Abstract
Chitosan foams with promising mechanical properties, heat-insulating ability, and flame retardancy were produced through oven drying. The chitosan foams were reinforced with cellulose, boric acid, and different ratios of activated carbon. The foams showed desirable low density (80.2 to 109.8 kg/m3) and compression properties. The compression resistance and compression modulus of foams ranged between 53.6 and 98.5 KPa and 214 to 394 KPa, respectively. Thermal conductivity tests revealed that the foams endowed low thermal conductivity values (0.035 to 0.051 W/mK). The limiting oxygen index (LOI) of the foams was as high as 32.9% for activated carbon (20 g/L). The activated carbon reinforcement produced higher thermal properties and decreased the mass loss 48.1% at 600 °C. The produced foams exhibited good biodegradability (39% degradation in 15 days). The overall test results showed that the chitosan foams can be utilized as a promising environmentally friendly material in thermal insulation fields.
Download PDF
Full Article
Activated Carbon and Cellulose-reinforced Biodegradable Chitosan Foams
Mehmet Emin Ergun *
Chitosan foams with promising mechanical properties, heat-insulating ability, and flame retardancy were produced through oven drying. The chitosan foams were reinforced with cellulose, boric acid, and different ratios of activated carbon. The foams showed desirable low density (80.2 to 109.8 kg/m3) and compression properties. The compression resistance and compression modulus of foams ranged between 53.6 and 98.5 KPa and 214 to 394 KPa, respectively. Thermal conductivity tests revealed that the foams endowed low thermal conductivity values (0.035 to 0.051 W/mK). The limiting oxygen index (LOI) of the foams was as high as 32.9% for activated carbon (20 g/L). The activated carbon reinforcement produced higher thermal properties and decreased the mass loss 48.1% at 600 °C. The produced foams exhibited good biodegradability (39% degradation in 15 days). The overall test results showed that the chitosan foams can be utilized as a promising environmentally friendly material in thermal insulation fields.
DOI: 10.15376/biores.18.1.1215-1231
Keywords: Chitosan foams; Activated carbon; Cellulose; Thermal properties; Biodegradability
Contact information: Department of Forestry, Akseki Vocational School, Alanya Alaaddin Keykubat University, Antalya, Turkey; *Corresponding author: mehmet.ergun@alanya.edu.tr
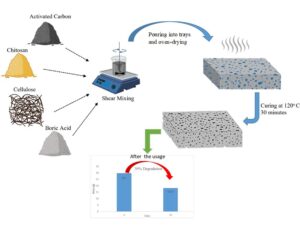
GRAPHICAL ABSTRACT

INTRODUCTION
Because of their cellular structure, foams allow the use of less raw materials, and they are extensively utilized in food packaging, insulation, packaging of valuable goods, and cushioning (Kuhnigk et al. 2022). The worldwide polymer foam market size was appraised at USD 123.1 billion in 2021 and is anticipated to grow at a compound annual growth rate of 3.6% from 2022 to 2030. The building and construction fields kept the highest revenue share of more than 30.0% in 2021 (Fortune Business Insights 2022).
Buildings and construction are required to utilize foam thermal insulation materials as energy resources become more precious. To maintain a comfortable building environment, thermal insulation solutions help to reduce reliance on heating, ventilation, and air conditioning systems. As a result, insulation saves energy and uses fewer natural resources. Additionally, foam insulation materials have some benefits such as energy efficiency, indoor thermal comfort, and lowering noise levels (Hung Anh and Pásztory 2021).
Polystyrene, polyolefin, polyurethane, and polyvinyl chloride-based foam insulation materials that are manufactured from petrochemical-based raw materials are mostly utilized in the building and construction industry (Aditya et al. 2017). However, petroleum-derivate products need a long period to decompose in nature (Bülbül and Büyük 2022).
The massive pollution caused by these products has greatly increased people’s environmental concerns (Bülbül and Ergün 2022). In addition, common insulation materials present a high risk in indoor applications because of their easy flammability (Yildirim et al. 2020). Considering all of these issues, many researchers have focused on producing from renewable resources because they have attractive properties, such as biodegradability, recyclability, environmental acceptability, and sustainability (Qiu et al. 2013). Different types of biomaterials have been investigated for the preparation of bio-foams, such as starch, polyhydroxyalkanoates, polybutyleneadipate-co-terephthalate, polylactic acid (Nofar et al. 2015), cellulose (Nechita and Năstac 2022), and chitosan (Hao et al. 2022). Because chitosan and cellulose are produced from wood, they have a safe, simple to handle, and aesthetic structure (Ergun 2021), and both are versatile and the most abundant biopolymers in nature. These biopolymers have been utilized in food, health, cosmetics, paper, textile, and water treatment industries (Elsabee and Abdou 2013; Muxika et al. 2017; Ottenhall et al. 2018). Although chitosan and cellulose-based foams were the focus of many studies, their application areas were constrained because of some insufficient properties (Xie et al. 2011; Niu et al. 2014). The chitosan and cellulose-based foams are flammable-like synthetic foams. Therefore, it is necessary to add fire retardants to the foams (Mai and Militz 2004).
Halogen-based fire retardants that are detrimental to human and environmental health have been prohibited. Thus, environmentally friendly fire retardants are of great interest in recent times (Wang and Sánchez-Soto 2015; Berglund et al. 2021). The boron compounds have been used in the wood industry (İstek et al. 2017; Can et al. 2018) and the metal industry because they are non-toxic and have a high thermal and biological resistance (Kartal et al. 2009). Boric acid prevents the release of inflammable gases when cellulosic materials, such as wood, cotton, fiber-based products, are burned. The pyrolytic fuel fragment concentration is diluted by the release of chemically bound water from boric acid, and combustion is further prevented by the formation of carbon char (Ullah et al. 2017). Activated carbon is obtained by improving pore volume and the inner surface area because of physical or chemical activation from various organic precursors. Activated carbon is utilized in food and beverage sectors, recovering different solvents, controlling mercury vapor emissions, energy storage devices (González-García 2018), and fireproof materials (Lei et al. 2020).
Generally, supercritical drying or freeze-drying methods are utilized for production of bio-based foams. However, these time-consuming and more expensive drying methods demand harsh conditions and special equipment to accommodate vacuum, high-pressure environments, or high temperatures (Liao et al. 2022). Thus, the practicability of this methodology for commercial production has been discussed. Therefore, it is more suitable for commercialization of the product by simple mechanical mixing to create foam and use common and fast drying methods such as air impingement, microwave, and ovens (Yu et al. 2021).
In this study, chitosan was dissolved with boric acid and then used as a matrix. Cellulose and activated carbon-reinforced produced foams were designed and manufactured using oven drying. This research aimed to examine the effect of the activated carbon reinforcement on the compression and thermal properties of the foams. Finally, the biodegradability of the foams was evaluated by a soil landfill experiment.
EXPERIMENTAL
Materials
Cellulose was provided from EUROPAP (İzmir, Turkey). The density of cellulose that was manufactured from Eastern Spruce was 0.55 g/cm3. According to the information acquired from the EUROPAP tissue factory, the cellulose bleached with peroxide had a kappa number of 3.5. The ISO brightness of the cellulose was specified as 90%. Fiber length and coarseness of cellulose were 2.18 mm and 0.14 mg/m, respectively. Boric acid was purchased from Kimetsan Chemistry (Ankara, Turkey). The density of boric acid was 1.43 g/cm3. The high molecular weight chitosan (density: 0.40 g/cm3) was supplied from Sigma-Aldrich (Schnelldorf, Germany) (molecular weights: 310,000 to 375,000 Da). Activated carbon (AC), which was produced from coconut waste via physical activation method, and sodium dodecyl sulfate (SDS) were supplied from Aromel Chemistry (Konya, Turkey). The density of activated carbon was 2.12 g/cm3.
Methods
Chitosan can dissolve in acidic conditions, so 40 g/L stock solution of boric acid was prepared in distilled water. Initially, chitosan was stirred at 1200 rpm in boric acid for 2 h at pH 4.8. Respectively, cellulose, and different rates of activated carbon were separately added and stirred at 600 rpm for 15 min. Then, 5 g/L SDS was added to the mixture and stirred at 2500 rpm for 10 min to form bubbles. A vortex developed in all mixing processes. Compositions of the foam are given in Table 1.
Table 1. Composition of the Foam
Fig. 1. Manufacturing process of foams
The prepared wet foams were poured into a chrome mold and dried at 75 ℃ for 6 h in the oven. Finally, all samples were cured at 120 ℃ for 30 min. The preparation process of foams is shown in Fig. 1.
Characterization
Fourier transform infrared (FT-IR) spectra were measured directly on fragments of foam using a Nicolet IS FT-IR (iS10, Thermo Fisher Scientific, Waltham, MA, USA) spectrometer. An ATR accessory was used. Sixteen scans were accumulated from 400 to 4000 cm-1 with a resolution of 4 cm-1. The experiments were carried out at 20 °C. The morphologies of foam were examined using Field Emission Scanning Electron Microscopy (FE-SEM) (ZEISS GeminiSEM 500). Foam was coated with iridium with a thickness of 5 nm. All foams were conditioned at 20 ℃ and 65% relative humidity (RH) for 24 h before the density and compression properties measurement.
The density of foam samples was evaluated according to the ASTM C303 (2010) standard. Compression properties were tested on a Marestek universal test device (MARES Engineering Research Electronic Systems, İstanbul, Turkey) according to the ASTM C165-07 (2017) standard.
The foams were produced in a square cross-section of approximately 10 × 10 × 2 cm3. The compression speed was adjusted to 10 mm/min, and the final strain was set to be 25% of the original sample height.
Thermal conductivity was evaluated according to ASTM C518 (2021) with a heat flux (KEM QTM 500, Kyoto Electronics, Kyoto, Japan). The limiting oxygen index (LOI) test was conducted with a Dynisco brand (Dynisco, Heilbronn, Germany) instrument according to ASTM D2863-19 (2019). Thermogravimetric analysis of foams was performed according to ASTM E1131-20 (2020) standard and conducted from 30 °C to 600 °C with a 10 °C increase per minute under nitrogen atmosphere using a Mettler Toledo thermal gravimetric analyzer (Mettler-Toledo, Barcelona, Spain). The degradation of the foams was tested using the soil landfill experiment. Its macroscopic view and change of weight were examined.
Density, compression, thermal conductivity, and LOI values of foams containing different ratios of activated carbon were evaluated with one-way analysis of variance at 95% confidence level (p<0.05) using the SPSS 16 program, and statistically significant homogeneity groups were detected using the Duncan homogeneity test.
RESULTS AND DISCUSSION
The FT-IR spectra of activated carbon (AC) and foams at 400 to 4000 cm-1 were investigated. Both the FT-IR spectra and produced foams are shown in Fig. 2. The spectra of AC samples displayed a band at 1420 cm−1 that could be assigned to the C–H group. The presence of bands in the 1012 cm−1 wavenumber ranges could be due to C–O stretching vibrations (Demiral et al. 2011).
Fig. 2. a) FT-IR results of activated carbon, b) the produced foams, c) SEM images of A-3, d) activated carbon particle in the foam
When the FT-IR results of the foam were evaluated, the bands at 3200 cm–1 could be assigned to O-H stretching. Increase of the absorption peaks in the FT-IR spectra in the range of 3800 to 3000 cm-1 demonstrates an apparent increase in intramolecular and inter intramolecular hydrogen bonding (Liao et al. 2022). In addition, the stretching and bending vibration of the C-H bonds at 2900 and 1420 cm–1 was observed, respectively. The N-H bond was seen at 1650 cm–1, and the band at 1040 cm–1 may occur because of the stretching vibration of the C-O bond. The intensity of the peaks increased as the addition of activated carbon increased in the foams, because most of the activated carbon consists of carbon and oxygen (Tsai and Jiang 2018).
The results showed that chitosan foams reinforced with cellulose and activated carbon had a low density, and additives contributed to improving strength. Table 2 shows the densities, compressive resistance, and modulus of chitosan foams with different amounts of activated carbon addition.
Table 2. Density and Compression Tests Results of Foams
The density of the foams depending on concentration ranged from 80.2 to 109.8 kg/m3. Due to attractive forces, fibers clump, and particles engage together during solvent removal. This phenomenon caused more dense foams (Sehaqui et al. 2011). In contrast, the intrinsic density of the foam material produced with the addition of activated carbon, which has the highest density among the polymers used in production, is directly proportional to increases. It was also observed that with an increase in the solid content the density of foams increased. Density values of foams were in the acceptable range. The density of biodegradable foams changed between 12 and 1000 kg/m3 for application in insulation purposes (Zhao and Li 2022). Densities of PVAc-based foams reinforced with cellulose and chitosan changed between 120 and 210 kg/m3 (Yildirim et al. 2022). When biopolymers, such as chitosan and cellulose, were mixed with surfactants, density values were decreased (Czakaj et al. 2022).
The compression resistance values of foams ranged between 53.6 and 98.5 KPa. The highest value was obtained from A-2 with activated carbon at 10 g/L concentration. A-3 gave the lowest compression resistance value with 53.6 KPa. Additionally, the compression modulus values ranged from 214 to 394 KPa. The A-2 showed the highest compression modulus value. The lowest compression modulus value was obtained from A-3 with 214 KPa. Compressive resistance and modulus depended widely on the density of the foams. The network structure of foams involves more interlocking cellulose fibers, chemical crosslinks, and fiber-to-fiber contact points which explains the strong dependence of resistance and modulus on density (Liao et al. 2022). The compression resistance of commercial foams such as polyurethane foam, extruded polystyrene foam and expanded polystyrene foam has been reported to range from 20 to 700 KPa (Bedarf et al. 2021).
The improved interfacial interactions caused by the polar-polar and hydrogen bond interactions between the reinforcement’s hydroxy group and the matrix could explain the reinforcing effect of cellulose (Murmu 2022). Additionally, the amino groups and hydroxyl groups in the chitosan and cellulose formed intermolecular hydrogen and covalent bonds (Kaisangsri et al. 2012). These situations enhanced the compression properties. In contrast, boric acid can be used to crosslink molecules of chitosan and cellulose that have free hydroxyl groups (Awada et al. 2014). Boric acid is an electrophile and attracts nucleophiles that then combine to create complexes because the trivalent boron atom’s empty p orbital is available. When boric acid is dissolved in water, it dissociates to create tetrahydro borate ions (Prosanov et al. 2018). Crosslinks are created as a result of the reaction between the tetrahydro borate ions and the nucleophilic diols of chitosan and cellulose (Gadhave et al. 2021). Therefore, foams were more robust and resistant to compression in this study.
There have been many studies about chitosan and cellulose-based or reinforced foams. Compression modulus of chitosan and cellulose-based foams that had different densities were determined to range from 10 to 1300 KPa (Wang et al. 2017; Michailidou et al. 2019; Ozen et al. 2021) and 15 to 2800 KPa (Gibson and Ashby 1997; Liu et al. 2017), respectively. In contrast, activated carbon-reinforced foam has been evaluated in few studies and could not notably enhance the mechanical properties of foams (Ketkul et al. 2017). In another study, the compressive resistance of resol-type phenolic resin foam that was reinforced with activated carbon was improved by 30% (Song et al. 2013). However, there has been no study related to foam reinforced with activated carbon. In the current study, while the concentration of activated carbon was up to 10 g/L in the foam, compression values of foam were increased. The oxygen-containing surface and carboxylic groups on activated carbons (Vargas et al. 2012) can improve the compression properties. Additionally, activated carbon, an amphoteric material, was thought to have a positive effect on compression properties (González-García 2018). However, when the concentration of activated carbon was 20 g/L in the foam, compression properties decreased. One of the reasons for the decrease in compression properties was the formation of a brittle surface between the polymers and activated carbon (Suppakarn and Jarukumjorn 2009; Arao et al. 2014). Further, as in the addition of large amounts of inorganic reinforcements (Xie et al. 2010), it was thought that aggregation could occur due to the excessive amount of additional activated carbon (Fig. 2c) and could prevent bond formation (Wang et al. 2016). Also, the pores of the activated carbon were still accessible after production of foam, as can be seen in Fig. 2 d.
The low thermal conductivity of foam is one of the most important features desired in insulation materials. The KEM QTM 500 was utilized to obtain the thermal conductivity of foams through the transient hot wire method. Thermal conductivity of cellulose and activated carbon reinforced foams are given in Fig. 3.
Fig. 3. Thermal conductivity of foams reinforced with cellulose and activated carbon
The thermal conductivity of the presented foams was in the adequate range for insulation purposes, with values of 0.035 to 0.051 W/mK. Chitosan- (Takeshita and Yoda 2015), starch- (Yildirim et al. 2014), cellulose- (Seantier et al. 2016), and wood- (Sun et al. 2020) based foams have similar thermal conductivity results, and they have been reported between 0.022 and 0.054 W/mK. Foams with a porous structure give good heat-insulating qualities (Li et al. 2019). Because atmospheric air has a low thermal conductivity and fills the pores of the foams when heat is applied to foams, pores and the pore walls can generate air layers, which can prevent heat conduction (Jiang et al. 2021).
When AC content increased in the foams, the thermal conductivities of foams were considerably increased. This situation was related to the increase in the density of foam, which led to a rapid increase in the thermal conductivity of the solid form (Yan et al. 2021).
The thermal conductivities of the present produced foams were compared with the commercial or reported foams. Heat blocking ability of freeze-dried or oven-dried bio-based and commercial foams has been accepted as a middle class. In contrast, the foams produced with the supercritical dried technique have equally distributed nanopores; such form has superior low thermal conductivity in comparison to oven-dried or freeze-dried foams. However, the supercritical drying technique is not preferred in commercial production because it has difficulty in production and limited production capacity (Ferreira et al. 2021). Therefore, considering the production capacity, drying in the oven, which is the authors’ production technique, is superior to the freeze-dried or supercritical drying technique.
The thermal behavior of the foams that were reinforced with and without activated carbon under a nitrogen atmosphere was evaluated using TGA and differential thermogravimetry (DTG). The results are shown in Table 3 and Fig. 4. Ti% and Tmax% values were found within the temperature range of 227 to 274 °C and 328 to 332 °C, respectively. As a result of thermal degradation at 600 °C, the least mass loss was obtained from A-1 with 48.1%, and the highest mass loss was obtained from A-0 with 54.6%. It was found that the use of boric acid to dissolve chitosan improved the thermal stability of the samples. While the mass loss of the A-0 coded sample was 54.6% in the present study, Ozen et al. (2021) reported the mass loss of chitosan foams reinforced with microcrystalline cellulose, prepared by dissolving in acidic acid, were changed between 74.6% and 85.6%. The main reason for superior thermal properties is that the boric acid prevented the diffusion of oxygen and heat so that the combustion could be effectively retarded during pyrolysis (Yu et al. 2017). The boric acid feature could create a melted B2O3 film at a high temperature, which could cover the surface of the foams and effectively insulate the fire and oxygen (Yang and Qing 2014). The other reason was that boron compounds caused the formation of water and oxide in the environment during thermal decomposition. This endothermic reaction cooled the material surface and increased charring (Rallini et al. 2018).
Table 3. Initial and Maximum Temperature of Thermal Degradation and the Mass Loss (%) after Thermogravimetric Analysis
The TGA and DTG curves of foams reinforced with cellulose and activated carbon are given in Fig. 4. The DTG curves indicate the velocity of mass loss as a function of temperature during thermal degradation.
Fig. 4. a) TGA and b) DTG results of foams
As the amount of activated carbon increased, the temperature at 10% mass loss increased. In contrast, temperature values corresponding to maximum mass loss were not remarkably changed with or without activated carbon reinforcement. While the mass loss of aerogels produced from neat chitosan was found at approximately 90% (Wang et al. 2015), the current study found that foams had a lower mass loss with the inclusion of boric acid and activated carbon.
As can be seen in Fig. 4a, when AC was added to the foams, mass loss of the foams decreased at the end of 600 °C. Activated carbon, which is composed of graphitized carbon layers, can serve as a physical barrier that diminishes the diffusion of oxygen and heat during the combustion process (Zhang et al. 2018). In Fig. 4b, foams produced from chitosan, boric acid, cellulose, and AC indicated that the degradation of the samples occurred in three main steps. The first step was observed between 80 and 150 °C, the second step was within the temperature range of 240 to 290 °C, and the third step was between 290 and 400 °C. The first peak of the DTG curves was due to moisture removal from the foams. The second peak was relevant to the decomposition (thermal and oxidative) of chitosan. Neto et al. (2005) found that DTG temperature of chitosan was between 230 and 350 °C. It was shown that the length of DTG peaks decreased compared to foam produced with acidic acid in literature (Hong et al. 2007) because boric acid led to a slowdown in the rate of mass loss during thermal degradation. It was concluded that cellulose was disrupted in the third peak. Lignocellulosic decomposition was related to cellulose and hemicellulose at temperatures ranging from 200 to 350 °C (Altuntas et al. 2017). While the amount of activated carbon increased in the foam, the maximum decomposition temperature decreased (Zhang et al. 2018).
The LOI test is a simple method for determining the flammability of polymers. In general, the materials that have a higher LOI value, are resistant to flame (Wang et al. 2019). The LOI values of cellulose and different ratios of AC-reinforced foams are given in Fig. 5.
Fig. 5. LOI values of foams reinforced with cellulose and activated carbon
In this study, LOI values were found between 24.7% and 32.9%, and the highest LOI value was obtained from the A-3 foam. The lowest LOI value was obtained from A-0. It was determined that as the amount of activated carbon increased in the samples, LOI values also increased.
In this study, foams were found to have high LOI values. The first reason is dehydration and other oxygen-eliminating reactions that took place at a comparatively low temperature with the addition of boric acid in the foams (100 to 300 °C). When the isomerization of the polymeric materials takes place in a catalyzed process, aromatic structures can be formed (Wang et al. 2004). This reaction occurs to diminish amounts of a volatile organic compound and increase the charring layer. Additionally, boric acid lessens smoldering and glowing combustion (Pedieu et al. 2012). In previous studies, the LOI values of foams that were reinforced with between 0.5% and 15% boric acid ranged from 18% to 39.5% (Jiang et al. 2021; Kaya 2022). Contrastingly, LOI values of materials produced from chitosan were investigated in previous studies and when chitosan and ammonium phytate were utilized for fire resistance, the LOI value of materials improved 9% (Li et al. 2020). In another study, polyester fibers were reinforced with ammonium polyphosphate and chitosan, where they enhanced fire retardant properties (Fang et al. 2019). When materials included chitosan, they were detected to be more durable at the fire. Materials produced without chitosan broke down as the temperature increased, releasing combustible gas that produced a lot of heat and smoke. However, when materials included chitosan, the production of flammable gas was suppressed, and the production of smoke and heat was reduced. Compounds that decomposed during thermal degradation were not mixed with oxygen for burning directly because of reaction with the amino group of chitosan (Jiao et al. 2020).
In the current study, as the amount of activated carbon was increased in the foam, the LOI values of foams were enhanced. In other words, foams were more resistant to fire. The addition of activated carbon contributed to the physical barrier and catalytic function (Wang et al. 2019) that occurred in the foam. In addition, activated carbon was supported by the formation of the charring layer (He et al. 2018). In some studies, the LOI values of composite materials reinforced with activated carbon were found between 26.4% and 29.1% (Zhang et al. 2018; Wang et al. 2019). Eventually, the LOI values of the present study gave promising results.
Biodegradability of the foams was assessed with the soil burial test. Their weight and macroscopic morphology changes were examined. As shown in Fig. 6, the foams had become degraded at end of the 15 days, and the dry weight of foam decreased from 29.70 g to 18.23 g after 15 days.
Fig. 6. The photograph of the foams (A-2) before (a) and after degradation (b)
According to these results, chitosan foams showed much better degradability than commercial foams such as polystyrene and polyethylene foams. Because of its biodegradability, non-toxic qualities, and low cost, the foam produced from bio-based polymers has the potential to replace foam made from petrochemicals. Polysaccharides, such as cellulose, starch, chitosan, and heparin, can degrade in nature in a short period (14 days). In contrast, it was determined that this period was prolonged with the addition of inorganic polymers, such as titanium dioxide, into corn starch (Anugrahwidya et al. 2021). Polylactic acid, which is frequently used in scientific studies, degrades between 6 months and 24 months (Tian and Bilal 2020). In another study, it was determined that 93% of the foam material produced from cellulose with polyamide epichlorohydrin degraded at the end of 85 days (Liao et al. 2022).
CONCLUSIONS
- While boric acid provides an acidic environment for the dissolution of chitosan, it not only causes crosslinking for cellulose and chitosan but also improves mechanical properties and contributes to increasing the fire resistance of the foams.
- The foam produced with activated carbon has a low density.
- Compression resistance and modulus were 98.5 KPa and 394 KPa, respectively.
- All-produced foams were utilized for thermal insulation purposes.
- When the activated carbon content increased, the LOI values were improved. They were categorized as self-extinguishing.
- As a result of the biodegradability test, the dry weight of foam decomposed approximately 39% after 15 days.
ACKNOWLEDGMENTS
The author would like to thank Associate Professor Şaban BÜLBÜL who helped with laboratory studies from Faculty of Engineer Seydisehir Ahmet Cengiz, Necmettin Erbakan University.
REFERENCES CITED
Aditya, L., Mahlia, T. M. I., Rismanchi, B., Ng, H. M., Hasan, M. H., Metselaar, H. S. C., Muraza, O., and Aditiya, H. B. (2017). “A review on insulation materials for energy conservation in buildings,” Renewable and Sustainable Energy Reviews 73, 1352-1365. DOI: 10.1016/j.rser.2017.02.034
Altuntas, E., Narlioglu, N., and Alma, M. H. (2017). “Investigation of the fire, thermal, and mechanical properties of zinc borate and synergic fire retardants on composites produced with PP-MDF wastes,” BioResources 12(4), 6971-6983. DOI: 10.1536/biores.12.4.6971-6983
Anugrahwidya, R., Armynah, B., and Tahir, D. (2021). “Bioplastics starch-based with additional fiber and nanoparticle: Characteristics and biodegradation performance: A review,” Journal of Polymers and the Environment 29(11), 3459-3476. DOI: 10.1007/s10924-021-02152-z
Arao, Y., Nakamura, S., Tomita, Y., Takakuwa, K., Umemura, T., and Tanaka, T. (2014). “Improvement on fire retardancy of wood flour/polypropylene composites using various fire retardants,” Polymer Degradation and Stability 100, 79-85. DOI: 10.1016/j.polymdegradstab.2013.12.022
ASTM C165-07 (2017). “Standard test method for measuring compressive properties of thermal insulations,” ASTM International, West Conshohocken, PA, USA.
ASTM C303 (2010). “Standard test method for dimensions and density of preformed block and board-type thermal insulation,” ASTM International, West Conshohocken, PA, USA.
ASTM C518 (2021). “Standard test method for steady-state thermal transmission properties by means of the heat flow meter apparatus,” ASTM International, West Conshohocken, PA, USA.
ASTM D2863-19 (2019). “Standard test method for measuring the minimum oxygen concentration to support candle-like combustion of plastics (oxygen index),” ASTM International, West Conshohocken, PA, USA.
ASTM E1131-20 (2020). “Standard test method for compositional analysis by thermogravimetry,” ASTM International, West Conshohocken, PA, USA.
Awada, H., Montplaisir, D., and Daneault, C. (2014). “The development of a composite based on cellulose fibres and polyvinyl alcohol in the presence of boric acid,” BioResources 9(2), 3439-3448. DOI: 10.1536/biores.9.2.3439-3448
Bedarf, P., Dutto, A., Zanini, M., and Dillenburger, B. (2021). “Foam 3D printing for construction: A review of applications, materials, and processes,” Automation in Construction, 130, article ID 103861. DOI: 10.1016/j.autcon.2021.103861
Berglund, L., Nissilä, T., Sivaraman, D., Komulainen, S., Telkki, V.-V., and Oksman, K. (2021). “Seaweed-derived alginate–cellulose nanofiber aerogel for insulation applications,” ACS Applied Materials & Interfaces 13(29), 34899-34909. DOI: 10.1021/acsami.1c07954
Bülbül, Ş., and Büyük, S. S. (2022). “The crosslinking and mechanical properties of SBR compounds with the addition of carburized pine nut cone ash,” Journal of Elastomers & Plastics 54(6), 906-921. DOI: 10.1177/00952443221102585
Bülbül, S., and Ergün, M. E. (2022). “Effect of mica powder-filled styrene-butadiene rubber compounds on crosslink density and mechanical properties,” Thermal Science 26(4A), 3019-3028. DOI: 10.2298/TSCI2204019B
Can, A., Grzeskowiak, W., and Özlüsoylu, İ. (2018). “Improving the fire resistance of heat-treated wood by using environment-friendly substance,” Bartın Orman Fakültesi Dergisi” 20(3), 519-524.
Czakaj, A., Krzan, M., and Warszyński, P. (2022). “The effect of electrolytes and urea on the ethyl lauroyl arginate and cellulose nanocrystals foam stability,” Applied Sciences 12(6), article no. 2797. DOI: 10.3390/app12062797
Demiral, H., Demiral, İ., Karabacakoğlu, B., and Tümsek, F. (2011). “Production of activated carbon from olive bagasse by physical activation,” Chemical Engineering Research and Design 89(2), 206-213. DOI: 10.1016/j.cherd.2010.05.005
Elsabee, M. Z., and Abdou, E. S. (2013). “Chitosan based edible films and coatings: A review,” Materials Science and Engineering: C 33(4), 1819-1841. DOI: 10.1016/j.msec.2013.01.010
Ergun, H. (2021). “Segmentation of wood cell in cross-section using deep convolutional neural networks,” Journal of Intelligent & Fuzzy Systems 41(6), 7447-7456. DOI: 10.3233/JIFS-211386
Fang, Y., Liu, X., and Tao, X. (2019). “Intumescent flame retardant and anti-dripping of PET fabrics through layer-by-layer assembly of chitosan and ammonium polyphosphate,” Progress in Organic Coatings 134, 162-168. DOI: 10.1016/j.porgcoat.2019.05.010
Ferreira, E. S., Rezende, C. A., and Cranston, E. D. (2021). “Fundamentals of cellulose lightweight materials: Bio-based assemblies with tailored properties,” Green Chemistry 23(10), 3542-3568. DOI: 10.1039/D1GC00326G
Fortune Business Insights (2022). “Activated carbon market size, share & COVID-19 impact analysis, by type (powdered, granular, and others), by application (water treatment, air & gas purification, food & beverage, pharmaceutical & healthcare treatment, and others), and regional forecast, 2022-2030,” Fortune Business Insights, (https://www.fortunebusinessinsights.com/activated-carbon-market-102175), Accessed 11 Sep 2022.
Gadhave, R. V., Vineeth, S. K., Mahanwar, P. A., and Gadekar, P. T. (2021). “Effect of addition of boric acid on thermo-mechanical properties of microcrystalline cellulose/polyvinyl alcohol blend and applicability as wood adhesive,” Journal of Adhesion Science and Technology 35(10), 1072-1086. DOI: 10.1080/01694243.2020.1832775
Gibson, L. J., and Ashby, M. F. (1997). Cellular Solids: Structure and Properties, Cambridge University Press, Cambridge, MA, USA. DOI: 10.1017/CBO9781139878326
González-García, P. (2018). “Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications,” Renewable and Sustainable Energy Reviews 82, 1393-1414. DOI: 10.1016/j.rser.2017.04.117
Hao, D., Fu, B., Zhou, J., and Liu, J. (2022). “Efficient particulate matter removal by metal-organic frameworks encapsulated in cellulose/chitosan foams,” Separation and Purification Technology 294, article ID 120927. DOI: 10.1016/j.seppur.2022.120927
He, S., Wu, W., Zhang, M., Han, H., Jiao, Y., Qu, H., and Xu, J. (2018). “Reduction in smoke emitted and fire hazard presented by flexible poly (vinyl chloride) through novel synthesis of SnO2 supported by activated carbon spheres,” Polymers for Advanced Technologies 29(9), 2505-2514. DOI: 10.1002/pat.4362
Hong, P.-Z., Li, S.-D., Ou, C.-Y., Li, C.-P., Yang, L., and Zhang, C.-H. (2007). “Thermogravimetric analysis of chitosan,” Journal of Applied Polymer Science 105(2), 547-551. DOI: 10.1002/app.25920
Hung Anh, L. D., and Pásztory, Z. (2021). “An overview of factors influencing thermal conductivity of building insulation materials,” Journal of Building Engineering 44, article ID 102604. DOI: 10.1016/j.jobe.2021.102604
İstek, A., Özlüsoylu, İ., Çelik, S., and Gönül, Ş. (2017). “Ahşap esasli levha sektöründe kullanilan yanma geciktiriciler,” İleri Teknoloji Bilimleri Dergisi 6(3), 389-399.
Jiang, S., Zhang, M., Li, M., Zhu, J., Ge, A., Liu, L., and Yu, J. (2021). “Cellulose-based composite thermal-insulating foams toward eco-friendly, flexible and flame-retardant,” Carbohydrate Polymers 273, article ID 118544. DOI: 10.1016/j.carbpol.2021.118544
Jiao, C., Li, M., Chen, X., and Li, S. (2020). “Flame retardancy and thermal decomposition behavior of TPU/chitosan composites,” Polymers for Advanced Technologies 31(1), 178-188. DOI: 10.1002/pat.4752
Kaisangsri, N., Kerdchoechuen, O., and Laohakunjit, N. (2012). “Biodegradable foam tray from cassava starch blended with natural fiber and chitosan,” Industrial Crops and Products 37(1), 542-546. DOI: 10.1016/j.indcrop.2011.07.034
Kartal, S. N., Yoshimura, T., and Imamura, Y. (2009). “Modification of wood with Si compounds to limit boron leaching from treated wood and to increase termite and decay resistance,” International Biodeterioration & Biodegradation 63(2), 187-190. DOI: 10.1016/j.ibiod.2008.08.006
Kaya, A. I. (2022). “Determination of chemical structure, mechanical properties and combustion resistance of polyurethane doped with boric acid,” Journal of Thermoplastic Composite Materials 35(5), 720-739. DOI: 10.1177/08927057211019444
Ketkul, K., Threepopnatkul, P., Aussawasathien, D., and Hrimchum, K. (2017). “Poly(lactic acid)-polybutylene succinate-activated carbon composite foams,” Key Engineering Materials 751, 344-349. DOI: 10.4028/www.scientific.net/KEM.751.344
Kuhnigk, J., Standau, T., Dörr, D., Brütting, C., Altstädt, V., and Ruckdäschel, H. (2022). “Progress in the development of bead foams – A review,” Journal of Cellular Plastics 58(4), 707-735. DOI: 10.1177/0021955X221087603
Lei, Q., Xie, Q., and Ding, Y. (2020). “Fire hazard evaluation of activated carbons: Pyrolysis kinetic parameters analyses and model development,” Journal of Thermal Analysis and Calorimetry 139(1), 441-449. DOI: 10.1007/s10973-019-08417-z
Li, P., Wang, B., Liu, Y.-Y., Xu, Y.-J., Jiang, Z.-M., Dong, C.-H., Zhang, L., Liu, Y., and Zhu, P. (2020). “Fully bio-based coating from chitosan and phytate for fire-safety and antibacterial cotton fabrics,” Carbohydrate Polymers 237, article ID 116173. DOI: 10.1016/j.carbpol.2020.116173
Li, Y., Liu, X., Nie, X., Yang, W., Wang, Y., Yu, R., and Shui, J. (2019). “Multifunctional organic–inorganic hybrid aerogel for self-cleaning, heat-insulating, and highly efficient microwave absorbing material,” Advanced Functional Materials 29(10), article ID 1807624. DOI: 10.1002/adfm.201807624
Liao, J., Luan, P., Zhang, Y., Chen, L., Huang, L., Mo, L., Li, J., and Xiong, Q. (2022). “A lightweight, biodegradable, and recyclable cellulose-based bio-foam with good mechanical strength and water stability,” Journal of Environmental Chemical Engineering 10(3), article ID 107788. DOI: 10.1016/j.jece.2022.107788
Liu, Y., Lu, P., Xiao, H., Heydarifard, S., and Wang, S. (2017). “Novel aqueous spongy foams made of three-dimensionally dispersed wood-fiber: Entrapment and stabilization with NFC/MFC within capillary foams,” Cellulose 24(1), 241-251. DOI: 10.1007/s10570-016-1103-y
Mai, C., and Militz, H. (2004). “Modification of wood with silicon compounds. Treatment systems based on organic silicon compounds — A review,” Wood Science and Technology 37(6), 453-461. DOI: 10.1007/s00226-004-0225-9
Michailidou, G., Christodoulou, E., Nanaki, S., Barmpalexis, P., Karavas, E., Vergkizi-Nikolakaki, S., and Bikiaris, D. N. (2019). “Super-hydrophilic and high strength polymeric foam dressings of modified chitosan blends for topical wound delivery of chloramphenicol,” Carbohydrate Polymers 208, 1-13. DOI: 10.1016/j.carbpol.2018.12.050
Murmu, S. B. (2022). “Alternatives derived from renewable natural fibre to replace conventional polyurethane rigid foam insulation,” Cleaner Engineering and Technology 8, article ID 100513. DOI: 10.1016/j.clet.2022.100513
Muxika, A., Etxabide, A., Uranga, J., Guerrero, P., and de la Caba, K. (2017). “Chitosan as a bioactive polymer: Processing, properties and applications,” International Journal of Biological Macromolecules 105, 1358-1368. DOI: 10.1016/j.ijbiomac.2017.07.087
Nechita, P., and Năstac, S. M. (2022). “Overview on foam forming cellulose materials for cushioning packaging applications,” Polymers 14(10), Article Number 1963. DOI: 10.3390/polym14101963
Neto, C. G. T., Giacometti, J. A., Job, A. E., Ferreira, F. C., Fonseca, J. L. C., and Pereira, M. R. (2005). “Thermal analysis of chitosan based networks,” Carbohydrate Polymers 62(2), 97-103. DOI: 10.1016/j.carbpol.2005.02.022
Niu, M., Hagman, O., Wang, X., Xie, Y., Karlsson, O., and Cai, L. (2014). “Effect of Si-Al compounds on fire properties of ultra-low density fiberboard,” BioResources 9(2), 2415-2430. DOI: 10.15376/biores.9.2.2415-2430
Nofar, M., Ameli, A., and Park, C. B. (2015). “A novel technology to manufacture biodegradable polylactide bead foam products,” Materials & Design 83, 413-421. DOI: 10.1016/j.matdes.2015.06.052
Ottenhall, A., Seppänen, T., and Ek, M. (2018). “Water-stable cellulose fiber foam with antimicrobial properties for bio based low-density materials,” Cellulose 25(4), 2599-2613. DOI: 10.1007/s10570-018-1738-y
Ozen, E., Yildirim, N., Dalkilic, B., and Ergun, M. E. (2021). “Effects of microcrystalline cellulose on some performance properties of chitosan aerogels,” Maderas. Ciencia y Tecnología 23(26), 1-10. DOI: 10.4067/S0718-221X2021000100426
Pedieu, R., Koubaa, A., Riedl, B., Wang, X.-M., and Deng, J. (2012). “Fire-retardant properties of wood particleboards treated with boric acid,” European Journal of Wood and Wood Products 70(1), 191-197. DOI: 10.1007/s00107-011-0538-y
Prosanov, I. Y., Abdulrahman, S. T., Thomas, S., Bulina, N. V., and Gerasimov, K. B. (2018). “Complex of polyvinyl alcohol with boric acid: Structure and use,” Materials Today Communications 14, 77-81. DOI: 10.1016/j.mtcomm.2017.12.012
Qiu, J. F., Zhang, M. Q., Rong, M. Z., Wu, S. P., and Karger-Kocsis, J. (2013). “Rigid bio-foam plastics with intrinsic flame retardancy derived from soybean oil,” Journal of Materials Chemistry A 1(7), article ID 2533. DOI: 10.1039/c2ta01404a
Rallini, M., Puri, I., Torre, L., and Natali, M. (2018). “Boron based fillers as char enhancers of EPDM based heat shielding materials for SRMs: A comparative analysis,” Composite Structures 198, 73-83. DOI: 10.1016/j.compstruct.2018.03.102
Seantier, B., Bendahou, D., Bendahou, A., Grohens, Y., and Kaddami, H. (2016). “Multi-scale cellulose based new bio-aerogel composites with thermal super-insulating and tunable mechanical properties,” Carbohydrate Polymers 138, 335-348. DOI: 10.1016/j.carbpol.2015.11.032
Sehaqui, H., Zhou, Q., and Berglund, L. A. (2011). “High-porosity aerogels of high specific surface area prepared from nanofibrillated cellulose (NFC),” Composites Science and Technology 71(13), 1593-1599. DOI: 10.1016/j.compscitech.2011.07.003
Song, S. A., Oh, H. J., Kim, B. G., and Kim, S. S. (2013). “Novel foaming methods to fabricate activated carbon reinforced microcellular phenolic foams,” Composites Science and Technology 76, 45-51. DOI: 10.1016/j.compscitech.2012.12.018
Sun, H., Bi, H., Lin, X., Cai, L., and Xu, M. (2020). “Lightweight, anisotropic, compressible, and thermally-insulating wood aerogels with aligned cellulose fibers,” Polymers 12(1), article no. 165. DOI: 10.3390/polym12010165
Suppakarn, N., and Jarukumjorn, K. (2009). “Mechanical properties and flammability of sisal/PP composites: Effect of flame retardant type and content,” Composites Part B: Engineering 40(7), 613-618. DOI: 10.1016/j.compositesb.2009.04.005
Takeshita, S., and Yoda, S. (2015). “Chitosan aerogels: Transparent, flexible thermal insulators,” Chemistry of Materials 27(22), 7569-7572. DOI: 10.1021/acs.chemmater.5b03610
Tian, K., and Bilal, M. (2020). “Research progress of biodegradable materials in reducing environmental pollution,” in: Abatement of Environmental Pollutants, P. Singh, A. Kumar, and A. Borthakur (eds.), Elsevier, Amsterdam, Netherlands, pp. 313-330. DOI: 10.1016/B978-0-12-818095-2.00015-1
Tsai, W.-T., and Jiang, T.-J. (2018). “Mesoporous activated carbon produced from coconut shell using a single-step physical activation process,” Biomass Conversion and Biorefinery 8(3), 711-718. DOI: 10.1007/s13399-018-0322-x
Ullah, S., Ahmad, F., Shariff, A. M., Bustam, M. A., Gonfa, G., and Gillani, Q. F. (2017). “Effects of ammonium polyphosphate and boric acid on the thermal degradation of an intumescent fire retardant coating,” Progress in Organic Coatings 109, 70-82. DOI: 10.1016/j.porgcoat.2017.04.017
Vargas, A. M. M., Cazetta, A. L., Martins, A. C., Moraes, J. C. G., Garcia, E. E., Gauze, G. F., Costa, W. F., and Almeida, V. C. (2012). “Kinetic and equilibrium studies: Adsorption of food dyes Acid Yellow 6, Acid Yellow 23, and Acid Red 18 on activated carbon from flamboyant pods,” Chemical Engineering Journal 181-182, 243–250. DOI: 10.1016/j.cej.2011.11.073
Wang, Q., Li, J., and Winandy, J. E. (2004). “Chemical mechanism of fire retardance of boric acid on wood,” Wood Science and Technology 38(5), 375-389. DOI: 10.1007/s00226-004-0246-4
Wang, J., Zhou, Q., Song, D., Qi, B., Zhang, Y., Shao, Y., and Shao, Z. (2015). “Chitosan–silica composite aerogels: Preparation, characterization and Congo red adsorption,” Journal of Sol-Gel Science and Technology 76(3), 501-509. DOI: 10.1007/s10971-015-3800-7
Wang, L., and Sánchez-Soto, M. (2015). “Green bio-based aerogels prepared from recycled cellulose fiber suspensions,” RSC Advances 5(40), 31384-31391. DOI: 10.1039/C5RA02981C
Wang, L., Sánchez-Soto, M., Abt, T., Maspoch, M. L., and Santana, O. O. (2016). “Microwave-crosslinked bio-based starch/clay aerogels,” Polymer International 65(8), 899-904. DOI: 10.1002/pi.5104
Wang, Y., Uetani, K., Liu, S., Zhang, X., Wang, Y., Lu, P., Wei, T., Fan, Z., Shen, J., Yu, H., et al. (2017). “Multifunctional bionanocomposite foams with a chitosan matrix reinforced by nanofibrillated cellulose,” ChemNanoMat 3(2), 98-108. DOI: 10.1002/cnma.201600266
Wang, Y.-H., Wu, W.-H., Meng, W.-H., Liu, H., Yang, G., Jiao, Y.-H., Xu, J.-Z., and Qu, H.-Q. (2019). “Activated carbon spheres@NiCo2(CO3)1.5(OH)3 hybrid material modified by ionic liquids and its effects on flame retardant and mechanical properties of PVC,” Composites Part B: Engineering 179, article ID 107543. DOI: 10.1016/j.compositesb.2019.107543
Xie, Y., Hill, C. A. S., Xiao, Z., Militz, H., and Mai, C. (2010). “Silane coupling agents used for natural fiber/polymer composites: A review,” Composites Part A: Applied Science and Manufacturing 41(7), 806-819. DOI: 10.1016/j.compositesa.2010.03.005
Xie, Y., Tong, Q., Chen, Y., Liu, J., and Lin, M. (2011). “Manufacture and properties of ultra-low densıty fıbreboard from wood fıbre,” BioResources 6(4), 4055-4066. DOI: 10.15376/biores.6.4.4055-4066
Yan, M., Pan, Y., Cheng, X., Zhang, Z., Deng, Y., Lun, Z., Gong, L., Gao, M., and Zhang, H. (2021). “‘Robust–soft’ anisotropic nanofibrillated cellulose aerogels with superior mechanical, flame-retardant, and thermal insulating properties,” ACS Applied Materials & Interfaces 13(23), 27458-27470. DOI: 10.1021/acsami.1c05334
Yang, S., Wu, Y., Qing, Y., and Yao, C. (2014). “Effect of typical boron compounds on the thermal degradation and combustion properties of Phyllostachys pubescens,” Engineering Sciences 16(4), 51-55.
Yildirim, N., Erdonmez, F. S., Ozen, E., Avci, E., Yeniocak, M., Acar, M., Dalkilic, B., and Ergun, M. E. (2020). “4 – Fire-retardant bioproducts for green buildings,” in: Bio-Based Materials and Biotechnologies for Eco-Efficient Construction, F. Pacheco-Torgal, V. Ivanov, and D. C. W. Tsang (eds.), Elsevier Ltd.- Woodhead Publishing, Duxford, UK, pp. 67-79. DOI: 10.1016/B978-0-12-819481-2.00004-0
Yildirim, N., Ozen, E., Ergun, M. E., and Dalkilic, B. (2022). “A study on physical, morphological and antibacterial properties of bio polymers reinforced polyvinyl acetate foams,” Materials Research 25, article ID e20210579. DOI: 10.1590/1980-5373-MR-2021-0579
Yildirim, N., Shaler, S. M., Gardner, D. J., Rice, R., and Bousfield, D. W. (2014). “Cellulose nanofibril (CNF) reinforced starch insulating foams,” MRS Online Proceedings Library (OPL) 1621, 177-189. DOI: 10.1557/opl.2014.1
Yu, K., Li, B., Zhang, H., Wang, Z., Zhang, W., Wang, D., Xu, H., Harbottle, D., Wang, J., and Pan, J. (2021). “Critical role of nanocomposites at air–water interface: From aqueous foams to foam-based lightweight functional materials,” Chemical Engineering Journal 416, article ID 129121. DOI: 10.1016/j.cej.2021.129121
Yu, L., Cai, J., Li, H., Lu, F., Qin, D., and Fei, B. (2017). “Effects of boric acid and/or borax treatments on the fire resistance of bamboo filament,” BioResources 12(3), 5296-5307. DOI: 10.15376/biores.12.3.5296-5307
Zhang, M., Wu, W., He, S., Wang, X., Jiao, Y., Qu, H., and Xu, J. (2018). “Synergistic flame retardant effects of activated carbon and molybdenum oxide in poly(vinyl chloride),” Polymer International 67(4), 445-452. DOI: 10.1002/pi.5526
Zhao, J., and Li, S. (2022). “Life cycle cost assessment and multi-criteria decision analysis of environment-friendly building insulation materials – A review,” Energy and Buildings 254, article ID 111582. DOI: 10.1016/j.enbuild.2021.111582
Article submitted: October 9, 2022; Peer review completed: December 8, 2022; Revised version received and accepted: December 14, 2022; Published: December 21, 2022.
DOI: 10.15376/biores.18.1.1215-1231
