Abstract
Download PDF
Full Article
Assessment of the Growth and Fruiting of 19 Oyster Mushroom Strains for Indoor Cultivation on Lignocellulosic Wastes
Olena Myronycheva,a Iryna Bandura,b Nina Bisko,c Andrii P. Gryganskyi,d and Olov Karlsson a*
Twelve Pleurotus ostreatus (Jacq. ex Fr.) P. Kumm and six Pleurotus pulmonarius (Fr.) Quél. strains were characterized from the National Culture Collection of Mushrooms, Institute of Botany Kholodny, National Academy of Science, Kyiv, Ukraine (IBK). The strains were grown under commercial conditions on a mixture of wheat straw and sunflower shells under both winter and summer temperatures typical for those climatic conditions. The strains were divided into three groups according to their growing patterns. Important characteristics were compared with a commercial analogue, HK-35, such as vegetative growth, generative growth, and biological efficiency (1.9- to 3.1-fold), and were recorded for strains 2251, 2292, 2316, 2319, and 2320 of P. ostreatus and 2314 of P. pulmonarius. Strains 2251, 2292, 2301, 2321 and 2323 were the most suitable for commercial production, while strains 2319 and 2320 could satisfy processing industry requirements with their high biological efficiency. Strains 2287 and 2317 produced high-quality fruit bodies but probably required a higher temperature for cultivation. Strain 2318 might be attractive for some consumers due to its unique and unusual fruit body shape. Strain 2314 was the most promising for summer cultivation, while strain 537 produced the highest quality fruit bodies.
Keywords: Oyster mushroom; Strain; Vegetative growth; Fruit body; Biological efficiency; Conversion Factor (CF); Asymmetry of the Fruit Body Cap (Cas)
Contact information: a: Department of Engineering Sciences and Mathematics Wood Science and Engineering Division, Luleå University of Technology, Forskargatan 1, Skellefteå, 931 87 Sweden; b: Department of Technology Processing and Storage of Agricultural Products, Tavria State Agrotecnological University, Khmelnytskoho Ave 18, Melitopol, 72310 Ukraine; c: Department of Mycology, Institute of Botany, National Academy of Sciences of Ukraine, Tereschenkivska St 2, Kyiv, 01601 Ukraine; d: LF Lambert Spawn Co., 1507 Valley Rd, Coatesville, PA 19320, USA;
* Corresponding author: olov.karlsson@ltu.se
INTRODUCTION
Oyster mushrooms (fungi of genus Pleurotus) are the second largest cultivated mushroom species group in the world, constituting approximately 27% of the total global production (Royse 2014). The Pleurotus species were adapted for cultivation on small- and large-scale farms as well as in tropical and temperate climates on various lignocellulosic agro- and forest wastes (Miles and Chang 2004). The main requirement for the successful development of oyster mushroom cultivation is reliable high yields during seasonal and off-season production.
Currently, commercial oyster strains produce stable yields under particular controlled micro-climate conditions, significantly influencing the cost/benefit ratio of production (Celik and Peker 2009). The variation in the chemical composition of substrate mixtures from plant residues depends significantly on the origin of plant species (Tao et al. 2012), the ratio of component tissues (Collins et al. 2014), and the location and soil properties (Xiong et al. 2010), which are the main challenges for selecting mushroom strains for cultivation on lignocellulosic wastes (Cohen et al. 2002). Moreover, micro-climatic conditions regulate fungal culture homeostasis, affect the duration of fruit body morphogenesis, and define the transition from incubation (vegetative stage of growth) to fruiting (development of sporocarps) (Moore et al. 2008). Therefore, the selection and breeding of strains that are malleable and resistant to temperature fluctuations and substrate variations are extremely important for the production of Pleurotus.
Currently, spawn companies offer only a few commercial oyster mushroom strains. However, our knowledge of the biological origin of these strains does not guarantee a stable yield due to the lack of studies concerning the control of fungal growth by environmental and genetic factors (Osiewacz 2002). Spawn companies run their own breeding programs, and the intellectual property rights usually protect their new strains. They are selected for the particular climate/substrate conditions, which complicates their further use in various environments. Different mycological culture collections preserve a large amount of fungal genetic material collected in all climatic zones. However, they do not provide continuous selection programs to introduce new oyster mushroom strains for commercial cultivation due to differences in research interests.
The assessment of Pleurotus species heterogeneity (Pawlik et al. 2012), classification of new oyster mushroom strains, and evaluation of vegetative and generative growth stages are needed to increase the availability of genetic material for indoor cultivation in different climatic zones (Nurmetov and Devochkina 2010). Consistent studies of variations in ecological, physiological, morphological, and genetic properties of Pleurotus strains accumulated from different culture collections create an appropriate basis for high-quality strain selection, breeding programs (Rao et al. 2001), and further commercial use of the strains.
The aim of this study was to evaluate the temperature sensitivity, vegetative growth, and generative (fruit body development and flushing) responses, as well as the biological efficiency (criteria for substrate conversion/mushroom productivity estimation) of several oyster mushroom strains cultivated indoors on agro-waste that is typical for temperate climate zones of the European continent.
EXPERIMENTAL
Materials
The study was conducted for 12 Pleurotus ostreatus mushroom strains grown at 11±2 °C and six P. pulmonarius strains grown at 26±2 °С in the southeastern region of Ukraine; these temperatures are typical of temperate continental climates. Growth was observed in a growing room with a controlled microclimate from November 2007 to February 2012.
The oyster mushroom strains (Table 1) were obtained from international culture collections and deposited in the National Culture Collection of Mushrooms, Institute of Botany, National Academy of Sciences, Kyiv, Ukraine (IBK). Strain HK-35 (Sylvan, USA) was used for comparison due to its worldwide commercial distribution. All cultures were stored on solidified malt extract agar at 4±1°C. The cultures were transferred to the new medium after visual control of vegetative mycelium, and microbiological purity was evaluated on an annual basis (Nakasone et al. 2004).
The composition of the substrate (wheat straw (Triticum aestivum) and sunflower husks (Helianthus annuus)) at a 2:3 ratio was prepared according to standard procedures to provide a 45-55/1 C/N ratio optimal for Pleurotus cultivation (Royse 2002; Zied et al. 2011). The substrate was treated by aerobic fermentation in time mode: temperature increase to 65 °С, 36 h; fermentation, 12 h; aerial cooling to 25 °С, 24 h. The prepared substrate was inoculated with 4.75±0.10% of mushroom spawn. Spawn mixing and substrate block formation were completed by mechanical vibration of polyethylene bags (370 × 1000 mm in size with a film thickness of 75±5 µm). The substrate bag had a diameter of 250 mm and a height of 850 mm. The average weight of each substrate block was 12.2±0.2 kg (Oei 2003; Oei et al. 2005).
Table 1. Origin of Oyster Mushroom Strains in the IBK Collection

-//- indicates the same.
The substrate was incubated in a one-stage cultivation system at Tavria State Agrotechnological University, Melitopol, Ukraine. The substrate bags were positioned randomly in two to three rows. The bags were perforated for air exchange with eight to 12 slits in each 150±50 mm by 70±10 mm bag section (chess order) with a total open surface less than 2.5% from the total bag surface (Oei et al. 2005). Vegetative growth was monitored visually every 2 to 3 days. Substrate quality was estimated in two ways: the presence of molds and bacteria that infected some areas of the substrate surface, and the absence of fruit body formation. The amount of contaminated substrate was determined by calculating the ratio of infected bags weight to the total substrate weight.
Fruit body formation was initiated by changing the environmental conditions in the growing rooms. The following items were recorded: the duration of cluster formation, initiation of harvesting, and total amount and weight of the mushrooms for each day of the harvested flush in each sampling area.
Methods
Strain productivity was evaluated according to the duration of the technological cycle and biological efficiency (BE). The following technological parameters of the growth cycle were examined by visual observations and estimated in days (e.g., 24 h):
- duration of vegetative mycelia growth before primordium appearance;
- duration of fruit body development to harvest in the mature stage, characterizing the process of individual generative development of mushroom strains;
- and duration of fruiting flushes from the moment of the first to the last mature fruit body harvested in a particular sampling area.
The biological efficiency (%) was determined using the ratio of the total weight of fresh mushrooms to the absolute dry weight of the substrates. The mushroom growth period was evaluated by calculating the first mushroom flush that was 80±5% from the total yield by weight. Morphological characterizations of mature clusters of fruit bodies, individual fruit bodies, and growth patterns were determined by direct measurements of the following parameters:
- weight of fruit bodies in the clusters;
- size (width and height);
- quantity of fruit bodies in the clusters;
- weight of individual fruit bodies;
- width and height of the mushroom cap (taking into account the cap asymmetry);
- weight of the pileus;
- and height of the fruit body stipe.
For variability analysis, a sample size of 80 to 100 fruit bodies was used. The weight and size of the fruit bodies were analyzed according to UNECE standard FFV-24 (2012). Fruit bodies and their clusters were characterized morphologically (Bilaj 1982). Packages that were eligible for commercial mushroom use were assessed using plastic boxes with dimensions of 400×300×125 mm and a total capacity of 2 kg (DSTU 7786 2015).
Data were collected from three growing cycles. Complete data comprising 1523 observations were available for 19 strains. This dataset included 13 variables (vegetative growth, fruit body development time, fruiting pattern, biological efficiency, weight, width and height of the clusters, fruit body quantity, fruit body weight, cap weight, cap width, cap height, and stipe height), producing a 1523×13 data matrix. The univariant analysis was completed to evaluate the differences between multiple groups (i.e., strain or temperature) using IBM SPSS Statistics 20.0 (Armonk, NY, USA).
RESULTS AND DISCUSSION
Growth Stage and Biological Efficiency
Combined data (assessment of vegetative growth, fruit body development, and fruiting pattern as a general technological cycle) were found to be most helpful for making decisions regarding strain selection for further mushroom production. Complete colonization of the bags under winter conditions was observed over 10 days for all studied strains, and no significant differences were observed. Table 2 lists the experimental results for the growth stages and biological efficiency (BE) of 19 Pleurotus strains. During the vegetative growth stage, the substrate inoculated with strains 2317 and 2287 was spatially contaminated with molds with a total substrate weight of 6.6% and 5.1%, respectively. This result revealed a low resistance to competitive microbiota under the studied cultivation conditions. In almost all strains, the first primordia was observed within 14 to 16 days of the spawn run, which is significantly longer than that observed in similar studies conducted in Bangladesh and China (Ahmed et al. 2013; Yang et al. 2013; Owaid 2014) due to the bag size, substrate processing, growth temperature, and different substrates. The exceptions were strain 2322 with the shortest vegetative and fruiting period of 12 days, and HK-35 with the longest vegetative growth stage of 28 days (Table 2).
Strains 2322 and 2319 had the shortest period of fruit body development with an average of three days. Strains 2322 and 2319 developed very rapidly under winter temperatures, while strains 2323 and HK-35 exhibited the longest periods of morphogenesis (Table 2). The short fruiting flush helped to reduce the entire production cycle and decrease labor costs during harvesting. Thus, it is important to record the duration of fruit body development for strain characterization. The shortest fruiting flush of three days was observed for strains 2322, 2251, 2292, 2320, and HK-35 (despite the long developmental stage of the last strain). A medium wave of 4-5 days was observed for strains 2287, 2301, and 2318. A long wave of more than five days was observed for strains 2319, 2321, 2317, 22316, and 2323. During the winter season, we grouped the strains by summing the vegetative and generative stages of growth: strains 2322, 2251, 2320, 2292, and 2319: up to 23 days; strains 2287, 2301, 2316, 2323, 2321, and 2318: approximately 27 days; and 2317 and HK-35: more than 31 days.
The biological efficiency reflects the transfer of substrate nutrients to the fruit body and characterizes mushroom growth. As shown in Table 2, strains HK-35, 2287, 2301, and 2317 were more sensitive to low temperature, as previously noted (Dudka 1983; Buhalo 1990; Yakovlev et al. 2000). However, the biological efficiency was significantly higher (p≤0.05) for the other studied strains, suggesting variations in their resistance to cold. The highest biological efficiency was calculated for strain 2320 (65.51%), which was 3.1 times higher than that of HK-35 (21.18%). The other strains formed a more or less homogenous biological efficiency group, with the exception of strain 2301. In general, the biological efficiency of the studied strains was significantly lower compared with a similar study conducted in Pakistan examining wheat and barley straw (Dahmardeh et al. 2010), presumably due to the different climatic conditions.
Another important feature related to biological efficiency is that strains with low variability are characterized by their uniform fruit body formations on all substrate blocks as well as their resistance to contamination. The characteristics of fruit body morphology are described in Tables 2 and 3. Strains 2320 and 2319 displayed a maximum biological efficiency and low variability (Table 2). Unfortunately, their fruit bodies had an undesirable size and shape, thus limiting their marketing suitability according to UNECE Standard FFV-24 (2012). Strains 2251, 2316, 2292, and 2301 had a slightly lower biological efficiency, but their fruit bodies appeared more attractive for prospective consumers (Figs. 1 and 2). Despite the relatively high biological efficiency of strain 2318, its fruit body characteristics (shape and size) were worse than those of other strains (Figs. 1 and 2).
Table 2. Growth and Biological Efficiency of Oyster Mushroom Strains (2007-2012)
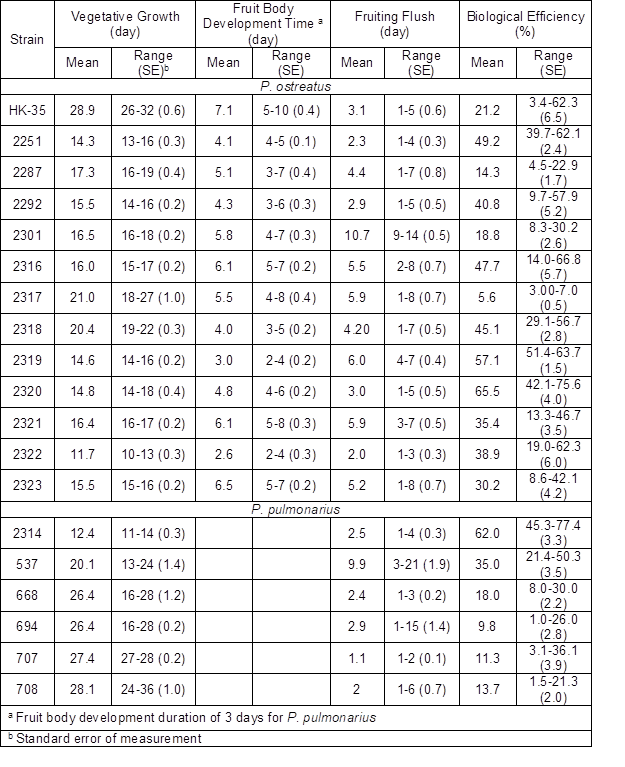
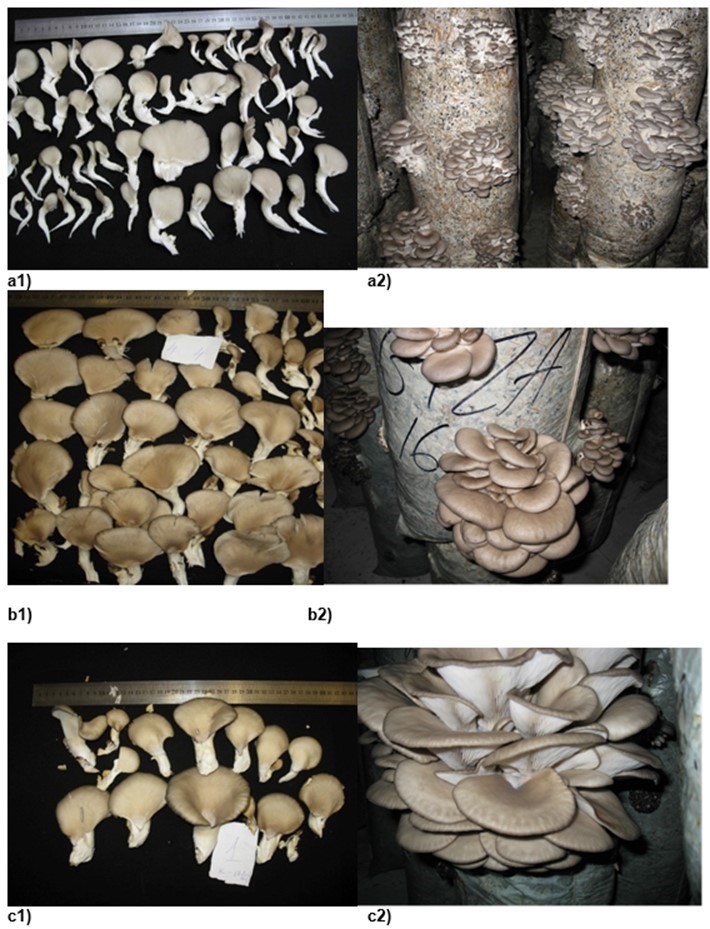
Fig. 1. The morphological characteristics of P. ostreatus strains fruit bodies and clusters: strain 2251 (a1-2); strain 2292 (b1-2); strain 2301 (c1-2).
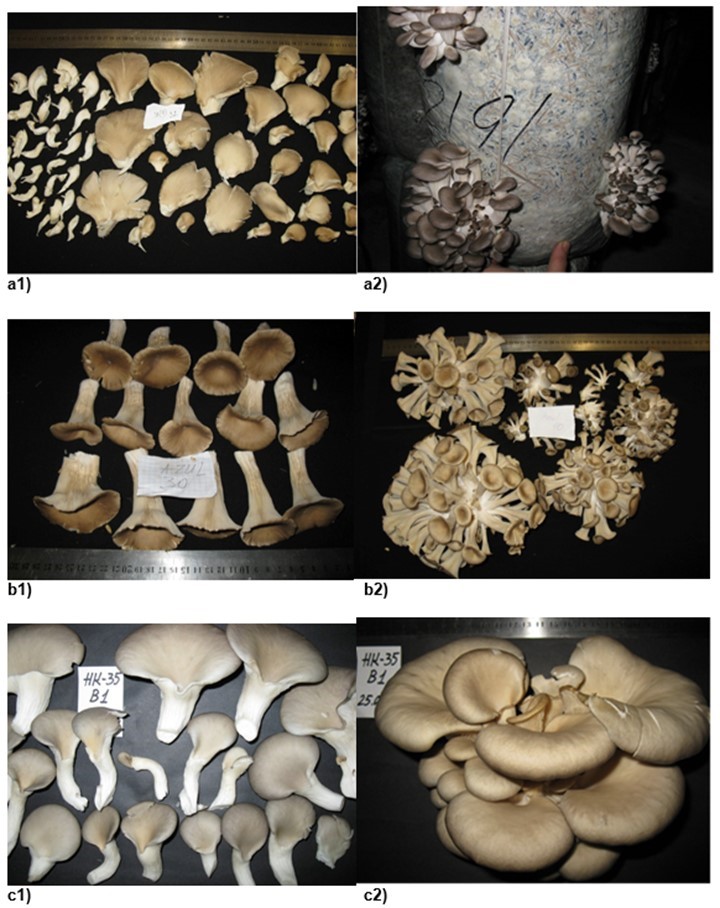
Fig. 2. The morphological characteristics of P. ostreatus strains fruit bodies and clusters: strain 2316 (a1-2); strain 2318 (b1-2); strain HK35 (c1-2).
The vegetative growth of HK-35 strain did not result in fruiting under the critical summer temperature after 40 days of the experiment. Therefore, we stopped evaluating this strain at that time. The strains of P. pulmonarius exhibited no significant differences (p≥0.05) during the vegetative growth stage (Table 2). The beginning of substrate colonization by the spawn was recorded on the second day of incubation for all strains. The colony diameter around all grain spawns was 50±20 mm on the third day of cultivation. Despite their similar developmental stage, the hyphae formed by strain 2314 were thinner compared with the other strains. Strain HK-35 was compared to the first cycle of the study. Consequently, the spawn run was found to be denser and thicker than the other studied strains. Full colonization of substrate bags by mycelia were recorded for all strains after seven days of cultivation. All bags had a bright white color with single glassy drops of metabolic liquid. The only substrate block with a more grayish shade was strain 2314.
After 10 days of cultivation, strain 2314 had formed sclerotia. The morphogenesis of the other strains occurred very rapidly in the absence of sclerotium formation. The first clusters of mature fruit bodies were harvested after 11 to 13 days subsequent to the substrate incubation. The duration of this developmental stage was not significantly different among the strains and was approximately one day. The vegetative growth rate was fastest in strain 2314 and slowest in strain 708. The whole technological cycle (vegetative growth, fruit body development, and fruiting flush) was two times shorter in strains 2314 and 668 (12 to 13 days). Compared with the other variants assessed herein, strain 708 had the longest cycle of 28 days. Therefore, the data for vegetative growth, fruit body development, and fruiting pattern were combined to derive a general technological cycle to estimate the usability of each particular strain for commercial production.
In contrast to vegetative growth, the biological efficiency of all strains overall was low, with the exception of strain 2314 (Table 2). Strain 2314 had 62% efficiency over an average of three cycles of growth, which is almost 2 to 5.5 times higher than the other strains. The efficiency of mushroom growth should be lower than 40% for industrial cultivation (personal communication with mushroom growers). The efficiency of harvesting depends on the duration of fruiting. The shortest fruiting time (three days) was observed in all P. pulmonarius strains with the exception of strain 537 (10 days). The biological efficiency of strain 2314 was 1.8 to 5.7 times higher than the other strains. Based on these data, it is suggested that strain 2314 of P. pulmonarius could prospectively be introduced into commercial culture (Table 2). In general, the present data are similar to other studies examining the cultivation of different Pleurotus species despite differences in substrate, locality, or cultivation techniques (Moonmoon et al. 2010; Pathmashini L. et al. 2008; Uddin et al. 2010; Szarvas et al. 2011).
The variability of the experimental data were estimated using univariate analysis. Levene’s test for comparing the homogeneity of the population variance showed a significant difference (p≤0.05 for all variables) within each group (strain) for all variables (columns) in Table 2. This result was confirmed using the robust Welch test for all groups (strains) to reject any assumption concerning the presence of relatively equal variance between measurements (p≤0.05 for all variables). This result was confirmed using the robust Welch test for all groups (strains) to reject any assumption concerning the presence of relatively equal variance between measurements. According to the non-parametric Mann-Whitney U test, the distribution differed significantly within the P. ostreatus and the P. pulmonarius strains (p≤0.05), with the exception of the cap height (p=0.198) and stipe width (p=0.237). The generative stage morphology is described below.
Morphological Characteristics
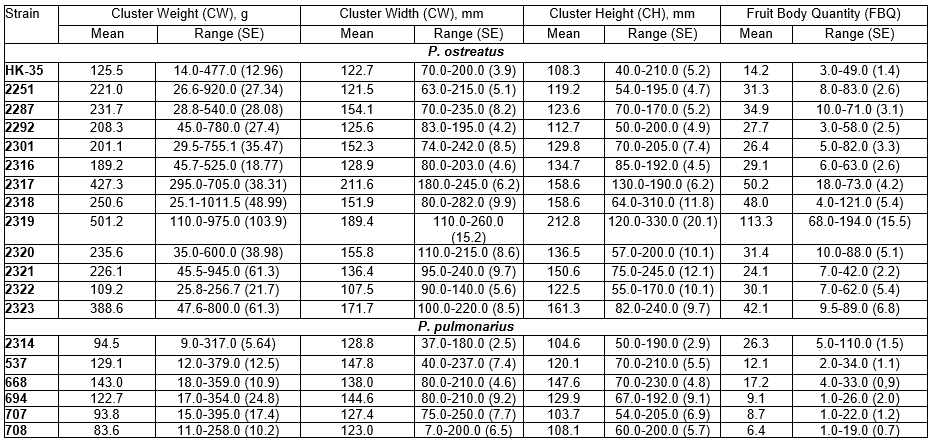
The morphological characteristics of fruit bodies have a significant influence on harvesting and packing routines. Table 3 shows the properties of the studied Pleurotus strains during the formation of the fruit body clusters. Multiple comparisons of clusters were performed using the two-sided Dunnett’s test (Dunnett 1955) and are presented in Table 3 using strain HK-35 as a control. Differences were noted with respect to the cluster weight in P. ostreatus strains 2251 (p=0.022), 2287 (p=0.032), 2317, 2318, 2319, and 2323 (p=0.001). The Dunnett’s T3 test for multiple comparisons was performed for the smallest clusters in strain 2322, and the results revealed no statistically significant differences in comparison to strains HK-35 (p=0.808), 2301 (p=0.377), 2320 (p=0.171), and 2321 (p=0.435) (Table 3). The test for strain 2319 with the highest fruit body cluster weight revealed no significant differences compared with strains 2251, 2287, 2292, 2301, 2317, 2318, 2320, and 2323 (p>0.05). The index for strain 2316 differed only from strains 2317, 2319, and 2322 (p<0.05).
The measurements for the cluster sizes (width and height) revealed a larger width compared with height for strains 2251, 2287, 2292, 2301, 2317, and HK-35 than for the other P. ostreatus strains. Dunnett’s test for multiple comparisons for strains 2251, 2292, 2316, 2321, and 2322, which formed clusters with widths up to 140 mm, did not indicate any differences in comparison to strain HK-35, while the other strains were significantly different in terms of the cluster width from 152 mm (2301 and 2318) to 212 mm (strain 2317). Thus, strains 2251, 2287, 2292, 2301, 2316, and 2320 grown at 11±2°C and with weights ranging from 200 to 250 g and a compact cluster size, were best suited for technologies in which the harvested mushrooms are immediately placed in their packaging boxes for sale, which represents a clear trend in global mushroom production aimed at higher standards from the consumer perspective.
Further characterization of P. ostreatus strains was conducted using Duncan’s multiple range post hoc test (Duncan 1955). The largest cluster width was measured in strain 2317, which was significantly different from the other strains and formed a homogeneous subset. The strains were also grouped according to the cluster height. With a height of 201 mm, strain 2319 differed significantly from the other strains and formed a single homogeneous subset.
Other strains had cluster height measurements ranging from 108 to 137 mm. In contrast, growth at a low temperature (11±2°C) resulted in the largest cluster size in strains 2317 and 2319. Thus, the use of these two strains is limited due to the need for pre-sale processing for commercial distribution in countries like Ukraine.
In summary, morphological characterization of P. ostreatus cluster-forming properties revealed the lowest variability in strain 2317 and the highest in strain 2301. Thus, a search for other climatic conditions is needed to determine an optimal temperature range for the development of fruit bodies specific for pleurotoid mushrooms (Boddy and Frankland 2008).
The cluster weight for P. pulmonarius assessed by Dunnett’s T3 test demonstrated no differences among the strains with the exception of strain 668 (p=0.027). The variability in cluster weight in P. pulmonarius ranged from 54% (strain 668) to 95% (strain 707). The cluster width was equivalent for all P. pulmonarius strains, and the cluster height was significantly different for strain 668 (p=0.001). The stability of the fruit body morphological parameters was very significant for commercial production. Assessment of fruit bodies parameters allows the use of standard packaging material during harvesting and pre-sale packing, and efficiently simplifies further mushroom processing.
Fruit Body Quantity
The quantity of fruit bodies in the cluster is particularly important for canning and further processing of the harvested mushrooms. Based on an evaluation of the data by Dunnett’s (2-sided) test, there were significant differences in the quantity of fruit bodies between almost all strains compared with strain HK-35 as a control, excluding strain 2321 (p=0.111). The largest number of fruit bodies was estimated for strain 2319 and was 8.0 times higher than that for strain HK-35 and 2.3 times higher than the average for groups 2317, 2318, and 2323, for which the quantity of fruit bodies varied from 42 to 50 (Table 3). It is interesting that the number of fruit bodies obtained in this study was significantly higher than that in similar studies conducted in Bangladesh on various substrates, potentially due to the differences in their counts (Mondal et al. 2011). However, studies conducted in Ethiopia have reported an average quantity of fruit bodies similar to the present results for most strains (Girmay et al. 2016).
Table 3. Characteristics of Pleurotus spp. Fruit Body Clusters
Characteristics of the Fruit Bodies
The fruit body parameters are presented in Table 4. Duncan’s multiple range post hoc test (Duncan 1955) revealed a significant difference for strain 2322 in fruit body weight and did not exceed 3 g. Strains 2251, 2287, 2292, 2317, and 2319 did not significantly differ in terms of fruit body weight, and this parameter varied from 6 to 9 g but was significantly different from the other group of strains HK-35, 2301, 2316, 2318, and 2323, for which the fruit body weight ranged from 9 to 11 g (Table 4). Strains 2320 (15 g) and 2321 (14.7 g) did not display significant differences between each other but were significantly different from the other strains and produced a fruit body weight more than five times greater than that of strain 2322 with the lowest fruit body weight. The average fruit body weight of strain 2320 was 1.4 times higher than that of strain HK-35.
Analysis of the cap width (Table 4) revealed the significantly lowest average for strain 2322 (27.4 mm). The other strains were divided into three groups according to the size of their cap width:
- up to 50 mm (2251, 2287, 2292, 2316, 2317, 2318, 2319);
- from 50 to 60 mm (HK-35, 2301, 2323);
- greater than 60 mm (2320 and 2321).
The average cap width in the third group was 2.5 times greater than that in the smallest strain 2322, and up to 1.7 times higher in comparison to the first group (≤50 mm). The highest value was found for strain 2320, which was 9.5 mm wider than strain HK-35 (Table 4).
The Pleurotus strains were divided into groups to evaluate parameters of fruit body weight and size. Based on the fruit body parameters, the strains were further divided into two groups: the first group had a soft texture and medium-sized fruit bodies (2287, 2301, and 2317) but they required particular conditions for cultivation; the second group had satisfactory morphological properties and taste, and they also had good growing indexes such as the high rate of vegetative growth, resistance to contaminants and flexibility in terms of various substrate mixtures (strains 2251, 2292, 2316, 2321, 2322, and HK-35). Other low-temperature-growing strains assessed in the study had unsatisfactory properties. For example, strain 2320 had a good texture and fruit body size, as well as a pale pink cap color, which is valued by consumers in Asian countries (Osma et al. 2011).
The present observations of mushroom growth at high temperatures revealed significant differences in the weight and width of fruit bodies for strain 2314 (p=0.001) compared with other strains (537, 668, 694, 707, and 708) (Fig. 3). There were no significant differences in fruit body weight among strains 537, 668, and 708. Strains 694 and 707 had significantly lower fruit body weights but did not differ in terms of cap width in comparison to strains 537, 668, and 708. The cap asymmetry for strains 668, 694, 707, and 708 was significantly
Table 4. Characteristics of the Fruit Bodies of Pleurotus Strains
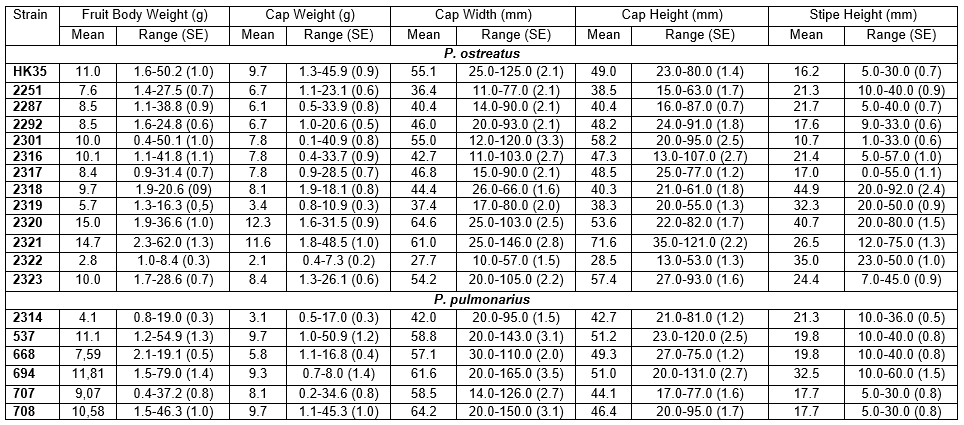

Fig. 3. The morphological characteristics of P. pulmonarius strain fruit bodies and clusters: (a1) strain 2314; (a2) strain 537; (b1) strain 668; (b2) strain 694; (c1) strain 707; (c2) strain 708.
greater that that for strains 2314 and 537 (1.2- to 1.5-fold) due to the prolonged fan-shaped form of the developed fruit bodies.
The weight of the obtained fruit bodies is one of the main parameters determining the success of the completely growing cycle to achieve mushrooms with sufficient quality. Mushrooms are sold by weight, which is therefore one of the main characteristics evaluated for mushroom strains (Jeznabadi et al. 2016). The variability in Pleurotus fruit body weight exceeded 33%, which indicated the heterogeneity of the population. This result might be explained by the influence of artificial conditions during industrial cultivation controlled by ventilation. Pleurotus clusters usually develop to provide free access to fresh air to all fruit bodies; therefore, the size and weight of fruit bodies gradually increased from the bottom to the top of their clusters. Strains 2287, 2301, and 2316 displayed fruit body weight variation exceeding 90% and had dense cluster structures.
The high temperature strains (Fig. 3) were nominally divided into two groups based on the pattern of fruit body development. In the first group, which contained strains 537, 668, 707, and 708, significant variability (no significant differences within the group) was observed in terms of the formation of mixed large and small sizes as well as unusually dark-colored fruit bodies. The second group, which included strains 2314 and 694, exhibited significantly smaller fruit body sizes and a lighter color (Fig. 3).
The fruit bodies of strains 2314 and 537 had the best organoleptic properties. The last one also had dark, dense caps and a pleasant mushroom aroma. To evaluate the high-temperature strains, all their characteristics were summarized: duration of technological maturation, biological efficiency, and qualitative characteristics of the clusters. Strain 2314 developed the most promising fruit bodies for industrial cultivation, while strain 537 might be successfully used for growing mushrooms with high marketing quality during the summer.
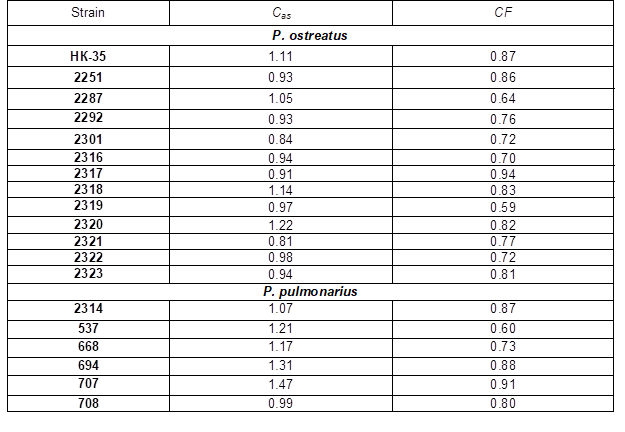
The structure and form of fruit bodies are parameters that influence the marketing properties of commercial mushroom strains. The EU market is focused on the consumption of mushrooms caps, while stipes and other parts of the cluster are usually processed into a powder as a flavoring for food products. Therefore, the conversion factor (CF) of the total weight yield to the obtained cap weight could serve as an additional characteristic that simplifies the evaluation of new Pleurotus strain marketing properties. Instead of the generally accepted measurement of the cap diameter according to the UNECE FFV-24 standard (2012), which is almost unusable for pleurotoid mushrooms due to their elongated fruit body form, we designed an asymmetry index (Cas) as a better application for the evaluation of new Pleurotus strains. The index can be determined by the ratio of the cap width to the cap height and defines the form of the fruit body. If Cas>1, the cap is expanded against the stipe; if Cas=1, the cap is symmetrically round; if Cas<1, the cap is elongated to approximate an ear shape. The CF and Cas indexes are presented in Table 5.
Strains HK-35, 2287, 2318, 2320 had a tendency to develop caps with a more expanded shape (Cas>1), while the fruit bodies of strains 2301 and 2321 were the most ear-like (Cas<0.9). Strains 2319 and 2322 had a rounded cap shape. The index of conversion of total yield weight (CF) to cap weight allows the determination of the mushroom weight to be sold on European markets. The minimum weight loss was obtained for strain 2317 (CF=0.94).
The fruit bodies of strain 2319 (CF=0.59) had long stipes, spoiling the fruit body appearance. Recalculation of the total biological efficiency to determine the biological efficiency of cap production reduced the initial index of strain 2319 from 81 to 48%. This strain might be of interest for the processing industry. The low-temperature growing conditions decreased the physiological activity of the mushroom culture. Therefore, at this temperature, the strain developed smaller fruit bodies in comparison to its optimal growing parameters (Yakovlev et al. 2000). In this case, most strains grown at a low temperature had satisfactory marketing properties due to the high conversion factor. The exceptions were strains 2319 and 2322, which had smaller caps and, consequently, the lowest conversion factor index of fruit body weight to caps.
Table 5. Conversion Factor (CF) and Asymmetry of the Fruit Body Cap (Cas)
CONCLUSIONS
- To assess prospective oyster mushroom strain properties, strains with properties superior to the commercial analogue HK-35 were selected. Three groups of ostreatus strains displayed differences in response to climatic and substrate conditions. One strain of P. pulmonarius was suitable for indoor commercial cultivation during the summer. The biological efficiency of the selected strains 2251, 2292, 2316, 2319, 2320, and 2314 were superior to HK-35 by 1.9- to 3.1-fold, which suggested that those strains could be used commercially.
- The variability in vegetative growth, fruit body formation, and fruiting duration for the studied strains opens possibilities for flexible technological growth cycles that could be focused either on fresh or processed mushroom product development.
- Based on the morphological characterization of low-temperature strains, the best marketing properties were found for strains 2251, 2292, 2301, 2321, and 2323, while strains 2319 and 2320 satisfied processing industry requirements with the maximal efficiency. Strains 2287 and 2317 had high quality fruit bodies but required higher cultivation temperatures. Strain 2318 might be attractive for some consumers due to the unusual fruit body formation.
- Among the “summer” group of strains, the speed of technological maturation and biological efficiency, qualitative features of the clusters and fruit bodies for industrial cultivation were recorded for strain 2314, while strain 537 might be used to obtain mushrooms with high marketing quality during the summer.
- It was found that strain 2317 had the highest cap yield and could satisfy EU market requirements.
- The proposed assessment procedure allows the primary selection of new oyster strains for introduction into commercial culture. However, a multivariate analysis of mushroom properties for pattern recognition in assessment procedures must be developed to confirm this statement.
ACKNOWLEDGMENTS
This work was supported financially by the Hubert H. Humphrey Fellowship Award administrated by the Bureau of Educational and Cultural Affairs, U.S. Department of the State, with the cooperation of the Institute of International Education (31110139). Other support was provided by the Visby Scholarship, administrated by the Swedish Institute (00311/2014), and research programs from Tavria State Agrotechnological University, funded by the Ministry of Agricultural Policy and Food of Ukraine (state registration 0107U008969 and 0111U002553). The authors thank Vija Wilkinson, Vitaliy Sevastyanovich, Antonina Gromakova, and Sergey Makogon for technical assistance and support, and Scarlett Geunes-Boyer and Felicia Anike for the help with our manuscript.
REFERENCES CITED
Ahmed, M., Abdullah, N., Ahmed, K. U., and Bhuyan, M. H. M. B. (2013). “Yield and nutritional composition of oyster mushroom strains newly introduced in Bangladesh,” Pesquisa Agropecuária Brasileira, 48(2), 197-202. DOI: 10.1590/S0100-204X2013000200010
Bilaj, V. J. (1982). Metody Eksperimental‘noj Mikologii (Methods of Experimental Mycology), Naukova dumka, Kiev, Ukraine.
Boddy, L., and Frankland, J. C. (Eds.). (2008). Ecology of Saprotrophic Basidiomycetes, British Mycological Society symposia series, Elsevier, Acad. Press, Amsterdam.
Buhalo, A.S. (1990). “Suchasni tendentsiyi kulʹtivuvannya hribiv iz rodu Pleurotus (Current trends culturing fungi of the genus Pleurotus),” Ukrainskiy botanicheskiy Zhurnal, 47(2), 101-104.
Celik, Y., and Peker, R. (2009). “Benefit/cost analysis of mushroom production for diversification of income in developing countries,” Bulgarian Journal of Agricultural Science 15(3), 228-237.
Cohen, R., Persky, L., and Hadar, Y. (2002). “Biotechnological applications and potential of wood-degrading mushrooms of the genus Pleurotus,” Applied Microbiology and Biotechnology, 58(5), 582-594. DOI: 10.1007/s00253-002-0930-y
Collins, S. R., Wellner, N., Bordonado, I. M., Harper, A. L., Miller, C. N., Bancroft, I., and Waldron, K. W. (2014). “Variation in the chemical composition of wheat straw: the role of tissue ratio and composition,” Biotechnology for Biofuels 7(1), 121. DOI: 10.1186/s13068-014-0121-y
Dahmardeh, M., Dahmardeh, M., Hossienabadi, R., Safarpoor, H., and Majid Dahmardeh. (2010). “Comparative study on cultivation and yield performance of Pleurotus ostreatus (oyster mushroom) grown on different substrates (wheat straw and barley straw) and supplemented at various levels of spawn,” Journal of Food Agriculture and Environment, 8(3-4), 996-998.
Dudka, I. A. (1983). Vysshiye s”yedobnyye Bazidiomitsety v Poverkhnostnoy i Glubinnoye Kul’ture (Higher Edible Basidiomycetes in Surface and Submerged Culture), Naukova dumka, Kiev.
Duncan, D. B. (1955). “Multiple range and multiple F tests,” Biometrics, 11(1), 1-42. DOI: 10.2307/3001478
Dunnett, C. W. (1955). “A multiple comparison procedure for comparing several treatments with a control,” Journal of the American Statistical Association 50(272), 1096-1121. DOI: 10.2307/2281208
Girmay, Z., Gorems, W., Birhanu, G., and Zewdie, S. (2016). “Growth and yield performance of Pleurotus ostreatus (Jacq. Fr.) Kumm (oyster mushroom) on different substrates,” AMB Express, 6(1). DOI: 10.1186/s13568-016-0265-1
DSTU 7786 (2015). Gry`by`. Gly`va zvy`chajna. Texnichni umovy. (Mushrooms. Pleurotus ostreatus. Specification), Ukrmetrteststandard, Kyiv, Ukraine.
Jeznabadi, E. K., Jafarpour, M., and Eghbalsaied, S. (2016). “King oyster mushroom production using various sources of agricultural wastes in Iran,” International Journal of Recycling of Organic Waste in Agriculture, 5(1), 17–24. DOI: 10.1007/s40093-015-0113-3
Miles, P. G., and Chang, S.-T. (2004). Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact, CRC Press, Boca Raton, FL, USA
Mondal, S., Rehana, J., Noman, M., and Adhikary, S. (2011). “Comparative study on growth and yield performance of oyster mushroom (Pleurotus florida) on different substrates,” Journal of the Bangladesh Agricultural University 8(2). DOI: 10.3329/jbau.v8i2.7928
Moonmoon, M., Uddin, M. N., Ahmed, S., Shelly, N. J., and Khan, M. A. (2010). “Cultivation of different strains of king oyster mushroom (Pleurotus eryngii) on saw dust and rice straw in Bangladesh,” Saudi Journal of Biological Sciences 17(4), 341-345. DOI: 10.1016/j.sjbs.2010.05.004
Moore, D., Gange, A. C., Gange, E. G., and Boddy, L. (2008). “Chapter 5. Fruit bodies: Their production and development in relation to environment,” in: British Mycological Society Symposia Series, Elsevier, Amsterdam, Netherlands, pp. 79-103.
Nakasone, K. K., Peterson, S. W., and Jong, S.-C. (2004). “Preservation and distribution of fungal culture,” in: Biodiversity of Fungi: Inventory and Monitoring Methods, M.s S. Foster, G. F. Bills, and G. M. Mueller (eds.), Academic Press, Cambridge, MA, USA, pp. 37-47.
Nurmetov, R., and Devochkina, N. (2010). Growing Champignon and Oyster Mushroom. Manual, Russian Academy of Agricultural Sciences, Moscow.
Oei, P. (2003). Mushroom cultivation: appropriate technology for mushroom growers, Backhuys, Leiden.
Oei, P., Nieuwenhuijzen, B. van, Feijter, J. de, and Zylva, N. de. (2005). Small-scale Mushroom Cultivation: Oyster, Shiitake and Wood Ear Mushrooms, Agromisa; CTA, Wageningen; Wageningen.
Osiewacz, H. D. (2002). “Aging in fungi: Role of mitochondria in Podospora anserina,” Mechanisms of Ageing and Development 123(7), 755-764. DOI: 10.1016/S0047-6374(01)00421-3
Osma, J. F., Moilanen, U., Toca-Herrera, J. L., and Rodríguez-Couto, S. (2011). “Morphology and laccase production of white-rot fungi grown on wheat bran flakes under semi-solid-state fermentation conditions,” FEMS Microbiology Letters 318(1), 27-34. DOI: 10.1111/j.1574-6968.2011.02234.x
Owaid, M. N. (2014). “Growth performance and cultivation of four oyster mushroom species on sawdust and rice bran substrates,” Journal of Advances in Biotechnology 4(3), 424-429.
Pathmashini, V. A., Arulnandhy V., and Wilson, W. R. S. (2008). “Cultivation of oyster mushroom (Pleurotus ostreatus) on sawdust,” Ceylon Journal of Science (Biological Sciences), 37, 177-182.
Pawlik, A., Janusz, G., Koszerny, J., Małek, W., and Rogalski, J. (2012). “Genetic diversity of the edible mushroom Pleurotus sp. by amplified fragment length polymorphism,” Current Microbiology 65(4), 438-445. DOI: 10.1007/s00284-012-0175-7
Rao, V. R., Brown, A. H. D., and Jackson, M. (2001). Managing Plant Genetic Diversity, CABI Publishing, Oxford.
Royse, D. J. (2002). “Influence of spawn rate and commercial delayed release nutrient levels on Pleurotus cornucopiae (oyster mushroom) yield, size, and time to production,” Applied Microbiology and Biotechnology 58(4), 527-531. DOI: 10.1007/s00253-001-0915-2
Royse, D. J. (2014). “A global perspective on the high five: Agaricus, Pleurotus, Lentinula, Auricularia and Flammulina,” in: Proceedings of the 8th International Conference on Mushroom Biology and Mushroom Products, New Delhi, India, p. 1-6.
Szarvas, J., Pal, K., Geosel, A., and Gyorfi, J. (2011). “Comparative studies on the cultivation and phylogenetics of king oyster mushroom (Pleurotus eryngii (DC.: Fr.) Qu´el.) strains,” Acta Universitatis Sapientiae Agriculture and Environment, 3, 18-34.
Tao, G., Lestander, T. A., Geladi, P., and Xiong, S. (2012). “Biomass properties in association with plant species and assortments I: A synthesis based on literature data of energy properties,” Renewable and Sustainable Energy Reviews 16(5), 3481-3506. DOI: 10.1016/j.rser.2012.02.039
Uddin, M. N., Yesmin, S., Khan, M. A., Tania, M., Moonmoon, M., and Ahmed, S. (2010). “Production of oyster mushrooms in different seasonal conditions of Bangladesh,” Journal of Scientific Research 3(1), 161. DOI: 10.3329/jsr.v3i1.6130
UNECE standard FFV-24 (2012). “Concerning the marketing and commercial quality control of cultivated mushrooms,” Working Party on Agricultural Quality Standards of the United Nations Economic Commission for Europe (UNECE), Geneva, Switzerland, (http://www.unece.org/fileadmin/DAM/trade/agr/standard/fresh/FFV-Std/English/24Cultivated_mushrooms_2012.pdf)
Xiong, S., Zhang, Y., Zhuo, Y., Lestander, T., and Geladi, P. (2010). “Variations in fuel characteristics of corn (Zea mays) stovers: General spatial patterns and relationships to soil properties,” Renewable Energy 35(6), 1185-1191. DOI: 10.1016/j.renene.2009.11.032
Yakovlev, A. U., Borovskiy, G .B., Penzina, T. A., Petrov, A. N., and Voynikov, V. K. (2000). “Vliyaniye otritsatel’nykh temperatur na rost mitseliya i zhiznesposobnost’ plodovykh tel nekotorykh vysshikh ksilotrofnykh bazidiomitsetov (The impact of negative temperatures on the growth of mycelium and viability of fruiting bodies of some higher xylotrophic Basidiomycetes),” Mycology and Phytopathology 6(34), 56-63.
Yang, W., Guo, F., and Wan, Z. (2013). “Yield and size of oyster mushroom grown on rice/wheat straw basal substrate supplemented with cotton seed hull,” Saudi Journal of Biological Sciences, 20(4), 333-338. DOI: 10.1016/j.sjbs.2013.02.006
Zied, D. C., Savoie, J.-M., and Pardo-Giméne, A. (2011). “Soybean, the main nitrogen source in cultivation substrates of edible and medicinal mushrooms,” in: Soybean and Nutrition, 22, PHE-S (ed.), inTech, pp. 433-452.
Article submitted: August 17, 2016; Peer review completed: October 24, 2016; Revised version accepted: April 27, 2017; Published: May 9, 2017.
DOI: 10.15376/biores.12.3.4606-4626
