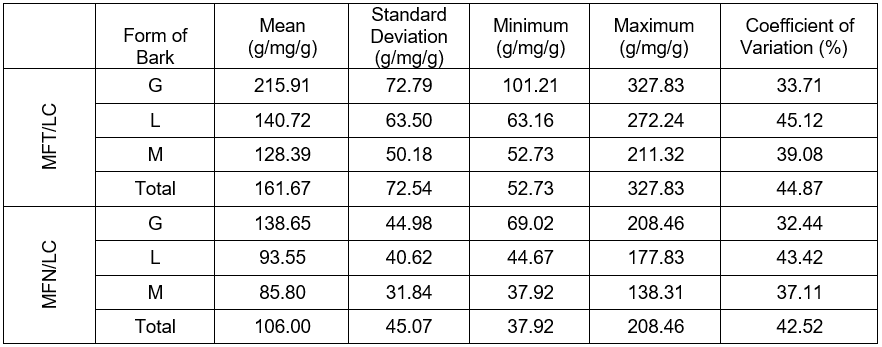
*Note: ropy bark (G), shell-type bark (M), and scaly bark (L)

Fig. 4. Characteristic of the lignification marker MFT/LC Fig. 5. Characteristic of the lignification marker MFN/LC
depending on the dead bark form depending on the dead bark form
The variance analysis of the lignification markers discussed in Table 3 demonstrated the occurrence of statistically significant differences in (MFT/LC), and (MFN/LC), depending on the form of dead bark of the Scots pine.
Table 3. Analysis of Variance of the Lignification Markers MFT/LC and MFN/LC in Pines with Various Forms of Dead Bark
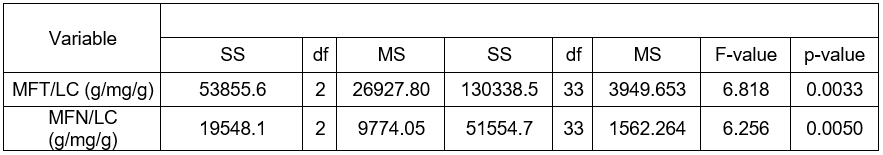
*Note: differences statistically significant at the significance level p < 0.05
The following was established on the basis of the Tukey NIR test. Statistically, higher markers of MFT/LC and MFN/LC manifested themselves in the pines with ropy bark rather than in the trees with shell-type or scaly dead bark. The pines with the shell and scaly forms did not vary significantly in the range of the two analyzed markers.
Analyzing the values of (MFT/LC) and (MFN/LC), one should notice one particular element that special attention needs to be paid to the observed statistically significant diversification of values of both markers (MFT/LC, MFN/LC) depending on the form of dead bark analyzed in the paper. In both cases, statistically significant higher values of the markers were determined in the pines with ropy bark, while the lowest value was ascertained in the trees with scaly bark. A slightly higher value of both markers was determined in the trees with shell-type bark, while in the case of the pines with scaly bark and shell-type bark, no significant difference was determined between the values of both markers taken into consideration. It seemed that the differentiation of both markers (MFT/LC and MFN/LC) in the pines characterized by different forms of dead bark were connected with physiological, physical, and structural conditioning of water transportation with mineral salts in the stems of P. sylvestris. The trees with ropy bark possessed the highest values of the analyzed markers (MFT/LC) and (MFN/LC). Their mean value equaled 215.9 (g/mg/g) and 138.6 (g/mg/g), respectively. Conversely, it seems that it was the result of markedly larger fresh-needled twigs mass, higher conifer needles mass of these trees, and smaller lignin content in the tracheid walls of the circumferential zone (Jelonek et al. 2009b). It also seemed that the noted values of both markers were determined by a reduced intensity of the assimilation and transpiration processes. Due to this, it possibly resulted in a reduced efficiency of water transportation. The pines with the scaly and shell-type dead bark formed with smaller crowns more intensively assimilated and transpired. Because of this, the efficiency of water transportation in the xylem must have been significantly (p < 0.05) higher than in the trees with ropy bark. The resulting force, coupled with the evaporation of water from the surface of intracellular spaces, pulled the water up in the tracheid elements due to cohesion. When the pressure, resulting from this pulling up, was higher in comparison to the absolute value than the root pressure, the under-pressure was created in the water of the apoplast, i.e., inside the tracheidal elements, which achieve a very high absolute value (Zimmermann 1966). For these reasons, a statistically significant higher lignin content was determined in the tracheid walls of the circumferential zone of the pines’ stem with the scaly and shell-type forms of dead bark than in the trees with ropy bark. The results published by Jelonek et al. (2009a) were a confirmation of this principle pertaining to the subject matter. To be more specific, it pertained to the lignin content in the tracheid walls of the pines with varied forms of dead bark from timber forests.
CONCLUSIONS
- This research illustrated that the shaping of the lignification markers described by the quotients of fresh-needled twig mass and fresh conifer needles mass with the lignification content in the circumferential zone of Scots pines stems, did present a distinct differentiation in the values of the analyzed markers dependent on the form of dead bark.
- The variance analysis and Tukey NIR test demonstrated that the pines with the ropy dead bark possessed statistically significant (p < 0.05) higher values of markers than the trees with scaly and shell-type bark. The pines from the former group’s mean values of both markers were 215.9 g/mg/g and 138.6 g/mg/g, respectively. The scaly bark trees mean values were 128.4 and 85.9 g/mg/g. In the pines with shell-type bark the resulting mean values were 140.7 and 93.6 g/mg/g.
- During this study, diversification was ascertained of the values of both markers of the Scots pine, which were characterized by varied forms of dead bark. They were connected with physiological, physical, and structural conditioning of water transportation with mineral salts in the tree trunks, and the dead bark form was their marker.
REFERENCES CITED
Abreu, H. D., do Nascimento, A. M., and Maria, M. A. (1999). “Lignin structure and wood properties,” Wood and Fiber Science 31(4), 426-433.
Antonova, G. F., Varaksina, T. N., Zheleznichenko, T. V., and Stasova, V. V. (2014). “Lignin deposition during earlywood and latewood formation in Scots pine stems,” Wood Science and Technology 48(5), 919-936. DOI: 10.1007/s00226-014-0650-3
Austin, A. T., and Ballaré, C. L. (2010). “Dual role of lignin in plant litter decomposition in terrestrial ecosystems,” Proceedings of the National Academy of Sciences 107(10), 4618-4622. DOI: 10.1073/pnas.0909396107
Balatinecz, J. J., and Kennedy, R. W. (1967). “Mechanism of earlywood-latewood differentiation in Larix deciduas,” in: Proceedings of the Fourth Forest Biology Conference, Pointe Claire, Quebec, pp. 36-55.
Boerjan, W., Ralph, J., and Baucher, M. (2003). “Lignin biosynthesis,” Annual Review of Plant Biology 54(1), 519-546. DOI: 10.1146/annurev.arplant.54.031902.134938
Boudet, A. M., Lapierre, C., and Grima-Pettenati, J. (1995). “Biochemistry and molecular biology of lignification,” New Phytology 129(2), 203-236. DOI: 10.1111/j.1469-8137.1995.tb04292.x
Cantiani, P., and Chiavetta, U. (2015). “Estimating the mechanical stability of Pinus nigra (Arn.) using an alternative approach across several plantations in central Italy,” iForest-Biogeosciences and Forestry 8(6), 846-852. DOI: 10.3832/ifor1300-007
De Stevens, G., and Nord, F. F. (1952). “A study of lignin formation based on the oxidation of native and enzymatically liberated lignins,” Proceedings of the National Academy of Sciences U.S.A. 39(2), 80-84.
Dengler, A. (1937). “Kronengrösse, Nadelmenge und Zuwachsleistung von Altkiefern,” Zeitschrift für Forst- und Jagdwesen 69(321), 321-336.
Doster, M. A., and Bostock, P. M. (1988). “Quantification of lignin formation in almond bark in response to wounding and infection by Phytophtotra species,” Phytopathology 78(4), 473-477. DOI: 10.1094/Phyto-78-473
Fengel, D., and Wegener, G. (1989). Wood: Chemistry, Ultrastructure, Reactions, Walter de Gruyter, Berlin, Germany. DOI: 10.1002/pol.1985.130231112
Freudenberg, K. (1959). “Biosynthesis and constitution of lignin,” Nature 183(4669), 1152-1155. DOI: 10.1038/1831152a0
Gardiner, B., Berry, P., and Moulia, B. (2016). “Review: Wind impacts on plant growth, mechanics, and damage,” Plant Science 245(4), 94-118. DOI: 10.1016/j.plantsci.2016.01.006
Gibbs, R. D. (1958). “The Mäule reaction, lignins, and the relationships between woody plants,” in: Physiology of Forest Trees, K. V. Thimann (ed.), Ronald Press, New York, NY, pp. 269-312.
Hasegawa, M. (1962). “Alicyclic precursors of polyphenols,” in: Wood Extractives and their Significance to the Pulp and Paper Industries, W. E. Hillis (ed.), Elsevier, New York, NY, pp. 263-276. DOI: 10.1016/B978-1-4832-3321-5.50012-2
Hasegawa, M., Higuchi, T., and Ishikawa, H. (1960). “Formation of lignin in tissue culture of Pinus strobus,” Plant & Cell Physiology 1(3), 173-182. DOI: 10.1093/oxfordjournals.pcp.a075764
Hatfield, R., and Vermerris, W. (2001). “Lignin formation in plant. The dilemma of linkage specificity,” Plant Physiology 126(4), 1351-1357. DOI: 10.1104/pp.126.4.1351
Hellgren, J. M., Olofsson, K., and Sundberg, B. (2004). “Patterns of auxin distribution during gravitational induction of reaction wood in poplar and pine,” Plant Physiology 135(1), 212-220. DOI: 10.1104/pp.104.038927
Hon, D. N. -S., and Shiraishi, N. (1991). Wood and Cellulosic Chemistry, Marcel Dekker, New York, NY. DOI: 10.1021/ja015237p
Jelonek, T., Pazdrowski, W., Arasimowicz-Jelonek, M., Gzyl, J., Tomczak, A., and Floryszak-Wieczorek, J. (2009a). “The relationship between the form of dead bark and lignin content in Scots pine (Pinus sylvestris L.),” Turkish Journal of Agriculture and Forestry 33, 455-462. DOI: 10.3906/tar-0903-42
Jelonek, T., Pazdrowski, W., and Tomczak, A. (2009b). “The effect of biological class and age on physical and mechanical properties of European larch (Larix decidua MILL.) in Poland,” Wood Research 54(1), 1-14.
Jelonek, T., Pazdrowski, W., Tomczak, A., and Grzywiński, W. (2012). “Biomechanical stability of pines growing on former farm land in Northern Poland,” Wood Research 57(1), 31-44.
Kende, H., and Zeewart, J. A. D. (1997). “The five ”classical” plant hormones,” Plant Cell 9(7), 1197-1210. DOI: 10.1105/tpc.9.7.1197
Kim, J. S., and Daniel, G. (2014). “Distributional variation of lignin and non-cellulosic polysaccharide epitopes in different pit membranes of Scots pine and Norway spruce seedlings,” IAWA Journal 35(4), 407-429.
Kraft, G. (1884). “Durchfarstungen Schlagstellungen und Lichtungshieben,” in: Beiträge zur Lehre, Klindworth’s Verlag, Hannover, Germany, pp. 18-58
Kramer, E. M., Lewandowski, M., Beri, S., Bernard, J., Borkowski, M., Borkowski, M. H., Burchfield, L. A., Mathisen, B., and Normanly, J. (2008). “Auxin gradients are associated with polarity changes in trees,” Science 320(5883), 1610-1610. DOI: 10.1126/science.1156130
Kroon, J., Andersson, B., and Mullin, T. J. (2008). “Genetic variation in the diameter-height relationship in Scots pine (Pinus sylvestris),” Canadian Journal of Forest Research 38(6), 1493-1503. DOI: 10.1139/X07-233
Larson, P. R. (1964). “Some indirect effects of environment on wood formation,” in: Formation of Wood in Forest Trees, M. H. Zimmermann (ed.), Elsevier, New York, NY, pp. 345-365.
Lemke, J. (1966). “Korona jako kryterium oceny dynamiki wzrostowej drzew w drzewostanie sosnowym [The crown as a criterion of evaluating the growth rate of trees in a pine tree stand],” Folia Forestalia Polonica A 12, 185-236.
Lewin, M., and Goldstein, J. S. (1991). Wood Structure and Composition, I. S. Goldstein (ed.), Marcel Dekker, New York, NY. DOI: 10.1002/prac.19923340821
Little, C. H. A., and Pharis, R. P. (1995). “Hormonal control of radial and longitudinal growth in the tree stem,” in: Plant Stems: Physiology and Functional Morphology, B. L. Gartner (ed.), Academic Press, San Diego, CA, pp. 281-319. DOI: 10.1016/b978-012276460-8/50015-1
Little, C. H. A., and Savidge, R. A. (1987). “The role of plant-growth regulators in forest tree cambial growth,” Plant Growth Regulation 6(1-2), 137-169. DOI: 10.1007/BF00043953
Mayer, R. (1958). “Kronengrösse und zuwachsleistung der traubeneiche auf süddeutschen standorten,” Allgemeine Forst- und Jagdzeitung 129:105-14, 151-163, 191-201
Mellerowicz, E. J., and Sundberg, B. (2008). “Wood cell walls: Biosynthesis, developmental dynamics, and their implications for wood properties,” Current Opinion in Plant Biology 11(3), 293-300. DOI: 10.1016/j.pbi.2008.03.003
Mellerowicz, E. J., Baucher, M., Sundberg, B., and Boerjan, W. (2001). “Unravelling cell wall formation in the woody dicot stem,” Plant Molecular Biology 47(1), 239-274. DOI: 10.1023/A:1010699919325
Miidla, H. (1989). “Biochemistry of lignin formation,” in: The Formation of Lignin in Wheat Plants and its Connection with Mineral Nutrition, E. Padu (ed.), Tartu State University, Tartu, Estonia, pp. 11-23.
Monties, B. (1989). “Lignins,” in: Methods in Plant Biochemistry, Vol. 1 Plant Phenolics, P. M. Dey and J. B. Harborne (eds.), Academic Press, London, UK, pp. 113-157.
Motallebi, A., and Kangur, A. (2016). “Are allometric relationships between tree height and diameter dependent on environmental conditions and management?” Trees 30(4), 1429-1443. DOI: 10.1007/s00468-016-1379-4
Neish, A. C. (1964). “Cinnamic acid derivatives as intermediates in the biosynthesis of lignin and related compounds,” in: The Formation of Wood in Forest Trees, M. H. Zimmermann (ed.), Academic Press, New York, NY.
Pazdrowski, W., Jelonek, T., Arasimowicz-Jelonek, M., and Tomczak, A. (2016). “The form of dead bark in Scots pine (Pinus sylvestris L.) and selected biometric features and indicators,” Annals of Warsaw University of Life Sciences-SGGW Forestry and Wood Technology 95, 12-19.
Pereira, H., Graca, J., and Rodrigues, J. (2003). “Wood chemistry in relation to quality,” in: Wood Quality and its Biological Basis, J. R. Barnett and G. Jeronimidis (eds.), Blackwell, Oxford, UK, pp. 53-86.
Sarkanen, K. V., and Ludwig, C. H. (1971). Lignins: Occurrence, Formation, Structure, and Reactions, John Wiley & Sons, New York, NY. DOI: 10.1002/pol.1972.110100315
Schubert, W. J. (1965). Lignin Biochemistry, Academy Press, New York, NY.
Shmulsky, R., and Jones, D. P. (2011). Forest Products and Wood Science: An Introduction, 6th ed., Wiley-Blackwell, West Sussex, UK, pp. 426-433. DOI: 10.1002/9780470960035
Tomczak, A., Redzimska, M., and Jelonek, T. (2015). “Allometric relationships between trunk slenderness and crown dimensions in Scots pine,” Forestry Letters 108, 20-26.
Tsuyama, T., and Takabe, K. (2014). “Distribution of lignin and lignin precursors in differentiating xylem of Japanese cypress and poplar,” Journal of Wood Science 60(5), 353-361. DOI: 10.1007/s10086-014-1417-z
Tukey, J. W. (1949). “Comparing individual means in the analysis of variance,” Biometrics 5(2), 99-114. DOI: 10.2307/3001913
Uggla, C., Moritz, T., Sandberg, G., and Sundberg, B. (1996). “Auxin as a positional signal in pattern formation in plants,” Proceedings of the National Academy of Sciences 93(17), 9282-9286. DOI: 10.1073/pnas.93.17.9282
Üner, B., Karaman, I., Tanriverdi, H., and Özdemir, D. (2011). “Determination of lignin and extractive content of Turkish Pine (Pinus brutia (Ten.)) trees using near infrared spectroscopy and multivariate calibration,” Wood Science and Technology 45(1), 121-134. DOI: 10.1007/s00226-010-0312-z
Van Laar, A., and Akça, A. (2007). Forest Mensuration, Springer, Dordrecht, The Netherlands.
Vanholme, R., Demedts, B., Morreel, K., Ralph, J., and Boerjan, W. (2010). “Lignin biosynthesis and structure,” Plant Physiology 153(3), 895-905. DOI: 10.1104/pp.110.155119
Wareing, P. F., Haney, C. E. A., and Digby, J. (1964). “The role of endo genous hormones in cambial activity and xylem differentiation,” in: The Formation of Wood in Forest Trees, M. H. Zimmermann (ed.), Academic Press, New York, NY, pp. 323-344.
Wąsik, R. (2010). “Relationships between selected crown parameters and the macrostructure properties and density of Douglas fir,” Sylwan 154(11), 783-790.
Wodzicki, T. J. (1964). “Photoperiodic control of natural growth substances and wood formation in larch (Larix decidua (D.C.)),” Journal of Experimental Botany 15(45), 584-589. DOI: 10.1093/jxb/15.3.584
Wodzicki, T. J. (1965). “Annual ring of wood formation and seasonal changes of natural growth-inhibitors in larch,” Acta Societatis Botanicorum Poloniae 34(1), 117-151. DOI: 10.5586/asbp.1965.008
Woodward, A. W., and Bartel, B. (2005). “A receptor for auxin,” The Plant Cell 17(9), 2425-2429. DOI: 10.1105/tpc.105.036236
Zimmermann, M. H. (1966). “Translocation of water and nutrients in palms,” Journal of the International Palm Society 10, 105-114.
Article submitted: November 1, 2016; Peer review completed: February 6, 2017; Revised version received and accepted: March 13, 2017; Published: April 17, 2017.
DOI: 10.15376/biores.12.2.3992-4003
