Abstract
Alkali ratios and cooking time of the alkaline sulfite anthraquinone (AQ) and methanol (ASAM) pulping process of bamboo (Gigantochloa scortechinii) were studied. Bamboo chips were cooked at three different levels of sodium hydroxide and cooking time, namely 14, 16, or 18% for 60, 90, or 120 minutes. Pulping parameters that remained constant were the use of 0.5% ethylene diamine tetraacetic acid (EDTA), with an 80/20 ratio Na2SO3/NaOH, 0.1% anthraquinone, 15% methanol, and a temperature of 170 °C in the cooking process. Samples prepared using 14% NaOH and 90 min of cooking time resulted in the highest pulp yield, 52.4%, and a Kappa number of 18.1. It seems that 16% sodium hydroxide and 90 min of cooking time are the most appropriate cooking conditions, giving a 49.1% pulp yield and 14.2 Kappa number. The quality of bamboo pulp produced by the ASAM pulping process was found to be beneficial for the use in paper and board manufacturing.
Download PDF
Full Article
Alkaline Sulfite Anthraquinone and Methanol Pulping of Bamboo (Gigantochloa scortechinii)
Amin Moradbak,a,* Paridah Md. Tahir,a Ainun Zuriyati Mohamed,a,* and Rasmina Halis a,b
Alkali ratios and cooking time of the alkaline sulfite anthraquinone (AQ) and methanol (ASAM) pulping process of bamboo (Gigantochloa scortechinii) were studied. Bamboo chips were cooked at three different levels of sodium hydroxide and cooking time, namely 14, 16, or 18% for 60, 90, or 120 minutes. Pulping parameters that remained constant were the use of 0.5% ethylene diamine tetraacetic acid (EDTA), with an 80/20 ratio Na2SO3/NaOH, 0.1% anthraquinone, 15% methanol, and a temperature of 170 °C in the cooking process. Samples prepared using 14% NaOH and 90 min of cooking time resulted in the highest pulp yield, 52.4%, and a Kappa number of 18.1. It seems that 16% sodium hydroxide and 90 min of cooking time are the most appropriate cooking conditions, giving a 49.1% pulp yield and 14.2 Kappa number. The quality of bamboo pulp produced by the ASAM pulping process was found to be beneficial for the use in paper and board manufacturing.
Keywords: Gigantochloa scortechinii bamboo; Fiber dimensions; Chemical composition; ASAM pulping;
Pulp properties
Contact information: a: Institute of Tropical Forestry and Forest Products (INTROP) – Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia; b: Department of Forest Production, Faculty of Forestry, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia;
* Corresponding authors: ainun.introp@gmail.com; aminmoradbak@gmail.com
INTRODUCTION
The use of non-wood materials in the production of pulp and paper has attracted interest worldwide since 1970 (Saijonkari-Pahkala 2001). Bamboo as a non-wood fiber is a viable alternative source for this kind of industry.
There are more than 70 genera of bamboo available worldwide, and these can be divided into about 1,450 species (Gratani et al. 2008). Bamboo species are found in diverse climates, from cold mountains to hot tropical regions. They occur across East Asia, to Northern Australia, and west to India and the Himalayas (Bystriakova et al. 2003). They also occur in sub-Saharan Africa, and in the Americas from the Mid-Atlantic United States (Mohlenbrock 1989) to Argentina and Chile, reaching their southernmost point anywhere, at 47°S latitude. Continental Europe is not known to have any native species of bamboo (Levy 1992).
Bamboo is a hygroscopic type of plant, meaning that it is able to obtain water from the environment and hold onto it (Erakhrumen and Ogunsanwo 2009). This is a very good property because, in the chemical pulping processes, cooking liquor needs to penetrate throughout the chips of a sample such as bamboo. Improper penetration of chemicals in the chips may lead to uncooked chip centers, high screen rejects, low pulp yield, and high dirt count. The penetration of chemicals into the chips is considered to take place by two separate processes: 1) mass flow of liquid and solute into the chips and 2) diffusion of solute through the liquid-saturated chip. These two movements act under different processes (Smook 1992; Sixta 2006). The movements of liquid into chips is governed by capillary forces and applied pressure, while the diffusion of chemical into liquid-filled chip is dependent on the concentration gradient between the liquor surrounding the chip and that inside the chip (Smook 1992).
Ogunwusi (2001) noted that the efficiency degree of wood and non-woody fibers to perform excellently in pulp and papermaking is much related to their biometric characteristics. The characteristics such as fiber length, lumen diameter, and fiber diameter may influence the performance of pulp and paper properties, namely bulk, apparent density, and inter-fiber bonding (Dinwoodie 2000). According to Oluwadara and Ashimiyn (2007) fiber length affects paper tear strength. Longer fiber length generally results in stronger paper, which leads to higher tear strength. Lumen diameter influences the effectiveness of beating. Larger lumen diameter of fibers offer better beating effects in comparison to smaller lumen diameter due to the easiness of liquid penetration into both the inner and outer part of fibers as the beating process is being carried out (Smook 1992; Sixta 2006; Reyier 2008).
Patt and Kordsachia in 1986 invented a new pulping process based on alkaline sulfite anthraquinone with methanol pulping known as the ASAM process. Chemical materials that used in the ASAM process are: sodium sulfite (Na2SO3), sodium hydroxide (NaOH), anthraquinone (AQ), and methanol (CH3OH). These materials have key role in places such as sodium sulfite changed lignin to solvable material, AQ has catalytic effects on delignification, and methanol improves the solubility of the AQ (Shukry et al. 1999; Knoblauch et al. 2000; Miranda and Pereira 2002; Jahan et al. 2003; Khristova et al. 2002, 2004; Kordsachia et al. 2004; Sridach 2010).
Few studies have considered the application of ASAM in the cooking process of both hard and soft wood. Kordsachia et al. (1992) reported that Eucalypt wood and E. globulus had been cooked by ASAM pulping process, which resulted in 56.9% and 53.6% pulp yield, and 14.3 and 10 kappa number, respectively. Pulp yield and kappa number of Pinus sylvestris were found to be from 52.9% to 52.5% and 31.8 to 27, respectively (Kordsachia and Patt 1988).
The ASAM pulping process is an alternative cooking process option for kraft and soda pulping processes. Some advantages of ASAM are listed as: avoidance of air pollution, high delignification rate, high brightness of pulp, high pulp yield, and it is easier to bleach the pulps (Kordsachia and Patt 1988; Patt and Kordsachia 1986; Patt et al. 1987; Miranda and Pereira 2002).
Parameters with the most significance, which affect delignification and polysaccharide removal, are alkali charge and cooking time (Khider et al. 2012a; Mertoglu-Elmas et al. 2012). In addition, ASAM pulping process has better selectivity in comparison to kraft or soda pulping processes in terms of delignification, which leads to low kappa number and high viscosity (Knoblauch et al. 2000).
The effects of methanol and AQ in this process are: a) stabilization of carbohydrates, b) increased solubility of AQ, c) better penetration of chemical materials into the chips, d) pulp with higher viscosity, and e) better dissolving of lignin. AQ also has effects on pulp yield, increasing delignification, decreasing carbohydrate degradation, and consumption of alkali charge. In other words, AQ has a role of accelerant for dissolution of lignin (Shukry et al. 1999; Sixta 2006; Sridach 2010).
AQ and methanol are two additives that can improve the pulp yield by saving polysaccharides. In ASAM, since there are AQ and methanol, pulp can be produced with high yield, low Kappa number, and suitable paper strengths (Khristova et al. 2004). Another advantage of this pulping process is to produce pulp with a low proportion of rejected material. Due to the use of methanol in the cooking liquor, more penetration of chemicals into the chips occurs. Raising pressure of the digester and more air removal of chips are the two main ways of improving the penetration of chemical materials into wood or non-wood chips. Therefore, adding methanol while an ASAM cooking liquor is in the process of penetrating the chips leads to a higher pressure of the digester. As a result, the entrapped air of the chips is displaced, and chemical materials can penetrate into both wood and non-wood chips. Consequently, more delignification happens and fibers can be easily separated (Patt and Kordsachia 1988; Paik et al. 1988).
On the other hand, AQ has the role of effective stabilizer in both wood and non-wood polysaccharides. Anthraquinone is effective at extremely lower dosage levels of 0.05 to 0.1% on oven-dry wood, giving good results in most cases (Sixta 2006). Aldonic acid, which is present as a result of isolation from the pulp hydrolyzates, indicates that stabilization takes place through conversion of the end group to the acid out of an oxidation reaction (Fiserova et al 2006; Hart and Rudie 2014). Therefore, according to role of AQ and methanol in ASAM pulping process, one can expect high yields of ASAM bamboo unbleached pulp.
There is no or a little information on ASAM pulping process of bamboo chips. Therefore, the objectives of this study are to investigate the effects of ASAM pulping parameters on bamboo pulp properties.
EXPERIMENTAL
Materials
Gigantochloa scortechinii (bamboo culms), which was used in this study, was collected from 3- to 4-year-old bamboo plants of Pahang state. The whole of the bamboo culms was converted to chips generally within the range 2 to 2.5 cm by a chipper machine at the Universiti Putra Malaysia (UPM). The moisture content of bamboo chips was measured after they had been dried in ambient air.
Methods
Biometric measurement
The Franklin method (1954) was used to determine the biometric characteristic of bamboo such as fiber length, fiber diameter, lumen diameter, and cell wall thickness.
Chemical composition
The bamboo meal for chemical compositions analysis was prepared according to T 257 cm-85 and T 264 cm-97 of TAPPI standards method. The bamboo meal had been passed through BS 40-mesh and collected on BS 60-mesh.
The analysis included ethanol-benzene solvent extractives, hot and cold-water solubility, lignin content, ash content, silicate content, and one percent NaOH solubility based on TAPPI standards method, namely, T 204 cm-97, T 207cm-99, T 222 om-98, T 211 om-93, T 244 cm-99, and T 212 om-98.
Extractive holocellulose
Five grams of air-dried bamboo meal, which had been screened to select particles in the 40 to 60-mesh range, were transferred to a 250 mL Erlenmeyer flask. The bamboo extractive materials were removed using an ethanol-benzene mixture according to T 204 cm-97 of TAPPI standard methods.
Distilled water (160 mL), glacial acetic acid (0.5 mL), and sodium chlorite (1.5 1 g) were added successively. A 25 mL Erlenmeyer flask was inverted in the neck of the 250 mL Erlenmeyer flask and the flask was placed in a water bath, which was adjusted to temperature of 70 to 80C in the flask.
The flask was heated for 1 h at the above-mentioned temperature, and the content were mixed by occasional swirling. Then, without cooling, glacial acetic acid (0.5 mL) was added, followed by sodium chlorite (1.5 g). The heating was continued at 70 to 80C for an additional hour. At the end of the second and third hours, adding of acetic acid and sodium chloride are repeated respectively. Acid was always added first.
At the end of the third or fourth hour of chlorite treatment, the flask was placed in an ice bath until the contents had cooled below 10 °C. The holocellulose was filtered on a coarse-porosity fritted-glass extraction crucible with a minimum of ice water to transfer all the holocellulose and to remove the color and odor of chlorine dioxide.
Extractive – cellulose
The holocellulose used for subsequent isolation of a-cellulose was prepared by treating 2 g of bamboo meal OD at 76C with 80 mL of buffer solution (48 g sodium hydroxide and 144 g acetic acid) and 2 mL of sodium chlorite solution (27% w/v). 2 mL sodium chlorite solution was added per hour and repeated for five more hours. The hollocellulose was filtered and washed with 500 mL of distilled water and 15 mL of acetone, then dried by oven to determine the a-cellulose content.
Pulping of bamboo chips
The ASAM pulping process was conducted in a 5-L digester, which was heated electrically with liquor circulation at three levels of alkali charge and cooking time. The parameters selected included an 80/20 ratio of Na2SO3 / NaOH, 15% methanol, 9/1 liquor-to-wood (L/W), and 0.5% EDTA according to Miranda and Pereira (2002), Khider et al. (2012a), Khristova et al. (2004), and Granholm et al. (2010).
Ethylene diamine tetraacetic acid (EDTA) is known as a chelating agent for heavy metal ions such as Mn, Mg, Zn, and Fe. These heavy metals cause some difficulties for the hydrogen peroxide bleaching processes known as totally chlorine free (TCF) and elemental chlorine free (ECF); such problems are generally believed to result from the decomposition of hydrogen peroxide (Colodette et al. 1998; Lachenal et al. 1997; Yuan et al 1997; Granholm et al. 2010). As noted by Gupta (1970), Granholm et al. (2010), and Forsskåhl (2000), heavy metals such as iron (Fe) adversely affect the color of pulp and optical properties of the finished paper product. On the other hand, during the cooking the calcium ion can result in scale formation in the digester (Granholm et al. 2010). Table 1 shows the cooking conditions for bamboo chips.
Kappa number and freeness of pulp (CSF) of ASAM bamboo pulp were measured based on T 236 om-99 and T 227 om-99 of TAPPI standards method, respectively.
Table 1. Pulp Conditions of ASAM Process of Bamboo
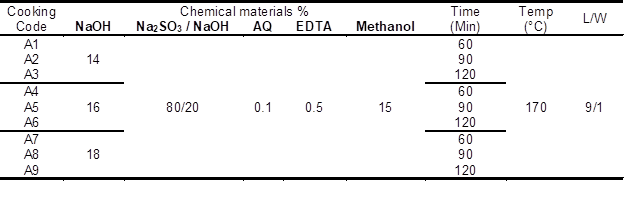
NaOH= Sodium hydroxide/Na2SO3= Sodium sulfite/ AQ= Anthraquinone/ EDTA= Ethylene diamine tetraacetic acid/Temp= Temperature/ L/W= Liquor to wood ratio
RESULTS AND DISCUSSION
Biometrics Characteristics
Table 2 shows the biometric characteristics of bamboo and other species such as B. tuda, E. globulus, and cotton stalks.
Average of bamboo fiber length was 1980 µm. The length was the highest among 1890, 840, and 810 µm. The results indicate that bamboo is beneficial to be used in the making of industrial papermaking such as specialty paper (Adamopoulos et al. 2007; Strelis and Kennedy 1967; Panshin and De Zeeuw 1980). It is expected that the bamboo’s final paper products will have stronger properties. According to Oluwadara and Ashimiyn (2007), longer fiber length contributes to hear tear resistance.
Bamboo has less fiber diameter compared with B. tuda, E. globulus, and cotton stalks. Kennedy et al. (1993) reported that fiber with smaller cells tends to provide lower opacity due to fewer air-fiber interfaces and more fiber-fiber interfaces.
Table 2. Biometric Characteristics of Bamboo and Other Species
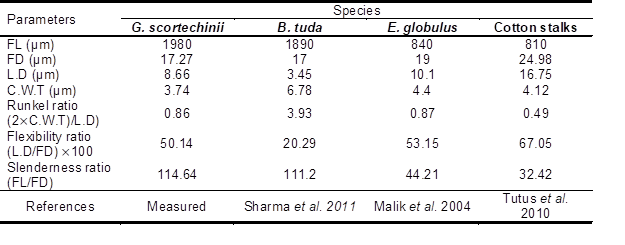
FL= Fiber length/ FD= Fiber diameter/ L.D= Lumen diameter/ C.W.T= Cell wall thickness
As Table 2 shows, bamboo fibers have a suitable lumen diameter, and this has a positive impact on the effectiveness of beating. A larger lumen diameter of fibers offers better beating effects in comparison with a smaller lumen diameter due to the easiness of liquid penetration in the inner and outer part of fibers during the beating process (Smook 1992; Sixta 2006; Reyier 2008).
High Runkel ratios of fibers generally give rise to stiffer, less flexible, and bulkier paper (Binotto and Nicholls 1977). The results show that bamboo fiber has a similar Runkel ratio in comparison with E. globulus. Fiber with higher Runkel ratio could produce paper with greater bulk, i.e. a lower apparent density. Kpikpi (1992) noted that the best Runkel ratio to produce higher pulp quality is less than 1.
Chemical Composition
The chemical composition of bamboo and other species are shown in Table 3. The ash and silica contents were high but within the range of tropical hardwood, 1 to 3% (Khristova et al. 2004). Bamboo chips had high contents of holocellulose and – cellulose, which were 68.33% and 47.67%, respectively. The ash content of bamboo chips was 1.98%, which is very low when compared with other bamboo species such as D. hamiltonii, with 2.6% (Sharma et al. 2011).
Table 3. Chemical Composition of Bamboo and Other Species
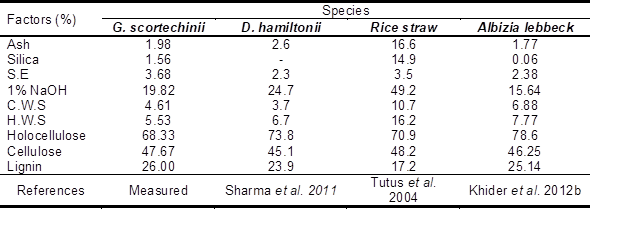
S.E = Solvent extractives (ethanol-benzene)/ H.W.S = Hot water solubility/ C.W.S = Cold water solubility/ 1% NaOH = 1% Sodium Hydroxide Solubility
Extractive materials have effects on final pulp yield and brightness. An increase in the percentage of these materials results in decreases of pulp yield and brightness. On the other hand, the consumption of alkali charge is increased (Khristova et al. 2004).
According to Robinson (1988), Dutt et al. (2004), and Shakhes et al. (2011) the amount of cellulose content has a positive effect on mechanical properties of paper. On the other hand, the pulp yield of chemical pulping is related to contents of cellulose and holocellulose present in the raw material. Therefore, it could be expected that unbleached bamboo ASAM pulp will have high pulp yield according to high cellulose and holocellulose contents.
Bamboo has less lignin content (26%) in comparison to tropical hardwoods (28% to 33%) (Savard et al. 1954).
ASAM Pulping Process
ASAM pulping is a type of sulfite pulping that is carried out under alkaline conditions. According to Khristova et al. (2002), high pulp yield, high paper strength properties, and easy bleaching in TCF sequences are the greatest advantages of the ASAM pulping process. The ASAM pulping process can be regarded as leading to a combination of the pulp properties of kraft and sulfite processes.
In ASAM pulping with the usage of alkali and sodium sulfite (Na2SO3), the depolymerization properties of kraft process and the hydrophilicity-creating properties of sulfite process were illustrated (Shukry et al. 1999). In this study, the effect of cooking parameters on bamboo pulp yield, kappa number, and initial CSF have been studied.
Effect of Cooking Parameters on Pulp Yield
According to Fig. 1, the rate of dissolution of bamboo fibers is accelerated based on the increasing alkali charge and cooking time. This shows that the amount of carbohydrate that dissolves during cooking process depends on the alkalinity and effective alkali charge.
At 14% NaOH, the highest pulp yield of ASAM bamboo pulp was measured. Probably the most important type of base degradation is the so-called peeling reaction. The peeling reaction is started from 2C and the formation of the ketones, which has equilibrium with the corresponding enediols. The next step involves the elimination of the replaced 4C. This leads to a new formation and reducing end of a dicarbony1 compounds (Sixta 2006). The cellulose chain, which is eliminated, contains a new reducing end. Therefore, this process will be repeated with progressive shortening of cellulose.
Ninety minutes of cooking was found to be the most suitable time to maximize the pulp yield. Overall it occurs as a result of chemical penetration into bamboo chips, which can consequently lead to optimum separation of fibers in the bamboo chips.
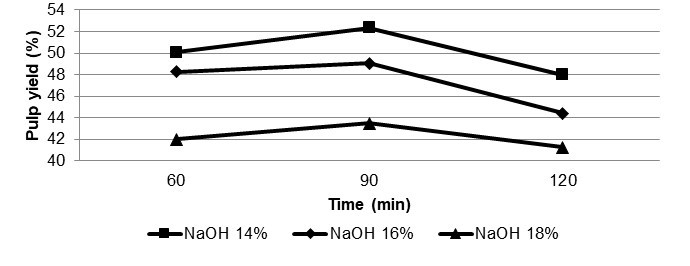
Fig. 1. Relationship between cooking parameters and pulp yield of bamboo
Effect of Cooking Parameters on Kappa Number
The relationship between cooking parameters and kappa number is shown in Fig. 2. At 14% NaOH and 60 min cooking time the highest kappa number was obtained. However, the best cooking time with low kappa number was obtained at 120 min and 18% NaOH. This result occurred due to more penetration of chemical material into the bamboo chips. During the ASAM cooking, methanol increases the pressure inside the digester. Increasing digester’s pressure displaces more air from the bamboo chips. By removing air from the chips, cooking liquor can easily penetrate to the materials and more delignification happens.
In addition, as noted by Holton (1977), AQ tends to promote the reduction of the kappa number and increase of the delignification rate. AQ is soluble only in alkali liquor. During cooking time, two formations of AQ occur. If AQ loses one electron, the anthrahydrosemiquinone (AHSQ) is formed, and if it loses two electrons, the anthrahydroquinone (AHQ) is formed. The AHQ is the primary form of AQ that is responsible for delignification. AHQ and quinine methides (QM) of -aryl ether enter into the procedure afterward.
The mechanism of AQ in the delignification process involves the reduced forms of AQ (particularly the AHQ) reacting with quinine methides (QM) and resulting in b-aryl ether fragmentation and in the improved rate of delignification associated with AQ pulping. The usual mechanism proposes that C-10 carbon of the anthrahydroquinone forms a bond to the C-a carbon of the b-aryl ether dimer. The b-aryl ether and AHQ separate producing a C- / C- olefin. Forming the double bond on the styrene-like fragment requires two electrons, oxidizing AHQ back to the AQ starting material (Hart and Rudie 2014).
Therefore, according to the role of AQ and methanol in the ASAM pulping process, AQ can keep the alkali concentration for bulk phase of delignification process. About 60% of the main lignin portion was dissolved in the bulk phase. (Smook 1992; Sixta 2006).
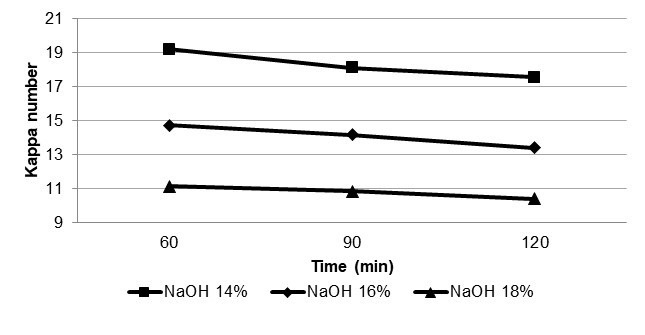
Fig. 2. Relationship between cooking parameters and kappa number of bamboo
Effect of Cooking Parameters on Pulp Freeness
The results show that the highest NaOH led to the lowest fiber freeness. Longer cooking times led to much lower fiber freeness. In alkaline cooking, the middle lamella is removed, and this removal allows the fibrils to extend from the surface of primary wall. When the structure of the fiber is loosened during the cooking, additional water is able to penetrate the fibers. The water is avidly attracted by the available surfaces of the amorphous, hydrophilic hemicellulose material.
Hemicellulose between the elemental threads of the fibers not only provides cleavage planes, but it also attracts water even more strongly than cellulose. Hemicellulose is most responsible for the swelling mechanism (Iwamoto et al. 2008; Sun et al. 2012; Heijnesson-Hultén et al. 2013).
However, besides the possible inclusion of carboxyl groups, hemicellulose is hydrophilic because of the relative shortness of their molecular chains and the presence of a polar group situated at one of their ends.
Therefore, with increasing cooking parameters, more delignification happens, and more fibrils were released on the fiber surface. And also increasing the delignification rate causes more hemicellulose, cellulose, and amorphous materials to be released on the fiber surface. Increasing these materials causes of swelling and increasing mutual bonding. The relationship between cooking parameters and CSF of unbleached bamboo ASAM pulp is shown in Fig 3.
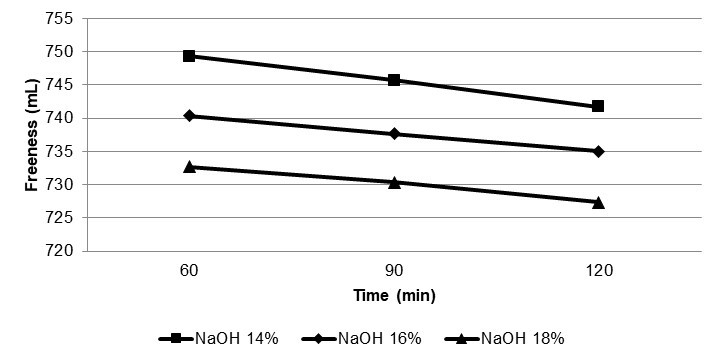
Fig. 3. Relationship between cooking parameters and CSF of bamboo
CONCLUSIONS
- Based on the findings in this work, the average fiber length of bamboo, of the type studied, is similar to that of softwood. The Runkel, flexibility, and slenderness ratios indicated that the pulp and paper of high quality likely could be produced.
- The chemical composition of the bamboo, having a 47.67% content of -cellulose and a 1.98% content of ash, indicates a good potential to produce pulp with suitable yield in comparison with other non-wood species
- The optimum cooking parameters were found to be 16% NaOH and 90 min cooking time with 49.1% pulp yield and 14.2 kappa number, giving a freeness of 737 mL CSF before refining.
ACKNOWLEDGEMENTS
The authors are grateful for the support of the Institute of Tropical Forestry and Forest Products (INTROP) and Research Management Center (RMC) – Universiti Putra Malaysia (UPM), Grant. No. PB 9413400.
REFERENCES CITED
Adamopoulos, S., Martinez, M., and Ramirez, D. (2007). “Characterization of packaging grade papers from recycled raw materials through the study of fiber morphology and composition,” Global NEST Journal 1(9), 20-28.
Bektas, I., Tutus, A., and Eroglu, H. (1999). “A study of the suitability of Calabrian pine (Pinus brutiaten) for pulp and paper manufacture,” Turk. J. Agri. For. 23, 589-599.
Binotto, A. P., and Nicholls, G. A. (1977). “Correlation of fiber morphological variation and wet mat compressibility of loblolly pine bleached kraft pulp,” TAPPI J. 60(6), 91-94.
Bystriakova, N., Bystriakova, N., Kapos, V., Lysenko, I., and Stapleton, C. M. A. (2003). “Distribution and conservation status of forest bamboo biodiversity in the Asia-Pacific Region,” Biodiversity and Conservation 12(9), 1833-1841.
Colodette, J. L., Rothenberg, S., and Dence, C. W. (1998). “Factors affecting hydrogen peroxide stability in the brightening of mechanical and chemimechanical pulps. Part 1: Hydrogen peroxide stability in the absence of stabilizing systems,” J. Pulp Pap. Sci. 14(6), J126-J132.
Dinwoodie, J. M. (2000). “The influence of extractive on tree properties California reed wood Sesquoia sempervirens,” Journal Institute of Wood Science 8, 14-34.
Dutt, D., Upadhyaya, J. S., Malik, R. S., and Tyagi, C. H. (2004). “Studies on pulp and paper-making characteristics of some Indian non-woody fibrous raw materials, Part II,” Journal of Scientific and Industrial Research 63(2), 58-67.
Erakhrumen, A. A., and Ogunsanwo, O. Y. (2009). “Water absorption, anti-swell efficiency, and dimensional stability properties of neem seed-oil treated wild grown Bambusa vulgaris Schrad. ex j.c. Wendl. in southwest Nigeria,” BioResources 4(4), 1417-1429.
Franklin, G. L. (1954). “A rapid method for softening wood for anatomical analysis,” Trop. Woods 88, 35-36.
Fišerová, M., Gigac, J., and Melník, P. (2006). “Application of anthraquinone in kraft pulping of beech wood,” Wood Research 51(4), 55-68.
Forsskåhl, I. (2000). Papermaking Science and Technology, Book 3, Forest Products Chemistry, Stenius, P. (ed.), Fapet Oy, 279-332.
Granholm, K., Harju, L., and Ivaska, A. (2010). “Desorption of metal ions from kraft pulps. Part 1. Chelation of hardwood and softwood kraft pulp with EDTA,” BioResources 5(1), 206-226.
Gratani, L., Crescente, M. F., Varone, L., Fabrini, G., and Digiulio, E. (2008). “Growth pattern and photosynthetic activity of different bamboo species growing in the Botanical Garden of Rome,” Flora 203, 77-84.
Gupta, V. N. (1970). “Effect of metal ions on brightness, bleachability and colour reversion of groundwood,” Pulp Pap. Can. 71(16), 69-77.
Hart, P. W., and Rudie, A. W. (2014). “Anthraquinone – A review of the rise and fall of a pulping catalyst,” Proceedings of the TAPPI PEERS Conference, Tacoma, WA, September 14-17, 2014, Preprint 24-1, TAPPI Press, Atlanta, 2014.
Heijnesson-Hultén, A., Guo, S., Basta, J., Daniel, G., Zhan, H., and Ulf Germgård, U. (2013). “Impact of drying on the quality of bamboo kraft pulps,” BioResources 8(1), 1245-1257.
Holton, H. H. (1977). “Soda additive softwood pulping; a major new process,” Pulp and Paper Canada 78(10), T218-T223.
Iwamoto, S., Abe, K., and Yano, H. (2008). “The effect of hemicelluloses on wood pulp nanofibrillation and nanofiber network characteristics.” Biomacromolecules 9(3), 1022–1026.
Jahan, M. S., Chowdhury, D. A. N., and Islam, M. K. (2003). “ASAM pulping of jute,” WPP. Pulp & Paper Science and Technology: Alternative Papermaking Fibres.
Kennedy, J. F., Phillips, G. O., and Williams, P. A. (1993). Cellulosics: Pulp, Fiber and Environmental Aspects, Ellis Horwood, UK.
Khider, O. T., Omer. S. T., and Shomeina, S. K. (2012a). “Ecologically friendly alkaline pulping of pigeon pea stalks from Sudan,” Researcher 4(4), 88-95.
Khider. O. T., Omer, S., Taha, O., and Shomeina, S. K. (2012b). “Suitability of Sudanese cotton stalks for alkaline pulping with additives,” Iranica Journal of Energy & Environment 3(2), 167-172.
Khristova, P., Kordsachia, O., Patt, R., Khider, T., and Karrar, I. (2002). “Alkaline pulping with additives of kenaf from Sudan,” Industrial Crops and Products 15, 229-235.
Khristova, P., Kordsachia, O., and Daffalla, S. (2004). “Alkaline pulping of Acacia seyal,” Tropical Science 44(4), 207-215. DOI: 10.1002/ts.170
Knoblauch, J., Röderweg, L. W., Zollner-Croll, H., and Kordsachia, O. (2000). “Comparison of pulp properties from the kraft, sulfite and ASAM process,” Das Papier, IPW (1), T1-T5.
Kordsachia, O., and Patt, R. (1988). “Full bleaching of ASAM pulps without chlorine compounds,” Holzforschung 42 (3), 203-209.
Kordsachia, O., Wandinger, B., and Patt, R. (1992). “Some investigations on ASAM pulping and chlorine free bleaching of Eucalyptus from Spain,” Holz Roh-Werkstoff 50, 85-91.
Kordsachia, O., Roßkopf, O., and Patt, R. (2004). “Production of spruce dissolving pulp with the prehydrolysis-alkaline sulfite process (PH-ASA),” Lenzinger Berichte 83, 24-34.
Kpikpi, W. M. (1992). “Wood structure and paper making potentials of Ricinodendron heudelotii and Albizia zygia in relation to Gmelina arborea in Nigeria,” Journal of Botany 5, 41-50.
Lachenal, D., Nguyen Thi, N. B., Chirat, C., and Soria, L. (1997). “Optimum use of H2O2 in kraft pulp delignification,” Paperi ja Puu 79(4), 252-256.
Levy, M. (1992). Dictionary of Gardening, New RHS Dictionary of Gardening, Huxley, A., and Griffiths, M. (eds), RHS. Macmillan New ISBN 0-333-47494-5.
Malik, R .S., Dutt, D., Tyagi, C. H., Jindal, A. K., and Lakharia, L. K. (2004). “Morphological, anatomical and chemical characteristics of Leucaena leucocephala and its impact on pulp and paper making properties,” Journal of Scientific & Industrial Research l(63), 125-133.
Mertoglu- Elmas, G., Gunaydin, K., and Ozden. O. (2012). “Environmental friendly alkaline sulfite anthraquinone-methanol (ASAM) pulping with Rumex crispus plant extract of woody materials,” J. Environ. Biol. 33(5), 941-944.
Miranda, I., and Pereira, H. (2002). “Kinetics of ASAM and kraft pulping of Eucalyptus wood (Eucalyptus globulus),” Holzforschung 5(1), 85-90.
Mohlenbrock, R. H. (1989). “Midwest wetland flora: Field office illustrated guide to plant species. Arundinaria gigantea (Walt.) Muhl. giant cane,” PLANTS Database. Midwest National Technical Center, Lincoln. USDA.
Ogunwusi, A. A. (2001). “Variation in pulp characteristic of Pinus caribaea,” Department of Resources Management, University of Ibadan, Ibadan, Nigeria, 103 pp.
Oluwadara, A. O., and Ashimiyu, O. S. (2007). “The relationship between fibre characteristics and pulp-sheet properties of Leucaena leucocephala (Lam.) De Wit,” Middle-East Journal of Scientific Research 2(2), 63-68.
Paik, K. H., SH, O. H., and Koo, J. O. (1988). “Alkaline sulfite pulping with the additions of anthraquinone and methanol,” J. Tappik 20(2), 24-32. (ABIPST 60:502).
Panshin, A. J., and De Zeeuw, C. (1980). Textbook of Wood Technology, Ed.4, McGraw-Hill, New York.
Patt, R., and Kordsachia, O. (1986). “Herstellung von Zellstoffen unter Verwendung von alkalischen Sulfitlosungen mit Zusatz von Anthrachinon und Methanol,” Papier 40(10A), V1-V8.
Patt, R., Kordsachia, O., and Knoblauch, J. (1987). “The ASAM process – Alkaline sulfite, anthraquinone, methanol pulping,” In: International Symposium on Wood and Pulping Chemistry, Paris, Proceedings, pp. 355-360.
Patt, R., and Kordsachia, O. (1988). “Anforderungen an neue Zellstoffherstellungsverfah-ren,” Wochenbl Papierfabr. 116(17), 709-713.
Reyier, S. (2008). “Bonding ability distribution of fibers in mechanical pulp furnishes”. (Degree of Licentiate of Technology). Retrieved from http://miun.diva-portal.org/smash/get/diva2:133065/FULLTEXT01.pdf.
Robinson, F. E. (1988). “Kenaf: A new fiber crop for paper production,” California Agriculture 42, 31-32.
Saijonkari-Pahkala, K. (2001). “Non-wood plants as raw material for pulp and paper,” (ACADEMIC DISSERTATION) Faculty of Agriculture and Forestry, University of Helsinki, for public criticism at Infokeskus Korona, Auditorium 1, on November 30.
Savard, J., Benson, A., and Morize, S. (1954). “Chemical analysis of tropical timber,” Publication No. 5, Centre Technique Forestier Tropical, Nogent-sur-Marne, France.
Shakhes, J., Zeinaly, F., Marandi, M. A. B., and Saghafi, T. (2011). “The effects of processing variables on the soda and soda-AQ pulping of kenaf bast fiber,” BioResources 6(4), 4626-4639.
Sharma, A. K., Dutt, D., Upadhyaya, J. S., and Roy, T. K. (2011). “Anatomical, morphological, and chemical characterization of Bambusa tulda, Dendrocalamus hamiltonii, Bambusa balcooa, Malocana baccifera, Bambusa arundinacea and Eucalyptus tereticornis,” BioResources 6(4), 5062-5073.
Shukry, N., EI-Kalyoubi, S. F., and EI-barbary, M. H. (1999). “Pulping of Casuarina glauca with ASAM – An environmental friendly process,” Journal of Scientific & Industrial Research 1(58), 799-806.
Sixta, H. (2006). Handbook of Pulp, Vol. 1, Wiley-VCH, Weinheim.
Smook, G.A. (1992). Handbook for Pulp and Paper Technologists, 2nd Edn. Angus Wilde Publications, Vancouver, pp. 419.
Sridach, W. (2010). “The environmentally benign pulping process of non-wood fibers,” Suranaree Journal of Science and Technology 17(2), 105-123.
Strelis, I., and Kennedy, R. W. (1967). Identification of North American Commercial Pulpwoods and Pulp Fibers, University of Toronto Press, Toronto, Canada.
Sun, S. N., Yuan, T. Q., Li, M. F., Cao, X. F., Xu, F., and Liu, Q. Y. (2012). “Structural characterization of hemicelluloses from bamboo culms (Neosinocalamus affinis),” Cellulose Chem Technol 46 (3-4): 165-176.
TAPPI Test Method T 204 cm-97 (1997). “Solvent extractives of wood and pulp,” TAPPI Press, Atlanta, GA, USA.
TAPPI Test Method T 257 cm-85 (1985). “Sampling and preparing wood for analysis,” TAPPI Press, Atlanta, GA, USA
TAPPI Test Method T 207 cm-99 (1999). “Water solubility of wood and pulp,” TAPPI Press, Atlanta, GA, USA.
TAPPI Test Method T 222 om-98 (1998). “Acid-insoluble lignin in wood and pulp,” TAPPI Press, Atlanta, GA, USA.
TAPPI Test Method T 211 om-93 (1993). “Ash in wood, pulp, and paperboard: Combustion at 525 C,” TAPPI Press, Atlanta, GA, USA.
TAPPI Test Method T 244 cm-99 (1999). “Acid-insoluble ash in wood, pulp, paper, and paperboard,” TAPPI Press, Atlanta, GA, USA.
TAPPI Test Method T 212 om-98 (1998). “One percent sodium hydroxide solubility of wood and pulp,” TAPPI Press, Atlanta, GA, USA.
TAPPI Test Method T 236 om-99 (1999). “Kappa number of pulp,” TAPPI Press, Atlanta, GA, USA.
TAPPI Test Method T 227 om-99 (1999). “Freeness of pulp (Canadian standard method),” TAPPI Press, Atlanta, GA, USA.
TAPPI Test Method T 264 cm-97 (1997). “Preparation of wood for chemical analysis,” TAPPI Press, Atlanta, GA, USA.
Tutus, A., Alma, H. M., and Bektas, I. (2004). “The effect of service age on various chemical properties of Scots pine and Crimean juniper wood used indoor constructions,” Wood Res. 49(4), 25-31.
Tutus, A., Ezici, A. C., and Ates, S. (2010). “Chemical, morphological and anatomical properties and evaluation of cotton stalks (Gossypium hirsutum L.) in pulp industry,” Scientific Research and Essays 5(12), 1553-1560.
Ververis, C., Georghiou, K., Christodoulakis, N., Santas, P., and Santas, R. (2004). “Fiber dimensions, lignin and cellulose content of various plant materials and their suitability for paper production,” Industrial Crops and Products (19), 245-254.
Yuan, M., d´Entremont, M. D., Ni, Y., and van Heiningen, A. R. P. (1997). “The role of transition metal ions during peracetic acid bleaching of chemical pulps,” Pulp Paper Canada 98(11), T408-T413.
Article submitted: January 9, 2015; Peer review completed: March 23, 2015; Revised version received and tentatively accepted: July 15, 2015; Further corrections: August 20, 2015; Further corrections and acceptance: October 25, 2015; Published: November 13, 2015.
DOI: 10.15376/biores.11.1.235-248
